Differential Physiological Responses to Different Drought Durations among a Diverse Set of Sugarcane Genotypes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials, Experimental Design, and Growing Conditions
2.2. Preparation of Seedlings
2.3. Field Preparation, Transplanting, and Crop Management
2.4. Data Collection
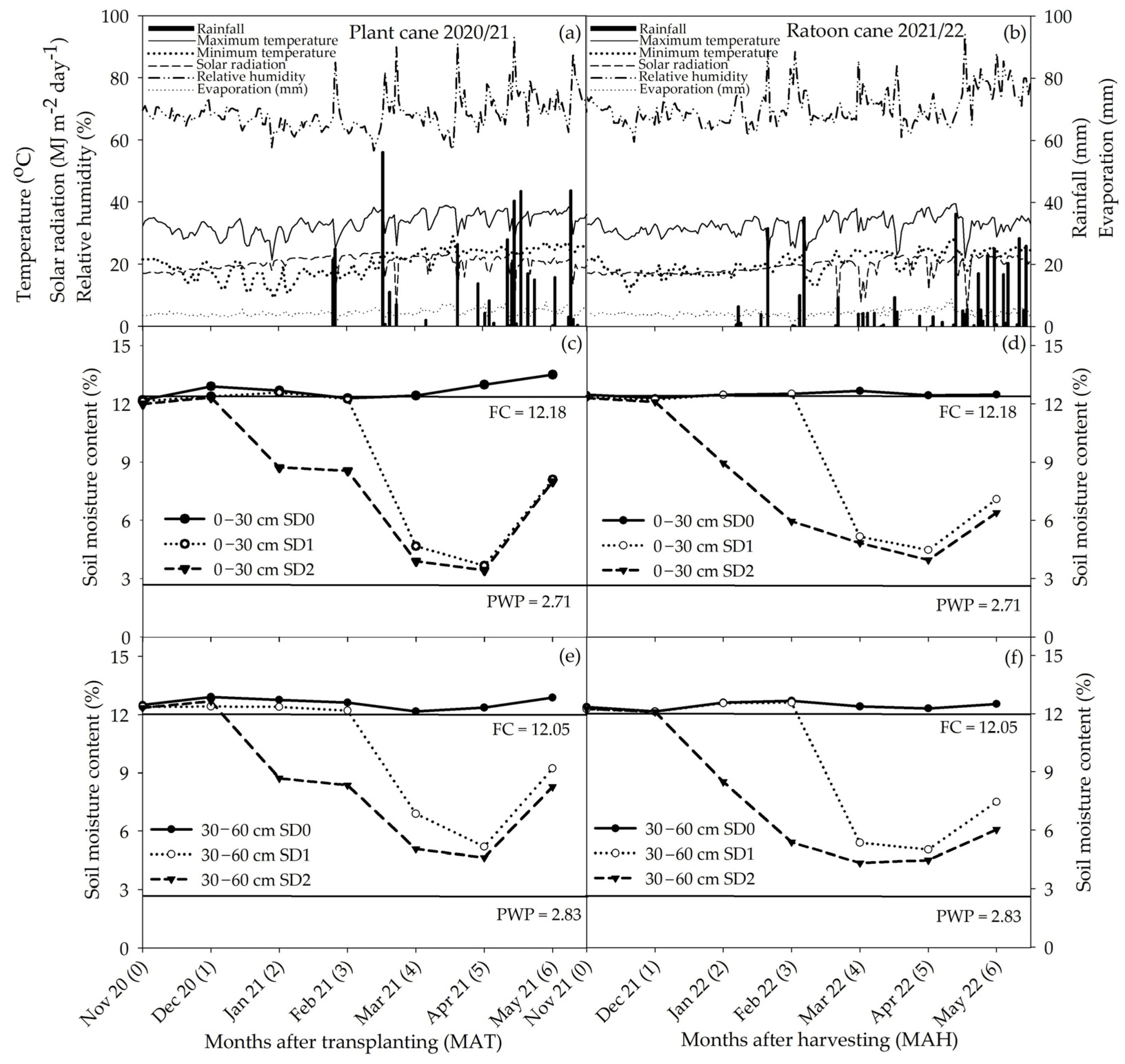
2.4.1. Growing Conditions and Soil Moisture Content
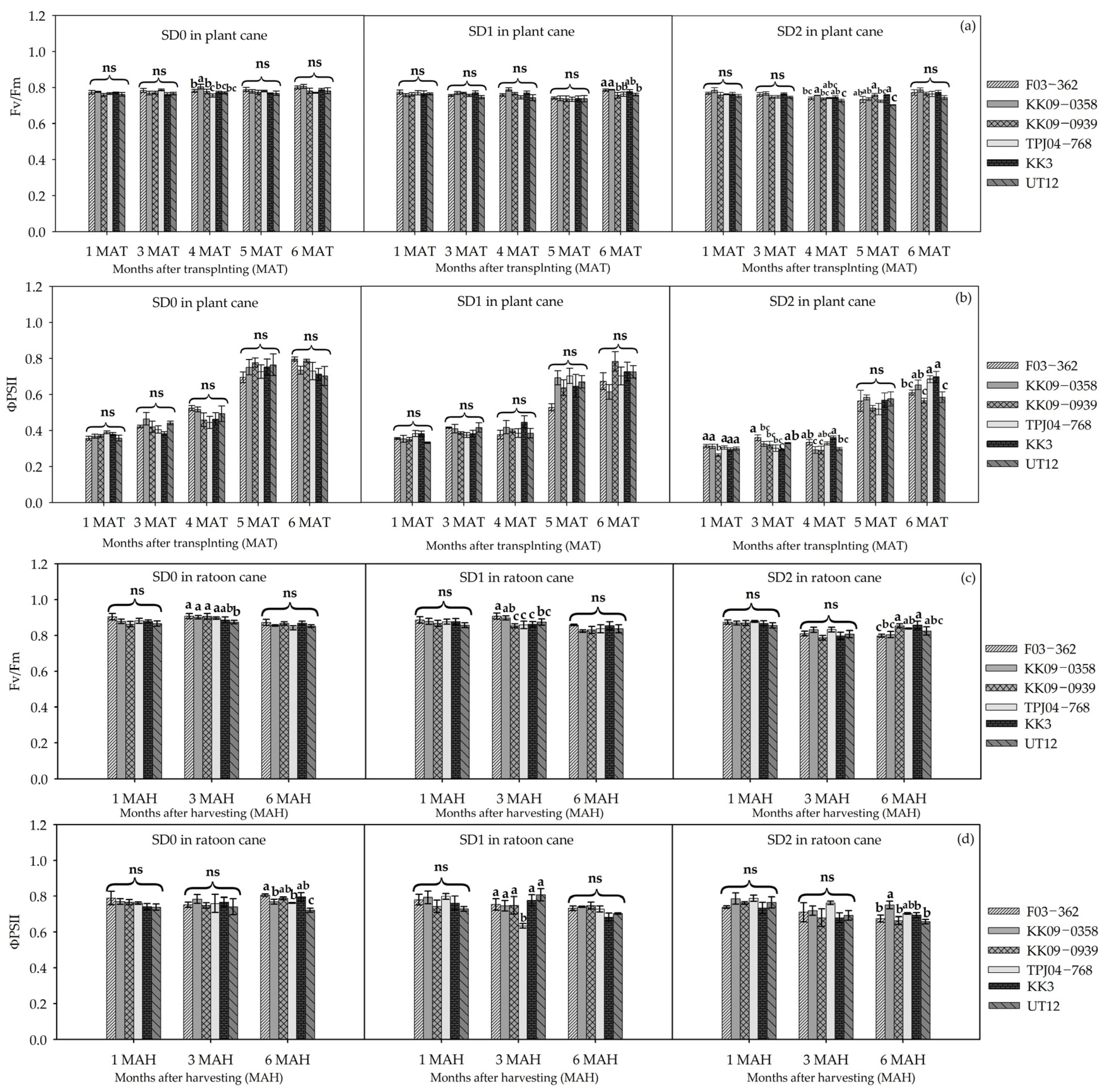
2.4.2. Leaf Gas Exchange and Chlorophyll Fluorescence
2.4.3. Leaf Chlorophyll and Relative Water Contents (RWC)
2.4.4. Biomass and Leaf Area Index (LAI)
2.4.5. Statistical Analyses
3. Results
3.1. Plant Water Status
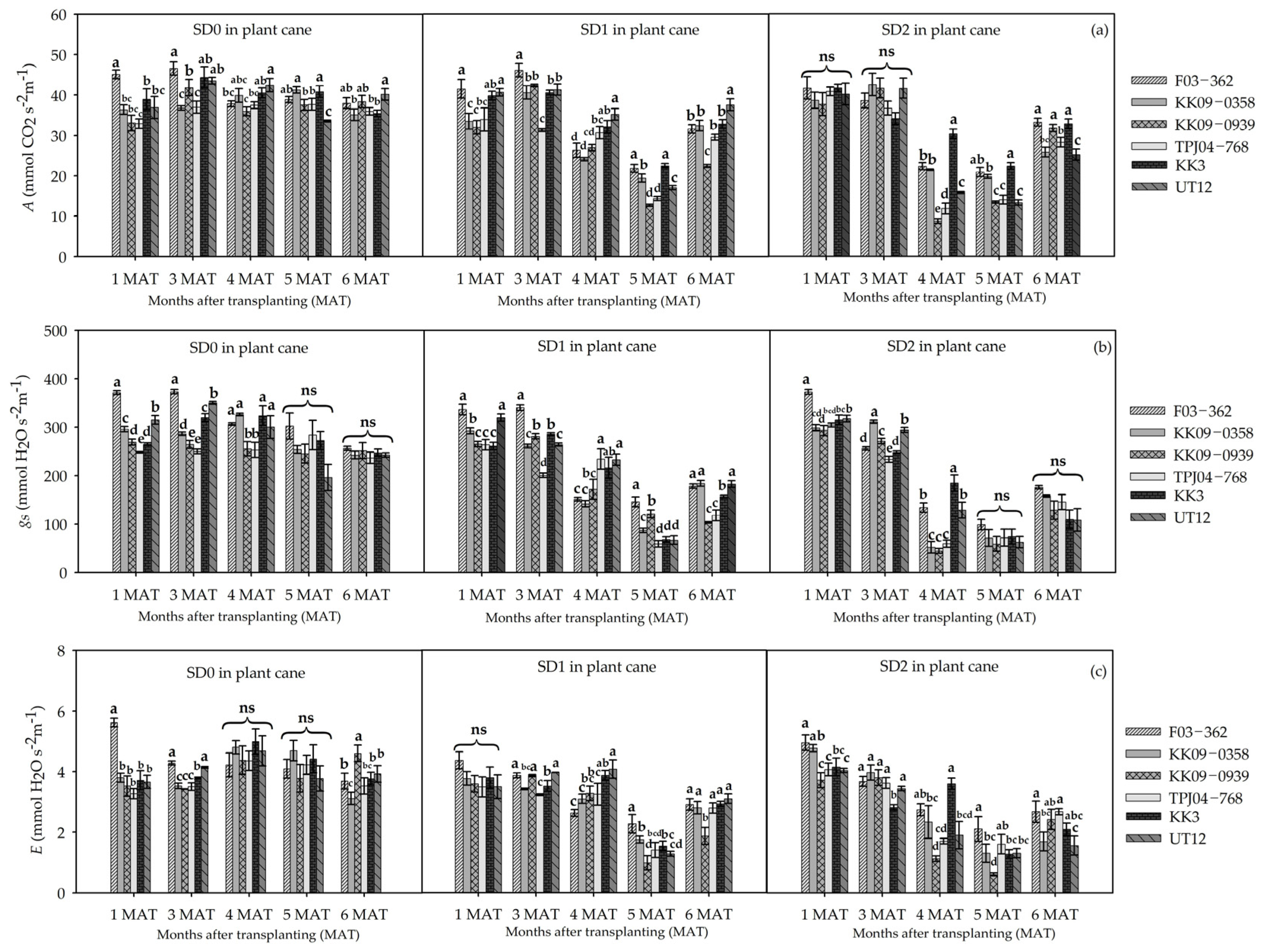
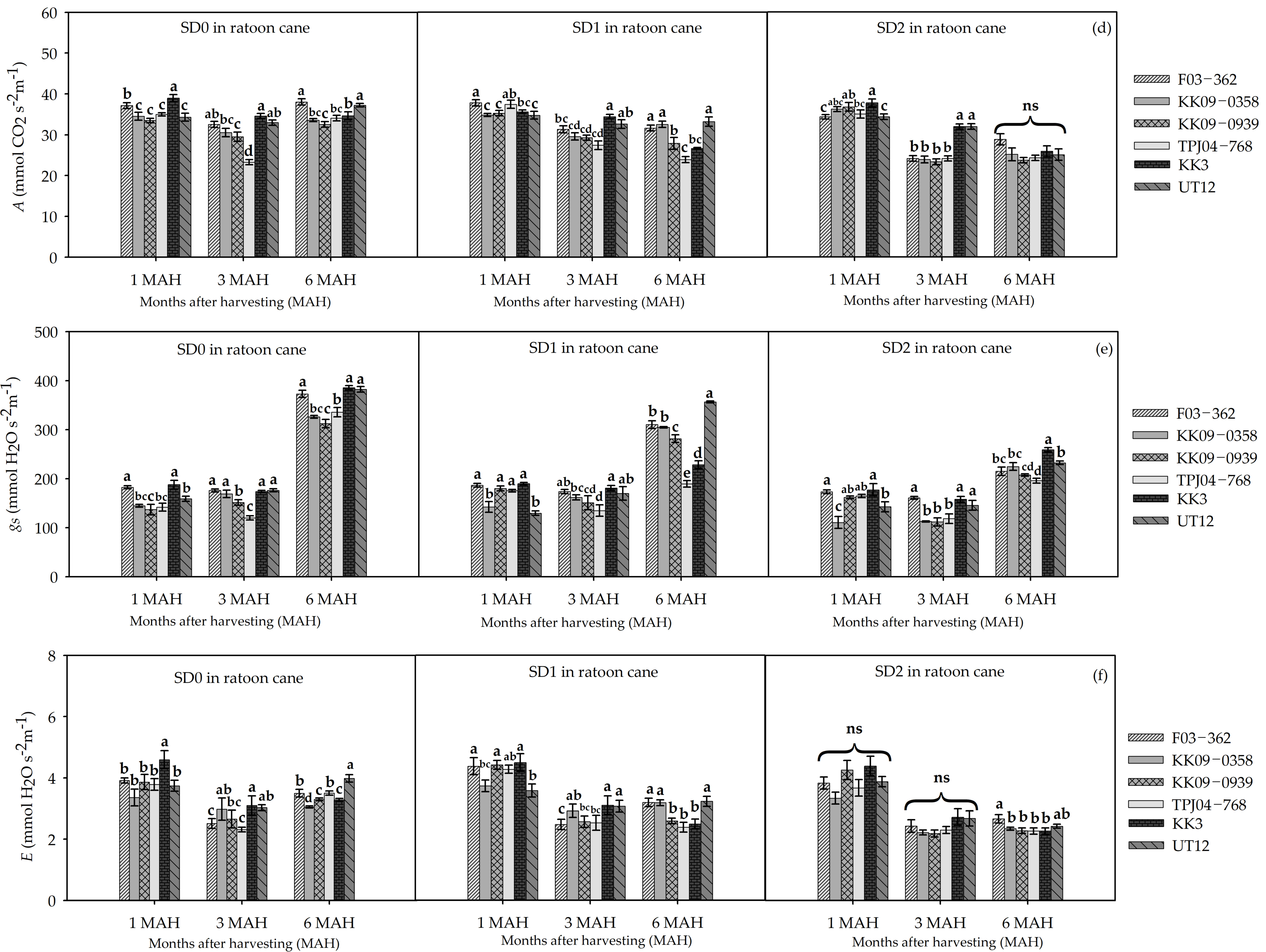
3.2. Leaf Gas Exchange and Physiological Responses to Drought Durations
3.3. Biomass
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, C.; Jackson, P.; Lu, X.; Xu, C.; Cai, Q.; Basnayake, J.; Lakshmanan, P.; Ghannoum, O.; Fan, Y. Genotypic variation in transpiration efficiency due to differences in photosynthetic capacity among sugarcane–related clones. J. Exp. Bot. 2017, 68, 2377–2385. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. FAOSTAT: Crops and Livestock Products; Food and Agriculture Organization of the United Nations: Québec City, QC, Canada, 2021; Available online: https://www.fao.org/faostat/en/#data/QCL/visualize (accessed on 10 September 2021).
- De Aquino, G.S.; de Contri Medina, C.; da Costa, D.C.; Shahab, M.; Santigo, A.D. Sugarcane straw management and its impact on production and development of ratoons. Ind. Crops Prod. 2017, 102, 58–64. [Google Scholar] [CrossRef]
- Pipitpukdee, S.; Attavanich, W.; Bejranonda, S. Climate change impacts on sugarcane production in Thailand. Atmosphere 2020, 11, 408. [Google Scholar] [CrossRef]
- Jangpromma, N.; Thammasirirak, S.; Jaisil, P.; Songsri, P. Effects of drought and recovery from drought stress on above ground and root growth, and water use efficiency in sugarcane (Saccharum officinarum L.). Aust. J. Crop Sci. 2012, 6, 1298–1304. [Google Scholar]
- Wolde, Z.; Adane, A. Determination of planting season of sugarcane at Kuraz sugar development project, Southern Ethiopia. ASRJETS 2014, 7, 44–49. [Google Scholar]
