Abstract
In order to provide a theoretical basis and technical approach for the construction and regulation of medium- and high-yield population cultivation practice of wheat after rice, agronomic and physiological characteristics in medium-high yielding populations were investigated by setting different basic seedlings and cutting leaves and ears with isotope tracing method in week-gluten wheat (Ningmai 29). The results showed that the medium-high yield (yield above 7500 kg/km2) group could be achieved at medium densities (150 × 104/hm2 and 225 × 104/hm2), whose populations own suitable number of spikes, higher grain number per spike and thousand-grain weight (the larger and stronger ‘sink’). Meanwhile, these two medium-high yielding populations had higher leaf area index and suitable light-transmission rate after anthesis; thus, the leaf net photosynthetic rate after anthesis was higher, and the capacity of carbon assimilates was stronger. From the 15N test, it can be seen that the relationship between individuals in the medium-high yielding population (medium-density) is more harmonious, and the plant had higher nitrogen utilization efficiency. More nitrogen is concentrated in the spike at maturity. The results of the 13C pot trials showed that the top-three functional leaves had a higher capacity for source-production, which was also the main source of post-flowering assimilates. Increasing their area to improve the ‘source–sink’ ratio would help coordinate the ‘source–sink’ relationship in the group with a stronger ‘sink’. The main technical approach is to increase the area and duration of the upper-three functional leaves after anthesis on the basis of a larger sink, thus ensuring a higher source–sink ratio and a harmonious ‘source–sink’ relationship after flowering.
1. Introduction
Wheat is one of the world’s most important food crops as the main source of protein and calories [1,2]. China is the world’s largest wheat producer and consumer. With the growth of the population, people’s demand for food is also increasing [3]. At present, the planting area of wheat is restricted by cultivated land resources and planting structure adjustment. Therefore, increasing wheat yield to ensure total yield is one of the ways to ensure food security in China and the world.
In cultivation practice, reasonable population structure can alleviate the contradiction between population and individual, coordinate the relationship among yield components, improve the canopy structure to ensure the full use of light energy, and ultimately achieve high yield [4]. Previous studies indicated that a reasonable high-yield population structure could be constructed by adjusting the basic seedling density, nitrogen fertilizer management, or a combination of the two [5,6]. This could promise the coordinated relationship of the source–sink in wheat, and photosynthetic assimilate production capacity to a certain extent improved and relatively stable. Liu et al. [7] found that adjusting the appropriate row spacing under high-density conditions is conducive to alleviating the contradiction between groups and individuals. The population leaf area index is higher while maintaining a longer green leaf period during the filling process. When nitrogen application is insufficient, the dry matter accumulation of the soft wheat population can be increased by increasing planting density to achieve high quality and high yield [8]. The number of dominant tillers in the early stage of wheat growth is closely related to yield (Cai et al. [9]). Selecting suitable varieties for early sowing in low-yield fields can increase yield by increasing spike number and grain number per spike [10]. The formation of a high-yield wheat population needs to be regulated from many aspects, and its physiological regulation mechanism is also complex, involving physiological activities such as plant carbon and nitrogen metabolism, endogenous hormone production and action, and changes in various enzyme activities in leaves.
The formation of a high-yield wheat population needs to be regulated by a variety of management measures. The underlying physiological regulation mechanism is also complicated, involving physiological activities such as plant carbon and nitrogen metabolism, endogenous hormone production and action, and changes in various enzyme activities in leaves. Carbon and nitrogen metabolism are the two most important metabolic processes in wheat growth and development. Photosynthesis is the key process of carbon metabolism in wheat, contributing more than 90% of biomass [11,12]. During grain filling, photosynthetic assimilates produced by photosynthesis (most closely related to yield) stored in vegetative organs or after flowering are mainly transported to grains in the form of sucrose and form amylose or amylopectin under a series of enzymatic reactions and finally stored in grains to form yield. Therefore, various enzyme activities in carbon metabolism reflect the physiological characteristics of yield formation [13,14]. Nitrogen metabolism is mainly a process of synthesis, catabolism, and re-synthesis of proteins and amino acids. The process of carbon and nitrogen metabolism also reflects the transfer and distribution of C and N elements in different organs after they enter the plant. The isotope tracing method can well show the moving path. Therefore, we constructed different yield population structures and different source–sink characteristic populations, and we used methods including isotope tracing to study the physiological characteristics of yield formation.
In this study, a new wheat variety Ningmai 29, was selected and grown under the condition of total-rice-straw returning. Through setting different basic seedlings for the construction of the different wheat populations, the characteristics of different yield populations of new wheat varieties after rice harvest were studied, and the transport and distribution characteristics of C and N substances were explored by isotope tracer method, which provided a theoretical basis for the construction of high yield populations of new wheat varieties. The purpose of this study is (1) to explore the characteristics and physiological characteristics of different yield groups of wheat after rice; (2) to explore the physiological characteristics of wheat source–sink under leaf-cutting conditions; (3) to clarify the breeding measures and technical route of high-yield population of new wheat varieties.
2. Materials and Methods
2.1. Experimental Site and Weather Conditions
2.1.1. Field Assay
The experiments were conducted during November 2020 and June 2021 and settled in Garden Village, Xinji Town, Yizheng City (32°23′ N, 119°25′ E), with the rice as the previous crop and all the straw returned to the field after harvesting. The precipitation and temperature data during wheat growth are shown in Figure 1. Soil organic matter content in 0–20 cm was 28.22 g/kg. The content of available phosphorus, alkaline nitrogen, phosphorus, and potassium was 18.50 mg/kg, 177.96 mg/kg, 18.50 mg/kg, and 80.68 mg/kg, respectively.
