Role of Temporal Zn Fertilization along with Zn Solubilizing Bacteria in Enhancing Zinc Content, Uptake, and Zinc Use Efficiency in Wheat Genotypes and Its Implications for Agronomic Biofortification
Abstract
:1. Introduction
2. Material and Methods
2.1. Location and Soil
2.2. Experimental Details
2.3. Crop Husbandry
2.4. Observations and Measurements
- GYZn: represent the grain yield with applied Zn (kg ha−1).
- GY0: represent the grain yield (kg ha−1) in a control treatment where no Zn was applied.
- FZn: represent the amount of Zn fertilizer applied (kg ha−1).
- GUZn: represent the Zn uptake in the grain (kg ha−1), in a plot that received Zn.
- GU0: represent the Zn uptake in the grain (kg ha−1), in a plot that did not receive Zn.
2.5. Statistical Analysis
3. Results
3.1. Grain and Straw Zn Content µg g−1
3.2. Grain, Straw and Total Zn Uptake g ha−1
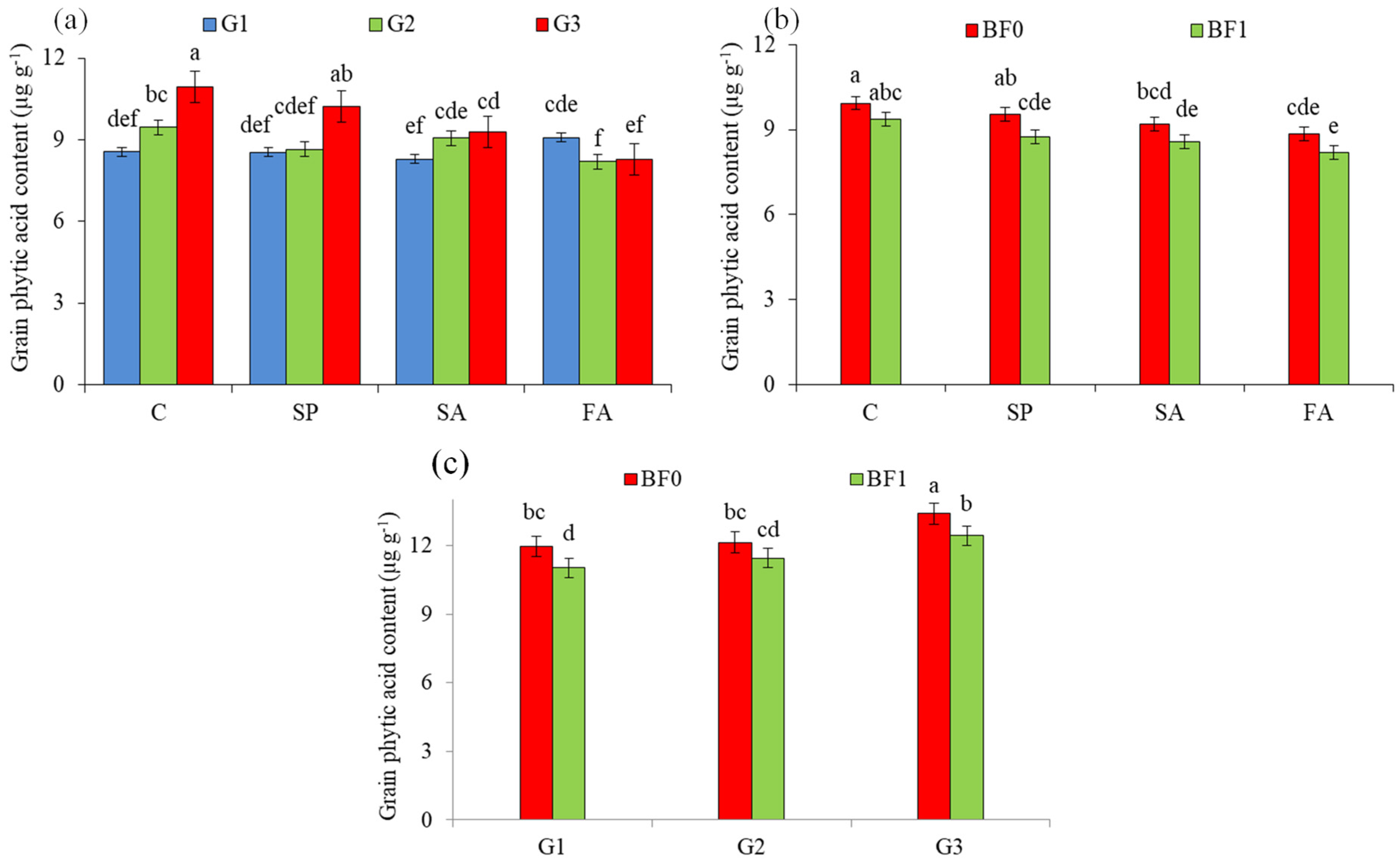
3.3. Grain Phytic Acid µg g−1
3.4. Grains Yield t ha−1
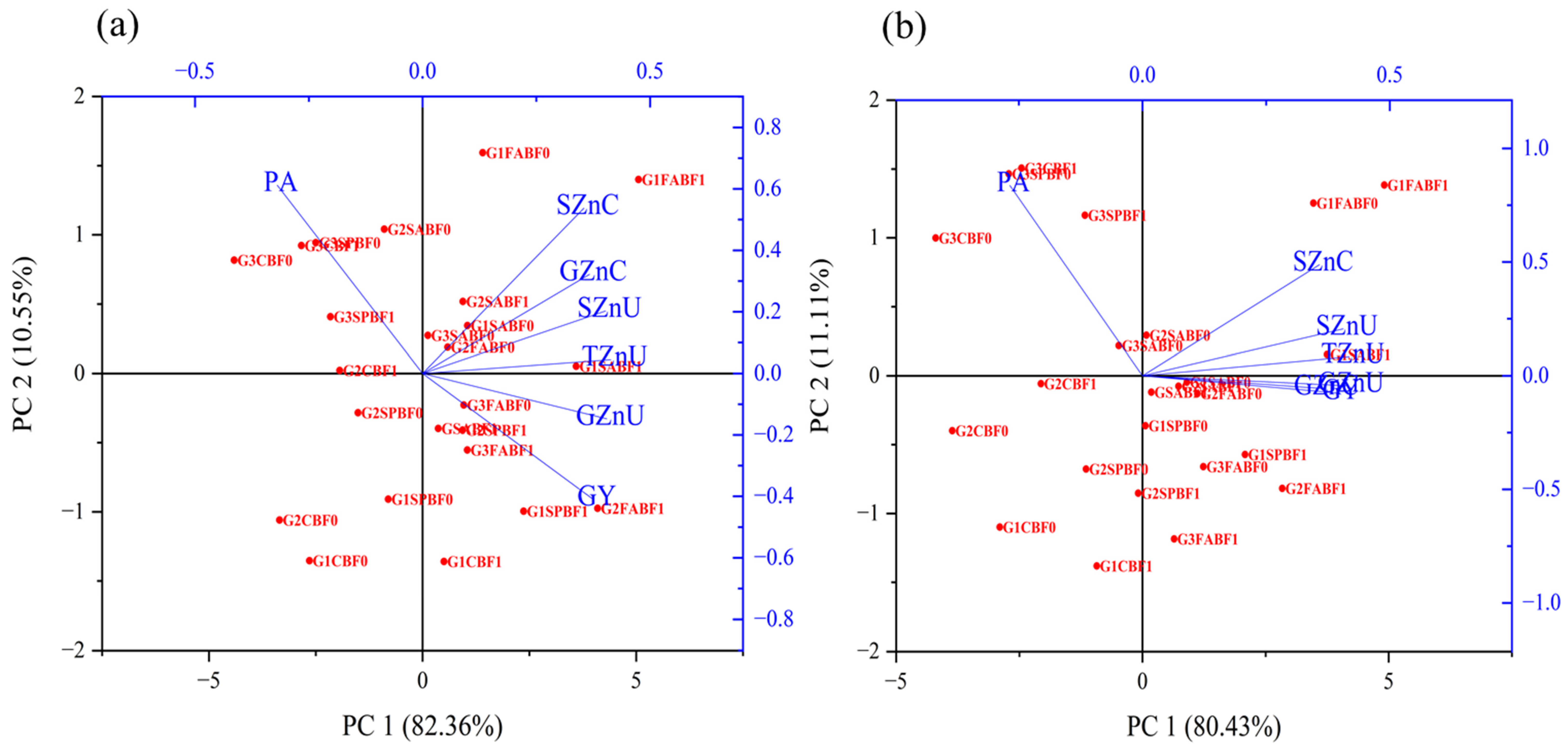
3.5. Correlations and PCA
3.6. Zn Use Efficiency
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fanzo, J. Healthy and sustainable diets and food systems: The key to achieving Sustainable Development Goal 2? Food Ethics 2019, 4, 159–174. [Google Scholar] [CrossRef]
- Biesalski, H.K. Obesity, vitamin D deficiency and old age a serious combination with respect to coronavirus disease-2019 severity and outcome. Curr. Opin. Clin. Nutr. Metab. Care 2021, 24, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on iron and its importance for human health. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2014, 19, 164. [Google Scholar]
- Krężel, A.; Maret, W. The biological inorganic chemistry of zinc ions. Arch. Biochem. Biophys. 2016, 611, 3–19. [Google Scholar] [CrossRef]
- Zulfiqar, U.; Hussain, S.; Ishfaq, M.; Matloob, A.; Ali, N.; Ahmad, M.; Alyemeni, M.N.; Ahmad, P. Zinc-induced effects on productivity, zinc use efficiency, and grain biofortification of bread wheat under different tillage permutations. Agronomy 2020, 10, 1566. [Google Scholar] [CrossRef]
- Manzeke, M.G.; Mtambanengwe, F.; Watts, M.J.; Hamilton, E.M.; Lark, R.M.; Broadley, M.R.; Mapfumo, P. Fertilizer management and soil type influence grain zinc and iron concentration under contrasting smallholder cropping systems in Zimbabwe. Sci. Rep. 2019, 9, 6445. [Google Scholar] [CrossRef]
- Prasad, A.S. Impact of the discovery of human zinc deficiency on health. J. Trace Elem. Med. Biol. 2014, 28, 357–363. [Google Scholar] [CrossRef]
- Alloway, B.J. Zn in Soil and Crop Nutrition, 2nd ed.; International Zinc Association (IZA) and International Fertilizer Association (IFA): Brussels, Belgium; Paris, France, 2008; p. 33. [Google Scholar]
- Bhatt, R.; Hossain, A.; Sharma, P. Zinc biofortification as an innovative technology to alleviate the zinc deficiency in human health: A review. Open Agric. 2020, 5, 176–187. [Google Scholar] [CrossRef]
