Root Causes of Flowering: Two Sides of Bolting in Sugar Beet
Abstract
:1. Introduction
2. Developmental Stages of Sugar Beet
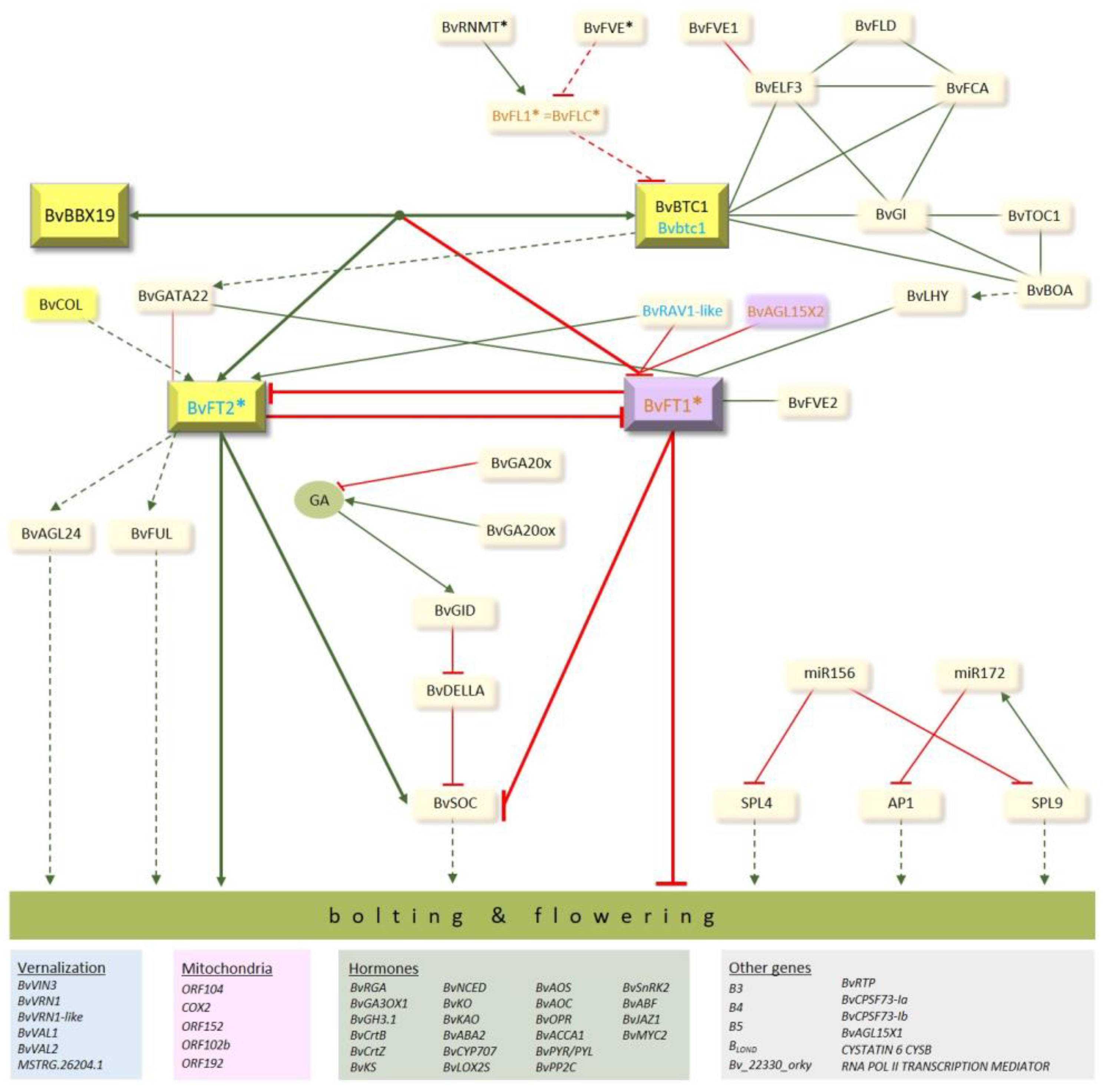
3. Genetic Mechanisms of Transition to Flowering in Sugar Beet
3.1. Genes Regulating Transition to Flowering in Sugar Beet
3.2. Hormonal Control of the Transition to Flowering in Sugar Beet
4. Bolting of Sugar Beet in the Field and Prevention Methods
4.1. Prerequisites for Sugar Beet Bolting in the Field
4.2. Preventing Bolting in Field-Grown Sugar Beet Using Agrotechnical Methods
4.3. Breeding for Bolting Resistance
4.3.1. Conventional Breeding for Bolting Resistance
4.3.2. Marker-Assisted and Genomic Selection for Bolting Resistance
4.3.3. Mutagenesis and Genetic Engineering for Bolting Resistance
5. Bolting as a Tool for Accelerating Breeding and Seed Production
6. Conclusions
- Fundamental scientific issues:
- (a)
- The development of comprehensive flowering regulatory networks for sugar beet based on the analysis of DEGs, DMRs, proteomes, and hormones in various tissues and organs. This analysis should involve comparing leaves of different ages and the apical meristem before, after, and during vernalization and/or long-day exposure in genotypes that are tolerant and sensitive to bolting.
- (b)
- The development of models for the co-expression of genes involved in cold stress (short-term chilling), vernalization (long-term chilling), and devernalization.
- (c)
- The study of recently discovered genes that regulate the floral transition using novel plant materials, including (i) lines with dysfunctional genes developed through RNA silencing or CRISPR/Cas9 knockout; (ii) RILs and NILs with different alleles/haplotypes; and (iii) diverse collections of cultivated and wild beet species.
- Practical issues:
- (a)
- The study of the allelic diversity of bolting-regulating genes in collections from various climatic zones.
- (b)
- The expansion of the allele/haplotype diversity of bolting-regulating genes can be achieved through various methods, such as genome editing, mutagenesis, and wide hybridization.
- (c)
- The improvement of the conventional, marker-assisted, and genomic selection of bolting-resistant genotypes.
- (d)
- The development of bolting-resistant cultivars and hybrids.
- (e)
- The improvement of agricultural techniques for preventing bolting.
- Speed-breeding issues:
- (a)
- The optimization of a set of conditions to accelerate the transition to the reproductive stage, namely, temperature, light intensity, duration, and spectral composition during different phases (pre-vernalization, vernalization, acclimatization, and post-vernalization).
- (b)
- The optimization of chemical treatments, such as those involving mineral nutrition, epimutagens, and hormones, to accelerate the transition to the reproductive stage.
- (c)
- The optimization of artificial conditions for the development of a sufficient amount of viable seeds.
- (d)
- The possibility of the vernalization of embryos in vitro using the embryo rescue technique.
- (e)
- The possibility of positive selection for bolting in segregating populations under speed-breeding conditions.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Niklyaev, V.S.; Kosinsky, V.S.; Tkachev, V.V.; Suchilina, A.A. Fundamentals of Agricultural Production Technology. In Agriculture and Crop Production; Niklyaev, V.S., Ed.; Bylina: Moscow, Russia, 2000. (In Russian) [Google Scholar]
- Mall, A.K.; Misra, V.; Santeshwari; Pathak, A.D.; Srivastava, S. Sugar Beet Cultivation in India: Prospects for Bio-Ethanol Production and Value-Added Co-Products. Sugar Tech 2021, 23, 1218–1234. [Google Scholar] [CrossRef]
- Vavilov, P.P.; Gritsenko, V.V.; Kuznetsov, V.S. Crop Production; Vavilov, P.P., Ed.; Kolos: Moscow, Russia, 1986. (In Russian) [Google Scholar]
- Abekova, A.M.; Yerzhebayeva, R.S.; Bastaubaeva, S.O.; Konysbekov, K.T. Molecular analysis of sugar beet samples for the presence of a resistance gene to bolting. Her. Sci. S. Seifullin Kazakh Agro Tech. Univ. 2019, 3, 92–100. [Google Scholar]
- Bulatov, R.K. History of Origin of Sugar Beet. Agric. Sci. 2016, 1, 67–69. [Google Scholar]
- Shahbandeh, M. Sugar Beet Production Worldwide from 1965 to 2021 (in Million Metric Tons). Available online: https://www.statista.com/statistics/249609/sugar-beet-production-worldwide/ (accessed on 19 October 2023).
