Estimation of the Enzymatic Activity of Haplic Chernozem under Contamination with Oxides and Nitrates of Ag, Bi, Te and Tl
Abstract
:1. Introduction
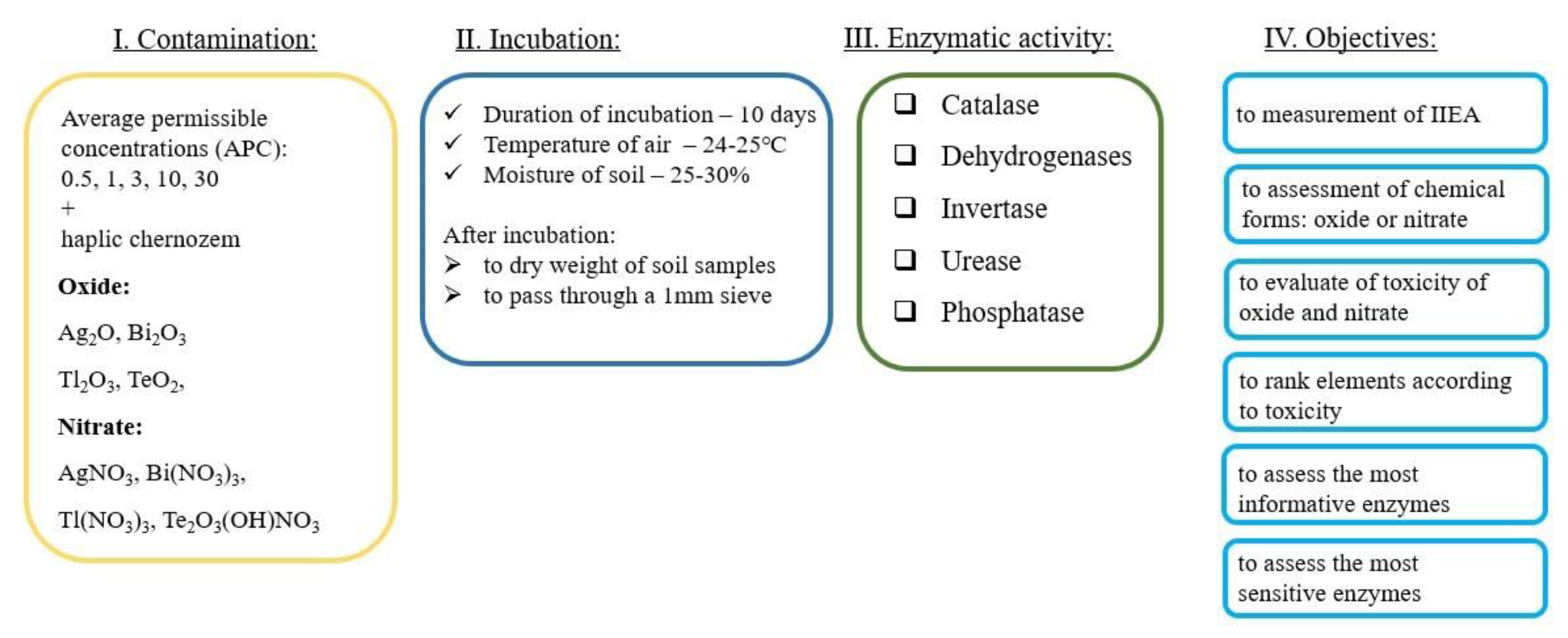
2. Materials and Methods
2.1. Soil Selection
2.2. Experiment Simulation
2.3. Methods for Assessing Enzymatic Activity
2.4. Measurement
2.5. Statistical Processing
3. Results
3.1. Changes in Activity of Oxidoreductases
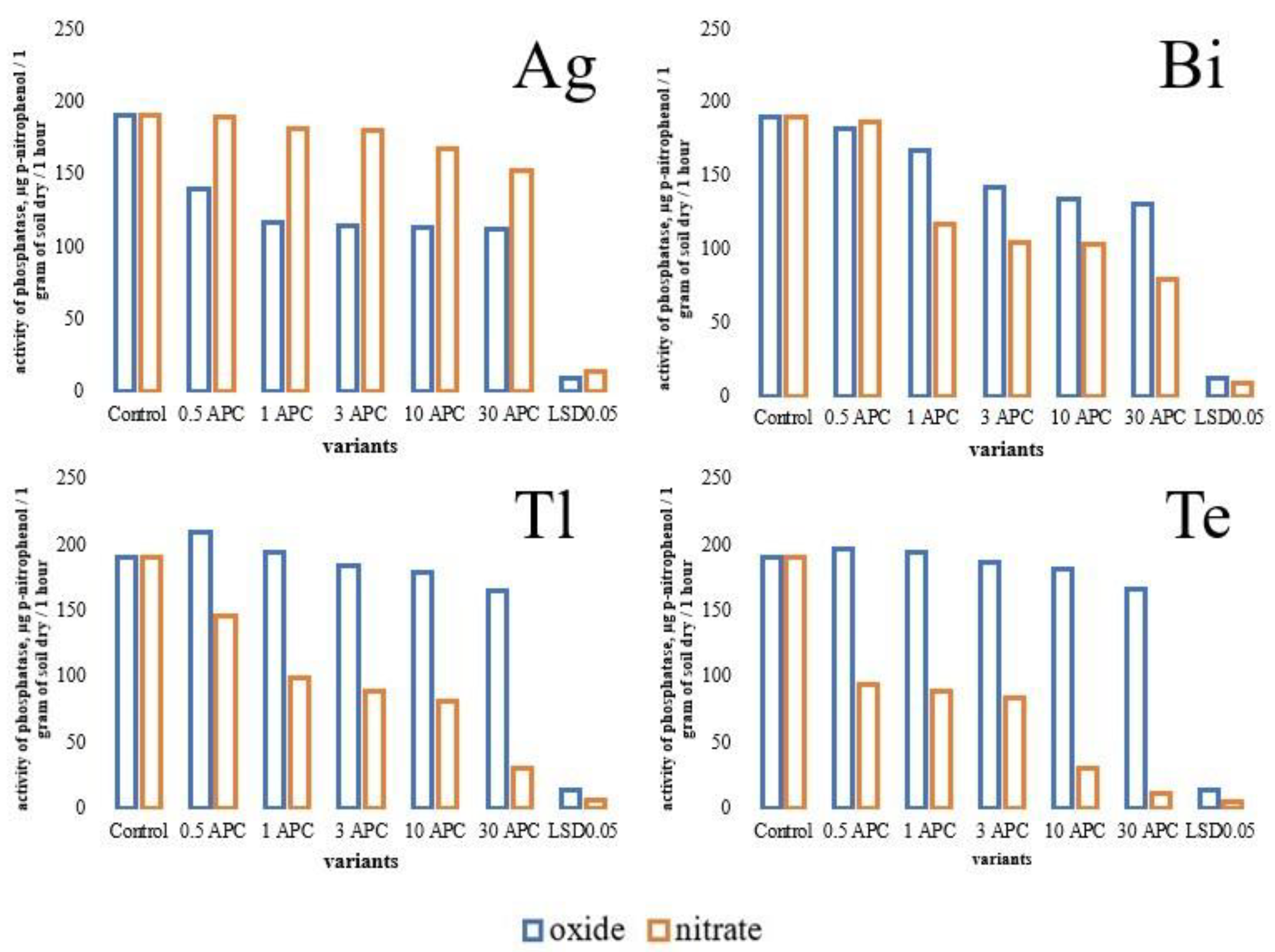
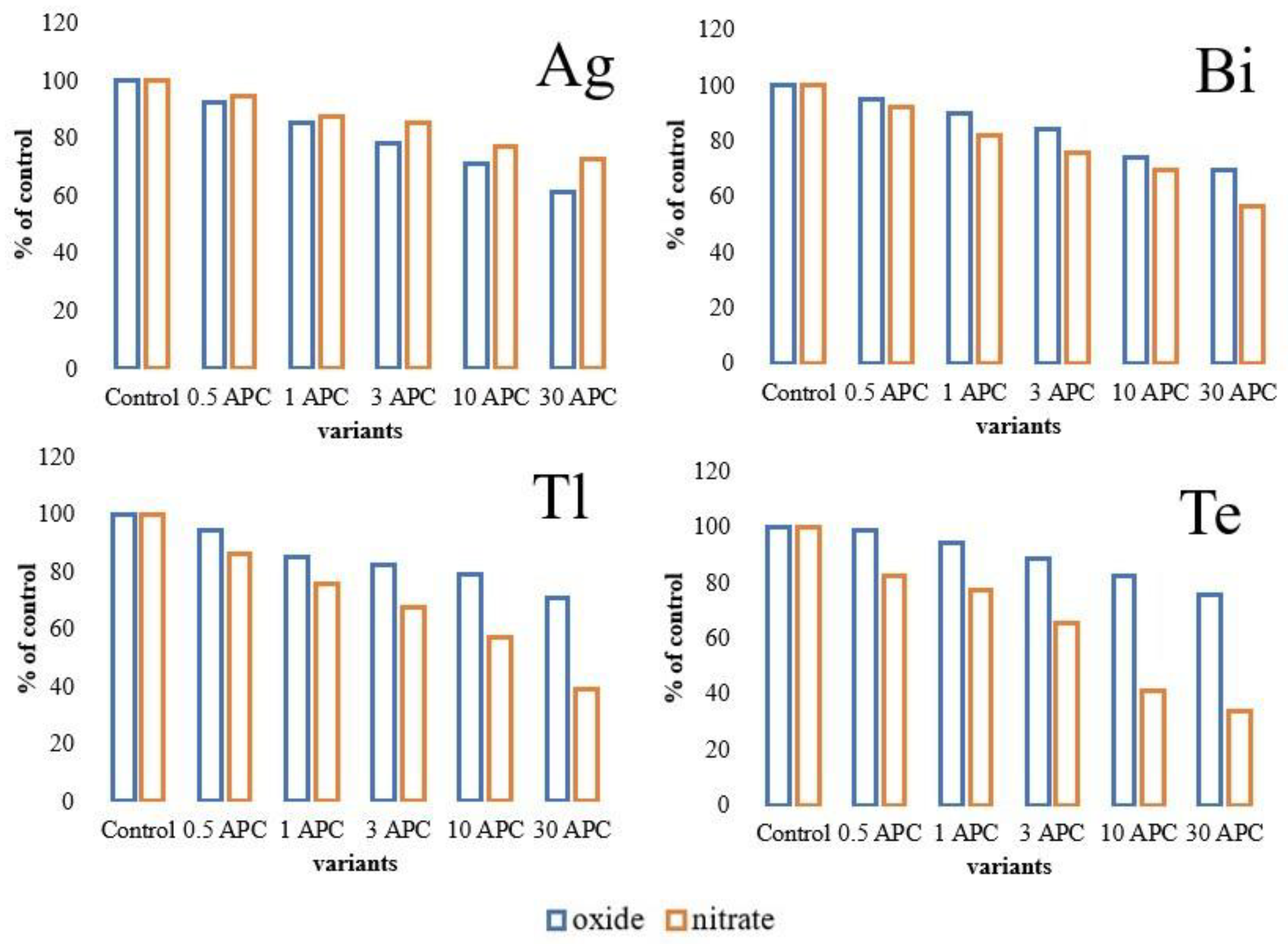
3.2. Changes in Hydrolase Activity
3.3. Changes in the Integral Index of Soil Enzymatic Activity (IIEA)
4. Discussion
4.1. Informative Value of Biological Indicators
4.2. Sensitivity of Biological Indicators
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Montanarella, L.; Ed, V.; Yagi, K.; Krasilnikov, P.; Alavi, P.; Seyed, K.; Mendonça Santos, M.; McKenzie, N.; Ed, D.; Nachtergaele, F. The Status of the World’s Soil Resources. In FAO: Chapter 4 Soils and Humans Main Report; FAO: Rome, Italy, 2015; pp. 50–87. [Google Scholar]
- Science Communication Unit, University of the West of England. Bristol (Science for Environment Policy in-Depth Report: Soil Contamination: Impacts on Human Health; Report produced for the European Commission DG Environment; University of the West of England: Bristol, England, 2013; pp. 3–29. [Google Scholar]
- Steffan, J.J.; Brevik, E.C.; Burgess, L.C.; Cerdà, A. The effect of soil on human health: An overview. Eur. J. Soil. Sci. 2018, 69, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Kolesnikov, S.I.; Kazeev, K.S.; Akimenko, Y.V. Development of regional standards for pollutants in the soil using biological parameters. Environ. Monit. Assess. 2019, 191, 544. [Google Scholar] [CrossRef] [PubMed]
- Husejnović, M.Š.; Janković, S.; Nikolić, D.; Antonijević, B. Human health risk assessment of lead, cadmium, and mercury co-exposure from agricultural soils in the Tuzla Canton (Bosnia and Herzegovina). Arh. Hig. Rada Toksikol. 2021, 72, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.; Mansour, S.N.; Najafi, M.L.; Toolabi, A.; Abdolahnejad, A.; Faraji, M.; Miri, M. Probabilistic risk assessment of soil contamination related to agricultural and industrial activities. Environ. Res. 2022, 203, 111837. [Google Scholar] [CrossRef] [PubMed]
- Kolesnikov, S.; Minnikova, T.; Kazeev, K.; Akimenko, Y.; Evstegneeva, N. Assessment of the Ecotoxicity of Pollution by Potentially Toxic Elements by Biological Indicators of Haplic Chernozem of Southern Russia (Rostov region). Water Air Soil Pollut. 2022, 233, 18. [Google Scholar] [CrossRef] [PubMed]
- Rogulina, L.I.; Kropotin, V.A.; Voropaeva, E.N. Distribution of rare elements, bismuth and silver in ores and concentrates of the Nikolaev skarn-polmetal deposit (Dalnegorsk, Primorye). Litosfera 2007, 3, 109–115. [Google Scholar]
