Identification, Pathogenicity, and Sensitivity to Fungicide of Colletotrichum Species That Causes Walnut Anthracnose in Beijing
Abstract
:1. Introduction
2. Materials and Methods
3. Results
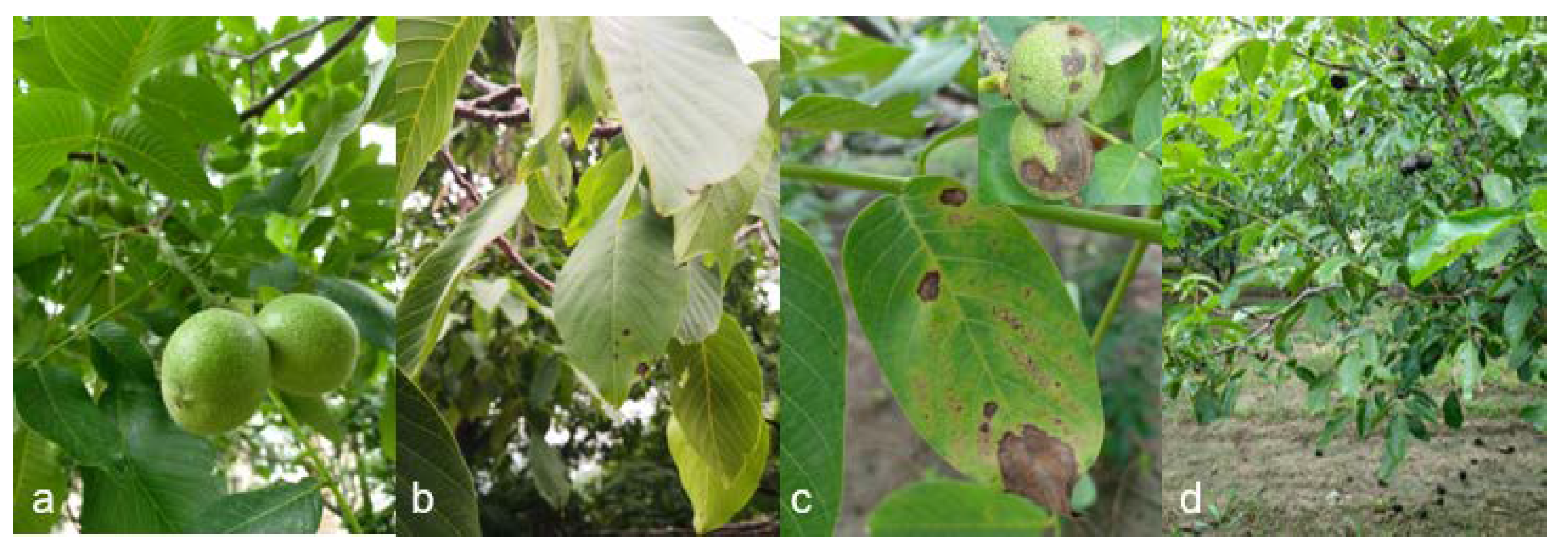
3.1. Field Investigations and Description of Symptoms
3.2. Isolation, Collection and Identification of Walnut Anthracnose
3.3. Phylogenetic Analysis
3.4. Morphological Characteristics
3.5. Pathogen Diversity Analysis
3.6. Effects of Temperature on Pathogen Growth
3.7. Pathogenicity Test
3.8. In Vitro Sensitivity of Isolates to Seven Fungicides
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pei, D.; Guo, B.G.; Li, P.J.; Zhang, Q.; Xu, Y.J. Investigation and analysis report on walnut market and industry in China. Agric. Prod. Mark. 2021, 19, 54–56. [Google Scholar]
- He, C.Y.; Cai, C.; Li, X.K. Occurrence and control of walnut bacterial black spot. Chin. Yunnan. Agric. Sci. 2008, 4, 49–50. [Google Scholar] [CrossRef]
- Niu, Y.S. Investigation and fungicide control of walnut powdery mildew. Chin. Gansu. Agric. 2005, 7, 117. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Liu, Y.; Yan, A.H.; Wang, Z.G. The first report of Juglans regia leaf spot cause by Fusarium proliferatum in China. Plant Dis. 2022, 106, 2265. [Google Scholar] [CrossRef]
- Wang, F.F.; Dun, C.Y.; Tang, T.; Guo, X.L.; Duan, Y.Y.; You, J.M. Boeremia exigua causes leaf spot of walnut trees (Juglans regia) in China. Plant Dis. 2022, 106, 1993. [Google Scholar] [CrossRef]
- Zheng, C.P.; Wang, Y.C. Comprehensive control of walnut anthracnose. J. Chin. Taishan. Univ. 2018, 40, 107–109. [Google Scholar]
- Dean, R.; Van, K.J.; Pretorius, Z.; Hammond-Kosack, K.; Pietro, A.; Spanu, P.; Rudd, J.; Dickman, M.; Kahmann, R.; Ellis, J. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [Green Version]
- Miles, T.D.; Gillett, J.M.; Jarosz, A.M.; Schilder, A. The effect of environmental factors on infection of blueberry fruit by Colletotrichum acutatum. Plant Pathol. 2013, 62, 1238–1247. [Google Scholar] [CrossRef]
- Talhinhas, P.; Gonçalves, E.; Sreenivasaprasad, S.; Oliveira, H. Virulence diversity of anthracnose pathogens (Colletotrichum acutatum and C. gloeosporioides species complexes) on eight olive cultivars commonly grown in Portugal. Eur. J. Plant Pathol. 2014, 142, 73–83. [Google Scholar] [CrossRef]
- Abera, A.; Lemessa, F.; Muleta, D. The antifungal activity of some medicinal plants against coffee berry disease caused by Colletotrichum kahawae. Int. J. Agric. Res. 2011, 6, 268–279. [Google Scholar] [CrossRef] [Green Version]