- FAOSTAT. Food and Agriculture Organization. Available online: https://www.fao.org/faostat (accessed on 19 October 2023).
- Webster, T.M.; Grey, T.L.; Scully, B.T.; Johnson, W.C.; Davis, R.F.; Brenneman, T.B. Yield Potential of Spring-Harvested Sugar Beet (Beta vulgaris) Depends on Autumn Planting Time. Ind. Crops Prod. 2016, 83, 55–60. [Google Scholar] [CrossRef]
- Deihimfard, R.; Rahimi-Moghaddam, S.; Chenu, K. Risk Assessment of Frost Damage to Sugar Beet Simulated under Cold and Semi-Arid Environments. Int. J. Biometeorol. 2019, 63, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, J.; Fasahat, P. Autumn-Sown Sugar Beet Cultivation in Semiarid Regions. In Sugar Beet Cultivation, Management and Processing; Misra, V., Srivastava, S., MallSingapore, A.K., Eds.; Springer: Singapore, 2023; pp. 275–290. [Google Scholar]
- Reinsdorf, E.; Koch, H.-J.; Märländer, B. Phenotype Related Differences in Frost Tolerance of Winter Sugar Beet (Beta vulgaris L.). F. Crop. Res. 2013, 151, 27–34. [Google Scholar] [CrossRef]
- Ertürk, E.; Ağır, H.B. Yield and Quality Characteristics, and Profitability of Some Winter–Summer Sugar Beet Varieties in Kahramanmaraş Conditions. Sugar Tech 2022, 24, 1461–1469. [Google Scholar] [CrossRef]
- Reinsdorf, E.; Koch, H.-J. Modeling Crown Temperature of Winter Sugar Beet and Its Application in Risk Assessment for Frost Killing in Central Europe. Agric. For. Meteorol. 2013, 182–183, 21–30. [Google Scholar] [CrossRef]
- Romano, A. Seed Production and Certification in Sugar Beet. In Sugar Beet Cultivation, Management and Processing; Springer Nature Singapore: Singapore, 2022; pp. 91–120. [Google Scholar]
- Kukharev, O.N.; Starostin, I.A.; Semov, I.N. On the Issue of Technical and Technological Support for Selection and Seed Production of Sugar Beets. Bull. Kazan State Agrar. Univ. 2019, 4, 25–30. [Google Scholar]
- Logvinov, A.V.; Suslov, V.I. Characteristics of Selection Samples of Sugar Beet Based on Floweriness. In Proceedings of the Materials of the International (correspondence) Scientific and Practical Conference “Science of the 21st Century: Current Issues, Problems and Prospects”, Neftekamsk, Russia, 16 December 2021; pp. 30–39. [Google Scholar]
- Bosemark, N.O. Genetics and Breeding. In Sugar Beet; Blackwell Publishing Ltd.: Oxford, UK, 2006; pp. 50–88. [Google Scholar]
- Pylnev, V.V.; Konovalov, Y.B.; Khupatsaria, V.I. Special Breeding of Field Crops; Pylnev, V.V., Ed.; KolosS: Moscow, Russia, 2005. [Google Scholar]
- Jung, C. Genetics and Breeding of Sugar Beet. By E. Biancardi, L. G. Campbell, M. de Biaggi and G. N. Skarakis. Enfield, NH, USA: Science Publishers (2005), pp. 367, £48.40. ISBN 1-57808-366-4. Exp. Agric. 2006, 42, 254. [Google Scholar] [CrossRef]
- Longden, P.; Scott, R.K.; Tyldesley, J.B. Bolting of Sugar Beet Grown in England. Outlook Agric. 1975, 8, 188–193. [Google Scholar] [CrossRef]
- Jaggard, K.W.; Wickens, R.; Webb, D.J.; Scott, R.K. Effects of Sowing Date on Plant Establishment and Bolting and the Influence of These Factors on Yields of Sugar Beet. J. Agric. Sci. 1983, 101, 147–161. [Google Scholar] [CrossRef]
- Tayyab, M.; Wakeel, A.; Mubarak, M.U.; Artyszak, A.; Ali, S.; Hakki, E.E.; Mahmood, K.; Song, B.; Ishfaq, M. Sugar Beet Cultivation in the Tropics and Subtropics: Challenges and Opportunities. Agronomy 2023, 13, 1213. [Google Scholar] [CrossRef]
- Taleghani, D.F.; Moharamzadeh, M.; Hemayati, S.S.; Mohammadian, R.; Farahmand, R. Effect of Sowing and Harvest Time on Yield of Autumn-Sown Sugar Beet in Moghan Region in Iran. Seed Plant Prod. J. 2011, 27, 355–371. [Google Scholar] [CrossRef]
- Mulet, J.M. Shaping the Sugar Beet of Tomorrow: Current Advances in Sugar Beet Biotechnology and New Breeding Techniques. In Sugar Beet Cultivation, Management and Processing; Springer Nature Singapore: Singapore, 2022; pp. 49–74. [Google Scholar]
- Kuroda, Y.; Takahashi, H.; Okazaki, K.; Taguchi, K. Molecular Variation at BvBTC1 Is Associated with Bolting Tolerance in Japanese Sugar Beet. Euphytica 2019, 215, 43. [Google Scholar] [CrossRef]
- Stephan, H.; Böttcher, U.; Sieling, K.; Kage, H. Yield Potential of Non-Bolting Winter Sugar Beet in Germany. Eur. J. Agron. 2020, 115, 126035. [Google Scholar] [CrossRef]
- Hoffmann, C.M.; Kluge-Severin, S. Growth Analysis of Autumn and Spring Sown Sugar Beet. Eur. J. Agron. 2011, 34, 1–9. [Google Scholar] [CrossRef]
- Melzer, S.; Müller, A.E.; Jung, C. Genetics and Genomics of Flowering Time Regulation in Sugar Beet. In Genomics of Plant Genetic Resources Volume 2. Crop Productivity, Food Security and Nutritional Quality; Tuberosa, R., Graner, A., Frison, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 3–26. [Google Scholar]
- Zhuzhzhalova, T.; Znamenskaya, V.V.; Podvigina, O.A.; Yarmolyuk, G.I. Reproductive Biology of Sugar Beet; Sotrudnichestvo Ltd.: Voronezh, Russia, 2007. [Google Scholar]
- Meier, V.U.; Bachmann, L.; Buhtz, E.; Hack, H.; Klose, R.; Märländer, B.; Weber, E. Phänologische Entwicklungsstadien der Beta-Rüben (Beta vulgaris L. sspp.). Nachrichtenbl. Deut. Pflanzenschutzd 1993, 45, 37–41. [Google Scholar]
- Artschwager, E. Anatomy of the Vegetative Organs of the Sugar Beet. J. Agric. Res. 1926, 33, 143–176. [Google Scholar]
- Rodrigues, C.M.; Müdsam, C.; Keller, I.; Zierer, W.; Czarnecki, O.; Corral, J.M.; Reinhardt, F.; Nieberl, P.; Fiedler-Wiechers, K.; Sommer, F.; et al. Vernalization Alters Sink and Source Identities and Reverses Phloem Translocation from Taproots to Shoots in Sugar Beet. Plant Cell 2020, 32, 3206–3223. [Google Scholar] [CrossRef]
- Van Dijk, H.; Boudry, P. Genetic Variation for Life Histories in Beta maritima. Int. Board Plant Genet. Res. 1991, 7, 44–55. [Google Scholar]
- Dijk, H.V.; Boudry, P.; McCombre, H.; Vernet, P. Flowering Time in Wild Beet (Beta vulgaris ssp. maritima) along a Latitudinal Cline. Acta Oecologica 1997, 18, 47–60. [Google Scholar] [CrossRef]
- Misra, V.; Shrivastava, A.K. Understanding the Sugar Beet Crop and Its Physiology. In Sugar Beet Cultivation, Management and Processing; Springer Nature Singapore: Singapore, 2022; pp. 11–25. [Google Scholar]
