Abstract
Bacteria and fungi are good indicators for soil health as well as soilless growing media (SGM) health. However, there is very limited information about the fungal and bacterial communities for SGM. In the present study, coir substrate and peat-based substrate were used as typical SGM under drip irrigation and tidal irrigation to understand the situation of fungal and bacterial communities by high-throughput sequencing technology. In this study, both environmental factors and microbial communities were significantly affected by SGM type and irrigation pattern, in which SGM type played a major role and irrigation pattern played a minor role. The bacterial phyla Actinobacteriota and Proteobacteria and the fungal phyla Ascomycota were more closely related to environmental factors including EC, pH, NO3−, NH4+ and ω as well as urease and phosphatase. The bacterial and fungal communities in the two SGM had some similarities with those in soil. In addition, the functions of the soil, including key soil organisms, carbon mineralization, wood decomposition, nitrification, denitrification, carbon fixation, nitrogen fixation and methanotrophy, could be basically performed by the two SGM. In general, the SGM should possess common soil capabilities according to bacterial and fungal analyses, but there are numerous fungi of unknown function that need be addressed in the future. Meanwhile, these results improve our understanding of the correlation between the environmental factors and the microbiome, and provide basic guidance for management and research on SGM in the future.
1. Introduction
Urbanization has contributed to a rapid development of greenhouse vegetable production in China, resulting in serious problems in soil due to the overuse of agricultural inputs [1]. In order to overcome the soil problems, soilless culture systems (SCS) used as substitutes are garnering more and more attention [2]. Currently, the common SCSs include soilless growing media (SGM) and hydroponic systems. However, the SGM are more applied because of the suitable characteristics for the growth of most plants, such as adequate porosity, low soluble salt content and high content of available water [3]. The SGM with many advantages are developing rapidly in urban and peri-urban areas to take the role of soil. Correspondingly, while research on SGM is increasing, most of it focuses on their physical and chemical properties rather than their biological properties [4]. Since the microbiomes in SGM have not been studied as extensively as soil, there is limited information about the microbiomes in SGM. Therefore, it is necessary to explore microbiomes in SGM that may play an important role in plant growth.
Many studies have shown that the microbiome in soil is closely related to the health and biomass of plants [5,6,7]. Soil stores not only the organic matter but also the live heterotrophic soil organisms, including protists, bacteria, archaea, fungi and animals. The diverse microbiome living in soil is critical for organic matter decomposition and nutrient cycling. They, especially rhizosphere microbes, can manage the soil fertility by regulating the absorption and release of the main elements of soil [5]. This is mainly related to the fact that soil microbiomes can transform organic matter into inorganic matter and provide effective nutrients for plants [8]. Some rhizosphere microbes can also secrete vitamins and plant growth-stimulating hormones to promote plant growth and improve the tolerance of adverse environmental conditions [9,10]. Among them, bacteria and fungi dominate soil habitats in biomass, biodiversity and essential-soil processes [11]. Community analysis based on bacteria and fungi reveals that soil microbiomes vary in structure and function in different ecosystems [12]. Many studies have proposed that soil type might be the determinant factor that shapes the composition of bacterial and fungal communities, as each soil has its own physicochemical characteristics and thus maintains a specific community under equilibrium conditions [13]. In addition, the structure and function of bacteria and fungi (for a specific soil) are also highly dependent on abiotic environmental factors in the soil. Previous studies have investigated the effects of soil physicochemical characteristics on microbial communities, such as water input, pH, organic matter content, salinity and soil particle size [14,15].
However, the bacterial and fungal communities in SGM should be largely affected by the material of SGM [13]. In theory, any material that meets plant growth can be used as an SGM, such as peat, coir, mineral wool and sand. This leads to the essential differences in physical, chemical and biological properties among different SGM, and between SGM and soil. Therefore, it is difficult to study SGM because of their complexity and diversity. Currently, coir and peat substrates are the most widely used and representative organic SGM in SCSs in China as well as in the world, accounting for over 80% of the organic SGM [2,16]. With the development of soilless culture technology, these substrates will be used more and more in the future, and so they should be studied first. There may be differences in pH, electrical conductivity (EC), organic matter (OM), water content and nutrient adsorption between the two SGM due to different material composition—which may be the main abiotic environmental factors affecting bacteria and fungi. Remarkably, the rhizosphere microbiome may play a key role in SGM, wherein roots tend to fill due to limited space. Consequently, a study of microbial communities of SGM may contain the rhizosphere microbiome. In addition, it is also very vital that irrigation as an agronomic management practice impacts bacterial and fungal preference. In SCSs, drip irrigation and tidal irrigation are common irrigation patterns. Therefore, the objectives of this study are (1) to evaluate the bacterial and fungal communities of the coir and peat substrates under drip irrigation and tidal irrigation; (2) to clarify the effect of the physicochemical properties of the two SGM under the two irrigation patterns on bacteria and fungi; and (3) to reveal the structure and function of bacteria and fungi for the SGM in replacing soil. The results will help to understand the structure and function of the microbiome in the typical SGM under common irrigation patterns, and to establish guidance towards optimal management practices for plant cultivation in SCSs.
2. Materials and Methods
2.1. Experimental Design
Greenhouse trials were conducted at the Chengdu Academy of Agricultural and Forestry Sciences (139° E, 30.7° N), situated in Chengdu, Sichuan Province, China, in 2020. Two typical SGM, including a coir substrate imported from a foreign source (Galuku Pty Ltd., Sydney, Australia) and a peat-based substrate including 60% peat, 20% perlite, 18% vermiculite and 2% plant fiber (China patent No. CN202011106379.6), were evaluated with an equal volume on the planting of tomatoes (Solanum lycopersicum cv. SV7846TH) under drip irrigation and tidal irrigation systems. The peat-based substrate with drip irrigation and tidal irrigation were named DL and TL treatments, and the coir substrate with drip irrigation and tidal irrigation were named DY and TY treatments, respectively. There were 4 replicates (plots) for each treatment. The water and nutrients were supplied with the irrigation systems to meet plant growth needs (see Supplementary Table S1). Initial characteristics of both coir substrate and peat-based substrate were listed in Supplementary Table S2.
2.2. Cultivation and Sampling
Seeds were sown into expanded polystyrene trays with an inverted pyramid design and filled with the two SGM on 12 March 2020. The uniform seedlings were transplanted from a growth chamber (125 cm × 50 cm × 128 cm), after 16 h in the light and 8 h in the dark, to a U-shaped slot in a greenhouse (60 m × 40 m) on 9 April 2020. After being cultivated for 50 days, the plants were hand-pulled from the SGM, and the roots were shaken vigorously to obtain SGM samples. The SGM samples were carefully collected with a sterile tube. After removing residual plant residues (e.g., roots) and passing the samples through a 2 mm sieve, one portion of the samples was stored in a −80 °C freezer for future microbial analysis, and the other portion was extracted directly or air-dried for chemical analysis.