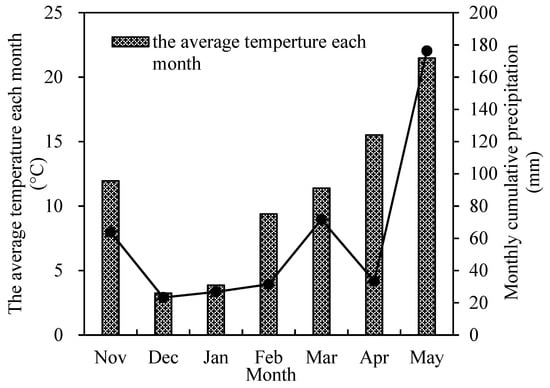
Figure 1.
The precipitation (mm) and daily average temperature (°C) of wheat growth period between 2020 and 2021.
2.1.2. Pot Assay
The pot experiment was conducted at the potting trial site on Wenhuilu campus, Yangzhou University (32°23′ N, 119°25′ E). Ningmai 29 was chosen as the test material. The seeds were sown in November 2021. The texture of the soil was light loam. The content of soil organic matter, total nitrogen, available phosphorus, available potassium, and available nitrogen was 13.56 g/kg, 0.75 mg/g, 31.12 mg/kg, 64.64 mg/kg, and 73.19 mg/kg, respectively.
2.2. Experimental Assays
2.2.1. Field Experiment
Plots were established in a split-plot randomized block design with five treatments (five densities: 75 × 104/hm2, 150 × 104/hm2, 225 × 104/hm2, 300 × 104/hm2, and 375 × 104/hm2). These treatments are abbreviated as M1, M2, M3, M4, and M5, respectively. The applied concentration of the pure nitrogen was 270 kg/hm2. The ratio was 5:1:2:2 (base fertilizer: tiller fertilizer: jointing fertilizer: booting fertilizer). The phosphorus pentoxide and potassium oxide were 120 kg/hm2, which were applied as base fertilizer and jointing fertilizer in the ratio of 1:1. The fertilizers in the forms of urea (N 46%), potassium oxide (K2O 60%) and compound fertilizer (N, P2O5, and K2O content were 15%). The replicates were included in each treatment, with 15 plots of 15 m2 (3 m × 5 m). The experiment was sown on 3rd November, using manual strip sowing with a row 25 cm spacing, and inter-planting and replanting were carried out after the wheat was fully seeded to achieve the expected number of basic seedlings for each treatment. Test treatment and corresponding numbers are shown in Table 1.

Table 1.
Field treatment and corresponding number.
2.2.2. Pot Experiment
The pot size was 26 cm in diameter on top, 18 cm in diameter on the bottom, and 26 cm in height. Each pot was filled with 11 kg of soil. The soil was mixed with the base fertilizer and loaded into the pot, watered in equal amounts after loading and compacted naturally, and mulched with 1 kg of soil after sowing. The soil was passed through an 8 mm sieve. Twelve seeds were laid evenly in each pot. The pure nitrogen was 270 kg/hm2, which was applied as base fertilizer: tiller fertilizer: jointing fertilizer: booting fertilizer in the ratio of 5:1:2:2. The base fertilizer application rate was 0.72 g/pot; the tiller fertilizer application rate was 0.144 g/pot; the application amount of jointing fertilizer was 0.288 g/pot; application amount of booting fertilizer was 0.288 g/pot. Phosphorus and potassium fertilizer were 120 kg/hm2; basal fertilizer and jointing fertilizer accounted for 50%, 0.32 g/pot. Its management measures with field high-yield cultivation and weed manual removal. Marking of plants flowering on the same day at flowering and leaf cutting. Test treatment and corresponding numbers are shown in Table 2.

Table 2.
Field treatment and corresponding number.
2.3. Measurements of Agronomical and Physiological Characteristics
2.3.1. Yield and Yield Component
In the field experiment, 50 spikes were continuously collected for seed test at milky stage to investigate the number of fertile spikes, the number of un-pregnant spikes, and the number of grains per panicle. A 1 m2 yield measurement area was selected at the maturity stage, with 3 replicates per plot. After investigating the number of ears, artificial harvest, threshing, weighing after drying, and 1000 grains of 3 groups were selected to measure thousand-grain weight. The thousand-grain weight and yield at 13% water content were measured by a near infrared spectrometer.
2.3.2. Tiller Dynamics, Leaf Area Index, and Dry Matter Accumulation
Leaf area index and dry matter weight in field experiment, the length of 1 m, were selected according to the walking direction in the post-tiller of wheat to investigate the dynamics of tillers, with 3 replicates per treatment. Samples were taken at the overwintering stage, jointing stage, booting stage, flowering stage, and maturity stage, and 20 representative plants were taken from each treatment. After root removal, the samples were divided into leaf, stem sheath, and panicle (only leaf and stem sheath before flowering). The leaf area of each wheat plant was measured by a leaf area meter (LI-6400) (LI-COR, Lincoln, NE, USA). After packing into the oven, 105 °C kill out 60 min, 80 °C drying to constant weight after weighing dry weight.
