Waste-Derived Fertilizer Acts as Biostimulant, Boosting Tomato Quality and Aroma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site and Soil Details
2.2. Sample Preparation
2.3. Preparation of Ethanol and Water Extracts
2.4. Total Soluble Proteins
2.5. Total Available Carbohydrates
2.6. Total Water-Soluble Phenols, Ascorbic Acid, Total Carotenoids, Total Flavonoids, and Vitamin E
2.7. Determination of Antioxidant Activities
2.8. Ultra-Fast Gas Chromatography Analysis
2.9. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations (FAO). Available online: http://www.fao.org/faostat/en/#home (accessed on 1 January 2021).
- Ilahya, R.; Hdiderb, C.; Lenucci, M.S.; Tlili, I.; Dalessandro, G. Phytochemical composition and antioxidant activity of high-lycopene tomato (Solanum lycopersicum L.) cultivars grown in Southern Italy. Sci. Hortic. 2011, 127, 255–261. [Google Scholar] [CrossRef]
- Pinela, J.; Barros, L.; Carvalho, A.M.; Ferreira, I.C.F.R. Nutritional composition and antioxidant activity of four tomato (Lycopersicon esculentum L.) farmer’ varieties in Northeastern Portugal homegardens. Food Chem. Toxicol. 2012, 50, 829–834. [Google Scholar] [PubMed]
- Martirosyan, D.; Miller, E. Bioactive Compounds: The Key to Functional Foods. Bioact. Compd. Health Dis. 2018, 1, 36–39. [Google Scholar] [CrossRef]
- Agarwal, S.; Rao, A.V. Tomato lycopene and its role in human health and chronic diseases. Can. Med. Assoc. J. 2000, 163, 739–744. [Google Scholar]
- Habibi, A.; Heidari, G.; Sohrabia, Y.; Badakhshan, H.; Mohammadi, K. Influence of bio, organic and chemical fertilizers on medicinal pumpkin traits. J. Med. Plants Res. 2011, 523, 5590–5597. [Google Scholar]
- Dumas, Y.; Dadomo, M.; Di Lucca, G.; Grolier, P. Effects of environmental factors and agricultural techniques on antioxidant content of tomatoes. J. Sci. Food Agric. 2003, 83, 369–382. [Google Scholar]
- Young, J.W.; Mau, J.L.; Ko, P.T.; Huang, L.C. Antioxidant properties of fermented soybean broth. Food Chem. 2000, 71, 249–254. [Google Scholar] [CrossRef]
- Verma, A.; Sharma, R.; Kumar, C.; Kaur, A.; Arora, R.; Shah, L. Nain Improvement of antioxidant and defense properties of Tomato (var. Pusa rohini) by application of bioaugmented compost. Saudi J. Biol. Sci. 2015, 22, 256–264. [Google Scholar] [CrossRef]
- Jin, N.; Jin, L.; Wang, S.; Li, J.; Liu, F.; Liu, Z.; Luo, S.; Wu, Y.; Lyu, J.; Yu, J. Reduced Chemical Fertilizer Combined with Bio-Organic Fertilizer Affects the Soil Microbial Community and Yield and Quality of Lettuce. Front. Microbiol. 2022, 21, 863325. [Google Scholar] [CrossRef]
- Moradzadeh, S.; Moghaddam, S.S.; Rahimi, A.; Pourakbar, L.; Sayyed, R.Z. Combined bio-chemical fertilizers ameliorate agro-biochemical attributes of black cumin (Nigella sativa L.). Sci. Rep. 2021, 11, 11399. [Google Scholar] [CrossRef]
- Akiyama, T.; Shimo, Y.; Yanai, H.; Qin, J.; Ohshima, D.; Maruyama, Y.; Asaumi, Y.; Kitazawa, J.; Takayanagi, H.; Penninger, J.M.; et al. The tumor necrosis factor family receptors RANK and CD40 cooperatively establish the thymic medullary microenvironment and self-tolerance. Immunity 2008, 29, 423–437. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.L.d.S.; Trevizam, A.R.; Piccolo, M.C.; Furlan, G. Tomato production in function of sulfur doses application. Rev. Bras. Tecnol. Apl. Ciências Agrárias 2014, 7, 47–54. [Google Scholar] [CrossRef]
- FAO-UNESCO. Word Soil Map, Revised Legend; FAO-UNESCO: Rome, Italy, 1999. [Google Scholar]