2.3. Determination of Soilless Growing Media Characteristics
A part of the fresh SGM samples was used to measure mass water content (ω). The remainder was shaken with 2M KCl solution (1:10 w/v) for 1 h. The content of NH4+ and NO3− in KCl extracts was measured using a continuous flow analyzer (QuikChem8000, LACHAT, Milwaukee, USA). The air-dried SGM samples were extracted with de-ionized water (1:20, w/v) by shaking for 1 h. The pH and electrical conductivity (EC) in the water extracts were measured using a pH meter (FE20; Mettler Toledo, Schwerzenbach, Switzerland) and a conductivity meter (SG3, Mettler Toledo, Schwerzenbach, Switzerland), respectively. Urease and neutral phosphatase activities that are closely related to substrate fertility were also determined using soil enzyme testing kits (Solarbio, Beijing, China) according to the manufacturer’s instructions. In addition, we obtained the tomato yield by weighing.
2.4. Extraction of DNA and Illumina MiSeq DNA Sequencing
The total microbial genomic DNA of the different SGM samples was extracted. The DNA extraction was performed using the Fast DNA SPIN Kit for soil (MP Biomedicals, Santa Ana, CA, USA) according to the manufacturer’s protocols. The extracted all-parallel samples for each treatment were mixed together before sequencing [17,18]. The DNA concentration and quality were determined using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Wilmington, NC, USA). The V4–V5 hypervariable regions of the bacterial 16S rRNA gene were amplified with universal primers 515F (5′-GTGCCAGCMGCCGCGGTAA-3′) and 909R (5′-CCCCGYCAATTCMTTTRAGT-3′) as described previously [19]. The internal transcribed spacer (ITS) regions of ribosomal RNA derived from fungi were amplified using primers ITS1F (5′-CTTGGTCATTTAGAGGAAGTAA-3′) and ITS2R (5′-TGCGTTCTTCATCGATGC-3′) [20]. PCR product purification and Illumina sequencing were performed as reported previously [21].
2.5. Data Processing and Analysis
Raw fastq files were quality-filtered by Trimmomatic and merged by FLASH; low-quality sequences and chimeras were removed [22,23]. Then, the sequences were clustered into operational taxonomic units (OTUs, 97% similarity) using Uparse [24]. The taxonomy of each 16S rRNA gene sequence was analyzed by the RDP Classifier algorithm (http://rdp.cme.msu.edu/, accessed on 20 July 2022) against the Silva 16S rRNA database [25] and the Unite database [26] for bacteria and fungi, respectively. Moreover, chloroplasts and mitochondria were removed for downstream analysis.
The sequence was normalized to obtain the minimum read counts of all samples. The alpha-diversity index was calculated using MOTHUR software [27]. The circus was created using R (version 4.0.2) to show the bacteria and fungi at the phylum level. The hierarchically clustered heatmap, based on Bray-Curtis similarity index, was created using R to show the bacteria and fungi at the genus level (top 30 genera). Principal co-ordinates analysis (PCoA) was also analyzed using the R package vegan. The correlation network analysis based on Spearman’s correlation was conducted using Cytoscape (version 3.9.1) to evaluate the correlation between the environmental factors and primary genera (mean relative abundance > 1%). The bacterial and fungal functional profiles were predicted by PICRUSt 2 depending on the KEGG pathway database and by FunGuild according to an open fungal taxa annotation, respectively. One-way and two-way analyses of variance (ANOVA) were used to assess differences between treatment groups in terms of the physicochemical characteristics of the SGM using R. Differences were considered significant at p < 0.05, with a separation of mean values by a Tukey test.
3. Results
3.1. Characteristics of the Different Treatments
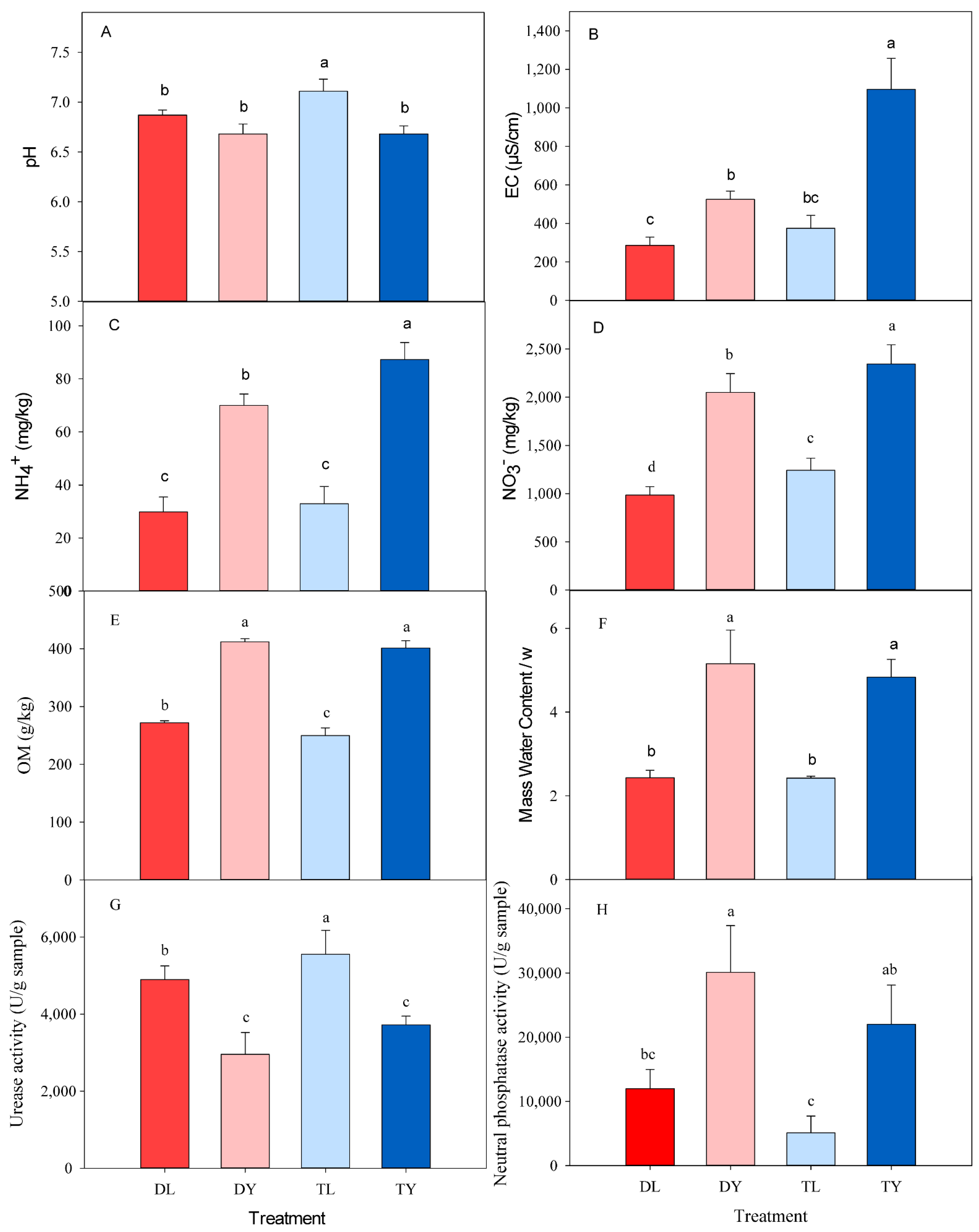
The pH values for all treatments were circumneutral, ranging from 6.46 to 7.19 (Figure 1A). In addition, the EC, OM, NO3−, NH4+, ω, urease activity and phosphatase activity varied in the different treatments, ranging from 272 to 1207 μS/cm, 220.4 to 415.9 g/kg, 878.5 to 2566.6 mg/kg, 22.9 to 95.0 mg/kg, 2.2 to 5.8, 2192 to 6434 U/g, and 2426 to 36799 U/g, respectively (Figure 1B–H). The values for EC, OM, NO3−, NH4+, ω and phosphatase activity in the DY and TY treatments were significantly larger than that in the DL and TL treatments, while the values for pH and urease activity were opposite (Table 1, p < 0.01). There were interactive effects on pH, EC, NO3− and NH4+ between SGM type and irrigation pattern (Table 1, p < 0.05).