2.3.3. Leaf Layer Transmittance
In the field experiment, leaf transmittance was measured by ACCuPAR LP-80 (DECAGON, pullman, DC, USA) photometer on a sunny, windless morning during flowering. During the operation, the probe rod of the crop canopy analyzer was diagonally crossed between wheat rows at a 45° angle with wheat planting row, and the photosynthetic effective radiation of the canopy top, flag leaf layer, second leaf layer, and third leaf layer was measured. The transmittance of different leaf layers was calculated. Ten single stems of wheat plants were marked in each treatment at the flowering stage, and the height from the middle to the ground of the flag leaf, second leaf, and third leaf was measured and recorded, respectively. The average value was taken to determine the height of each leaf layer.
2.3.4. Net Photosynthetic Rate
The single stem of wheat flowering on the same day was marked at the flowering stage. The net photosynthetic rate of flag leaf of wheat was measured by LI-6400 photosynthetic apparatus at flowering and milk-ripen stages, repeated five times and measured at 9:00–11:00 a.m. on sunny days.
2.3.5. 15N Abundance by Organ
In the field experiment of 15N abundance in different organs at the maturity stage, 15N urea base fertilizer + 15N urea jointing fertilizer was applied to each treatment. The wheat samples were collected at the maturity stage, divided into stems and leaves, bagged and placed in an oven at 105 °C for 60 min, dried at 80 °C to constant weight, and then weighed. The samples were sent to the Shanghai Research Institute of Chemical Industry for determination of 15N abundance.
2.3.6. Sucrose Synthase
In the pot experiment, we selected the spikes of wheat marked on the same day. Spikes were taken at 7 days, 14 days, and 21 days after post anthesis. Grains were frozen in liquid nitrogen for 1 min and then stored at −80 °C for enzymatic measurement. The plants were ground under liquid nitrogen, and 0.1 g fresh samples were weighed in 2 mL centrifuge tubes. All chemicals and enzymes used for enzymatic measurement were from (Suzhou, China) Ke-min Biotechnology Company. Sucrose synthase catalyzed the reaction of free fructose with glucose donor UDPG to produce sucrose, and the reaction of sucrose with resorcinol showed a color change. The absorbance at 480 nm was measured by a UV spectrophotometer.
2.3.7. Sucrose Content
In the pot experiment, we selected the spikes of wheat marked on the same day. Spikes were taken at 7 days, 14 days, and 21 days after post anthesis. One pot was a repeat, and three pots were taken from each treatment. The samples were killed at 105 °C for 60 min and dried at 80 °C to constant weight. After grinding the dried spike samples, 0.1 g was weighed and placed in a 10 mL centrifuge tube. Extraction of sucrose with 80% alcohol three times, Sucrose and resorcinol generated colored substances in hydrochloric acid solution, and the absorbance was measured at 500 nm by UV spectrophotometer. The content of sucrose was calculated from the standard curve of sucrose.
2.3.8. 13C Abundance in Mature Organs
In the pot sowing experiment, the plants that blossomed on the same day were marked with sunny weather after flowering. The plants were placed in a closed space constructed with plastic film, and 13CO2 was injected into the space with a syringe. The amount of mark was 200 mL per plant. The mark mouth was sealed with a rubber sheet, and the reaction time was 2 h. After the reaction time, the mark shed was removed to allow the normal growth of the plants. The plants were sampled at the mature stage, dried after sampling, and the 13C abundance of each organ was measured.
2.4. Statistical Analyses
Leaf area index = leaf area per unit land area/unit land area.
Light transmittance = (light intensity of a leaf layer/natural light intensity at the top of canopy) × 100% stem-sheath material conversion rate = (single stem dry weight at flowering stage—single stem dry weight at maturity stage)/panicle dry weight at maturity stage × 100%.
Tiller spike rate = tiller number at jointing stage/spike number at maturity × 100%.
15N labeled fertilizer nitrogen efficiency calculation method:
15N labeled urea nitrogen use efficiency% = crop uptake 15N labeled urea nitrogen content Ndfs (kg/hm2)/15N labeled urea total nitrogen content × 100%.
Excel 2016 was used to process and map data. DPS7.05 was used for statistical analysis, and the LSD method was used for dominance analysis.
3. Results and Analysis
3.1. Yield and Yield Components
The data on yield and its components are shown in Table 3; different densities had significant effects on yield, each yield component factor. Among all treatments, the M2 and M3 density levels produced the highest yields, which were 1.36–9.04% higher than other treatments, but the reasons for the high yields obtained by these two treatments were not entirely consistent. M2 had the highest number of grains per spike and thousand-grain weight, and M3 had a lower thousand-grain weight than M2, but the number of spikes per unit area and population glumes were relatively high, while the number of grains per spike of these two treatments were not significantly different, so the M1 had a lower number of spikes per unit area, and the M4 and M5 had lower grain number per spike and thousand-grain weight, therefore both yields were lower.

Table 3.
Effects of plant density on yield and yield components.
Different densities had significant effects on the number of fruiting spikelets, total number of spikelets, and stem-tiller rate of spike (Table 4). The spikelet number increased first and then decreased with the increase in density. M2 had the highest number of spikelets, and M5 had the lowest number of spikelets. The number of spikes was 9.01% higher in M2 than in M5. The total number of spikelets in the M2 was also higher than in the other treatments. In terms of tiller spikelet rate, M1 had the highest tiller spikelet rate because the population was too small and had good ventilation and light penetration, while both M2 and M3 had higher tiller spikelet rates than M4 and M5.

