Nitrogen Source Preference in Maize at Seedling Stage Is Mainly Dependent on Growth Medium pH
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Collection
2.2. Soil Culture Experiment
2.3. Hydroponic Experiment
2.4. Analytical Methods
2.5. Calculation Methods
2.6. Statistical Analyses
3. Results
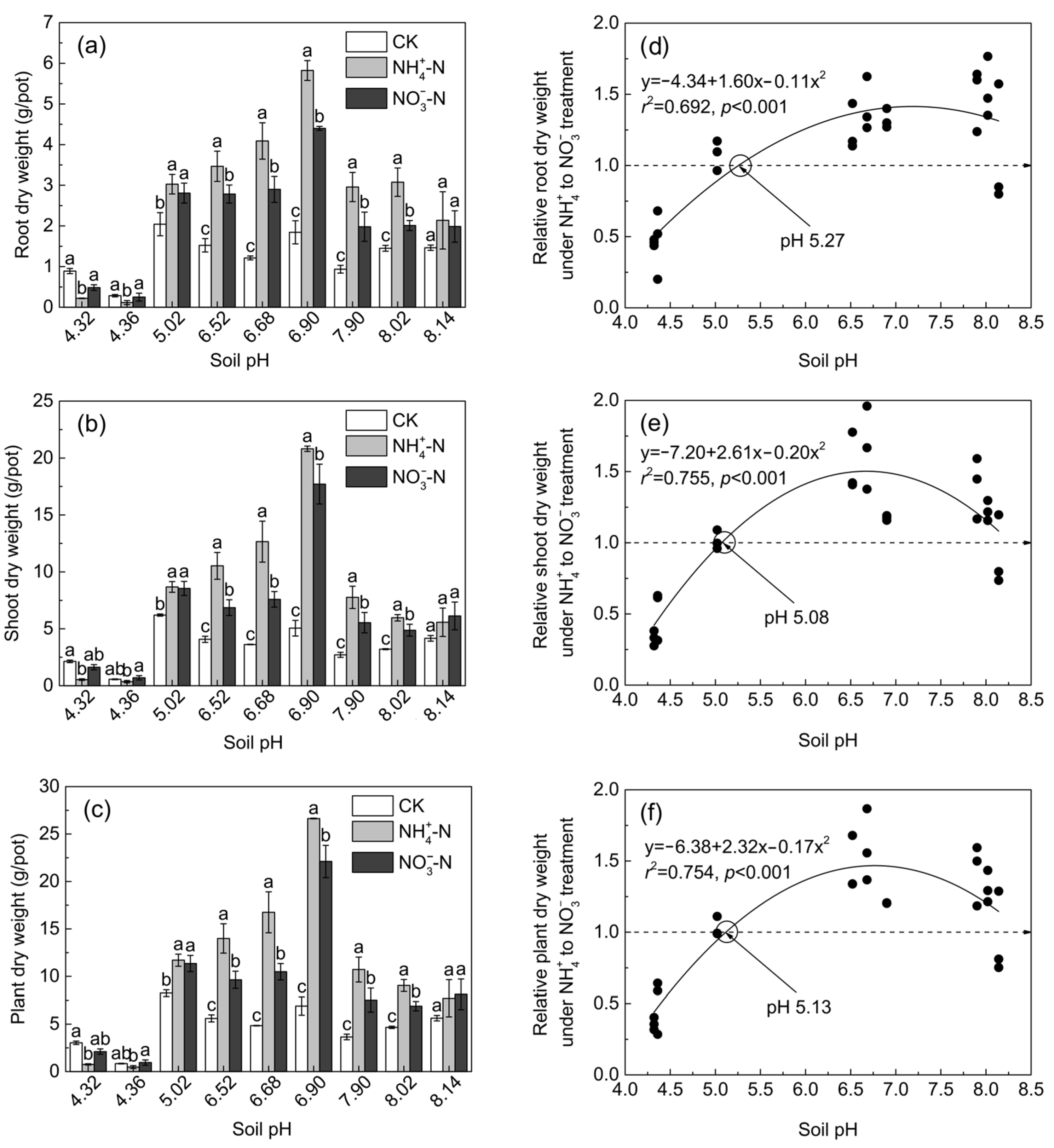
3.1. Maize Growth in Soil Culture
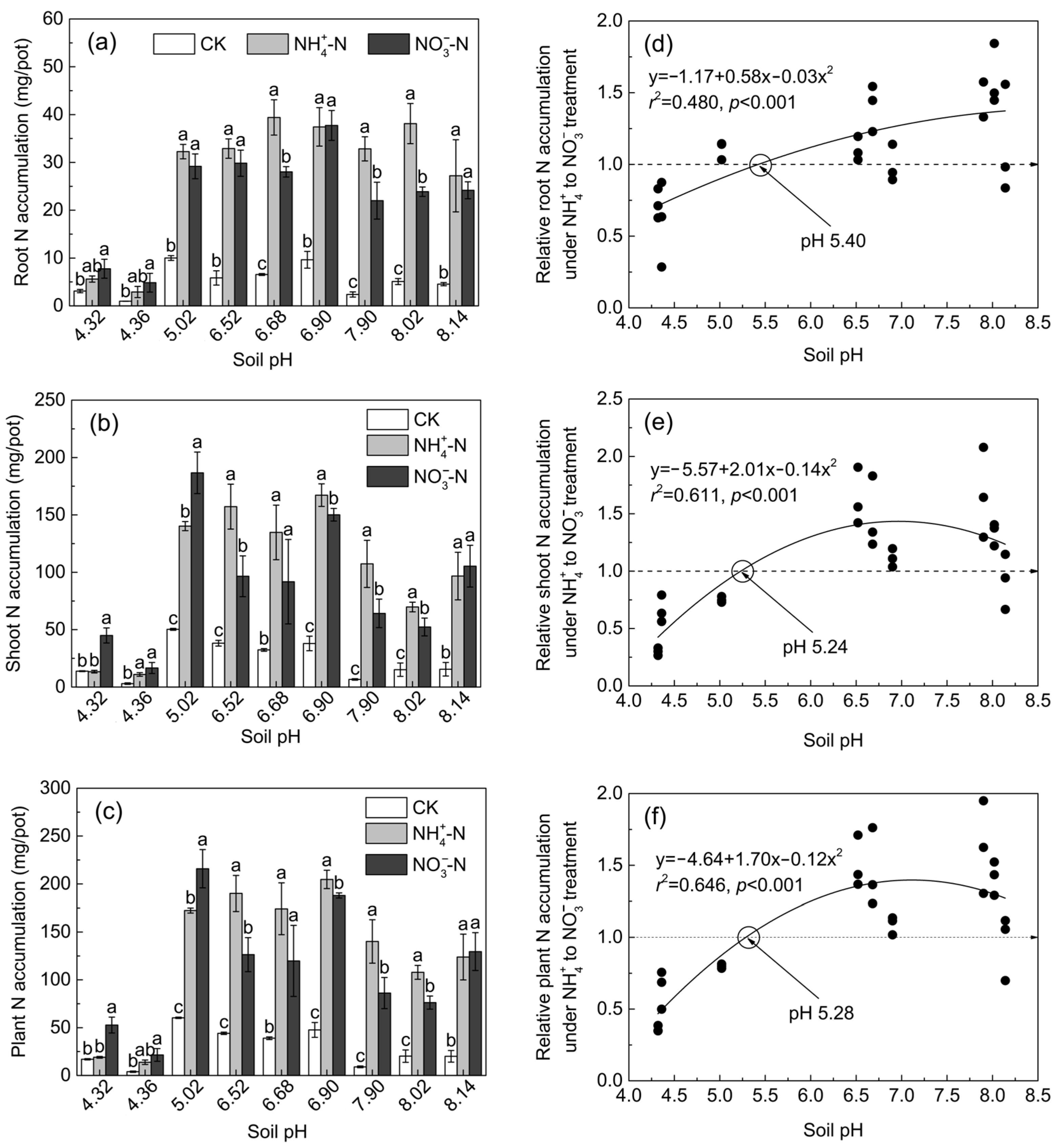
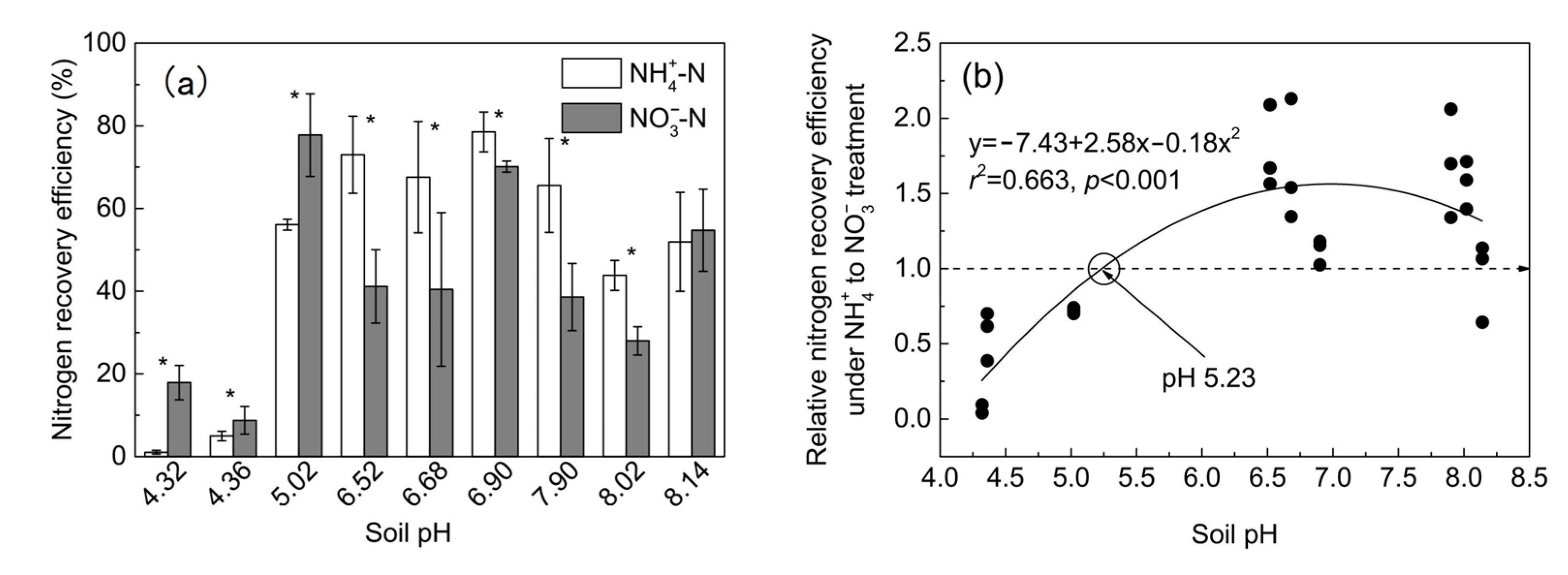
3.2. Nitrogen Accumulation and NRE of Maize in Soil Culture
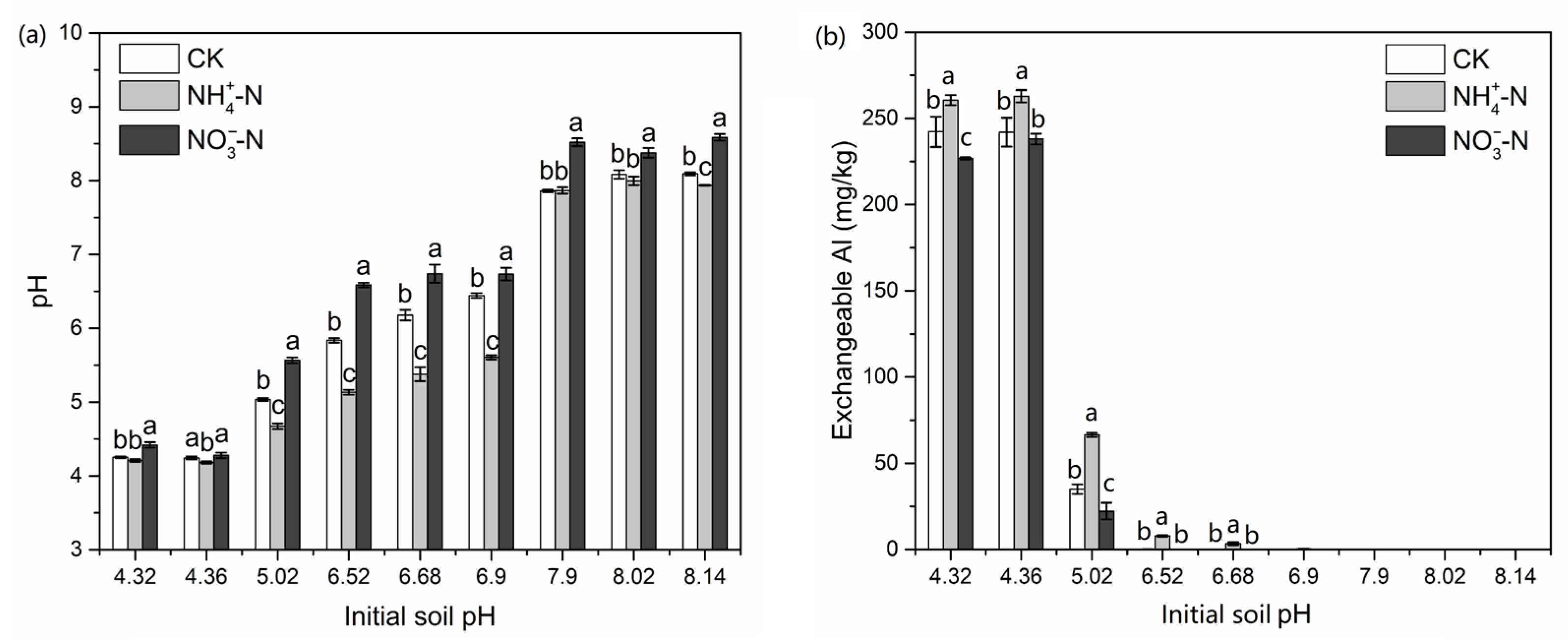
3.3. Soil pH and Exchangeable Aluminum Content
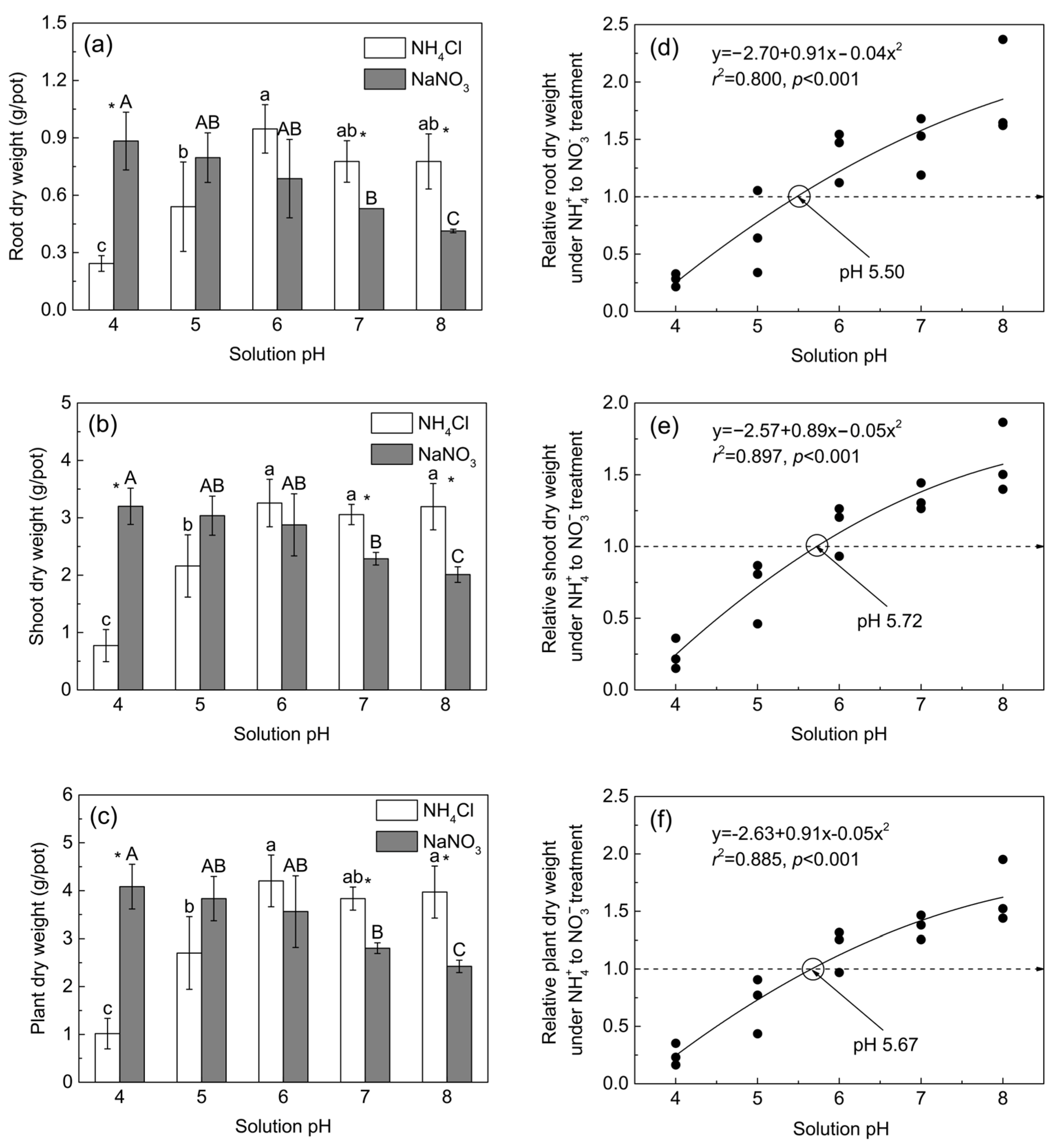
3.4. Maize Growth in the Hydroponic Experiment
3.5. Nitrogen Accumulation by Maize in the Hydroponic Experiment
4. Discussion
4.1. Growth and N Accumulation of Maize under Ammonium and Nitrate Treatments at Different pHs
4.2. The Critical pH Changing the N Accumulation and Growth Preference of Maize for NH4+ and NO3−
4.3. Implications for Improvement of Maize NRE and Growth
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ladha, J.K.; Tirol-Padre, A.; Reddy, C.K.; Cassman, K.G.; Verma, S.; Powlson, D.S.; van Kessel, C.; de BRichter, D.; Chakraborty, D.; Pathak, H. Global nitrogen budgets in cereals: A 50-year assessment for maize, rice, and wheat production systems. Sci. Rep. 2016, 6, 19355. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Xu, M.; Wang, B.; Yang, X.; Huang, S.; Gao, S. Long-term evaluation of manure application on maize yield and nitrogen use efficiency in China. Soil Sci. Soc. Am. J. 2011, 75, 1562–1573. [Google Scholar] [CrossRef]
