Effect of Biochar on Soil Physiochemical Properties and Bacterial Diversity in Dry Direct-Seeded Rice Paddy Fields
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site and Materials
2.2. Experimental Design
2.3. Soil Sampling Collection
2.4. Soil Trait Determination
2.5. Determination of Soil Bacterial Diversity
2.6. Bioinformatics and Statistical Analyses
3. Results
3.1. Soil Water-Stable Aggregates
3.2. Soil Particle Content
3.3. Soil Nutrients
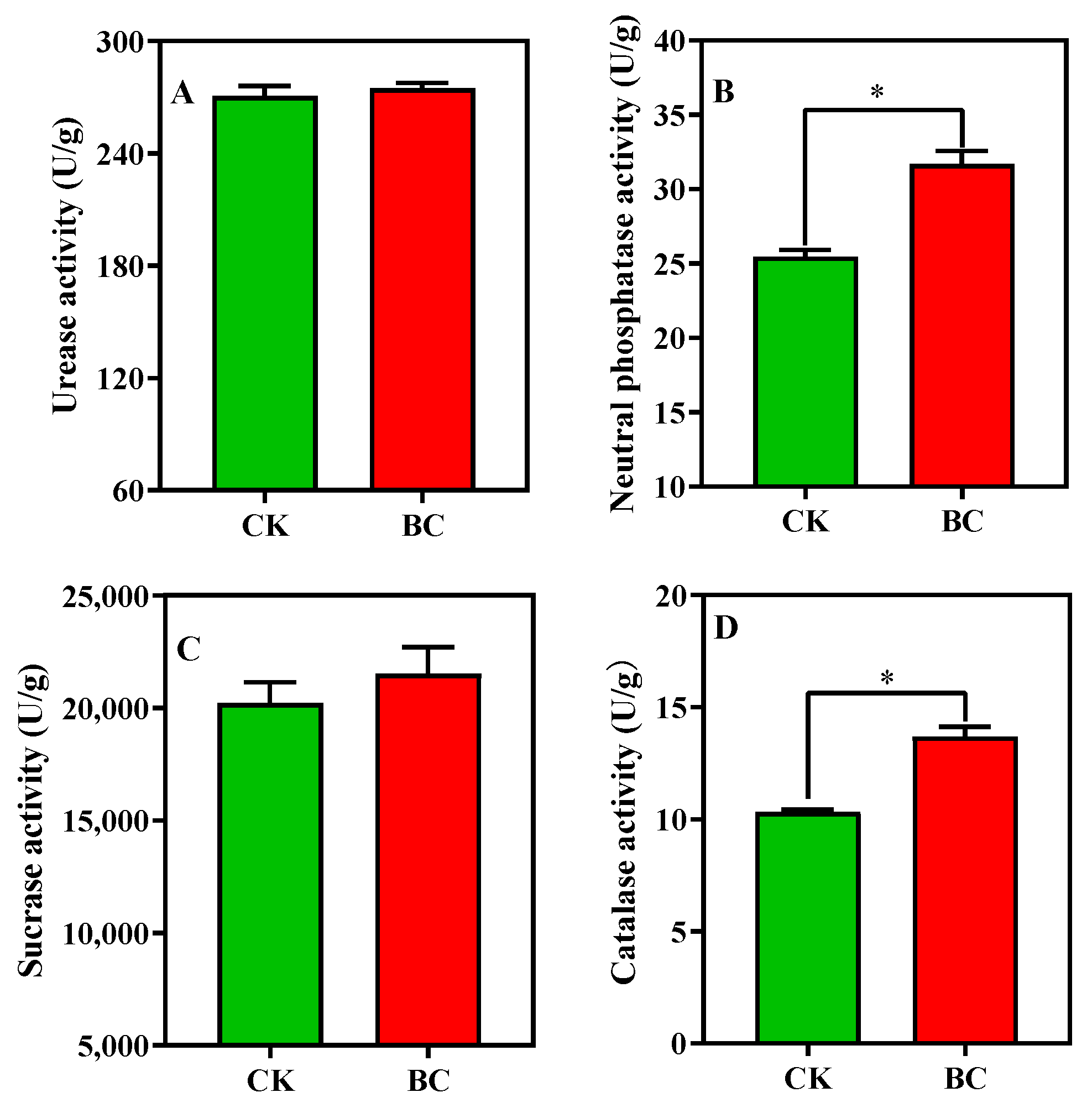
3.4. Soil Enzyme Activity
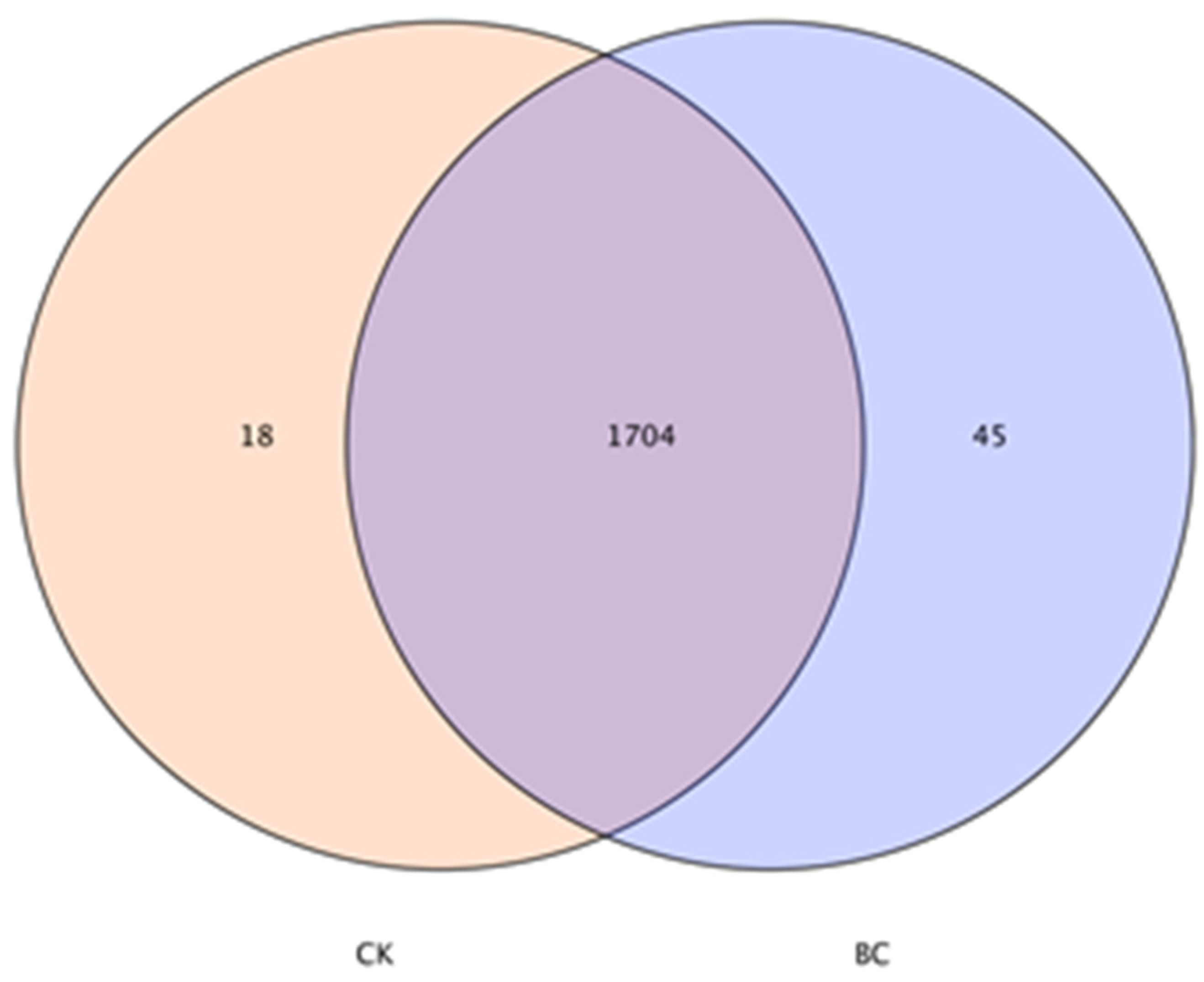
3.5. Richness and Diversity of Soil Bacterial Community
3.6. Soil Bacterial Community Composition
3.7. Analysis of Bacterial Taxa
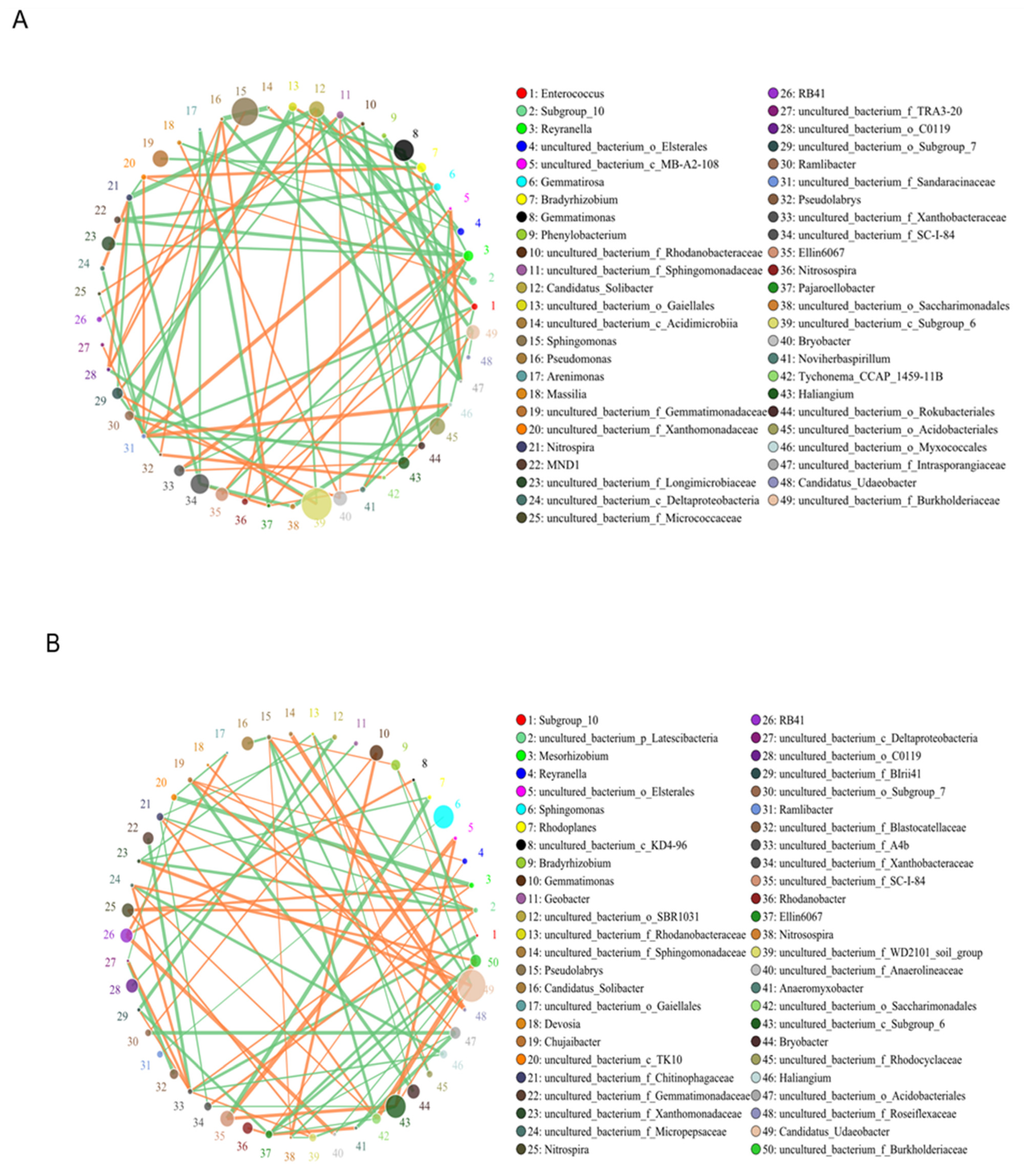
3.8. Species-Correlation Networks
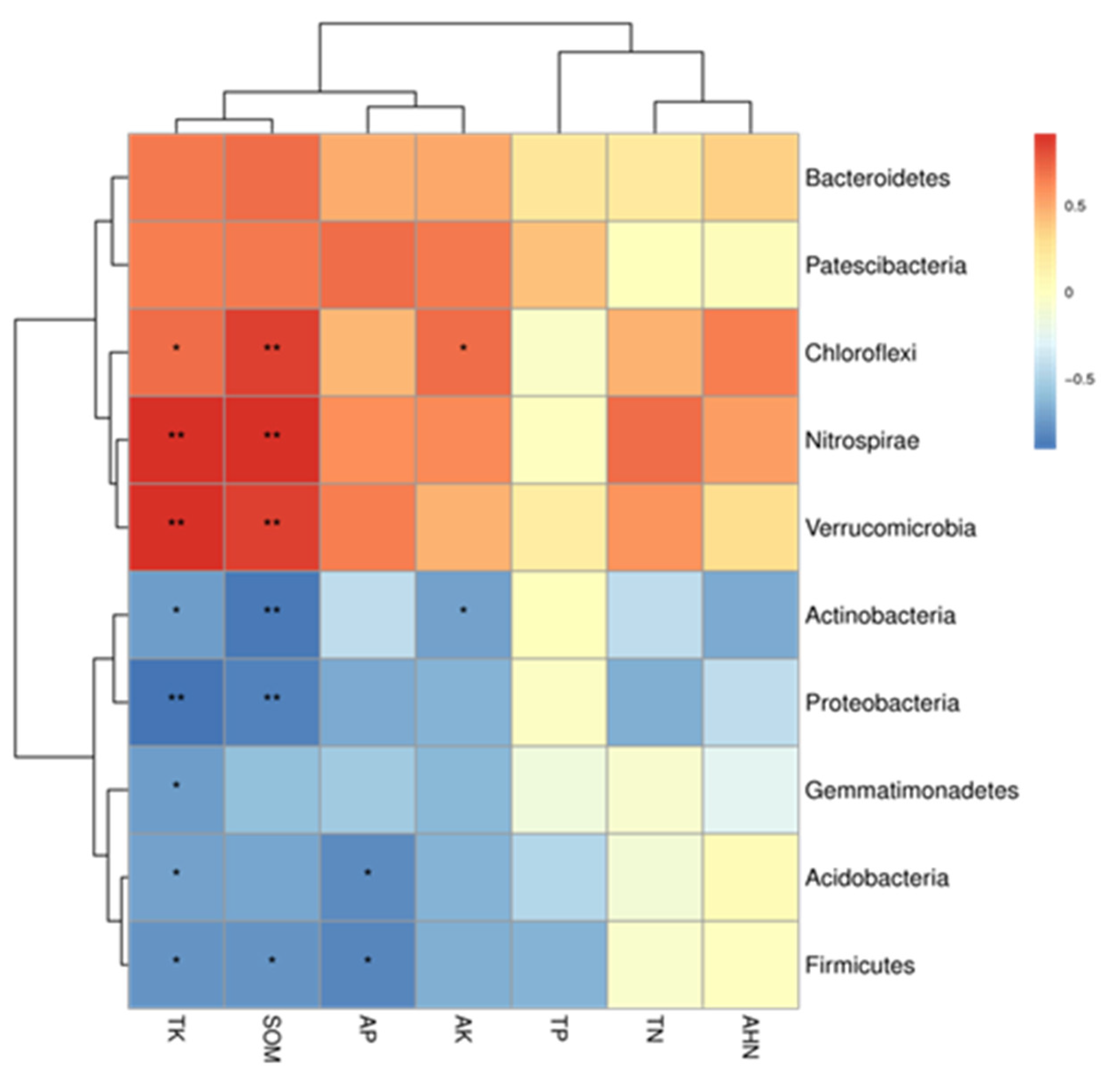
3.9. Correlation Analysis of Soil Bacterial Community and Soil Nutrients
4. Discussion
4.1. Correlation Analysis of Soil Bacterial Community and Soil Nutrients
4.2. Correlation Analysis of Soil Bacterial Community and Soil Nutrients
4.3. Effect of Biochar on Soil Bacterial Community Composition
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alexandratos, N.; Bruinsma, J. World Agriculture towards 2030/2050: The 2012 Revision; ESA E Working Paper; FAO: Rome, Italy, 2012. [Google Scholar]
- Xu, L.Q.; Guo, X.H.; Zhang, J.N.; Zhao, Y.; Li, X.L.; Liu, S.F.; Cui, Z.Y.; An, Y.L.; Lv, Y.D. The effect of organic fertilizer on the quality of dry direct-seeding rice. ACTA Agric. Boreali Sincia 2022, 37, 137–146. (In Chinese) [Google Scholar]
- Tang, Z.Q.; Zhang, L.Y.; He, N.; Ma, Z.B.; Zhao, M.Z.; Wang, C.H.; Zheng, W.J.; Yin, Y.A.; Wang, H. Effects of mechanical direct dry seeding on rice growth, photosynthetic characteristics and yield. Crops 2021, 5, 87–94. (In Chinese) [Google Scholar]
- Cao, G.L.; Zhang, X.Y.; Wang, Y.Q.; Zheng, F.C. Estimation of emissions from field burning of crop straw in China. Chin. Sci. Bull. 2008, 53, 784–790. [Google Scholar] [CrossRef]
- Sun, R.B.; Zhang, X.X.; Guo, X.S.; Wang, D.Z.; Chu, H.Y. Bacterial diversity in soils subjected to long-term chemical fertilization can be more stably maintained with the addition of livestock manure than wheat straw. Soil Biol. Biochem. 2015, 88, 9–18. [Google Scholar] [CrossRef]