- Jaiphong, T.; Tominaga, J.; Watanabe, K.; Nakabaru, M.; Takaragawa, H.; Suwa, R.; Ueno, M.; Kawamitsu, Y. Effects of duration and combination of drought and flood conditions on leaf photosynthesis, growth and sugar content in sugarcane. Plant Prod. Sci. 2016, 19, 427–437. [Google Scholar] [CrossRef]
- Leanasawat, N.; Kosittrakun, M.; Lontom, W.; Songsri, P. Physiological and agronomic traits of certain sugarcane genotypes grown under field conditions as influenced by early drought stress. Agronomy 2021, 11, 2319. [Google Scholar] [CrossRef]
- Hemaprabha, G.; Nagarajan, R.; Alarmelu, S. Response of sugarcane genotypes to water deficit stress. Sugar Tech 2004, 6, 165–168. [Google Scholar] [CrossRef]
- Devi, K.; Gomathi, R.; Arun Kumar, R.; Manimekalai, R.; Selvi, A. Field tolerance and recovery potential of sugarcane varieties subjected to drought. Indian J. Plant Physiol. 2018, 23, 271–282. [Google Scholar] [CrossRef]
- Dinh, H.T.; Watanable, K.; Takaragawa, H.; Kawamitsu, Y. Effects of drought stress at early growth stage on response of sugarcane to different nitrogen application. Sugar Tech 2018, 20, 420–430. [Google Scholar] [CrossRef]
- Khonghintaisong, J.; Songsri, P.; Jongrungklang, N. Understanding growth rate patterns among different drought resistant sugarcane cultivars during plant and ratoon crops encountered water deficit at early growth stage under natural field conditions. Agronomy 2021, 11, 2083. [Google Scholar] [CrossRef]
- Silva, F.L.; Pedrozo, C.Â.; Barbosa, M.H.P.; Resende, M.D.V.; Peternelli, L.A.; Costa, P.M.A.; Vieira, M.S. Análise de trilha para os componentes de produção de cana-de-açúcar via blup. Rev. Ceres 2009, 56, 308–314. [Google Scholar]
- Robertson, M.J.; Inman-Bamber, N.G.; Muchow, R.C.; Wood, A.W. Physiology and productivity of sugarcane with early and mid–season water deficit. Field Crops Res. 1999, 64, 211–227. [Google Scholar] [CrossRef]
- Som–Ard, J.; Atzberger, C.; Izquierdo–Verdiguier, E.; Vuolo, F.; Immitzer, M. Remote sensing applications in sugarcane cultivation. Remote Sens. 2021, 13, 4040. [Google Scholar] [CrossRef]
