Genome-Wide Analysis Revealed NBS-LRR Gene Candidates Associated with Bacterial Wilt Resistance in Eggplant (Solanum melongena L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Growth Conditions and Treatments
2.2. Identification and Categorization of NBS Genes in Eggplant
2.3. Gene Characteristics, Structure, and Chromosomal Distribution Analysis
2.4. Phylogenetic, Conserved Motifs, and Cis-Acting Elements Analysis
2.5. Gene Duplication and Syntenic Analysis
2.6. Expression Analysis of SmNBSs
3. Results
3.1. Identification and Categorization of the NBS Gene Family
3.2. Gene Characteristics and Structure
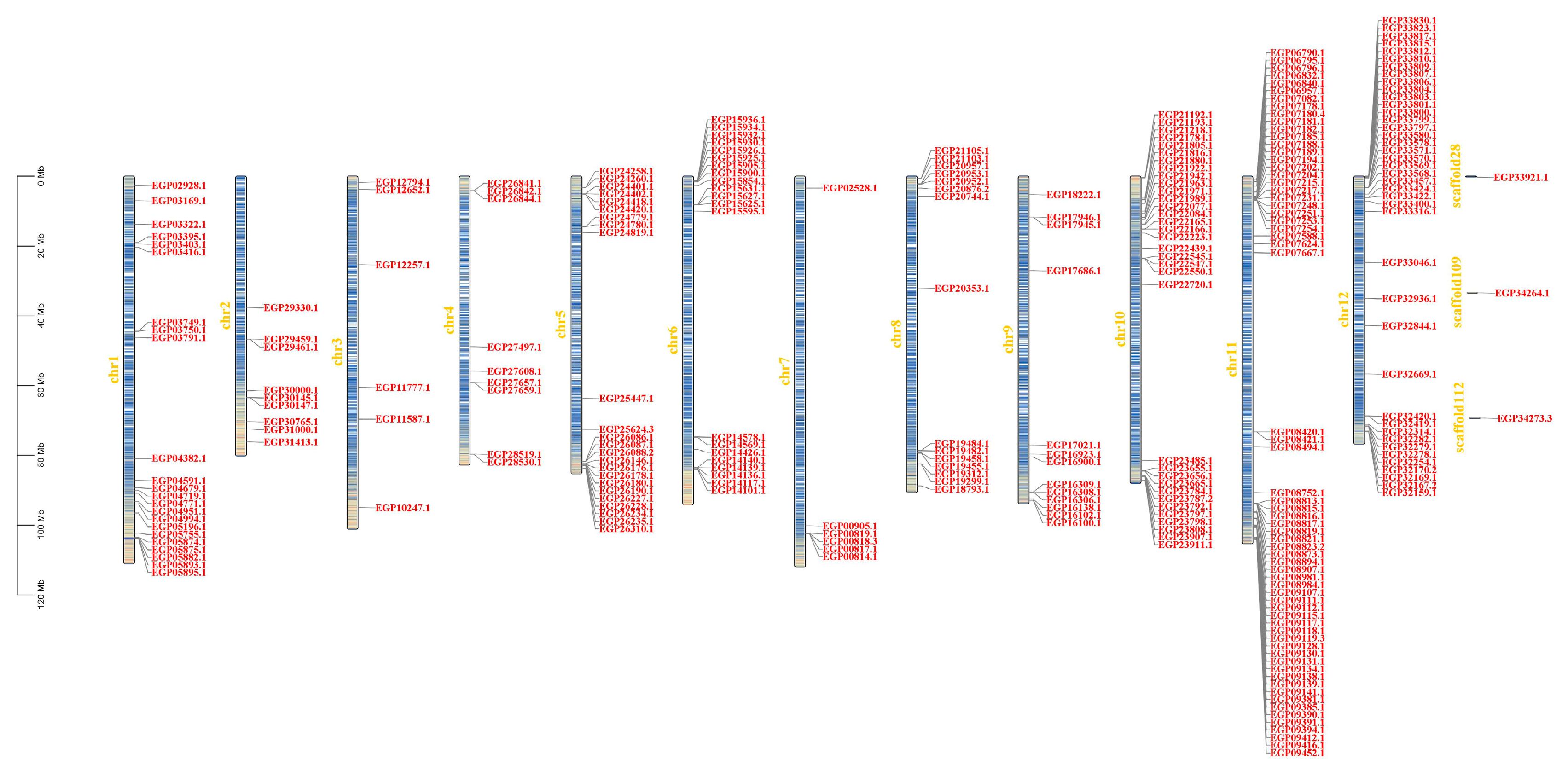
3.3. Genomic Distribution on Chromosomes
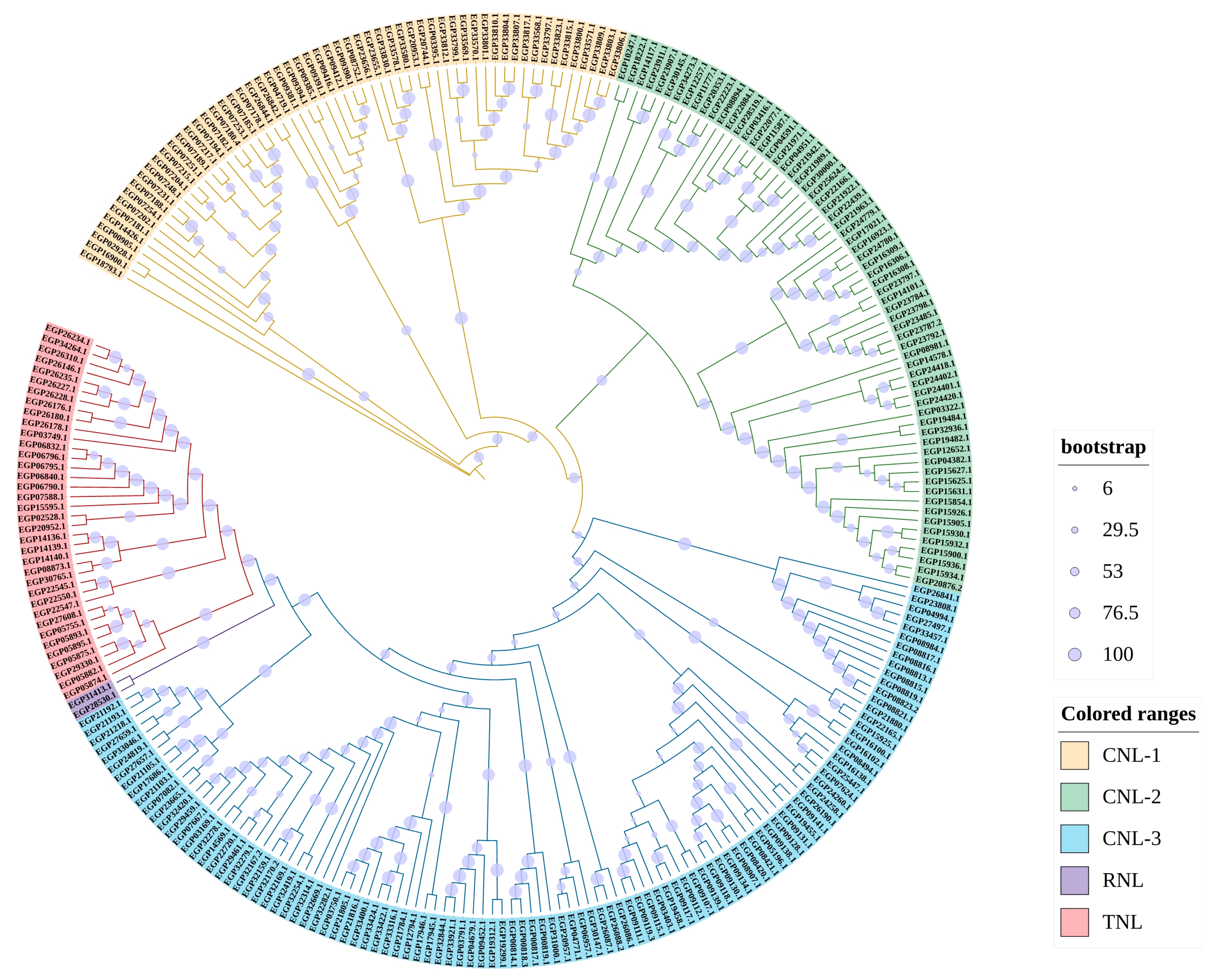
3.4. Phylogenetic Relationships and Conserved Motifs
3.5. Cis-Acting Regulatory Elements in the Promoters
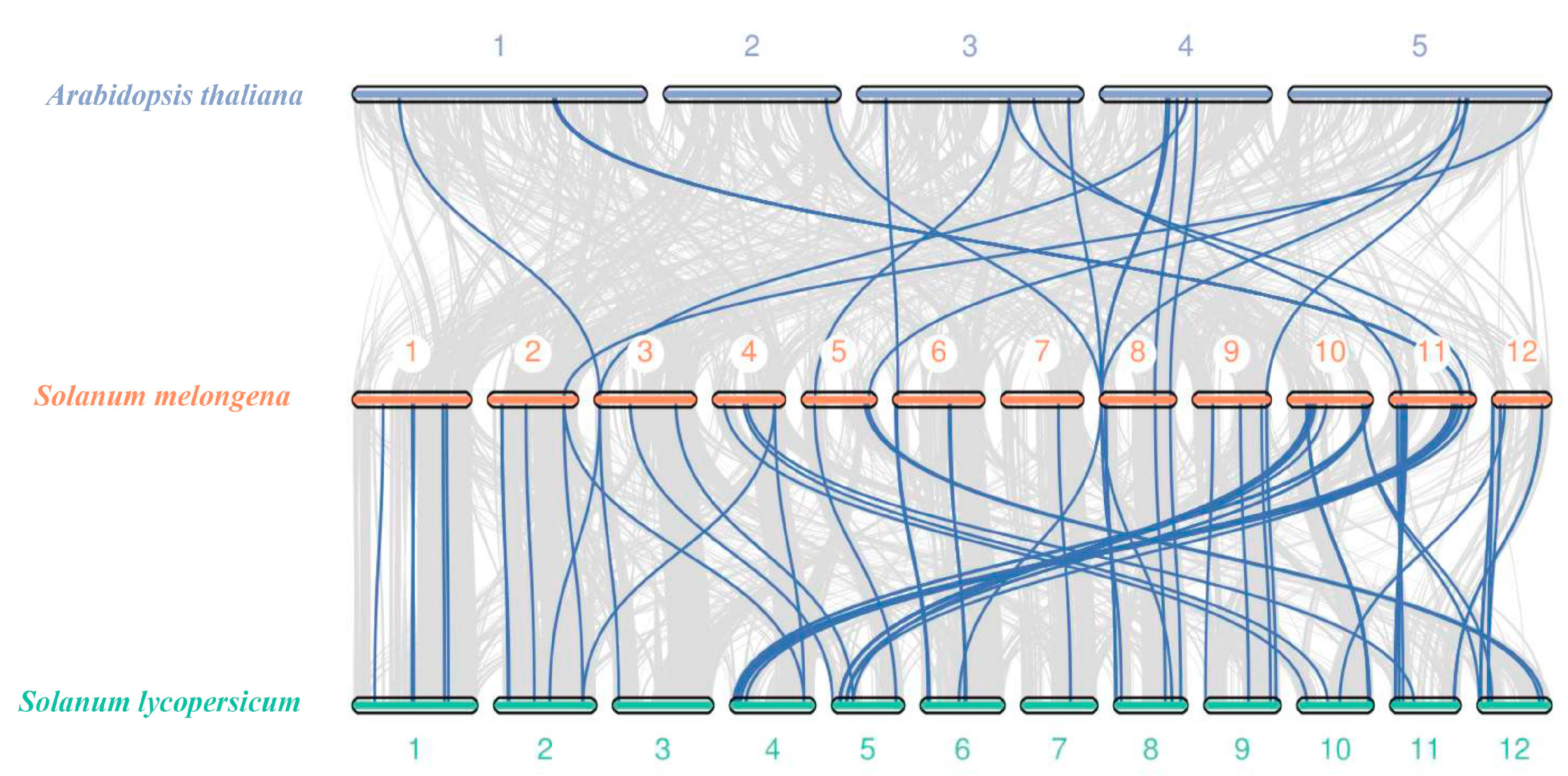
3.6. Gene Duplication and Synteny Analysis
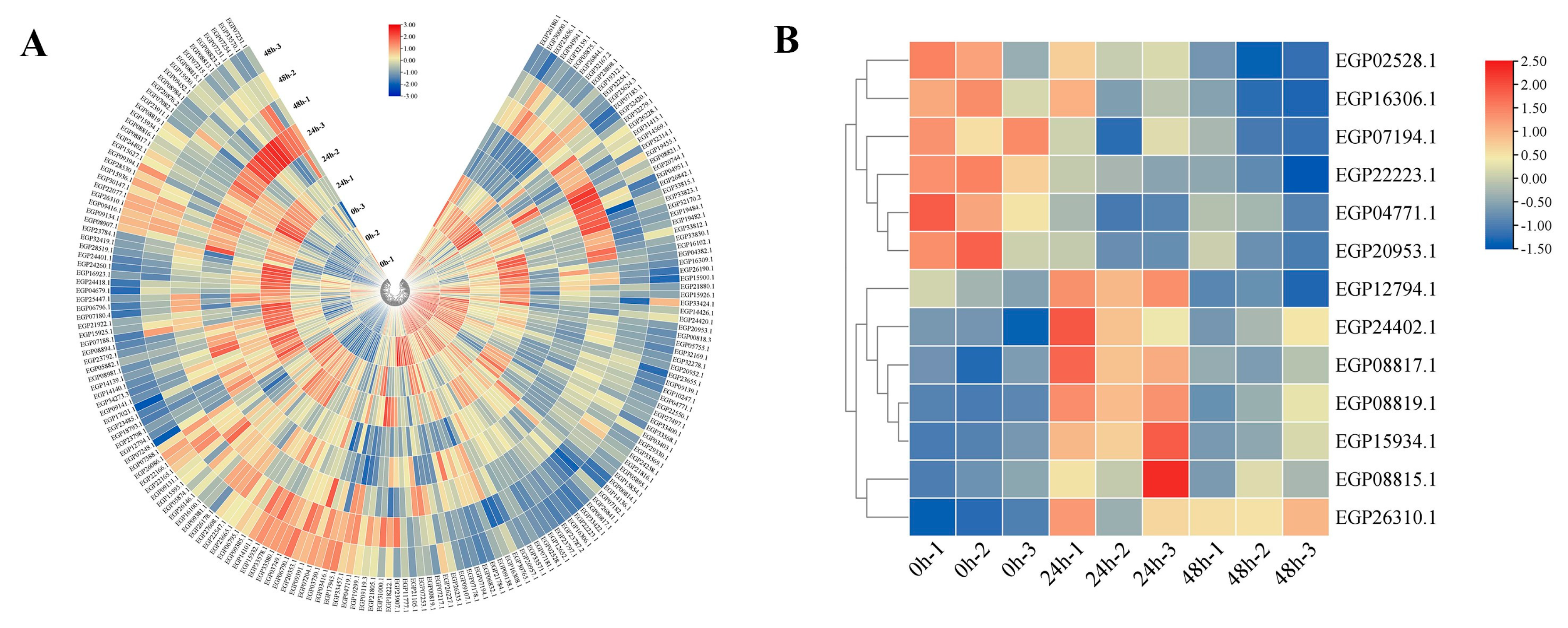
3.7. Expression Pattern of SmNBSs under Pathogen Treatment
3.8. Responses to R. solanacearum Pathogen Infection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jones, J.D.G.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef]
- Dangl, J.L.; Horvath, D.M.; Staskawicz, B.J. Pivoting the plant immune system from dissection to deployment. Science 2013, 341, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.X.; Meyers, B.C.; Chen, J.Q.; Tian, D.; Yang, S. Tracing the origin and evolutionary history of plant nucleotide-binding site-leucine-rich repeat (NBS-LRR) genes. New Phytol. 2012, 193, 1049–1063. [Google Scholar] [CrossRef]
- Dangl, J.L.; Jones, J.D. Plant pathogens and integrated defence responses to infection. Nature 2001, 411, 826–833. [Google Scholar] [PubMed]
- McHale, L.; Tan, X.; Koehl, P.; Michelmore, R.W. Plant NBS-LRR proteins: Adaptable guards. Genome Biol. 2006, 7, 212. [Google Scholar] [CrossRef] [PubMed]
- Kourelis, J.; van der Hoorn, R.A.L. Defended to the nines: 25 years of resistance gene cloning identifies nine mechanisms for R protein function. Plant Cell 2018, 30, 285–299. [Google Scholar] [CrossRef]
- Shao, Z.Q.; Xue, J.Y.; Wu, P.; Zhang, Y.M.; Wu, Y.; Hang, Y.Y.; Wang, B.; Chen, J.Q. Large-scale analyses of angiosperm nucleotide-binding site-leucine-rich repeat genes reveal three anciently diverged classes with distinct evolutionary patterns. Plant Physiol. 2016, 170, 2095–2109. [Google Scholar] [CrossRef] [PubMed]