- Ghorbani, M.; Amirahmadi, E.; Zamanian, K. In-situ biochar production associated with paddies: Direct involvement of farmers in greenhouse gases reduction policies besides increasing nutrients availability and rice production. Land Degrad. Dev. 2021, 32, 3893–3904. [Google Scholar] [CrossRef]
- Major, J.; Steiner, C.; Downie, A.; Lehmann, J. Biochar effects on nutrient leaching. In Biochar for Environmental Management: Science and Technology; Lehmann, J., Joseph, S., Eds.; Earthscan Publishers: London, UK, 2009; pp. 271–287. [Google Scholar]
- Ma, N.N.; Zhang, L.L.; Zhang, Y.L.; Yang, L.J.; Yu, C.X.; Yin, G.H.; Doane, T.A.; Wu, Z.J.; Zhu, P.; Ma, X.Z. Biochar improves soil aggregate stability and water availability in a mollisol after three years of field application. PLoS ONE 2016, 11, e0154091. [Google Scholar] [CrossRef] [Green Version]
- Abulaiti, A.; She, D.L.; Liu, Z.P.; Sun, X.Q.; Wang, H.D. Application of biochar and polyacrylamide to revitalize coastal saline soil quality to improve rice growth. Environ. Sci. Pollut. Res. 2022, 11, 1–17. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Tang, G.M.; Long, X.S.; Ge, C.H.; Xu, W.L. Effects of one-time biochar input on soil properties and corn yield in irrigation sandy soil. Agric. Res. Arid Areas 2021, 39, 137–141. (In Chinese) [Google Scholar]
- Joseph, S.D.; Camps-Arbestain, M.; Lin, Y.; Munroe, P.; Chia, C.H.; Hook, J.; Van Zwieten, L.; Kimber, S.; Cowie, A.; Singh, B.P.; et al. An investigation into the reactions of biochar in soil. Aust. J. Soil Res. 2010, 48, 501–515. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Wong, J.T.; Fung, H.Y.; Huang, L.B.; Rinklebe, J.; Chang, S.X.; Bolan, N.; Wang, H.L.; Ok, Y.S. Response of microbial communities to biochar-amended soils: A critical review. Biochar 2019, 1, 3–22. [Google Scholar] [CrossRef] [Green Version]
- Butnan, S.; Deenik, J.L.; Toomsan, B.; Vityakon, P. Biochar properties affecting carbon stability in soils contrasting in texture and mineralogy. Agric. Nat. Resour. 2017, 51, 492–498. [Google Scholar] [CrossRef]