- Marino, S. Horticultural Crop Response to Different Environmental and Nutritional Stress. Horticulturae 2021, 7, 240. [Google Scholar] [CrossRef]
- Anisimova, G.S.; Kondratieva, L.A.; Kardashevskaya, V.N.; Mustafin, S.K. Features of gold-silver-bismuth-tellurium mineralization of the Allah-Yun metallogenic zone (North-East of Russia). Nat. Resour. Arct. Subarct. 2021, 26, 5–30. [Google Scholar] [CrossRef]
- Wieser, P.E.; Jenner, F.E. Chalcophile Elements: Systematics and Relevance. In Encyclopedia of Geology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 5, pp. 67–80. [Google Scholar] [CrossRef]
- Xing, G.; Zhu, J.; Xiong, Z. Ag, Ta, Ru, and Ir enrichment in surface soil: Evidence for land pollution of heavy metal from atmospheric deposition. Global Biogeochem. Cycles. 2004, 18, 1–5. [Google Scholar] [CrossRef]
- Matusiewicz, H.; Krawczyk, M. Determination of tellurium by hydride generation with in situ trapping flame atomic absorption spectrometry. Spectrochim. Acta 2007, 62, 309–316. [Google Scholar] [CrossRef]
- Anton, M.A.L.; Spears, D.A.; Somoano, M.D.; Tarazona, M.R.M. Thallium in coal: Analysis and environmental implications. Fuel 2013, 105, 13–18. [Google Scholar] [CrossRef]
- Karbowska, B.; Zembrzuski, W.; Jabukowska, M.; Wojtkowiak, T.; Pasieczna, A.; Lukaszewski, Z. Translocation and mobility of thallium from zinc-lead ores. J. Geochem. Explor. 2014, 143, 127–135. [Google Scholar] [CrossRef]
- Krylov, D.A.; Sidorova, G.P. Ways to reduce the environmental impact of coal-fired thermal power plants. Min. Inf. Anal. Bull. 2015, 11, 277–286. [Google Scholar]
- Nelson, B.; Chen, Y.-W. Tellurium in the environment: A critical review focused on natural waters, soils, sediments and airborne particles. Appl. Geochem. 2015, 63, 83–92. [Google Scholar]
- Krylov, D.A. Negative impact of impurity elements from coal thermal power plants on the environment. Min. Inf. Anal. Bull. 2017, 12, 77–87. [Google Scholar] [CrossRef]
- Baceva, K.; Stafilov, T.; Sajn, R.; Tanaselia, C.; Makerski, P. Distribution of chemical elements in soils and stream sediments in the area of abandoned Sb-As-Tl Allchar mine, Republic of Macedonia. Environ. Res. 2014, 133, 77–89. [Google Scholar] [CrossRef]
- Bootjomchai, C.; Laopaiboon, J.; Yenchai, C.; Laopaiboon, R. Gamma-ray shielding and structural properties of barium-bismuth-borosilicate glasses. Radiat. Phys. Chem. 2012, 81, 785–790. [Google Scholar] [CrossRef]
- Shcherbakova, E.V. Ecological state of soils and technogenic soils of the landfill of the city of Slavyansk-on-Kuban. In Ecological Problems of Industrial Cities: Coll. Scientific tr. Saratov; Saratov State Technical University: Saratov, Russia, 2013; pp. 106–107. Available online: https://www.elibrary.ru/item.asp?id=30576776 (accessed on 20 July 2022).
- Michels, C.; Perazzoli, S.; Soares, M. Inhibition of the enriched culture of ammonium-oxidizing bacteria by two different nanoparticles: Silver and magnetite. Common Environ. Sci. 2017, 586, 995–1002. [Google Scholar] [CrossRef]
