Abstract
To clarify the contribution of the bridging effect from three metal cations (K+, Mn2+, and Fe3+) on the humification of lignin-rich Tilia wood shavings and further enrich the theory of lignin humification, an indoor incubation method with constant temperature and humidity was adopted. K+, Mn2+, and Fe3+ served as additives, with CK as the control for studying the differential influence of metal cations with different valences on the humus composition of dark-brown soil mixed with Tilia wood shavings. The change in the C contents of water-soluble substance (CWSS), humic-extracted acid (CHE) and humin (CHu), ∆logK value of HE, atomic ratio and FTIR spectra of humic acid (HA), and the ratio of C content of humic acid to fulvic acid (CHA/CFA) of dark-brown soil mixed with Tilia wood shavings were analyzed after 0, 30, 80, and 150 days of incubation, and the following conclusions were reached: (1) The addition of metal cations, regardless of their valence, could effectively improve the microbial utilization and consumption of WSS, and the effect was as follows: Fe3+ > Mn2+ > K+. The addition of three metal cations could effectively inhibit mineralization and reduce the loss of TOC, and the effect could be seen as follows: Fe3+ > Mn2+ > K+. (2) Although the CHE content first decreased and then increased with incubation, the addition of Fe3+ and Mn2+ ions increased the CHE content, showing that Fe3+ > Mn2+, and K+ ions had no significant effect. Throughout the incubation, the structure of HE molecules changed first via a complex process and then through a simple process. Comparing the change before and after the incubation, the overall structure of HE molecules tended to be simpler with the CK control, and HE became more complicated with the addition of Fe3+ and Mn2+; however, the addition of K+ had little effect on the structure of HE molecules. (3) At the end of the incubation, the addition of Fe3+, Mn2+, and K+ ions strengthened the molecular condensation of HA and its aromatization degree, while the CK control without any added metal cations caused HA molecules to decompose and obtain a greater aliphatic degree. In addition, the number of O-containing functional groups and N-containing compounds in HA molecules increased to varying degrees regardless of which metal cation was added. The decomposition of Tilia wood chips led to a partial entry of the decomposition products into the HA component, which was then reconsumed by continuous mineralization. After incubation, the polysaccharides in HA molecules were consumed only with the addition of Mn2+ ions. Fe3+ and Mn2+ ions had greater advantages in increasing the CHA/CFA ratio and improving the humus quality than K+ ions. (4) The addition of metal cations could effectively inhibit the mineralization and decomposition of the Hu component, among which Fe3+ ions had the most significant effect, followed by Mn2+ ions. Compared to monovalent cations (K+), polyvalent cations (Fe3+ and Mn2+) had the advantage of a bridging effect, and their addition promoted the microbial utilization of WSS, effectively reduced the loss of TOC, increased the CHE content, complicated its molecular structure, improved the humus quality, and inhibited the decomposition of Hu. Regardless of which metal cation was added, the degree of molecular polycondensation and aromatization of HA was enhanced, and the number of O-functional groups and N-containing compounds in HA molecules increased.
1. Introduction
Interactions between metal cations and soil organic matter (SOM) are central to the stability of organic matter, the formation of supramolecular SOM structures, the formation of organo-mineral associations, and soil aggregation [1]. The strong interaction of carboxylic groups of SOM with dissolved metal cations is thought to be the leading chemical interaction in SOM supramolecular aggregation [2]. The effect of multivalent cations on the environmental functionalities of SOM strongly depends on the relative importance between intramolecular complexation and intermolecular cross-linking, the degree of which will be determined by the spatial arrangement of the hydrophilic functional groups in SOM [1]. SOM tends to form aggregates of large molecular size due to the presence of metal cations commonly found in soils. The cations serve as seeds to agglomerate SOM and thus give rise to aggregates. The absence of cations leads to SOM destabilization and potentially also to the dispersion of the organic matter components, mainly due to electrostatic repulsion among them [3]. Common cations such as Na+, Mg2+, and Ca2+ are thought to play an important role in the supramolecular aggregation of SOM molecules into colloidal particles, the formation of SOM surface coatings, and the aggregation of mineral particles. Ca2+ forms the strongest inner-sphere complexes with SOM, and Na+ forms weak outer-sphere complexes. It is the least strongly held to carboxylic groups and, thus, is likely to have little effect on SOM aggregation [2].
Polyvalent cations such as Al3+, Fe3+, Ca2+, or Mg2+, which are naturally abundant in soils, are able to form cross-linking cation bridges in humic acid (HA), which usually results in an increasing rigidity of these compounds. With increasing Al3+ concentrations, C mineralization was reduced up to 80% due to the inhibitory effect [4]. Kraal et al. [5] reported that the addition of Al3+ suppressed soil respiration, nitrification, and the microbial uptake of NH4+ in pine litter. Here, the stabilization of OM via the formation of Al-humus complexes and resistance to microbial attack was considered the key mechanism controlling OM accumulation in soils [6]. The enhanced fulvic acid (FA) adsorption by divalent cations was ascribed to cation bridging, the decreased negative charge of dissolved organic matter (DOM) by the formation of dissolved metal-DOM complexes, and the shrinkage of the macromolecular configuration of DOM. The enhancement of FA adsorption decreased in the order of Ca2+, Mg2+, and Na+, correlating with their binding strength to FA [7]. Sutton et al. [8] reported that multivalent cations played a more significant role in the formation of organo-mineral complexes in soil systems than monovalent cations due to the formation of strong organo-mineral cation bridges. Rakhsh et al. [9] pointed out that in the cation bridging mechanism, the exchangeable polyvalent cations of soil clay, such as Ca2+, Mg2+, Fe3+, and Al3+, acted as bridges to neutralize both the negative charges of the clay surfaces and organic anions and connected organic biopolymers to the clay surfaces. Fukuchi et al. [10] pointed out that zeolite could serve as an effective catalyst for enhancing polycondensation reactions between glycine, catechol, and glucose. These observed differences in catalytic activity could be attributed to the transition metal content (Fe, Mn, and Ti) in the zeolite samples, which can function as Lewis acids. Takashi et al. [11] showed that high levels of soluble and exchangeable Al and Al/Fe-humus complexes in acidic soils protected organic carbon (OC) from degradation via Al toxicity and reduced the availability of OM, respectively. The major polyvalent cations present in neutral and alkaline soils are Ca2+ and Mg2+, which could act as bridges between negatively charged clay surfaces and negatively charged organic groups [12]. Cheng et al. [13] studied Mn2+ as a cation bridge linking both negatively charged MnOx and humic acid (HA). HA is characterized by a large number of functional groups with high affinity for metals such as Fe3+ and Fe2+, which can form complexes with different stability levels [14]. FeSO4 had an inhibitory effect on microbial degradation activities, which improved the compost humification process: the humus substance (HS) contents in the compost product with FeSO4 were 109.82~129.86 g/kg, higher than 106.31 g/kg in the control group [15]. Phenols are one of the essential precursors of humus formation. By serving as electron acceptors, polyvalent metals, including Cu, Mn, and Fe, from diverse biotic and abiotic catalysts could accelerate the transformation of phenolic compounds [16]. The active coordination between Fe3+ and catechol strengthened the catalysis of iron phases in the oxidative polymerization of the humification precursors [17].
Plant derivatives in the soil were partially degraded into smaller molecules such as phenols, phenylpropene units, amino acids, peptides, amino sugars, and sugars. These smaller molecules further react, polymerize, and polycondense partially with the aid of soil oxidoreductases or mineral surfaces and are partly supported by soil microorganisms to form medium- to high-molecular-weight organic substances, specifically humic substances (HS). Humic molecules of different masses form supramolecular bonds under the action of cation bridging, eventually resulting in a humic network [18]. Plant inputs drove decomposition and SOM formation and could be viewed as the controlling pump in the C cycle [19]. The polyvalent metals in abiotic catalysts played a critical role in enhancing the humification reaction. These metals could function as Lewis acids and oxides to promote nucleophilic addition and polycondensation by obtaining electrons from micromolecular precursors [20]. Although cations with different valences usually coexist in the natural soil environment, their effects on the mineralization of plant residues and the formation of humus are different [21]. In view of this, we were interested in two topics: How do metal cations with different valences behave in biotic humification processes? Could polyvalent metal cation bridging promote the humification process of lignin-rich plant residues in soil? To answer these two scientific questions, we used lignin-rich Tilia wood shavings as an organic material and typical dark-brown soil of Jilin Province as a test soil based on an incubation experiment to explore the effects of metal cations with different valences (Fe3+, Mn2+, and K+) on the humus composition and structural characteristics of humic acid (HA) molecules of dark-brown soil mixed with Tilia wood shavings to provide a theoretical reference for accelerating the humification process of plant residues in soil catalyzed by cation bridging and a practical basis for research on catalytic decomposition agents for agricultural and forestry straw substances.
2. Materials and Methods
2.1. Materials
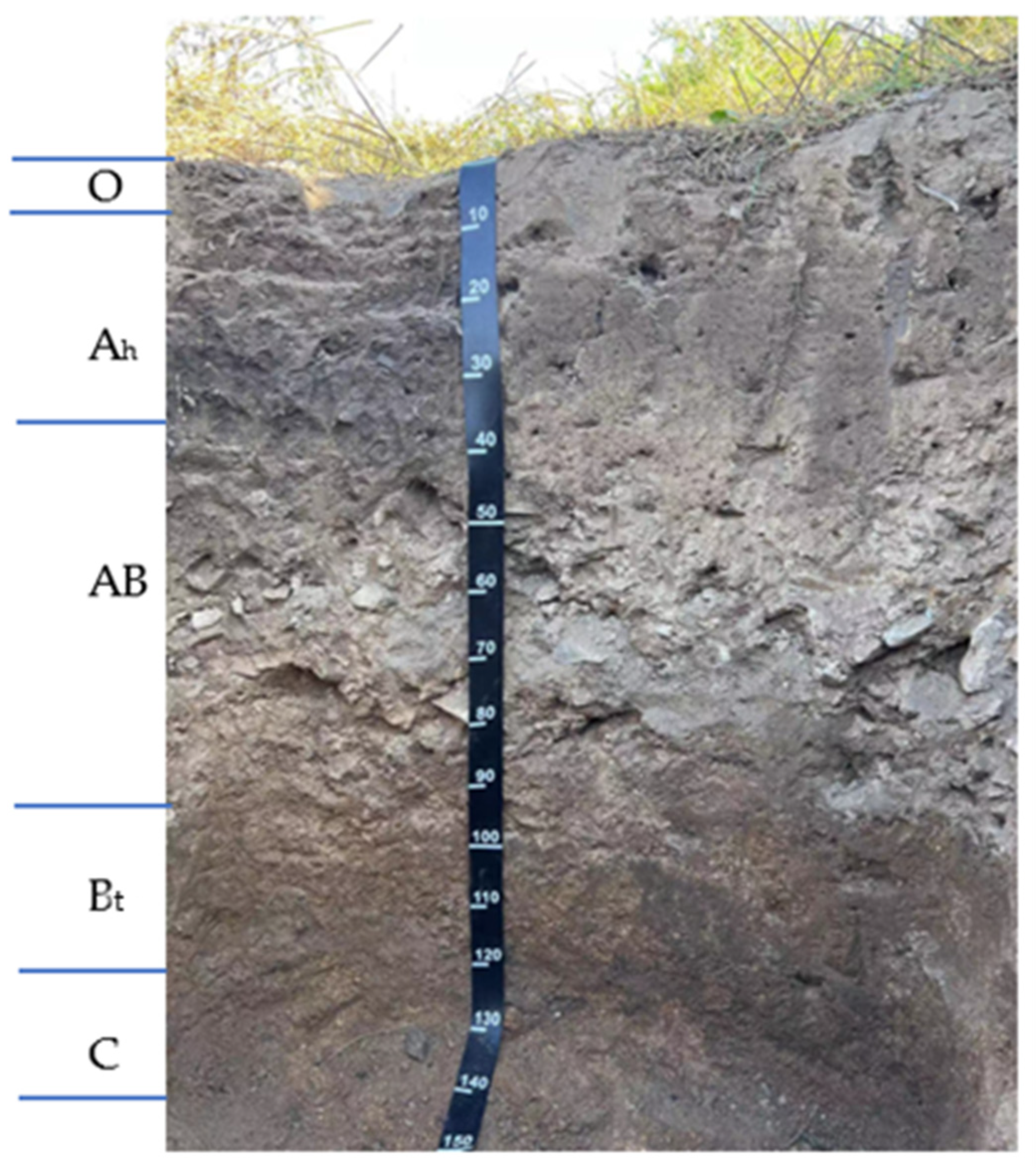
The tested dark-brown soil (Alfisols; WRB classification system-FAO, 2022) was taken from a mixed broad leaf-conifer forest located 243 m above the sea level of the Houshi mountain in the Economic and Technological Development Zone, Jilin city. The sampling depth was 0–18 cm, loam soil (proportion of sand particles, silt particles, and clay particles in the fine earth fracion were 29.9%, 46.6%, and 23.5%, respectively), soil organic matter 28.9 g/kg, total N 1.55 g/kg, total P 1.01 g/kg, total K 11.9 g/kg, pH value 5.76, CEC 20.6 cmol(+)/kg, no calcareous reaction. The configuration of the dark-brown soil profile was O-Ah-AB-Bt-C, Figure 1. The accumulation of weakly acidic humus and the mild leaching and agglutination processes were its main soil-forming processes. After removing the visible roots from the dark-brown soil, the dark-brown soil was air-dried, ground, and passed through a 1.0 mm sieve, retaining part of the grainy soil to ensure soil ventilation and water permeability during the incubation process.
