Evaluation of Soil Fertility Quality under Biochar Combined with Nitrogen in an Irrigated Wheat Field in Northern Xinjiang, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Study Design
2.3. Sampling and Analysis of Soil and Crop
2.4. Evolution of Soil Fertility
2.5. Statistical Analysis
3. Results
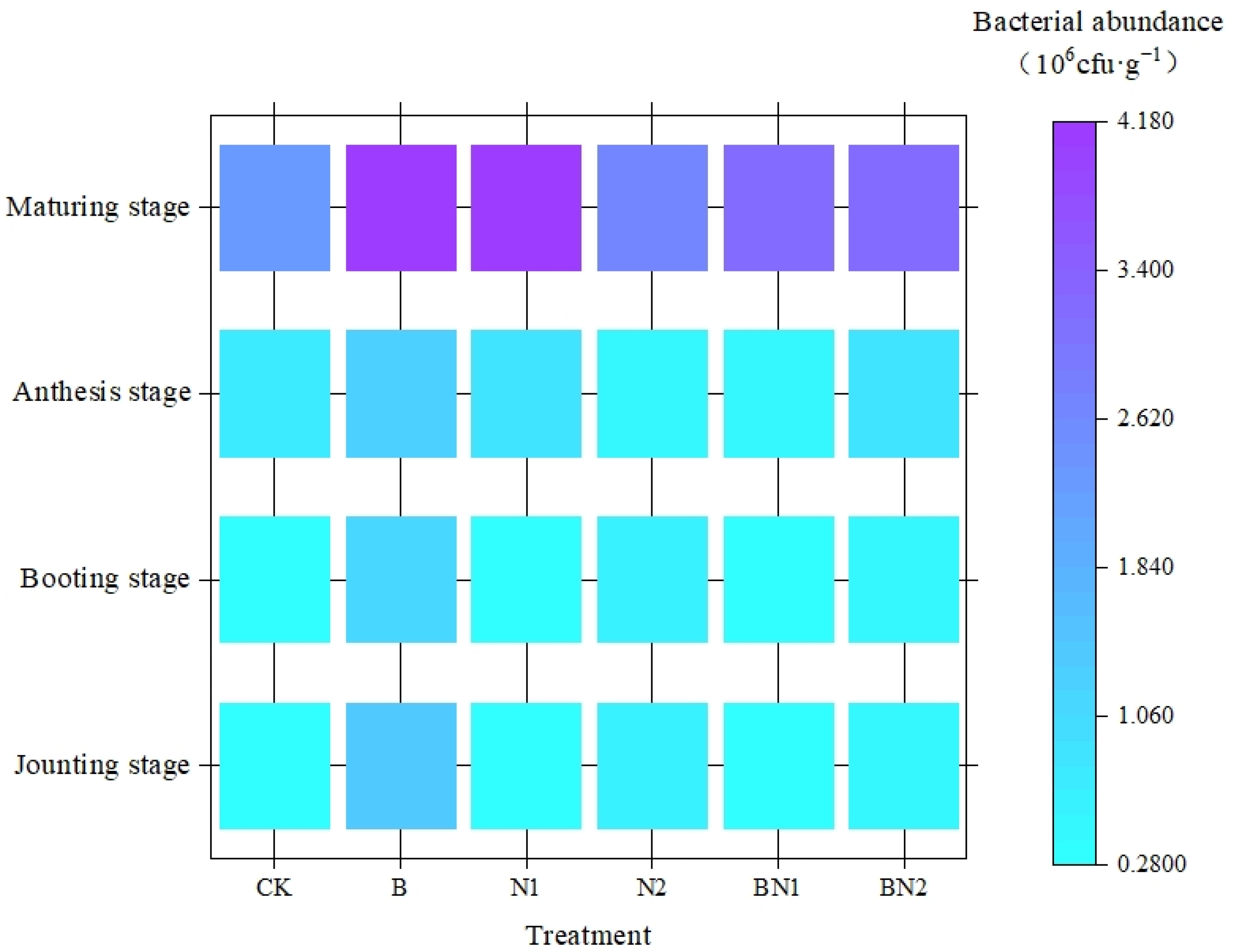
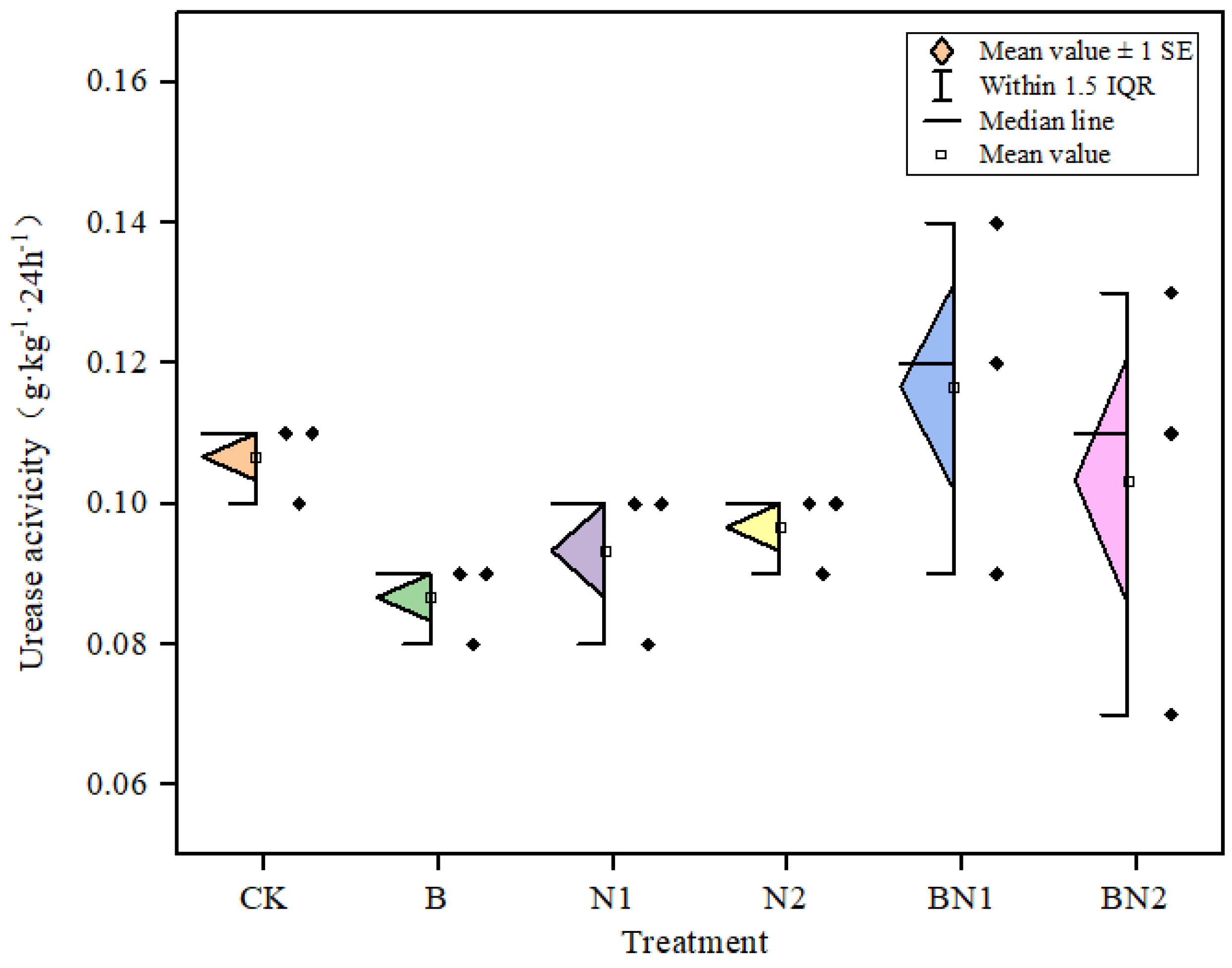
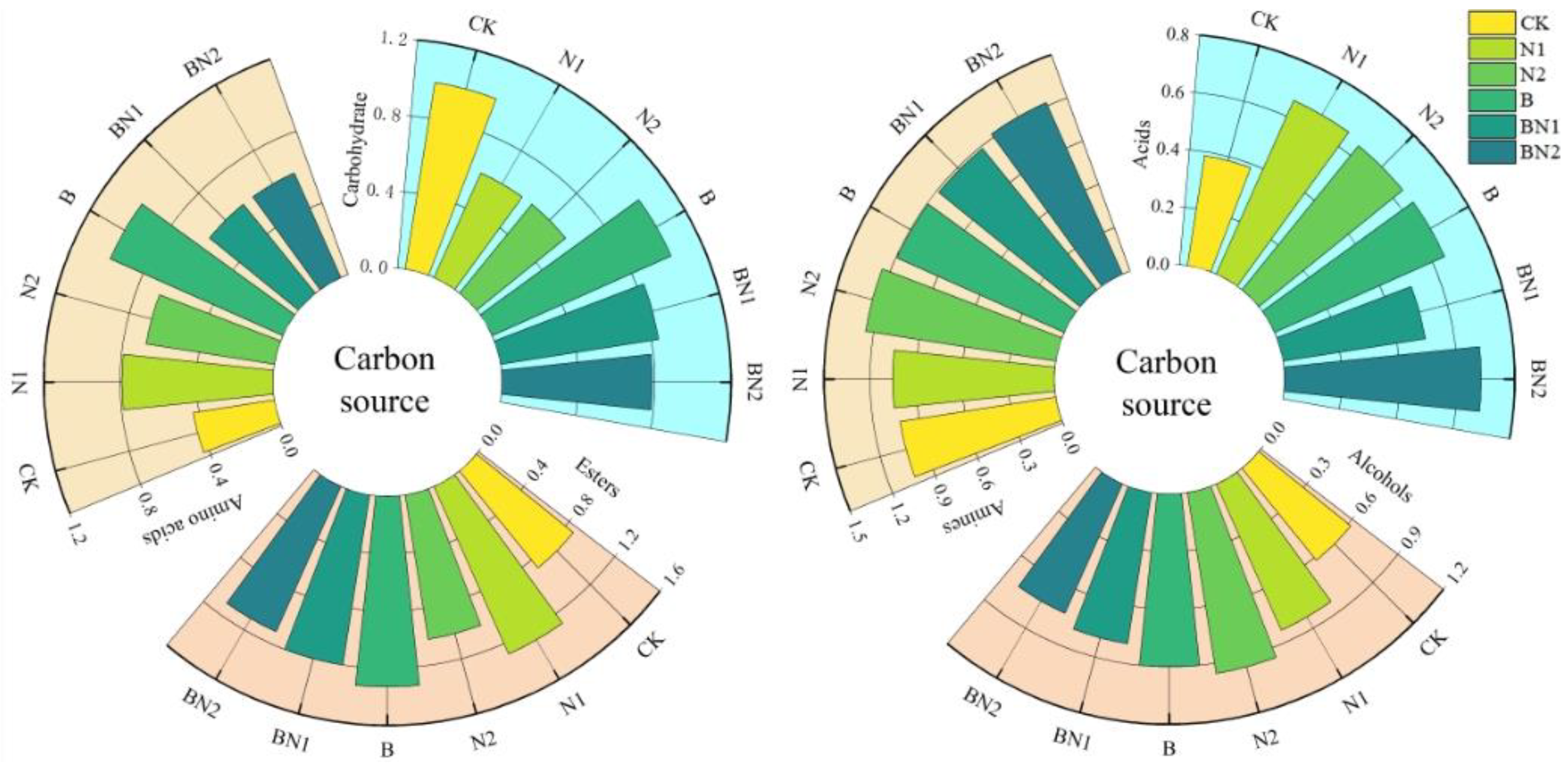
3.1. Effects of Biochar Application on Basic Soil Fertility and Microbial Activity
3.2. Selection of Soil Fertility Evaluation Indicators during Biochar Application
3.3. Factor Analysis of Soil Fertility Quality
3.4. Scores and Ranking of Soil Quality under Different Treatments
3.5. Yield Data
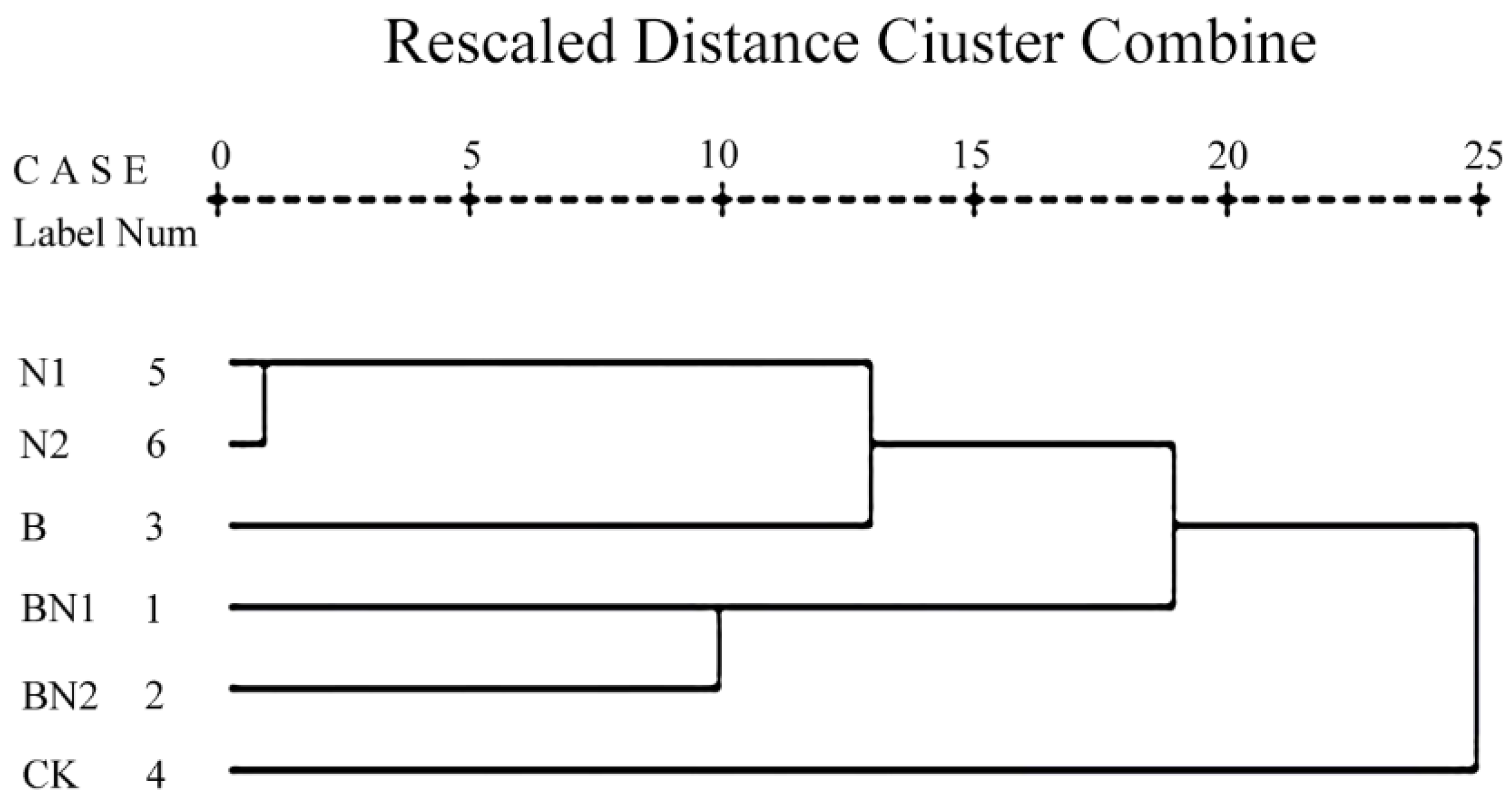
3.6. Cluster Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bezerra, J.; Turnhout, E.; Vasquez, I.M.; Rittl, T.F.; Arts, B.; Kuyper, T.W. The promises of the Amazonian soil: Shifts in discourses of Terra Preta and biochar. J. Environ. Policy Plan. 2019, 21, 623–635. [Google Scholar] [CrossRef]
- Hossain, M.Z.; Bahar, M.M.; Sarkar, B.; Donne, S.W.; Ok, Y.S.; Palansooriya, K.N.; Kirkham, M.B.; Chowdhury, S.; Bolan, N. Biochar and its importance on nutrient dynamics in soil and plant. Biochar 2020, 2, 379–420. [Google Scholar] [CrossRef]
- Spokas, K.A.; Novak, J.M.; Venterea, R.T. Biochar’s role as an alternative N-fertilizer: Ammonia capture. Plant Soil. 2012, 350, 35–42. [Google Scholar] [CrossRef]
- Biederman, L.A.; Harpole, W.S. Biochar and its effects on plant productivity and nutrient cycling: A meta-analysis. GCB Bioenergy 2013, 5, 202–214. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, A.; Ji, C.; Joseph, S.; Bian, R.; Li, L.; Pan, G.; Paz-Ferreiro, J. Biochar’s effect on crop productivity and the dependence on experimental conditions—A meta-analysis of literature data. Plant Soil 2013, 373, 583–594. [Google Scholar] [CrossRef]
- Gale, N.V.; Halim, M.A.; Horsburgh, M.; Thomas, S.C. Comparative responses of early-successional plants to charcoal soil amendments. Ecosphere 2017, 8, e01933. [Google Scholar] [CrossRef]
- Thomas, S.C.; Gale, N. Biochar and forest restoration: A review and meta-analysis of tree growth responses. N. For. 2015, 46, 931–946. [Google Scholar] [CrossRef]
- Gao, S.; DeLuca, T.H.; Cleveland, C.C. Biochar additions alter phosphorus and nitrogen availability in agricultural ecosystems: A meta-analysis. Sci. Total Environ. 2019, 654, 463–472. [Google Scholar] [CrossRef]
- Dai, Y.; Zheng, H.; Jiang, Z.; Xing, B. Combined effects of biochar properties and soil conditions on plant growth: A meta-analysis. Sci. Total Environ. 2020, 713, 136635. [Google Scholar] [CrossRef]