- Hautekèete, N.; Piquot, Y.; Van Dijk, H. Life Span in Beta vulgaris ssp. maritima: The Effects of Age at First Reproduction and Disturbance. J. Ecol. 2002, 90, 508–516. [Google Scholar] [CrossRef]
- Hautekèete, N.-C.; Piquot, Y.; Van Dijk, H. Investment in Survival and Reproduction along a Semelparity-Iteroparity Gradient in the Beta Species Complex. J. Evol. Biol. 2001, 14, 795–804. [Google Scholar] [CrossRef]
- Pi, Z.; Xing, W.; Zhu, X.; Long, J.; Zou, Y.; Wu, Z. Temporal Expression Pattern of Bolting-Related Genes During Vernalization in Sugar Beet. Sugar Tech 2021, 23, 146–157. [Google Scholar] [CrossRef]
- Liang, N.; Cheng, D.; Cui, J.; Dai, C.; Luo, C.; Liu, T.; Li, J. Vernalisation Mediated LncRNA-like Gene Expression in Beta Vulgaris. Funct. Plant Biol. 2017, 44, 720. [Google Scholar] [CrossRef] [PubMed]
- Dally, N.; Xiao, K.; Holtgräwe, D.; Jung, C. The B2 Flowering Time Locus of Beet Encodes a Zinc Finger Transcription Factor. Proc. Natl. Acad. Sci. USA 2014, 111, 10365–10370. [Google Scholar] [CrossRef]
- Pin, P.A.; Zhang, W.; Vogt, S.H.; Dally, N.; Büttner, B.; Schulze-Buxloh, G.; Jelly, N.S.; Chia, T.Y.P.; Mutasa-Göttgens, E.S.; Dohm, J.C.; et al. The Role of a Pseudo-Response Regulator Gene in Life Cycle Adaptation and Domestication of Beet. Curr. Biol. 2012, 22, 1095–1101. [Google Scholar] [CrossRef]
- Hébrard, C.; Peterson, D.G.; Willems, G.; Delaunay, A.; Jesson, B.; Lefèbvre, M.; Barnes, S.; Maury, S. Epigenomics and Bolting Tolerance in Sugar Beet Genotypes. J. Exp. Bot. 2016, 67, 207–225. [Google Scholar] [CrossRef]
- Shojaei, E.; Mirzaie-Asl, A.; Mahmoudi, S.B.; Nazeri, S. Identification of Sugar Beet Flowering Genes Based on Arabidopsis Homologous Gene. J. Agr. Sci. Tech. 2018, 19, 719–729. [Google Scholar]
- Asgari, M.; Mirzaie-asl, A.; Abdollahi, M.R.; Khodaei, L. Flowering Time Regulation by the MiRNA156 in the Beet (Beta vulgaris ssp. maritima). Res. Sq. 2022, 150, 361–370. [Google Scholar] [CrossRef]
- Mutasa-Göttgens, E.S.; Joshi, A.; Holmes, H.F.; Hedden, P.; Göttgens, B. A New RNASeq-Based Reference Transcriptome for Sugar Beet and Its Application in Transcriptomescale Analysis of Vernalization and Gibberellin Responses. BMC Genom. 2012, 13, 99. [Google Scholar] [CrossRef]
- Liang, N.; Cheng, D.; Zhao, L.; Lu, H.; Xu, L.; Bi, Y. Identification of the Genes Encoding B3 Domain-Containing Proteins Related to Vernalization of Beta vulgaris. Genes 2022, 13, 2217. [Google Scholar] [CrossRef]
- Felkel, S.; Dohm, J.C.; Himmelbauer, H. Genomic Variation in the Genus Beta Based on 656 Sequenced Beet Genomes. Sci. Rep. 2023, 13, 8654. [Google Scholar] [CrossRef]
- Zhao, L.; Li, S.; Yu, Q.; Zhang, C.; Wang, L.; Jiang, Y.; Wu, Z.; Pi, Z. Vernalization Promotes GA-Mediated Bolting Initiation via the Inhibition of ABA and JA Biosynthesis. Agronomy 2023, 13, 1251. [Google Scholar] [CrossRef]
- Song, Y.H.; Estrada, D.A.; Johnson, R.S.; Kim, S.K.; Lee, S.Y.; MacCoss, M.J.; Imaizumi, T. Distinct Roles of FKF1, GIGANTEA, and ZEITLUPE Proteins in the Regulation of CONSTANS Stability in Arabidopsis Photoperiodic Flowering. Proc. Natl. Acad. Sci. USA 2014, 111, 17672–17677. [Google Scholar] [CrossRef]
- Matías-Hernández, L.; Jiang, W.; Yang, K.; Tang, K.; Brodelius, P.E.; Pelaz, S. AaMYB1 and Its Orthologue AtMYB61 Affect Terpene Metabolism and Trichome Development in Artemisia annua and Arabidopsis thaliana. Plant J. 2017, 90, 520–534. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zheng, H.; Zhang, F.; Wang, S.; Ji, X.; Xu, C.; He, Y.; Ding, Y. PRC2 Recruitment and H3K27me3 Deposition at FLC Require FCA Binding of COOLAIR. Sci. Adv. 2019, 5, eaau7246. [Google Scholar] [CrossRef]
- Qi, P.-L.; Zhou, H.-R.; Zhao, Q.-Q.; Feng, C.; Ning, Y.-Q.; Su, Y.-N.; Cai, X.-W.; Yuan, D.-Y.; Zhang, Z.-C.; Su, X.-M.; et al. Characterization of an Autonomous Pathway Complex That Promotes Flowering in Arabidopsis. Nucleic Acids Res. 2022, 50, 7380–7395. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Li, T.; Xu, P.-B.; Li, L.; Du, S.-S.; Lian, H.-L.; Yang, H.-Q. DELLA Proteins Physically Interact with CONSTANS to Regulate Flowering under Long Days in Arabidopsis. FEBS Lett. 2016, 590, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.; Hua, C.; Shen, L.; Yu, H. New Insights into Gibberellin Signaling in Regulating Flowering in Arabidopsis. J. Integr. Plant Biol. 2020, 62, 118–131. [Google Scholar] [CrossRef]
- Rieu, I.; Ruiz-Rivero, O.; Fernandez-Garcia, N.; Griffiths, J.; Powers, S.J.; Gong, F.; Linhartova, T.; Eriksson, S.; Nilsson, O.; Thomas, S.G.; et al. The Gibberellin Biosynthetic Genes AtGA20ox1 and AtGA20ox2 Act, Partially Redundantly, to Promote Growth and Development throughout the Arabidopsis Life Cycle. Plant J. 2007, 53, 488–504. [Google Scholar] [CrossRef] [PubMed]
- Osnato, M.; Matias-Hernandez, L.; Aguilar-Jaramillo, A.E.; Kater, M.M.; Pelaz, S. Genes of the RAV Family Control Heading Date and Carpel Development in Rice. Plant Physiol. 2020, 183, 1663–1680. [Google Scholar] [CrossRef]
- Wu, G.; Park, M.Y.; Conway, S.R.; Wang, J.-W.; Weigel, D.; Poethig, R.S. The Sequential Action of MiR156 and MiR172 Regulates Developmental Timing in Arabidopsis. Cell 2009, 138, 750–759. [Google Scholar] [CrossRef]
- Schepens, I.; Duek, P.; Fankhauser, C. Phytochrome-Mediated Light Signalling in Arabidopsis. Curr. Opin. Plant Biol. 2004, 7, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Hayama, R.; Sarid-Krebs, L.; Richter, R.; Fernández, V.; Jang, S.; Coupland, G. PSEUDO RESPONSE REGULATORs Stabilize CONSTANS Protein to Promote Flowering in Response to Day Length. EMBO J. 2017, 36, 904–918. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H. Current Understanding of Flowering Pathways in Plants: Focusing on the Vernalization Pathway in Arabidopsis and Several Vegetable Crop Plants. Hortic. Environ. Biotechnol. 2020, 61, 209–227. [Google Scholar] [CrossRef]
- Takagi, H.; Hempton, A.K.; Imaizumi, T. Photoperiodic Flowering in Arabidopsis: Multilayered Regulatory Mechanisms of CONSTANS and the Florigen FLOWERING LOCUS T. Plant Commun. 2023, 4, 100552. [Google Scholar] [CrossRef]