- Zhang, X.; Davidson, E.A.; Mauzerall, D.L.; Searchinger, T.D.; Dumas, P.; Shen, Y. Managing nitrogen for sustainable development. Nature 2015, 528, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Chi, Q.; Cheng, Y.; Zhu, B.; Li, W.; Zhang, X.; Huang, Y.; Müller, C.; Cai, Z.; Zhang, J. Importance of matching soil N transformations, crop N form preference, and climate to enhance crop yield and reducing N loss. Sci. Total Environ. 2019, 657, 1265–1273. [Google Scholar] [CrossRef]
- Zhang, J.; Cai, Z.; Müller, C. Terrestrial N cycling associated with climate and plant-specific N preferences: A review. Eur. J. Soil Sci. 2018, 69, 488–501. [Google Scholar] [CrossRef]
- Britto, D.T.; Kronzucker, H.J. Ecological significance and complexity of N-source preference in plants. Ann. Bot. 2013, 112, 957–963. [Google Scholar] [CrossRef]
- Zhao, X.Q.; Shen, R.F. Aluminum–nitrogen interactions in the soil–plant system. Front. Plant Sci. 2018, 9, 807. [Google Scholar] [CrossRef]
- Kováčik, P.; Wiśniowska-Kielian, B.; Smoleń, S.; Škarpa, P.; Olšovská, K.; Urminská, J. Dependence of quantitative and qualitative parameters of radish yield on contents of ammonium and nitrate nitrogen in soil substrate. J. Ecol. Eng. 2021, 22, 68–77. [Google Scholar] [CrossRef]
- Claussen, W.; Lenz, F. Effect of ammonium or nitrate nutrition on net photosynthesis, growth, and activity of the enzymes nitrate reductase and glutamine synthetase in blueberry, raspberry and strawberry. Plant Soil 1999, 208, 95–102. [Google Scholar] [CrossRef]