- Loureiro, A.; Guerra-Guimarães, L.; Lidon, F.C.; Bertrand, B.; Silva, M.C.; Várzea, V. Isoenzymatic characterization of Colletotrichum kahawae isolates with different levels of aggressiveness. Trop. Plant Pathol. 2011, 36, 287–293. [Google Scholar] [CrossRef] [Green Version]
- Rahimlou, S.; Babaeizad, V.; Sayari, M. First report of fruit spot of pomegranate caused by Colletotrichum gloeosporioides in Iran. J. Plant Pathol. 2014, 96, 605. [Google Scholar] [CrossRef]
- Palou, L.; Montesinos-Herrero, C.; Tarazona, I.; Taberner, V. Postharvest anthracnose of persimmon fruit caused by Colletotrichum gloeosporioides first reported in Spain. Plant Dis. 2013, 97, 691. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Hyde, K.D.; Taylor, P.; Weir, B.S.; Waller, J.M.; Abang, M.M.; Zhang, J.Z.; Yang, Y.L.; Phoulivong, S.; Liu, Z.Y. A polyphasic approach for studying Colletotrichum. Fungal Divers. 2009, 39, 183–204. [Google Scholar] [CrossRef]
- Dar, R.A.; Rai, A.N.; Shiekh, I.A. Isolation and mycotaxonomic characterization of Colletotrichum lillacola: A novel species causing anthracnose of Bergenia ligulata. Proc. Indian Natl. Sci. Acad. Part B 2018, 88, 1615–1620. [Google Scholar] [CrossRef]
- De Silva, D.D.; Groenewald, J.Z.; Crous, P.W.; Ades, P.K.; Nasruddin, A.; Mongkolporn, O.; Taylor, P.W.J. Identification, prevalence and pathogenicity of Colletotrichum species causing anthracnose of Capsicum annuum in Asia. IMA Fungus 2019, 10, 1–32. [Google Scholar] [CrossRef]
- Douanla-Meli, C.; Unger, J.G. Phylogenetic study of the Colletotrichum species on imported citrus fruits uncovers a low diversity and a new species in the Colletotrichum gigasporum complex. Fungal Biol. 2017, 121, 858–868. [Google Scholar] [CrossRef]
- Gan, P.; Tsushima, A.; Hiroyama, R.; Narusaka, M.; Takano, Y.; Narusaka, Y.; Kawaradani, M.; Damm, U.; Shirasu, K. Colletotrichum shisoi sp. nov Oxford, England an anthracnose pathogen of Perilla frutescens in Japan: Molecular phylogenetic, morphological and genomic evidence. Sci. Rep. 2019, 9, 13349. [Google Scholar] [CrossRef] [Green Version]
- Jayawardena, R.S.; Camporesi, E.; Elgorban, A.M.; Bahkali, A.H.; Yan, J.Y.; Hyde, K.D. A new species of Colletotrichum from Sonchus sp. in Italy. Phytotaxa 2017, 314, 55–63. [Google Scholar] [CrossRef]
- Jayawardena, R.S.; Hyde, K.D.; Damm, U.; Cai, L.; Liu, M.; Li, X.H.; Zhang, W.; Zhao, W.S.; Yan, J.Y. Notes on currently accepted species of Colletotrichum. Mycosphere 2016, 7, 1192–1260. [Google Scholar] [CrossRef]