- Dodds, P.N.; Lawrence, G.J.; Catanzariti, A.M.; Teh, T.; Wang, C.I.; Ayliffe, M.A.; Kobe, B.; Ellis, J.G. Direct protein interaction underlies gene-for-gene specificity and coevolution of the flax resistance genes and flax rust avirulence genes. Proc. Natl. Acad. Sci. USA 2006, 103, 8888–8893. [Google Scholar] [CrossRef]
- Kroj, T.; Chanclud, E.; Michel-Romiti, C.; Grand, X.; Morel, J.B. Integration of decoy domains derived from protein targets of pathogen effectors into plant immune receptors is widespread. New Phytol. 2016, 210, 618–626. [Google Scholar] [CrossRef]
- Wu, C.H.; Krasileva, K.V.; Banfield, M.J.; Terauchi, R.; Kamoun, S. The “sensor domains” of plant NLR proteins: More than decoys? Front. Plant Sci. 2015, 6, 134. [Google Scholar] [CrossRef]
- Andersen, E.J.; Ali, S.; Byamukama, E.; Yen, Y.; Nepal, M.P. Disease resistance mechanisms in plants. Genes 2018, 9, 339. [Google Scholar] [CrossRef]
- Maekawa, T.; Kufer, T.A.; Schulze-Lefert, P. NLR functions in plant and animal immune systems: So far and yet so close. Nat. Immunol. 2011, 12, 817–826. [Google Scholar]
- Meyers, B.C.; Dickerman, A.W.; Michelmore, R.W.; Sivaramakrishnan, S.; Sobral, B.W.; Young, N.D. Plant disease resistance genes encode members of an ancient and diverse protein family within the nucleotide-binding superfamily. Plant J. 1999, 20, 317–332. [Google Scholar] [CrossRef]
- Meyers, B.C.; Kozik, A.; Griego, A.; Kuang, H.; Michelmore, R.W. Genome-wide analysis of NBS-LRR-encoding genes in Arabidopsis. Plant Cell 2003, 15, 809–834. [Google Scholar] [CrossRef] [PubMed]
- Monosi, B.; Wisser, R.J.; Pennill, L.; Hulbert, S.H. Full-genome analysis of resistance gene homologues in rice. Theor. Appl. 2004, 109, 1434–1447. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Wang, Y.; Chen, J.Q.; Araki, H.; Jing, Z.; Jiang, K.; Shen, J.; Tian, D. Genome-wide identification of NBS genes in japonica rice reveals significant expansion of divergent non-TIR NBS-LRR genes. Mol. Genet. Genom. 2004, 271, 402–415. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xie, J.; Wang, H.; Zhong, X.; Li, H.; Yu, J.; Kang, J. Identification and expression profiling analysis of NBS-LRR genes involved in Fusarium oxysporum f.sp. conglutinans resistance in cabbage. 3 Biotech 2019, 9, 202. [Google Scholar] [CrossRef]