- Xiang, Y.; Deng, Q.; Duan, H.; Guo, Y. Effects of biochar application on root traits: A meta-analysis. GCB Bioenergy 2017, 9, 1563–1572. [Google Scholar] [CrossRef]
- Xiao, Q.; Zhu, L.X.; Zhang, H.P.; Li, X.Y.; Shen, Y.F.; Li, S.Q. Soil amendment with biochar increase maize yields in a semi-arid region by improving soil quality and root growth. Crop Pasture Sci. 2016, 67, 495–507. [Google Scholar] [CrossRef]
- Li, Y.F.; Hu, S.D.; Chen, J.H.; Müller, K.; Li, Y.C.; Fu, W.J.; Lin, Z.W.; Wang, H.L. Effects of biochar application in forest ecosystems on soil properties and greenhouse gas emissions—A review. J. Soils Sediments 2018, 18, 546–563. [Google Scholar] [CrossRef]
- Zhao, W.; Zhou, Q.; Tian, Z.Z.; Cui, Y.T.; Liang, Y.; Wang, H.Y. Apply biochar to ameliorate soda saline-alkali land, improve soil function and increase corn nutrient availability in the Songnen Plain. Sci. Total Environ. 2020, 722, 137428. [Google Scholar] [CrossRef]
- Bao, S.D. Soil Agrochemical Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2000; pp. 14–21. [Google Scholar]
- Faithfull, N.T. Methods in Agricultural Chemical Analysis: A Practical Handbook, 1st ed.; CABI Publishing: Wallingford, UK, 2002; Volume 140, pp. 245–249. [Google Scholar] [CrossRef]
- Herigstad, B.; Hamilton, M.; Heersink, J. How to optimize the drop plate method for enumerating bacteria. J. Microbiol. Methods 2001, 44, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Garland, J.L.; Mills, A.L. Classification and characterization of heterotrophic microbial communities on the basis of patterns of community-level sole-carbon-source utilization. Appl. Environ. Microbiol. 1991, 57, 2351–2359. [Google Scholar] [CrossRef]
- Lever, J.; Krzywinski, M.; Altman, N. Points of significance: Principal component analysis. Nat. Methods 2017, 14, 641–642. [Google Scholar] [CrossRef]
- Gianluca Alaimo, G.; Auricchio, F.; Marfia, S.; Sacco, E. Optimization clustering technique for PieceWise Uniform Transformation Field Analysis homogenization of viscoplastic composites. Comput. Mech. 2019, 64, 1495–1516. [Google Scholar] [CrossRef]
- Wagg, C.; Schlaeppi, K.; Banerjee, S.; Kuramae, E.E.; Heijden, M.G.A. Fungal-bacterial diversity and microbiome complexity predict ecosystem functioning. Nat. Commun. 2019, 10, 4841. [Google Scholar] [CrossRef]
- Khan, Z.; Zhang, K.K.; Khan, M.N.; Fahad, S.; Xu, Z.H.; Hu, L.Y. Coupling of Biochar with Nitrogen Supplements Improve Soil Fertility, Nitrogen Utilization Efficiency and Rapeseed Growth. Agronomy 2020, 10, 1661. [Google Scholar] [CrossRef]
- Zhang, C.; Li, X.Y.; Yan, H.; Ullah, I.; Zuo, Z.Y.; Li, L.; Yu, J.J. Effects of irrigation quantity and biochar on soil physical proerties, growth characteristics, yield and quality of greenhouse tomato. Agric. Water Manag. 2022, 241, 106243. [Google Scholar] [CrossRef]