- Zhang, Q.Z.; Dijkstra, F.A.; Liu, X.R.; Wang, Y.D.; Huang, J.; Lu, N.; Singer, A.C. Effects of biochar on soil microbial biomass after four years of consecutive application in the north China plain. PLoS ONE 2014, 9, e102062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinbeiss, S.; Gleixner, G.; Antonietti, M. Effect of biochar amendment on soil carbon balance and soil microbial activity. Soil Biol. Biochem. 2009, 41, 1301–1310. [Google Scholar] [CrossRef]
- Azeem, M.; Hale, L.; Montgomery, J.; Crowley, D.; McGiffen, M.E.J. Biochar and compost effects on soil microbial communities and nitrogen induced respiration in turfgrass soils. PLoS ONE 2020, 15, e0242209. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota-A review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Ghorbani, M.; Amirahmadi, E.; Neugschwandtner, R.W.; Konvalina, P.; Kopecký, M.; Moudrý, J.; Perná, K.; Murindangabo, Y.T. The impact of pyrolysis temperature on biochar properties and its effects on soil hydrological properties. Sustainability 2022, 14, 14722. [Google Scholar] [CrossRef]
- Ghorbani, M.; Asadi, H.; Abrishamkesh, S. Effects of rice husk biochar on selected soil properties and nitrate leaching in loamy sand and clay soil. Int. Soil Water Conserv. Res. 2019, 7, 258–265. [Google Scholar] [CrossRef]
- Ameloot, N.; Sleutel, S.; Das, K.C.; Kanagaratnam, J.; De Neve, S. Biochar amendment to soils with contrasting organic matter level: Effects on N mineralization and biological soil properties. Glob. Chang. Biol. Bioenergy 2015, 7, 135–144. [Google Scholar] [CrossRef]
- Bao, S.D. Soil Agrochemical Analysis, 3rd ed.; China Agricultural Press: Beijing, China, 2005. (In Chinese) [Google Scholar]
- Zheng, B.Z. Technical Guide for Soil Analysis; China Agricultural Press: Beijing, China, 2013. (In Chinese) [Google Scholar]