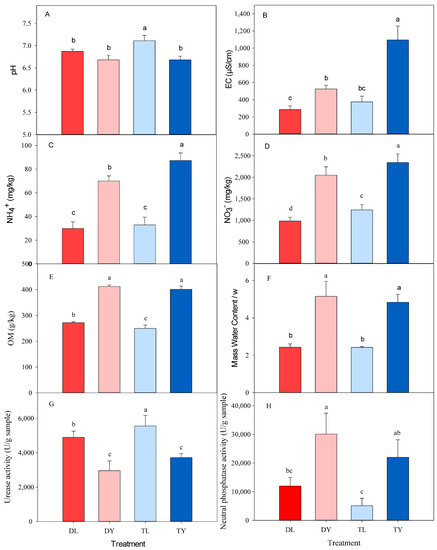
Figure 1.
Characteristics parameters of different treatments. (A) pH; (B) EC; (C) NH4+ content; (D) NO3− content; (E) OM content; (F) mass water content/ω; (G) urease activity; (H) neutral phosphatase activity; DL, drip irrigation + peat-based substrate; DY, drip irrigation + coir substrate; TL, tidal irrigation + peat-based substrate; TY, tidal irrigation + coir substrate. The same below. Different lowercase letters indicate statistically significant differences at 5% level. Results are average values of the treatments ± SD (n = 4).

Table 1.
Mean values of different treatments in pH, EC, OM, NO3−, NH4+, ω, urease and neutral phosphatase.
3.2. Bacterial and Fungal Diversity and Structure of the Different Treatments
A total of 195,431 bacterial 16S rRNA (DL: 50,756; TL: 48,096; DY: 50,392; TY: 46,187) and 282,035 fungal ITS (DL: 66,420; TL: 73,427; DY: 70,279; TY: 71,909) high-quality sequences were obtained from the four treatments, which respectively were given into 1830 and 397 OTUs (Table 2). The coverage was close to 1, showing that the results were reliable (Table 2). The OTU numbers and Chao1 richness in the TL and DL treatments were obvious larger than that in the TY and DY treatments for both bacteria and fungi, while Simpson diversity showed this trend only for bacteria and Shannon diversity showed the same result only for fungi (Table 2). In addition, each treatment had some common and unique bacterial and fungal OTUs based on the Venn diagram (Figure S1).

Table 2.
MiSeq sequencing coverage and alpha diversity of bacterial 16S rRNA gene and fungal ITS.
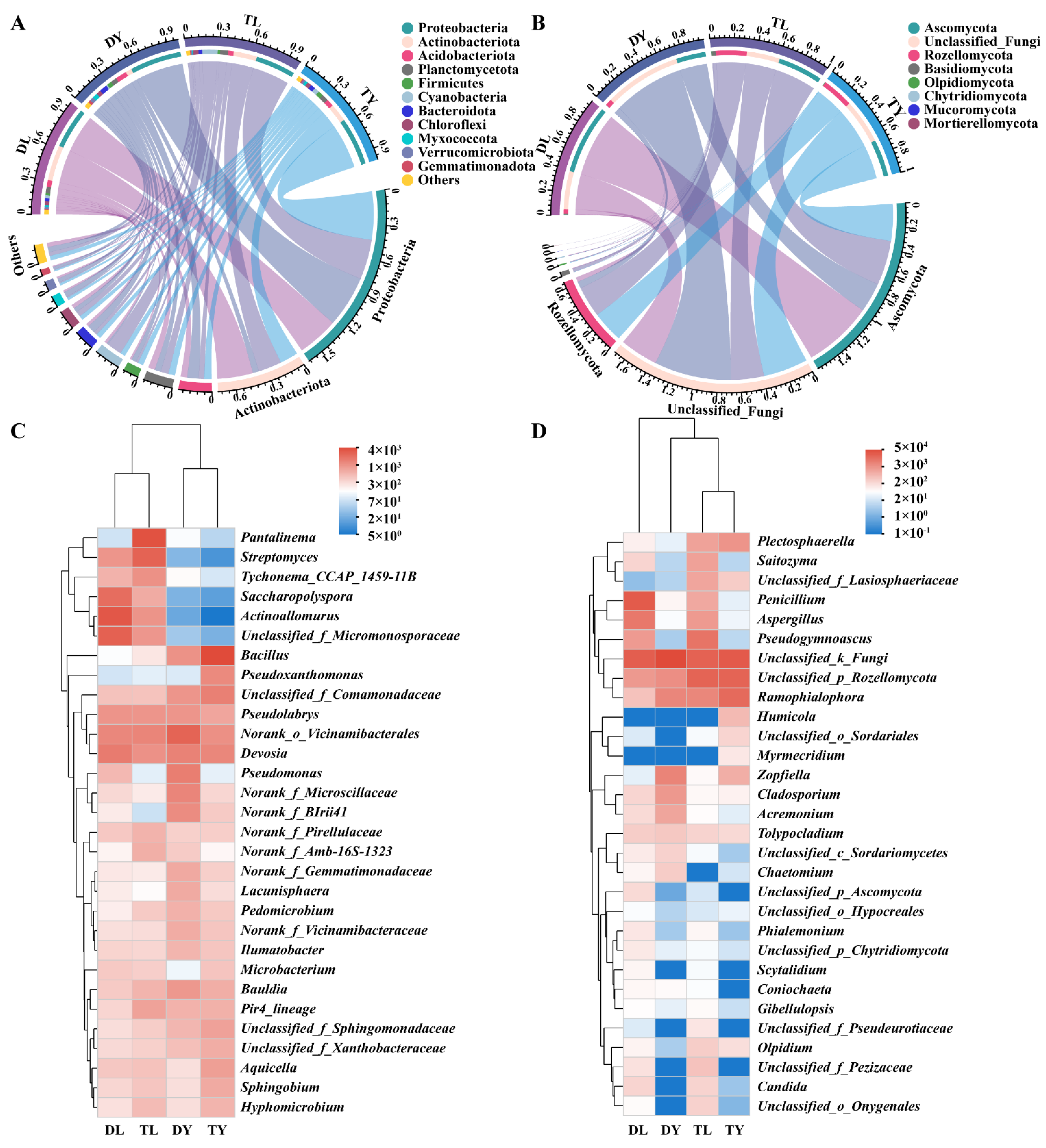
As shown in Figure 2A,B, the relative abundance was analyzed for bacteria and fungi at the phylum level. For bacteria, Proteobacteria, as the largest phylum in the DL (37.09%) and TL (36.05%) treatments, were lower than that in the DY (46.69%) and TY (54.19%) treatments. However, Actinobacteria and Planctomycetes in the DL (32.16%, 6.07%) and TL (21.12%, 7.22%) treatments were higher compared to in the DY (5.52%, 5.55%) and TY (6.97%, 5.24%) treatments. In addition, Acidobacteria in the DY treatment, Firmicutes in the TY treatment and Cyanobacteria in the TL treatment had higher relative abundance than other treatments. For fungi, Ascomycota was the dominant phylum in all treatments, especially in the DL treatment, followed by Rozellomycota in the TL and TY treatments, which was obviously higher than that in the DL and DY treatments. Remarkably, there were many unclassified fungal phyla in the four treatments, with the DY treatment having the most.