- Kang, Y.J.; Kim, K.H.; Shim, S.; Yoon, M.Y.; Sun, S.; Kim, M.Y.; Van, K.; Lee, S.H. Genome-wide mapping of NBS-LRR genes and their association with disease resistance in soybean. BMC Plant Biol. 2012, 12, 139. [Google Scholar] [CrossRef]
- Gu, L.; Si, W.; Zhao, L.; Yang, S.; Zhang, X. Dynamic evolution of NBS-LRR genes in bread wheat and its progenitors. Mol. Genet. Genom. 2015, 290, 727–738. [Google Scholar] [CrossRef]
- Andersen, E.J.; Nepal, M.P.; Purintun, J.M.; Nelson, D.; Mermigka, G.; Sarris, P.F. Wheat disease resistance genes and their diversification through integrated domain fusions. Front. Genet. 2020, 11, 898. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, X.; Jiang, H.; Ma, W.; Miao, W.; Yamada, T.; Zhang, M. Systematic analysis and comparison of nucleotide-binding site disease resistance genes in maize. FEBS J. 2012, 279, 2431–2443. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.H.; Zhou, G.C.; Sun, X.Q.; Lei, Z.; Zhang, Y.M.; Xue, J.Y.; Hang, Y.Y. Distinct patterns of gene gain and loss: Diverse evolutionary modes of nbs-encoding genes in three solanaceae crop species. G3 2017, 7, 1577–1585. [Google Scholar] [CrossRef] [PubMed]
- Jupe, F.; Pritchard, L.; Etherington, G.J.; Mackenzie, K.; Cock, P.J.; Wright, F.; Sharma, S.K.; Bolser, D.; Bryan, G.J.; Jones, J.D.; et al. Identification and localisation of the NB-LRR gene family within the potato genome. BMC Genom. 2012, 13, 75. [Google Scholar] [CrossRef]
- Shi, J.L.; Zai, W.S.; Xiong, Z.L.; Wan, H.J.; Wu, W.R. NB-LRR genes: Characteristics in three Solanum species and transcriptional response to Ralstonia solanacearum in tomato. Planta 2021, 254, 96. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.Q.; Xue, J.Y.; Wang, Q.; Wang, B.; Chen, J.Q. Revisiting the origin of plant NBS-LRR genes. Trends Plant Sci. 2019, 24, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Andersen, E.J.; Ali, S.; Reese, R.N.; Yen, Y.; Neupane, S.; Nepal, M.P. Diversity and evolution of disease resistance genes in Barley (Hordeum vulgare L.). Evol. Bioinform. Online 2016, 12, 99–108. [Google Scholar] [CrossRef]
- Shao, Z.Q.; Zhang, Y.M.; Hang, Y.Y.; Xue, J.Y.; Zhou, G.C.; Wu, P.; Wu, X.Y.; Wu, X.Z.; Wang, Q.; Wang, B.; et al. Long-term evolution of nucleotide-binding site-leucine-rich repeat genes: Understanding gained from and beyond the legume family. Plant Physiol. 2014, 166, 217–234. [Google Scholar] [CrossRef]