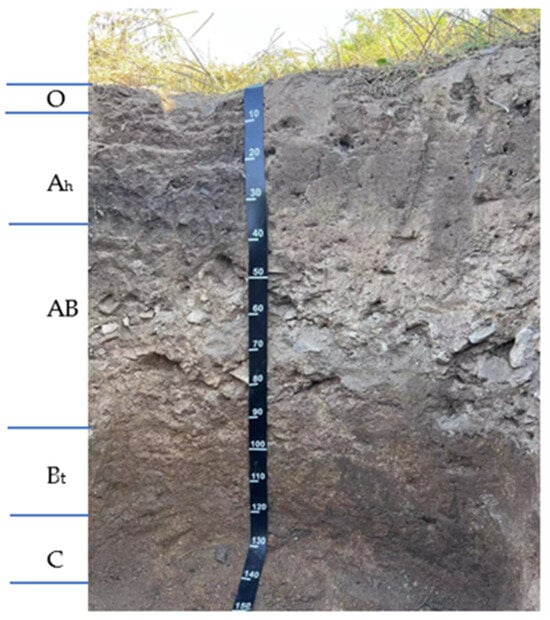
Figure 1.
The photo shows a dark-brown soil profile. (The letters O, Ah, AB, Bt and C stand for the dead soil covering, humus layer, AB transition layer, argillic horizon, parent rock horizon, respectively).
Tilia (Tilia tuan Szyszyl) wood shavings were purchased from an online mall called Mulinsen Wood. After drying and grinding through a 0.25 mm sieve, the contents of total organic C, N, P2O5, and K2O were determined by the K2Cr2O7 external heating method, Kjeldahl method, molybdenum-antimony colorimetric method, and flame photometer for 37.8%, 1.93%, 2.33%, and 0.76%, respectively. The analytical reagents Fe2(SO4)3, MnSO4, and K2SO4 were purchased from Sinopharm Chemical Reagent Co., Ltd., Shanghai, China.
Preparation of microbial suspension: A total of 1.0 g of straw decomposing agent (effective viable microbial number ≥ 2 × 1010 cfu/g, composed of Trichoderma, Bacillus, Actinomyces, Pseudomonas, lactic acid bacteria, yeast, cellulase, protease, etc.) was accurately weighed and produced by Tianjin Huanwei Biotechnology Co., Ltd., Tianjin, China, in 100 mL sterile water. Extraction was carried out by oscillating at 120 r/min in a gas bath oscillator at 28 °C for 2 h, stationary deposition for 30 min, and centrifugation at 3500 r/min for 15 min, and the supernatant was collected for use.
2.2. Methods
A method of indoor incubation that maintained a constant temperature and humidity was adopted. After being ground through a 0.10 mm sieve, 45.0 g of dark-brown soil and 5.0 g of Tilia wood shavings were accurately weighed and mixed evenly. A certain concentration of (NH4)2SO4 solution (N 27.2%) was evenly sprayed in the dark-brown soil mixed with Tilia wood shavings, adjusting the mixture to an appropriate water content (60%) and C/N ratio (25:1). Three treatments were designed for this experiment. Five milliliters of 0.5 mol/L Fe2(SO4)3, MnSO4, and K2SO4 solutions were added to a triangle bottle containing dark-brown soil with Tilia wood shavings, represented by Fs, Ms, and Ps, respectively, and the same volume of distilled water served as the CK control. Then, 10 mL of the microbial suspension was inoculated into a triangle bottle under aseptic operating conditions, sealed with a sterile breathable membrane, and maintained at constant temperature and humidity. The total incubation period was designed for 150 days. During the incubation period, dynamic sampling was performed at 0, 30, 80, and 150 days for the analysis of humus composition.
2.3. Analysis
The total organic C content of the sample was determined by an external heating oxidation method for K2Cr2O7. The humus composition of the samples was analyzed according to a modified method for reducing the possibility of soil humus structure change in the extraction process [22], using a mixture of relatively mild Na4P2O7·10H2O and NaOH to replace the strong alkali solution as the extraction agent. The brief process was as follows: A total of 5.0 g of the sample was weighed accurately in a 100-mL polyethylene centrifuge tube, and 30 mL of distilled water was added to the tube and stirred evenly. The centrifuge tubes with the sample were extracted in a water bath oscillator, maintained at 70 °C for 1 h, and centrifuged (3500 r/min, 15 min). The obtained supernatant was filtered into a 50-mL volumetric flask. The sample was extracted again using 20 mL of distilled water, and the extracts from two extractions were pooled together to yield the water-soluble substance (WSS). The residue in the centrifuge tube was extracted again with a mixed solution of 0.1 mol/L Na4P2O7·10H2O and 0.1 mol/L NaOH using the same procedure as above, and the extracted solution was humic-extracted acid (HE). The residue in the tube was rinsed with distilled water many times until the eluent was nearly neutral, then transferred into a drying oven at 50 °C until a constant weight was achieved, which was defined as humin (Hu), and ground through a 0.10 mm sieve.
The HE solution was extracted (5 mL) and diluted to 50 mL to prepare a HE liquid sample. The absorbances of the HE liquid sample at 400 and 600 nm (A400 and A600) were measured by a UV-visible light spectrophotometer (TU-1900, Beijing Purkinje General Instrument Co., Ltd., Beijing, China), and then ∆logK was calculated by the following equation: ∆logK = lgA400 − lgA600 [14]. Then, the HE solution (30 mL) was adjusted to pH 1.0–1.5 using H2SO4 at a concentration of 0.5 mol/L, transferred to a water bath, maintained at 70 °C for 1.5 h, and then kept overnight. The solution containing the flocculent matter was filtered into a volumetric flask at a constant volume of 50 mL. The diluted filtrate was fulvic acid (FA). The flocculent matter retained on the filter paper was rinsed repeatedly with a dilute acid solution and dissolved in a 50-mL volumetric flask with a hot NaOH solution with a concentration of 0.05 mol/L. The solution obtained was humic acid (HA). The carbon concentrations (g/kg) of the WSS, HE, HA, and Hu components (CWSS, CHE, CHA, and CHu) were all determined by the TOC analyzer, in which the CFA (CFA = CHE − CHA) and the degree of polymerization (CHA/CFA ratio) were calculated [23]. All analyses were carried out in triplicate, and the mean values are presented.
The extracted HA liquid samples were freeze-dried to prepare solid HA samples for further analysis. The percentages of C, H, O, and N in the solid HA samples were measured by an elemental analyzer (PE 2400II Series II CHNS/O, Perkin Elmer, Waltham, MA, USA), and the C/N, H/C, and O/C ratios were calculated. The spectroscopic characterization of solid HA samples was measured by FTIR spectrophotometry (FTIR-850, Tianjin Guangdong Sci and Tech Development Co., Ltd., Tianjin, China). FTIR spectra were acquired over the wavelength region from 400 to 4000 cm−1, analyzed with FTIR 850 software, and presented as graphs with Origin 8.0 software.
2.4. Statistical Analysis of the Data
Data analysis and spectrum processing were performed using Excel 2003 and Origin 8.0. All statistical tests were performed using SPSS 18.0 software (SPSS Inc., Chicago, IL, USA). The differences among the treatment means were determined using a one-way analysis of variance (ANOVA) with the least significant difference (LSD) test. The significance was set at the p < 0.05 level.