- Singh, B.; Dheeravathu, S.N.; Usha, K. Micronutrient deficiency: A global challenge and physiological approach to improve grain productivity under low zinc availability. Plant Stress 2010, 4, 76–93. [Google Scholar]
- FAOSTAT. Food and Agriculture Organization of the United Nations FAOSTAT Database; FAOSTAT: Rome, Italy, 2021. [Google Scholar]
- Li, P.; Ma, B.; Palta, J.A.; Ding, T.; Cheng, Z.; Lv, G.; Xiong, Y. Wheat breeding highlights drought tolerance while ignores the advantages of drought avoidance: A meta-analysis. Eur. J. Agron. 2021, 122, 126196. [Google Scholar] [CrossRef]
- Rabieyan, E.; Alipour, H. NGS-based multiplex assay of trait-linked molecular markers revealed the genetic diversity of Iranian bread wheat landraces and cultivars. Crop Pasture Sci. 2021, 72, 173–182. [Google Scholar] [CrossRef]
- Rabieyan, E.; Bihamta, M.R.; Moghaddam, M.E.; Mohammadi, V.; Alipour, H. Genome-wide association mapping and genomic prediction of agronomical traits and breeding values in Iranian wheat under rain-fed and well-watered conditions. BMC Genom. 2022, 23, 831. [Google Scholar] [CrossRef]
- Rehman, A.; Farooq, M.; Ullah, A.; Nadeem, F.; Im, S.Y.; Park, S.K.; Lee, D.J. Agronomic biofortification of zinc in Pakistan: Status, benefits, and constraints. Front. Sustain. Food Syst. 2020, 4, 591–722. [Google Scholar] [CrossRef]
- Szerement, J.; Szatanik-Kloc, A.; Mokrzycki, J.; Mierzwa-Hersztek, M. Agronomic biofortification with Se, Zn, and Fe: An effective strategy to enhance crop nutritional quality and stress defense—A review. J. Soil Sci. Plant Nutr. 2022, 22, 1129–1159. [Google Scholar] [CrossRef]
- Velu, G.; Ortiz-Monasterio, I.; Cakmak, I.; Hao, Y.; Singh, R.Á. Biofortification strategies to increase grain zinc and iron concentrations in wheat. J. Cereal Sci. 2015, 59, 365–372. [Google Scholar] [CrossRef]
- Bashir, A.; Khan, Q.U.; Alem, A.; Hendi, A.A.; Zaman, U.; Khan, S.U.; ur Rehman, K.; Ali Khan, A.; Ullah, I.; Anwar, Y.; et al. Zinc and Potassium Fertilizer Synergizes Plant Nutrient Availability and Affects Growth, Yield, and Quality of Wheat Genotypes. Plants 2023, 12, 2241. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.K.; Seth, A.; Kumar, V.; Datta, A. Agronomic biofortification of wheat through proper fertilizer management to alleviate zinc malnutrition: A review. Commun. Soil Sci. Plant Anal. 2023, 54, 154–177. [Google Scholar] [CrossRef]
- Cakmak, I. Enrichment of cereal grains with zinc: Agronomic or genetic biofortification? Plant Soil 2008, 302, 1–17. [Google Scholar] [CrossRef]
- Jan, B.; Bhat, T.A.; Sheikh, T.A.; Wani, O.A.; Bhat, M.A.; Nazir, A.; Fayaz, S.; Mushtaq, T.; Farooq, A.; Wani, S.; et al. Agronomic bio-fortification of rice and maize with iron and zinc: A review. Int. Res. J. Pure Appl. Chem. 2020, 21, 28–37. [Google Scholar] [CrossRef]
- Ramzan, Y.; Hafeez, M.B.; Khan, S.; Nadeem, M.; Batool, S.; Ahmad, J. Biofortification with zinc and iron improves the grain quality and yield of wheat crop. Int. J. Plant Prod. 2020, 14, 501–510. [Google Scholar] [CrossRef]