- Silva, P.P.; Soares, L.; Costa, J.G.; Viana, L.S.; Andrade, J.C.F.; Goncalves, E.R.; Santos, J.M.; Barbosa, G.V.S.; Nascimento, V.X.; Todaro, A.R.; et al. Path analysis for selection of drought tolerant sugarcane genotypes through physiological components. Ind Crops Prod. 2012, 37, 11–19. [Google Scholar] [CrossRef]
- Ramesh, P.; Mahadevaswamy, M. Effect of formative phase drought on different classes of shoots, shoot mortality, cane attributes, yield and quality of four sugarcane cultivars. J. Agron. Crop Sci. 2000, 185, 249–258. [Google Scholar] [CrossRef]
- Silva, M.A.; Jifon, J.L.; Santos, C.M.; Jadoski, C.J.; Silva, J.A.G. Photosynthetic capacity and water use efficiency in sugarcane genotypes subject to water deficit during early growth phase. Braz Arch Biol. Technol. 2013, 56, 735–748. [Google Scholar] [CrossRef]
- Castro, P.R.C.; Zambon, S.; Sansigolo, M.A.; Beltrame, J.A.; Nogueira, M.C.S. Ação comparada de ethrel, fuzilade e roundup, em duas épocas de aplicação, na maturação e produtividade da cana-de-açúcar SP 70-1143. Rev. Agric. 2002, 77, 23–38. [Google Scholar] [CrossRef]
- Ferreira, T.H.S.; Tsunada, M.S.; Bassi, D.; Araujo, P.; Mattiello, L.; Guidelli, G.V.; Righetto, G.L.; Goncalves, R.V.; Lakshmanan, P.; Menossi, M. Sugarcane water stress tolerance mechanisms and its implications on developing biotechnology solutions. Front. Plant Sci. 2017, 8, 1077–1095. [Google Scholar] [CrossRef]
- Reyes, J.A.O.; Carpentero, A.S.; Santos, P.J.A.; Delfin, E.F. Effects of water regime, genotype, and formative stages on the agro–physiological response of sugarcane (Saccharum officinarum L.) to drought. Plants 2020, 9, 661. [Google Scholar] [CrossRef]
- Azevedo, R.A.; Carvalho, R.F.; Cia, M.C.; Gratão, P.L. Sugarcane under pressure: An overview of biochemical and physiological studies of abiotic stress. Trop. Plant Biol. 2011, 4, 42–51. [Google Scholar] [CrossRef]
- Luo, J.; Pan, Y.B.; Xu, L.; Zhang, Y.; Zhang, H.; Chen, R.; Que, Y. Photosynthetic and canopy characteristics of different varieties at the early elongation stage and their relationships with the cane yield in sugarcane. Sci. World J. 2014, 2014, 707095. [Google Scholar] [CrossRef]
