Enhancing Efficiency of Enzymatic-Assisted Extraction Method for Evaluating Bioactive Compound Analysis in Mulberry: An Optimization Approach
Abstract
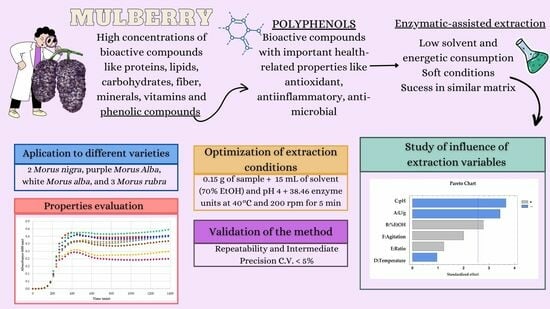
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Chemicals and Solvents
2.3. Enzyme-Assisted Extraction
2.3.1. Enzymatic Extraction Procedure
2.3.2. Optimization of the Process
2.4. Identification of Anthocyanins
2.5. Quantification of Anthocyanins
2.6. Identification and Quantification of Phenolic Compounds
2.7. Antioxidant Activity Assay
2.8. Antimicrobial Activity Assay
2.9. Statistical Analysis
3. Results and Discussion
3.1. Determining the Influencing Variables
3.1.1. Influential Variables for Anthocyanins
3.1.2. Influential Variables for Phenolic Compounds
3.2. Box–Behnken Design
3.2.1. Optimization for Anthocyanins
3.2.2. Optimization for Phenolic Compounds
3.3. Optimal Extraction Conditions
3.4. Ethanol Percentage Influence
3.5. Study of the Optimum Extraction Time
3.6. Validation of the Extraction Method
3.6.1. Repeatability and Intermediate Precision Evaluation
3.6.2. Enzyme-Assisted Extraction Evaluation
3.7. Application to Real Samples
3.8. Antioxidant Activity
3.9. Antimicrobial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tortora, C.; Pisano, L.; Vergine, V.; Ghirga, F.; Iazzetti, A.; Calcaterra, A.; Marković, V.; Botta, B.; Quaglio, D. Synthesis, Biosynthesis, and Biological Activity of Diels–Alder Adducts from Morus Genus: An Update. Molecules 2022, 27, 7580. [Google Scholar] [CrossRef]
- Liao, B.-Y.; Li, L.; Tanase, C.; Thakur, K.; Zhu, D.-Y.; Zhang, J.-G.; Wei, Z.-J. The Rheological Behavior of Polysaccharides from Mulberry Leaves (Morus alba L.). Agronomy 2020, 10, 1267. [Google Scholar] [CrossRef]
- Wang, R.-S.; Dong, P.-H.; Shuai, X.-X.; Chen, M.-S. Evaluation of Different Black Mulberry Fruits (Morus nigra L.) Based on Phenolic Compounds and Antioxidant Activity. Foods 2022, 11, 1252. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-X.; Liu, S.-Y.; Zerega, N.J.C.; Stull, G.W.; Gardner, E.M.; Tian, Q.; Gu, W.; Lu, Q.; Folk, R.A.; Kates, H.R.; et al. Phylogeny and Biogeography of Morus (Moraceae). Agronomy 2023, 13, 2021. [Google Scholar] [CrossRef]
- Singhal, B.; Khan, M.A.U.; Dhar, A.; Baqual, F.M.; Bindroo, B.B. Approaches to Industrial Exploitation of Mulberry (Mulberry Sp.) Fruits. J. Fruit Ornam. Plant Res. 2010, 18, 83–99. [Google Scholar]
- Sánchez-Salcedo, E.M.; Mena, P.; García-Viguera, C.; Hernández, F.; Martínez, J.J. (Poly)Phenolic Compounds and Antioxidant Activity of White (Morus alba) and Black (Morus nigra) Mulberry Leaves: Their Potential for New Products Rich in Phytochemicals. J. Funct. Foods 2015, 18, 1039–1046. [Google Scholar] [CrossRef]