- Yurgenson, G.A.; Gorban, D.N. Features of the distribution of bismuth in soils, technozems and plants of the Sherlovogorsk ore region. Int. J. Appl. Fundam. Res. 2017, 7, 111–116. [Google Scholar]
- Calder, A.J.; Dimkpa, C.O.; McLean, J.E.; Britt, D.W.; Johnson, W.; Anderson, A.J. Soil components mitigate the antimicrobial effects of silver nanoparticles towards a beneficial soil bacterium, Pseudomonas chlororaphis O6. Sci. Total Environ. 2012, 429, 215–222. [Google Scholar] [CrossRef]
- Beddow, J.; Stolpe, B.; Cole, P.; Lead, J.R.; Sapp, M.; Lyons, B.P.; Colbeck, I.; Whitby, C. Effects of engineered silver nanoparticles on the growth and activity of ecologically important microbes. Environ. Microbiol. Rep. 2014, 6, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Samarajeewa, A.D.; Velicogna, J.R.; Princz, J.I.; Subasinghe, R.M.; Scroggins, R.P.; Beaudette, L.A. Effect of silver nano-particles on soil microbial growth, activity and community diversity in a sandy loam soil. Environ. Pollut. 2017, 220, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shu, K.; Zhang, L.; Si, Y. Effects of Silver Nanoparticles on Soil Microbial Communities and Bacterial Nitrification in Suburban Vegetable Soils. Pedosphere 2017, 27, 482–490. [Google Scholar] [CrossRef]
- Eivazi, F.; Afrasiabi, Z.; Jose, E. Pedosphere Effects of Silver Nanoparticles on the Activities of Soil Enzymes Involved in Carbon and Nutrient Cycling. Pedosphere 2018, 28, 209–214. [Google Scholar] [CrossRef]
- Schlich, K.; Hoppe, M.; Kraas, M.; Schubert, J.; Chanana, M.; Hund-Rinke, K. Long-term effects of three different silver sulfide nanomaterials, silver nitrate and bulk silver sulfide on soil microorganisms and plants. Environ. Pollut. 2018, 242, 1850–1859. [Google Scholar] [CrossRef]
- Singh, H.; Dua, J.; Singh, P.; Yi, T.H. Extracellular synthesis of silver nanoparticles by Pseudomonas sp. THG-LS1.4 and their antimicrobial application. J. Pharm. Anal. 2018, 8, 258–264. [Google Scholar] [CrossRef]
- Wei, C.; Deng, Q.; Wu, F.; Fu, Z.; Xu, L. Arsenic, antimony, and bismuth uptake and accumulation by plants in an old antimony mine, China. Biol. Trace Elem. Res. 2011, 144, 1150–1158. [Google Scholar] [CrossRef]
- Chernyakhov, V.B.; Kalinina, O.N.; Alekseev, M.I. Distribution of Heavy Metals in the Vegetation Cover of the Yaman-Kasinsky Deposit University Complex as a Regional Center of Education, Science and Culture; OGU: Orenburg, Russia, 2012; pp. 844–851. (In Russian) [Google Scholar]
- Shevchenko, V.P.; Starodymova, D.P.; Afanas’eva, A.A.; Bychkov, A.Y.; Bychkova, Y.V.; Koneva, V.V.; Savvichev, A.S. Features of the accumulation of heavy metals by fruticose epiphytic lichens in the Republics of Altai and Khakassia. Basic Res. Biol. Sci. 2014, 12, 2373–2377. [Google Scholar]