- Nadeem, F.; Farooq, M.; Ullah, A.; Rehman, A.; Nawaz, A.; Naveed, M. Influence of Zn nutrition on the productivity, grain quality and grain biofortification of wheat under conventional and conservation rice–wheat cropping systems. Arch. Agron. Soil Sci. 2020, 66, 1042–1057. [Google Scholar] [CrossRef]
- Kalyani, S.F.; Siosemardeh, A.; Hosseinpanahi, F.; Jalali-Honarmand, S.; Ghobadi, M.E. Effect of seed priming on grain yield, protein content, and concentration of some nutrients in dryland wheat grain. Cereal Biotechnol. Biochem. 2022, 1, 87–99. [Google Scholar]
- Kumar, S.; Verma, G.; Dhaliwal, S.S.; Sharma, V. Influence of zinc fertilization levels and frequencies on crop productivity, zinc uptake and buildup of soil zinc in maize–wheat system. J. Plant Nutr. 2022, 45, 1774–1785. [Google Scholar] [CrossRef]
- Rasul, M.; Yasmin, S.; Suleman, M.; Zaheer, A.; Reitz, T.; Tarkka, M.T.; Islam, E.; Mirza, M.S. Glucose dehydrogenase gene containing phosphobacteria for biofortification of Phosphorus with growth promotion of rice. Microbiol. Res. 2019, 223, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Khalid, S.; Amanullah; Ahmed, I. Enhancing Zinc Biofortification of Wheat through Integration of Zinc, Compost, and Zinc-Solubilizing Bacteria. Agriculture 2022, 12, 968. [Google Scholar] [CrossRef]
- Rehman, A.; Farooq, M.; Naveed, M.; Nawaz, A.; Shahzad, B. Seed priming of Zn with endophytic bacteria improves the productivity and grain biofortification of bread wheat. Eur. J. Agron. 2018, 94, 98–107. [Google Scholar] [CrossRef]
- Ullah, A.; Farooq, M.; Nadeem, F.; Rehman, A.; Hussain, M.; Nawaz, A.; Naveed, M. Zinc application in combination with zinc solubilizing Enterobacter sp. MN17 improved productivity, profitability, zinc efficiency, and quality of desi chickpea. J. Soil Sci. Plant Nutr. 2020, 20, 2133–2144. [Google Scholar] [CrossRef]
- Wright, R.J.; Stuczynski, T. Atomic absorption and flame emission spectrometry. Methods Soil Anal. Part 3 Chem. Methods 1996, 5, 65–90. [Google Scholar]
- Haug, W.; Lantzsch, H.J. Sensitive method for the rapid determination of phytate in cereals and cereal products. J. Sci. Food Agric. 1983, 34, 1423–1426. [Google Scholar] [CrossRef]
- Shivay, Y.S.; Kumar, D. A Practical Manual of Field Crops Management; Division of Agronomy, Indian Agricultural Research Institute: New Delhi, India, 2008; 120p. [Google Scholar]
- Fageria, N.K. The Use of Nutrients in Crop Plants; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Nozoye, T.; Nagasaka, S.; Kobayashi, T.; Takahashi, M.; Sato, Y.; Sato, Y.; Uozumi, N.; Kobayashi, T.; Nishizawa, N.K. Iron uptake, translocation, and regulation in higher plants. Annu. Rev. Plant Biol. 2012, 63, 131–152. [Google Scholar]
- Ghasal, P.C.; Shivay, Y.S.; Pooniya, V.; Choudhary, M.; Verma, R.K. Response of wheat genotypes to zinc fertilization for improving productivity and quality. Arch. Agron. Soil Sci. 2017, 63, 1597–1612. [Google Scholar] [CrossRef]