3. Results
3.1. Total Organic C Content and C Content of Water-Soluble Substances (CWSS)
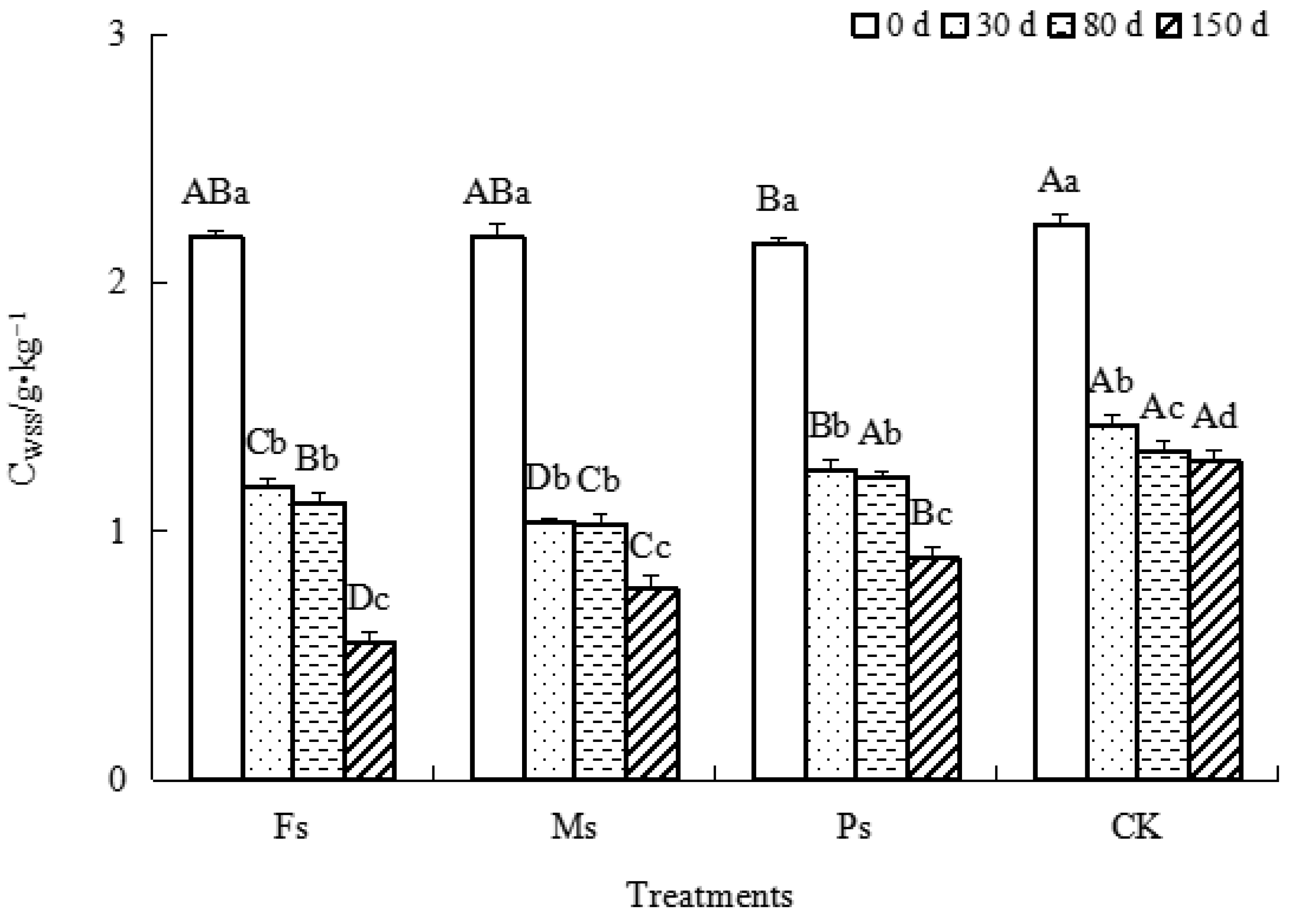
As shown in Figure 2, with incubation, the CWSS contents in the three treatments and CK showed a gradual decrease. Compared with the results at 0 d, after incubation for 150 d, the decreases in CWSS contents under the Fs, Ms, and Ps treatments and the CK were 74.7%, 65.0%, 58.4%, and 42.8%, respectively. It could be inferred that the effects of adding metal cations with different valences on the CWSS decrease were in the following order: Fs > Ms > Ps > CK control. The addition of metal cations could effectively promote the utilization and consumption of WSS by microorganisms, as follows: Fe3+ > Mn2+ > K+.
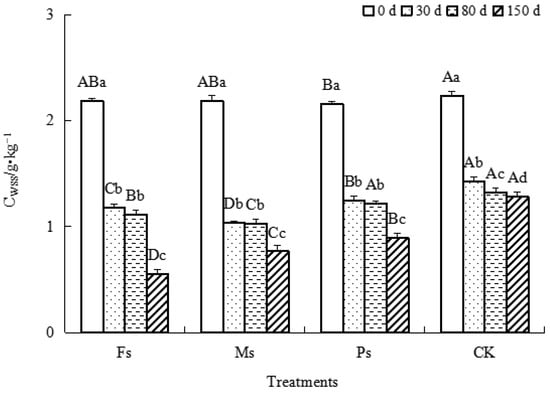
Figure 2.
Effect of metal cations with different valence additions on the CWSS of dark-brown soil mixed with Tilia wood shavings. Note: The treatments of adding Fe2(SO4)3, MnSO4, K2SO4 solutions, and CK control are represented by Fs, Ms, Ps, and CK, respectively. Different uppercase letters indicate significant differences among different treatments at the same incubation time at the 0.05 level, while different lowercase letters indicate significant differences among different incubation times at the same treatment at the 0.05 level.
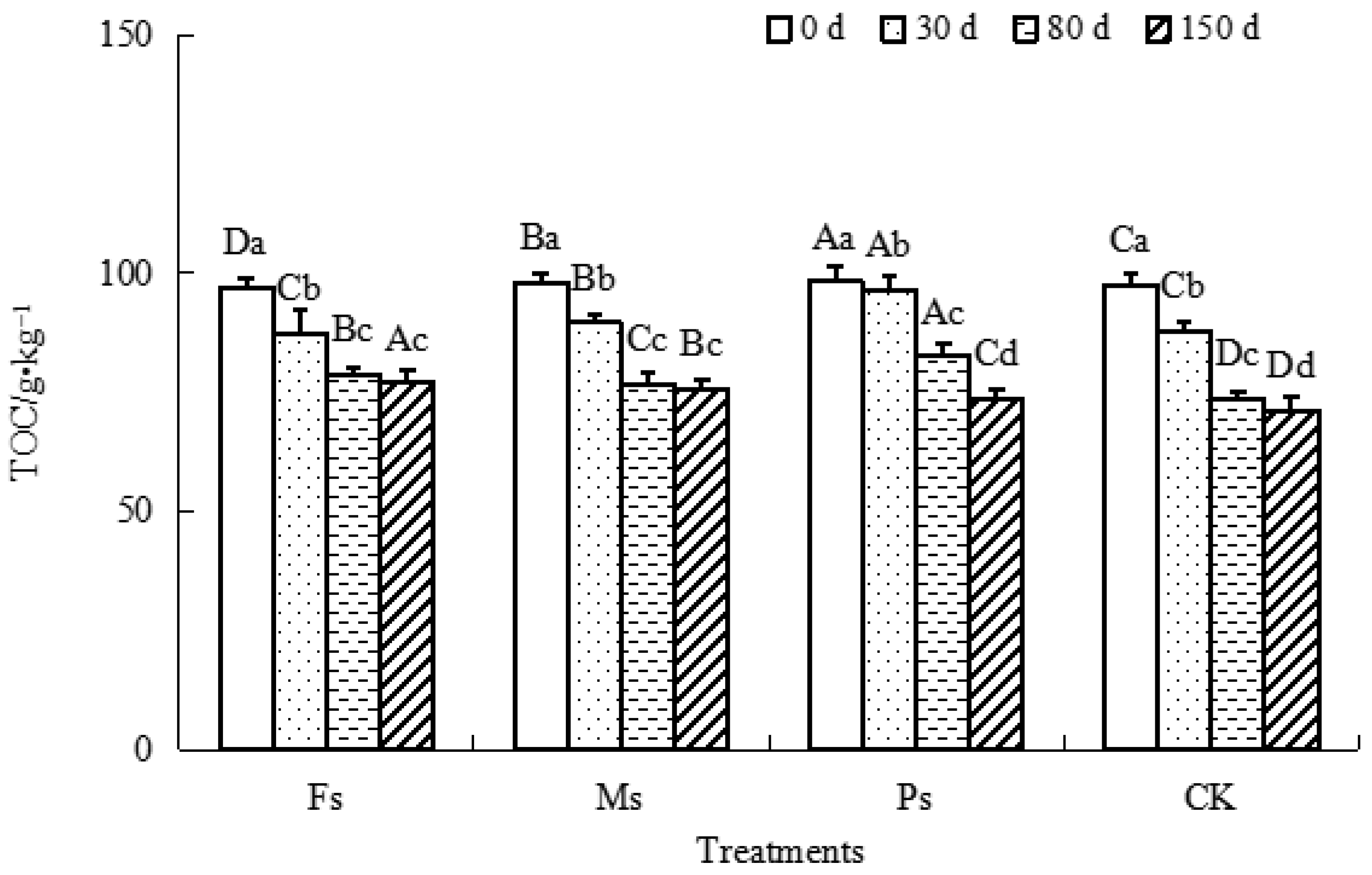
As shown in Figure 3, all the TOC of dark-brown soil mixed with Tilia wood shavings in each treatment decreased gradually as the incubation progressed. Compared with the result at 0 d, after incubation for 150 d, the TOC of dark-brown soil under the Fs, Ms, and Ps treatments and the CK decreased by 20.6%, 22.7%, 25.2%, and 26.8%, respectively, with the CK having the largest decrease. The TOC content decreased the least under Fs treatment, and the overall performance was CK control > Ps > Ms > Fs. The addition of three metal cations could effectively inhibit mineralization and reduce TOC loss, and the effect was as follows: Fe3+ > Mn2+ > K+.
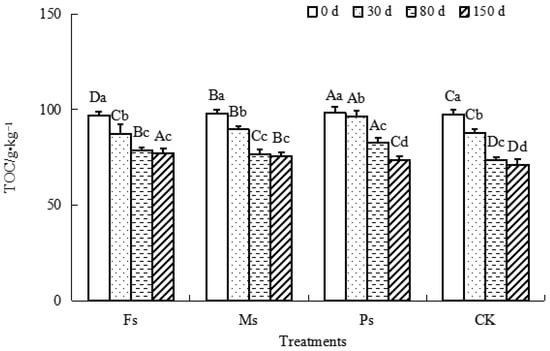
Figure 3.
Effect of metal cations with different valence additions on the TOC of dark-brown soil mixed with Tilia wood shavings. Different uppercase letters indicate significant differences among different treatments at the same incubation time at the 0.05 level, while different lowercase letters indicate significant differences among different incubation times at the same treatment at the 0.05 level.