- Brambilla, V.; Fornara, F. Y Flowering? Regulation and Activity of CONSTANS and CCT-Domain Proteins in Arabidopsis and Crop Species. BBA—Gene Regul. Mech. 2016, 1860, 655–660. [Google Scholar] [CrossRef]
- Shim, J.S.; Kubota, A.; Imaizumi, T. Circadian Clock and Photoperiodic Flowering in Arabidopsis: CONSTANS Is a Hub for Signal Integration. Plant Physiol. 2017, 173, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Geuten, K.; Giri, B.S.; Varma, A. The Molecular Mechanism of Vernalization in Arabidopsis and Cereals: Role of Flowering Locus C and Its Homologs. Physiol. Plant. 2020, 170, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Munerati, O. L’eredita Della Tendenza Alla Annualita Nella Commune Barbabietola Coltivata. Ztschr Züchtung Reihe A Pflanzenzüchtung 1931, 17, 84–89. [Google Scholar]
- Mutasa-Göttgens, E.S.; Qi, A.; Zhang, W.; Schulze-Buxloh, G.; Jennings, A.; Hohmann, U.; Müller, A.E.; Hedden, P. Bolting and Flowering Control in Sugar Beet: Relationships and Effects of Gibberellin, the Bolting Gene B and Vernalization. AoB Plants 2010, 2010, plq012. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Bouillet, S.; Stock, A.M. Structural Basis of Response Regulator Function. Annu. Rev. Microbiol. 2019, 73, 175–197. [Google Scholar] [CrossRef]
- Omolade, O.; Müller, A.E.; Jung, C.; Melzer, S. BvPRR7 Is a Cold Responsive Gene with a Clock Function in Beet. Biol. Plant. 2016, 60, 95–104. [Google Scholar] [CrossRef]
- Höft, N.; Dally, N.; Hasler, M.; Jung, C. Haplotype Variation of Flowering Time Genes of Sugar Beet and Its Wild Relatives and the Impact on Life Cycle Regimes. Front. Plant Sci. 2018, 8, 2211. [Google Scholar] [CrossRef] [PubMed]
- Höft, N.; Dally, N.; Jung, C. Sequence Variation in the Bolting Time Regulator BTC 1 Changes the Life Cycle Regime in Sugar Beet. Plant Breed. 2018, 137, 412–422. [Google Scholar] [CrossRef]
- Dally, N.; Eckel, M.; Batschauer, A.; Höft, N.; Jung, C. Two CONSTANS-LIKE Genes Jointly Control Flowering Time in Beet. Sci. Rep. 2018, 8, 16120. [Google Scholar] [CrossRef]
- Pin, P.A.; Benlloch, R.; Bonnet, D.; Wremerth-Weich, E.; Kraft, T.; Gielen, J.J.L.; Nilsson, O. An Antagonistic Pair of FT Homologs Mediates the Control of Flowering Time in Sugar Beet. Science 2010, 330, 1397–1400. [Google Scholar] [CrossRef]
- Reeves, P.A.; He, Y.; Schmitz, R.J.; Amasino, R.M.; Panella, L.W.; Richards, C.M. Evolutionary Conservation of the FLOWERING LOCUS C -Mediated Vernalization Response: Evidence from the Sugar Beet (Beta vulgaris). Genetics 2007, 176, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Trap-Gentil, M.-V.; Hébrard, C.; Lafon-Placette, C.; Delaunay, A.; Hagège, D.; Joseph, C.; Brignolas, F.; Lefebvre, M.; Barnes, S.; Maury, S. Time Course and Amplitude of DNA Methylation in the Shoot Apical Meristem Are Critical Points for Bolting Induction in Sugar Beet and Bolting Tolerance between Genotypes. J. Exp. Bot. 2011, 62, 2585–2597. [Google Scholar] [CrossRef]
- Frerichmann, S.L.; Kirchhoff, M.; Müller, A.E.; Scheidig, A.J.; Jung, C.; Kopisch-Obuch, F.J. EcoTILLING in Beta vulgaris Reveals Polymorphisms in the FLC-like Gene BvFL1that Are Associated with Annuality and Winter Hardiness. BMC Plant Biol. 2013, 13, 52. [Google Scholar] [CrossRef] [PubMed]
- Vogt, S.H.; Weyens, G.; Lefèbvre, M.; Bork, B.; Schechert, A.; Müller, A.E. The FLC-like Gene BvFL1 Is Not a Major Regulator of Vernalization Response in Biennial Beets. Front. Plant Sci. 2014, 5, 146. [Google Scholar] [CrossRef] [PubMed]
- Mateos, J.L.; Madrigal, P.; Tsuda, K.; Rawat, V.; Richter, R.; Romera-Branchat, M.; Fornara, F.; Schneeberger, K.; Krajewski, P.; Coupland, G. Combinatorial Activities of SHORT VEGETATIVE PHASE and FLOWERING LOCUS C Define Distinct Modes of Flowering Regulation in Arabidopsis. Genome Biol. 2015, 16, 31. [Google Scholar] [CrossRef] [PubMed]
- Abou-Elwafa, S.F.; Büttner, B.; Chia, T.; Schulze-Buxloh, G.; Hohmann, U.; Mutasa-Göttgens, E.; Jung, C.; Müller, A.E. Conservation and Divergence of Autonomous Pathway Genes in the Flowering Regulatory Network of Beta vulgaris. J. Exp. Bot. 2011, 62, 3359–3374. [Google Scholar] [CrossRef] [PubMed]
- Chia, T.Y.P.; Müller, A.; Jung, C.; Mutasa-Göttgens, E.S. Sugar Beet Contains a Large CONSTANS-LIKE Gene Family Including a CO Homologue That Is Independent of the Early-Bolting (B) Gene Locus. J. Exp. Bot. 2008, 59, 2735–2748. [Google Scholar] [CrossRef]
- Xie, Y.; Zhou, Q.; Zhao, Y.; Li, Q.; Liu, Y.; Ma, M.; Wang, B.; Shen, R.; Zheng, Z.; Wang, H. FHY3 and FAR1 Integrate Light Signals with the MiR156-SPL Module-Mediated Aging Pathway to Regulate Arabidopsis Flowering. Mol. Plant 2020, 13, 483–498. [Google Scholar] [CrossRef]
- Hébrard, C.; Trap-Gentil, M.-V.; Lafon-Placette, C.; Delaunay, A.; Joseph, C.; Lefèbvre, M.; Barnes, S.; Maury, S. Identification of Differentially Methylated Regions during Vernalization Revealed a Role for RNA Methyltransferases in Bolting. J. Exp. Bot. 2013, 64, 651–663. [Google Scholar] [CrossRef]
- Gutschker, S.; Corral, J.M.; Schmiedl, A.; Ludewig, F.; Koch, W.; Fiedler-Wiechers, K.; Czarnecki, O.; Harms, K.; Keller, I.; Martins Rodrigues, C.; et al. Multi-Omics Data Integration Reveals Link between Epigenetic Modifications and Gene Expression in Sugar Beet (Beta vulgaris subsp. vulgaris) in Response to Cold. BMC Genom. 2022, 23, 144. [Google Scholar] [CrossRef]
- Hohmann, U.; Jacobs, G.; Jung, C. An EMS Mutagenesis Protocol for Sugar Beet and Isolation of Non-Bolting Mutants. Plant Breed. 2005, 124, 317–321. [Google Scholar] [CrossRef]
- Büttner, B.; Abou-Elwafa, S.F.; Zhang, W.; Jung, C.; Müller, A.E. A Survey of EMS-Induced Biennial Beta Vulgaris Mutants Reveals a Novel Bolting Locus Which Is Unlinked to the Bolting Gene B. Theor. Appl. Genet. 2010, 121, 1117–1131. [Google Scholar] [CrossRef]
- Abou-Elwafa, S.F.; Büttner, B.; Kopisch-Obuch, F.J.; Jung, C.; Müller, A.E. Genetic Identification of a Novel Bolting Locus in Beta vulgaris Which Promotes Annuality Independently of the Bolting Gene B. Mol. Breed. 2012, 29, 989–998. [Google Scholar] [CrossRef]