- Gorban, D.N. The content of bismuth in the Gmelin wormwood in the natural and technogenic landscape of the Sherlovogorsk ore region. In Proceedings of the International Scientific and Practical Conference Ecology, Health, Sport, Chita, Russia, 16 June 2015; pp. 16–21. (In Russian). [Google Scholar]
- Yurgenson, G.A.; Gorban, D.N. Plant Biogeochemistry and the Problem of Reclamation of Tailings. Evolution and Current State of Landscapes and Biota of Inner Asia; East Siberian State University of Technology and Management (Ulan-Ude): Ulan-Ude, Russia, 2016; pp. 284–291. [Google Scholar]
- Kudin, M.V. Trace element composition of hair and nails in children living in conditions of exposure to cement dust. Quest. Child. Dietol. 2010, 8, 47–50. [Google Scholar]
- Lyzhina, A.V.; Buzinov, R.V.; Unguryanu, T.N.; Gudkov, A.B. Chemical contamination of food products and its impact on the health of the population of the Arkhangelsk region. Hum. Ecology. Ser. Environ. 2012, 12, 3–9. [Google Scholar] [CrossRef]
- Rybkin, V.S.; Bogdanov, A.N.; Chuikov, Y.S.; Teplaya, G.A. Heavy metals as a factor of possible environmentally caused diseases in the Astrakhan region. Hyg. Sanit. 2014, 2, 27–31. [Google Scholar]
- Li, Z.Y.; Ma, Z.W.; Van der Kuijp, T.J.; Yuan, Z.W.; Huang, L. A review of soil heavy metal pollution from mines in China: Pollution and health risk assessment. Sci. Total Environ. 2014, 468, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.V.; Vekovshinina, S.A.; Balashov, S.Y.; Khoroshavin, V.A.; Ukhabov, V.M. Hygienic assessment of carcinogenic risk to the health of the population living in the zone of influence of waste storage sites of the mining and processing plant. Hyg. Sanit. 2018, 97, 10–14. [Google Scholar] [CrossRef]
- Chasteen, T.G.; Bentley, R. Biomethylation of selenium and tellurium: Microorganisms and plants. Chem. Rev. 2003, 103, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; Crc Presspp: Boca Raton, FL, USA, 2010; p. 548. [Google Scholar]
- Vodyanitskii, Y.N. Status and behavior of natural and technogenic forms of As, Sb, Se, and Te in ore tailings and contaminated soils: A review. Eurasian Soil Sci. 2010, 43, 30–38. [Google Scholar] [CrossRef]
- Vodyanitskii, Y.N. Contamination of soils with heavy metals and metalloids and its ecological hazard (analytic review). Eurasian Soil Sci. 2013, 46, 793–801. [Google Scholar] [CrossRef]
- Perkins, W.T. Extreme selenium and tellurium contamination in soils—An eighty year-old industrial legacy surrounding a Ni refinery in the Swansea Valley. Sci. Total Environ. 2011, 412–413, 162–169. [Google Scholar] [CrossRef]
- Yang, G.; Zheng, J.; Tagami, K.; Uchida, S. Rapid and sensitive determination of tellurium in soil and plant samples by sector-field inductively coupled plasma mass spectrometry. Talanta 2013, 116, 181–187. [Google Scholar] [CrossRef]
- Tremel, A.; Masson, P.; Sterckeman, T.; Baize, D.; Mench, M. Thallium in French agrosystems. Thallium contents in arable soils. Environ. Pollut. 1997, 95, 293–302. [Google Scholar] [CrossRef]