- Liu, Y.H.; Ma, Z.T.; Chen, R.; Jiang, W.T.; Yin, C.M.; Mao, Z.Q.; Wang, Y.F. Biochar promotes the growth of apple seedlings by adsorbing phloridzin. Sci. Hortic. 2022, 303, 111187. [Google Scholar] [CrossRef]
- Ali, I.; He, L.; Ullah, S.; Quan, Z.; Wei, S.Q.; Iqbal, A.; Munsif, F.; Shah, T.; Xuan, Y.; Luo, Y.Q.; et al. Biochar addition coupled with nitrogen fertilization impacts on soil quality, crop productivity, and nitrogen uptake under double-cropping system. Food Energy Secur. 2020, 9, e208. [Google Scholar] [CrossRef]
- Ali, I.; Ullah, S.; He, L.; Zhao, Q.; Iqbal, A.; Wei, S.Q.; Shah, T.; Ali, N.; Bo, Y.; Adnan, M.M.; et al. Combined application of biochar and nitrogen fertilizer improves rice yield, microbial activity and N-metabolism in a pot experiment. PeerJ 2020, 8, e10311. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Zhao, Q.; Wu, K.; Ullah, S.; Iqbal, A.; Liang, H.; Zhang, J.; Muhammad, I.; Amanullah; Khan, A.; et al. Biochar in combination with nitrogen fertilizer is a technique: To enhance physiological and morphological traits of rice (Oryza sativa L.) by improving soil physiobiochemical properties. J. Plant Growth Regul. 2021, 41, 2406–2420. [Google Scholar] [CrossRef]
- Song, D.L.; Xi, X.Y.; Zheng, Q.; Liang, G.Q.; Zhou, W.; Wang, X.B. Soil nutrient and microbial activity responses to two years after maize straw biochar application in a calcareous soil. Ecotoxicol. Environ. Saf. 2019, 180, 348–356. [Google Scholar] [CrossRef]
- Głodowska, M.; Wozniak, M. Changes in Soil Microbial Activity and Community Composition as a Result of Selected Agricultural Practices. Agric. Sci. 2019, 10, 330–351. [Google Scholar] [CrossRef]
- Lu, W.W.; Ding, W.X.; Zhang, J.H.; Li, Y.; Luo, J.F.; Balan, N.; Xie, Z.B. Biochar suppressed the decomposition of organic carbon in a cultivated sandy loam soil: A negative priming effect. Soil Biol. Biochem. 2014, 76, 12–21. [Google Scholar] [CrossRef]
- Yu, Z.Y.; Ling, L.; Singh, B.P.; Luo, Y.; Xu, J.M. Gain in carbon: Deciphering the abiotic and biotic mechanisms of biochar-induced negative priming effects in contrasting soils. Sci. Total Environ. 2020, 746, 141057. [Google Scholar] [CrossRef]
- Tian, X.; Li, Z.; Wang, Y.; Li, B.; Wang, L. Evaluation on soil fertility quality under biochar combined with nitrogen reduction. Sci. Rep. 2021, 11, 13792. [Google Scholar] [CrossRef]
- Hussain, M.; Farooq, M.; Nawaz, A.; Abdullah, M.A.; Solaiman, Z.M.; Alghamdi, S.S.; Ammara, U.; Sik, O.Y.; Siddique, K.H.M. Biochar for crop production: Potential benefits and risks. J. Soils Sediments 2017, 17, 685–716. [Google Scholar] [CrossRef]