- Wang, L.; Gao, H.; Sun, C.; Huang, L. Protective Application of Morus and Its Extracts in Animal Production. Animals 2022, 12, 3541. [Google Scholar] [CrossRef]
- Erden, Y. Sour Black Mulberry (Morus nigra L.) Causes Cell Death by Decreasing Mutant P53 Expression in HT-29 Human Colon Cancer Cells. Food Biosci. 2021, 42, 101113. [Google Scholar] [CrossRef]
- Negro, C.; Aprile, A.; De Bellis, L.; Miceli, A. Nutraceutical Properties of Mulberries Grown in Southern Italy (Apulia). Antioxidants 2019, 8, 223. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, Z.F.; Luo, X.; Li, X. Effects of Mulberry Fruit (Morus alba L.) Consumption on Health Outcomes: A Mini-Review. Antioxidants 2018, 7, 69. [Google Scholar] [CrossRef]
- Li, H.; Xiao, C.; Wang, F.; Guo, X.; Zhou, Z.; Jiang, Y. Blueberry–Mulberry Extract Alleviates Cognitive Impairment, Regulates Gut Metabolites, and Inhibits Inflammation in Aged Mice. Foods 2023, 12, 860. [Google Scholar] [CrossRef] [PubMed]
- Memete, A.R.; Miere (Groza), F.; Laslo, V.; Purcarea, C.; Vicas, L.; Ganea, M.; Antonescu, A.; Vicas, S.I. An In Vitro Study of the Healing Potential of Black Mulberry (Morus nigra L.) Extract in a Liposomal Formulation. Appl. Sci. 2023, 13, 1041. [Google Scholar] [CrossRef]
- Jung, S.; Lee, M.-S.; Chang, E.; Kim, C.-T.; Kim, Y. Mulberry (Morus alba L.) Fruit Extract Ameliorates Inflammation via Regulating MicroRNA-21/132/143 Expression and Increases the Skeletal Muscle Mitochondrial Content and AMPK/SIRT Activities. Antioxidants 2021, 10, 1453. [Google Scholar] [CrossRef]
- Birt, D.F.; Hendrich, S.; Wang, W. Dietary Agents in Cancer Prevention: Flavonoids and Isoflavonoids. Pharmacol. Ther. 2001, 90, 157–177. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Kim, R.; Son, S.-R.; Kang, K.S.; Jang, D.S.; Lee, S. Oddioside A, a New Phenolic Glycoside Isolated from the Fruits of Morus Alba (Mulberry), Protects TNF-α-Induced Human Dermal Fibroblast Damage. Antioxidants 2022, 11, 1894. [Google Scholar] [CrossRef] [PubMed]
- Naz, R.; Saqib, F.; Awadallah, S.; Wahid, M.; Latif, M.F.; Iqbal, I.; Mubarak, M.S. Food Polyphenols and Type II Diabetes Mellitus: Pharmacology and Mechanisms. Molecules 2023, 28, 3996. [Google Scholar] [CrossRef]
- Engelhart, M.J. Dietary Intake of Antioxidants and Risk of Alzheimer Disease. JAMA-J. Am. Med. Assoc. 2002, 287, 3223. [Google Scholar] [CrossRef]
- Chen, J.; Shu, Y.; Chen, Y.; Ge, Z.; Zhang, C.; Cao, J.; Li, X.; Wang, Y.; Sun, C. Evaluation of Antioxidant Capacity and Gut Microbiota Modulatory Effects of Different Kinds of Berries. Antioxidants 2022, 11, 1020. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Y.; Xiang, J.; Wang, C.; Johnson, J.B.; Beta, T. Diverse Polyphenol Components Contribute to Antioxidant Activity and Hypoglycemic Potential of Mulberry Varieties. LWT 2023, 173, 114308. [Google Scholar] [CrossRef]