- Basnayake, J.; Jackson, P.A.; Inman–Bamber, N.G.; Lakshmanan, P. Sugarcane for water–limited environments. Variation in stomatal conductance and its genetic correlation with crop productivity. J. Exp. Bot. 2015, 66, 3945–3958. [Google Scholar] [CrossRef]
- Rodrigues, F.A.; Laia, M.L.; Zingaretti, S.M. Analysis of gene expression profiles under water stress in tolerant and sensitive sugarcane plants. Plant Sci. 2009, 176, 286–302. [Google Scholar] [CrossRef]
- Bunphan, D.; Sinsiri, N.; Wanna, R. Application of SCMR and fluorescence for chlorophyll measurement in sugarcane. Int. J. GEOMATE 2019, 16, 33–38. [Google Scholar] [CrossRef]
- Zhao, D.; Glaz, B.; Comstock, J.C. Sugarcane response to water-deficit stress during early growth on organic and sandy soils. Am. J. Agric. Biol. Sci. 2010, 5, 403–414. [Google Scholar] [CrossRef]
- Endres, L.; Silva, J.V.; Ferreira, V.M.; Barbosa, G.V.D.S. Photosynthesis and water relations in Brazilian sugarcane. Open Agric. J. 2010, 4, 31–37. [Google Scholar] [CrossRef]
- Zhang, H.; Kaiuki, S.; Schroder, J.L.; Payton, M.E.; Focht, C. Interlaboratory validation of the Mehlich 3 method for extraction of plant–available phosphorus. J. AOAC Int. 2009, 92, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J. Sugarcane nutrition and fertilization. In Good Management Practices Manual for the Cane Sugar Industry; Meyer, J., Ed.; The International Finance Corporation (IFC): Johannesburg, South Africa, 2011; pp. 173–226. [Google Scholar]
- Santanoo, S.; Vongcharoen, K.; Banterng, P.; Vorasoot, N.; Jogloy, S.; Roytrakul, S.; Theerakulpisut, P. Seasonal variation in diurnal photosynthesis and chlorophyll fluorescence of four genotypes of cassava (Manihot esculenta Crantz) under irrigation conditions in a tropical savanna climate. Agronomy 2019, 9, 206. [Google Scholar] [CrossRef]
- Moran, R. Formulae for determination of chlorophyllous pigments extracted with N, N–dimethylformamide. Plant Physiol. 1982, 69, 1376–1381. [Google Scholar] [CrossRef]
- Statistix-Data Analysis Software for Researchers, Version 10.0; Analytical Software: Tallahassee, FL, USA, 2013. Available online: https://www.statistix.com/ (accessed on 1 September 2022).