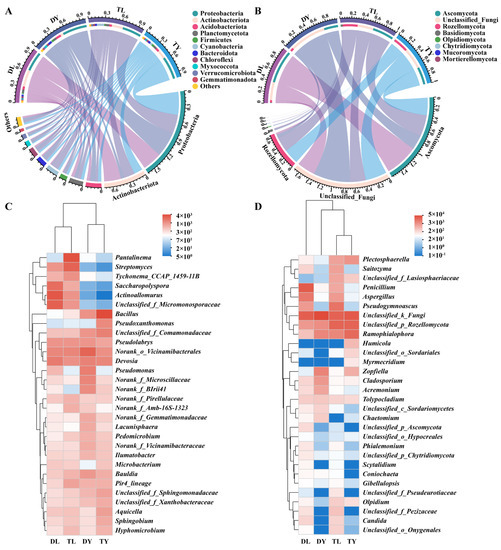
Figure 2.
Microbial community structure of the soilless growing media. (A) Circus analysis for bacteria at phylum level; (B) circus analysis for fungi at phylum level; (C) hierarchically clustered heatmap for bacteria at genus level; (D) hierarchically clustered heatmap for fungi at genus level. Data show as mean value. The same below.
In addition, the hierarchically clustered heatmap showed the relationship of bacteria and fungi among the four treatments (Figure 2C,D). For bacteria, there were close relationships between DL and TL treatments and between DY and TY treatments. For fungi, a close relationship was found between TL and TY treatments and between DL and DY treatments. It can be also observed that the Devosia, Bacillus, Actinoallomurus, Pseudolabrys, Pantalinema, Streptomyces, Saccharopolyspora, Bauldia, Pir4_lineage and Pseudomonas were the representative bacterial genera, and Penicillium, Ramophialophora, Pseudogymnoascus, Aspergillus, Zopfiella and Plectosphaerella were the representative fungal genera.
3.3. Effects of Environmental Parameters on Microbial Community under Different Treatments
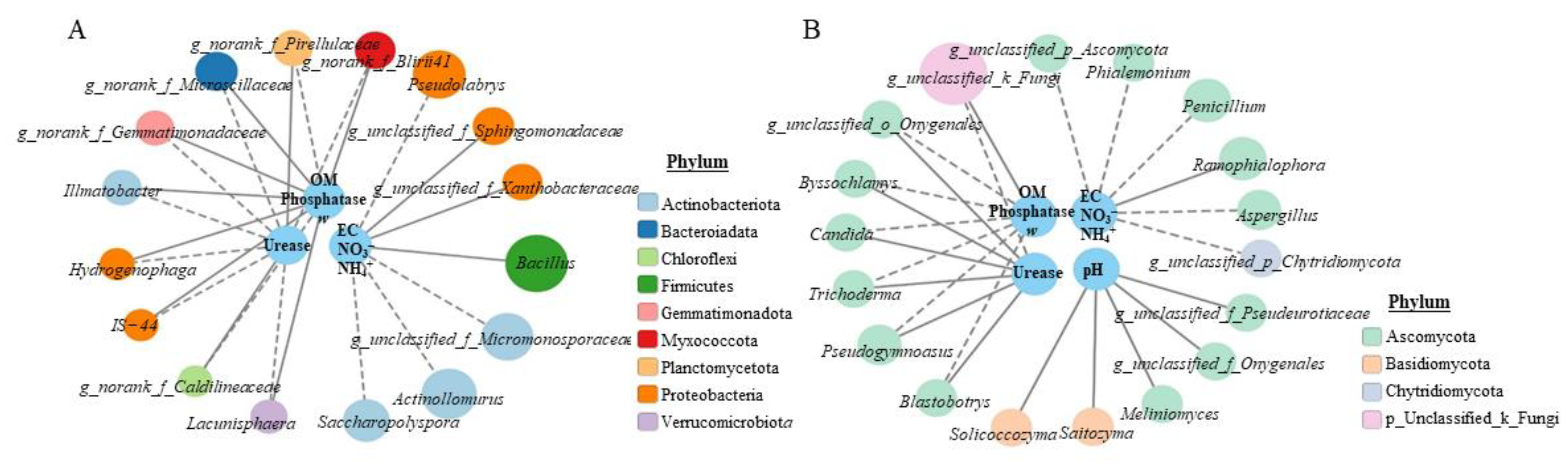
To reveal the correlation between environmental parameters and primary genera, correlation network analysis was performed based on Spearman’s correlation coefficients (p < 0.01). As shown in Figure 3A,B, the urease, phosphatase, OM and ω were significantly correlated with norank_f__Microscillaceae, norank_f__BIrii41, norank_f__Pirellulaceae, Ilumatobacter, norank_f__Gemmatimonadaceae, Lacunisphaera, Hydrogenophaga and IS-44 (p < 0.05), but there was a reverse correlation between urease and phosphatase, and OM and ω. The EC, NO3− and NH4+ were significantly correlated with Bacillus, Actinoallomurus, Pseudolabrys, unclassified_f__Micromonosporaceae, Saccharopolyspora, unclassified_f__Sphingomonadaceae and unclassified_f__Xanthobacteraceae (p < 0.01), while pH was not significantly correlated with these bacterial genera at p < 0.01 level. It was also found that actinobacteriota containing four influential genera and proteobacteria containing five influential genera were the core bacterial phyla in SGM. For fungi, the urease, phosphatase, OM and ω were significantly correlated with unclassified_k__Fungi, Pseudogymnoascus, Candida, unclassified_o__Onygenales, Blastobotrys, Trichoderma and Byssochlamys (p < 0.05), but there was an opposite correlation between the urease and phosphatase, OM and ω. In addition, EC, NO3− and NH4+ were significantly correlated with Penicillium, Ramophialophora, Aspergillus, unclassified_p__Ascomycota, Phialemonium and unclassified_p__Chytridiomycota (p < 0.05); pH was significantly correlated with Saitozyma, unclassified_f__Pezizaceae, unclassified_f__Pseudeurotiaceae, Solicoccozyma and Meliniomyces (p < 0.05). The core fungal phyla were ascomycota containing 14 influential genera.
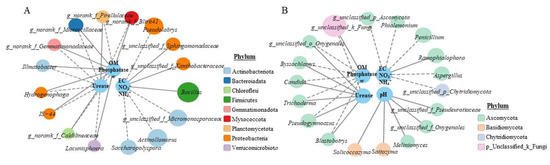
Figure 3.
The correlation between environmental parameters and genera for different treatments. (A) Network analysis for bacteria; (B) network analysis for fungi. The grey solid line indicates a positive correlation, whereas the grey dotted line indicates a negative correlation (A,B). The bigger the circle, the higher relative abundance (A,B).