- Ozdal, T.; Kamiloglu, S. 1.02—Polyphenols, Bioavailability and Potency. In Comprehensive Gut Microbiota; Glibetic, M., Ed.; Elsevier: Oxford, UK, 2022; pp. 3–19. ISBN 978-0-12-822036-8. [Google Scholar]
- Chhoden, T.; Singh, A.; Aggarwal, P.; Sharma, S. Chapter 7—Anthocyanins and Its Health Benefits. In Functionality and Application of Colored Cereals; Punia Bangar, S., Kumar, M., Eds.; Academic Press: New York, NY, USA, 2023; pp. 161–184. ISBN 978-0-323-99733-1. [Google Scholar]
- Cassidy, A. Berry Anthocyanin Intake and Cardiovascular Health. Mol. Asp. Med. 2018, 61, 76–82. [Google Scholar] [CrossRef]
- Wu, Y.; Han, T.; Yang, H.; Lyu, L.; Li, W.; Wu, W. Known and Potential Health Benefits and Mechanisms of Blueberry Anthocyanins: A Review. Food Biosci. 2023, 55, 103050. [Google Scholar] [CrossRef]
- Shu, C.; Wu, S.; Li, H.; Tian, J. Health Benefits of Anthocyanin-Containing Foods, Beverages, and Supplements Have Unpredictable Relation to Gastrointestinal Microbiota: A Systematic Review and Meta-Analysis of Random Clinical Trials. Nutr. Res. 2023, 116, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Escalante-Aburto, A.; Mendoza-Córdova, M.Y.; Mahady, G.B.; Luna-Vital, D.A.; Gutiérrez-Uribe, J.A.; Chuck-Hernández, C. Consumption of Dietary Anthocyanins and Their Association with a Reduction in Obesity Biomarkers and the Prevention of Obesity. Trends Food Sci. Technol. 2023, 140, 104140. [Google Scholar] [CrossRef]
- Huo, J.; Ni, Y.; Li, D.; Qiao, J.; Huang, D.; Sui, X.; Zhang, Y. Comprehensive Structural Analysis of Polyphenols and Their Enzymatic Inhibition Activities and Antioxidant Capacity of Black Mulberry (Morus nigra L.). Food Chem. 2023, 427, 136605. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Wang, S.; Wang, S.; Cao, S.; Wang, X.; Lü, X. Extraction Optimization and Characterization of Persimmon Peel Pectin Extracted by Subcritical Water. Food Chem. X 2022, 16, 100486. [Google Scholar] [CrossRef]
- Wang, P.-Y.; Shuang, F.-F.; Yang, J.-X.; Jv, Y.-X.; Hu, R.-Z.; Chen, T.; Yao, X.-H.; Zhao, W.-G.; Liu, L.; Zhang, D.-Y. A Rapid and Efficient Method of Microwave-Assisted Extraction and Hydrolysis and Automatic Amino Acid Analyzer Determination of 17 Amino Acids from Mulberry Leaves. Ind. Crop. Prod. 2022, 186, 115271. [Google Scholar] [CrossRef]
- Mustafa, A.M.; Mazzara, E.; Abouelenein, D.; Angeloni, S.; Nunez, S.; Sagratini, G.; López, V.; Cespi, M.; Vittori, S.; Caprioli, G.; et al. Optimization of Solvent-Free Microwave-Assisted Hydrodiffusion and Gravity Extraction of Morus nigra L. Fruits Maximizing Polyphenols, Sugar Content, and Biological Activities Using Central Composite Design. Pharmaceuticals 2022, 15, 99. [Google Scholar] [CrossRef]