- Xiao, T.; Yang, F.; Li, S.; Zheng, B.; Ning, Z. Thallium pollution in China: A geo-environmental perspective. Sci. Total Environ. 2012, 421, 51–58. [Google Scholar] [CrossRef]
- He, L.B. An Environmental Geochemistry Study on Uptake of Thallium by Green Cabbage (Brassica oleracea L. var. capitata L.). Ph.D. Thesis, Chinese Academy of Sciences, Beijing, China, 2008; p. 103. [Google Scholar]
- Aponte, H.; Meli, P.; Butler, B.M.; Paolini, J.E.; Matus, F.; Merino, C.; Cornejo, P.; Kuzyakov, Y. Meta-analysis of heavy metal effects on soil enzyme activities. Sci. Total Environ. 2020, 737, 139744. [Google Scholar] [CrossRef] [PubMed]
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.-Q. Heavy Metals and Pesticides Toxicity in Agricultural Soil and Plants: Ecological Risks and Human Health Implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Quan, Q.; Gan, Y.; Dong, J.; Fang, J.; Wang, L.; Liu, J. Effects of heavy metals on microbial communities in sediments and establishment of bioindicators based on microbial taxa and function for environmental monitoring and management. Sci. Total Environ. 2020, 749, 141555. [Google Scholar] [CrossRef] [PubMed]
- Tóth, G.; Montanarella, L.; Stolbovoy, V.; Máté, F.; Bódis, K.; Jones, A.; Panagos, P.; van Liedekerke, M. Soils of the European Union; Office for Official Publications of the European Communities: Luxembourg, 2008; p. 85. [Google Scholar]
- Kazeev, K.S.; Kolesnikov, S.I.; Akimenko, Y.V.; Dadenko, E.V. Methods for Biodiagnostics of Terrestrial Ecosystems; Southern Federal University: Rostov-on-Don, Russia, 2016; p. 356. [Google Scholar]
- Akimenko, Y. Influence of pollution by antibiotics on biological properties of soils (through the example of ordinary chernozem). Water Air Soil Pollut. 2021, 232, 6. [Google Scholar] [CrossRef]
- Kolesnikov, S.; Tsepina, N.; Minnikova, T.; Kazeev, K.; Mandzhieva, S.; Sushkova, S.; Minkina, T.; Mazarji, M.; Singh, R.K.; Rajput, V.D. Influence of Silver Nanoparticles on the Biological Indicators of Haplic Chernozem. Plants 2021, 10, 1022. [Google Scholar] [CrossRef]
- Kolesnikov, S.; Minnikova, T.; Minkina, T.; Rajput, V.D.; Tsepina, N.; Kazeev, K.; Zhadobin, A.; Nevedomaya, E.; Ter-Misakyants, T.; Akimenko, Y.; et al. Toxic Effects of Thallium on Biological Indicators of Haplic Chernozem Health: A Case Study. Environments 2021, 8, 119. [Google Scholar] [CrossRef]
- Kolesnikov, S.I.; Sudina, L.V.; Kuzina, A.A.; Minnikova, T.V.; Tsepina, N.I.; Kazeev, K.S.; Akimenko, Y.V. The effect of bismuth contamination on the soil biological properties. Agric. Nat. Resour. 2022, 56, 205–216. [Google Scholar] [CrossRef]
- Minnikova, T.; Kolesnikov, S.; Minkina, T.; Mandzhieva, S. Assessment of Ecological Condition of Haplic Chernozem Calcic Contaminated with Petroleum Hydrocarbons during Application of Bioremediation Agents of Various Natures. Land 2021, 10, 169. [Google Scholar] [CrossRef]