- Lee, S.Y.; Jung, H.Y. Colletotrichum kakivorum sp nov., a new leaf spot pathogen of persimmon in Korea. Mycol. Prog. 2018, 17, 1113–1121. [Google Scholar] [CrossRef]
- Marin-Felix, Y.; Groenewald, J.Z.; Cai, L.; Chen, Q.; Marincowitz, S.; Barnes, I.; Bensch, K.; Braun, U.; Camporesi, E.; Damm, U.; et al. Genera of phytopathogenic fungi: GOPHY 1. Stud. Mycol. 2017, 86, 99–216. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Maymon, M.; Freeman, S. Epidemiology, pathology and identification of Colletotrichum including a novel species associated with avocado (Persea americana) anthracnose in Israel. Sci. Rep. 2017, 7, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.C.; Damm, U.; Huang, C.J. Colletotrichum plurivorum, the causal agent of anthracnose fruit rot of Papaya in Taiwan. Plant Dis. 2019, 103, 1041–1104. [Google Scholar] [CrossRef]
- Xu, H.; Zhou, R.; Fu, J.; Yuan, Y.; Ge, X.; Damm, U. Colletotrichum atractylodicola sp nov.: The anthracnose pathogen of Atractylodes chinensis in China. Mycol. Prog. 2018, 17, 393–402. [Google Scholar] [CrossRef]
- Qu, W.W.; Yang, K.Q.; Liu, H.X.; Hou, L.Q. Main diseases of walnut and integrated management in Shandong. Plant Prot. 2011, 44, 136–140. [Google Scholar] [CrossRef]
- Weir, B.S.; Johnston, P.R.; Damm, U. The Colletotrichum gloeosporioides species complex. Stud. Mycol. 2012, 73, 115–180. [Google Scholar] [CrossRef] [Green Version]
- He, X.W.; Lin, X.L.; Pu, Y.L.; Yan, H.; Wu, M.N. Walnut brown spot and anthracnose–main diseases endangering walnut producing areas in Chongqing. Plant Doc. 2003, 2, 28–29. [Google Scholar] [CrossRef]
- Huang, X.; Wang, L.Y.; Xiao, Q.W.; Pu, G.L.; He, W.C. Pathogen identification of walnut anthracnose and fungicide screening. J. China Agric. Univ. 2016, 21, 41–48. [Google Scholar] [CrossRef]
- He, L.F.; Li, X.X.; Gao, Y.Y.; Wei, B.X.; Feng, M. Characterization and fungicide sensitivity of Colletotrichum spp. from different hosts in Shandong, China. Plant Dis. 2019, 103, 34. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.H.; Liu, X.; Wang, R.; Lei, F.A.; Hou, X. First Report of Colletotrichum aenigma Causing Walnut Anthracnose in China. Plant Dis. 2020, 105, 225. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.Z.; Liao, W.J.; Zou, D.X.; Wu, Y.J.; Zhou, Y. First report of leaf spot disease on walnut caused by Colletotrichum fioriniae in China. Plant Dis. 2015, 99, 289. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.H.; Li, D.W.; Duan, C.H.; Liu, X.H.; Niu, S.G.; Hou, L.Q.; Wu, X.Q. First report of walnut anthracnose caused by Colletotrichum fructicola in China. Plant Dis. 2018, 102, 247. [Google Scholar] [CrossRef]