- Gomez, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research; John Wiley and Sons: Singapore, 1984. [Google Scholar]
- Waclawovsky, A.J.; Sato, P.M.; Lembke, C.G. Sugarcane for bioenergy production: An assessment of yield and regulation of sucrose content. Plant Biotechnol. J. 2010, 8, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Meena, M.R.; Kumar, R.; Chinnaswamy, A.; Karuppaiyan, R.; Kulshreshtha, N.; Ram, B. Current breeding and genomic approaches to enhance the cane and sugar productivity under abiotic stress conditions. 3 Biotech 2020, 10, 440. [Google Scholar] [CrossRef]
- Lugojan, C.; Ciulca, S. Evaluation of relative water content in winter wheat. J. Hortic. For. Biotechnol. 2011, 15, 173–177. [Google Scholar]
- Jahan, E.; Sharwood, R.E.; Tissue, D.T. Effects of leaf age during drought and recovery on photosynthesis, mesophyll conductance and leaf anatomy in wheat leaves. Front. Plant Sci. 2023, 14, 1091418. [Google Scholar] [CrossRef] [PubMed]
- Varone, L.; Ribas–Carbo, M.; Cardona, C.; Galle, A.; Medrano, H.; Gratani, L. Stomatal and non-stomatal limitations to photosynthesis in seedlings and saplings of Mediterranean species pre-conditioned and aged in nurseries: Different response to water stress. Environ. Exp. Bot. 2012, 75, 235–247. [Google Scholar] [CrossRef]
- Endres, L.; dos Santos, C.M.; Silva, J.V.; Barbosa, G.V.D.S.; Silva, A.L.J.; Froehlich, A.; Teixeira, M.M. Inter-relationship between photosynthetic efficiency, Δ13C, antioxidant activity and sugarcane yield under drought stress in field conditions. J. Agron. Crop Sci. 2019, 205, 433–446. [Google Scholar] [CrossRef]
- Verstraeten, W.W.; Veroustraete, F.; Feyen, J. Assessment of evapotranspiration and soil moisture content across different scales of observation. Sensors 2008, 8, 70–117. [Google Scholar] [CrossRef]
- Mir, R.R.; Zaman–Allah, M.; Sreenivasulu, N.; Trethowan, R.; Varshney, R.K. Integrated genomics, physiology and breeding approaches for improving drought tolerance in crops. Theor. Appl. Genet. 2012, 125, 625–645. [Google Scholar] [CrossRef]
- Dinh, T.H.; Takaragawa, H.; Watanabe, K.; Nakabaru, M.; Kawamitsu, Y. Leaf photosynthesis response to change of soil moisture content in sugarcane. Sugar Tech 2019, 21, 949–958. [Google Scholar] [CrossRef]
- Natarajan, S.; Basnayake, J.; Lakshmanan, P.; Fukai, S. Genotypic variation in intrinsic transpiration efficiency correlates with sugarcane yield under rainfed and irrigated field conditions. Physiol. Plant 2020, 172, 976–989. [Google Scholar] [CrossRef]
- da Graca, J.P.; Rodrigues, F.A.; Farias, J.R.B.; da Oliveira, M.C.N.; Hoffmann-Campo, C.B.; Zingaretti, S.M. Physiological parameters in sugarcane cultivars submitted to water deficit. Braz. J. Plant Physiol. 2010, 22, 189–197. [Google Scholar] [CrossRef]
- Slatyer, R.O.; Bierhuizen, J.F. The influence of several transpiration suppressants on transpiration, photosynthesis, and water-use efficiency of cotton leaves. Aust. J. Biol. Sci. 1964, 17, 131–146. [Google Scholar] [CrossRef]
- Pallardy, S.G. Photosynthesis. In Physiology of Woody Plants, 3rd ed.; Pallardy, S.G., Ed.; Academic Press: San Diego, CA, USA, 2010; pp. 107–167. [Google Scholar]
- Bunce, J.A. Low humidity effects on photosynthesis in single leaves of C4 plants. Oecologia 1982, 54, 233–235. [Google Scholar] [CrossRef] [PubMed]
- Suriyan, C.U.; Chalermpol, K. Proline accumulation, photosynthetic abilities and growth characters of sugarcane (Saccharum officinarum L.) plantlets in response to iso-osmotic salt and water-deficit stress. Agric. Sci. China 2009, 8, 51–58. [Google Scholar] [CrossRef]
- Silva, A.L.C.; Costa, W.A.J.M. Varietal variation in stomatal conductance, transpiration and photosynthesis of commercial sugarcane varieties under two contrasting water regimes. Trop. Agric. Res. Ext. 2009, 12, 97–102. [Google Scholar] [CrossRef]