- Akyüz, A.; Ersus, S. Optimization of Enzyme Assisted Extraction of Protein from the Sugar Beet (Beta vulgaris L.) Leaves for Alternative Plant Protein Concentrate Production. Food Chem. 2021, 335, 127673. [Google Scholar] [CrossRef]
- Abdullah, S.; Pradhan, R.C.; Pradhan, D.; Mishra, S. Modeling and Optimization of Pectinase-Assisted Low-Temperature Extraction of Cashew Apple Juice Using Artificial Neural Network Coupled with Genetic Algorithm. Food Chem. 2021, 339, 127862. [Google Scholar] [CrossRef]
- Alavarsa-Cascales, D.; Aliaño-González, M.J.; Palma, M.; Barbero, G.F.; Carrera, C. Optimization of an Enzyme-Assisted Extraction Method for the Anthocyanins Present in Açai (Euterpe oleracea Mart.). Agronomy 2022, 12, 2327. [Google Scholar] [CrossRef]
- Jung, S.; Moura, J.; Campbell, K.; Johnson, I. Enzyme-Assisted Aqueous Extraction of Oilseeds. In Enhancing Extraction Processes in the Food Industry; Lebovka, N., Vorobiev, E., Chemat, F., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 477–518. ISBN 978-0-429-10867-9. [Google Scholar]
- Aliaño González, M.J.; Carrera, C.; Barbero, G.F.; Palma, M. A Comparison Study between Ultrasound–Assisted and Enzyme–Assisted Extraction of Anthocyanins from Blackcurrant (Ribes nigrum L.). Food Chem. X 2022, 13, 100192. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Rodríguez, G.; Marina, M.L.; Plaza, M. Enzyme-Assisted Extraction of Bioactive Non-Extractable Polyphenols from Sweet Cherry (Prunus avium L.) Pomace. Food Chem. 2021, 339, 128086. [Google Scholar] [CrossRef] [PubMed]
- Bandara, S.; Devereaux, S.; Weerasooriya, A. Methods to Evaluate the Antiobesity Effects of Medicinal Plants Using Enzyme Assays. Obesities 2023, 3, 13–35. [Google Scholar] [CrossRef]
- Quitério, E.; Grosso, C.; Ferraz, R.; Delerue-Matos, C.; Soares, C. A Critical Comparison of the Advanced Extraction Techniques Applied to Obtain Health-Promoting Compounds from Seaweeds. Mar. Drugs 2022, 20, 677. [Google Scholar] [CrossRef] [PubMed]
- Chávez-González, M.L.; López-López, L.I.; Rodríguez-Herrera, R.; Contreras-Esquivel, J.C.; Aguilar, C.N. Enzyme-Assisted Extraction of Citrus Essential Oil. Chem. Pap. 2016, 70, 412–417. [Google Scholar] [CrossRef]
- Yilmaz, E. Use of Hydrolytic Enzymes as Green and Effective Extraction Agents for Ultrasound Assisted-Enzyme Based Hydrolytic Water Phase Microextraction of Arsenic in Food Samples. Talanta 2018, 189, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhu, J.; Wang, T.; Sun, J.; Guo, T.; Zhang, L.; Yu, G.; Xia, X. Antidiabetic Activity of Armillaria Mellea Polysaccharides: Joint Ultrasonic and Enzyme Assisted Extraction. Ultrason. Sonochem. 2023, 95, 106370. [Google Scholar] [CrossRef]
- Kuhlman, B.; Hansen, J.; Jørgensen, B.; du Toit, W.; Moore, J.P. The Effect of Enzyme Treatment on Polyphenol and Cell Wall Polysaccharide Extraction from the Grape Berry and Subsequent Sensory Attributes in Cabernet Sauvignon Wines. Food Chem. 2022, 385, 132645. [Google Scholar] [CrossRef]