- Khan, H.R.; McDonald, G.K.; Rengel, Z. Zn fertilization improves water use efficiency, grain yield and seed Zn content in chickpea. Plant Soil 2003, 249, 389–400. [Google Scholar] [CrossRef]
- Shivay, Y.S.; Prasad, R.; Singh, R.K.; Pal, M. Relative efficiency of zinc-coated urea and soil and foliar application of zinc sulphate on yield, nitrogen, phosphorus, potassium, zinc and iron biofortification in grains and uptake by basmati rice (Oryza sativa L.). J. Agric. Sci. 2015, 7, 161. [Google Scholar] [CrossRef]
- Paramesh, V.; Dhar, S.; Dass, A.; Kumar, B.; Kumar, A.; El-Ansary, D.O.; Elansary, H.O. Role of integrated nutrient management and agronomic fortification of zinc on yield, nutrient uptake and quality of wheat. Sustainability 2020, 12, 3513. [Google Scholar] [CrossRef]
- Maheshwari, D.K.; Kumar, S.; Maheshwari, N.K.; Patel, D.; Saraf, M. Nutrient availability and management in the rhizosphere by microorganisms. In Bacteria in Agrobiology: Stress Management; Springer: Berlin/Heidelberg, Germany, 2012; pp. 301–326. [Google Scholar]
- Naz, I.; Ahmad, H.; Khokhar, S.N.; Khan, K.; Shah, A.H. Impact of zinc solubilizing bacteria on zinc contents of wheat. Am. Euras. J. Agric. Environ. Sci 2016, 16, 449–454. [Google Scholar]
- Alloway, B.J. Soil factors associated with zinc deficiency in crops and humans. Environ. Geochem. Health 2009, 31, 537–548. [Google Scholar] [CrossRef]
- Zou, C.Q.; Zhang, Y.Q.; Rashid, A.; Ram, H.; Savasli, E.; Arisoy, R.Z.; Ortiz-Monasterio, I.; Simunji, S.; Wang, Z.G.; Sohu, V.; et al. Biofortification of wheat with zinc through zinc fertilization in seven countries. Plant Soil 2012, 361, 119–130. [Google Scholar] [CrossRef]
- Wu, C.Y.; Lu, L.L.; Yang, X.E.; Feng, Y.; Wei, Y.Y.; Hao, H.L.; Stoffella, P.J.; He, Z.L. Uptake, translocation, and remobilization of zinc absorbed at different growth stages by rice genotypes of different Zn densities. J. Agric. Food Chem. 2010, 58, 6767–6773. [Google Scholar] [CrossRef]
- Li, M.; Yang, X.W.; Tian, X.H.; Wang, S.X.; Chen, Y.L. Effect of nitrogen fertilizer and foliar zinc application at different growth stages on zinc translocation and utilization efficiency in winter wheat. Cereal Res. Commun. 2014, 42, 81–90. [Google Scholar] [CrossRef]
- Ramesh, A.; Sharma, S.K.; Sharma, M.P.; Yadav, N.; Joshi, O.P. Inoculation of zinc solubilizing Bacillus aryabhattai strains for improved growth, mobilization and biofortification of zinc in soybean and wheat cultivated in Vertisols of central India. Appl. Soil Ecol. 2014, 73, 87–96. [Google Scholar] [CrossRef]
- Abaid-Ullah, M.; Nadeem, M.; Hassan, M.; Ganter, J.; Muhammad, B.; Nawaz, K.; Sessitsch, A.; Hafeez, F.Y. Plant growth promoting rhizobacteria: An alternate way to improve yield and quality of wheat (Triticum aestivum). Int. J. Agric. Biol. 2015, 17, 51–60. [Google Scholar]