- Ruan, J.; Gerendás, J.; Härdter, R.; Sattelmacher, B. Effect of nitrogen form and root-zone pH on growth and nitrogen uptake of tea (Camellia sinensis) plants. Ann. Bot. 2007, 99, 301–310. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.Q.; Guo, S.W.; Shinmachi, F.; Sunairi, M.; Noguchi, A.; Hasegawa, I.; Shen, R.F. Aluminium tolerance in rice is antagonistic with nitrate preference and synergistic with ammonium preference. Ann. Bot. 2013, 111, 69–77. [Google Scholar] [CrossRef]
- Cox, W.J.; Reisenauer, H.M. Growth and ion uptake by wheat supplied nitrogen as nitrate, or ammonium, or both. Plant Soil 1973, 38, 363–380. [Google Scholar] [CrossRef]
- Guo, J.; Jia, Y.; Chen, H.; Zhang, L.; Yang, J.; Zhang, J.; Hu, X.; Ye, X.; Li, Y.; Zhou, Y. Growth, photosynthesis, and nutrient uptake in wheat are affected by differences in nitrogen levels and forms and potassium supply. Sci. Rep. 2019, 9, 1248. [Google Scholar] [CrossRef]
- Ali, S.; Farooq, M.A.; Jahangir, M.M.; Abbas, F.; Bharwana, S.A.; Zhang, G.P. Effect of chromium and nitrogen form on photosynthesis and anti-oxidative system in barley. Biol. Plant. 2013, 57, 758–763. [Google Scholar] [CrossRef]
- Houdusse, F.; Garnica, M.; García-Mina, J.M. Nitrogen fertiliser source effects on the growth and mineral nutrition of pepper (Capsicum annum L.) and wheat (Triticum aestivum L.). J. Sci. Food Agric. 2007, 87, 2099–2105. [Google Scholar] [CrossRef]
- Wang, C.; Zheng, M.M.; Hu, A.Y.; Zhu, C.Q.; Shen, R.F. Diazotroph abundance and community composition in an acidic soil in response to aluminum-tolerant and aluminum-sensitive maize (Zea mays L.) cultivars under two nitrogen fertilizer forms. Plant Soil 2018, 424, 463–478. [Google Scholar] [CrossRef]