- Kang, M.C.; Kim, S.-Y.; Kim, E.-A.; Lee, J.-H.; Kim, Y.-S.; Yu, S.-K.; Chae, J.B.; Choe, I.-H.; Cho, J.H.; Jeon, Y.-J. Antioxidant activity of polysaccharide purified from Acanthopanax koreanum Nakai stems in vitro and in vivo zebrafish model. Carb. Polym. 2015, 127, 38–46. [Google Scholar] [CrossRef]
- Muscolo, A.; Calderao, A.; Papalia, T.; Settineri, G.; Mallamaci, C.; Panuccio, M.R. Soil salinity improves nutritional and health promoting compounds in three varieties of lentil (Lens culinaris Med.). Food Biosci. 2020, 35, 100571. [Google Scholar] [CrossRef]
- Velioglu, Y.S.; Mazza, M.; Gao, L.; Oomah, B.D. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J. Agric. Food Chem. 1998, 46, 4113–4117. [Google Scholar] [CrossRef]
- Davies, S.H.R.; Masten, S.J. Spectrophotometric method for ascorbic acid using dichlo-rophenolindophenol: Elimination of the interference due to iron. Anal. Chim. Acta 1991, 248, 225–227. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Djeridane, A.; Yousfi, M.; Nadjemi, B.; Boutassouna, D.; Stocker, P.; Vidal, N. Antioxidant activity of some Algerian medicinal plants extracts containing phenolic compounds. Food Chem. 2006, 97, 654–660. [Google Scholar] [CrossRef]
- Zhang, B.; Deng, Z.; Tang, Y.; Chen, P.; Liu, R.; Ramdath, D.D.; Liu, Q.; Hernandez, M.; Tsao, R. Fatty acid, carotenoid and tocopherol compositions of 20 Canadian lentil cultivars and synergistic contribution to antioxidant activities. Food Chem. 2014, 161, 296–304. [Google Scholar] [CrossRef]
- Narayan, O.P.; Kumar, P.; Yadav, B.; Dua, M.; Atul Kumar Johri, A.K. Sulfur nutrition and its role in plant growth and development. Plant Sign. Behav. 2022, 2030082. [Google Scholar] [CrossRef]
- Zenda, T.; Liu, S.; Dong, A.; Duan, H. Revisiting Sulphur—The Once Neglected Nutrient: It’s Roles in Plant Growth, Metabolism, Stress Tolerance and Crop Production. Agriculture 2021, 11, 626. [Google Scholar] [CrossRef]
- Ranadev, P.; Revanna, A.; Bagyaraj, D.J.; Shinde, A.H. Sulfur oxidizing bacteria in agro ecosystem and its role in plant productivity—A review. J. Appl. Microbiol. 2023, 134, lxad161. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.A.H.; Sultana, T.; Rahman, M.A.; Saha, B.K.; Chowdhury, T.; Tarafder, S. Sulphur fertilization enhanced yield, its uptake, use efficiency and economic returns of Aloe vera L. Heliyon 2020, 18, e05726. [Google Scholar] [CrossRef] [PubMed]
- De Pascale, S.; Maggio, A.; Pernice, R.; Fogliano, V.; Barbieri, G. Sulphur fertilization may improve the nutritional value of Brassica rapa L. subsp. sylvestris. Eur. J. Agron. 2007, 26, 418–424. [Google Scholar] [CrossRef]
- Yanar, D.; Geboloğlu, N.; Yanar, Y.; Aydin, M.; Çakmak, P. Effect of different organic fertilizers on yield and fruit quality of ındeterminate tomato (Lycopersicon esculentum). Sci. Res. Essays 2011, 6, 3623–3628. [Google Scholar] [CrossRef]
- Ibañez, T.B.; de Melo Santos, L.F.; de Marcos, A.; Igor, L.; Ribeiro, V.; Ribeiro, F.V.; dos Reis, A.R.; Adônis Moreira, A.; Heinrichs, R. Sulfur modulates yield and storage proteins in soybean grains. Soil Plant Nutr. 2021, 78, 1–9. [Google Scholar] [CrossRef]
- Degryse, F.; Baird, R.; da Silva, R.C.; Holzapfel, C.B.; Kappes, C.; Tysko, M.; McLaughlin, M.J. Sulfur Uptake from Fertilizer Fortified with Sulfate and Elemental S in Three Contrasting Climatic Zones. Agronomy 2020, 10, 1035. [Google Scholar] [CrossRef]