- Wang, Q.H.; Fan, K.; Li, D.W.; Niu, S.G.; Hou, L.Q.; Wu, X.Q. Walnut anthracnose caused by Colletotrichum siamense in China. Australas. Plant Pathol. 2017, 46, 585–595. [Google Scholar] [CrossRef]
- Fang, Z.D. Research Methods in Plant Pathology. Agriculture 1979. [Google Scholar]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes–application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef]
- Carbone, I.; Kohn, L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 1999, 91, 553–556. [Google Scholar] [CrossRef]
- O’Donnell, K.; Nirenberg, H.I.; Aoki, T.; Cigelnik, E. A Multigene phylogeny of the Gibberella fujikuroi species complex: Detection of additional phylogenetically distinct species. Mycoscience 2000, 41, 61–78. [Google Scholar] [CrossRef]
- Templeton, M.D.; Rikkerink, E.H.A.; Solon, S.L.; Crowhurst, R.N. Cloning and molecular characterization of the glyceraldehyde-3-phosphate dehydrogenase-encoding gene and cDNA from the plant pathogenic fungus Glomerella cingulata. Gene 1992, 122, 225–230. [Google Scholar] [CrossRef]
- O’Donnell, K.; Cigelnik, E. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Mol. Phylogenet. Evol. 1997, 7, 103–116. [Google Scholar] [CrossRef]
- Glass, N.L.; Donaldson, G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol. 1995, 61, 1323–1330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, T.; Yamada, K.D.; Tomii, K.; Katoh, K. Parallelization of MAFFT for large-scale multiple sequence alignments. Bioinformatics 2018, 14, 2490–2492. [Google Scholar] [CrossRef] [PubMed]
- Huelsenbeck, J.P.; Ronquist, F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Yang, K.Q.; Zhu, Y.F.; Yin, Y.F. In vitro virulence of eight fungicides to Colletotrichum gloeosporioides of walnut anthracnose. Chin. J. Pestic. Sci. 2013, 15, 412–420. [Google Scholar] [CrossRef]
- Wang, F.; Hu, K.; Jiang, H.; Li, L.; Fang, Y.; Li, K.J. Inhibitory effect and combined virulence of nine fungicides on walnut anthracnose. Agric. Sci. 2015, 6, 127–129. [Google Scholar] [CrossRef]
- Fu, X.L.; Zhao, R.; Min, S.B.; Zhu, Z.F. Study on chemical control of walnut anthracnose. Chin. J. Sichuan Sci. 2016, 37, 107–109. [Google Scholar] [CrossRef]
- Zhang, L.Y. Investigation and control effect of several walnut diseases and pests in Shaanxi province. Northwest AF Univ. 2017. [Google Scholar]
- Wang, X.X.; Wei, J.G.; Yang, Z.D.; Luo, J.; Wu, Y.J.; Yang, X.H.; Luo, J.T.; Xie, D.Z. Investigation of walnut diseases and control of walnut anthracnose in northwest Guangxi. Agric. Sci. 2018, 49, 1531–1540. [Google Scholar] [CrossRef]