- Li, M.; Liu, M.; Li, Z.P.; Jiang, C.Y.; Wu, M. Soil N transformation and microbial community structure as affected by adding biochar to a paddy soil of subtropical China. J. Integr. Agric. 2016, 15, 209–219. [Google Scholar] [CrossRef]
- Max, K.; Ellen, R.; Ludmila, T.; Yigal, E.; Eddie, C. Biochar-stimulated plant performance is strongly linked to microbial diversity and metabolic potential in the rhizosphere. New Phytol. 2017, 213, 1393–1404. [Google Scholar] [CrossRef]
- Liu, S.M.; Li, Y.W.; Xu, J.Z.; Ma, W.J.; Liu, B.Y.; Wang, H.Y.; Liu, X.Y.; Luan, Y.J. Biochar partially offset the increased ammonia volatilization from salt-affected soil. Arch. Agron. Soil Sci. 2021, 67, 1202–1216. [Google Scholar] [CrossRef]
- Lehmann, J.; Cowie, A.; Masiello, C.A.; Kammann, C.; Woolf, D.; Amonette, J.; Cayuela, M.; Camps, A.M.; Whitman, T. Biochar in climate change mitigation. Nat. Geosci. 2021, 14, 883–892. [Google Scholar] [CrossRef]
- Huang, K.; Zhang, J.; Tang, G.; Da, B.; Tangyu, W.; Deping, K. Impacts and mechanisms of biochar on soil microorganisms. Plant, Soil and Environment. 2023, 69, 45–54. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, X.; Chen, L.; Wang, Z.Y.; Xia, Y.; Zhang, Y.P.; Wang, H.F.; Luo, X.X.; Xing, B.S. Enhanced growth of halophyte plants in biochar-amended coastal soil: Roles of nutrient availability and rhizosphere microbial modulation. Plant Cell Environ. 2018, 41, 517–532. [Google Scholar] [CrossRef]
- Luo, X.X.; Liu, G.C.; Xia, Y.; Lei, C.; Jiang, Z.X.; Zheng, H.; Wang, Z.Y. Use of biochar-compost to improve properties and productivity of the degraded coastal soil in the Yellow River Delta, China. J. Soils Sediments 2017, 17, 780–789. [Google Scholar] [CrossRef]
- Xu, X.Y.; Zhao, Y.H.; Sima, J.K.; Zhao, L.; Ondřej, M.; Cao, X.D. Indispensable role of biochar-inherent mineral constituents in its environmental applications: A review. Bioresour. Technol. 2017, 241, 887–899. [Google Scholar] [CrossRef]
- Zheng, S.; Wang, Y.W.; Lai, J.L.; Zhang, Y.; Luo, X.G. Effects of long-term herbaceous plant restoration on microbial communities and metabolic profiles in coal gangue-contaminated soil. Environ. Res. 2023, 234, 116491. [Google Scholar] [CrossRef]
- Zhu, X.M.; Chen, B.L.; Zhu, L.Z.; Xing, B.S. Effects and mechanisms of biochar-microbe interactions in soil improvement and pollution remediation: A review. Environ. Pollut. 2017, 227, 98–115. [Google Scholar] [CrossRef]
- Liao, H.K.; Zheng, C.L.; Long, J.; Ivette, G. Effects of biochar amendment on tomato rhizosphere bacterial communities and their utilization of plant-derived carbon in a calcareous soil. Geoderma 2021, 396, 115082. [Google Scholar] [CrossRef]