Table 4.
Effects of plant density on the number of fruiting spike, total spikelet, and stem-tiller rate of spike.
3.2. LAI
LAI of different treatments was variable at each fertility stage (Table 5). Before flowering, the LAI of wheat increased with increasing density at all fertility stages. The LAI of M5 was the highest at the overwintering, jointing, and booting stages and was significantly higher by 360.00%, 96.98%, and 32.37% than M1. At flowering and milk-ripe stage, the LAI showed a trend of increasing and then decreasing with increasing density, with higher LAI in both M2 and M3, and an increase of 18.66%, 13.68%, and 14.56%, 9.49% at flowering and milk-ripe stage compared to the lowest density group M5.

Table 5.
Effects of plant density on leaf area index (LAI).
3.3. Dry Matter Accumulation
Dry matter accumulation in all treatments were significant differences (Table 6). From the period of emergence to overwintering and from overwintering to jointing, dry matter accumulation showed an increasing trend with increasing density. From jointing to maturity, dry matter accumulation after flowering of M2 and M3 was significantly higher than that of M1, M4, and M5. The result suggested that M2 and M3 treatments have stronger photosynthetic material production capacity after anthesis.

Table 6.
Effects of plant density on dry matter accumulation at the reproductive stage.
3.4. Canopy Light Transmittance
The light transmission rate of different leaf layers was significantly positively correlated with different densities (Figure 2). With the increase in density, the light transmittance of the flag leaf layer, the second leaf layer, and the third leaf layer showed a downward trend. The overall downward trend of the flag leaf layer is the most obvious. It is worth noting that the density increased from M4 to M5, the decrease in light transmittance in the three leaf layers slowed down, and there was no significant difference between the two treatments. This shows that when the density reaches a certain level, it is easy to cause shade and poor light transmission in the late growth stage of wheat, which is not conducive to the better use of light energy by the population.
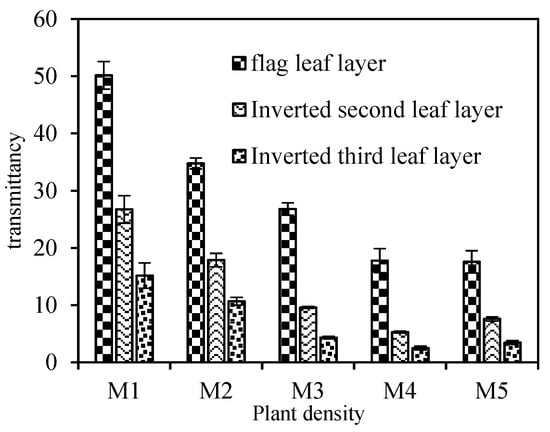
Figure 2.
Effects of plant density on canopy transmittance. M1, 75 × 104/hm2; M2, 150 × 104/hm2; M3, 225 × 104/hm2; M4, 300 × 104/hm2; M5, 375 × 104/hm2. Vertical bars represent ± SE of the mean.
3.5. Net Photosynthetic Rate
It can be seen from Figure 3 that different densities had significant effects on the net photosynthetic rate of flag leaf at the flowering and milk-ripe stage. With the increase in basic seedlings, the net photosynthetic rate of flag leaf showed a downward trend, and the performance of the flowering and milk-ripe stage was consistent. The net photosynthetic rate of flag leaf in M2 and M3 was always higher than that in M4 and M5, and the net photosynthetic rate of flag leaf in M1 was the highest in the two periods. From the flowering stage to the milk-ripe stage, the net photosynthetic rate of each treatment decreased, among which M2 and M3 decreased slightly from flowering stage to milk-ripe stage, and the decrease was smaller than that of M1, M4, and M5, and the milk-ripe stage still maintained a high level.
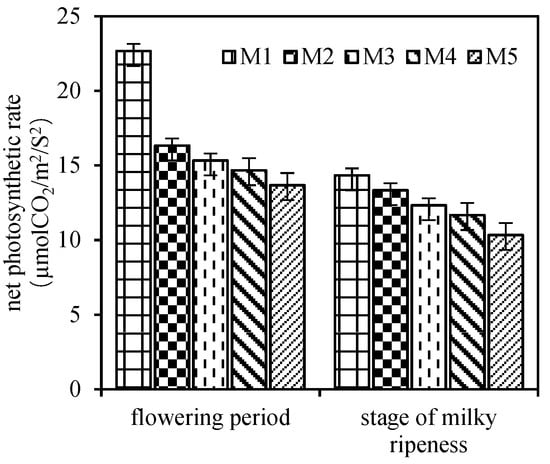
Figure 3.
Effects of plant density on net photosynthetic rate of flag leaves after flowering. M1, 75 × 104/hm2; M2, 150 × 104/hm2; M3, 225 × 104/hm2; M4, 300 × 104/hm2; M5, 375 × 104/hm2. Vertical bars represent ± SE of the mean.
3.6. Nitrogen Fertilizer Utilization Rate
It can be seen from Table 7 that there were significant differences in 15N-labeled urea uptake in different organs under different densities. From the perspective of 15N-labeled urea nitrogen uptake, under the same nitrogen application rate, the nitrogen uptake of M2 was higher than that of M4. Combined with nitrogen accumulation, it also showed that the medium-density population M2 could better absorb nitrogen. From the perspective of the 15N marker urea nitrogen utilization rate, the utilization rate of nitrogen in stems, leaves, and ears of the medium-density population was higher. This shows that the construction of M2 is more reasonable so that plants can better absorb and utilize nitrogen and increase yield.