- Ercisli, S.; Orhan, E. Chemical Composition of White (Morus alba), Red (Morus rubra) and Black (Morus nigra) Mulberry Fruits. Food Chem. 2007, 103, 1380–1384. [Google Scholar] [CrossRef]
- Sánchez-Salcedo, E.M.; Sendra, E.; Carbonell-Barrachina, Á.A.; Martínez, J.J.; Hernández, F. Fatty Acids Composition of Spanish Black (Morus nigra L.) and White (Morus alba L.) Mulberries. Food Chem. 2016, 190, 566–571. [Google Scholar] [CrossRef]
- Li, X.; Chen, F.; Li, S.; Jia, J.; Gu, H.; Yang, L. An Efficient Homogenate-Microwave-Assisted Extraction of Flavonols and Anthocyanins from Blackcurrant Marc: Optimization Using Combination of Plackett-Burman Design and Box-Behnken Design. Ind. Crop. Prod. 2016, 94, 834–847. [Google Scholar] [CrossRef]
- Ayyubi, S.N.; Purbasari, A.; Kusmiyati. The Effect of Composition on Mechanical Properties of Biodegradable Plastic Based on Chitosan/Cassava Starch/PVA/Crude Glycerol: Optimization of the Composition Using Box Behnken Design. Mater. Today 2022, 63, S78–S83. [Google Scholar] [CrossRef]
- Dejaegher, B.; Heyden, Y.V. Experimental Designs and Their Recent Advances in Set-up, Data Interpretation, and Analytical Applications. J. Pharm. Biomed. Anal. 2011, 56, 141–158. [Google Scholar] [CrossRef]
- Khatib, I.; Chow, M.Y.T.; Ruan, J.; Cipolla, D.; Chan, H.-K. Modeling of a Spray Drying Method to Produce Ciprofloxacin Nanocrystals inside the Liposomes Utilizing a Response Surface Methodology: Box-Behnken Experimental Design. Int. J. Pharm. 2021, 597, 120277. [Google Scholar] [CrossRef] [PubMed]
- González-de-Peredo, A.V.; Vázquez-Espinosa, M.; Espada-Bellido, E.; Ferreiro-González, M.; Carrera, C.; Barbero, G.F.; Palma, M. Development of Optimized Ultrasound-Assisted Extraction Methods for the Recovery of Total Phenolic Compounds and Anthocyanins from Onion Bulbs. Antioxidants 2021, 10, 1755. [Google Scholar] [CrossRef] [PubMed]
- V. González de Peredo, A.; Vázquez-Espinosa, M.; Espada-Bellido, E.; Jiménez-Cantizano, A.; Ferreiro-González, M.; Amores-Arrocha, A.; Palma, M.; Barroso, C.G.; Barbero, G.F. Development of New Analytical Microwave-Assisted Extraction Methods for Bioactive Compounds from Myrtle (Myrtus communis L.). Molecules 2018, 23, 2992. [Google Scholar] [CrossRef]
- Aliaño-González, M.J.; Espada-Bellido, E.; Ferreiro-González, M.; Carrera, C.; Palma, M.; Ayuso, J.; Álvarez, J.Á.; Barbero, G.F. Extraction of Anthocyanins and Total Phenolic Compounds from Açai (Euterpe oleracea Mart.) Using an Experimental Design Methodology. Part 2: Ultrasound-Assisted Extraction. Agronomy 2020, 10, 326. [Google Scholar] [CrossRef]