- Li, Y.F.; Li, G.H. Mechanisms of straw biochar’s improvement of phosphorus bioavailability in soda saline-alkali soil. Environ. Sci. Pollut. Res. 2022, 29, 47867–47872. [Google Scholar] [CrossRef]
- Liang, J.F.; Li, Q.W.; Gao, J.Q.; Feng, J.G.; Zhang, X.Y.; Wu, Y.Q.; Yu, F.H. Biochar rhizosphere addition promoted phragmites australis growth and changed soil properties in the yellow river delta. Sci. Total Environ. 2021, 761, 143291. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.P.; Zheng, H.N.; Wang, X.J. Effects of soil amendments on fractions and stability of soil organic matter in saline-alkaline paddy. J. Environ. Manag. 2021, 294, 112993. [Google Scholar] [CrossRef] [PubMed]
- Masiello, C.A.; Chen, Y.; Gao, X.D.; Liu, S.; Cheng, H.-Y.; Bennett, M.R.; Rudgers, J.A.; Wagner, D.S.; Zygourakis, K.; Silberg, J.J. Biochar and microbial signaling: Production conditions determine effects on microbial communication. Environ. Sci. Technol. 2013, 47, 11496–11503. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.N.; Meng, J.; Jiang, L.L.; Yang, X.; Lan, Y.; Cheng, X.; Chen, W. Rice husk biochar impacts soil phosphorous availability, phosphatase activities and bacterial community characteristic s in three different soil types. Appl. Soil Ecol. 2017, 116, 12–22. [Google Scholar] [CrossRef]
- El-naggar, A.; Lee, S.S.; Rinklebe, J.; Muhammad, F.; Song, H.; Ajit, K.S.; Andrew, R.Z.; Mahtab, A.; Sabry, M.S.; Yong, S.O. Biochar application to low fertility soils: A review of current status, and future prospects. Geoderma 2019, 337, 536–554. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, Z.Y.; Deng, X.; Zhao, J.; Luo, Y.; Novak, J.; Herbert, S.; Xing, B.S. Characteristics and nutrient values of biochars produced from giant reed at different temperatures. Bioresour. Technol. 2013, 130, 463–471. [Google Scholar] [CrossRef]
- Xu, W.; Wang, G.; Deng, F.; Zou, X.; Ruan, H.; Chen, H.Y.H. Responses of soil microbial biomass, diversity and metabolic activity to biochar applications in managed poplar plantations on reclaimed coastal saline soil. Soil Use Manag. 2018, 34, 597–605. [Google Scholar] [CrossRef]
- Rehab, H.; Hegab, A. Evaluation of nitrogen sources and polymer coated fertilizers on wheat yield in sandy soil. J. Soil Sci. Plant Nutr. 2018, 3, 1–12. [Google Scholar] [CrossRef]
- Nasim, W.; Ahmad, A.; Amin, A.; Tariq, M.; Awais, M.; Saqib, M.; Jabran, K.; Shah, G.M.; Sultana, S.R.; Hammad, H.M.; et al. Radiation efficiency and nitrogen fertilizer impacts on sunflower crop in contrasting environments of Punjab, Pakistan. Environ. Sci. Pollut. Res. 2018, 25, 1822–1836. [Google Scholar] [CrossRef] [PubMed]