3.2. C Content (CHE) and ∆logK Value of Humic-Extracted Acid
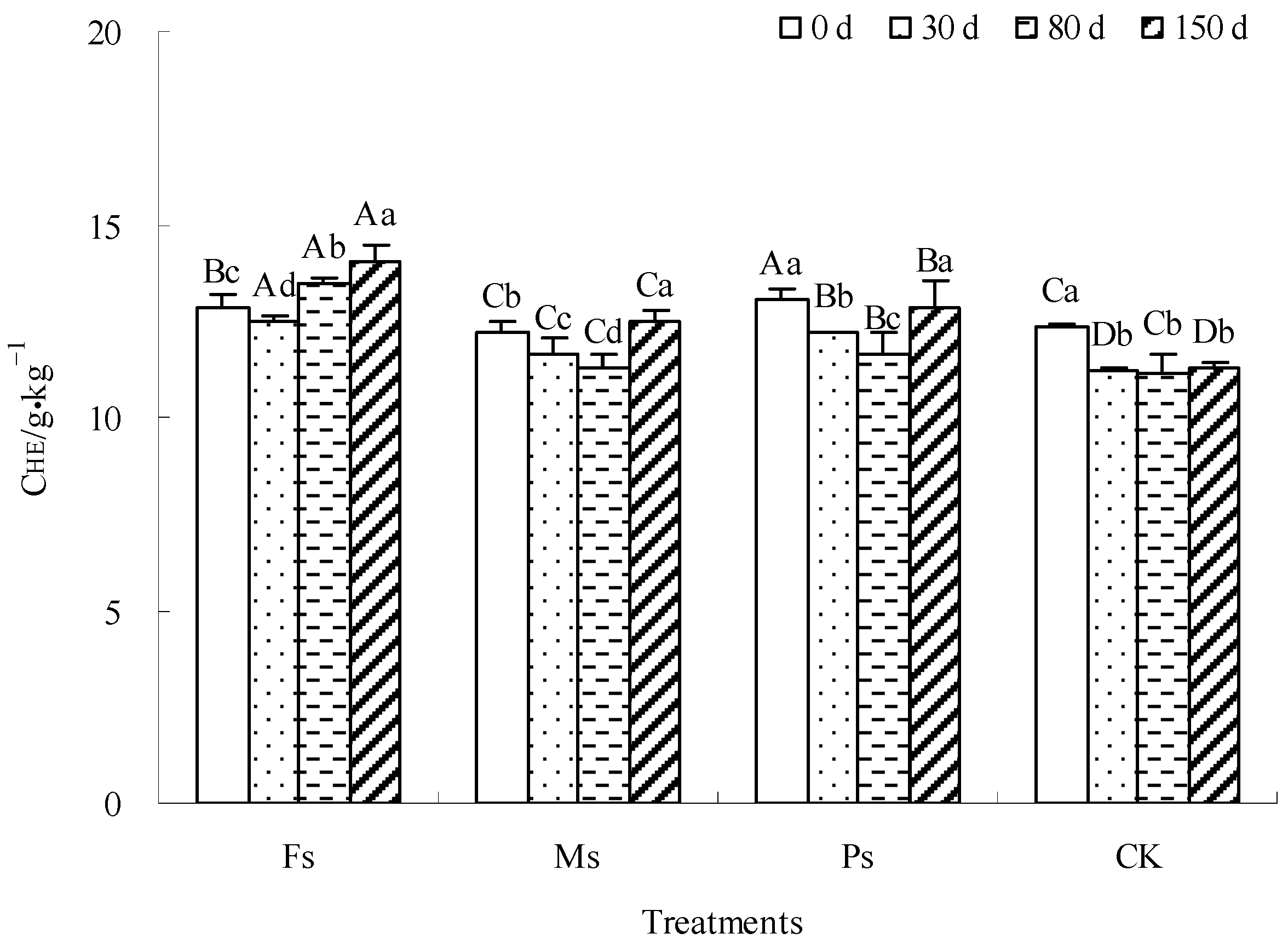
As shown in Figure 4, the CHE content in the CK control decreased first and then stabilized until the incubation was completed. The CHE contents in the Fs, Ms, and Ps treatments first decreased and then increased. After incubation for 150 days, the CHE contents in the Fs and Ms treatments increased by 9.1% and 2.5%, respectively, while the CHE content in the CK control decreased by 8.6%. There was no significant change in the CHE content of the Ps treatment. Under the influence of adding metal cations with different valences, the mineralization of dark-brown soil mixed with Tilia wood shavings was dominant in the early stage of incubation; the HE tended to decompose, and then the humification was gradually enhanced. The HE tended to accumulate; therefore, the CHE content first decreased and then increased. Finally, the addition of Fe3+ and Mn2+ ions could increase the CHE content, showing a rule that Fe3+ > Mn2+, and K+ ions had no significant effect.
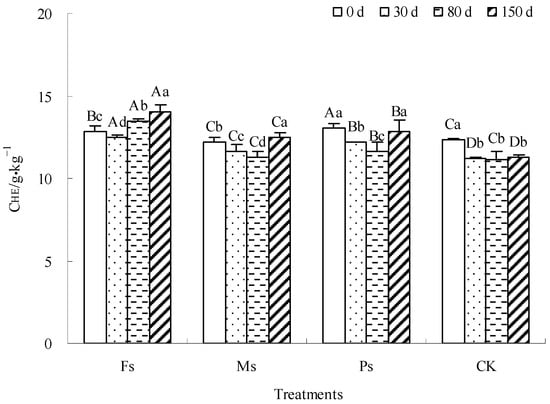
Figure 4.
Effect of metal cations with different valence additions on the CHE of dark-brown soil mixed with Tilia wood shavings. Different uppercase letters indicate significant differences among different treatments at the same incubation time at the 0.05 level, while different lowercase letters indicate significant differences among different incubation times at the same treatment at the 0.05 level.
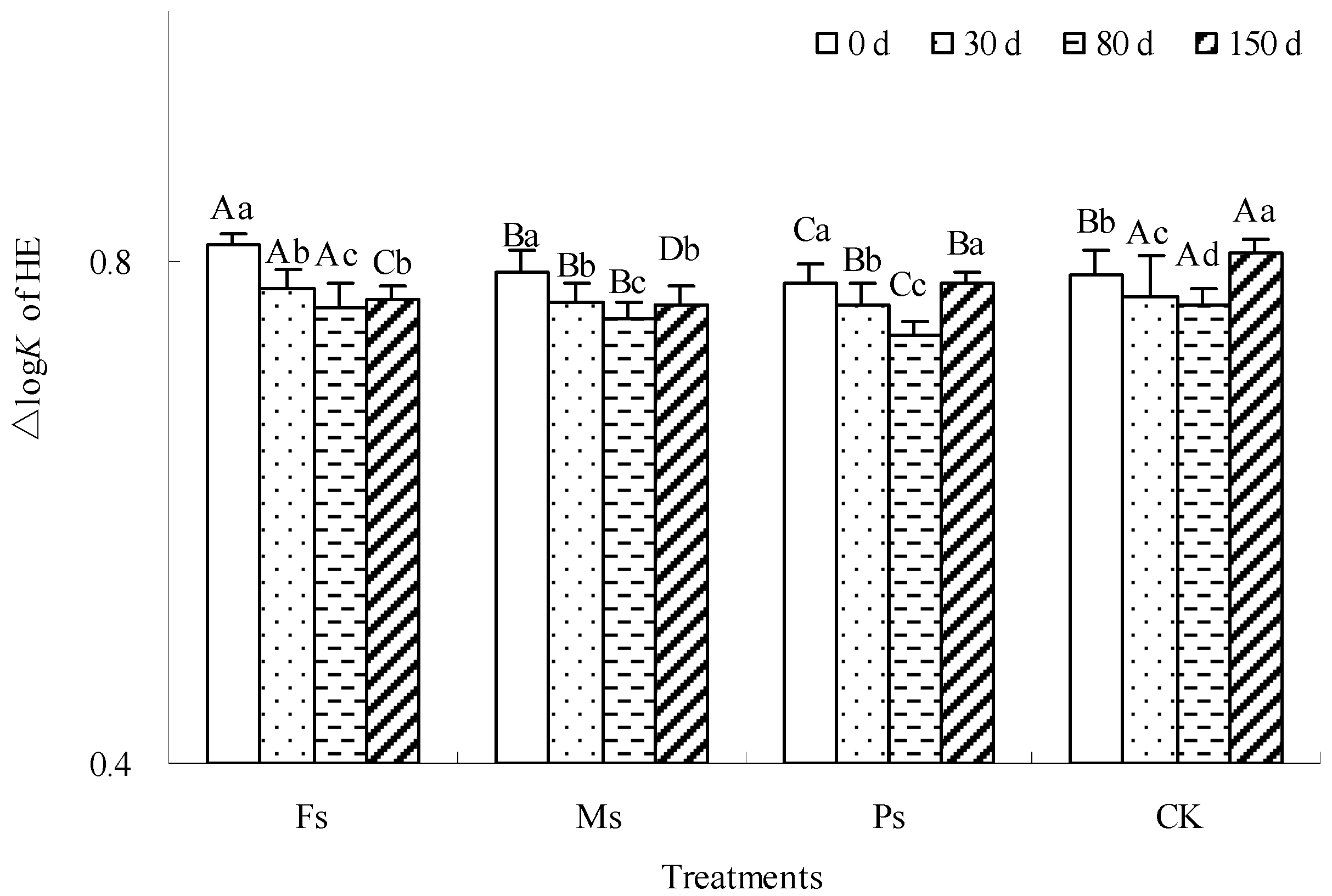
The ∆logK value is an important parameter to measure the optical properties of humic components, which is inversely proportional to the molecular complexity of humus components. As shown in Figure 5, with incubation, the ∆logK value of the HE alkaline extract under each treatment showed a rule of first decreasing and then increasing, indicating that the molecular complexity of the HE alkaline extract experienced a complex process first and then a simple process. After incubation for 150 d, the ∆logK value of the HE alkaline extract treated with Fs and Ms decreased by 5.3% and 3.2%, respectively. However, the ∆logK value of the HE alkaline extract under Ps treatment had no significant change before and after incubation, while that of the CK control increased by 2.1%. Early mineralization of the dark-brown soil mixed with Tilia wood shavings promoted the decomposition of relatively simple HE molecules, complicating the overall structure of the remaining HE molecules. Later, HE molecules with relatively complex structures also tended to mineralize, making the molecular structure simpler. Compared with the result at 0 d, the addition of Fe3+ and Mn2+ made the overall structure of HE molecules more complex after incubation, and the addition of K+ had little effect on the structure of HE molecules. However, under CK control, the newly formed molecules had not been condensed to their initial state; therefore, the overall structure of HE molecules tended to be simple.
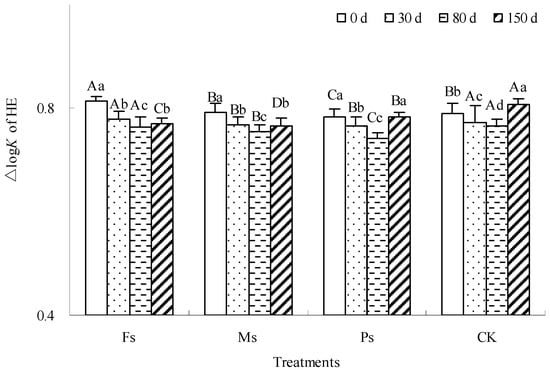
Figure 5.
Effect of metal cations with different valence additions on the ∆logK value of HE extracted from dark-brown soil mixed with Tilia wood shavings. Different uppercase letters indicate significant differences among different treatments at the same incubation time at the 0.05 level, while different lowercase letters indicate significant differences among different incubation times at the same treatment at the 0.05 level.
3.3. Atomic Ratio and FTIR Spectra of Humic Acid (HA)
As could be seen from Table 1, with incubation, the H/C ratio of HA molecules under the CK control slightly increased, whereas the H/C ratio of HA molecules from the Ps, Ms, and Fs treatments decreased gradually, indicating that the condensation degree of HA molecules increased under the influence of cation addition. The O/C ratio of HA molecules treated by the CK control, Ps, Ms, and Fs treatments increased to different degrees. The results showed that the molecular condensation of HA was enhanced after incubation with the addition of Fe3+, Mn2+, and K+ ions, while the molecular condensation of HA was decreased under the CK control without any metal cations. In addition, the number of O-containing functional groups in HA molecules increased to varying degrees after incubation, regardless of whether metal cations were added. The C/N ratio of HA molecules in the CK control had a slight increase, while the C/N ratio in the Fs, Ms, and Ps treatments showed a gradual decrease with incubation. Without the addition of any metal cations, the mineralization process caused a loss of C in the HA molecules of the CK control, while the N-containing compounds in the HA molecules were consumed to a greater extent, ultimately resulting in a slight increase in the C/N ratio of the HA molecules. The addition of Fe3+, Mn2+, and K+ ions could alleviate the mineralization of C in HA molecules to a certain extent, and the enhancement of humification resulted in the accumulation of N-containing compounds in HA molecules.

Table 1.
Atomic ratio of HA extracted from the decomposing material of rice straw at different incubation temperatures.