- Wang, J.L.; Zhao, X.Q.; Zhang, H.Q.; Shen, R.F. The preference of maize plants for nitrate improves fertilizer N recovery efficiency in an acid soil partially because of alleviated Al toxicity. J. Soils Sediments 2021, 21, 3019–3033. [Google Scholar] [CrossRef]
- Anderson, D.S.; Teyker, R.H.; Rayburn, A.L. Nitrogen form effects on early corn root morphological and anatomical development. J. Plant Nutr. 1991, 14, 1255–1266. [Google Scholar] [CrossRef]
- Zhang, H.Q.; Zhao, X.Q.; Chen, Y.L.; Zhang, L.Y.; Shen, R.F. Case of a stronger capability of maize seedlings to use ammonium being responsible for the higher 15N recovery efficiency of ammonium compared with nitrate. Plant Soil 2019, 440, 293–309. [Google Scholar] [CrossRef]
- Teyker, R.H.; Hobbs, D.C. Growth and root morphology of corn as influenced by nitrogen form. Agron. J. 1992, 84, 694–700. [Google Scholar] [CrossRef]
- Soon, Y.K.; Miller, M.H. Changes in the rhizosphere due to NH4+ and NO3− fertilization and phosphorus uptake by corn seedlings (Zea mays L.). Soil Sci. Soc. Am. J. 1977, 41, 77–80. [Google Scholar] [CrossRef]
- Barber, K.L.; Maddux, L.D.; Kissel, D.E.; Pierzynski, G.M.; Bock, B.R. Corn responses to ammonium- and nitrate-nitrogen fertilization. Agron. J. 1992, 56, 1166–1171. [Google Scholar] [CrossRef]
- Tang, C.; Conyers, M.K.; Nuruzzaman, M.; Poile, G.J.; Liu, D.L. Biological amelioration of subsoil acidity through managing nitrate uptake by wheat crops. Plant Soil 2011, 338, 383–397. [Google Scholar] [CrossRef]
- Bao, S.D. Soil Agrochemical Analysis; China Agricultural Press: Beijing, China, 2002. [Google Scholar]