- Veysel, T.; Shahbaz, A.K.; Mahmood, R.M.; Muhammad, I.; Pia, M.A.R.; Maryam, F. Promoting the productivity and quality of brinjal aligned with heavy metals immobilization in a wastewater irrigated heavy metal polluted soil with biochar and chitosan. Ecotox. Environ. Saf. 2018, 161, 409–419. [Google Scholar] [CrossRef]

| Index | Stage | Treatment | |||||
|---|---|---|---|---|---|---|---|
| CK | B | N1 | N2 | BN1 | BN2 | ||
| Total nitrogen (g·kg−1) | Jounting | 1.0 ± 0.01 b | 1.0 ± 0.01 b | 0.9 ± 0.01 b | 1.0 ± 0.05 b | 1.1 ± 0.04 a | 1.1 ± 0.03 a |
| Booting | 1.0 ± 0.05 c | 1.0 ± 0.03 c | 1.0 ± 0.02 b | 1.0 ± 0.01 b | 1.1 ± 0.04 a | 1.1 ± 0.02 a | |
| Anthesis | 1.0 ± 0.02 b | 1.0 ± 0.01 b | 1.0 ± 0.02 b | 1.0 ± 0.03 a | 1.0 ± 0.02 a | 1.1 ± 0.02 a | |
| Maturing | 0.9 ± 0.04 c | 1.0 ± 0.03 c | 0.9 ± 0.00 b | 0.9 ± 0.02 a | 1.0 ± 0.02 a | 1.0 ± 0.02 a | |
| Organic matter (g·kg−1) | Jounting | 9.0 ± 0.39 a | 9.4 ± 0.92 a | 8.6 ± 1.14 a | 8.8 ± 0.49 a | 10.6 ± 0.77 a | 10.6 ± 0.68 a |
| Booting | 8.7 ± 0.20 b | 10.9 ± 0.83 a | 8.4 ± 0.30 b | 8.9 ± 0.56 a | 9.7 ± 1.25 a | 10.1 ± 0.08 a | |
| Anthesis | 8.6 ± 0.09 b | 9.5 ± 0.40 a | 7.2 ± 0.50 c | 7.3 ± 0.14 c | 9.9 ± 0.56 a | 9.8 ± 0.45 a | |
| Maturing | 7.3 ± 0.15 b | 7.7 ± 0.04 b | 7.1 ± 0.20 b | 6.5 ± 0.38 c | 9.0 ± 0.60 a | 7.8 ± 0.39 b | |
| Total potassium (g·kg−1) | Jounting | 22.4 ± 0.15 b | 22.1 ± 0.07 c | 22.4 ± 0.06 b | 22.3 ± 0.13 b | 22.7 ± 0.06 a | 22.5 ± 0.07 a |
| Booting | 22.4 ± 0.05 a | 22.0 ± 0.18 a | 22.6 ± 0.21 a | 22.4 ± 0.16 a | 22.2 ± 0.29 a | 22.3 ± 0.07 a | |
| Anthesis | 23.5 ± 0.24 a | 21.1 ± 0.17 b | 21.8 ± 0.59 b | 21.2 ± 0.14 b | 21.2 ± 0.41 b | 21.7 ± 0.11 b | |
| Maturing | 19.6 ± 0.15 b | 19.6 ± 0.22 b | 19.5 ± 0.23 b | 20.0 ± 0.21 b | 21.7 ± 0.10 a | 21.9 ± 0.15 a | |
| Total phosphorous (mg·kg−1) | Jounting | 37.1 ± 2.53 a | 32.8 ± 2.33 a | 32.2 ± 1.84 a | 32.0 ± 2.82 a | 30.7 ± 1.57 a | 30.7 ± 0.61 a |
| Booting | 30.5 ± 0.25 a | 30.9 ± 0.23 a | 29.5 ± 0.18 a | 27.1 ± 0.28 a | 33.3 ± 0.15 a | 32.7 ± 0.06 a | |
| Anthesis | 31.5 ± 2.29 a | 34.5 ± 4.81 a | 30.3 ± 4.08 a | 30.2 ± 5.70 a | 32.6 ± 3.00 a | 30.7 ± 4.05 a | |
| Maturing | 29.6 ± 1.43 ab | 26.4 ± 1.51 b | 30.1 ± 0.20 ab | 26.6 ± 0.36 b | 30.0 ± 0.70 ab | 32.5 ± 1.49 a | |
| Available potassium (g·kg−1) | Jounting | 397.4 ± 7.81 b | 497.7 ± 30.38 a | 372.9 ± 22.36 b | 390.5 ± 11.41 b | 531.5 ± 38.81 a | 507.9 ± 16.06 a |
| Booting | 382.5 ± 0.92 b | 497.5 ± 33.31 a | 374.5 ± 15.34 b | 390.4 ± 11.73 b | 480.1 ± 26.00 a | 518.7 ± 27.21 a | |
| Anthesis | 390.3 ± 13.85 b | 512.4 ± 31.70 a | 382.4 ± 16.01 a | 372.1 ± 11.66 b | 464.8 ± 29.90 a | 496.0 ± 18.01 a | |