- Meng, K.; Zhang, Y.B.; Chang, J.; Li, Z.H.; Wang, D.; Zhai, F.Y.; Shu, J.P. Virulence of eight fungicides to nine anthracnose pathogens of thin shell Carya cathayensis. For. Res. 2021, 34, 153–164. [Google Scholar] [CrossRef]
- Li, Y.; Liu, C.Y.; Chen, X.R.; Zhao, K.H.; Miao, Z.Y.; Liang, C.H.; Wang, H. Identification of grape anthracnose and sensitivity to carbendazim in Liaoning province. Plant Prot. 2009, 35, 74–77. [Google Scholar] [CrossRef]
- Ye, J.; Zhang, C.Q. Detection of susceptibility of grape anthracnose to thiophanate-methyl, tebuconazole and kresoxim-methyl. J. Pestic. Sci. 2012, 14, 111–114. [Google Scholar] [CrossRef]
- Sanders, G.M.; Korsten, L.; Wehner, F.C. Survey of fungicide sensitivity in Colletotrichum gloeosporioides from different avocado and mango production areas in South Africa. Eur. J. Plant Pathol. 2000, 10, 745–752. [Google Scholar] [CrossRef]
- Wen, X.Y. Study on the occurrence and control of grape anthracnose. Agric. Sci. 1983, 2, 1–7. [Google Scholar] [CrossRef]
- Avila, A.C.; Olaya, G.; Koller, W. Characterization of Colletotrichum graminicola isolates resistant to strobilurin-related QoI fungicides. Plant Dis. 2003, 87, 1426–1432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inada, M.; Ishii, H.; Chung, W.; Yamada, T.; Yamaguchi, J.; Furuta, A. Occurrence of strobilurin-resistant strains of Colletotrichum gloeosporioides (Glomerella cingulata), the causal fungus of strawberry anthracnose. Jpn. J. Phytopathol. 2008, 74, 114–117. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.H.; Michailides, T.J. Advances in understanding molecular mechanisms of fungicide resistance and molecular detection of resistant genotypes in phytopathogenic fungi. Crop. Prot. 2005, 24, 853–863. [Google Scholar] [CrossRef]
- Deng, W.P.; Yang, M.; Du, F.; Liu, Y.X.; Zhang, L.T.; Yan, Y.Y.; Wang, S.Q.; Yang, J.Z.; He, X.H.; Zhu, S.S. Sensitivity of Colletotrichum gloeosporioides to three ergosterol demethylation inhibitors. Chin. J. Pestic. Sci. 2011, 13, 245–252. [Google Scholar] [CrossRef]






| Target | Primer | Sequence (5′to 3′) | Direct | Initial Denaturation | Denaturation | Annealing | Extension | Final Extension | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| °C | Sec | °C | Sec | °C | Sec | °C | Sec | °C | Sec | ||||
| ACT | ACT-512F | ATG TGC AAG GCC GGT TTC GC | F | 94 | 120 | 94 | 10 | 58 | 10 | 72 | 60 | 72 | 300 |
| ACT-783R | TAC GAG TCC TTC TGG CCC AT | R | 94 | 120 | 94 | 10 | 58 | 10 | 72 | 60 | 72 | 300 | |