- Zhang, H.Q.; Zhao, X.Q.; Shi, Y.; Liang, Y.; Shen, R.F. Changes in soil bacterial communities with increasing distance from maize roots affected by ammonium and nitrate additions. Geoderma 2021, 398, 115102. [Google Scholar] [CrossRef]
- Fageria, N.K.; Baligar, V.C. Enhancing nitrogen use efficiency in crop plants. Adv. Agron. 2005, 88, 97–185. [Google Scholar]
- Schubert, S.; Yan, F. Nitrate and ammonium nutrition of plants: Effects on acid/base balance and adaptation of root cell plasmalemma H+ ATPase. Z. Pflanzenernaehr. Bodenk. 1997, 160, 275–281. [Google Scholar] [CrossRef]
- Yan, F.; Schubert, S.; Mengel, K. Effect of low root medium pH on net proton release, root respiration, and root growth of corn (Zea mays L.) and broad bean (Vicia faba L.). Plant Physiol. 1992, 99, 415–421. [Google Scholar] [CrossRef]
- Schortemeyer, M.; Feil, B.; Stamp, P. Root morphology and nitrogen uptake of maize simultaneously supplied with ammonium and nitrate in a split-root system. Ann. Bot. 1993, 72, 107–115. [Google Scholar] [CrossRef]
- Li, S.X.; Wang, Z.H.; Stewart, B.A. Responses of crop plants to ammonium and nitrate N. Adv. Agron. 2013, 118, 205–397. [Google Scholar]
- Ma, Q.; Wu, L.; Cao, X.; Mi, W. Effects of pH and nitrogen forms of hydroponic nutrient solution on the growth, nitrogen absorption and edible quality of pakchoi (Brassica chinensis L.) (in Chinese). J. Soil Water Conserv. 2015, 29, 64–68. [Google Scholar]
- Jampeetong, A.; Konnerup, D.; Piwpuan, N.; Brix, H. Interactive effects of nitrogen form and pH on growth, morphology, N uptake and mineral contents of Coix lacryma-jobi L. Aquat. Bot. 2013, 111, 144–149. [Google Scholar] [CrossRef]