- Guo, X.H.; Zou, B.; Xu, L.Q.; Zhang, J.N.; Zheng, G.P.; Wang, H.Z.; Yin, D.W.; Li, H.Y.; Zhang, W.Z.; Lv, Y.D.; et al. Changes in the physical, chemical, and bacterial community characteristics of soil in response to short-term combined organic–inorganic fertilizers in a dry direct-seeded paddy field. Agronimy 2022, 12, 2808. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [Green Version]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Six, J.; Bossuyt, B.; Degryze, H.; Denef, K. A history of research on the link between (micro) aggregates, soil biota, and soil organic matter dynamics. Soil Tillage Res. 2004, 79, 7–31. [Google Scholar] [CrossRef]
- Liu, Z.L.; Yu, W.T. Review of researches on soil aggregate and soil organic carbon. Chin. J. Eco-Agric. 2011, 19, 447–455. (In Chinese) [Google Scholar] [CrossRef]
- Han, L.F.; Sun, K.; Yang, Y.; Xia, X.H.; Li, F.B.; Yang, Z.F.; Xing, B.S. Biochar’s stability and effect on the content, composition and turnover of soil organic carbon. Geoderma 2020, 364, 114184. [Google Scholar] [CrossRef]
- Liu, X.H.; Han, F.P.; Zhang, X.C. Effect of biochar on soil aggregates in the loess plateau: Results from incubation experiments. Int. J. Agric. Biol. 2012, 6, 975–979. [Google Scholar]
- Du, Z.L.; Zhao, J.K.; Wang, Y.D.; Zhang, Q.Z. Biochar addition drives soil aggregation and carbon sequestration in aggregate fractions from an intensive agricultural system. J. Soils Sediments 2017, 17, 581–589. [Google Scholar] [CrossRef]
- Situ, G.M.; Zhao, Y.L.; Zhang, L.; Yang, X.Q.; Chen, D.; Li, S.H.; Wu, Q.F.; Xu, Q.F.; Chen, J.H.; Qin, H. Linking the chemical nature of soil organic carbon and biological binding agent in aggregates to soil aggregate stability following biochar amendment in a rice paddy. Sci. Total Environ. 2022, 847, 157460. [Google Scholar] [CrossRef]
- Li, J.Z.; Dai, K.; Zhang, L.M.; Ji, S.G.; Qiao, Z.X.; Jiao, Y.G.; Meng, J.; Lan, Y. Effects of biochar application on soil organic carbon distribution and soil aggregate composition of red soils in Yunnan tobacco planting area. Acta Sci. Circumstantiae 2016, 36, 2114–2120. (In Chinese) [Google Scholar]
- Glaser, B.; Lehmann, J.; Zech, W. Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal—A review. Biol. Fertil. Soils 2002, 35, 219–230. [Google Scholar] [CrossRef]
- Zhu, Q.L.; Zeng, D.P.; Wang, C.; Tong, C.; Wang, W.Q. Effects of waste applications on the distribution and stability of soil aggregates in the paddy field of Fuzhou plain. Acta Sci. Circumstantiae 2016, 8, 3000–3008. (In Chinese) [Google Scholar]