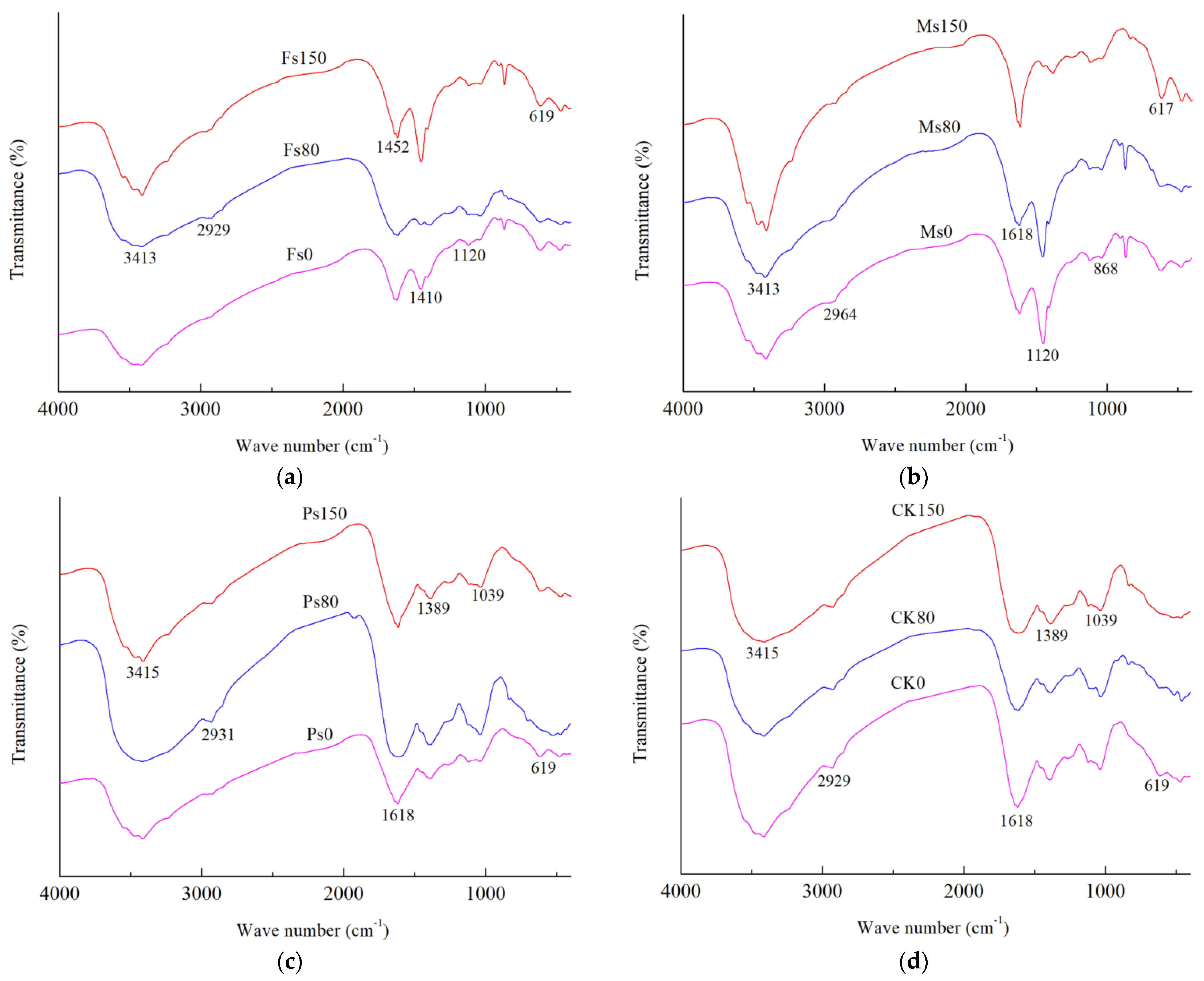
As shown in Figure 6, the HA extracted from the dark-brown soil mixed with Tilia wood shavings showed a broad absorption at 3413~3415 cm−1, indicating a strong –OH absorption peak (O–H group stretching for phenols and alcohols). In contrast, the –OH vibration frequency of HA molecules obtained from the addition of Fe3+ was much higher than that of Mn2+ and K+. The peak in the range of 2927~2966 cm−1 was attributed to the stretching vibrations of –CH3 and –CH2– in aliphatic groups. The absorption peak at 1618~1620 cm−1 was caused by the stretching vibration of aromatic C=C and conjugated carbonyl C=O stretching, indicating the presence of aromatic conjugated double bonds [24]. The peak at 1450~1452 cm−1 likely represented C–H of –CH2– and –CH3 stretching. The band at 1119~1039 cm−1 probably represented the C=O stretching of polysaccharides and carbohydrates [4].
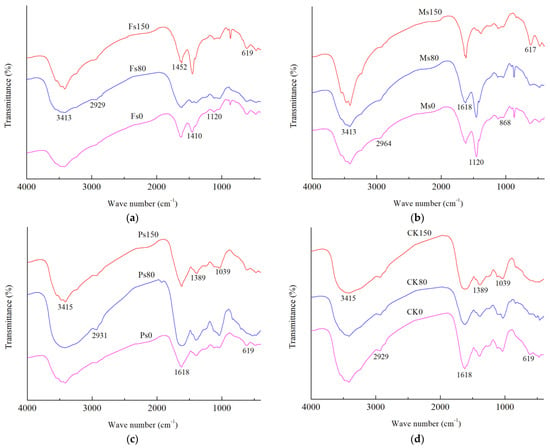
Figure 6.
FTIR spectra of HA extracted from dark-brown soil mixed with Tilia wood shavings. Note: The addition of Fe2(SO4)3, MnSO4, K2SO4 solution and CK control were represented by Fs (a), Ms (b), Ps (c) and CK (d) respectively, and the numbers 0, 80 and 150 after the above letter combination indicated the incubation days under the corresponding treatment, respectively.
As shown in Table 2, regardless of the incubation day, under the condition of adding Fe3+, the intensity of the peak at 3413~3415 cm−1 of HA molecules was much higher than that under the additions of Mn2+, K+, and CK control. Since both of the absorption peaks at 2927~2966 cm−1 and 1450~1452 cm−1 represented aliphatic groups, represented by a and b, respectively, and 1618~1620 cm−1 represented aromatic groups, denoted by c, we could use the ratio of (a + b)/c to compare the ratio of aliphatic C to aromatic C in HA molecules under different incubation days at the same treatment. With incubation, the (a + b)/c ratio of HA molecules had a downward trend under the CK control, while the (a + b)/c ratio of HA molecules increased gradually in the Ps, Ms, and Fs treatments. Compared with the CK control, the addition of metal cations (Fe3+, Mn2+, and K+ ions) made the aromaticity of the HA molecules stronger after incubation. During the whole incubation process, the polysaccharides in HA molecules first increased and then decreased, regardless of whether metal cations were added. Compared with the result at 0 d, the polysaccharide content of HA molecules was slightly decreased after incubation under the condition of adding Mn2+ only.

Table 2.
FTIR relative intensities (% of total area) for the HA extracted from the dark-brown soil mixed with Tilia wood shavings.
3.4. Humification Index (CHA/CFA Ratio)
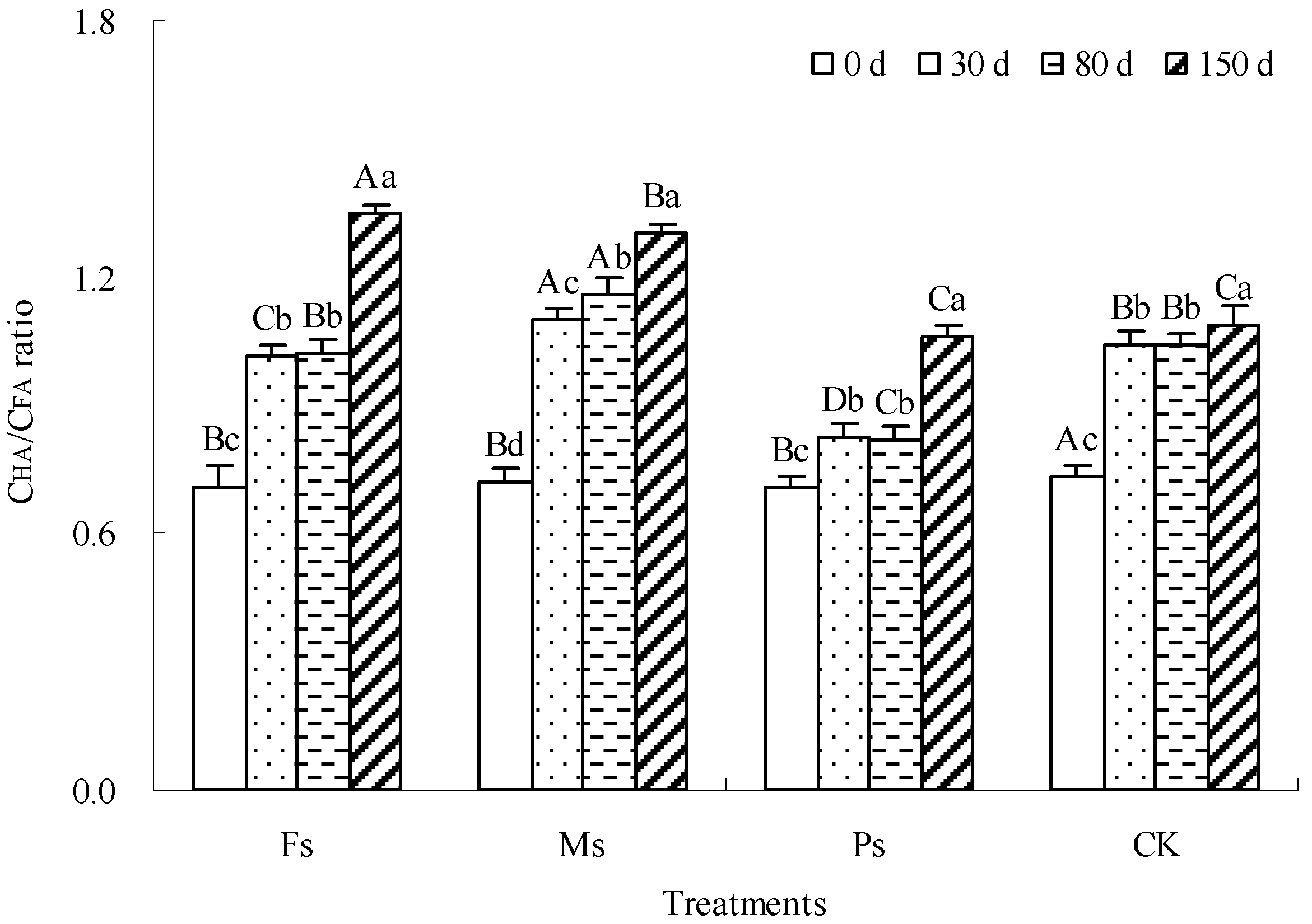
The CHA/CFA ratio is widely used to describe the relative sequestration speed of HA and FA and evaluate the complexity of the HS structure, humification degree, and polymerization degree [22]. The increase in the CHA/CFA ratio suggested the enhancement of the structural complexity and quality of HS [5]. As shown in Figure 7, with the progress of incubation, the CHA/CFA ratio of dark-brown soil mixed with Tilia wood shavings showed a gradual increase under the Fs, Ms, and Ps treatments and the CK control. Compared with the result at 0 d, after the end of incubation, the CHA/CFA ratios of the Fs, Ms, and Ps treatments and CK increased by 90.3%, 81.8%, 49.4%, and 48.5%, respectively, showing that Fs > Ms > Ps > CK. Fe3+ and Mn2+ ions had greater advantages in increasing the CHA/CFA ratio and improving humus quality than K+ ions.