- Bloom, A.J.; Meyerhoff, P.A.; Taylor, A.R.; Rost, T.L. Root development and absorption of ammonium and nitrate from the rhizosphere. J. Plant Growth Regul. 2002, 21, 416–431. [Google Scholar] [CrossRef]
- Gu, R.; Duan, F.; An, X.; Zhang, F.; von Wirén, N.; Yuan, L. Characterization of AMT-mediated high-affinity ammonium uptake in roots of maize (Zea mays L.). Plant Cell Physiol. 2013, 54, 1515–1524. [Google Scholar] [CrossRef]
- Hao, D.L.; Zhou, J.Y.; Yang, S.Y.; Huang, Y.N.; Su, Y.H. Functional and regulatory characterization of three AMTs in maize roots. Front. Plant Sci. 2020, 11, 884. [Google Scholar] [CrossRef]
- Colombo, C.; Palumbo, G.; He, J.Z.; Pinton, R.; Cesco, S. Review on iron availability in soil: Interaction of Fe minerals, plants, and microbes. J. Soils Sediments 2014, 14, 538–548. [Google Scholar] [CrossRef]
- Hoffmann, C.; Ladewig, E.; Claassen, N.; Jungk, A. Phosphorus uptake of maize as affected by ammonium and nitrate nitrogen - Measurements and model calculations. Z. Pflanzenernähr Bodenk 1994, 157, 225–232. [Google Scholar] [CrossRef]
- Jing, J.; Zhang, F.; Rengel, Z.; Shen, J. Localized fertilization with P plus N elicits an ammonium-dependent enhancement of maize root growth and nutrient uptake. Field Crop Res. 2012, 133, 176–185. [Google Scholar] [CrossRef]
- Duan, Z.; Xiao, H. Effects of soil properties on ammonia volatilization. Soil Sci. Plant Nutr. 2000, 46, 845–852. [Google Scholar]
- Wang, J.; Tu, X.; Zhang, H.; Cui, J.; Ni, K.; Chen, J.; Cheng, Y.; Zhang, J.; Chang, S.X. Effects of ammonium-based nitrogen addition on soil nitrification and nitrogen gas emissions depend on fertilizer-induced changes in pH in a tea plantation soil. Sci. Total Environ. 2020, 747, 141340. [Google Scholar] [CrossRef]