- Fang, B.; Li, X.Q.; Zhao, B.; Zhong, L. Influence of biochar on soil physical and chemical properties and crop yields in rainfed field. Ecol. Environ. Sci. 2014, 23, 1292–1297. (In Chinese) [Google Scholar]
- Yao, Q. Effect of Biochar Addition on Soil Physicochemical Properties and Microbial Diversity in a Black Soil of Northeast China; Changchun: University of Chinese Academy of Sciences (Northeast Institute of Geography and Agroecology, Chinese Academy of Sciences): Changchun, China, 2017. (In Chinese) [Google Scholar]
- Yang, H.H.; Li, M.S.; Wang, L.Y.; Feng, H.L.; Liu, F.T.; Du, J.; Ren, T.B.; Gao, W.K. Effects of biochar on soil nutrients and rhizosphere bacterial community structure of flue-cured tobacco at maturity stage. J. Huazhong Agric. Univ. 2021, 40, 62–71. (In Chinese) [Google Scholar]
- Gao, T.Y.; Li, N.; Peng, J.; Gao, M.H.; Luo, P.Y.; Han, X.R. Effect of consecutive application of biochar on phosphate morphology and availability in brown soil. J. Plant Nutr. Fertil. 2019, 25, 1451–1460. (In Chinese) [Google Scholar]
- Angst, T.E.; Sohi, S.P. Establishing release dynamics for plant nutrients from biochar. Glob. Change Biol. Bioenergy 2013, 5, 221–226. [Google Scholar] [CrossRef]
- Zhan, X.M.; Peng, J.; Wang, Y.; Liu, Y.F.; Chen, K.; Han, X.R.; Wang, H.F.; Lin, W.C.; Li, X.Y. Influences of application of biochar and biochar-based fertilizer on brown soil physiochemical properties and peanut yields. J. Plant Nutr. Fertil. 2015, 21, 1633–1641. (In Chinese) [Google Scholar]
- Wang, N.; Jiao, X.Y.; Wu, A.L.; Wang, J.S.; Dong, E.W.; Guo, J.; Ding, Y.C.; Wang, L.G. Research advances on effects of biochar application on soil phosphorus and potassium. J. Shanxi Agric. Sci. 2016, 44, 1402–1405, 1420. (In Chinese) [Google Scholar]
- Ji, L.D.; Liu, X.T.; Si, H.L.; Sun, Q.; Wang, R. Effects of biomass charcoal on soil physicochemical properties and corn growth. Agric. Res. Arid Areas 2021, 39, 114–120. (In Chinese) [Google Scholar]