- Irvine, J.E. Relations of photosynthetic rate and leaf and canopy characters of sugarcane yield. Crop Sci. 1975, 15, 671–676. [Google Scholar] [CrossRef]
- Lawson, T.; Vialet–Chabrand, S. Speedy stomata, photosynthesis and plant water use efficiency. New Phytol. 2019, 221, 93–98. [Google Scholar] [CrossRef]
- Sampaio–Filho, I.J.; Jardine, K.J.; de Oliveira, R.C.A.; Gimenez, B.O.; Cobello, L.O.; Piva, L.R.O.; Candido, L.A.; Higuchi, N.; Chambers, J.Q. Below versus above ground plant sources of abscisic acid (ABA) at the heart of tropical forest response to warming. Int. J. Mol. Sci. 2018, 19, 2023. [Google Scholar] [CrossRef]
- Reynold, M.; Langridge, P. Physiological breeding. Curr. Opin. Plant Biol. 2016, 31, 162–171. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence: A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Silva, M.A.; Jifon, J.L.; Sharma, V.; da Silva, J.A.G.; Caputo, M.M.; Damaj, M.B. Use of physiological parameters in screening drought tolerance in sugarcane genotypes. Sugar Tech 2011, 13, 178–184. [Google Scholar] [CrossRef]
- Souza, A.P.; Grandis, A.; Arenque–Musa, B.C.; Buckeridge, M. Diurnal variation in gas exchange and nonstructural carbohydrates throughout sugarcane development. Funct. Plant Biol. 2018, 45, 865–876. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, R.V.; Machado, R.S.; Machado, E.C.; Machado, D.F.S.P.; Filho, R.M.; Landell, M.G.A. Revealing drought-resistance and productive patterns in sugarcane genotypes by evaluating both physiological responses and stalk yield. Exp. Agric. 2013, 49, 212–224. [Google Scholar] [CrossRef]
- Torres, N.A.; Campostrini, E.; Oliveira, J.G.; Bressan-Smith, R.E. Photosynthetic pigments, nitrogen, chlorophyll a fluorescence and SPAD–502 readings in coffee leaves. Sci. Hort. 2005, 104, 199–209. [Google Scholar] [CrossRef]
- Sugiura, D.; Watanabe, C.K.A.; Betsuyak, E.; Terashima, I. Sink–source balance and down–regulation of photosynthesis in Raphanus sativus: Effects of grafting, N and CO2. Plant Cell Physiol. 2017, 58, 2043–2056. [Google Scholar] [CrossRef] [PubMed]
- Ellis, R.D.; Lankford, B.A. The tolerance of sugarcane to water stress during its main development phases. Agric. Water Manag. 1990, 17, 117–128. [Google Scholar] [CrossRef]



| Genotypes | Chl a (μg cm−2) | Chl b (μg cm−2) | Chl total (μg cm−2) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 MAT | SD0 | SD1 | SD2 | SD0 | SD1 | SD2 | SD0 | SD1 | SD2 |
| F03-362 | 11.69Aa | 9.88 Ab | 10.32 Aab | 3.58 Aa | 2.98 Ab | 2.64 Aa | 15.28 Aa | 12.87 Ab | 12.96 Aa |
| KK09-0358 | 9.39 Aa | 10.32 Ab | 10.52 Aab | 2.75 Aa | 2.88 Ab | 3.03 Aa | 12.14 Aa | 13.20 Ab | 13.57 Aa |
| KK09-0939 | 10.77 Aa | 9.28 Ab | 8.73 Ab | 3.60 Aa | 2.84 Ab | 2.66 Aa | 14.38 Aa | 12.12 Ab | 11.39 Aa |
| TPJ04-768 | 11.48 Aa | 13.35 Aa | 11.21 Aab | 3.69 Aa | 4.14 Aa | 3.37 Aa | 15.17 Aa | 17.50 Aa | 14.59 Aa |
| KK3 | 9.43 Aa | 11.16 Ab | 10.99 Aab | 2.54 Aa | 3.60 Aab | 2.88 Aa | 11.99 Aa | 14.76 Ab | 13.87 Aa |
| UT12 | 9.92 Aa | 10.33 Ab | 10.53 Aab | 3.12 Aa | 2.86 Ab | 3.17 Aa | 13.04 Aa | 13.20 Ab | 13.71 Aa |
| Mean | 10.45 | 10.72 | 10.38 | 3.21 | 3.21 | 2.96 | 13.67 | 13.94 | 13.35 |
| F-test | ns | ** | ns | ns | ** | ns | ns | ** | ns |
| CV (%) | 17.22 | 12.47 | 15.62 | 24.52 | 16.70 | 20.70 | 17.42 | 12.75 | 16.05 |
| 3 MAT | SD0 | SD1 | SD2 | SD0 | SD1 | SD2 | SD0 | SD1 | SD2 |
| F03-362 | 8.84 Aab | 8.82 Aab | 9.27 Aab | 2.73 Aab | 2.67 Aab | 2.65 Aab | 11.58 Aab | 11.49 Aab | 11.93 Aab |
| KK09-0358 | 9.55 Aa | 9.16 Aa | 8.93 Aab | 3.06 Aa | 2.70 Aab | 3.51 Aa | 12.61 Aa | 11.86 Aab | 12.44 Aa |
| KK09-0939 | 6.92 Bc | 8.17 Aab | 7.02 Bb | 2.61 Aab | 2.53 Aab | 2.25 Ab | 9.53 Ac | 10.70 Aab | 9.27 Ab |
| TPJ04-768 | 9.03 Aab | 9.17 Aa | 9.61 Aa | 2.49 Ab | 2.79 Aa | 2.65 Aab | 11.53 Aab | 11.97 Aa | 12.27 Aab |