- Farooq, A.; Hussain, S.Z.; Bhat, T.A.; Naseer, B.; Shafi, F. Walnut Fruit: Impact of Ethylene Assisted Hulling on in Vitro Antioxidant Activity, Polyphenols, PUFAs, Amino Acids and Sensory Attributes. Food Chem. 2023, 404, 134763. [Google Scholar] [CrossRef]
- Lopez-Romero, J.C.; González-Ríos, H.; Borges, A.; Simões, M. Antibacterial Effects and Mode of Action of Selected Essential Oils Components against Escherichia coli and Staphylococcus aureus. Evid. Based Complement Altern. Med. 2015, 2015, 795435. [Google Scholar] [CrossRef]
- Simões, M.; Rocha, S.; Coimbra, M.A.; Vieira, M.J. Enhancement of Escherichia coli and Staphylococcus aureus Antibiotic Susceptibility Using Sesquiterpenoids. Med. Chem. 2008, 4, 616–623. [Google Scholar] [CrossRef]
- Jiang, Y.; Ding, Y.; Wang, D.; Deng, Y.; Zhao, Y. Radio Frequency-Assisted Enzymatic Extraction of Anthocyanins from Akebia trifoliata (Thunb.) Koidz. Flowers: Process Optimization, Structure, and Bioactivity Determination. Ind. Crop. Prod. 2020, 149, 112327. [Google Scholar] [CrossRef]
- Kumar, M.; Dahuja, A.; Sachdev, A.; Tomar, M.; Lorenzo, J.M.; Dhumal, S.; Radha; Chandran, D.; Varghese, E.; Saha, S.; et al. Optimization of the Use of Cellulolytic Enzyme Preparation for the Extraction of Health Promoting Anthocyanins from Black Carrot Using Response Surface Methodology. LWT 2022, 163, 113528. [Google Scholar] [CrossRef]
- Meini, M.-R.; Cabezudo, I.; Boschetti, C.E.; Romanini, D. Recovery of Phenolic Antioxidants from Syrah Grape Pomace through the Optimization of an Enzymatic Extraction Process. Food Chem. 2019, 283, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Chamorro, S.; Viveros, A.; Alvarez, I.; Vega, E.; Brenes, A. Changes in Polyphenol and Polysaccharide Content of Grape Seed Extract and Grape Pomace after Enzymatic Treatment. Food Chem. 2012, 133, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Verbeyst, L.; Oey, I.; Van der Plancken, I.; Hendrickx, M.; Van Loey, A. Kinetic Study on the Thermal and Pressure Degradation of Anthocyanins in Strawberries. Food Chem. 2010, 123, 269–274. [Google Scholar] [CrossRef]
- Özgen, M.; Serçe, S.; Kaya, C. Phytochemical and Antioxidant Properties of Anthocyanin-Rich Morus nigra and Morus rubra Fruits. Sci. Hortic. 2009, 119, 275–279. [Google Scholar] [CrossRef]
- Espada-Bellido, E.; Ferreiro-González, M.; Carrera, C.; Palma, M.; Barroso, C.G.; Barbero, G.F. Optimization of the Ultrasound-Assisted Extraction of Anthocyanins and Total Phenolic Compounds in Mulberry (Morus nigra) Pulp. Food Chem. 2017, 219, 23–32. [Google Scholar] [CrossRef]
- Ruiz-Rodríguez, B.M.; Sánchez-Moreno, C.; Ancos, B.D.; Sánchez-Mata, M.d.C.; Fernández-Ruiz, V.; Cámara, M.; Tardío, J. Wild Arbutus Unedo L. and Rubus Ulmifolius Schott Fruits Are Underutilized Sources of Valuable Bioactive Compounds with Antioxidant Capacity. Fruits 2014, 69, 435–448. [Google Scholar] [CrossRef]