- Wexler, P. Encyclopedia of Toxicology, 3rd ed.; Academic Press: Cambridge, MA, USA, 2014; p. 5220. [Google Scholar]
- Khaziev, F.K. Functional role of enzymes in soil processes. Bull. Acad. Sci. Repub. Bashkortostan 2015, 20, 14–24. [Google Scholar]
- Nannipieri, P.; Giagnoni, L.; Renella, G.; Puglisi, E.; Ceccanti, B.; Masciandaro, G.; Fornasier, F.; Moscatelli, M.C.; Marinari, S. Soil enzymology: Classical and molecular approaches. Biol. Fertil. Soils 2012, 48, 743–762. [Google Scholar] [CrossRef]
- Terekhova, V.A. Biotesting of soil ecotoxicity under chemical pollution: Modern approaches to integration for assessing the ecological state (review). Eurasian Soil Sci. 2022, 5, 586–599. [Google Scholar] [CrossRef]
- Titova, K.; Shvakova, E.; Popova, L.; Trofimova, A.; Nikitina, M.; Popov, S. Enzymatic activity as a diagnostic indicator of soil pollution in Solovetsky settlement. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Arkhangelsk, Russian, 26–27 November 2019; Volume 263, p. 012026. [Google Scholar] [CrossRef]
- Alef, K.; Nannipieri, P. Catalase activity. In Methods in Applied Soil Microbiology and Biochemistry; Academic Press: London, UK, 1995; pp. 362–363. [Google Scholar]
- Gong, W.-J.; Niu, Z.-F.; Wang, X.-R.; Zhao, H.-P. How the Soil Microbial Communities and Activities Respond to Long-Term Heavy Metal Contamination in Electroplating Contaminated Site. Microorganisms 2021, 9, 362. [Google Scholar] [CrossRef] [PubMed]
- Tyler, G. Heavy metal pollution, phosphatase activity, and mineralization of organic phosphorus in forest soils. Soil Biol. Biochem. 1976, 8, 327–332. [Google Scholar] [CrossRef]
- Singh, J.P.; Ojinnaka, E.U.; Krumins, J.A.; Goodey, N.M. Abiotic factors determine functional outcomes of microbial inoculation of soils from a metal contaminated brownfield. Ecotoxicol. Environ. Saf. 2019, 168, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, B.P.; Hagmann, D.F.; Balacco, J.; Passchier, S.; Krumins, J.A.; Goodey, N.M. Plants mitigate restrictions to phosphatase activity in metal contaminated soils. Environ. Pollut. 2020, 265, 114801. [Google Scholar] [CrossRef]
- Pan, J.; Yu, L. Effects of Cd or/and Pb on soil enzyme activities and microbial community structure. Ecol. Eng. 2011, 37, 1889–1894. [Google Scholar] [CrossRef]
- Tang, J.; Zhang, J.; Ren, L.; Zhou, Y.; Gao, J.; Luo, L.; Yang, Y.; Peng, Q.; Huang, H.; Chen, A. Diagnosis of soil contamination using microbiological indices: A review on heavy metal pollution. J. Environ. Manag. 2019, 242, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Kolesnikov, S.I.; Tsepina, N.I.; Sudina, L.V.; Minnikova, T.V.; Kazeev, K.S.; Akimenko, Y.V. Silver ecotoxicity estimation by the soil state biological indicators. Appl. Environ. Soil Sci. 2020, 2020, 1207210. [Google Scholar] [CrossRef]