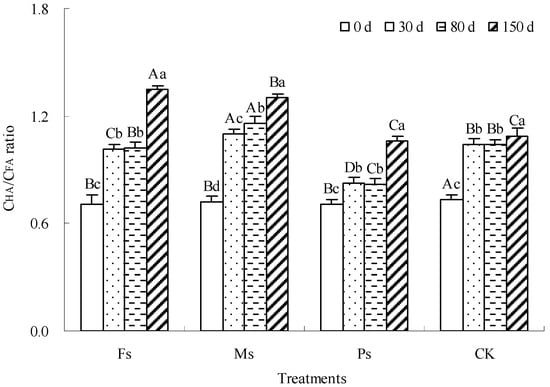
Figure 7.
Effect of metal cations with different valence additions on the CHA/CFA ratio of dark-brown soil mixed with Tilia wood shavings. Different uppercase letters indicate significant differences among different treatments at the same incubation time at the 0.05 level, while different lowercase letters indicate significant differences among different incubation times at the same treatment at the 0.05 level.
3.5. C Content of Humin (CHu)
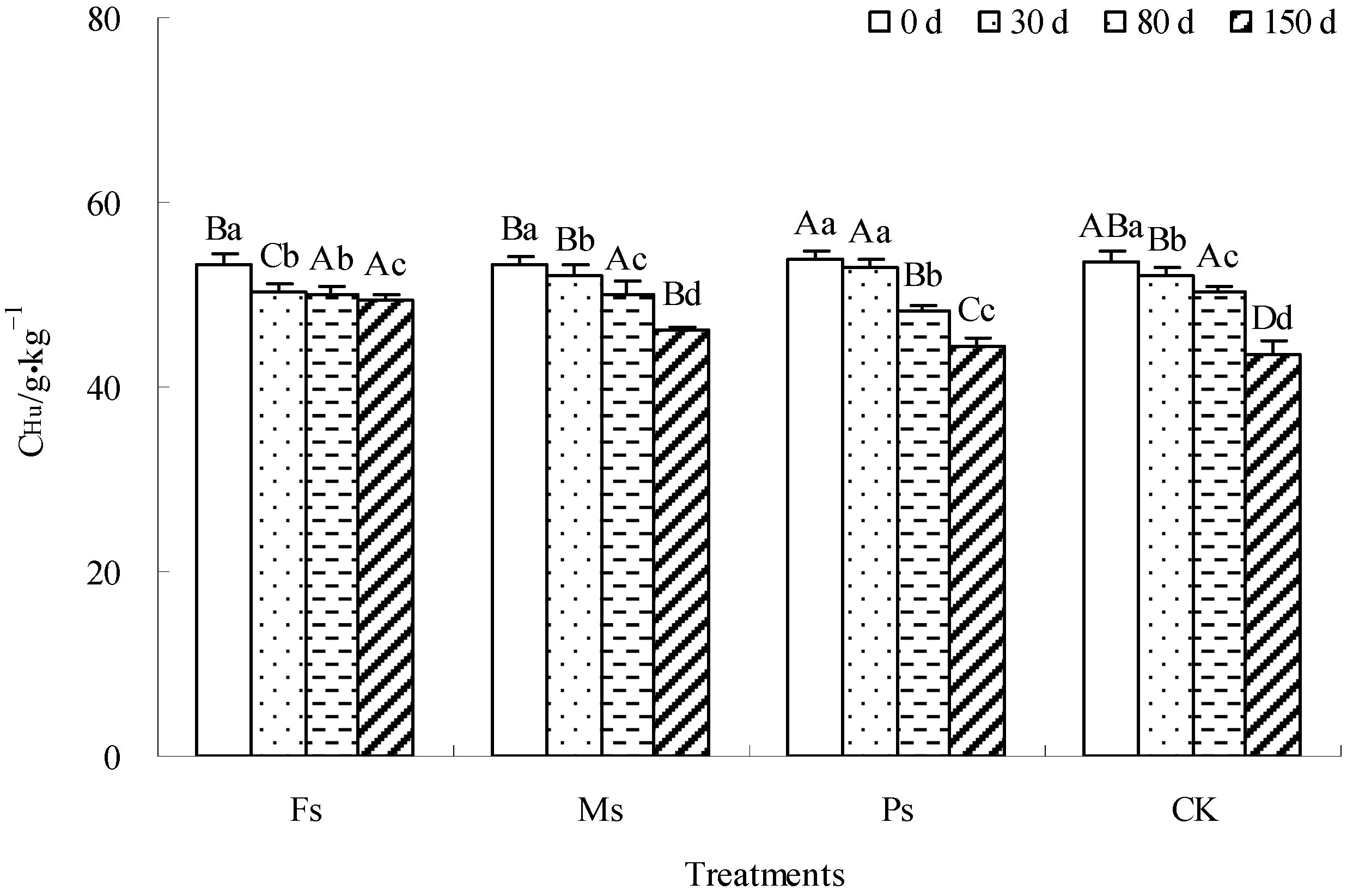
As illustrated in Figure 8, with incubation, the CHu content of dark-brown soil mixed with Tilia wood shavings under Fs, Ms, and Ps treatments and the CK control showed a gradual decrease. Compared with the result at 0 d, after incubation for 150 d, the CHu contents of the Fs, Ms, and Ps treatments and CK decreased by 7.5%, 13.5%, 17.5%, and 19.1%, respectively, and the reduced ranges of CHu content under each treatment were higher than that of the CK control. The addition of metal cations effectively inhibited the mineralization and decomposition of the Hu component, among which Fe3+ ions had the most significant effect, followed by Mn2+ ions.
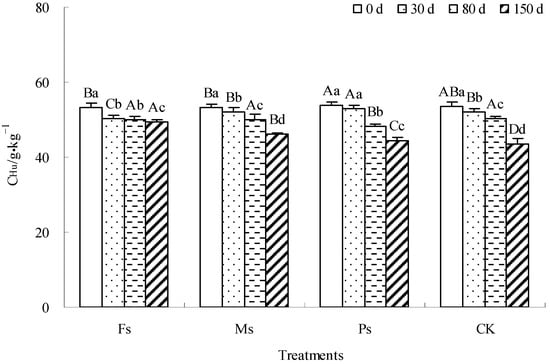
Figure 8.
Effect of metal cations with different valence additions on the CHu of dark-brown soil mixed with Tilia wood shavings. Different uppercase letters indicate significant differences among different treatments at the same incubation time at the 0.05 level, while different lowercase letters indicate significant differences among different incubation times at the same treatment at the 0.05 level.
4. Discussion
4.1. TOC and CWSS
WSS is widely recognized as the main driver of the humification process during organic matter degradation because it contains abundant bioavailable carbohydrate or protein components and can directly provide energy or essential nutrients for microbial metabolism [25]. The addition of Fe3+, Mn2+, or K+ could effectively enhance the utilization and consumption of WSS by microorganisms regardless of their valence, and the degree of utilization was Fe3+ > Mn2+ > K+. Tilia wood shavings as organic inputs could stimulate microbial decomposition of SOM in dark-brown soil (known as the priming effect) while being microbially decomposed and transformed into SOM [26]. Meanwhile, the degradation of Tilia wood shavings in dark-brown soil also produced small-molecule compounds that were humus precursors and promoted humus formation [27]. In this process, microbial reproduction required a large amount of WSS as a carbon and energy source [28], and the intervention of cations could increase the affinity of microorganisms to WSS and reduce the energy consumption of microorganisms utilizing WSS. However, cations could effectively inhibit the further decomposition of Tilia wood shavings in dark-brown soil and reduce the loss of TOC, and the effect could be seen as follows: Fe3+ > Mn2+ > K+. TOC change depended on the trade-off between primed SOM decomposition and the transformation of inputted materials into SOM. Inputs of fresh organic materials (Tilia wood shavings) could also stimulate SOM decomposition, which is known as the priming effect. Aquino et al. [29] reported that polyvalent cations (e.g., Ca2+) could establish more effective cation bridges than monovalent cations, such as Na+. Polyvalent cations such as Al3+, Fe3+, Ca2+, or Mg2+, which are naturally abundant in soils, were able to form cross-linking cation bridges in WSS, which usually results in an increasing rigidity of these compounds [30].
4.2. CHE and ∆logK Value of HE
Although the CHE content first decreased and then increased with incubation, the addition of Fe3+ and Mn2+ ions increased the CHE content, showing that Fe3+ > Mn2+ and that K+ ions had no significant effect. The activities of the cations Fe3+, Mn2+, and K+ exhibit a pronounced gradation according to their charge, which was consistent with the findings reported by Aquino et al. [30]. The presence of Fe3+ or Mn2+ largely enhanced HE sorption on clay particles in comparison to Na+ [12], where a significant contribution of cation bridging was identified in addition to ligand exchange and van der Waals interactions [31]. Interactions between carboxyl and/or phenolic groups in HE and Al3+/Fe3+ enhance HE accumulation [32]. By judging that the ∆logK value of the HE alkaline extract under each treatment decreased first and then increased throughout the incubation, the structure of HE molecules experienced a complex first and then a simple process. Whereas the overall structure of HE molecules tended to be simpler than that of the CK control, HE became more complicated with the addition of Fe3+ and Mn2+; however, the addition of K+ had little effect on the structure of HE molecules. The polyvalent cations (Fe3+ and Mn2+) could cause condensation of HE molecules. This might increase the amount of CHE adsorbed to the clay surface and affect the conformation and orientation of sorbed organic molecules [33]. Direct complexes of carboxylate groups with Fe3+ and Fe2+ would have high directional stability and, once formed, would not be easily broken by water molecules [30]. K+ was not considered to bind specifically to HE, unlike Fe3+ or Mn2+. K+ was considered to control the ionic strength and to affect other metal ion complexation by HE only via electrostatic effects [34].
4.3. Structural Characteristics of HA Molecules
After incubation, the addition of Fe3+, Mn2+, or K+ ions strengthened the molecular condensation of HA and its aromatization degree, while the CK control without any cations tended to decompose HA molecules and obtain a greater degree of aliphatic content. This might be related to the cation binding efficiency of the HA macromolecule. The presence of cations led to the formation of HA-cation coordination complexes and resulted in a decrease in the magnitude of the negative HA surface charge, which decreased the surface negative charge and intermolecular repulsion of HA and thereby increased the polycondensation of HA molecules [35]. The interaction of HA with metal ions induced strong HA-metal complexation. These cations might also enhance aggregation by bridging interactions between different HAs [36]. The addition of polyvalent cations (e.g., Cu2+, Mn2+, and Fe3+) could induce the aggregation of HA particles, and the degree of aggregation was related to the number of cations bonded [37]. Divalent cations exhibited relatively weak bonding with HA compared to trivalent cations. Regarding monovalent cations, they did not bind to HA, and even their outer-sphere coordination with the carboxylate groups was statistically weak [38]. Polyvalent cations have been found to effectively enhance HA coagulation, which could form stable inner-sphere complexes and showed a higher effect than monovalent cations [39]. Multivalent cations were more capable of colloidal aggregation of HA molecules than monovalent cations and could lead to a more compact network of HA with the formation of intra- or intermolecular bridges between negatively charged HA molecules [40].