- Rana, A.; Joshi, M.; Prasanna, R.; Shivay, Y.S.; Nain, L. Biofortification of wheat through inoculation of plant growth promoting rhizobacteria and cyanobacteria. Eur. J. Soil Biol. 2012, 50, 118–126. [Google Scholar] [CrossRef]
- Wang, C.; Yuan, N.; Pei, Y. Effect of pH on metal lability in drinking water treatment residuals. J. Environ. Qual. 2014, 43, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Rajawat, M.V.S.; Kaushik, R.; Prasanna, R.; Saxena, A.K. Beneficial role of endophytes in biofortification of Zn in wheat genotypes varying in nutrient use efficiency grown in soils sufficient and deficient in Zn. Plant Soil 2017, 416, 107–116. [Google Scholar] [CrossRef]
- Erdal, I.; Yilmaz, A.; Taban, S.; Eker, S.; Torun, B.; Cakmak, I. Phytic acid and phosphorus concentrations in seeds of wheat cultivars grown with and without zinc fertilization. J. Plant Nutr. 2002, 25, 113–127. [Google Scholar] [CrossRef]
- Velu, G.; Singh, R.P.; Crespo-Herrera, L.; Juliana, P.; Dreisigacker, S.; Valluru, R.; Stangoulis, J.; Sohu, V.S.; Mavi, G.S.; Mishra, V.K.; et al. Genetic dissection of grain zinc concentration in spring wheat for mainstreaming biofortification in CIMMYT wheat breeding. Sci. Rep. 2019, 8, 13526. [Google Scholar] [CrossRef]
- Huang, C.; Barker, S.J.; Langridge, P.; Smith, F.W.; Graham, R.D. Zinc deficiency up-regulates expression of high-affinity phosphate transporter genes in both phosphate-sufficient and-deficient barley roots. Plant Physiol. 2000, 124, 415–422. [Google Scholar] [CrossRef]
- Cakmak, I.; Kalayci, M.; Kaya, Y.; Torun, A.A.; Aydin, N.; Wang, Y.; Arisoy, Z.; Erdem, H.; Yazici, A.; Gokmen, O.; et al. Biofortification and localization of zinc in wheat grain. J. Agric. Food Chem. 2010, 58, 9092–9102. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, H.; Tu, C.; Hu, X.; Li, L.; Luo, Y.; Christie, P. Phytotoxicity of ZnO nanoparticles and the released Zn (II) ion to corn (Zea mays L.) and cucumber (Cucumis sativus L.) during germination. Environ. Sci. Pollut. Res. 2015, 22, 11109–11117. [Google Scholar] [CrossRef]
- Genc, Y.; McDonald, G.K.; Graham, R.D. Critical deficiency concentration of zinc in barley genotypes differing in zinc efficiency and its relation to growth responses. J. Plant Nutr. 2002, 25, 545–560. [Google Scholar] [CrossRef]
- Fageria, N.K.; Moreira, A. The role of mineral nutrition on root growth of crop plants. Adv. Agron. 2011, 110, 251–331. [Google Scholar]
- Khalili, A.; Dhar, S.; Dass, A.; Mukhar, A.F.; Eldo, V. Agronomic indices of nitrogen use efficiency and maize yield response to various rates, time of application and their interaction effect in Kandahar region of Afghanistan. Ann. Agric. Res. New Ser. 2018, 39, 347–353. [Google Scholar]
- Ullah, A.; Farooq, M.; Hussain, M. Improving the productivity, profitability and grain quality of kabuli chickpea with co-application of zinc and endophyte bacteria Enterobacter sp. MN17. Arch. Agron. Soil Sci. 2020, 66, 897–912. [Google Scholar] [CrossRef]


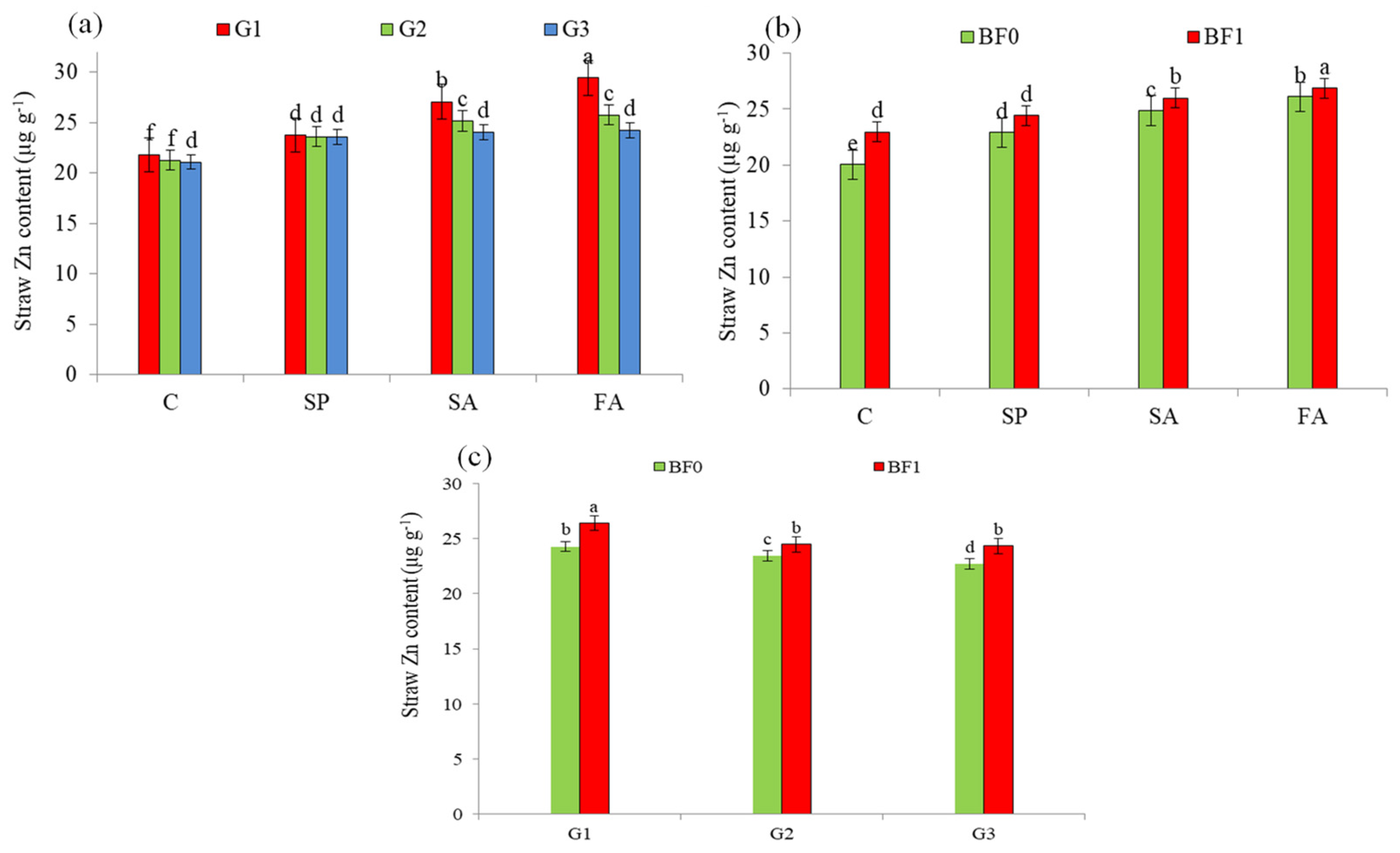