- Tsepina, N.I.; Sudyina, L.V.; Minnikova, T.V.; Kolesnikov, S.I. The effect of silver pollution on catalase activity in chernozems, brown forest soils and seropes. Sci. Notes Crime. Fed. Univ. Vernadsky Biol. Chem. 2020, 6, 259–266. (In Russian) [Google Scholar] [CrossRef]
- Schlich, K.; Klawonn, T.; Terytze, K.; Hund-Rinke, K. Effects of silver nanoparticles and silver nitrate in the earthworm reproduction test. Environ. Toxicol. Chem. 2013, 32, 181–188. [Google Scholar] [CrossRef]
- Langdon, K.A.; McLaughlin, M.J.; Kirby, J.K.; Merrington, G. The effect of soil properties on the toxicity of silver to the soil nitrification process. Environ. Toxicol. Chem. 2014, 33, 1170–1178. [Google Scholar] [CrossRef] [PubMed]
- Sudina, L.; Kolesnikov, S.I.; Minnikova, T.V.; Kazeev, K.S.; Sushkova, S.N.; Minkina, T.M. Assessment of ecotoxicity of the bismuth by biological indicators of soil condition. Eurasian J. Soil Sci. 2021, 10, 236–242. [Google Scholar] [CrossRef]
- Kolesnikov, S.I. Impact of contamination with tellurium on biological properties of ordinary chernozem. Soil Sediment Contam. 2019, 28, 792–800. [Google Scholar] [CrossRef]
- Jia, Y.; Xiao, T.; Zhou, G.; Ning, Z. Thallium at the interface of soil and green cabbage (Brassica oleracea L. var. capitata L.): Soil-plant transfer and influencing factors. Sci. Total. Environ. 2013, 450, 140–147. [Google Scholar] [CrossRef]
- Presentato, A.; Turner, R.J.; Vásquez, C.C.; Yurkov, V.; Zannoni, D. Tellurite-dependent blackening of bacteria emerges from the dark ages. Environ. Chem. 2019, 16, 266–288. [Google Scholar] [CrossRef]




| № | List of Determined Enzymes | EC | Methods of Measurement |
|---|---|---|---|
| Class of oxidoreductases | |||
| 1. | activity of catalase | EC 1.11.1.6. | by the volume of decomposed oxygen during the decomposition of hydrogen peroxide, mL O2/1 g of soil dry/1 min |
| 2. | activity of dehydrogenases | EC 1.1.1 | for the reduction of tetrazolium salts to formazan, mg triphenylformazan (TPF)/10 g of soil dry/24 h |
| Class of hydrolases | |||
| 3. | activity of invertase (β-fructofuranosidase) | EC 3.2.1.26 | by the amount of glucose during the hydrolysis of sucrose, colorimetrically, using Felling’s reagent, mg glucose/10 g of soil dry/24 h |
| 4. | activity of urease (amidohydrolase) | EC 3.5.1.5. | by the amount of ammonia with Nessler’s reagent, with hydrolysis of carbamide, mg NH4+/10 g of soil dry/24 h |
| 5. | activity of phosphatase | EC 3.1.3.1-2 | by the amount of inorganic phosphorus during hydrolysis of sodium nitrophenol phosphate, µg p-nitrophenol/1 g of soil dry/1 h |
| Biological Indicators | Ag | Bi | Tl | Te | ||||
|---|---|---|---|---|---|---|---|---|
| Oxide | Nitrate | Oxide | Nitrate | Oxide | Nitrate | Oxide | Nitrate | |
| Catalase activity | −0.81 * | −0.67 * | −0.89 * | −0.51 | −0.65 | −0.81 * | −0.88 * | −0.76 * |