- Liu, J.; Liu, X.; Dai, L.; Wang, G. Recent progress in elucidating the structure, function and evolution of disease resistance genes in plants. J. Genet. Genom. 2007, 34, 765–776. [Google Scholar] [CrossRef]
- Bent, A.F.; Kunkel, B.N.; Dahlbeck, D.; Brown, K.L.; Schmidt, R.; Giraudat, J.; Leung, J.; Staskawicz, B.J. RPS2 of Arabidopsis thaliana: A leucine-rich repeat class of plant disease resistance genes. Science 1994, 265, 1856–1860. [Google Scholar] [CrossRef]
- Grant, M.R.; Godiard, L.; Straube, E.; Ashfield, T.; Lewald, J.; Sattler, A.; Innes, R.W.; Dangl, J.L. Structure of the Arabidopsis RPM1 gene enabling dual specificity disease resistance. Science 1995, 269, 843–846. [Google Scholar] [CrossRef]
- Ma, J.; Lei, C.; Xu, X.; Hao, K.; Wang, J.; Cheng, Z.; Ma, X.; Ma, J.; Zhou, K.; Zhang, X.; et al. Pi64, Encoding a novel CC-NBS-LRR protein, confers resistance to leaf and neck blast in rice. Mol. Plant Microbe Interact. 2015, 28, 558–568. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, R.; Shi, Z.; Zhao, Y.; Su, A.; Wang, Y.; Xing, J.; Ge, J.; Li, C.; Wang, X.; et al. Identification and fine mapping of rppm, a southern corn rust resistance gene in maize. Front. Plant Sci. 2020, 11, 1057. [Google Scholar] [CrossRef]
- Whitham, S.; Dinesh-Kumar, S.P.; Choi, D.; Hehl, R.; Corr, C.; Baker, B. The product of the tobacco mosaic virus resistance gene N: Similarity to toll and the interleukin-1 receptor. Cell 1994, 78, 1101–1115. [Google Scholar] [CrossRef]
- Bendahmane, A.; Querci, M.; Kanyuka, K.; Baulcombe, D.C. Agrobacterium transient expression system as a tool for the isolation of disease resistance genes: Application to the Rx2 locus in potato. Plant J. 2000, 21, 73–81. [Google Scholar] [CrossRef]
- Tai, T.H.; Dahlbeck, D.; Clark, E.T.; Gajiwala, P.; Pasion, R.; Whalen, M.C.; Stall, R.E.; Staskawicz, B.J. Expression of the Bs2 pepper gene confers resistance to bacterial spot disease in tomato. Proc. Natl. Acad. Sci. USA 1999, 96, 14153–14158. [Google Scholar] [CrossRef]