- Yao, F.; Ren, T.B.; Xu, M.; Zhang, F.J.; Xu, C.S.; Ma, G.J.; Zhang, Z.Y.; Xie, T.X.; Liu, G.S. Research progress and mechanism of action analysis of biochar improving soil mineral nutrient absorption. Jiangsu Agric. Sci. 2020, 10, 46–51. (In Chinese) [Google Scholar]
- Czimczik, C.I.; Masiello, C.A. Controls on black carbon storage in soils. Glob. Biogeochem. Cycles 2007, 21, 113. [Google Scholar] [CrossRef]
- Feng, H.L.; Xu, C.S.; He, H.H.; Zeng, Q.; Chen, N.; Li, X.L.; Ren, T.B.; Ji, X.M.; Liu, G.S. Effect of biochar on soil enzyme activity and the bacterial community and its mechanism. Environ. Sci. 2021, 42, 422–432. (In Chinese) [Google Scholar]
- Forge, T.; Ehret, D.; Messiga, A.; Dorais, M. Influnences of nitrogen inputs on nematode populations under high bush blueberry. J. Nematol. 2020, 52, 1–14. [Google Scholar] [PubMed]
- He, X.F.; Zhao, F.Y.; Yu, K.; Yang, X.; Wang, J.W.; Yu, S.L. Effect of biochar on nutrient, enzyme activities and microbial diversity of rhizosphere soil of grape seedlings. Soil Fertil. Sci. China 2020, 6, 19–26. (In Chinese) [Google Scholar]
- Gui, Y.Y.; Li, H.B.; Wei, J.J.; Mao, L.Y.; Zhang, R.H.; Qu, H.P.; Zhu, K.; Zhao, P.F.; Zhou, H.; Liu, X.H. Effects of biochar on soil nutrients, enzyme activities and microbial diversity of ratoon sugarcane in dry slope land. J. South Agric. 2022, 53, 776–784. (In Chinese) [Google Scholar]
- Kennedy, A.C. Bacterial diversity in agroecosystems. Agric. Ecosyst. Environ. 1999, 74, 65–76. [Google Scholar] [CrossRef]
- Gu, L.S.; Whalen, J.K.; Thomas, B.W.; Sachdeva, V.; Deng, H.Y. Physico-chemical properties and microbial responses in biochar-amended soils: Mechanisms and future directions. Agric. Ecosyst. Environ. 2015, 206, 46–59. [Google Scholar] [CrossRef]
- Pietikäinen, J.; Kiikkilä, O.; Fritze, H. Charcoal as a habitat for microbes and its effect on the microbial community of the underlying humus. Oikos 2000, 89, 231–242. [Google Scholar] [CrossRef]
- Nguyen, T.T.N.; Wallace, H.M.; Xu, C.Y.; Zwieten, L.V.; Weng, Z.H.; Xu, Z.H.; Che, R.X.; Tahmasbian, I.; Hu, H.W.; Bai, S.H. The effects of short term, long term and reapplication of biochar on soil bacteria. Sci. Total Environ. 2018, 636, 142–151. [Google Scholar] [CrossRef]