| CAL | CL1C | GAA TTC AAG GAG GCC TTC TC | F | 94 | 120 | 94 | 10 | 55 | 10 | 72 | 60 | 72 | 300 |
| CL2C | CTT CTG CAT I GAG CTG GAC | R | 94 | 120 | 94 | 10 | 55 | 10 | 72 | 60 | 72 | 300 | |
| CHS-1 | CHS-79F | TGG GGC AAG GAT GCT TGG AAG AAG | F | 94 | 120 | 94 | 10 | 58 | 10 | 72 | 60 | 72 | 300 |
| CHS-345R | TGG AAG AAC CAT CTG TGA GAG TTG | R | 94 | 120 | 94 | 10 | 58 | 10 | 72 | 60 | 72 | 300 | |
| GAPDH | GDF1 | GCC GTC AAC GAC CCC TTC ATT GA | F | 94 | 120 | 94 | 10 | 60 | 10 | 72 | 60 | 72 | 300 |
| GDR1 | GGG TGG AGT CGT ACT TGA GCA TGT | R | 94 | 120 | 94 | 10 | 60 | 10 | 72 | 60 | 72 | 300 | |
| ITS | ITS-1 | TCC GTA GGT GAA CCT GCG G | F | 94 | 120 | 94 | 10 | 55 | 10 | 72 | 60 | 72 | 300 |
| ITS-4 | TCC Gerber GCT TAT TGA TAT GC | R | 94 | 120 | 94 | 10 | 55 | 10 | 72 | 60 | 72 | 300 | |
| TUB2 | T1 | AAC ATG CGT GAG ATT GTA AGT | F | 94 | 120 | 94 | 10 | 55 | 10 | 72 | 60 | 72 | 300 |
| Bt2b | ACC CTC AGT GTA GTG ACC CTT GGC | R | 94 | 120 | 94 | 10 | 55 | 10 | 72 | 60 | 72 | 300 | |
| Month | Part | Disease Incidence Rate (%) | Disease Index |
|---|---|---|---|
| June | Fruit | 0 | 0 |
| Leaf | 0 | 0 | |
| July | Fruit | 10.93 | 3.85 |
| Leaf | 15.1 | 4.84 | |
| August | Fruit | 24.53 | 26.7 |
| Leaf | 12.21 | 13.52 | |
| September | Fruit | 45.43 | 41.07 |
| Leaf | 41.44 | 27.32 |
| District | Orchard | Longitude and Latitude | C. a | C. f | C. g | C. si | C. l | C. so | Total |
|---|---|---|---|---|---|---|---|---|---|
| Changping | Shatuo village | N: 40°21′43.7″ E: 116°42′75.3″ | 3 | 3 | 6 | ||||
| Fangshan | Shawo village | N: 39°76′37.0″ E: 116°04′54.7″ | 24 | 33 | 57 | ||||
| Fangshan | N: 39°81′95.2″ E: 116°04′09.0″ | 10 | 10 | ||||||
| Haidian | Jiufeng mountain | N: 40°05′71.3″ E: 116°12′69.8″ | 3 | 3 | 20 | 68 | 94 | ||
| Xueqinglu road | N: 40°01′61.7″ E: 116°35′95.5″ | 38 | 38 | ||||||
| Huairou | Liuduhe village | N: 40°39′08.0″ E: 116°54′34.3″ | 11 | 3 | 14 | ||||
| Denggezhuang village | N: 40°43′47.6″ E: 116°70′89.8″ | 7 | 12 | 3 | 22 | ||||
| Mentougou | Mentougou | N: 39°96′01.4″ E: 115°70′75.8″ | 9 | 2 | 11 | ||||
| Miyun | Fengezhuang village | N: 40°45′30.9″ E: 116°79′20.4″ | 15 | 15 | |||||
| Jizhuang village | N: 40°39′68.7″ E: 116°82′64.0″ | 12 | 3 | 14 | 29 | ||||
| Pinggu | Pinggu | N: 40°19′58.7″ E: 117°12′29.1″ | 65 | 1 | 66 | ||||
| Dahuashan town | N: 40°27′91.0″ E: 117°08′65.9″ | 4 | 1 | 5 | |||||
| Xiagezhuang town | N: 40°14′28.9″ E: 117°14′87.4″ | 1 | 3 | 3 | 3 | 10 | |||
| Total | 33 | 19 | 163 | 156 | 3 | 3 | 377 | ||
| Species | Spot Diameter (mm) x | |
|---|---|---|