- Abou-Elwafa, S.F. A New Locus Suppresses Bolting under Shortening Daylength in Sugar Beet. World J. Agric. Res. 2015, 3, 179–184. [Google Scholar]
- Shavrukov, Y.N. Localization of New Monogerm and Late-Bolting Genes in Sugarbeet Using RFLP Markers. J. Sugar Beet Res. 2000, 37, 107–115. [Google Scholar] [CrossRef]
- Abou-Elwafa, S.F.; Hamada, A.; Mehareb, E.M. Genetic Identification of a Novel Locus (LB2) Regulates Bolting Time in Beta Vulgaris. Int. J. Agric. Sci. Technol. 2014, 2, 48. [Google Scholar] [CrossRef]
- Kuroda, Y.; Kuranouchi, T.; Okazaki, K.; Takahashi, H.; Taguchi, K. Biennial Sugar Beets Capable of Flowering Without Vernalization Treatment. Genetic Resources and Crop Evolution. 2023, 1–12. [Google Scholar] [CrossRef]
- Pfeiffer, N.; Tränkner, C.; Lemnian, I.; Grosse, I.; Müller, A.E.; Jung, C.; Kopisch-Obuch, F.J. Genetic Analysis of Bolting after Winter in Sugar Beet (Beta vulgaris L.). Theor. Appl. Genet. 2014, 127, 2479–2489. [Google Scholar] [CrossRef] [PubMed]
- Tränkner, C.; Lemnian, I.M.; Emrani, N.; Pfeiffer, N.; Tiwari, S.P.; Kopisch-Obuch, F.J.; Vogt, S.H.; Müller, A.E.; Schilhabel, M.; Jung, C.; et al. A Detailed Analysis of the BR1 Locus Suggests a New Mechanism for Bolting after Winter in Sugar Beet (Beta vulgaris L.). Front. Plant Sci. 2016, 7, 1662. [Google Scholar] [CrossRef]
- Pfeiffer, N.; Müller, A.E.; Jung, C.; Kopisch-Obuch, F.J. QTL for Delayed Bolting after Winter Detected in Leaf Beet (Beta vulgaris L.). Plant Breed. 2017, 136, 237–244. [Google Scholar] [CrossRef]
- Broccanello, C.; Stevanato, P.; Biscarini, F.; Cantu, D.; Saccomani, M. A New Polymorphism on Chromosome 6 Associated with Bolting Tendency in Sugar Beet. BMC Genet. 2015, 16, 142. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, Y. Key Quantitative Trait Loci Controlling Bolting Tolerance in Sugar Beet. Res. Sqare 2023. [Google Scholar] [CrossRef]
- Ravi, S.; Campagna, G.; Della Lucia, M.C.; Broccanello, C.; Bertoldo, G.; Chiodi, C.; Maretto, L.; Moro, M.; Eslami, A.S.; Srinivasan, S.; et al. SNP Alleles Associated With Low Bolting Tendency in Sugar Beet. Front. Plant Sci. 2021, 12, 693285. [Google Scholar] [CrossRef]
- Liang, N.; Cheng, D.; Liu, Q.; Cui, J.; Luo, C. Difference of Proteomics Vernalization-Induced in Bolting and Flowering Transitions of Beta vulgaris. Plant Physiol. Biochem. 2018, 123, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Chiurugwi, T.; Holmes, H.F.; Qi, A.; Chia, T.Y.P.; Hedden, P.; Mutasa-Göttgens, E.S. Development of New Quantitative Physiological and Molecular Breeding Parameters Based on the Sugar-Beet Vernalization Intensity Model. J. Agric. Sci. 2013, 151, 492–505. [Google Scholar] [CrossRef]
- Mutasa-Gottgens, E.; Qi, A.; Mathews, A.; Thomas, S.; Phillips, A.; Hedden, P. Modification of Gibberellin Signalling (Metabolism & Signal Transduction) in Sugar Beet: Analysis of Potential Targets for Crop Improvement. Transgenic Res. 2009, 18, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Kiba, T.; Naitou, T.; Koizumi, N.; Yamashino, T.; Sakakibara, H.; Mizuno, T. Combinatorial Microarray Analysis Revealing Arabidopsis Genes Implicated in Cytokinin Responses through the His→Asp Phosphorelay Circuitry. Plant Cell Physiol. 2005, 46, 339–355. [Google Scholar] [CrossRef]
- Köllmer, I.; Werner, T.; Schmülling, T. Ectopic Expression of Different Cytokinin-Regulated Transcription Factor Genes of Arabidopsis thaliana Alters Plant Growth and Development. J. Plant Physiol. 2011, 168, 1320–1327. [Google Scholar] [CrossRef] [PubMed]
- Smit, A.L. Influence of External Factors on Growth and Development of Sugar-Beet (Beta vulgaris L.); Wageningen University and Research: Wageningen, The Netherlands, 1983. [Google Scholar]
- Koda, Y.; Ohkawa-Takahashi, K.; Kikuta, Y. Stimulation of Root Thickening and Inhibition of Bolting by Jasmonic Acid in Beet Plants. Plant Prod. Sci. 2001, 4, 131–135. [Google Scholar] [CrossRef]
- Moliterni, V.M.C.; Paris, R.; Onofri, C.; Orrù, L.; Cattivelli, L.; Pacifico, D.; Avanzato, C.; Ferrarini, A.; Delledonne, M.; Mandolino, G. Early Transcriptional Changes in Beta vulgaris in Response to Low Temperature. Planta 2015, 242, 187–201. [Google Scholar] [CrossRef]
- Crosthwaite, S.K.; Jenkins, G.I. The Role of Leaves in the Perception of Vernalizing Temperatures in Sugar Beet. J. Exp. Bot. 1993, 44, 801–806. [Google Scholar] [CrossRef]
- Xu, S.; Chong, K. Remembering Winter through Vernalisation. Nat. Plants 2018, 4, 997–1009. [Google Scholar] [CrossRef] [PubMed]
- Stout, M. Relation of Temperature to Reproduction in Sugar Beets. J. Agric. Res. 1946, 72, 40–68. [Google Scholar]
- Chechetkina, I.; Gulyaka, M. Recommendations for Sowing Sugar Beets in 2021 Year. Our Agric. Agron. 2021, 17, 42–44. [Google Scholar]
- Milford, G.F.J.; Jarvis, P.J.; Walters, C. A Vernalization-Intensity Model to Predict Bolting in Sugar Beet. J. Agric. Sci. 2010, 148, 127–137. [Google Scholar] [CrossRef]
- Milford, G.F.J. Plant Structure and Crop Physiology. In Sugar Beet; Springer Nature Singapore: Singapore, 2006; pp. 30–49. [Google Scholar]
- Bernier, G.; Havelange, A.; Houssa, C.; Petitjean, A.; Lejeune, P. Physiological Signals That Induce Flowering. Plant Cell 1993, 5, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Wood, D.W.; Scott, R.K.; Longden, P.C. The Effects of Mother Plant Temperature on Seed Quality in Beta vulgaris L.; Hebblethwaite, P.D., Ed.; Seed Production: London, Butterworth, 1980; pp. 257–270. [Google Scholar]
- Sapronov, A.R. Sugar Production Technology; Kolos: Moscow, Russia, 1999. [Google Scholar]
- Abu-Ellail, F.F.B.; Salem, K.F.M.; Saleh, M.M.; Alnaddaf, L.M.; Al-Khayri, J.M. Molecular Breeding Strategies of Beetroot (Beta vulgaris Ssp. vulgaris var. conditiva Alefeld). In Advances in Plant Breeding Strategies: Vegetable Crops; Al-Khayri, J.M., Jain, S.M., Johnson, D.V., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 157–212. [Google Scholar]
- Chroboczek, E. A Study of Some Ecological Factors Influencing Seed-Stalk Development in Beets (Beta vulgaris L.). Cornell Univ. Agric. Exp. Stn. Mem. 1934, 154, 84. [Google Scholar]