- Thangasamy, A.; Kalyani, G.; Pranjali, H.; Ghodke, S.; Ahammed, T.P.; Manjusha, J.; Kaushik, B.; Major, S. Effects of sulfur fertilization on yield, biochemical quality, and thiosulfinate content of garlic. Sci. Hortic. 2021, 289, 110442. [Google Scholar] [CrossRef]
- Nawirska-Olszanska, A.; Biesiada, A.; Kita, A. Effect of Different Forms of Sulfur Fertilization on Bioactive Components andv Antioxidant Activity of White Cabbage (Brassica Oleracea L.). Appl. Sci. 2021, 11, 8784. [Google Scholar] [CrossRef]
- Judita, B.; Petra, K.; Alena, V.; Ján, T.; Matyáš, O. The role of sulphur on the content of total polyphenols and antioxidant activity in onion (Allium cepa L.). Potravinarstvo 2014, 8, 284–289. [Google Scholar]
- Muscolo, A.; Papalia, T.; Settineri, G.; Mallamaci, C.; Panuccio, M.R. Sulfur bentonite-organic-based fertilizers as tool for improving bio-compounds with antioxidant activities in red onion. J. Sci. Food Agric. 2019, 100, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Montesano, V.; Negro, D.; Sonnante, G.; Laghetti, G.; Urbano, M. Polyphenolic Compound Variation in Globe Artichoke Cultivars as Affected by Fertilization and Biostimulants Application. Plants 2022, 11, 2067. [Google Scholar] [CrossRef] [PubMed]
- Tietel, Z.; Yermiyahu, U.; Bar-Tal, A. Sulfate Fertilization Preserves Tomato Fruit Nutritional Quality. Agronomy 2022, 12, 1117. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 2018, 25, 93. [Google Scholar] [CrossRef]
- Mutha, R.E.; Tatiya, A.U.; Surana, S.J. Flavonoids as natural phenolic compounds and their role in therapeutics: An overview. Futur. J. Pharm. Sci. 2021, 7, 25. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef]
- Tchonkouang, R.D.N.; Antunes, M.D.C.; Vieira, M.M.C. Potential of Carotenoids from Fresh Tomatoes and Their Availability in Processed Tomato-Based Products. In Carotenoids—New Perspectives and Application; Martínez-Espinosa, R.M., Ed.; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Mozos, I.; Stoian, D.; Caraba, A.; Malainer, C.; Horbańczuk, J.O.; Atanasov Atanas, G. Lycopene and Vascular Health. Front. Pharmacol. 2018, 9, 521. Available online: https://www.frontiersin.org/articles/10.3389/fphar.2018.00521 (accessed on 23 May 2018). [CrossRef]
- Arballo, J.; Jaume, A.; John, W.E. Lycopene: A Critical Review of Digestion, Absorption, Metabolism, and Excretion. Antioxidants 2021, 10, 342. [Google Scholar] [CrossRef]
- Ferraz, C.R.; Carvalho, T.T.; Manchope, M.F.; Artero, N.A.; Rasquel-Oliveira, F.S.; Fattori, V.; Casagrande, R.; Verri, W.A., Jr. Therapeutic Potential of Flavonoids in Pain and Inflammation: Mechanisms of Action, Pre-Clinical and Clinical Data, and Pharmaceutical Development. Molecules 2020, 25, 762. [Google Scholar] [CrossRef]
- Feng, X.; Weng, D.; Zhou, F.; Owen, Y.D.; Qin, H.; Zhao, J.; Huang, Y.; Chen, J.; Fu, H.; Yang, N.; et al. Activation of PPARgamma by a Natural Flavonoid Modulator, Apigenin Ameliorates Obesity-Related Inflammation Via Regulation of Macrophage Polarization. EBioMedicine 2016, 9, 61–76. [Google Scholar] [CrossRef]
- Pan, L.; Ye, H.; Pi, X.; Liu, W.; Wang, Z.; Zhang, Y.; Zheng, J. Effects of several flavonoids on human gut microbiota and its metabolism by in vitro simulated fermentation. Front. Microbiol. 2023, 14, 1092729. [Google Scholar] [CrossRef] [PubMed]
- Rosa, S.I.; Rios-Santos, F.; Balogun, S.O.; Martins, D.T. Vitexin reduces neutrophil migration to inflammatory focus by down-regulating pro-inflammatory mediators via inhibition of p38, ERK1/2 and JNK pathway. Phytomedicine 2016, 23, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Han, Y.; Zhou, Q.; Jie, H.; He, Y.; Han, J.; He, J.; Jiang, Y.; Sun, E. Apigenin, a potent suppressor of dendritic cell maturation and migration, protects against collagen-induced arthritis. J. Cell Mol. Med. 2016, 20, 170–180. [Google Scholar] [CrossRef]