| Gloeosporioides | C. aenigma | 5.50 ± 0.87 a |
| C. fructicola | 10.42 ± 1.88 bc | |
| C. gloeosporioides | 12.33 ± 0.29 c | |
| C. siamense | 10.17 ± 1.23 bc | |
| Magnum | C. liaoningense | 7.83 ± 1.42 ab |
| Orchidearum | C. sojae | 6.83 ± 2.01 a |
| Fungicides | EC50 (μg/mL) x | |||||
|---|---|---|---|---|---|---|
| C. aenigma | C. fructicola | C. gloeosporioides | C. siamense | C. liaoningense | C. sojae | |
| Difenoconazole | 0.1850 ± 0.0775 A | 0.0945 ± 0.0251 AB | 0.1350 ± 0.0520 A | 0.1030 ± 0.0314 AB | 0.1308 ± 0.0309 AB | 0.2333 ± 0.0268 B |
| Fluazinam | 0.0676 ± 0.0198 A | 0.0535 ± 0.0135 A | 0.0590 ± 0.0117 A | 0.0548 ± 0.0181 AB | 0.1082 ± 0.0276 AB | 0.2570 ± 0.0452 B |
| Epoxiconazole | 0.3442 ± 0.1029 A | 0.2914 ± 0.0234 B | 0.3554 ± 0.1488 B | 0.1590 ± 0.0365 B | 0.2288 ± 0.0523 AB | 0.6782 ± 0.2494 C |
| Mefentriflucona-zole | 1.0523 ± 0.5442 B | 0.9210 ± 0.3815 C | 1.1524 ± 0.3415 D | 0.8433 ± 0.3192 C | 0.5701 ± 0.1733 B | 1.4666 ± 0.4278 D |
| Prochloraz | 0.0269 ± 0.0071 A | 0.0198 ± 0.0042 A | 0.0210 ± 0.0057 A | 0.0179 ± 0.0042 A | 0.0154 ± 0.0056 A | 0.0514 ± 0.0214 A |
| SYP-14288 | 0.0107 ± 0.0010 A | 0.0096 ± 0.0026 A | 0.0175 ± 0.0083 A | 0.0187 ± 0.0051 A | 0.0227 ± 0.0095 A | 0.0414 ± 0.0132 A |
| Tebuconazole | 0.8308 ± 0.5358 B | 0.2135 ± 0.1288 AB | 0.6698 ± 0.3985 C | 0.7244 ± 0.1216 C | 0.2516 ± 0.0148 AB | 0.6900 ± 0.3582 C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.; Chen, J.; Chen, Q.; Liu, Z.; Sun, J.; Yan, Y.; Zhang, H.; Bi, Y. Identification, Pathogenicity, and Sensitivity to Fungicide of Colletotrichum Species That Causes Walnut Anthracnose in Beijing. Agronomy 2023, 13, 214. https://doi.org/10.3390/agronomy13010214
Li F, Chen J, Chen Q, Liu Z, Sun J, Yan Y, Zhang H, Bi Y. Identification, Pathogenicity, and Sensitivity to Fungicide of Colletotrichum Species That Causes Walnut Anthracnose in Beijing. Agronomy. 2023; 13(1):214. https://doi.org/10.3390/agronomy13010214
Chicago/Turabian StyleLi, Fuxin, Jiawen Chen, Qian Chen, Ziyi Liu, Junyuan Sun, Yitong Yan, Hanxing Zhang, and Yang Bi. 2023. "Identification, Pathogenicity, and Sensitivity to Fungicide of Colletotrichum Species That Causes Walnut Anthracnose in Beijing" Agronomy 13, no. 1: 214. https://doi.org/10.3390/agronomy13010214
APA StyleLi, F., Chen, J., Chen, Q., Liu, Z., Sun, J., Yan, Y., Zhang, H., & Bi, Y. (2023). Identification, Pathogenicity, and Sensitivity to Fungicide of Colletotrichum Species That Causes Walnut Anthracnose in Beijing. Agronomy, 13(1), 214. https://doi.org/10.3390/agronomy13010214