| KK3 | 7.80 Abc | 8.53 Aab | 7.98 Aab | 2.25 Ab | 2.33 Ab | 2.90 Aab | 10.05 Abc | 10.87 Aab | 10.89 Aab |
| UT12 | 8.73 Aab | 7.83 Ab | 8.16 Aab | 2.72 Aab | 2.44 Aab | 2.71 Aab | 11.45 Aabc | 10.27 Ab | 10.87 Aab |
| Mean | 8.48 | 8.61 | 8.50 | 2.64 | 2.58 | 2.78 | 11.12 | 11.19 | 11.28 |
| F-test | * | ns | ns | ns | ns | ns | * | ns | ns |
| CV (%) | 12.95 | 9.72 | 19.65 | 12.75 | 11.10 | 26.90 | 11.76 | 9.50 | 17.97 |
| 4 MAT | SD0 | SD1 | SD2 | SD0 | SD1 | SD2 | SD0 | SD1 | SD2 |
| F03-362 | 8.48 Aa | 7.95 Aa | 7.87 Aa | 2.64 Aa | 1.81 Aa | 2.51 Aa | 11.13 Aa | 9.76 Aab | 10.39 Aa |
| KK09-0358 | 8.43 Aa | 7.98 Aa | 7.56 Aab | 2.65 Aa | 2.06 Aa | 2.25 Aab | 11.09 Aa | 10.04 Aa | 9.82 Aab |
| KK09-0939 | 7.37 Aab | 6.53 Aab | 5.82 Ac | 2.16 Aa | 2.27 Aa | 2.01 Aab | 8.92 Aab | 8.81 Aab | 7.84 Ab |
| TPJ04-768 | 7.02 Aab | 7.17 Aab | 5.95 Ac | 2.31 Aa | 2.39 Aa | 2.04 Aab | 9.68 Aab | 9.57 Aab | 7.99 Ab |
| KK3 | 6.76 Aab | 6.41 Ab | 6.03 Abc | 2.12 Aa | 2.39 Aa | 1.82 Ab | 9.16 Aab | 8.80 Aab | 7.85 Ab |
| UT12 | 6.07 Ab | 6.11 Ab | 6.56 Aabc | 1.98 Aa | 1.98 Aa | 2.16 Aab | 8.05 Ab | 8.10 Aab | 8.73 Aab |
| Mean | 7.36 | 7.02 | 6.63 | 2.31 | 2.15 | 2.13 | 9.67 | 9.18 | 8.77 |
| F-test | ns | ns | * | ns | ns | ns | ns | ns | ns |
| CV (%) | 18.24 | 14.40 | 15.35 | 20.17 | 34.10 | 16.00 | 18.61 | 12.95 | 15.34 |
| 5 MAT | SD0 | SD1 | SD2 | SD0 | SD1 | SD2 | SD0 | SD1 | SD2 |
| F03-362 | 9.78 Aa | 8.31 Aa | 8.56 Aa | 2.33 Aab | 2.18 Aa | 2.22 Aa | 12.11 Aa | 10.49 Aa | 10.78 Aa |
| KK09-0358 | 9.60 Aab | 8.26 Aa | 8.24 Aab | 2.25 Aab | 2.08 Aab | 2.07 Aab | 11.85 Aab | 10.35 Aa | 10.31 Aab |
| KK09-0939 | 8.33 Aab | 6.67 Bb | 6.35 Bc | 2.03 Aab | 1.81 Abc | 1.79 Abc | 10.36 Aab | 8.48 ABbc | 8.14 Bcd |
| TPJ04-768 | 10.03 Aa | 7.91 Bab | 7.11 Bbc | 2.46 Aa | 2.17 ABa | 1.94 Babc | 12.49 Aa | 10.08 Bab | 9.05 Bbcd |
| KK3 | 8.55 Aab | 6.79 Ab | 7.69 Aab | 1.96 Ab | 1.71 Ac | 1.90 Abc | 10.51 Aab | 8.50 Abc | 9.59 Aabc |
| UT12 | 7.93 Ab | 6.47 Bb | 6.27 Bc | 1.92 Ab | 1.61 Ac | 1.70 Ac | 9.85 Ab | 8.08 Abc | 7.98 Bd |
| Mean | 9.03 | 7.40 | 7.37 | 2.16 | 1.93 | 1.93 | 11.20 | 9.33 | 9.31 |
| F-test | ns | ns | ** | ns | ** | * | ns | * | ** |
| CV (%) | 12.88 | 12.98 | 11.71 | 13.77 | 11.70 | 10.90 | 12.95 | 12.61 | 11.15 |
| 6 MAT | SD0 | SD1 | SD2 | SD0 | SD1 | SD2 | SD0 | SD1 | SD2 |
| F03-362 | 8.81 ABab | 8.38 Ba | 9.56 Aa | 2.72 Aab | 2.56 Aa | 3.04 Aa | 11.53 ABabc | 10.95 Ba | 12.61 Aa |
| KK09-0358 | 9.01 Aab | 8.22 Aa | 8.62 Aa | 2.92 Aa | 2.59 Aa | 3.22 Aa | 11.93 Aab | 10.81 Aa | 11.85 Aab |
| KK09-0939 | 7.54 Ab | 7.80 Aa | 7.09 Ab | 2.63 Aab | 2.91 Aa | 2.39 Aa | 10.18 Abc | 10.71 Aa | 9.49 Ac |
| TPJ04-768 | 9.67 Aa | 8.53 Aa | 8.47 Aab | 3.26 Aa | 3.33 Aa | 2.82 Aa | 12.94 Aa | 11.86 Aa | 11.28 Ab |
| KK3 | 7.19 Ab | 8.58 Aa | 8.63 Aa | 2.11 Ab | 2.72 Aa | 3.02 Aa | 9.30 Ac | 11.30 Aa | 11.65 Aab |
| UT12 | 8.37 Aab | 8.11 Aa | 8.72 Aa | 2.63 Aab | 2.65 Aa | 2.67 Aa | 11.00 Aabc | 10.77 Aa | 11.40 Aab |
| Mean | 8.43 | 8.27 | 8.51 | 2.71 | 2.79 | 2.86 | 11.15 | 11.07 | 11.38 |
| F-test | ns | ns | * | ns | ns | ns | ns | ns | ** |
| CV (%) | 15.17 | 9.52 | 10.80 | 16.84 | 14.20 | 23.37 | 15.46 | 8.88 | 7.45 |
| Genotypes | Chl a (μg cm−2) | Chl b (μg cm−2) | Chl total (μg cm−2) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 MAH | SD0 | SD1 | SD2 | SD0 | SD1 | SD2 | SD0 | SD1 | SD2 |
| F03-362 | 9.80 Aa | 9.52 Aa | 8.49 Aa | 2.99 Aa | 2.95 Aa | 3.00 Aa | 12.79 Aa | 12.47 Aa | 11.49 Aa |
| KK09-0358 | 8.24 Aa | 9.14 Aa | 9.01 Aa | 3.27 Aa | 3.11 Aa | 3.07 Aa | 11.52 Aa | 12.26 Aa | 12.08 Aa |
| KK09-0939 | 8.63 Aa | 8.86 Aa | 8.55 Aa | 3.05 Aa | 2.99 Aa | 3.16 Aa | 11.68 Aa | 11.86 Aa | 11.71 Aa |
| TPJ04-768 | 8.85 Aa | 9.48 Aa | 9.55 Aa | 2.92 Aa | 3.17 Aa | 3.36 Aa | 11.78 Aa | 12.66 Aa | 12.92 Aa |
| KK3 | 8.23 Aa | 8.04 Aa | 8.72 Aa | 3.07 Aa | 3.25 Aa | 3.40 Aa | 11.31 Aa | 11.29 Aa | 12.12 Aa |