- Yang, H.; Wang, H.; Jiang, J.; Liu, M.; Liu, Z.; Tan, Y.; Zhao, T.; Zhang, H.; Chen, X.; Li, J.; et al. The Sm gene conferring resistance to gray leaf spot disease encodes an NBS-LRR (nucleotide-binding site-leucine-rich repeat) plant resistance protein in tomato. Theor. Appl. 2022, 135, 1467–1476. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Jiang, J.; Yang, H.; Zhao, T.; Xu, X.; Li, J. Virus-induced gene silencing (VIGS) of the NBS-LRR gene SLNLC1 compromises Sm-mediated disease resistance to Stemphylium lycopersici in tomato. Biochem. Biophys. Res. 2018, 503, 1524–1529. [Google Scholar] [CrossRef]
- Ernst, K.; Kumar, A.; Kriseleit, D.; Kloos, D.U.; Phillips, M.S.; Ganal, M.W. The broad-spectrum potato cyst nematode resistance gene (Hero) from tomato is the only member of a large gene family of NBS-LRR genes with an unusual amino acid repeat in the LRR region. Plant J. 2002, 31, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liu, J.; Bao, S.; Yang, Y.; Zhuang, Y. Molecular cloning and characterization of a wild eggplant Solanum aculeatissimum NBS-LRR gene, involved in plant resistance to Meloidogyne incognita. Int. J. Mol. Sci. 2018, 19, 583. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.O.; Lin, W.; Feng, E.; Ou, X. Transcriptome and metabolome response of eggplant against Ralstonia solanacearum infection. PeerJ 2023, 11, e14658. [Google Scholar] [CrossRef]
- Li, D.; Qian, J.; Li, W.; Yu, N.; Gan, G.; Jiang, Y.; Li, W.; Liang, X.; Chen, R.; Mo, Y.; et al. A high-quality genome assembly of the eggplant provides insights into the molecular basis of disease resistance and chlorogenic acid synthesis. Mol. Ecol. Resour. 2021, 21, 1274–1286. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.D.; Coggill, P.; Eberhardt, R.Y.; Eddy, S.R.; Mistry, J.; Mitchell, A.L.; Potter, S.C.; Punta, M.; Qureshi, M.; Sangrador-Vegas, A.; et al. The Pfam protein families database: Towards a more sustainable future. Nucleic Acids Res. 2016, 44, D279–D285. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; Debarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.H.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative pcr and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Gantasala, N.P.; Papolu, P.K.; Thakur, P.K.; Kamaraju, D.; Sreevathsa, R.; Rao, U. Selection and validation of reference genes for quantitative gene expression studies by real-time PCR in eggplant (Solanum melongena L.). BMC Res. Notes 2013, 6, 312. [Google Scholar] [CrossRef] [PubMed]
- Untergasser, A.; Nijveen, H.; Rao, X.; Bisseling, T.; Geurts, R.; Leunissen, J.A. Primer3Plus, an enhanced web interface to Primer3. Nucleic Acids Res. 2007, 35, W71–W74. [Google Scholar] [CrossRef]