- Xu, N.; Tan, G.C.; Wang, H.Y.; Gai, X.P. Effect of biochar additions to soil on nitrogen leaching, microbial biomass and bacterial community structure. Eur. J. Soil Biol. 2016, 74, 1–8. [Google Scholar] [CrossRef]
- Yuan, H.C.; Wu, H.; Ge, T.D.; Li, K.L.; Wu, J.S.; Wang, J.R. Effects of long-term fertilization on bacterial and archaeal diversity and community structure within subtropical red paddy soils. Chin. J. Appl. Ecol. 2015, 26, 1807–1813. (In Chinese) [Google Scholar]
- Song, Y.N.; Lin, Y.; Chen, Z.Q. Effect of nitrogen fertilizer level on bacterial community and N2O emission in paddy soil. Chin. J. Eco-Agric. 2017, 25, 1266–1275. (In Chinese) [Google Scholar]
- Chen, X.D.; Jiang, N.; Chen, Z.H.; Tian, J.H.; Sun, N.; Xu, M.G.; Chen, L.J. Response of soil phoD phosphatase gene to long-term combined applications of chemical fertilizers and organic materials. Appl. Soil Ecol. 2017, 119, 197–204. [Google Scholar] [CrossRef]
- Jones, R.T.; Robeson, M.S.; Lauber, C.L.; Hamady, M.; Knight, R.; Fierer, N. A comprehensive survey of soil acidobacterial diversity using pyrosequencing and clone library analyses. Int. Soc. Microb. Ecol. J. 2009, 3, 442–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, X.J.; Huang, Y.L.; Jing, R.Y.; Ma, F.Y.; An, R.; Tian, Q.; Chen, B.J. Bacterial structure and diversity of four plantations in the Yellow River Delta by high-throughput sequencing. Acta Ecol. Sin. 2018, 38, 5857–5864. (In Chinese) [Google Scholar]
- Li, P.; Wu, J.Q.; Sha, C.Y.; Ye, C.M.; Huang, S.F. Effects of manure and organic fertilizer application on soil microbial community diversity in paddy fields. Environ. Sci. 2020, 41, 4262–4272. (In Chinese) [Google Scholar]
- Li, Y.Y. Microbial Diversity and Enzyme Activity of Soil Reclaimed by Hydraulic Dredge Pump in Coal-Mining Subsidence Areas; Xuzhou: China University of Mining and Technology: Xuzhou, China, 2015. (In Chinese) [Google Scholar]
- Zhang, Y.J.; Wu, T.; Zhao, J.; Liu, S.L.; Yu, Y.G.; Zhang, H. Effect of biochar amendment on bacterial community structure and diversity in straw-amended soils. Acta Sci. Circumstantiae 2017, 37, 712–720. (In Chinese) [Google Scholar]