| Dehydrogenase activity | −0.89 | −0.70 * | −0.67 * | −0.64 | −0.73 * | −0.70 * | −0.82 * | −0.62 |
| Phosphatase activity | −0.45 | −0.95 * | −0.71 * | −0.68 | −0.83 * | −0.79 | −0.96 ** | −0.73 * |
| Invertase activity | −0.98 * | −0.90 ** | −0.80 | −1.00 ** | −0.62 | −0.99 ** | −0.84 * | −0.93 ** |
| Urease activity | −0.82 * | −0.79 * | −0.90 | −0.71 * | −0.95 ** | −0.89 * | −0.60 | −0.89 ** |
| IIEA | −0.85 * | −0.82 * | −0.84 * | −0.86 * | −0.80 * | −0.87 * | −0.89 * | −0.83 * |
| Biological Indicators | Ag | Bi | Tl | Te | ||||
|---|---|---|---|---|---|---|---|---|
| Oxide | Nitrate | Oxide | Nitrate | Oxide | Nitrate | Oxide | Nitrate | |
| Catalase activity | 74 | 62 | 81 | 65 | 60 | 51 | 70 | 50 |
| Dehydrogenase activity | 88 | 77 | 81 | 76 | 73 | 54 | 82 | 47 |
| Phosphatase activity | 63 | 91 | 80 | 62 | 98 | 47 | 97 | 33 |
| Invertase activity | 82 | 88 | 87 | 77 | 83 | 83 | 87 | 74 |
| Urease activity | 82 | 99 | 85 | 96 | 98 | 91 | 103 | 96 |
| IIEA | 78 | 83 | 83 | 75 | 82 | 65 | 88 | 60 |
| Metal | Rare Metal’s Content in Soil, mg/kg | |||
|---|---|---|---|---|
| Ag | <0.197 | 0.197–0.395 | 0.395–3.206 | >3.206 |
| Bi | <0.513 | 0.513–0.968 | 0.968–6.522 | >6.522 |
| Tl | <0.376 | 0.376–0.601 | 0.601–2.454 | >2.454 |
| Te | <0.999 | 0.999–1.475 | 1.475–4.753 | >4.753 |
| Decrease in soil IIEA | <5% | 5–10% | 10–25% | >25% |
| Disturbed ecological functions of the soil | – | Informational | Chemical, physico-chemical, biochemical, holistic | Physical |
| Degree of soil pollution | Not polluted | Weakly polluted | Medium polluted | Heavily polluted |
| Soil sanitation method | Not required | Phytoremediation, leaching | Chemical melioration | Removal of contaminated soil layer |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minnikova, T.; Kolesnikov, S.; Evstegneeva, N.; Timoshenko, A.; Tsepina, N. Estimation of the Enzymatic Activity of Haplic Chernozem under Contamination with Oxides and Nitrates of Ag, Bi, Te and Tl. Agronomy 2022, 12, 2183. https://doi.org/10.3390/agronomy12092183
Minnikova T, Kolesnikov S, Evstegneeva N, Timoshenko A, Tsepina N. Estimation of the Enzymatic Activity of Haplic Chernozem under Contamination with Oxides and Nitrates of Ag, Bi, Te and Tl. Agronomy. 2022; 12(9):2183. https://doi.org/10.3390/agronomy12092183
Chicago/Turabian StyleMinnikova, Tatiana, Sergey Kolesnikov, Natalia Evstegneeva, Alena Timoshenko, and Natalia Tsepina. 2022. "Estimation of the Enzymatic Activity of Haplic Chernozem under Contamination with Oxides and Nitrates of Ag, Bi, Te and Tl" Agronomy 12, no. 9: 2183. https://doi.org/10.3390/agronomy12092183
APA StyleMinnikova, T., Kolesnikov, S., Evstegneeva, N., Timoshenko, A., & Tsepina, N. (2022). Estimation of the Enzymatic Activity of Haplic Chernozem under Contamination with Oxides and Nitrates of Ag, Bi, Te and Tl. Agronomy, 12(9), 2183. https://doi.org/10.3390/agronomy12092183