- Porter, B.W.; Paidi, M.; Ming, R.; Alam, M.; Nishijima, W.T.; Zhu, Y.J. Genome-wide analysis of Carica papaya reveals a small NBS resistance gene family. Mol. Genet. Genom. 2009, 281, 609–626. [Google Scholar] [CrossRef]
- Yu, X.; Zhong, S.; Yang, H.; Chen, C.; Chen, W.; Yang, H.; Guan, J.; Fu, P.; Tan, F.; Ren, T.; et al. Identification and Characterization of NBS Resistance Genes in Akebia trifoliata. Front. Plant Sci. 2021, 12, 758559. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Chen, M.; Sun, L.; Wang, Y.; Yin, J.; Liu, J.; Sun, X.Q.; Hang, Y.Y. Genome-wide identification and evolutionary analysis of NBS-LRR genes from Dioscorea rotundata. Front. Genet. 2020, 11, 484. [Google Scholar] [CrossRef]
- Lozano, R.; Hamblin, M.T.; Prochnik, S.; Jannink, J.L. Identification and distribution of the NBS-LRR gene family in the Cassava genome. BMC Genom. 2015, 16, 360. [Google Scholar] [CrossRef]
- Kohler, A.; Rinaldi, C.; Duplessis, S.; Baucher, M.; Geelen, D.; Duchaussoy, F.; Meyers, B.C.; Boerjan, W.; Martin, F. Genome-wide identification of NBS resistance genes in Populus trichocarpa. Plant Mol. Biol. 2008, 66, 619–636. [Google Scholar] [CrossRef]
- Qian, L.H.; Wang, Y.; Chen, M.; Liu, J.; Lu, R.S.; Zou, X.; Sun, X.Q.; Zhang, Y.M. Genome-wide identification and evolutionary analysis of NBS-LRR genes from Secale cereale. Front. Genet. 2021, 12, 771814. [Google Scholar] [CrossRef]
- DeYoung, B.J.; Innes, R.W. Plant NBS-LRR proteins in pathogen sensing and host defense. Nat. Immunol. 2006, 7, 1243–1249. [Google Scholar] [PubMed]
- Liang, S.; Xu, S.; Qu, D.; Yang, L.; Wang, J.; Liu, H.; Xin, W.; Zou, D.; Zheng, H. Identification and functional analysis of the caffeic acid o-methyltransferase (COMT) gene family in rice (Oryza sativa L.). Int. J. Mol. Sci. 2022, 23, 8491. [Google Scholar] [CrossRef]
- Jacob, F.; Vernaldi, S.; Maekawa, T. Evolution and conservation of plant NLR functions. Front. Immunol. 2013, 4, 297. [Google Scholar] [CrossRef]
- Yang, S.; Li, J.; Zhang, X.; Zhang, Q.; Huang, J.; Chen, J.Q.; Hartl, D.L.; Tian, D. Rapidly evolving R genes in diverse grass species confer resistance to rice blast disease. Proc. Natl. Acad. Sci. USA 2013, 110, 18572–18577. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Sun, W.; Ma, Z.; Huang, L.; Wu, Q.; Tang, Z.; Bu, T.; Li, C.; Chen, H. Genome-wide identification of the SPL gene family in Tartary Buckwheat (Fagopyrum tataricum) and expression analysis during fruit development stages. BMC Plant Biol. 2019, 19, 299. [Google Scholar]