- Wood, D.W.; Scott, R.K. Sowing Sugar Beet in Autumn in England. J. Agric. Sci. 1975, 84, 97–108. [Google Scholar] [CrossRef]
- Logvinov, V.A.; Moiseev, V.V.; Mishchenko, V.N.; Logvinov, A.V.; Moiseev, A.V. Seed Production of Sugar Beet in Connection with New Directions of Breeding Work. Proc. Kuban State Agrar. Univ. 2018, 71, 45–52. [Google Scholar] [CrossRef]
- Devlikamov, K.S.; Devlikamov, D.K. Flowering of Sugar Beets: Causes and Methods of Control. Our Agric. Agron. 2016, 9, 10–14. [Google Scholar]
- Oksenenko, I.A.; Shuklina, I.A.; Grekov, V.E. A Method for Combating Flowering of Beet Plants. USSR Patent 646483, 30 September 1986. [Google Scholar]
- Sadeghi-Shoae, M.; Habibi, D.; Taleghani, D.F.; Paknejad, F.; Kashani, A. Evaluation the Effect of Paclobutrazol on Bolting, Qualitative and Quantitative Performance in Autumn Sown-Sugar Beet Genotypes in Moghan Region. Int. J. Biosci. 2014, 5, 346–354. [Google Scholar] [CrossRef]
- Sadeghi-Shoae, M.; Fatholah Taleghani, D.; Habibi, D. Some Reactions of Physiological and Morphological Characteristics to Foliar Application of Paclobutrazol in Autumn Sugar Beet (Beta vulgaris). Biosci. Biotechnol. Res. Asia 2017, 14, 225–231. [Google Scholar] [CrossRef]
- Bell, G.D.H.; Bauer, A.B. Experiments on Growing Sugar Beet under Continuous Illumination. J. Agric. Sci. 1942, 32, 112–141. [Google Scholar] [CrossRef]
- Bell, G.D.H. Induced Bolting and Anthesis in Sugar Beet and the Effect of Selection of Physiological Types. J. Agric. Sci. 1946, 36, 167–183. [Google Scholar] [CrossRef]
- Gaskill, J.O. Induction of Reproductive Development in Sugar Beets by Photothermal Treatment of Young Seedlings. Proc. Am. Soc. Sugar Beet Technol. 1952, 7, 112–120. [Google Scholar] [CrossRef]
- Curth, P. Der Übergang in Die Reproduktive Phase Bei Der Zuckerrübe in Abhängigkeit von Verschiedenen Umweltfaktoren. Wiss. Abh. Dt. Akad. Landwirtsch.-Wiss. Berlin 1960, 46, 7–80. [Google Scholar]
- Margara, J. Recherches Sur Le Déterminisme de l’élongation et de La Floraison Dans Le Genre Beta. Ann. Amélioration des Plantes 1960, 10, 362–471. [Google Scholar]
- Logvinov, A.V.; Tsatsenko, L.V.; Mishchenko, V.N.; Zhabatinskaya, Y.V. Test Results of Sugar Beet Breeding Material for Resistance to Flowering. Proc. Kazan State Agrar. Univ. 2022, 101, 168–174. [Google Scholar] [CrossRef]
- Jonsson, B.O. Development of a Winter Beet Alternative for South Europe. In Proceedings of the IIRB, Sevilla Spain, June 1999; pp. 69–76. [Google Scholar]
- Suslov, V.I.; Logvinov, V.A.; Shevchenko, A.G.; Mishchenko, V.N.; Suslov, A.V.; Logvinov, A.V.; Titarenko, A.I.; Kolganov, V.V. Evaluation of Sugar Beet Breeding Materials Based on Flowering Characteristics. Sugar Beet 2012, 6, 12–15. [Google Scholar]
- Kornienko, A.V.; Osadchiy, A.S.; Makogon, A.M. Method for Selecting Sugar Beet Plants for Resistance to Bolting. USSR Patent 993886, 7 February 1983. [Google Scholar]
- Kornienko, A.V.; Osadchiy, A.S.; Makogon, A.M.; Lyushnyak, V.P.; Osadchiy, A.S.; Makogon, A.M. Method for Selecting Sugar Beet Plants for Resistance to Bolting. USSR Patent 1237126, 15 June 1986. [Google Scholar]
- Shchepetnev, P.E.; Shchepetneva, A.S. A Method for Breeding Forms of Sugar Beet with Increased Sugar Content and Resistant to Bolting. USSR Patent 383435, 23 May 1973. [Google Scholar]
- Kutnyakhova, E.S.; Tsykalov, A.N. Disease Resistance and Yield Structure of Sugar Beet Hybrids Provided by Shchelkovo Agrokhim. In Proceedings of the Materials of the International Scientific and Practical Conference of Young Scientists and Specialists “Innovative Technologies and Technical Means for the Agro-Industrial Complex, Voronezh, Russia, 15–17 November 2016; pp. 51–54. [Google Scholar]
- Burenin, V.I.; Piskunova, T.M. Gene Pool for Sugar Beet Breeding. In Proceedings of the Scientific Support of the Beet Industry. Materials of the International Scientific and Practical Conference Dedicated to the 90th Anniversary of the Experimental Scientific Station on Sugar Beet, Nesvizh, Belarus, 5-6 September 2018; pp. 26–32. [Google Scholar]
- Hussein, A.S.; Nalbandyan, A.A.; Fedulova, T.P.; Cherepukhina, I.V.; Kryukova, T.I.; Mikheeva, N.R.; Rudenko, T.S. New Nucleotide Polymorphisms in the BTC1 Gene of Sugar Beet. Biotekhnologia 2020, 36, 49–54. [Google Scholar]
- Tränkner, C.; Pfeiffer, N.; Kirchhoff, M.; Kopisch-Obuch, F.J.; van Dijk, H.; Schilhabel, M.; Hasler, M.; Emrani, N. Deciphering the Complex Nature of Bolting Time Regulation in Beta vulgaris. Theor. Appl. Genet. 2017, 130, 1649–1667. [Google Scholar] [CrossRef]
- A Comprehensive Website for Beta Vulgaris Genome Sequence and Annotations. Available online: http://sugarbeets.msu.edu (accessed on 19 October 2023).
- McGrath, J.M.; Funk, A.; Galewski, P.; Ou, S.; Townsend, B.; Davenport, K.; Daligault, H.; Johnson, S.; Lee, J.; Hastie, A.; et al. A Contiguous de Novo Genome Assembly of Sugar Beet EL10 (Beta vulgaris L.). DNA Res. 2023, 30, dsac033. [Google Scholar] [CrossRef]
- Beta vulgaris Resource. Available online: https://bvseq.boku.ac.at/ (accessed on 19 October 2023).
- Dohm, J.C.; Minoche, A.E.; Holtgräwe, D.; Capella-Gutiérrez, S.; Zakrzewski, F.; Tafer, H.; Rupp, O.; Sörensen, T.R.; Stracke, R.; Reinhardt, R.; et al. The Genome of the Recently Domesticated Crop Plant Sugar Beet (Beta vulgaris). Nature 2014, 505, 546–549. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez del Río, Á.; Minoche, A.E.; Zwickl, N.F.; Friedrich, A.; Liedtke, S.; Schmidt, T.; Himmelbauer, H.; Dohm, J.C. Genomes of the Wild Beets Beta patula and Beta vulgaris ssp. maritima. Plant J. 2019, 99, 1242–1253. [Google Scholar] [CrossRef]
- Lehner, R.; Blazek, L.; Minoche, A.E.; Dohm, J.C.; Himmelbauer, H. Assembly and Characterization of the Genome of Chard (Beta vulgaris ssp. vulgaris var. cicla). J. Biotechnol. 2021, 333, 67–76. [Google Scholar] [CrossRef]
- Sugar Beet Microsatellite Database. Available online: http://webapp.cabgrid.res.in/sbmdb/ (accessed on 23 October 2023).