- Yang, H.; Huang, J.; Mao, Y.; Wang, L.; Li, R.; Ha, C. Vitexin alleviates interleukin-1beta-induced inflammatory responses in chondrocytes from osteoarthritis patients: Involvement of HIF-1alpha pathway. Scand. J. Immunol. 2019, 90, e12773. [Google Scholar] [CrossRef] [PubMed]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatonien, J. Flavonoids as Anticancer Agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef] [PubMed]
- Buttery, R.G.; Ling, L.C. Volatile Components of Tomato Fruit and Plant Parts. In Bioactive Volatile Compounds from Plants; Teranishi, R., Buttery, R.G., Sugisawa, H., Eds.; American Chemical Society: Washington, DC, USA, 1993; pp. 23–34. [Google Scholar]
- Wang, L.; Baldwin, E.A.; Bai, J. Recent Advance in Aromatic Volatile Research in Tomato Fruit: The Metabolisms and Regulations. Food Bioprocess Technol. 2016, 9, 203–216. [Google Scholar] [CrossRef]
- Zhan, P.; Tian, H.; Sun, B.; Zhang, Y.; Chen, H. Quality control of mutton by using volatile compound fingerprinting techniques and chemometric methods. J. Food Qual. 2017, 2017, 9273929. [Google Scholar] [CrossRef]
- Muscolo, A.; Papalia, T.; Settineri, G.; Mallamaci, C.; Carabetta, S.; Di Sanzo, R.; Russo, M.T. Effect of organic fertilizers on selected health beneficial bioactive compounds and aroma profile of Topepo sweet pepper. Foods 2020, 9, 1323. [Google Scholar] [CrossRef]
- Korčok, M.; Vietorisová, N.; Martišová, P.; Štefániková, J.; Mravcová, A.; Vietoris, V. Aromatic Profile of Hydroponically and Conventionally Grown Tomatoes. Appl. Sci. 2021, 11, 8012. [Google Scholar] [CrossRef]
- Wakai, J.; Kusama, S.; Nakajima, K. Effects of trans-2-hexenal and cis-3-hexenal on post-harvest strawberry. Sci. Rep. 2019, 9, 10112. [Google Scholar] [CrossRef]







 (CTR), nitrogen–phosphorous–potassium fertilized soil
(CTR), nitrogen–phosphorous–potassium fertilized soil  (NPK), soil with sulfur bentonite mixed with orange residue
(NPK), soil with sulfur bentonite mixed with orange residue  (SB), and soil with horse manure
(SB), and soil with horse manure  (HM). The chart discriminates based on the following odorous compounds: acetaldehyde (13,56-1-A), 3-heptanol (49,70-1-A), ethyl hexanoate (61,40-1-A), (Z)-2-octanal (66,63-1-A), unknown compound (10,68-2-A), butane-2,3-dione (38,74-2-A), hexanal (56,36-2-A), and 1-nonanol (90,80-2-A).
(HM). The chart discriminates based on the following odorous compounds: acetaldehyde (13,56-1-A), 3-heptanol (49,70-1-A), ethyl hexanoate (61,40-1-A), (Z)-2-octanal (66,63-1-A), unknown compound (10,68-2-A), butane-2,3-dione (38,74-2-A), hexanal (56,36-2-A), and 1-nonanol (90,80-2-A).
 (CTR), nitrogen–phosphorous–potassium fertilized soil
(CTR), nitrogen–phosphorous–potassium fertilized soil  (NPK), soil with sulfur bentonite mixed with orange residue
(NPK), soil with sulfur bentonite mixed with orange residue  (SB), and soil with horse manure
(SB), and soil with horse manure  (HM). The chart discriminates based on the following odorous compounds: acetaldehyde (13,56-1-A), 3-heptanol (49,70-1-A), ethyl hexanoate (61,40-1-A), (Z)-2-octanal (66,63-1-A), unknown compound (10,68-2-A), butane-2,3-dione (38,74-2-A), hexanal (56,36-2-A), and 1-nonanol (90,80-2-A).