- Zhang, Y.M.; Shao, Z.Q.; Wang, Q.; Hang, Y.Y.; Xue, J.Y.; Wang, B.; Chen, J.Q. Uncovering the dynamic evolution of nucleotide-binding site-leucine-rich repeat (NBS-LRR) genes in Brassicaceae. J. Integr. Plant Biol. 2016, 58, 165–177. [Google Scholar] [CrossRef]
- Yu, J.; Tehrim, S.; Zhang, F.; Tong, C.; Huang, J.; Cheng, X.; Dong, C.; Zhou, Y.; Qin, R.; Hua, W.; et al. Genome-wide comparative analysis of NBS-encoding genes between Brassica species and Arabidopsis thaliana. BMC Genom. 2014, 15, 3. [Google Scholar]
- Yang, X.; Wang, J. Genome-wide analysis of NBS-LRR genes in sorghum genome revealed several events contributing to NBS-LRR gene evolution in Grass species. Evol. Bioinform. Online 2016, 12, 9–21. [Google Scholar] [CrossRef]
- Traut, T.W. The functions and consensus motifs of nine types of peptide segments that form different types of nucleotide-binding sites. Eur. J. Biochem. 1994, 222, 9–19. [Google Scholar]
- Lv, D.; Wang, G.; Xiong, L.R.; Sun, J.X.; Chen, Y.; Guo, C.L.; Yu, Y.; He, H.L.; Cai, R.; Pan, J.S. Genome-wide identification and characterization of lectin receptor-like kinase gene family in cucumber and expression profiling analysis under different treatments. Genes 2020, 11, 1032. [Google Scholar] [CrossRef]


| Species | NBS Gene Subgroups | Total NBS Genes | Total Genes | Proportion of NBS Genes | Source | ||
|---|---|---|---|---|---|---|---|
| CNL | TNL | RNL | |||||
| Akebia trifoliata | 50 | 19 | 4 | 73 | 24,138 | 0.30% | [56] |
| Arabidopsis thaliana | 55 | 94 | − | 149 | 25,498 | 0.58% | [13] |
| Carica papaya | 33 | 20 | 1 | 54 | 24,746 | 0.22% | [55] |
| Dioscorea rotundata | 166 | − | 1 | 167 | 26,198 | 0.64% | [57] |
| Manihot esculenta | 181 | 146 | − | 327 | 30,666 | 1.07% | [58] |
| Oryza sativa | 467 | 63 | − | 535 | 37,544 | 1.42% | [16] |
| Populus trichocarpa | 279 | 123 | − | 402 | 45,555 | 0.79% | [59] |
| Secale cereale | 581 | − | 1 | 582 | 86,991 | 0.67% | [60] |
| Solanum melongena | 231 | 36 | 2 | 269 | 30,518 | 0.88% | In this study |
| Solanum lycopersicum | 222 | 31 | 2 | 255 | 34,074 | 0.75% | [22] |