- Iquebal, M.A.; Jaiswal, S.; Angadi, U.B.; Sablok, G.; Arora, V.; Kumar, S.; Rai, A.; Kumar, D. SBMDb: First Whole Genome Putative Microsatellite DNA Marker Database of Sugarbeet for Bioenergy and Industrial Applications. Database 2015, 2015, bav111. [Google Scholar] [CrossRef]
- van Roggen, P.M.; Debenham, B.; Hedden, P.; Phillips, A.L.; Thomas, S.G. A Model for Control of Bolting and Flowering in Sugar Beet and the Involvement of Gibberellins. Flower. Newsl. 1998, 25, 45–49. [Google Scholar]
- Yıldırım, K.; Kavas, M.; Küçük, İ.S.; Seçgin, Z.; Saraç, Ç.G. Development of Highly Efficient Resistance to Beet Curly Top Iran Virus (Becurtovirus) in Sugar Beet (B. Vulgaris) via CRISPR/Cas9 System. Int. J. Mol. Sci. 2023, 24, 6515. [Google Scholar] [CrossRef]
- Simpson, G.G.; Dean, C. Arabidopsis, the Rosetta Stone of Flowering Time? Science 2002, 296, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Maysenya, S.V.; Melentyeva, S.A. Methodological Recommendations for Growing Plants of Sugar Beet in Breeding-Greenhouse Complexes to Fasten Breeding Process; Sugar Beet Experimental Research Station: Nesvizh, Belarus, 2018. [Google Scholar]
- Tyldesley, J.B. Vernalisation of Sugar Beet Seed on the Mother Plant in Western European and Mediterranean Climates. Int. J. Biometeorol. 1980, 24, 203–209. [Google Scholar] [CrossRef]
- Wellensiek, S.J.; Verkerk, K. Annual Seed Growing of Beets. Neth. J. Agric. Sci. 1954, 2, 98–104. [Google Scholar] [CrossRef]
- Mah, J.J.; Llewellyn, D.; Zheng, Y. Morphology and Flowering Responses of Four Bedding Plant Species to a Range of Red to Far Red Ratios. HortScience 2018, 53, 472–478. [Google Scholar] [CrossRef]
- Ilias, I.F.; Rajapakse, N. The Effects of End-of-the-Day Red and Far-Red Light on Growth and Flowering of Petunia × hybrida `Countdown Burgundy’ Grown under Photoselective Films. HortScience 2005, 40, 131–133. [Google Scholar] [CrossRef]
- Cerny, T.A.; Faust, J.E.; Layne, D.R.; Rajapakse, N.C. Influence of Photoselective Films and Growing Season on Stem Growth and Flowering of Six Plant Species. J. Am. Soc. Hortic. Sci. 2003, 128, 486–491. [Google Scholar] [CrossRef]
- Runkle, E.S.; Heins, R.D. Specific Functions of Red, Far Red, and Blue Light in Flowering and Stem Extension of Long-Day Plants. J. Am. Soc. Hortic. Sci. 2001, 126, 275–282. [Google Scholar] [CrossRef]
- Lane, H.C.; Cathey, H.M.; Evans, L.T. The dependence of flowering in several long-day plants on the spectral composition of light extending the photoperiod. Am. J. Bot. 1965, 52, 1006–1014. [Google Scholar] [CrossRef]
- Li, J.; Li, G.; Wang, H.; Wang Deng, X. Phytochrome Signaling Mechanisms. Arab. B. 2011, 9, e0148. [Google Scholar] [CrossRef]
- Watson, A.; Ghosh, S.; Williams, M.J.; Cuddy, W.S.; Simmonds, J.; Rey, M.-D.; Asyraf Md Hatta, M.; Hinchliffe, A.; Steed, A.; Reynolds, D.; et al. Speed Breeding Is a Powerful Tool to Accelerate Crop Research and Breeding. Nat. Plants 2018, 4, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Divashuk, M.G.; Blinkov, A.O.; Kocheshkova, A.A.; Kroupin, P.Y.; Svistunova, N.Y.; Radzeniece, S.B. Practical Experience in Acceleration of Plant Development under Controlled Growing Conditions. In Proceedings of the Plant Genetics, Genomics, Bioinformatics and Biotechnology (PlantGen 2023), Kazan, Russia, 11–15 July 2023. [Google Scholar]
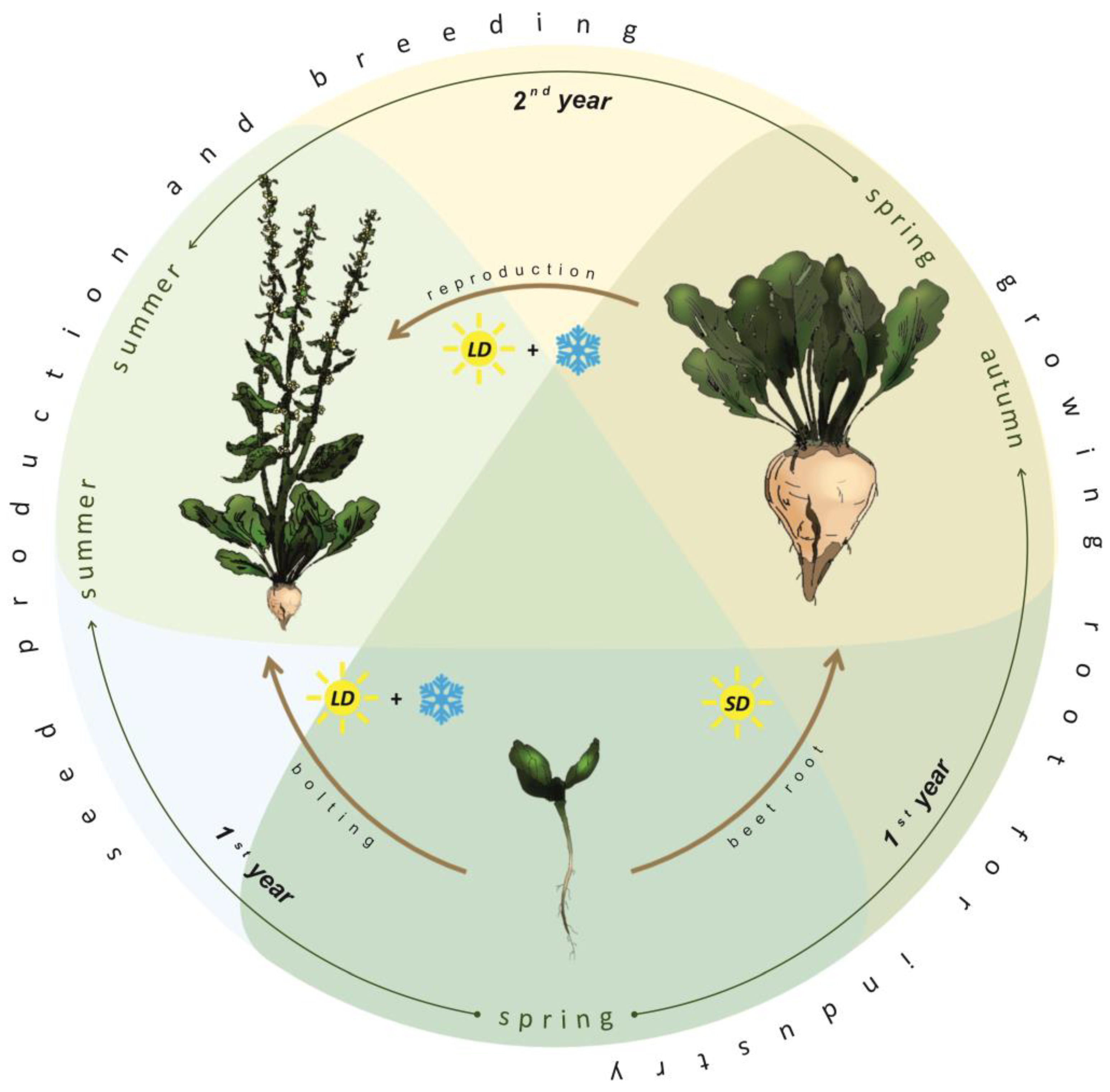
| Classification Developed by Zhuzhzhalova et al. (2007) [29] | Classification Developed Meier et al. (1993) [30] | ||||
|---|---|---|---|---|---|
| Age Period | Age State | Plant Development Phase | Stage of Organogenesis | Macrostage of Growth | |
| Phase | Duration, Days | ||||
| latent | dormant seeds | From 14 days to 7 years or more | - | ||
| pre-reproductive (vegetative) | Sprout | shoots “fork” | 6–10 | I | Macrostage 0 Germination/Seedling development |
| Macrostage 1 Leaf development (youth stage) | |||||
| 1–2 pairs of leaves | 12–14 | II | |||
| Juvenile | 3–5 pairs of leaves | 7–9 | |||
| immature | 11–30 leaves | 20–22 | |||
| Virginal | rosette of leaves | 90–100 | Macrostage 3 Rosette growth (crop cover) | ||
| Macrostage 4 Development of harvestable vegetative plant parts Beet root | |||||
| dormant root vegetables during storage | 95–200 | III | |||
| reproductive | Early | 8–17 | IV–V | ||
| regrowth of rosette leaves | 20–26 | VI | |||
| Bolting | 18–20 | Macrostage 5 Development of inflorescence/flower buds (2nd year of growth) | |||
| budding | 19–20 | VII, VIII | |||
| Middle | flowering | 25–27 | IX, X | Macrostage 6 Flowering | |
| Late | seed ripening and full maturity | 30–35 | XI, XII | Macrostage 7 Fruit development | |
| Macrostage 8 Seed ripening | |||||
| post-generative | Senile | plant senescence and dying | 30 | - | Macrostage 9 Dying-off |
| Temperature, °C | Number of Plants, % of Total Number | ||||
|---|---|---|---|---|---|