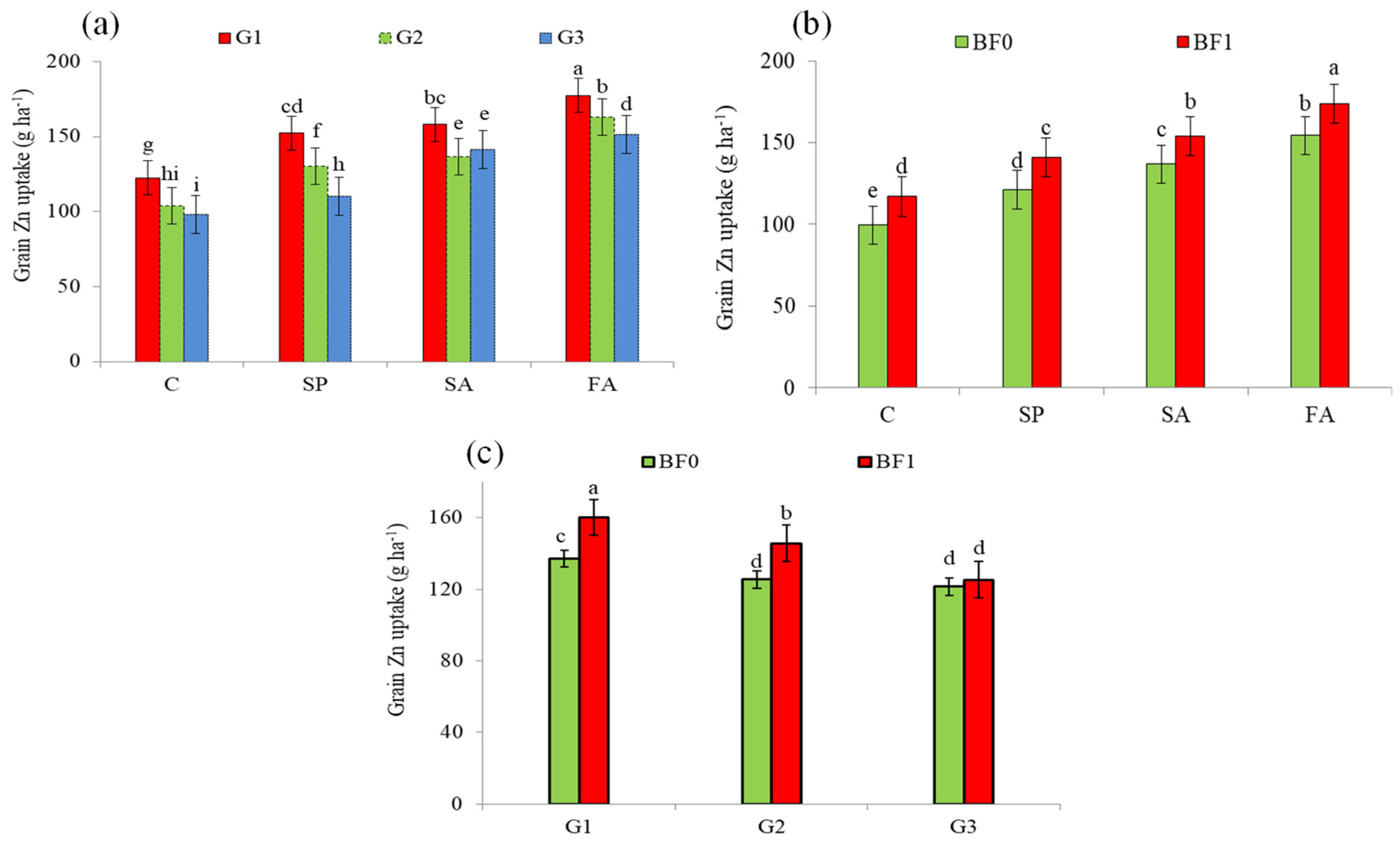
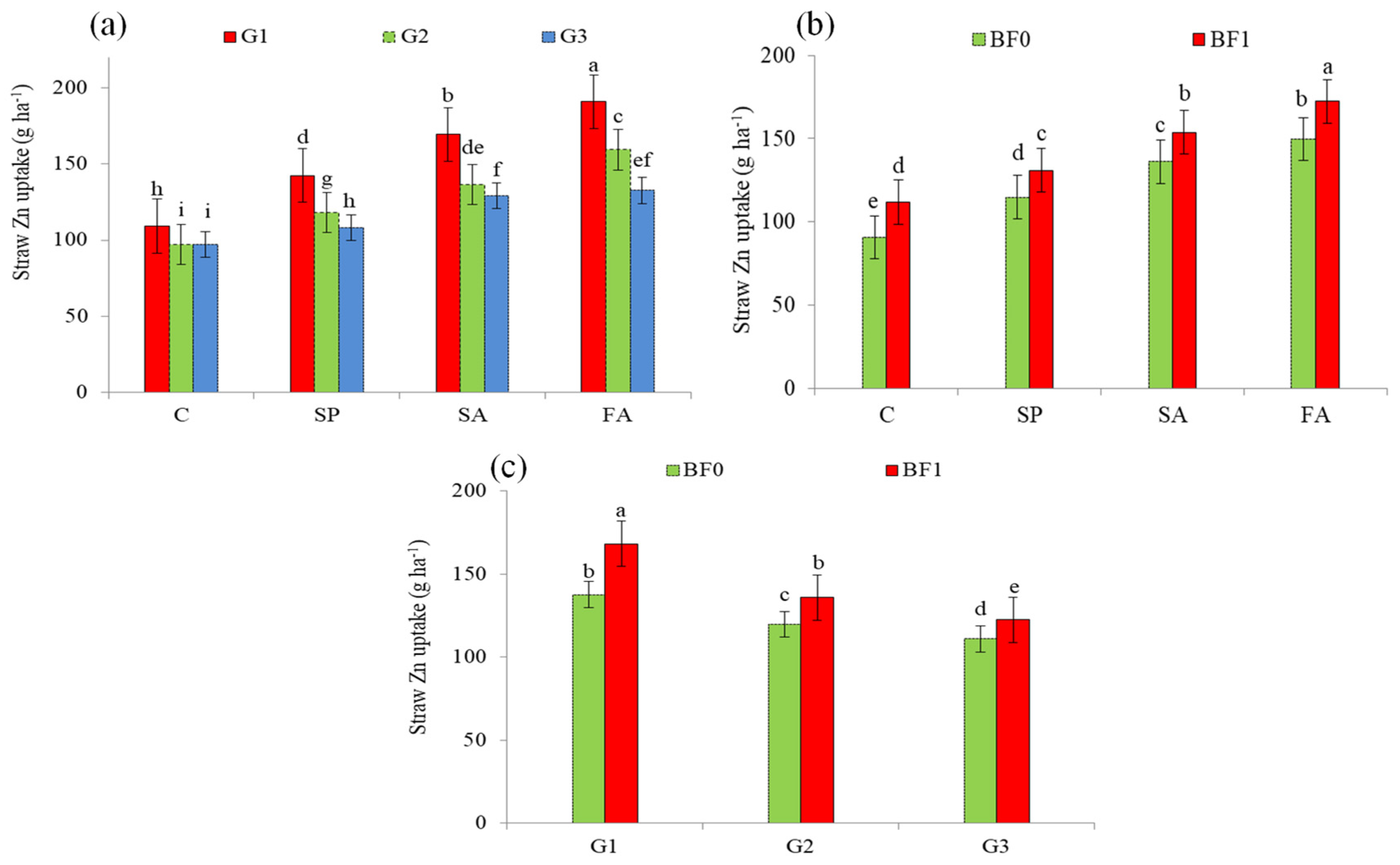
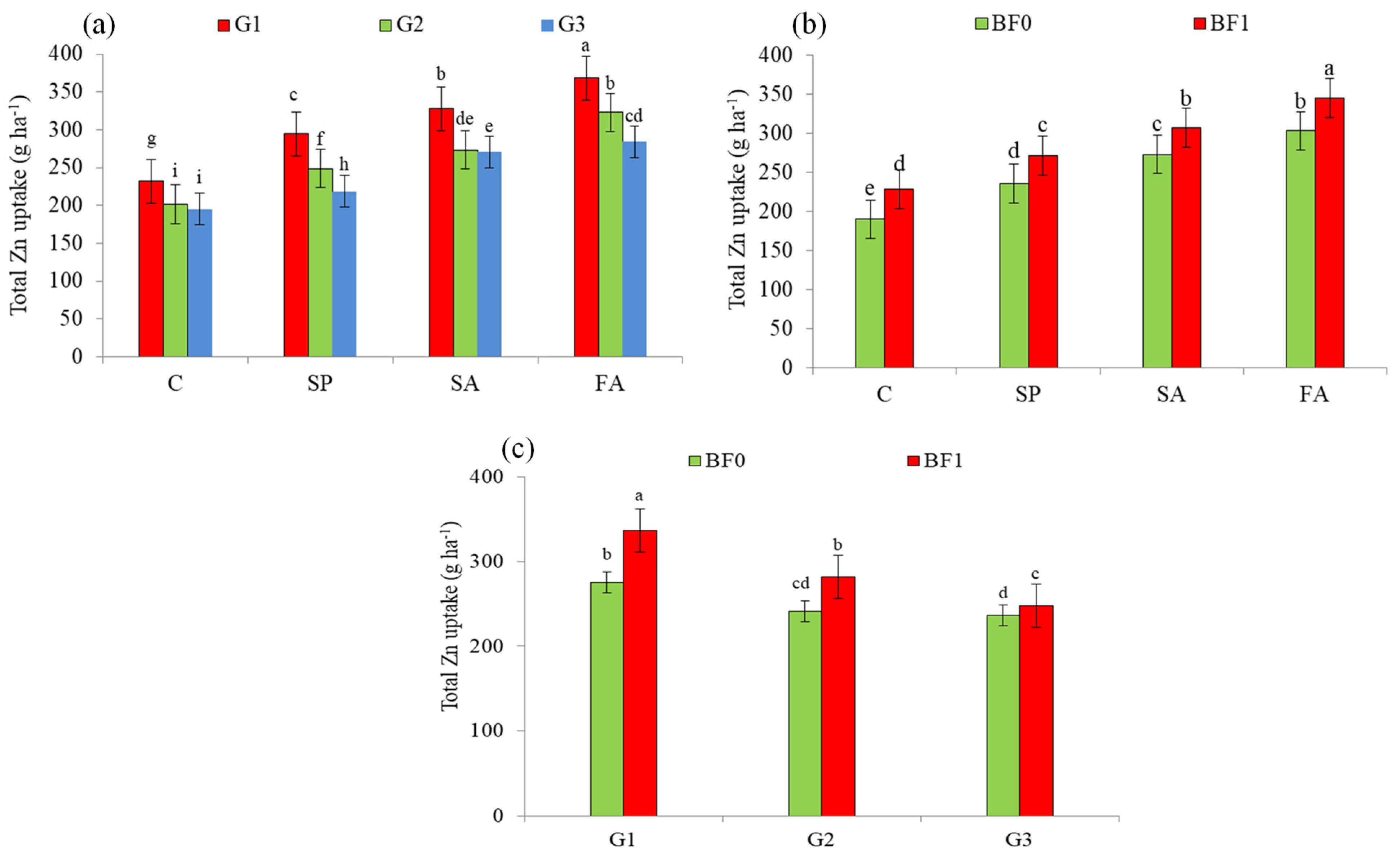


| Treatment | Grain Zn Content | Mean | Straw Zn Content | Mean | |||
|---|---|---|---|---|---|---|---|
| Year | Year | ||||||
| 2021–22 | 2022–23 | 2021–22 | 2022–23 | ||||
| Genotypes (G) | |||||||
| G1 | 33.10 a (4.1%) | 33.17 a (3.0%) | 33.14 a (3.5%) | 24.84 a (9.1%) | 25.84 a (6.5%) | 25.34 a (7.8 %) | |
| G2 | 32.52 b (2.2%) | 32.27 b (0.2%) | 32.45 b (1.4%) | 23.53 b (3.3%) | 24.37 b (0.5%) | 23.95 b (1.9%) | |
| G3 | 31.81 c | 32.20 c | 32.01 c | 22.77 c | 24.26 c | 23.51 c | |
| LSD (0.05) | 0.56 | 0.47 | 0.36 | 0.60 | 0.60 | 0.46 | |
| Application methods (AM) | |||||||
| C | 29.72 d | 29.44 d | 29.58 d | 20.75 d | 22.25 d | 21.50 d | |
| SP | 32.18 c (8.3%) | 32.53 c (10.5%) | 32.35 c (9.4%) | 22.55 c (8.7%) | 24.76 c (11.3%) | 23.66 c (10.0%) | |
| SA | 33.42 b (12.4%) | 33.26 b (13.0%) | 33.34 b (12.7) | 25.21 b (21.5%) | 25.64 b (15.2%) | 25.43 b (18.3%) | |
| FA | 34.60 a (16.4%) | 35.11 a (19.3%) | 34.85 a (17.8%) | 26.33 a (26.9%) | 26.63 a (19.7%) | 26.48 a (23.2%) | |
| LSD (0.05) | 0.65 | 0.54 | 0.41 | 0.70 | 0.69 | 0.53 | |
| Biofertilizer (ZSB) | |||||||
| BF0 | 31.49 b | 31.79 b | 31.64 b | 22.98 b | 23.96 b | 23.47 b | |
| BF1 | 33.47 a (6.3%) | 33.38 a (5.0%) | 33.42 a (5.6%) | 24.45 a (6.4%) | 25.68 a (7.2%) | 25.06 a (6.8%) | |
| LSD (0.05) | 0.46 | 0.38 | 0.29 | 0.49 | 0.49 | 0.38 | |
| Year means | 32.48 a | 32.58 a | NS | 23.71 b | 24.82 a | 0.000 | |
| Interactions effects | p value | p value | p value | p value | |||
| G × AM | 0.000 | Y × AM | NS | G × AM | 0.000 | Y × AM | 0.000 |
| G × ZSB | 0.000 | Y × G × AM | 0.000 | G × ZSB | 0.045 | Y × G × AM | NS |
| AM × ZSB | 0.000 | Y × ZSB | NS | AM × ZSB | 0.000 | Y × ZSB | NS |
| G × AM × ZSB | NS | Y × AM × ZSB | NS | G × AM × ZSB | 0.026 | Y × AM × ZSB | NS |
| Y × G | NS | Y × G × ZSB | 0.012 | Y × G | NS | Y × G × ZSB | NS |
| Y × G × AM × ZSB | 0.000 | Y × G × AM × ZSB | NS | ||||
| Treatment | Grain Zn Uptake | Mean | Straw Zn Uptake | Mean | |||
|---|---|---|---|---|---|---|---|
| Year | Year | ||||||
| 2021–22 | 2022–23 | 2021–22 | 2022–23 | ||||
| Genotypes (G) | |||||||
| G1 | 147.06 a (20.0%) | 158.31 a (23.5%) | 152.68 a (21.8%) | 148.50 a (32.0%) | 157.42 a (30.0%) | 152.96 a (31.0%) | |
| G2 | 131.58 b (7.4%) | 135.57 b (5.7%) | 133.58 b (6.6%) | 123.66 b (9.9%) | 132.01 b (9.0%) | 127.83 b (9.4%) | |
| G3 | 122.50 c | 128.22 c | 125.36 c | 112.49 c | 121.11 c | 116.80 c | |
| LSD (0.05) | 4.58 | 4.33 | 3.06 | 5.85 | 3.93 | 3.80 | |
| Application methods (AM) | |||||||
| C | 110.10 d | 106.51 d | 108.30 d | 96.07 d | 106.47 d | 101.27 d | |
| SP | 129.42 c (17.5%) | 132.56 c (24.5%) | 130.99 c (21.0%) | 116.50 c (21.3%) | 129.36 c (21.5%) | 122.93 c (21.4%) | |
| SA | 139.66 b (26.8%) | 151.27 b (42.0% | 145.46 b (34.3%) | 142.25 b (48.1%) | 147.67 b (38.7%) | 144.96 b (43.1%) | |