- Pan, X.; Baquy, M.A.; Guan, P.; Yan, J.; Wang, R.; Xu, R.; Xie, L. Effect of soil acidification on the growth and nitrogen use efficiency of maize in Ultisols. J. Soils Sediments 2020, 20, 1435–1445. [Google Scholar] [CrossRef]
- Kochian, L.V.; Hoekenga, O.A.; Piñeros, M.A. How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorous efficiency. Annu. Rev. Plant Biol. 2004, 55, 459–493. [Google Scholar] [CrossRef]
- Dong, D.; Xie, Z.; Du, Y.; Liu, C.; Wang, S. Influence of soil pH on aluminum availability in the soil and aluminum in tea leaves. Commun. Soil Sci. Plan. 1999, 30, 873–883. [Google Scholar] [CrossRef]
- Ma, J.F. Syndrome of aluminum toxicity and diversity of aluminum resistance in higher plants. Int. Rev. Cytol. 2007, 264, 225–252. [Google Scholar]
- von Uexküll, H.R.; Mutert, E. Global extent, development and economic impact of acid soils. Plant Soil 1995, 171, 1–15. [Google Scholar] [CrossRef]
- Dewi-Hayati, P.K.; Sutoyo Syarif, A.; Prasetyo, T. Performance of maize single-cross hybrids evaluated on acidic soils. Int. J. Adv. Sci. Eng. Inf. Technol. 2014, 4, 30–33. [Google Scholar]
- Huang, S.; Zhang, W.J.; Yu, X.C.; Huang, Q.R. Effects of long-term fertilization on corn productivity and its sustainability in an Ultisol of southern China. Agr. Ecosyst. Environ. 2010, 138, 44–50. [Google Scholar] [CrossRef]
- Zhang, H.M.; Wang, B.R.; Xu, M.G.; Fan, T.L. Crop yield and soil responses to long-term fertilization on a red soil in southern China. Pedosphere 2009, 19, 199–207. [Google Scholar] [CrossRef]


| City | Sampling Site | pH | NH4+-N (mg/kg) | NO3−-N (mg/kg) | Available P (mg/kg) | Available K (mg/kg) |