- Vukmirović, S.; Ilić, V.; Tadić, V.; Čapo, I.; Pavlović, N.; Tomas, A.; Paut Kusturica, M.; Tomić, N.; Maksimović, S.; Stilinović, N. Comprehensive Analysis of Antioxidant and Hepatoprotective Properties of Morus nigra L. Antioxidants 2023, 12, 382. [Google Scholar] [CrossRef]
- Krzykowski, A.; Dziki, D.; Rudy, S.; Polak, R.; Biernacka, B.; Gawlik-Dziki, U.; Janiszewska-Turak, E. Effect of Air-Drying and Freeze-Drying Temperature on the Process Kinetics and Physicochemical Characteristics of White Mulberry Fruits (Morus alba L.). Processes 2023, 11, 750. [Google Scholar] [CrossRef]
- Du, H.; Wang, X.; Yang, H.; Zhu, F.; Liu, J.; Cheng, J.; Lin, Y.; Tang, D.; Liu, X. Regulation on the Quality of Yogurt by Phenolic Fraction of Mulberry Pomace Supplemented before and after Fermentation. Food Control 2023, 144, 109333. [Google Scholar] [CrossRef]
- Herman, R.A.; Ayepa, E.; Fometu, S.S.; Shittu, S.; Davids, J.S.; Wang, J. Mulberry Fruit Post-Harvest Management: Techniques, Composition and Influence on Quality Traits—A Review. Food Control 2022, 140, 109126. [Google Scholar] [CrossRef]
- Sharifi, K.A.; Pirsa, S. Biodegradable Film of Black Mulberry Pulp Pectin/Chlorophyll of Black Mulberry Leaf Encapsulated with Carboxymethylcellulose/Silica Nanoparticles: Investigation of Physicochemical and Antimicrobial Properties. Mater. Chem. Phys. 2021, 267, 124580. [Google Scholar] [CrossRef]



| Variety | Location | Sampling Date | Code |
|---|---|---|---|
| Morus nigra | Puerto Real, Cádiz (Spain) | 6 January 2021 | Mn-PR |
| Morus nigra | Torrecera, Cádiz (Spain) | 7 June 2022 | Mn-T |
| Purple Morus alba | Torrecera, Cádiz (Spain) | 7 June 2022 | Ma-P |
| White Morus alba | Torrecera, Cádiz (Spain) | 7 June 2022 | Ma-B |
| Morus rubra | Torrecera, Cádiz (Spain) | 1 September 2022 | Mr-T |
| Morus rubra | Puerto Real, Cádiz (Spain) | 1 September 2022 | Mr-Pr 1 |
| Morus rubra | Puerto Real, Cádiz (Spain) | 11 September 2022 | Mr-Pr 2 |
| Sample | Anthocyanins (mg/100 g) | ||||
|---|---|---|---|---|---|
| Cyanidin 3-O-glucoside | Cyanidin 3-O-rutinoside | Pelargonidin 3-O-glucoside | Pelargonidin 3-O-rutinoside | Total | |
| Mn-PR | 144.00 ± 22.00 a | 13.00 ± 0.41 a | 5.00 ± 1.00 a | 18.00 ± 3.00 a | 181.00 ± 26.00 a |
| Ma-B | 60.00 ± 4.00 a | 12.00 ± 0.28 a | 1.00 ± 0.12 a | 8.00 ± 0.11 a | 82.00 ± 4.00 a |
| Ma-P | 166.00 ± 10.00 a | 17.00 ± 2.00 a | 3.00 ± 0.44 a | 18.00 ± 1.00 a | 204.00 ± 7.00 a |
| Mn-T | 2530.00 ± 192.00 d | 123.00 ± 11.00 c | 83.00 ± 7.00 d | 56.00 ± 5.00 d | 2790.00 ± 214.00 d |
| Mr-T | 1434.00 ± 118.00 c | 113.00 ± 10.00 c | 59.00 ± 4.00 c | 54.00 ± 4.00 cd | 1660.00 ± 135.00 c |
| Mr-Pr 1 | 1045.00 ± 84.00 b | 78.00 ± 5.00 b | 39.00 ± 3.00 b | 40.00 ± 10.00 b | 1202.00 ± 101.00 b |
| Mr-Pr 2 | 1113.00 ± 13.00 b | 95.00 ± 1.00 b | 60.00 ± 0.46 c | 42.00 ± 1.00 bc | 1310.00 ± 14.00 b |