| FA | 155.67 a (41.4%) | 172.46 a (61.9%) | 164.07 a (51.5%) | 158.05 a (64.5%) | 163.89 a (53.9%) | 160.97 a (59.0%) | |
| LSD (0.05) | 5.29 | 5.00 | 3.53 | 6.75 | 4.54 | 4.39 | |
| Biofertilizer (ZSB) | |||||||
| BF0 | 121.74 b | 134.29 b | 128.02 b | 117.62 b | 128.01 b | 122.82 b | |
| BF1 | 145.68 a (19.7%) | 147.11 a (9.5%) | 146.39 a (14.3%) | 138.81 a (18.0%) | 145.69 a (13.8%) | 142.25 a (15.8%) | |
| LSD (0.05) | 3.74 | 3.54 | 2.50 | 4.77 | 3.21 | 3.11 | |
| Year means | 133.71 | 140.70 | 0.000 | 128.21 | 136.84 | 0.000 | |
| Interactions effects | p value | p value | p value | p value | |||
| G × AM | 0.000 | Y × AM | 0.000 | G × AM | 0.000 | Y × AM | NS |
| G × ZSB | 0.000 | Y × G × AM | 0.001 | G × ZSB | 0.000 | Y × G × AM | 0.017 |
| AM × ZSB | NS | Y × ZSB | 0.000 | AM × ZSB | NS | Y × ZSB | NS |
| G × AM × ZSB | 0.000 | Y × AM × ZSB | 0.004 | G × AM × ZSB | 0.009 | Y × AM × ZSB | NS |
| Y × G | NS | Y × G × ZSB | 0.000 | Y × G | NS | Y × G × ZSB | NS |
| Y × G × AM × ZSB | 0.006 | Y × G × AM × ZSB | NS | ||||
| Treatment | Total Zn Uptake | Mean | Phytic Acid | Mean | |||
|---|---|---|---|---|---|---|---|
| Year | Year | ||||||
| 2021–22 | 2022–23 | 2021–22 | 2022–23 | ||||
| Genotypes (G) | |||||||
| G1 | 295.57 a (25.8%) | 315.73 a (26.6%) | 305.65 a (26.2%) | 8.58 c (−11.7%) | 8.66 c (−10.2%) | 8.62 c (−11.0%) | |
| G2 | 255.23 b (8.6%) | 267.59 b (7.3%) | 261.41 b (7.9%) | 8.87 b (−8.7%) | 8.81 b (−8.6%) | 8.84 b (−8.7%) | |
| G3 | 234.98 c | 249.33 c | 242.16 c | 9.72 a | 9.64 a | 9.68 a | |
| LSD (0.05) | 8.88 | 6.98 | 5.83 | 0.66 | 0.53 | 0.59 | |
| Application methods (AM) | |||||||
| C | 206.17 d | 212.97 d | 209.57 d | 9.74 a | 9.56 a | 9.65 a | |
| SP | 245.92 c (19.3%) | 261.92 c (23.0%) | 253.92 c (21.2%) | 9.16 b (−6.0%) | 9.11 b (−4.7%) | 9.13 b (−5.4%) | |
| SA | 281.90 b (36.7%) | 298.94 b (40.4%) | 290.42 b (38.6%) | 8.86 c (−9.0%) | 8.90 c (−6.9%) | 8.88 c (−8.0%) | |
| FA | 313.72 a (52.2%) | 336.36 a (57.9%) | 325.04 a (55.1%) | 8.47 d (−13.0%) | 8.57 d (−10.4%) | 8.52 d (−11.7%) | |
| LSD (0.05) | 10.26 | 8.06 | 6.74 | 0.77 | 0.61 | 0.68 | |
| Biofertilizer (ZSB) | |||||||
| BF0 | 239.37 b | 262.30 b | 250.84 b | 9.43 a | 9.31 a | 9.37 a | |
| BF1 | 284.49 a (18.8%) | 292.79 a (11.6%) | 288.64 a (15.1%) | 8.69 b (−7.8%) | 8.76 b (−5.9%) | 8.72 b (−6.9%) | |
| LSD (0.05) | 7.25 | 5.70 | 8.65 | 0.54 | 0.43 | 0.48 | |
| Year means | 261.92 | 277.54 | 0.000 | 9.05 | 9.03 | NS | |
| Interactions effects | p value | p value | p value | p value | |||
| G × AM | 0.000 | Y × AM | NS | G × AM | 0.000 | Y × AM | NS |
| G × ZSB | 0.000 | Y × G × AM | 0.001 | G × ZSB | NS | Y × G × AM | NS |
| AM × ZSB | NS | Y × ZSB | 0.002 | AM × ZSB | NS | Y × ZSB | NS |
| G × AM × ZSB | 0.000 | Y × AM × ZSB | 0.009 | G × AM × ZSB | NS | Y × AM ×ZSB | NS |
| Y × G | NS | Y × G × ZSB | 0.019 | Y × G | NS | Y × G × ZSB | NS |
| Y × G × AM × ZSB | NS | Y × G × AM × ZSB | NS | ||||