Table 7.
Effects of plant density on nitrogen accumulation, 15N accumulation, and distribution in each organ.
3.7. Sucrose Synthase Activity and Sucrose Content
The SS enzyme activity in the grains showed a trend of increasing first and then decreasing. At 14 days after flowering, the enzyme activity of each leaf-cutting treatment reached the maximum. It can be seen from Figure 4 that the enzyme activities of 7 days, 14 days, and 21 days after flowering were: CK > cutting the remaining leaves > cutting the upper three leaves > cutting the whole leaves. The grain activity of CK and cutting the remaining leaves after flowering was higher than that of cutting the functional leaves, indicating that the upper three leaves had a strong source function. The grain enzyme activity of the upper three-leaf cutting treatment was greater than that of the whole-leaf cutting treatment, indicating that the remaining leaves in the plant, except for the upper three leaves, also had a certain compensation effect after losing the functional leaves and also had a certain contribution to grain filling. It can be seen from Figure 5 that each treatment had a very significant effect on the sucrose content of wheat grains. At 10 days, 20 days, and 30 days after flowering, the sucrose content was CK > cutting the rest of leaves > cutting the top three leaves > cutting the whole leaves. The sucrose content of grain in the leaf-cutting treatment was lower than that in control. This shows that the upper three leaves have strong leaf source functions and a high contribution rate to grain sucrose accumulation.
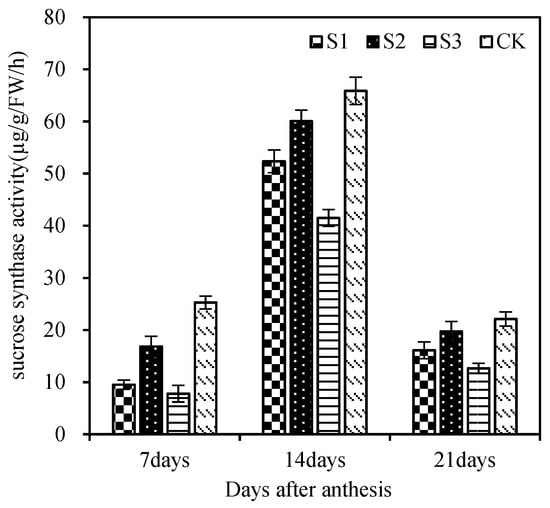
Figure 4.
Effects of different leaf cutting methods on grain SS enzyme activity. S1, cut three leaves; S2, cut the remaining leaves except for the upper three leaves; S3, cut whole leaves; CK, normal plants. Vertical bars represent ± SE of the mean.
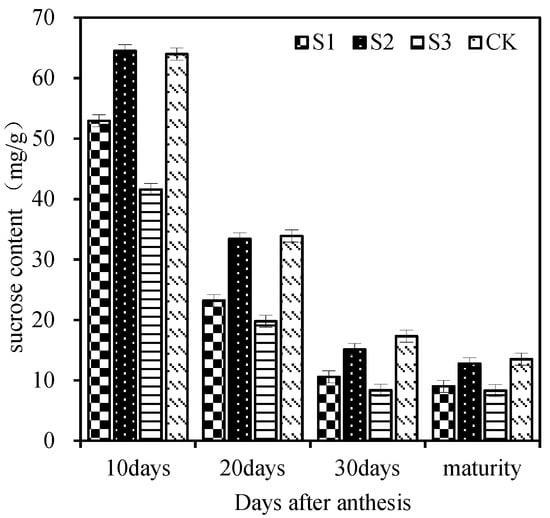
Figure 5.
Effects of different leaf cutting methods on sucrose content in grain. S1, cut three leaves; S2, cut the remaining leaves except for the upper three leaves; S3, cut whole leaves; CK, normal plants. Vertical bars represent ± SE of the mean.
3.8. C Content and Distribution Rate
It can be seen from Table 8 that the content of 13C in each organ of the plant was lower than that of the control in the treatment of cutting three leaves and cutting the whole leaf. It can be seen from Table 9 that the ear distribution rates of the treatment of cutting three leaves and cutting the whole leaf were 92.77% and 91.35%, respectively, which were higher than that of the control. This indicates that the reduction of the source of wheat in the treatment of cutting three leaves and cutting the whole leaf makes a large amount of material stored in the stem of wheat transported to the ear to supply the grain filling. There was no significant difference in the 13C accumulation in panicles between the treatment of cutting other leaves and the control, which indicated that cutting other leaves except the upper three leaves had no significant effect on the carbon accumulation in panicles. The leaf source of the three functional leaves on the plant was strong, and the contribution of the leaf source to grain filling could also be guaranteed when the other leaves were lost.

Table 8.
13C content of each organ of the plant under different source bank treatments at wheat maturity.

Table 9.
Distribution of 13C in each organ of the plant under different source bank treatments at wheat maturity.