3.4. Bacterial and Fungal Function Prediction
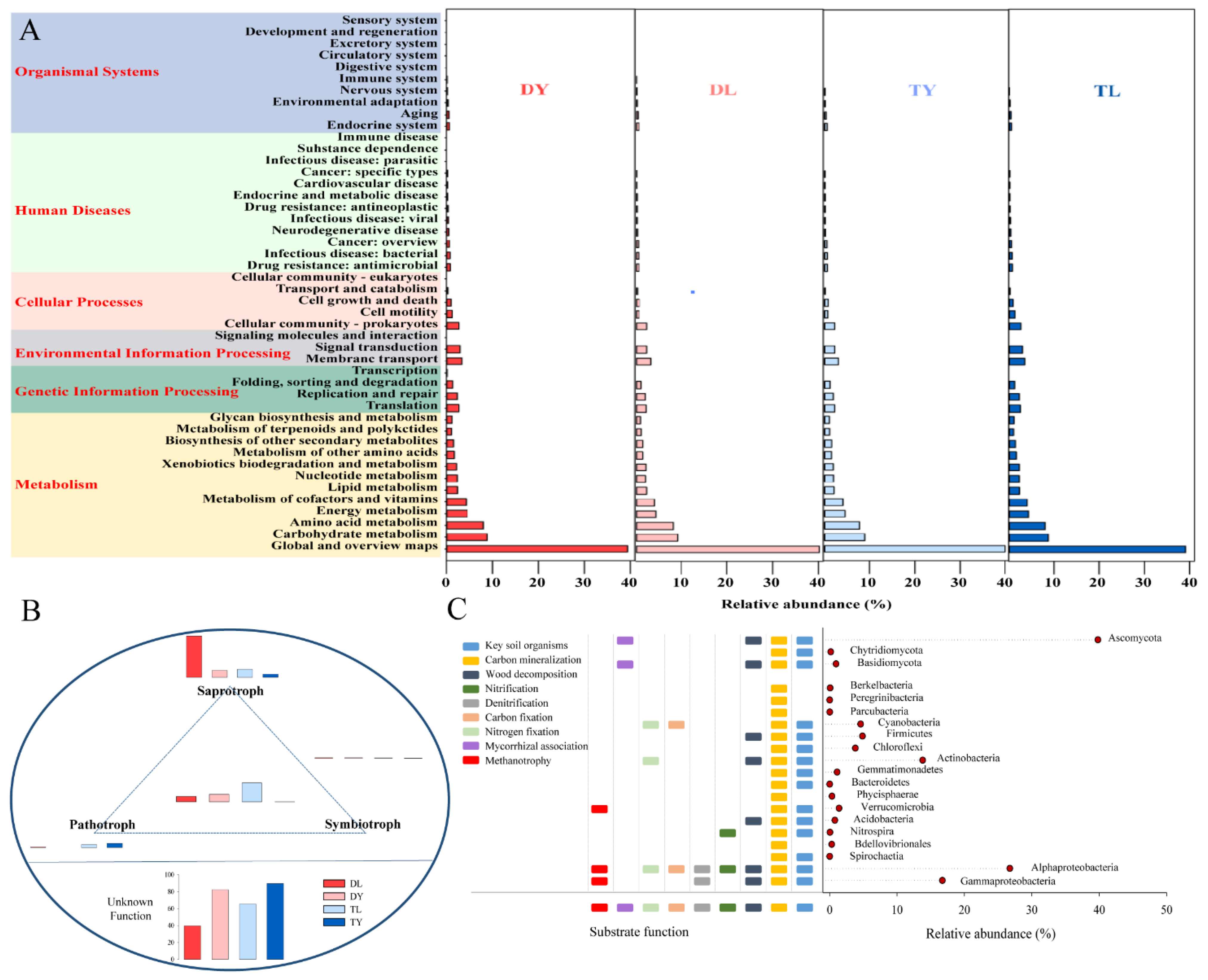
As shown in Figure 4A, the bacterial functions predicted metabolism (76.34~78.56%), genetic information processing (5.86~6.29%), environment information processing (5.70~6.32%), cellular processes (4.37~5.10%), human diseases (3.60~4.23%) and organismal systems (1.87~1.95%). In a deeper level, the global and overview maps (39.11~40.19%), carbohydrate metabolism (8.68~9.12%) and amino acid metabolism (7.89~8.24%) were main bacterial functions. Furthermore, there was no significant difference in these bacterial functions among the four treatments. The fungal functions were assigned into saprotrophy, symbiotrophy and pathotrophy. As shown in Figure 4B, the fungi could perform one, two or even three of the three functions. Apart from unknown functional fungi, fungi performing single saprotrophy had the highest abundance, with 4.3~53.14%, followed by fungi performing all three functions, with 2.8~18.9%. It also can be seen that there were obvious differences in these fungal functions among the four treatments.
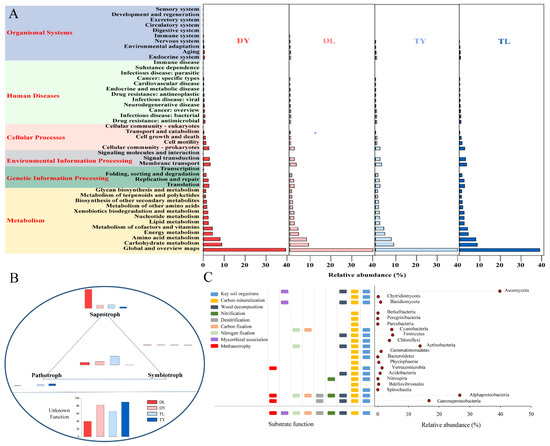
Figure 4.
Function prediction of microbial communities for soilless growing media. (A) Bacterial functional profiles; (B) fungal functional profiles; (C) common functional profiles.
In the four treatments, some common functions performed by soil organisms were also predicted based on the same bacteria or fungi with soil (Figure 4C) [28]. The bacterial functions involved key soil organisms, carbon mineralization, wood decomposition, nitrification, denitrification, carbon fixation, nitrogen fixation and methanotrophy. The fungal functions included key soil organisms, carbon mineralization, wood decomposition and mycorrhizal association.
4. Discussion
Most of the current research mainly focuses on soil rather than soilless growing media (SGM). Accordingly, irrigation patterns based on SGM in soilless culture systems (SCS) are less studied. Therefore, the peat-based substrate and coir substrate under drip irrigation and tidal irrigation were sampled and analyzed in this study. The EC, OM, NO3−, NH4+, ω and phosphatase activity in the coir substrate was higher than the peat-based substrate, whereas the pH and urease activity were the opposite (Figure 1). These distinct differences should be related to the components of the two SGM that come from different materials. The difference for EC, NO3− and NH4+ mainly is due to the fact that coir substrate contains or absorbs more inorganic salt compared to peat-based substrate [29]. A similar phenomenon is also found between different soil ecosystems with complex environmental conditions [30]. However, the SGM, as a kind of SCS, very much differs from soil ecosystems and are rarely studied. Therefore, we cannot obtain decisive information based on the different physicochemical properties of the two SGM. Furthermore, the pH, EC, OM, NO3−, NH4+, urease and neutral phosphatase were also significantly affected by irrigation patterns (Table 1, p < 0.05), indicating that the importance of irrigation pattern in SCS is the same as in many soil ecosystems [31]. In summary, the SGM type and irrigation pattern are important for the tomato-growing environment in SCSs, although tomato yield was not sensitive to different SGM types or irrigation patterns (Figure S2).