| Treatment | Grain Yield | Mean | |
|---|---|---|---|
| Year | |||
| 2021–22 | 2022–23 | ||
| Genotypes (G) | |||
| G1 | 4.42 a (15.4%) | 4.73 a (19.7%) | 4.58 a (17.7%) |
| G2 | 4.02 b (5.0%) | 4.17 b (5.6%) | 4.09 b (5.1%) |
| G3 | 3.83 c | 3.95 c | 3.89 c |
| LSD (0.05) | 0.11 | 0.12 | 0.08 |
| Application methods (AM) | |||
| C | 3.69 d | 3.61 d | 3.65 d |
| SP | 4.01 c (8.7%) | 4.07 c (12.7%) | 4.04 c (10.7%) |
| SA | 4.17 b (13.0%) | 4.55 b (26.0%) | 4.36 b (19.5%) |
| FA | 4.48 a (21.4%) | 4.91 a (36.0%) | 4.70 a (28.8%) |
| LSD (0.05) | 0.14 | 0.13 | 0.10 |
| Biofertilizer (ZSB) | |||
| BF0 | 3.86 b | 4.18 b | 4.02 b |
| BF1 | 4.32 a (11.9%) | 4.38 a (4.8%) | 4.35 a (8.2%) |
| LSD (0.05) | 0.09 | 0.09 | 0.06 |
| Year means | 4.08 b | 4.28 a | 0.000 |
| Interactions effects | p value | p value | |
| G × AM | 0.000 | Y × AM | 0.000 |
| G × ZSB | 0.000 | Y × G × AM | 0.000 |
| AM × ZSB | NS | Y × ZSB | 0.000 |
| G × AM × ZSB | 0.000 | Y × AM × ZSB | 0.016 |
| Y × G | 0.032 | Y × G × ZSB | NS |
| Y × G × AM × ZSB | 0.000 | ||
| Treatment | Year | Mean | Year | Mean | Year | Mean | Year | Mean | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2021 −22 | 2022 −23 | 2021 −22 | 2022 −23 | 2021 −22 | 2022 −23 | 2021 −22 | 2022 −23 | |||||
| AE (kg kg−1) | PE (kg kg−1) | AR efficiency (%) | PFP (kg kg−1) | |||||||||
| C | - | - | - | - | - | - | - | - | - | - | - | - |
| SP | 106.01 b | 152.67 b | 129.15 b | 26.05 b | 18.66 b | 22.35 b | 6.44 a | 8.68 b | 7.56 b | 1336.37 a | 1356.00 b | 1346.19 a |
| SA | 48.05 c | 93.54 c | 70.74 c | 35.96 a | 20.71 a | 28.33 a | 2.96 c | 4.47 c | 3.71 c | 417.15 c | 454.54 c | 435.85 b |
| FA | 226.62 a | 371.22 a | 298.76 a | 24.51 b | 19.82 a | 22.17 b | 10.32 a | 18.84 a | 15.93 a | 1281.22 b | 1402.65 a | 1341.93 a |
| LSD (0.05) | 38.97 | 42.38 | 31.91 | 9.55 | 1.87 | 4.99 | 3.36 | 1.54 | 1.16 | 31.82 | 36.26 | 25.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalili, A.; Qayyum, A.; Khan, S.U.; Ullah, I.; Khalofah, A. Role of Temporal Zn Fertilization along with Zn Solubilizing Bacteria in Enhancing Zinc Content, Uptake, and Zinc Use Efficiency in Wheat Genotypes and Its Implications for Agronomic Biofortification. Agronomy 2023, 13, 2677. https://doi.org/10.3390/agronomy13112677
Khalili A, Qayyum A, Khan SU, Ullah I, Khalofah A. Role of Temporal Zn Fertilization along with Zn Solubilizing Bacteria in Enhancing Zinc Content, Uptake, and Zinc Use Efficiency in Wheat Genotypes and Its Implications for Agronomic Biofortification. Agronomy. 2023; 13(11):2677. https://doi.org/10.3390/agronomy13112677
Chicago/Turabian StyleKhalili, Azizullah, Abdul Qayyum, Sami Ullah Khan, Iltaf Ullah, and Ahlam Khalofah. 2023. "Role of Temporal Zn Fertilization along with Zn Solubilizing Bacteria in Enhancing Zinc Content, Uptake, and Zinc Use Efficiency in Wheat Genotypes and Its Implications for Agronomic Biofortification" Agronomy 13, no. 11: 2677. https://doi.org/10.3390/agronomy13112677