The O-containing functional groups (e.g., carboxylic, phenolic, or hydroxyl) produced by the decomposition of Tilia wood chips led to a partial entry of the products into the HA component of dark-brown soil, increasing the number of O-containing functional groups of HA to varying degrees regardless of whether cations were added. As in the compost environment, the ultimate fate of HA possessed a range of active chemical functional groups, including phenolic and alcoholic hydroxyl carboxyl, amide, and aldehyde groups, and these groups had a higher content of O-containing functional groups [22]. Some studies have pointed out that chemical additives inhibit the ammoniating process, which could increase the organic nitrogen content (such as amino acids) in the material and promote the synthesis and conversion of intermediate degradation products, such as polyphenols and quinones, to HS [15]. According to this principle, the addition of metal cations could effectively inhibit the ammoniating process, increase N-compounds in HA components, and inhibit mineralization for the carbon fixation effect, so that C/N had different degrees of decline. With incubation, the condensation degree of HA molecules tended to increase under the influence of adding cations. The addition of polyvalent cations could induce the aggregation of HA particles, and the degree of aggregation was related to the number of cations bonded [37]. Transition metals (Fe and Mn) could act as Lewis acids to accept electrons from micromolecular precursors and facilitate nucleophilic addition and polycondensation reactions [20]. Polyvalent cations exhibited a greater capacity to induce aggregation of HA than K+ in terms of microscopic structures and energies [3]. Nevertheless, HA molecules are large, negatively charged polyelectrolytes. This led to an accumulation of cations in the vicinity of HA binding sites [14]. K+ could neutralize the negative charge on the surface of HA molecules to a certain extent and indirectly increase the polycondensation between HA molecules. In this process, the cation is primarily associated with the carboxylic groups of HA but hardly associated with the phenolic and other -OH groups [41].
The microorganisms could improve the conversion efficiency of lignin-rich Tilia wood shavings in dark-brown soil, thus providing more available polyphenolic precursors for humification, which was similar to the results of Zhou et al. [42], where polyphenols were important precursors for humification and had a positive correlation with HA yield. Compared with the CK control without any cation and the addition of Mn2+ and K+, the polyphenol humification pathway enhanced by the microorganisms resulted in a greater increase in the intensity of the peak at 3413~3415 cm−1 of HA molecules under the addition of Fe3+. The aggregations of HA were stabilized by hydrophobic interactions, hydrogen bonds, and the presence of metal ions, such as K+, Mn2+, or Fe3+. In the presence of multivalent cations, aggregation was promoted via charge neutralization and cation bridge formation between different HA molecules [43]. Under this action, with incubation, the condensation degree of HA molecules was further increased, increasing the proportion of aromatic C and decreasing the proportion of aliphatic C. The polysaccharides in HA molecules were mainly affected by microbial activity. At the beginning of the incubation, the mineralization of Tilia wood shavings in the dark-brown soil was dominant, allowing released sugars to enter the HA component, and the mineralization was weakened as the availability of microbial C sources in the substrate decreased. Meanwhile, continuous humification drove the microorganisms to begin utilizing the available polysaccharides in the HA molecules and reduce them somewhat. Compared with the result at 0 d, the polysaccharide content in HA molecules was consumed only when Mn2+ ions were added, while the polysaccharide content in HA molecules accumulated when Fe3+ and K+ ions were added or no cations were added. Under normal conditions, under the influence of humification driven by microorganisms, the degradation products of Tilia wood shavings would recondense and enter the HA component so that the polysaccharide content could be accumulated. In addition to the presence of polyvalent metals, the arrangement of active sites, where electrons are efficiently transferred, determines the activity of enzymes [16]. The transformation of phenolic compounds in laccase (a biotic catalyst related to humus formation) was governed by Mn2+ [23]. Under the addition of Mn2+, affected by laccase activity, the polysaccharides in HA molecules were partially consumed as microbial energy. The presence of K+ did not affect the interactions between HA and laccase molecules because they could not bind to HA [44]. Several Fe-HA interactions occur during Fe redox reactions, where Fe3+ could act as an acceptor of electrons for various oxidant agents, promoting humification and enabling the accumulation of polysaccharides in HA molecules [23].
4.4. CHA/CFA Ratio
FA was more reactive and unstable than HA [18]. FA in virgin materials could be used as energy by microorganisms during composting or could be converted to HA through polycondensation and polymerization reactions, leading to increased HS aromatization and humification [45]. Fe3+ and Mn2+ ions had greater advantages than K+ ions in promoting the conversion of FA to HA, increasing the CHA/CFA ratio, and improving humus quality. In addition, the enhancement of humification by multivalent cations also increased the transformation of FA to HA components, thus improving the humus quality. For monovalent cations such as K+, it was difficult to coordinate with SOM [41]. Therefore, it had no obvious effect on humus quality.
4.5. CHu
Hu is usually considered a major component of SOM; however, it is insoluble and nonreactive in nature, holding a greater degree of polymerization than HA or FA [18]. Therefore, increased Hu content in soil played a pivotal role in increasing the storage of HS and eventually increasing C sequestration [18]. The addition of metal cations could effectively inhibit the mineralization and decomposition of the Hu component, among which Fe3+ ions had the most significant effect, followed by Mn2+ ions. Fe3+ could restrain laccase activity because the formed Fe3+ complex bound with the laccase site, interrupted the binding site of the enzyme, lowered the catalytic activity of fungal laccase, and thus impeded the single-electron transfer system, inhibiting the degradation of Hu by microorganisms [46]. Mn2+ behaved as a cation bridge linking both the negatively charged minerals and humic acid, thereby increasing the stability of the mineral nanoparticles as a result of the steric repulsion of the adsorbed humic acid. These mechanisms promote the formation and accumulation of Hu. The polyvalent metal cations could increase the binding of clay to the Hu component in the dark-brown soil, protecting Hu against microbial degradation [47]. Thus, the protective effect of cation bridging on humic fractions was realized indirectly by means of minerals. Both “labile” and “recalcitrant” materials were physically protected through mineral-organic complexes [48]. Moreover, in the presence of Fe3+ or Mn2+ ions, such functional groups could form a complex with these metal ions, and HA or FA are then adsorbed to Hu by cation-bridging interactions [32]. Under these conditions, the metal bridging occurring between the negative surface sites of HA or FA and the clay in the dark-brown soil itself or the adsorption of HA or FA on the clay [35] might produce new Hu molecules, to a certain extent, making up for the loss in the C content of the Hu component caused by microbial mineralization and decomposition.
5. Conclusions
- (1)
- The addition of metal cations could effectively improve the utilization and consumption of WSS by microorganisms regardless of their valence state, as Fe3+ > Mn2+ > K+. The addition of three metal cations could effectively inhibit mineralization and reduce the loss of TOC, and the effect could be seen as follows: Fe3+ > Mn2+ > K+.
- (2)
- Although the CHE content first decreased and then increased with incubation, the addition of Fe3+ and Mn2+ ions increased the CHE content, showing that Fe3+ > Mn2+ and K+ ions had no significant effect. Throughout the incubation, the structure of HE molecules experienced a complex first and then a simple process. Comparing the change before and after the incubation, the overall structure of HE molecules tended to be simpler in the CK control, and HE became more complicated with the addition of Fe3+ and Mn2+; however, the addition of K+ had little effect on the structure of HE molecules.
- (3)
- After incubation, the addition of Fe3+, Mn2+, and K+ ions strengthened the molecular condensation of HA and its aromatization degree, while the CK control without any added metal cations caused HA molecules to decompose and obtain a greater degree of aliphatic content. In addition, the number of O-containing functional groups and N-containing compounds in HA molecules increased to varying degrees regardless of which metal cation was added. The decomposition of Tilia wood chips led to a partial entry of the decomposition products into the HA component, which was then reconsumed by continuous mineralization. After the end of incubation, the polysaccharides in HA molecules were consumed only with the addition of Mn2+. Fe3+ and Mn2+ ions had greater advantages in increasing the CHA/CFA ratio and improving humus quality than K+ ions.
- (4)
- The addition of metal cations could effectively inhibit the mineralization and decomposition of the Hu component, among which Fe3+ ions had the most significant effect, followed by Mn2+ ions.
Author Contributions
Conceptualization, N.W. and S.W.; methodology, Z.L. and C.B.; software, C.B. and M.L.; validation, N.W., M.L. and Y.W.; formal analysis, Z.L., W.Y. and C.B.; investigation, N.W., C.B., Y.W., S.W., P.L. and R.Y.; resources, S.W.; data curation, N.W., R.Y., P.L. and R.L.; writing—original draft preparation, N.W., Z.L., R.L., M.L., Y.W., W.Y., P.L., R.Y. and S.W.; writing—review and editing, Z.L., R.L., M.L., Y.W., W.Y., P.L., R.Y. and S.W.; visualization, N.W., R.L. and W.Y.; supervision, S.W., P.L. and R.Y.; project administration, N.W. and S.W.; funding acquisition, N.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Science Foundation Project of Jilin Province (grant number 20230101308JC) and the 18th Innovative and Entrepreneurial Talent Funding Project of Jilin Province (Excellence Category) (grant number 2022ZY18).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mouvenchery, Y.K.; Kučerík, J.; Diehl, D.; Schaumann, G.E. Cation-mediated cross-linking in natural organic matter: A review. Rev. Environ. Sci. Bio. 2012, 11, 41–54. [Google Scholar]
- Iskrenova-Tchoukova, E.; Kalinichev, A.G.; Kirkpatrick, R.J. Metal cation complexation with natural organic matter in aqueous solutions: Molecular dynamics simulations and potentials of mean Force. Langmuir 2010, 26, 15909–15919. [Google Scholar]