| Triticum aestivum | 2148 | 5 | 3 | 2151 | 107,891 | 1.99% | [20] |
| Subgroup | Predicted Domains | Number (Proportion) |
|---|---|---|
| CNL subgroup | 231 (85.87%) | |
| CC-NBS (CN) | CC, NBS | 111 |
| CC-NBS-LRR (CNL) | CC, NBS, LRR | 6 |
| NBS (NCC) | NBS | 112 |
| NBS-LRR (NCCL) | NBS, LRR | 2 |
| TNL subgroup | 36 (13.38%) | |
| TIR-NBS (TN) | NBS, LRR | 24 |
| TIR-NBS-LRR (TNL) | TIR, NBS, LRR | 6 |
| NBS (NTIR) | NBS | 6 |
| RNL subgroup | 2 (0.65%) | |
| RPW8-NBS (RN) | RPW8, NBS | 2 |
| Total | 269 |
| Motif | Sequence Tag | Conserved Sequence | Number |
|---|---|---|---|
| P-loop | 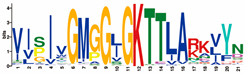 | VISIVGMGGJGKTTLARKVYN | 212 |
| GLPL | 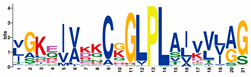 | VGKZIVKKCGGLPLAJVVLAG | 213 |
| Kinase-2 |  | LLKGKRYLIVLDDVWDTEA | 213 |
| RNBS-A | 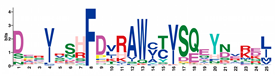 | DERVRSHFDVRAWCTVSQEYBEREJ | 173 |
| RNBS-B |  | NGSRIJLTTRNKEVA | 207 |
| RNBS-C |  | PHELRLLSEEESWELFEKKAF | 210 |
| RNBS-D | 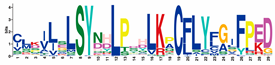 | CLKILSLSYNHLPDHLKPCFLYFGIFPED | 188 |
| MHDL | 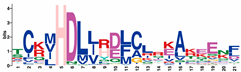 | TCKMHDLLRDLCLRKAKEENF | 181 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Li, Z.; Li, W.; Cheng, H.; Zhao, W.; Li, T.; Sun, B.; You, Q.; Zhou, D. Genome-Wide Analysis Revealed NBS-LRR Gene Candidates Associated with Bacterial Wilt Resistance in Eggplant (Solanum melongena L.). Agronomy 2023, 13, 2583. https://doi.org/10.3390/agronomy13102583
Jiang Y, Li Z, Li W, Cheng H, Zhao W, Li T, Sun B, You Q, Zhou D. Genome-Wide Analysis Revealed NBS-LRR Gene Candidates Associated with Bacterial Wilt Resistance in Eggplant (Solanum melongena L.). Agronomy. 2023; 13(10):2583. https://doi.org/10.3390/agronomy13102583
Chicago/Turabian StyleJiang, Yaolan, Zhiliang Li, Wenxiang Li, Hefen Cheng, Wei Zhao, Tao Li, Baojuan Sun, Qian You, and Dinggang Zhou. 2023. "Genome-Wide Analysis Revealed NBS-LRR Gene Candidates Associated with Bacterial Wilt Resistance in Eggplant (Solanum melongena L.)" Agronomy 13, no. 10: 2583. https://doi.org/10.3390/agronomy13102583
APA StyleJiang, Y., Li, Z., Li, W., Cheng, H., Zhao, W., Li, T., Sun, B., You, Q., & Zhou, D. (2023). Genome-Wide Analysis Revealed NBS-LRR Gene Candidates Associated with Bacterial Wilt Resistance in Eggplant (Solanum melongena L.). Agronomy, 13(10), 2583. https://doi.org/10.3390/agronomy13102583