| Maturing | 383.8 ± 18.76 a | 467.8 ± 42.13 a | 366.6 ± 23.71 b | 368.1 ± 8.32 b | 484.9 ± 53.74 a | 448.5 ± 15.08 a | |
| Available phosphorous (mg·kg−1) | Jounting | 13.2 ± 0.96 a | 16.9 ± 1.79 a | 14.5 ± 3.69 a | 19.8 ± 6.30 a | 16.2 ± 1.00 a | 17.3 ± 2.58 a |
| Booting | 24.8 ± 3.36 a | 16.5 ± 1.44 b | 22.1 ± 4.08 a | 20.4 ± 2.09 a | 24.8 ± 2.48 a | 25.6 ± 0.56 a | |
| Anthesis | 23.3 ± 5.58 a | 22.3 ± 1.27 a | 16.9 ± 0.74 b | 17.3 ± 0.43 a | 22.4 ± 1.71 a | 26.3 ± 0.97 a | |
| Maturing | 15.7 ± 0.97 a | 16.4 ± 3.14 a | 16.1 ± 1.94 a | 14.1 ± 0.50 a | 17.3 ± 0.95 a | 15.3 ± 0.96 a | |
| Treatment | AWCD | Shannon Index (H) | McIntosh Index (U) | Simpson Index (D) |
|---|---|---|---|---|
| CK | 0.57 ± 0.08 b | 2.67 ± 0.29 b | 4.29 ± 0.56 a | 0.95 ± 0.01 a |
| N1 | 0.86 ± 0.04 ab | 3.82 ± 0.12 a | 5.81 ± 0.25 a | 0.92 ± 0.01 a |
| N2 | 0.89 ± 0.14 a | 3.91 ± 0.47 a | 5.93 ± 0.73 a | 0.91 ± 0.02 a |
| B | 0.84 ± 0.02 ab | 3.71 ± 0.07 ab | 5.65 ± 0.14 a | 0.92 ± 0.00 a |
| BN1 | 0.77 ± 0.09 ab | 3.48 ± 0.29 ab | 5.33 ± 0.60 a | 0.93 ± 0.02 a |
| BN2 | 0.79 ± 0.14 ab | 3.53 ± 0.46 ab | 5.40 ± 0.80 a | 0.93 ± 0.02 a |
| Fertility Index | Treatments | |||||
|---|---|---|---|---|---|---|
| CK | B | N1 | N2 | BN1 | BN2 | |
| X1 (g·kg−1) | 7.3 | 7.7 | 7.1 | 6.5 | 9.0 | 7.8 |
| X2 (g·kg−1) | 0.9 | 1.0 | 0.9 | 0.9 | 1.0 | 1.0 |
| X3 (g·kg−1) | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 |
| X4 (g·kg−1) | 19.6 | 19.6 | 19.5 | 20.0 | 21.7 | 21.9 |
| X5 (g·kg−1) | 15.7 | 16.4 | 16.1 | 14.1 | 17.3 | 15.3 |
| X6 (g·kg−1) | 383.8 | 467.8 | 366.6 | 368.1 | 484.9 | 448.5 |
| X7 (×106 cfu·g−1) | 2.9 | 3.6 | 3.3 | 2.9 | 2.7 | 2.4 |
| X8 (g·kg−1·24 h−1) | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| X9 (H) | 2.7 | 3.8 | 3.9 | 3.7 | 3.5 | 3.5 |
| X10 (U) | 4.3 | 5.8 | 5.9 | 5.7 | 5.3 | 5.4 |
| X11 (D) | 1.0 | 0.9 | 0.9 | 0.9 | 0.9 | 0.9 |
| Item | F1 | F2 | F3 |
|---|---|---|---|
| Organic matter (X1) | −0.14 | 0.93 | 0.32 |
| Total nitrogen (X2) | 0.22 | 0.65 | 0.59 |
| Total phosphorous (X3) | −0.21 | 0.11 | 0.75 |
| Total potassium (X4) | 0.02 | 0.43 | 0.90 |
| Available phosphorous (X5) | −0.09 | 0.90 | −0.14 |
| Available potassium (X6) | 0.09 | 0.91 | 0.25 |
| Bacterial abundance (X7) | 0.28 | 0.08 | −0.95 |
| Urease activity (X8) | −0.62 | 0.33 | 0.59 |
| Shannon index (X9) | 0.99 | 0.06 | −0.08 |
| McIntosh index (X10) | 0.99 | 0.05 | −0.10 |
| Simpson index (X11) | −0.95 | 0.08 | 0.19 |
| Eigen value | 3.45 | 3.25 | 3.20 |
| % of variance | 31.35 | 29.53 | 29.07 |
| Cumulative % | 31.35 | 60.88 | 89.95 |
| Item | F1 | F2 | F3 |
|---|---|---|---|
| Organic matter (X1) | −0.04 | 0.30 | −0.04 |
| Total nitrogen (X2) | 0.12 | 0.14 | 0.16 |
| Total phosphorous (X3) | 0.02 | −0.08 | 0.27 |