The microbiomes of SGM in CSC remain unclear, but they are critical for the health and biomass of crops [32]. In this study, hence, bacteria and fungi as the main microbiome were analyzed to ascertain their situation in two typical SGM under two common irrigation patterns. From the OTU numbers and α diversity perspective, the bacteria and fungi of the SGM samples in the four treatments were distinctly lower than those in common soil ecosystems [33,34,35], and were larger than those in raw SGM (below detection limit). Therefore, most bacteria and fungi in the SGM samples might be adsorbed from exogenous sources, such as irrigation water and the atmosphere. However, different SGM types and irrigation patterns may also change the adsorption effect on the microbiome. As observed, the four treatments exhibited unique OTUs (Figure S1) and differed in diversity for bacteria and fungi (Table 2). At the phylum level, these differences caused by SGM were also embodied in the bacterial community, while the differences in the fungal community were more susceptible to irrigation patterns (Figure 2A,B). The analysis of the hierarchically clustered heatmap at the genus level showed to be more intuitive for this point (Figure 2C,D). In any case, the PCoA results can also prove these affinity relationships (Figure S3). Similarly, many studies on soil ecosystems also confirmed that bacteria are sensitive to soil type [5,36], and fungi are sensitive to irrigation pattern [11,37]. Moreover, Proteobacteria, Actinobacteria, and Acidobacteria were essential members for the bacterial phyla (Figure 2A), which is consistent with previous studies on soil [7,38]. The fungal phyla, including Ascomycota, Rozellomycota and Basidiomycota (Figure 2B), have been reported to be the majority groups in the many soil ecosystems [39,40]. What is noteworthy is that unclassified k Fungi accounted for an average of 42.49% of the fungal community in all treatments, which was quite different from previous studies on soil fungal communities [35,40]. This result suggested that the two SGM had numerous unknown fungi which may be associated with the material composition and fabrication process of coir substrate and peat-based substrate. Therefore, these unknown fungi need further investigation in the future. In addition, the bacterial genera and fungal genera from the two SGM can also be observed in many soil ecosystems [18,41], indicating that there may be some similarities in the microbial communities between the SGM and soil.
Previous studies demonstrated that microbial communities vary with environmental conditions such as pH [42,43], organic matter content [33,44], water content [45,46], various nutrients content [14], soil particle size [47] and other physicochemical properties of soil [12]. Compared to soil, there are more diverse physical and chemical characteristics among SGM, and thus different SGM may differ in the diversity and structure of their microbiome. In this study, the EC, pH, OM, NO3−, NH4+, ω, urease and phosphatase were analyzed as environmental parameters. According to the correlation network analysis between environmental parameters and primary genera, there was a similar effect among EC, NO3− and NH4+; among OM, ω and phosphatase; and between pH and urease. In addition, pH had a more significant effect on the fungal community, based on this network analysis. Although pH and EC are regarded as important factors on affecting the microbial community in the soil [48,49], they do not show such differences in this study. This might be associated with the widening of differences caused by salt accumulation in the SGM in this study—the same phenomenon is found in soil with salinity gradients [30]. Furthermore, Actinobacteria, Proteobacteria and Ascomycota were core phyla of this study, and they are also more important phyla in saline soil compared to non-saline soil [35,50]. However, high EC values were observed only in the coir substrate (Figure 1B). Therefore, we speculate that the salinization problems are more likely to occur in coir substrate. It also was clearly observed that there were many bacterial or fungal phyla significantly associated with urease and phosphatase, as well as EC, pH, OM, NO3−, NH4+ and ω, suggesting that the nutrient function of SGM influenced the multiple bacteria and fungi. Therefore, a comprehensive understanding of the microbiome helps to explore more function of SGM.
In this study, the functions performed by bacteria and fungi were predicted by PICRUSt 2 and FunGuild, respectively. For bacteria, metabolism was the primary function, while its abundance in the SGM was higher than that in common soil [51] and compost [18]. On a deeper level, overview maps, carbohydrate metabolism and amino acid metabolism were more abundant. Carbohydrate metabolism can degrade hemicellulose and cellulose [52], and amino acid metabolism can provide carbon and energy sources for microbial growth and activity [53]. Therefore, it can be inferred that the transformation of organic matter played vital roles in this study. For fungi, saprotrophy was the main function apart from an unknown function, especially in peat-based substrate, indicating a large number of dead organisms in the peat-based substrate and coir substrate. Similarly, key soil organisms, carbon mineralization and wood decomposition were observed based on the same bacteria and fungi from the previous study [28], which supports our judgment above. Meanwhile, other soil functions, such as nitrification, denitrification, carbon fixation, nitrogen fixation and methanotrophy, were also observed in the two SGM [28]. Furthermore, Pseudomonas and Bacillus were the most representative bacterial genera in the two SGM. They play a vital role in biocontrol and plant-growth-promoting activity in the soil [54]. In summary, either the peat-based substrate or the coir substrate can basically perform soil functions in terms of their microbiome.
5. Conclusions
Identifying the microbiome information is essential for improving mutually favorable relationships between soilless growing media (SGM) and plants, and to guide management practices in soilless culture systems (SCS). In this study, both environmental factors and microbial communities were significantly affected by SGM type and irrigation pattern, in which SGM type played a major role and irrigation pattern played a minor role. In addition, although there were some differences in bacterial and fungal community structures among different treatments, the microbial functions and tomato yield had no significant differences between the two SGM. We also found that the common soil functions can basically be performed by the two SGM. These results indicated that peat-based substrate and coir substrate are very good SGM and can be used to replace the soil. Furthermore, it is likely that the bacteria and fungi are primarily derived from exogenous adsorption rather than from the SGM itself. Therefore, it is speculated that other SGM can also perform similar functions. In addition, numerous unknown fungi and other environmental factors in the two SGM, such as porosity and nutrient content, need be investigated in later research. In general, the SGM should possess common soil capabilities, but there are some problems that need be addressed in the future.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy13010006/s1, Figure S1: Venn diagram of bacteria (a) and fungi (b) at OUT level for different treatment; Figure S2: The tomato yield of different treatments; Figure S3: Principal Co-ordinates Analysis (PCoA) of bacteria (a) and fungi (b) at OTU level for different treatments; Table S1: Composition of nutrient solution; Table S2: Initial characteristics of soilless growing media.
Author Contributions
J.L.: Conceptualization, methodology, investigation, data curation, formal analysis, writing, review and editing. W.L.: conceptualization, methodology, investigation, writing, review and editing, funding acquisition, project administration. W.Z.: data curation, writing, review and editing. R.Y.: data curation, writing, review and editing. H.W.: investigation, writing, review and editing. D.Z.: formal analysis, writing, review and editing. Y.L.: writing, review and editing. Z.Q.: writing, review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by The National Natural Science Foundation of China (42107040), The Agricultural Science and Technology Innovation Program (ASTIP), and the Local Financial of National Agricultural Science & Technology Center, Chengdu (NASC).
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its Supplementary Information files. The sequencing raw reads were deposited in the NCBI Sequence Read Archive (SRA) database with the following accession numbers: PRJNA695038 (https://www.ncbi.nlm.nih.gov).