- Galicia-Andrés, E.; Escalona, Y.; Oostenbrink, C.; Tunega, D.; Gerzabek, M.H. Soil organic matter stabilization at molecular scale: The role of metal cations and hydrogen bonds. Geoderma 2021, 401, 115237. [Google Scholar]
- Merino, C.; Fontaine, S.; Palma, G.; Matus, F. Effect of aluminium on mineralization of water extractable organic matter and microbial respiration in southern temperate rainforest soils. Eur. J. Soil Biol. 2017, 82, 56–65. [Google Scholar]
- Kraal, P.; Nierop, K.G.J.; Kaal, J.; Tietema, A. Carbon respiration and nitrogen dynamics in Corsican pine litter amended with aluminum and tannins. Soil Biol. Biochem. 2009, 41, 2318–2327. [Google Scholar]
- Percival, H.J.; Parfifitt, R.L.; Scott, N.A. Factors controlling soil carbon levels in New Zealand grasslands: Is clay content important? Soil Sci. Soc. Am. J. 2000, 64, 1623–1630. [Google Scholar]
- Wang, Q.; Yang, P.; Zhu, M.Q. Effects of metal cations on coupled birnessite structural transformation and natural organic matter adsorption and oxidation. Geochim. Cosmochim. Ac. 2019, 250, 292–310. [Google Scholar]
- Sutton, R.; Sposito, G. Molecular simulation of humic substance-Ca-montmorillonite complexes. Geochim. Cosmochim. Ac. 2006, 70, 3566–3581. [Google Scholar]
- Rakhsh, F.; Golchin, A.; Agha, A.B.A.; Alamdari, P. Effects of exchangeable cations, mineralogy and clay content on the mineralization of plant residue carbon. Geoderma 2017, 307, 150–158. [Google Scholar]
- Fukuchi, S.; Miura, A.; Okabe, R.; Fukushima, M.; Sasaki, M.; Sato, T. Spectroscopic investigations of humic-like acids formed via polycondensation reactions between glycine, catechol and glucose in the presence of natural zeolites. J. Mol. Struct. 2010, 982, 181–186. [Google Scholar]
- Takashi, K.; Ikuko, I.; Hirotaka, S.; Ho-Dong, P.; Hideshige, T.; Shigeto, O.; Kazunari, N.; Kazutoshi, S.; Keishi, S. Aluminum and acidity suppress microbial activity and biomass in acidic forest soils. Soil Biol. Biochem. 2016, 97, 23–30. [Google Scholar]
- Feng, X.J.; Simpson, A.J.; Simpson, M.J. Chemical and mineralogical controls on humic acid sorption to clay mineral surfaces. Org. Geochem. 2005, 36, 1553–1566. [Google Scholar] [CrossRef]
- Cheng, H.J.; Yang, T.; Jiang, J.; Lu, X.H.; Wang, P.X.; Ma, J. Mn2+ effect on manganese oxides (MnOx) nanoparticles aggregation in solution: Chemical adsorption and cation bridging. Environ. Pollut. 2020, 267, 115561. [Google Scholar] [CrossRef] [PubMed]
- Iorio, E.D.; Circelli, L.; Angelico, R.; Torrent, J.; Tan, W.F.; Colombo, C. Environmental implications of interaction between humic substances and iron oxide nanoparticles: A review. Chemosphere 2022, 303, 135172. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Ji, K.; Su, L.H.; Wu, M.T.; Zhou, X.J.; Duan, E.S. Effects of FeSO4 dosage on nitrogen loss and humification during the composting of cow dung and corn straw. Bioresour. Technol. 2021, 341, 125867. [Google Scholar] [CrossRef]
- Bui, V.K.H.; Truong, H.B.; Hong, S.; Li, X.W.; Hur, J. Biotic and abiotic catalysts for enhanced humification in composting: A comprehensive review. J. Clean. Prod. 2023, 402, 136832. [Google Scholar] [CrossRef]
- Zhang, X.M.; Zong, Y.; Xu, L.Q.; Mao, Y.F.; Wu, D.L. Enhanced abiotic integrated polyphenol-Maillard humification by Mg/Fe layered double hydroxide (LDH): Role of Fe(III)-polyphenol complexation. Chem. Eng. J. 2021, 425, 130521. [Google Scholar] [CrossRef]
- Tiwari, J.; Ramanathan, A.; Bauddh, K.; Korstad, J. Humic substances: Structure, function and benefits for agroecosystems—A review. Pedosphere 2023, 33, 237–249. [Google Scholar]
- Paul, E.A. The nature and dynamics of soil organic matter: Plant inputs, microbial transformations, and organic matter stabilization. Soil Biol. Biochem. 2016, 98, 109–126. [Google Scholar]
- Miura, A.; Okabe, R.; Izumo, K.; Fukushima, M. Influence of the physicochemical properties of clay minerals on the degree of darkening via polycondensation reactions between catechol and glycine. Appl. Clay Sci. 2009, 46, 277–282. [Google Scholar] [CrossRef]
- Liu, M.M.; Zhao, Z.Y.; Lu, Q.X.; Yu, W.Z. Release of dissolved organic carbon from biochar and formation of humic-like component during photoreaction: Effects of Ca2+ and pH. Water Res. 2022, 219, 118616. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.C.; Xu, Y.; Yu, X.L.; Li, J.K.; Chen, G.; Wang, S.J.; Xu, Y.P.; Xu, R.; Zhang, B.H.; Zhang, H.Q. Microbial metabolism and humic acid formation in response to enhanced copper and zinc passivation during composting of wine grape pomace and pig manure. Bioresour. Technol. 2023, 384, 129226. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.S.; Wang, J.L.; Zhang, L.Y.; Chen, L.H.; Zhao, Y.; Wei, Z.M. Activation effect of catechol on biotic and abiotic factors of humus formation during chicken manure composting. Waste Manag. 2022, 149, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.X.; Li, Z.C.; Sun, Y.; Zhang, J.M.; Ge, Y.Y.; Li, Z.L. A comprehensive review on biomass humification: Recent advances in pathways, challenges, new applications, and perspectives. Renew. Sust. Energy Rev. 2022, 170, 112984. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, R.N.; Li, D.Y.; Qi, C.R.; Han, L.N.; Chen, M.; Li, G.X. Effects of calcium magnesium phosphate fertilizer, biochar and spent mushroom substrate on compost maturity and gaseous emissions during pig manure composting. J. Environ. Manag. 2020, 267, 110649. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Yao, S.H.; Cao, X.Y.; Schmidt-Rohr, K.; Olk, D.C.; Mao, J.D.; Zhang, B. Structural evidence for soil organic matter turnover following glucose addition and microbial controls over soil carbon change at different horizons of a Mollisol. Soil Biol. Biochem. 2018, 119, 63–73. [Google Scholar] [CrossRef]
- Tao, Z.D.; Liu, X.C.; Sun, L.L.; He, X.X.; Wu, Z.S. Effects of two types nitrogen sources on humification processes and phosphorus dynamics during the aerobic composting of spent mushroom substrate. J. Environ. Manag. 2022, 317, 115453. [Google Scholar] [CrossRef]
- Zhu, N.; Zhu, Y.; Li, B.; Jin, H.; Dong, Y. Increased enzyme activities and fungal degraders by Gloeophyllum trabeum inoculation improve lignocellulose degradation efficiency during manure-straw composting. Bioresour. Technol. 2021, 337, 125427. [Google Scholar] [CrossRef]
- Aquino, A.J.A.; Tunega, D.; Pašalić, H.; Haberhauer, G.; Gerzabek, M.H.; Lischka, H. The thermodynamic stability of hydrogen bonded and cation bridged complexes of humic acid models—A theoretical study. Chem. Phys. 2008, 349, 69–76. [Google Scholar] [CrossRef]
- Aquino, A.J.A.; Tunega, D.; Schaumann, G.E.; Haberhauer, G.; Gerzabek, M.H.; Lischka, H. Proton transfer processes in polar regions of humic substances initiated by aqueous aluminum cation bridges: A computational study. Geoderma 2014, 213, 115–123. [Google Scholar] [CrossRef]
- Majzik, A.; Tombácz, E. Interaction between humic acid and montmorillonite in the presence of calcium ions I. Interfacial and aqueous phase equilibria: Adsorption and complexation. Org. Geochem. 2007, 38, 1319–1329. [Google Scholar] [CrossRef]
- Murano, H.; Suzuki, K.; Kayada, S.; Saito, M.; Yuge, N.; Arishiro, T.; Watanabe, A.; Isoi, T. Influence of humic substances and iron and aluminum ions on the sorption of acetamiprid to an arable Soil. Sci. Total Environ. 2018, 615, 1478–1484. [Google Scholar] [CrossRef] [PubMed]
- Martinez, R.E.; Sharma, P.; Kappler, A. Surface binding site analysis of Ca2+-homoionized clay–humic acid complexes. J. Colloid Interf. Sci. 2010, 352, 526–534. [Google Scholar] [CrossRef]
- Marsac, R.; Banik, N.L.; Lützenkirchen, J.; Catrouillet, C.; Marquardt, C.M.; Johannesson, K.H. Modeling metal ion-humic substances complexation in highly saline conditions. Appl. Geochem. 2017, 79, 52–64. [Google Scholar] [CrossRef]
- Akbour, R.A.; Amal, H.; Ait-Addi, A.; Douch, J.; Jada, A.; Hamdani, M. Transport and retention of humic acid through natural quartz sand:Influence of the ionic strength and the nature of divalent cation. Colloids Surf. A Physicochem. Eng. Asp. 2013, 436, 589–598. [Google Scholar] [CrossRef]
- Ai, Y.J.; Zhao, C.F.; Sun, L.; Wang, X.K.; Liang, L.J. Coagulation mechanisms of humic acid in metal ions solution under different pH conditions: A molecular dynamics simulation. Sci. Total Environ. 2020, 702, 135072. [Google Scholar] [CrossRef]
- Qi, Y.B.; Zhu, J.; Fu, Q.L.; Hu, H.Q.; Huang, Q.Y. Sorption of Cu by humic acid from the decomposition of rice straw in the absence and presence of clay minerals. J. Environ. Manag. 2017, 200, 304–311. [Google Scholar] [CrossRef]
- Adusei-Gyamfi, J.; Ouddane, B.; Rietveld, L.; Cornard, J.P.; Criquet, J. Natural organic matter-cations complexation and its impact on water treatment: A critical review. Water Res. 2019, 160, 130–147. [Google Scholar] [CrossRef]
- Wang, L.F.; Wang, L.L.; Ye, X.D.; Li, W.W.; Ren, X.M.; Sheng, G.P.; Yu, H.Q.; Wang, X.K. Coagulation kinetics of humic aggregates in mono- and di-valent electrolyte solutions. Environ. Sci. Technol. 2013, 47, 5042–5049. [Google Scholar] [CrossRef]
- Baalousha, M.; Motelica-Heino, M.; le Coustumer, P. Conformation and size of humic substances: Effects of major cation concentration and type, pH, salinity, and residence time. Colloids Surf. A Physicochem. Eng. Asp. 2006, 272, 48–55. [Google Scholar] [CrossRef]
- Xing, Y.H.; Li, X.; Wu, Z.D.; Feng, H.T.; Xue, X.; Xie, L.C.; Zhang, T.Y.; Zhang, J.G. Retention of organic matter on the surface of illite particle under the influence of different cations: A molecular dynamics simulation study. Appl. Clay Sci. 2023, 232, 106810. [Google Scholar] [CrossRef]
- Zhou, X.L.; Li, J.B.; Zhang, J.; Deng, F.; Chen, Y.C.; Zhou, P.; Li, D. Bioaugmentation mechanism on humic acid formation during composting of food waste. Sci. Total Environ. 2022, 830, 154783. [Google Scholar] [CrossRef] [PubMed]
- Kloster, N.; Brigante, M.; Zanini, G.; Avena, M. Aggregation kinetics of humic acids in the presence of calcium ions. Colloids Surf. A Physicochem. Eng. Asp. 2013, 427, 76–82. [Google Scholar] [CrossRef]
- Lu, J.H.; Shi, Y.Y.; Ji, Y.F.; Kong, D.Y.; Huang, Q.G. Transformation of triclosan by laccase catalyzed oxidation: The influence of humic acid-metal binding process. Environ. Pollut. 2017, 220, 1418–1423. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.L.; Deng, F.; Wang, R.X.; Li, J.B.; Liu, X.F.; Li, D. Bioaugmentation on humification during co-composting of corn straw and biogas slurry. Bioresour. Technol. 2023, 374, 128756. [Google Scholar] [CrossRef]
- Li, S.Y.; Liu, Q.Z.; Liu, J.; Sun, K.; Yang, W.; Si, Y.B.; Li, Y.C.; Gao, Y.Z. Inhibition mechanisms of Fe2+/Fe3+ and Mn2+ on fungal laccase-enabled bisphenol a polyreaction. Chemosphere 2022, 307, 135685. [Google Scholar] [CrossRef]
- Zech, W.; Senesi, N.; Guggenberger, G.; Kaiser, K.; Lehmann, J.; Miano, T.M.; Miltner, A.; Schroth, G. Factors controlling humification and mineralization of soil organic matter in the tropics. Geoderma 1997, 79, 117–161. [Google Scholar] [CrossRef]
- Keiluweit, M.; Bougoure, J.J.; Nico, P.S.; Pett-Ridge, J.; Weber, P.K.; Kleber, M. Mineral protection of soil carbon counteracted by root exudates. Nat. Clim. Change 2015, 5, 588–595. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).