| UT12 | 8.47 Aa | 7.87 Aa | 9.20 Aa | 2.61 Aa | 2.62 Aa | 2.60 Aa | 11.09 Aa | 10.49 Aa | 11.81 Aa |
| Mean | 8.70 | 8.82 | 8.92 | 2.98 | 3.01 | 3.10 | 11.70 | 11.84 | 12.02 |
| F-test | ns | ns | ns | ns | ns | ns | ns | ** | ns |
| CV (%) | 14.24 | 14.60 | 12.22 | 18.70 | 18.93 | 19.40 | 12.94 | 13.89 | 11.89 |
| 3 MAH | SD0 | SD1 | SD2 | SD0 | SD1 | SD2 | SD0 | SD1 | SD2 |
| F03-362 | 7.04 Aa | 6.69 Aa | 6.84 Aa | 2.85 Aab | 2.47 Aa | 2.70 Aa | 9.89 Aa | 9.16 Aa | 9.54 Aa |
| KK09-0358 | 7.39 Aa | 6.37 Aa | 6.97 Aa | 2.87 Aab | 2.68 Aa | 3.10 Aa | 10.26 Aa | 9.06 Aa | 10.08 Aa |
| KK09-0939 | 5.81 Aa | 6.39 Ba | 7.03 Aa | 2.43 Aab | 2.38 Aa | 2.74 Aa | 8.24 Ba | 8.77 ABa | 9.78 Aa |
| TPJ04-768 | 6.99 Aa | 6.66 Aa | 7.22 Aa | 2.55 Aab | 2.54 Aa | 2.44 Aa | 9.54 Aa | 9.20 Aa | 9.66 Aa |
| KK3 | 7.03 Aa | 6.94 Aa | 7.58 Aa | 1.90 Ab | 2.58 Aa | 2.75 Aa | 8.93 Aa | 9.52 Aa | 10.34 Aa |
| UT12 | 6.85 Aa | 6.68 Aa | 7.13 Aa | 3.65 Aa | 2.49 Aa | 2.68 Aa | 10.51 Aa | 9.17 Aa | 9.81 Aa |
| Mean | 6.85 | 6.62 | 7.13 | 2.71 | 2.52 | 2.73 | 9.56 | 9.14 | 9.87 |
| F-test | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| CV (%) | 24.14 | 14.41 | 16.73 | 31.65 | 20.97 | 16.30 | 21.96 | 15.45 | 14.37 |
| 6 MAH | SD0 | SD1 | SD2 | SD0 | SD1 | SD2 | SD0 | SD1 | SD2 |
| F03-362 | 7.00 Aa | 7.81 Aa | 8.26 Aa | 2.20 Aab | 2.48 Aa | 2.72 Aa | 9.21 Aa | 10.29 Aa | 10.98 Aa |
| KK09-0358 | 7.47 Aa | 7.39 Aab | 7.94 Aab | 2.15 Aab | 1.90 Ab | 2.32 Aab | 9.62 Aa | 9.30 Aab | 10.26 Aab |
| KK09-0939 | 5.64 Ab | 6.63 Abc | 6.70 Abc | 1.76 Bb | 2.08 Ab | 2.02 ABb | 7.40 Ab | 8.71 Abc | 8.72 Abc |
| TPJ04-768 | 6.34 Aab | 6.49 Abc | 6.66 Ac | 1.84 Aab | 1.92 Ab | 2.20 Aab | 8.18 Aab | 8.41 Abc | 8.87 Abc |
| KK3 | 6.75 Aab | 5.99 Ac | 5.91 Ac | 2.27 Aab | 1.94 Ab | 1.87 Ab | 9.03 Aab | 7.93 Ac | 7.79 Ac |
| UT12 | 7.27 Aa | 7.05 Aab | 6.96 Abc | 2.31 Aa | 2.14 Aab | 2.18 Aab | 9.58 Aa | 9.20 Aab | 9.15 Abc |
| Mean | 6.74 | 6.89 | 7.07 | 2.09 | 2.08 | 2.22 | 8.84 | 8.97 | 9.29 |
| F-test | ns | * | ** | ns | * | ns | ns | ** | ** |
| CV (%) | 12.88 | 10.11 | 11.80 | 16.70 | 12.21 | 17.33 | 13.21 | 9.25 | 12.27 |
| Genotypes | Biomass Yield in Plant Cane (tons ha−1) | Biomass Yield in Ratoon Cane (tons ha−1) | ||||
|---|---|---|---|---|---|---|
| SD0 | SD1 | SD2 | SD0 | SD1 | SD2 | |
| F03-362 | 31.85 a | 21.07 a | 14.64 a | 31.73 a | 24.14 a | 16.44 a |
| KK09-0358 | 28.62 b | 18.63 b | 12.08 b | 30.02 a | 17.05 b | 13.09 b |
| KK09-0939 | 27.05 b | 19.50 ab | 12.88 ab | 23.87 b | 24.35 a | 10.07 c |
| TPJ04-768 | 27.36 b | 19.74 ab | 13.85 ab | 23.92 b | 22.67 a | 10.59 c |
| KK3 | 21.97 c | 14.38 c | 7.46 c | 21.60 c | 12.67 c | 5.75 d |
| UT12 | 21.40 c | 16.25 c | 8.96 c | 22.55 bc | 14.01 c | 8.69 c |
| Mean | 26.37 | 18.26 | 11.64 | 25.61 | 19.15 | 10.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tippayawat, A.; Jogloy, S.; Vorasoot, N.; Songsri, P.; Kimbeng, C.A.; Jifon, J.L.; Janket, A.; Thangthong, N.; Jongrungklang, N. Differential Physiological Responses to Different Drought Durations among a Diverse Set of Sugarcane Genotypes. Agronomy 2023, 13, 2594. https://doi.org/10.3390/agronomy13102594
Tippayawat A, Jogloy S, Vorasoot N, Songsri P, Kimbeng CA, Jifon JL, Janket A, Thangthong N, Jongrungklang N. Differential Physiological Responses to Different Drought Durations among a Diverse Set of Sugarcane Genotypes. Agronomy. 2023; 13(10):2594. https://doi.org/10.3390/agronomy13102594
Chicago/Turabian StyleTippayawat, Amarawan, Sanun Jogloy, Nimitr Vorasoot, Patcharin Songsri, Collins A. Kimbeng, John L. Jifon, Anon Janket, Nuengsap Thangthong, and Nakorn Jongrungklang. 2023. "Differential Physiological Responses to Different Drought Durations among a Diverse Set of Sugarcane Genotypes" Agronomy 13, no. 10: 2594. https://doi.org/10.3390/agronomy13102594