| Formed a Vegetative Rosette | Entered the Reproductive Stage | Bolted | Flowered | Formed Seeds | |
| 20–23 | 100 | 0 | 0 | 0 | 0 |
| 15–18 | 90 | 10 | 10 | 0 | 0 |
| 8–12 | 0 | 100 | 100 | 75 | 25 |
| Pre-Vernalization | Vernalization | Acclimatization | Post-Vernalization | Bolting Phenotyping | Reference |
|---|---|---|---|---|---|
| 20 °C 119 days 22 h of light 315 μmol m−2 s−1 | 4 °C 90 days 22 h of light 315 μmol m−2 s−1 | – | 20 °C 22 h of light 315 μmol m−2 s−1 | Plants that did not bolt 16 weeks after vernalization were classified as “never bolting” | [69] |
| 32 days | 38 days 5 °C 16 h of light fluorescent illumination | 5 days outdoors in the shade (25–30 April 2014) | Plants were transplanted into a field (Memuro, Hokkaido) and grown from 30 April to 29 July 2014. Average temperature: May—15C June—20C July—23C Day length increases from 13 h 50 m to 15 h 01 m on 16 July and decreased to 14 h 37 m on 29 July | Most bolted plants had already appeared by 29 July | [25] |
| 20 °C 135 days | 4 °C 12 weeks | 12 °C 3 days | 20 °C 102 days | Every second day, the onset of bolting was recorded (BBCH scale code: 51) according to the method reported by Meier et al.: (1) Annual plants that bolted within 135 days; (2) Biennial plants that only bolted after cold treatment; (3) Plants that did not bolt until the end of the experiment after 325 days | [71] |
| 18 °C 2 weeks 12 h of light 200 µmol m−2 s−1 | 6 °C 15 weeks 12 h of light 200 µmol m−2 s−1 | stepwise temperature increases from 6 °C to 18 °C 2 weeks 12 h of light 200 µmol m−2 s−1 | 18 °C 18 h of light 200 µmol m−2 s−1 | – | [72] |
| 20 °C 16 h of light | 4 °C 3 months 16 h of light | 6 weeks temperature was increased from 4 to 25 °C during the light cycle and from 4 to 15 °C during the dark cycle 16 h of light | – | Plants were phenotyped for the occurrence and time of bolting three times per week until 6 months after vernalization | [41] |
| 22 °C 16 h of light 220 µmol m−2 s−1 for 15 h + tungsten light for 1 h (far-red) | 6 °C 8 weeks in annuals 18 weeks in biennials 10 µmol m−2 s−1 | 15 °C 1 week | 22 °C 16 h of light 220 µmol m−2 s−1 for 15 h + tungsten light for 1 h (far-red) | Plants initiated bolting within 2–3 weeks and flowered within 6 weeks of vernalization | [79] |
| 24 °C 30 days 24 h of light high-pressure sodium and metal halide lamps | 5 °C 90 days 24 h of light high-pressure sodium and metal halide lamps | no gradual temperature transition | 24 °C 24 h of light high-pressure sodium and metal halide lamps | – | [73] |
| 24 °C 24 days 16 h of light 200 µmol m−2 s−1 | 4 °C 16 weeks 16 h of light 200 µmol m−2 s−1 | – | 24 °C 16 h of light 200 µmol m−2 s−1 | After transferring vernalized seedlings to room temperature, the elongation of the stems was observed daily | [38] |
| 16 h 6 weeks 300 µmol m−2 s−1 | 5 °C 3 months 8 h of light 300 µmol m−2 s−1 | – | 16 h 6 weeks 300 µmol m−2 s−1 | – | [78] |
| 22 °C 8 weeks 16 h of light 700 μmol m−2 s−1 | 4 °C 18 weeks 16 h of light | – | 22 °C 6 weeks 16 h of light 1000 μmol m−2 s−1 | After 9 weeks at 4 °C BI = 100% and average BD < 25 d considered as sensitive to bolting BI = 60% and average BD > 30 d considered as resistant to bolting | [74,81] |
| 20 °C 16 h of light 900 μmol m−2 s−1 400–700 nm 32 days if bolted 94 days if bolting did not occur; then, additional 22 days 22 h 1200 μmol m−2 s−1 | 5 °C 16 weeks 22 h of light 200 μmol m−2 s−1 | 8 °C 1 week 22 h of light 200 μmol m−2 s−1 | 20 °C 22 h of light 1200 μmol m−2 s−1 | Additional vernalization was applied for 26 weeks for those plants that did nor bolt after the first round of vernalization and were then kept under post-vernalization conditions | [92] |
| 15 °C 14 h of light 14–15 days (two true leaves) | 3/7/11/15 °C 0/14/28/42/49 days 14 h of light | 10 °C 1 week 14 h of light | 10 °C/15 °C/25 °C 90 days 14/18/24 h of light | Proportion bolting was counted for 100–160 days after chilling Temperature and duration of chilling as well as photophase and temperature of postvernalization were the factors of different experiments | [101] |
| 20 °C 16 h of light 4/7 weeks | 5 °C 16 weeks 22 h of light | 8 °C 1 week 22 h of light | Planted in field nurseries | Bolting was scored when stem elongation was visible. | [91] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kroupin, P.Y.; Kroupina, A.Y.; Karlov, G.I.; Divashuk, M.G. Root Causes of Flowering: Two Sides of Bolting in Sugar Beet. Agronomy 2023, 13, 2671. https://doi.org/10.3390/agronomy13112671
Kroupin PY, Kroupina AY, Karlov GI, Divashuk MG. Root Causes of Flowering: Two Sides of Bolting in Sugar Beet. Agronomy. 2023; 13(11):2671. https://doi.org/10.3390/agronomy13112671
Chicago/Turabian StyleKroupin, Pavel Yu., Aleksandra Yu. Kroupina, Gennady I. Karlov, and Mikhail G. Divashuk. 2023. "Root Causes of Flowering: Two Sides of Bolting in Sugar Beet" Agronomy 13, no. 11: 2671. https://doi.org/10.3390/agronomy13112671