4. Discussion
4.1. ‘Sink’-Yield Performance between Medium-High and Low Yield Groups
In this study, because of the severe drought stress after sowing and cold stress during the overwintering period, the wheat yield was relatively low; thus, the yield above 7500 kg/hm2 was considered a medium-high yield population. The yield of M2 and M3 with moderate density exceeded 7500 kg/hm2, but the reasons for the above two treatments achieving medium-high yield were not consistent. In all treatments, M2 had the highest number of grains per spike and thousand-grain weight, while M3 had a higher effective number of spikes and grains per spike. For the low-yield population, the yield components were also inconsistent across the different densities. The effective spike number was too low in the low-density (M1); the high-density (M4 and M5) showed a greater decrease in grain number and thousand-grain weight despite the higher number of spikes. This confirmed the previous viewpoint that excessive reduction in grain number per spike and thousand-grain weight was the main reason for yield decrease in high-density populations [15]. Zheng et al. [8] found that yield continued to increase with increasing density from 180 × 104/hm2 to 300 × 104/hm2 at three levels of nitrogen application. The possible reasons may be the low average yield of the wheat varieties selected in the experiment, the small plant type, the poor tillering capacity, and the relatively harmonized population-individual relationship at high density. Therefore, it could be inferred that the way to achieve medium-high yields for different wheat varieties was mainly through ensuring a reasonable number of effective spikes and further increasing the number of grains per spike and thousand-grain weight [16], but the appropriate density for each variety remained to be further determined through trials.
4.2. ‘Source’-Photosynthetic Characteristics between Medium-High and Low Yield Groups
The photosynthetic capacity of leaves plays a crucial role in crop growth and yield formation [17,18]. Among them, dry matter accumulation after anthesis is most closely related to yield. Leaf area index, net photosynthetic rate of sword leaves, leaf green holding time, and population canopy structure after anthesis all have profound effects on the assimilate production capacity of the ‘source’ [19]. During the wheat grain filling process, the leaf area index tends to decline, but the medium-high yield population had a lower leaf area decay rate and larger green leaf area and maintained the net photosynthetic rate at a higher level [20]. Results of this experiment showed that M2 and M3 had larger green leaf areas from anthesis to the milky stage and also maintained higher sword leaf net photosynthetic rate, but the sword leaf net photosynthetic rate after anthesis showed a decreasing trend with increasing density. It has also been shown that excessive planting density tends to deteriorate the crop canopy structure, resulting in the ‘source’ being limited by light conditions [21], and in high-density populations, individual competition was intensified, the lower leaf group near the root accelerates senescence after anthesis [22]. In this experiment, it is the poor light transmission in the field of the high-density M4 and M5 populations, with the intense competition between individuals, resulting in the insufficient use of light energy, which may be closely related to the reduced leaf net photosynthetic efficiency and the relatively insufficient production of assimilates after anthesis. For the low-yield population under low density, the M1 light energy loss was larger due to a small “source” and high light transmittance, and the net photosynthetic rate of flag leaves was higher, but the production of photosynthate was insufficient. Under the condition of suitable medium density, the medium and high yield populations M2 and M3 realized that the “source” was stronger after anthesis, the grain filling was fuller, and the yield was higher.
4.3. Carbon and Nitrogen Transport and Partitioning between Medium-High and Low Yield Group
Sucrose metabolism mainly regulates wheat growth, stress response, and yield formation by producing a series of carbohydrate metabolites and synthesizing necessary chemicals [23]. There are reports that [24,25] saccharose synthase is the main enzyme that promotes the conversion of sucrose into starch when accumulating reserve carbohydrates in the “sink”. In wheat grain, carbohydrate transport and grain filling rate can be improved, or sink strength can be increased by modulating the activity of source synthase. In this study, cutting three functional leaves (S1) and cutting whole leaves (S3) on the plant reduced the source–sink ratio, decreased the sucrose synthase activity in the seeds after anthesis, and reduced the sucrose content in the seeds, both of which were significantly lower than those of CK and S2. In combination with the 13C isotope tracing, the reduction of the wheat ‘source’ in S1 and S3 resulted in a large amount of material stored in the wheat steam being transported to spike to supply the grains for filling. There was no significant effect of cutting the remaining leaves other than the functional leaves (S2) on carbon accumulation in spikes. The strong ‘source’ of three functional leaves on the plant ensures the contribution of the ‘source’ to grain filling, even in the state of losing the rest of the leaves. It appears that increasing the ‘source’ area of the functional leaves and improving the ‘source–sink’ ratio were beneficial to promoting carbohydrate accumulation in grains and improving yield. It is worth noting that both stem sheaths and spikes also had certain photosynthetic capacity, but their duration and specific contribution to yield could not be determined in this experiment. The reference value that could be given was to pay attention to the exploitation of this photosynthetic potential in breeding.
Nitrogen is an important nutrient for wheat plant growth and yield formation [26,27]. The 15N tracer technology can directly detect the absorption of N from different sources and the distribution of N in different parts of crops [28]. Previous studies [29] showed that the amount of nitrogen absorbed by plants from 15N-labeled urea decreased with increasing planting density. The results in this study were generally consistent with previous studies. In this experiment, the highest-yielding group (M2 with medium density) and the lowest-yielding group (M4 with high density) were selected. We found that the amount of fertilizer N absorbed by stems, leaves, and spikes in the high-yielding group was higher than that in the low-yielding group, and the utilization of fertilizer N by spikes in the high-yielding group was significantly higher than that in the low-yielding group. This indicates that the planting density at M2 can better regulate the ‘source–sink’ relationship, promote the plants to better absorb and utilize nitrogen, and maintain the wheat yield.