| Total potassium (X4) | 0.09 | 0.01 | 0.31 |
| Available phosphorous (X5) | −0.08 | 0.37 | −0.23 |
| Available potassium (X6) | 0.03 | 0.30 | −0.04 |
| Bacterial abundance (X7) | −0.02 | 0.19 | −0.38 |
| Urease activity (X8) | −0.14 | 0.05 | 0.12 |
| Shannon index (X9) | 0.31 | 0.002 | 0.07 |
| McIntosh index (X10) | 0.31 | 0.003 | 0.06 |
| Simpson index (X11) | −0.29 | 0.03 | −0.04 |
| Treatments | F1 | F2 | F3 | F | ||||
|---|---|---|---|---|---|---|---|---|
| Score | Order | Score | Order | Score | Order | Score | Order | |
| BN1 | −0.26 | 5 | 1.44 | 1 | 0.55 | 2 | 0.60 | 1 |
| BN2 | 0.26 | 4 | −0.08 | 3 | 1.64 | 1 | 0.56 | 2 |
| B | 0.65 | 2 | 0.87 | 2 | −1.24 | 6 | 0.11 | 3 |
| CK | −1.90 | 6 | −0.43 | 4 | −0.47 | 4 | −0.96 | 6 |
| N1 | 0.77 | 1 | −0.47 | 5 | −0.50 | 5 | −0.05 | 4 |
| N2 | 0.47 | 3 | −1.33 | 6 | 0.01 | 3 | −0.27 | 5 |
| Treatment | Spikes Number (×104·hm−2) | Grains Number per Spike | 1000—Grain Weight (g) | Yield (kg·hm−2) | Harvest Index |
|---|---|---|---|---|---|
| CK | 487 ± 10.8 bc | 33 ± 1.2 c | 42.0 ± 0.5 d | 6701 ± 166.2 c | 0.5 ± 0.01 b |
| N1 | 562 ± 44.7 ab | 33 ± 1.5 c | 45.4 ± 0.4 b | 8335 ± 667.1 b | 0.5 ± 0.01 c |
| N2 | 416 ± 19.9 c | 41 ± 0.2 a | 47.8 ± 0.3 a | 8133 ± 397.1 bc | 0.5 ± 0.01 c |
| B | 567 ± 2.7 ab | 34 ± 0.8 bc | 43.9 ± 0.3 c | 8380 ± 175.3 b | 0.5 ± 0.01 b |
| BN1 | 525 ± 14.0 ab | 36 ± 0.1 b | 46.7 ± 0.5 ab | 8896 ± 126.9 ab | 0.6 ± 0.01 a |
| BN2 | 591 ± 30.7 a | 36 ± 1.5 b | 46.5 ± 0.5 ab | 9986 ± 291.5 a | 0.5 ± 0.01 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, W.; Wang, Z.; Guo, S.; Yang, M.; Zhao, L.; Zhao, H.; Jia, H.; Xu, W. Evaluation of Soil Fertility Quality under Biochar Combined with Nitrogen in an Irrigated Wheat Field in Northern Xinjiang, China. Agronomy 2023, 13, 2518. https://doi.org/10.3390/agronomy13102518
Yang W, Wang Z, Guo S, Yang M, Zhao L, Zhao H, Jia H, Xu W. Evaluation of Soil Fertility Quality under Biochar Combined with Nitrogen in an Irrigated Wheat Field in Northern Xinjiang, China. Agronomy. 2023; 13(10):2518. https://doi.org/10.3390/agronomy13102518
Chicago/Turabian StyleYang, Weijun, Zilong Wang, Song Guo, Mei Yang, Lining Zhao, Hongmei Zhao, Hongtao Jia, and Wanli Xu. 2023. "Evaluation of Soil Fertility Quality under Biochar Combined with Nitrogen in an Irrigated Wheat Field in Northern Xinjiang, China" Agronomy 13, no. 10: 2518. https://doi.org/10.3390/agronomy13102518
APA StyleYang, W., Wang, Z., Guo, S., Yang, M., Zhao, L., Zhao, H., Jia, H., & Xu, W. (2023). Evaluation of Soil Fertility Quality under Biochar Combined with Nitrogen in an Irrigated Wheat Field in Northern Xinjiang, China. Agronomy, 13(10), 2518. https://doi.org/10.3390/agronomy13102518