| p-value | 7.57 × 10−14 | 1.11 × 10−12 | 3.98 × 10−14 | 1.45 × 10−8 | 1.08 × 10−13 |
| Sample | Phenolic Compounds (mg/100 g) | ||||
| Chlorogenic Acid | Vanillic Acid | Rutin | Resveratrol | Total | |
| Mn-PR | 678.00 ± 65.00 ab | 505.00 ± 55.00 a | 594.00 ± 75.00 ab | 2057.00 ± 85.00 ab | 3834.00 ± 396.00 a |
| Ma-B | 1357.00 ± 72.00 c | 2805.00 ± 83.00 b | 1825.00 ± 671.00 ab | 1997.00 ± 24.00 ab | 7984.00 ± 1202.00 b |
| Ma-P | 428.00 ± 40.00 a | 990.00 ± 94.00 ab | 504.00 ± 45.00 a | 2125.00 ± 108.00 ab | 4047.00 ± 406.00 a |
| Mn-T | 576.00 ± 37.00 ab | 2265.00 ± 79.00 ab | 830.00 ± 70.00 ab | 2392.00 ± 50.00 c | 6063.00 ± 334.00 ab |
| Mr-T | 1204.00 ± 94.00 c | 6682.00 ± 924.00 c | 2198.00 ± 314.00 c | 2026.00 ± 51.00 ab | 12110.00 ± 1956.00 c |
| Mr-Pr 1 | 841.00 ± 45.00 b | 5209.00 ± 449.00 c | 1706.00 ± 116.00 ab | 1969.00 ± 12.00 ab | 9725.00 ± 880.00 bc |
| Mr-Pr 2 | 560.00 ± 32.00 ab | 2873.00 ± 269.00 b | 892.00 ± 21.00 ab | 1864.00 ± 138.00 a | 6189.00 ± 651.00 ab |
| p-value | 0.000068 | 0.000123 | 0.020516 | 0.035933 | 0.000574 |
| Samples | Trolox Equivalent | Diff. Abs E. coli | Diff. Abs S. aureus |
|---|---|---|---|
| Mn-PR | 25.51 ± 1.04 | 0.36 | 0.56 |
| Ma-B | 9.74 ± 0.44 | 0.35 | 0.63 |
| Ma-P | 13.12 ± 0.72 | 0.40 | 0.66 |
| Mn-T | 56.04 ± 4.60 | 0.20 | 0.57 |
| Mr-T | 29.53 ± 2.52 | 0.25 | 0.59 |
| Mr-Pr 1 | 21.80 ± 3.47 | 0.36 | 0.58 |
| Mr-Pr 2 | 28.46 ± 2.07 | 0.32 | 0.57 |
| Control (bacteria) | - | 0.35 | 0.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tizón Alba, A.; Aliaño-González, M.J.; Palma, M.; Fernández Barbero, G.; Carrera, C. Enhancing Efficiency of Enzymatic-Assisted Extraction Method for Evaluating Bioactive Compound Analysis in Mulberry: An Optimization Approach. Agronomy 2023, 13, 2548. https://doi.org/10.3390/agronomy13102548
Tizón Alba A, Aliaño-González MJ, Palma M, Fernández Barbero G, Carrera C. Enhancing Efficiency of Enzymatic-Assisted Extraction Method for Evaluating Bioactive Compound Analysis in Mulberry: An Optimization Approach. Agronomy. 2023; 13(10):2548. https://doi.org/10.3390/agronomy13102548
Chicago/Turabian StyleTizón Alba, Ainara, María José Aliaño-González, Miguel Palma, Gerardo Fernández Barbero, and Ceferino Carrera. 2023. "Enhancing Efficiency of Enzymatic-Assisted Extraction Method for Evaluating Bioactive Compound Analysis in Mulberry: An Optimization Approach" Agronomy 13, no. 10: 2548. https://doi.org/10.3390/agronomy13102548
APA StyleTizón Alba, A., Aliaño-González, M. J., Palma, M., Fernández Barbero, G., & Carrera, C. (2023). Enhancing Efficiency of Enzymatic-Assisted Extraction Method for Evaluating Bioactive Compound Analysis in Mulberry: An Optimization Approach. Agronomy, 13(10), 2548. https://doi.org/10.3390/agronomy13102548