5. Conclusions
There were significant differences in ‘source’ and ‘sink’ characteristics as well as carbon and nitrogen transport and allocation characteristics among different yielding populations in this experiment. Because of the severe dry weather after sowing and the cold stress during winter, the yield of M2 and M3 is higher than 7500 kg/hm2, which are regarded as medium-high yield population. Preliminary suggestions on the construction of a medium-high yielding population with a coordinated relationship between ‘source’ and ‘sink’ of wheat Ningmai 29 in middle and lower reaches of Yangtze river: the planting density is 150 × 104/hm2 and 225 × 104/hm2, the amount of pure nitrogen is 270 kg/hm2, and the ratio of nitrogen fertilizer application is 5:1:2:2. Medium-high yielding populations had higher ‘sink’ strength, as demonstrated by higher grains per spike and 1000-grain weight based on the appropriate spike numbers. The population also had a strong ‘source’, which was characterized by the strong ‘source’ function of the upper-three functional leaves of the plant, less light leakage loss in the canopy of the population, high net photosynthetic rate of the flag leaves after flowering, and long green leaf retention time. The experiments on 15N and 13C showed that the medium and high yield population under medium density had high nitrogen utilization efficiency and more reasonable nitrogen distribution; the upper three leaves were the main photosynthetic organs after anthesis, and the spike and leaf sheath also had certain photosynthetic production capacity. Therefore, this experiment preliminary concluded that the main technical approach is to increase the area and duration of the upper-three functional leaves after the flowering stage on the basis of a larger ‘sink’ strength, thus ensuring a higher ‘source–sink’ ratio and a harmonious ‘source–sink’ relationship after flowering.
Author Contributions
Conceptualization, W.G.; Investigation, Q.Y.; Resources, J.W., J.D., C.L., X.Z., W.G. and M.Z.; Writing—original draft, J.W.; Writing—reviewing & editing, M.Z.; Supervision, W.G. and M.Z.; Funding acquisition, W.G. and M.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (3177100326 and 31901433), Jiangsu Modern Agricultural (Wheat) Industry Technology System.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are presented in the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Govindan, V.; Ravi, P.S.; Julio, H.; Carlos, G. Genetic impact of Rht dwarfing genes on grain micronutrients concentration in wheat. Field Crop. Res. 2017, 214, 373–377. [Google Scholar]
- Jacques, L.G.; François-Xavier, O.; Gilles, C. How changes in climate and agricultural practices influenced wheat production in Western Europe. J. Cereal Sci. 2020, 93, 102960. [Google Scholar] [CrossRef]
- Qin, X.; Zhang, F.X.; Liu, C.; Yu, H.; Cao, B.G.; Tian, S.Q.; Liao, Y.C.; Siddique, K.H.M. Wheat yield improvements in China: Past trends and future directions. Field Crop. Res. 2015, 177, 117–124. [Google Scholar] [CrossRef]
- Moghimi, M.M.; Shamshiri, G.; Shabani, A.; Kamgar-Haghighi, A.A.; Fateh, M.; Mahmoudi, M.R. Determining optimum applied water and seeding rates for winter wheat by using Aqua Crop and mathematical-economic analysis. Irrig. Drain. 2022, 71, 349–364. [Google Scholar] [CrossRef]
- Guan, X.J.; Chen, J.; Chen, X.M.; Xie, J.; Deng, G.Q.; Hu, L.Z.; Li, Y.; Qian, Y.F.; Qiu, C.F.; Peng, C.R. Root characteristics and yield of rice as affected by the cultivation pattern of strong seedlings with increased planting density and reduced nitrogen application. J. Integr. Agric. 2022, 21, 1278–1289. [Google Scholar] [CrossRef]
- Arduini, A.; Masoni, L.; Ercoli, M.; Mariotti. Grain yield, and dry matter and nitrogen accumulation and remobilization in durum wheat as affected by variety and seeding rate. Eur. J. Agron. 2006, 25, 309–318. [Google Scholar] [CrossRef]
- Liu, T.N.; Wang, Z.L.; Cai, T. Canopy Apparent Photosynthetic Characteristics and Yield of Two Spike-Type Wheat Cultivars in Response to Row Spacing under High Plant Density. PLoS ONE 2016, 11, e0148582. [Google Scholar] [CrossRef]
- Zheng, B.Q.; Zhang, X.Q.; Wang, Q.; Li, W.Y.; Huang, M.; Zhou, Q.; Cai, J.; Wang, X.; Cao, W.X.; Dai, T.B.; et al. Increasing plant density improves grain yield, protein quality and nitrogen agronomic efficiency of soft wheat cultivars with reduced nitrogen rate. Field Crop. Res. 2021, 267, 108145. [Google Scholar] [CrossRef]
- Cai, T.; Xu, H.; Peng, D.L.; Yin, Y.P.; Yang, W.B.; Ni, Y.L.; Chen, X.G.; Xu, C.L.; Yang, D.Q.; Cui, Z.Y.; et al. Exogenous hormonal application improves grain yield of wheat by optimizing tiller productivity. Field Crop. Res. 2014, 155, 172–183. [Google Scholar] [CrossRef]