| Treatment | Percentage Content of Water-Stable Aggregate (%) | |||||
|---|---|---|---|---|---|---|
| WSA1 5 mm | WSA 2 2 mm | WSA 3 1 mm | WSA 4 0.5 mm | WSA 5 0.25 mm | WSA 6 <0.25 mm | |
| CK | 27.80 ± 1.01 a | 7.73 ± 0.47 a | 7.34 ± 0.38 a | 9.243 ± 0.92 a | 9.44 ± 2.79 a | 40.62 ± 1.41 a |
| BC | 29.40 ± 4.54 a | 8.95 ± 0.54 a | 8.32 ± 2.15 a | 11.92 ± 0.61 a | 10.38 ± 0.23 a | 31.89 ± 1.13 b |
| Soil Sampling Depth | Treatment | pH | SOM (g kg−1) | TN (g kg−1) | TP (g kg−1) | TK (g kg−1) | AHN (mg kg−1) | AP (mg kg−1) | AK (mg kg−1) |
|---|---|---|---|---|---|---|---|---|---|
| 0–10 cm | CK | 6.53 ± 0.06 a | 21.30 ± 0.52 a | 1.09 ± 0.06 a | 0.92 ± 0.02 a | 19.24 ± 0.47 a | 110.45 ± 6.74 a | 37.74 ± 2.75 b | 235.67 ± 16.78 b |
| BC | 6.55 ± 0.17 a | 23.05 ± 0.83 a | 1.13 ± 0.04 a | 0.93 ± 0.02 a | 20.26 ± 0.22 a | 112.85 ± 4.78 a | 46.19 ± 4.47 a | 280.35 ± 17.22 a | |
| 10–20 cm | CK | 6.55 ± 0.11 a | 19.42 ± 0.99 a | 1.04 ± 0.05 a | 0.83 ± 0.02 a | 17.47 ± 0.74 a | 100.84 ± 5.11 a | 32.29 ± 2.67 b | 208.13 ± 13.49 b |
| BC | 6.60 ± 0.16 a | 22.83 ± 2.21 a | 1.09 ± 0.07 a | 0.89 ± 0.05 a | 18.76 ± 0.57 a | 109.37 ± 4.65 a | 39.42 ± 2.21 a | 230.77 ± 18.19 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, Y.; Xu, L.; Guo, X.; Liu, J.; Zou, B.; Guo, Y.; Zhang, Y.; Li, H.; Zheng, G.; Guo, Y.; et al. Effect of Biochar on Soil Physiochemical Properties and Bacterial Diversity in Dry Direct-Seeded Rice Paddy Fields. Agronomy 2023, 13, 4. https://doi.org/10.3390/agronomy13010004
Lv Y, Xu L, Guo X, Liu J, Zou B, Guo Y, Zhang Y, Li H, Zheng G, Guo Y, et al. Effect of Biochar on Soil Physiochemical Properties and Bacterial Diversity in Dry Direct-Seeded Rice Paddy Fields. Agronomy. 2023; 13(1):4. https://doi.org/10.3390/agronomy13010004
Chicago/Turabian StyleLv, Yandong, Lingqi Xu, Xiaohong Guo, Jiajun Liu, Bing Zou, Yukun Guo, Yanfei Zhang, Hongyu Li, Guiping Zheng, Yongxia Guo, and et al. 2023. "Effect of Biochar on Soil Physiochemical Properties and Bacterial Diversity in Dry Direct-Seeded Rice Paddy Fields" Agronomy 13, no. 1: 4. https://doi.org/10.3390/agronomy13010004