Acknowledgments
The authors would like to acknowledge Zheng Wang and Tao Huang for technical assistance in the field experiments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lv, H.; Zhao, Y.; Wang, Y.; Wan, L.; Wang, J.; Butterbach–Bahl, K.; Lin, S. Conventional flooding irrigation and over fertilization drives soil pH decrease not only in the top–but also in subsoil layers in solar greenhouse vegetable production systems. Geoderma 2020, 363, 114156. [Google Scholar] [CrossRef]
- Gruda, N.S. Advances in Horticultural Soilless Culture; Burleigh Dodds Science: Cambridge, UK, 2021; Available online: https://bdspublishing.com (accessed on 6 May 2022).
- Gruda, N.S. Increasing sustainability of growing media constituents and stand-alone substrates in soilless culture systems. Agronomy 2019, 9, 298. [Google Scholar] [CrossRef]
- Banitalebi, G.; Mosaddeghi, M.R.; Shariatmadari, H. Evaluation of physico-chemical properties of biochar-based mixtures for soilless growth media. J. Mater. Cycles Waste 2021, 23, 950–964. [Google Scholar] [CrossRef]
- Liu, L.; Huang, X.; Zhang, J.; Cai, Z.; Jiang, K.; Chang, Y. Deciphering the relative importance of soil and plant traits on the development of rhizosphere microbial communities. Soil Biol. Biochem. 2020, 148, 107909. [Google Scholar] [CrossRef]
- Lin, W.; Ding, J.; Xu, C.; Zheng, Q.; Zhuang, S.; Mao, L.; Li, Q.; Liu, X.; Li, Y. Evaluation of N2O sources after fertilizers application in vegetable soil by dual isotopocule plots approach. Environ. Res. 2020, 188, 109818. [Google Scholar] [CrossRef]
- Li, J.; Ren, G.; Jia, Z.; Dong, Y. Composition and activity of rhizosphere microbial communities associated with healthy and diseased greenhouse tomatoes. Plant Soil 2014, 380, 337–347. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, Q.; Yang, R.; Zhuang, S.; Lin, W.; Li, Y. Evaluating microbial role in reducing N2O emission by dual isotopocule mapping following substitution of inorganic fertilizer for organic fertilizer. J. Clean. Prod. 2021, 326, 129442. [Google Scholar] [CrossRef]
- Berendsen, R.L.; Pieterse, C.M.; Bakker, P.A. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef]
- el Zahar Haichar, F.; Santaella, C.; Heulin, T.; Achouak, W. Root exudates mediated interactions belowground. Soil Biol. Biochem. 2014, 77, 69–80. [Google Scholar] [CrossRef]
- Zhang, R.; Gu, J.; Wang, X. Responses of soil bacteria and fungi after 36 years fertilizer, straw cover and irrigation management practices in northwest China. Soil Use Manag. 2021, 37, 843–854. [Google Scholar] [CrossRef]
- Shao, T.; Zhao, J.; Liu, A.; Long, X.; Rengel, Z. Effects of soil physicochemical properties on microbial communities in different ecological niches in coastal area. Appl. Soil Ecol. 2020, 150, 103486. [Google Scholar] [CrossRef]
- Xun, W.; Huang, T.; Zhao, J.; Ran, W.; Wang, B.; Shen, Q.; Zhang, R. Environmental conditions rather than microbial inoculum composition determine the bacterial composition, microbial biomass and enzymatic activity of reconstructed soil microbial communities. Soil Biol. Biochem. 2015, 90, 10–18. [Google Scholar] [CrossRef]
- Tian, P.; Razavi, B.S.; Zhang, X.; Wang, Q.; Blagodatskaya, E. Microbial growth and enzyme kinetics in rhizosphere hotspots are modulated by soil organics and nutrient availability. Soil Biol. Biochem. 2020, 141, 107662. [Google Scholar] [CrossRef]
- Wan, W.; Tan, J.; Wang, Y.; Qin, Y.; He, H.; Wu, H.; Zuo, W.; He, D. Responses of the rhizosphere bacterial community in acidic crop soil to pH: Changes in diversity, composition, interaction, and function. Sci. Total Environ. 2020, 700, 134418. [Google Scholar] [CrossRef]
- Savvas, D.; Gruda, N. Application of soilless culture technologies in the modern greenhouse industry—A review. Eur. J. Hortic. Sci. 2018, 83, 280–293. [Google Scholar] [CrossRef]
- Zhong, X.; Li, X.; Zeng, Y.; Wang, S.; Sun, Z.; Tang, Y. Dynamic change of bacterial community during dairy manure composting process revealed by high-throughput sequencing and advanced bioinformatics tools. Bioresour. Technol. 2020, 306, 123091. [Google Scholar] [CrossRef]
- Wang, S.; Sun, Z.; Wang, S.; Yuan, H.; An, M.; Xia, Z.; Tang, Y.; Shen, C.; Kida, K. Bacterial Community Structure and Metabolic Function Succession During the Composting of Distilled Grain Waste. Appl. Biochem. Biotech. 2021, 194, 1479–1495. [Google Scholar] [CrossRef]
- Li, J.; Wang, H.; Yi, Y.; Gou, M.; Nobu, M.K.; Chen, Y.; Tang, Y. Response of isovalerate-degrading methanogenic microbial community to inhibitors. Appl. Biochem. Biotech. 2020, 191, 1010–1026. [Google Scholar] [CrossRef]
- Yao, Q.; Liu, J.; Yu, Z.; Li, Y.; Jin, J.; Liu, X.; Wang, G. Three years of biochar amendment alters soil physiochemical properties and fungal community composition in a black soil of northeast China. Soil Biol. Biochem. 2017, 110, 56–67. [Google Scholar] [CrossRef]
- Zheng, D.; Wang, H.; Gou, M.; Nobu, M.K.; Narihiro, T.; Hu, B.; Nie, Y.; Tang, Y. Identification of novel potential acetate-oxidizing bacteria in thermophilic methanogenic chemostats by DNA stable isotope probing. Appl. Microbiol. Biot. 2019, 103, 8631–8645. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microb. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, R.H.; Larsson, K.H.; Taylor, A.F.S.; Bengtsson-Palme, J.; Jeppesen, T.S.; Schigel, D.; Kennedy, P.; Picard, K.; Glöckner, O.F.; Tedersoo, L.; et al. The UNITE database for molecular identification of fungi: Handling dark taxa and parallel taxonomic classifications. Nucleic Acids Res. 2019, 47, D259–D264. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microb. 2009, 75, 7537–7541. [Google Scholar] [CrossRef]
- Crowther, T.W.; Van den Hoogen, J.; Wan, J.; Mayes, M.A.; Keiser, A.; Mo, L.; Averil, C.; Maynard, D.S. The global soil community and its influence on biogeochemistry. Science 2019, 365, eaav0550. [Google Scholar] [CrossRef]
- Robinson, B.L.; Feng, W.; Gulbis, N.; Hajdu, K.; Harrison, R.J.; Jeffries, P.; Xu, X. The use of arbuscular mycorrhizal fungi to improve strawberry production in coir substrate. Front. Plant Sci. 2016, 7, 1237. [Google Scholar] [CrossRef]
- Yang, C.; Lv, D.; Jiang, S.; Lin, H.; Sun, J.; Li, K.; Sun, J. Soil salinity regulation of soil microbial carbon metabolic function in the Yellow River Delta, China. Sci. Total Environ. 2021, 790, 148258. [Google Scholar] [CrossRef]