- Li, C.; Wang, X.S.; Guo, Z.K.; Huang, N.; Hou, S.B.; He, G.; William, D.B.; Kadambot, H.M.; Siddique; Wang, Z.H.; et al. Optimizing nitrogen fertilizer inputs and plant populations for greener wheat production with high yields and high efficiency in dryland areas. Field Crop. Res. 2022, 276, 108374. [Google Scholar] [CrossRef]
- Brestic, M.; Zivcak, M.; Hauptvogel, P.; Misheva, S.; Kocheva, K.; Yang, X.; Li, X.; Allakhverdiev, S.I. Wheat plant selection for high yields entailed improvement of leaf anatomical and biochemical traits including tolerance to non-optimal temperature conditions. Photosynth. Res. 2018, 136, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Makino, A. Photosynthesis, grain yield, and nitrogen utilization in rice and wheat. Plant Physiol. 2011, 155, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Zeeman, S.C.; Kossmann, J.; Smith, A.M. Starch: Its metabolism, evolution, and biotechnological modification in plants. Annu. Rev. Plant Biol. 2010, 61, 209–234. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Xu, S.; Zhang, Z.; Chen, G.; Zhong, Y.; Liu, L.; Zhang, R.; Xue, J.; Guo, D. Evolutionary, structural and expression analysis of core genes involved in starch synthesis. Sci. Rep. 2018, 8, 12736. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Y.; Ming, B.; Fan, P.P.; Liu, Y.; Wang, K.R.; Hou, P.; Xue, J.; Li, S.K.; Xie, R.Z. Quantifying contributions of leaf area and longevity to leaf area duration under increased planting density and nitrogen input regimens during maize yield improvement. Field Crop. Res. 2022, 283, 108551. [Google Scholar] [CrossRef]
- Nerson, H. Effects of population density and number of ears on wheat yield and its components. Field Crop. Res. 1980, 3, 225–234. [Google Scholar] [CrossRef]
- Piazza, P.; Jasinski, S.; Tsiantis, M. Evolution of leaf developmental mechanisms. New Phytol. 2005, 167, 693–710. [Google Scholar] [CrossRef]
- Rowan, F.S.; Robert, W.P. The Nitrogen Use Efficiency of C3 and C4 Plants: II. Leaf Nitrogen Effects on the Gas Exchange Characteristics of Chenopodium album (L.) and Amaranthus retroflexus (L.). Plant Physiol. 1987, 84, 953–959. [Google Scholar]
- Richard, A.; Richards, C.R.; Cavanagh, P.R. Selection for erect canopy architecture can increase yield and biomass of spring wheat. Field Crop. Res. 2019, 244, 107649. [Google Scholar] [CrossRef]
- Du, X.; Gao, Z.; Sun, X.N.; Bian, D.H.; Ren, J.H.; Yan, P.; Cui, Y.H. Increasing temperature during early spring increases winter wheat grain yield by advancing phenology and mitigating leaf senescence. Sci. Total Environ. 2022, 812, 152557. [Google Scholar] [CrossRef]
- Yang, H.W.; Chai, Q.; Yin, W.; Hu, F.L.; Qin, A.Z.; Fan, Z.L.; Yu, A.Z.; Zhao, C.; Fan, H. Yield photosynthesis and leaf anatomy of maize in inter- and mono-cropping systems at varying plant densities. Crop J. 2021, 10, 839–930. [Google Scholar] [CrossRef]
- Antonietta, M.; Fanello, D.D.; Acciaresi, H.A.; Guiamet, J.J. Senescence and yield responses to plant density in stay green and earlier-senescing maize hybrids from Argentina. Field Crop. Res. 2014, 155, 111–119. [Google Scholar] [CrossRef]
- Manisha, K.; Bavita, A. Transformation of Sucrose to Starch and Protein in Rice Leaves and Grains under Two Establishment Methods. Rice Sci. 2016, 23, 255–265. [Google Scholar]
- Yang, J.C.; Zhang, J.H.; Wang, Z.Q.; Zhu, Q.S.; Liu, L.J. Activities of enzymes involved in sucrose-to-starch metabolism in rice grains subjected to water stress during filling. Field Crop. Res. 2003, 81, 69–81. [Google Scholar] [CrossRef]
- Huang, M.; Chen, J.N.; Cao, F.B.; Liu, Y.; Xiao, Z.W.; Hu, L.Q.; Chen, G.H.; Zou, Y.B. The activity ratio of glutamine synthetase to sucrose synthase: A physiological feature explaining the variation in grain nitrogen-based protein content of rice. J. Cereal Sci. 2020, 91, 102902. [Google Scholar] [CrossRef]
- Lambert, D.M.; Lowenberg-DeBoer, J.; Malzer, G.L. Economic Analysis of Spatial-Temporal Patterns in Corn and Soybean Response to Nitrogen and Phosphorus. Agro J. 2006, 98, 43–54. [Google Scholar] [CrossRef]
- Lassaletta, L.; Billen, G.; Grizzetti, B.; Anglade, J.; Garnier, J. 50 year trends in nitrogen use efficiency of world cropping systems: The relationship between yield and nitrogen input to cropland. Environ. Res. Lett. 2014, 9, 105011. [Google Scholar] [CrossRef]
- Christopher, J.S.; Phillip, M.C. The role of 15N in tracing N dynamics in agro-ecosystems under alternative systems of tillage management: A review. Soil Till Res. 2020, 197, 104496. [Google Scholar] [CrossRef]
- Luo, Z.; Hu, Q.Y.; Tang, W.; Wang, X.W.; Lu, H.Q.; Zhang, Z.; Liu, T.; Kong, X.Q. Effects of N fertilizer rate and planting density on short-season cotton yield, N agronomic efficiency and soil N using 15N tracing technique. Eur. J. Agron. 2022, 138, 126546. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).