|---|---|---|---|---|---|---|
| Yingtan | 116°55′48″ E 28°07′44″ N | 4.32 | 12.48 | 9.70 | 7.24 | 168.14 |
| 116°55′48″ E 28°12′07″ N | 4.36 | 4.70 | 3.13 | 0.08 | 54.57 | |
| 116°55′37″ E 31°43′14″ N | 5.02 | 19.37 | 19.13 | 12.75 | 124.74 | |
| Nanjing | 118°46′37″ E 31°15′38″ N | 6.52 | 4.37 | 3.13 | 19.49 | 104.81 |
| 118°46′37″ E 31°05′27″ N | 6.68 | 6.37 | 3.00 | 13.77 | 143.31 | |
| 118°46′37″ E 31°05′27″ N | 6.91 | 4.97 | 3.07 | 66.16 | 147.22 | |
| Yangling | 108°04′02″ E 34°15′48″ N | 7.90 | 3.36 | 13.99 | 9.17 | 138.43 |
| Baotou | 109°49′05″ E 40°39′53″ N | 8.02 | 3.62 | 20.79 | 10.60 | 71.19 |
| Fengqiu | 114°33′07″ E 35°01′14″ N | 8.14 | 4.39 | 32.28 | 19.03 | 86.63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.-Q.; Shen, R.-F.; Zhao, X.-Q. Nitrogen Source Preference in Maize at Seedling Stage Is Mainly Dependent on Growth Medium pH. Agronomy 2022, 12, 2149. https://doi.org/10.3390/agronomy12092149
Zhang H-Q, Shen R-F, Zhao X-Q. Nitrogen Source Preference in Maize at Seedling Stage Is Mainly Dependent on Growth Medium pH. Agronomy. 2022; 12(9):2149. https://doi.org/10.3390/agronomy12092149
Chicago/Turabian StyleZhang, Hao-Qing, Ren-Fang Shen, and Xue-Qiang Zhao. 2022. "Nitrogen Source Preference in Maize at Seedling Stage Is Mainly Dependent on Growth Medium pH" Agronomy 12, no. 9: 2149. https://doi.org/10.3390/agronomy12092149
APA StyleZhang, H.-Q., Shen, R.-F., & Zhao, X.-Q. (2022). Nitrogen Source Preference in Maize at Seedling Stage Is Mainly Dependent on Growth Medium pH. Agronomy, 12(9), 2149. https://doi.org/10.3390/agronomy12092149