- Ding, J.; Fang, F.; Lin, W.; Qiang, X.; Xu, C.; Mao, L.; Li, Q.; Zhang, X.; Li, Y. N2O emissions and source partitioning using stable isotopes under furrow and drip irrigation in vegetable field of North China. Sci. Total Environ. 2019, 665, 709–717. [Google Scholar] [CrossRef]
- Fang, F.; Li, Y.; Yuan, D.; Zheng, Q.; Ding, J.; Xu, C.; Lin, W.; Li, Y. Distinguishing N2O and N2 ratio and their microbial source in soil fertilized for vegetable production using a stable isotope method. Sci. Total Environ. 2021, 801, 149694. [Google Scholar] [CrossRef]
- Zhou, J.; Guan, D.; Zhou, B.; Zhao, B.; Ma, M.; Qin, J.; Jiang, X.; Chen, S.; Cao, F.; Shen, D.; et al. Influence of 34-years of fertilization on bacterial communities in an intensively cultivated black soil in northeast China. Soil Biol. Biochem. 2015, 90, 42–51. [Google Scholar] [CrossRef]
- Ai, C.; Zhang, S.; Zhang, X.; Guo, D.; Zhou, W.; Huang, S. Distinct responses of soil bacterial and fungal communities to changes in fertilization regime and crop rotation. Geoderma 2018, 319, 156–166. [Google Scholar] [CrossRef]
- Maguire, V.G.; Bordenave, C.D.; Nieva, A.S.; Llames, M.E.; Colavolpe, M.B.; Gárriz, A.; Ruiz, O.A. Soil bacterial and fungal community structure of a rice monoculture and rice-pasture rotation systems. Appl. Soil Ecol. 2020, 151, 103535. [Google Scholar] [CrossRef]
- Bahram, M.; Hildebrand, F.; Forslund, S.K.; Anderson, J.L.; Soudzilovskaia, N.A.; Bodegom, P.M.; Bengtsson-Palme, J.; Anslan, S.; Coelho, L.P.; Harend, H.; et al. Structure and function of the global topsoil microbiome. Nature 2018, 560, 233–237. [Google Scholar] [CrossRef]
- Torres, N.; Yu, R.; Kurtural, S.K. Inoculation with mycorrhizal fungi and irrigation management shape the bacterial and fungal communities and networks in vineyard soils. Microorganisms 2021, 9, 1273. [Google Scholar] [CrossRef]
- Shao, P.; Liang, C.; Rubert-Nason, K.; Li, X.; Xie, H.; Bao, X. Secondary successional forests undergo tightly-coupled changes in soil microbial community structure and soil organic matter. Soil Biol. Biochem. 2019, 128, 56–65. [Google Scholar] [CrossRef]
- Rebekka, R.; Artz, E.; Anderson, I.C.; Chapman, S.J.; Hagn, A.; Schloter, M.; Potts, J.M.; Campbell, C.D. Changes in Fungal Community Composition in Response to Vegetational Succession During the Natural Regeneration of Cutover Peatlands. Microb. Ecol. 2007, 54, 508. [Google Scholar]
- Sun, H.; Terhonen, E.; Kovalchuk, A.; Tuovila, H.; Chen, H.; Oghenekaro, A.O.; Heinonsalo, J.; Kohler, A.; Kasanen, R.; Vasander, H.; et al. Dominant tree species and soil type affect the fungal community structure in a boreal peatland forest. Appl. Environ. Microb. 2016, 82, 2632–2643. [Google Scholar] [CrossRef]
- Zhao, S.; Qiu, S.; Xu, X.; Ciampitti, I.A.; Zhang, S.; He, P. Change in straw decomposition rate and soil microbial community composition after straw addition in different long-term fertilization soils. Appl. Soil Ecol. 2019, 138, 123–133. [Google Scholar] [CrossRef]
- Romanowicz, K.J.; Freedman, Z.B.; Upchurch, R.A.; Argiroff, W.A.; Zak, D.R. Active microorganisms in forest soils differ from the total community yet are shaped by the same environmental factors: The influence of pH and soil moisture. FEMS Microbiol. Ecol. 2016, 92, fiw149. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, J.; Yi, Y.; Nobu, M.K.; Narihiro, T.; Tang, Y. Response to inhibitory conditions of acetate-degrading methanogenic microbial community. J. Biosci. Bioeng. 2020, 129, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Bird, J.A.; Herman, D.J.; Firestone, M.K. Rhizosphere priming of soil organic matter by bacterial groups in a grassland soil. Soil. Biol. Biochem. 2011, 43, 718–725. [Google Scholar] [CrossRef]
- Na, X.; Yu, H.; Wang, P.; Zhu, W.; Niu, Y.; Huang, J. Vegetation biomass and soil moisture coregulate bacterial community succession under altered precipitation regimes in a desert steppe in northwestern China. Soil Biol. Biochem. 2019, 136, 107520. [Google Scholar] [CrossRef]
- Qin, K.; Dong, X.; Jifon, J.; Leskovar, D.I. Rhizosphere microbial biomass is affected by soil type, organic and water inputs in a bell pepper system. Appl. Soil Ecol. 2019, 138, 80–87. [Google Scholar] [CrossRef]
- Wang, L.; Luo, X.; Liao, H.; Chen, W.; Wei, D.; Cai, P.; Huang, Q. Ureolytic microbial community is modulated by fertilization regimes and particle-size fractions in a Black soil of Northeastern China. Soil Biol. Biochem. 2018, 116, 171–178. [Google Scholar] [CrossRef]
- Husson, O. Redox potential (Eh) and pH as drivers of soil/plant/microorganism systems: A transdisciplinary overview pointing to integrative opportunities for agronomy. Plant Soil 2013, 362, 389–417. [Google Scholar] [CrossRef]
- Ibekwe, A.; Poss, J.; Grattan, S.; Grieve, C.; Suarez, D. Bacterial diversity in cucumber (Cucumis sativus) rhizosphere in response to salinity, soil pH, and boron. Soil Biol. Biochem. 2010, 42, 567–575. [Google Scholar] [CrossRef]
- Zhang, W.; Chong, W.; Rui, X.; Wang, L. Effects of salinity on the soil microbial community and soil fertility. J. Integr. Agr. 2019, 18, 1360–1368. [Google Scholar] [CrossRef]
- Liu, S.; Jin, J.; Yang, H.; Wang, P.; Liu, Q.; Huang, Y.; Zhang, X.; Yin, K. Effect of feeding geese in cornfields on soil bacterial diversity and metabolic function. Appl. Soil Ecol. 2022, 175, 104448. [Google Scholar] [CrossRef]
- Toledo, M.; Gutiérrez, M.; Siles, J.; García-Olmo, J.; Martín, M. Chemometric analysis and NIR spectroscopy to evaluate odorous impact during the composting of different raw materials. J. Clean. Prod. 2017, 167, 154–162. [Google Scholar] [CrossRef]
- Liang, J.; Tang, S.; Gong, J.; Zeng, G.; Tang, W.; Song, B.; Zhang, P.; Yang, Z.; Luo, Y. Responses of enzymatic activity and microbial communities to biochar/compost amendment in sulfamethoxazole polluted wetland soil. J. Hazard. Mater. 2020, 385, 121533. [Google Scholar] [CrossRef]
- Santoyo, G.; Orozco-Mosqueda, M.d.C.; Govindappa, M. Mechanisms of biocontrol and plant growth-promoting activity in soil bacterial species of Bacillus and Pseudomonas: A review. Biocontrol Sci. Technol. 2012, 22, 855–872. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).