(HM). The chart discriminates based on the following odorous compounds: acetaldehyde (13,56-1-A), 3-heptanol (49,70-1-A), ethyl hexanoate (61,40-1-A), (Z)-2-octanal (66,63-1-A), unknown compound (10,68-2-A), butane-2,3-dione (38,74-2-A), hexanal (56,36-2-A), and 1-nonanol (90,80-2-A).

| CTR | NPK | HM | SB | |
|---|---|---|---|---|
| WC | 90.7 a* | 89.2 a | 90.7 a | 89.7 a |
| Dry weight | 9.3 a | 10.8 a | 9.3 a | 10.3 a |
| Fresh weight | 86 b | 95 a | 93 a | 92 a |
| TPRO | 1.2 b | 1.3 ab | 1.5 a | 1.7 a |
| TCAR | 17 c | 16 c | 21 b | 24 a |
| LIC | 14 d | 19 c | 23 b | 26 a |
| TCARB | 2.2 b | 2.4 ab | 2.6 a | 2.8 a |
| TPHE | 181.8 b | 190.2 b | 125.4 c | 204.7 a |
| TFLA | 361.8 d | 389.9 c | 511.3 b | 533.3 a |
| VITA | 132.5 b | 137.3 ab | 122.9 c | 180.4 a |
| VITC | 33 c | 35 b | 38 b | 44 a |
| VITE | 0.125 a | 0.116 a | 0.125 a | 0.124 a |
| TAC | 1.83 b | 1.91 b | 2.01 b | 2.25 a |
| ABTS | 0.018 b | 0.029 a | 0.032 a | 0.035 a |
| DPPH% | 43.9 a | 36.6 b | 45.5 a | 37.2 b |
| DPPH | 7.7 b | 5.4 c | 8.18 a | 5.5 c |
| Variables | TPRO | TCAR | LIC | TCARB | TPHE | TFLA | VITA | VITC | VITE | TAC | ABTS | DPPH % | DPPH |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TPRO | 1 | 0.956 | 0.969 | 0.990 | 0.044 | 0.961 | 0.700 | 0.987 | 0.293 | 1 | 0.882 | −0.273 | −0.273 |
| TCAR | 0.956 | 1 | 0.868 | 0.908 | −0.028 | 0.943 | 0.653 | 0.940 | 0.559 | 0.959 | 0.717 | −0.049 | −0.048 |
| LIC | 0.969 | 0.868 | 1 | 0.994 | −0.051 | 0.953 | 0.585 | 0.936 | 0.118 | 0.969 | 0.969 | −0.315 | −0.315 |
| TCARB | 0.990 | 0.908 | 0.994 | 1 | 0.014 | 0.957 | 0.656 | 0.969 | 0.178 | 0.989 | 0.940 | −0.323 | −0.324 |
| TPHE | 0.044 | −0.028 | −0.051 | 0.014 | 1 | −0.232 | 0.735 | 0.199 | −0.355 | 0.020 | −0.057 | −0.803 | −0.801 |
| TFLA | 0.961 | 0.943 | 0.953 | 0.957 | −0.232 | 1 | 0.481 | 0.907 | 0.400 | 0.968 | 0.865 | −0.034 | −0.035 |
| VITA | 0.700 | 0.653 | 0.585 | 0.656 | 0.735 | 0.481 | 1 | 0.804 | 0.050 | 0.684 | 0.494 | −0.684 | −0.683 |
| VITC | 0.987 | 0.940 | 0.936 | 0.969 | 0.199 | 0.907 | 0.804 | 1 | 0.255 | 0.984 | 0.843 | −0.379 | −0.378 |
| VITE | 0.293 | 0.559 | 0.118 | 0.178 | −0.355 | 0.400 | 0.050 | 0.255 | 1 | 0.309 | −0.113 | 0.694 | 0.695 |
| TAC | 1 | 0.959 | 0.969 | 0.989 | 0.020 | 0.968 | 0.684 | 0.984 | 0.309 | 1 | 0.880 | −0.250 | −0.249 |
| ABTS | 0.882 | 0.717 | 0.969 | 0.940 | −0.057 | 0.865 | 0.494 | 0.843 | −0.113 | 0.880 | 1 | −0.417 | −0.418 |
| DPPH% | −0.273 | −0.049 | −0.315 | −0.323 | −0.803 | −0.034 | −0.684 | −0.379 | 0.694 | −0.250 | −0.417 | 1 | 1 |
| DPPH | −0.273 | −0.048 | −0.315 | −0.324 | −0.801 | −0.035 | −0.683 | −0.378 | 0.695 | −0.249 | −0.418 | 1 | 1 |
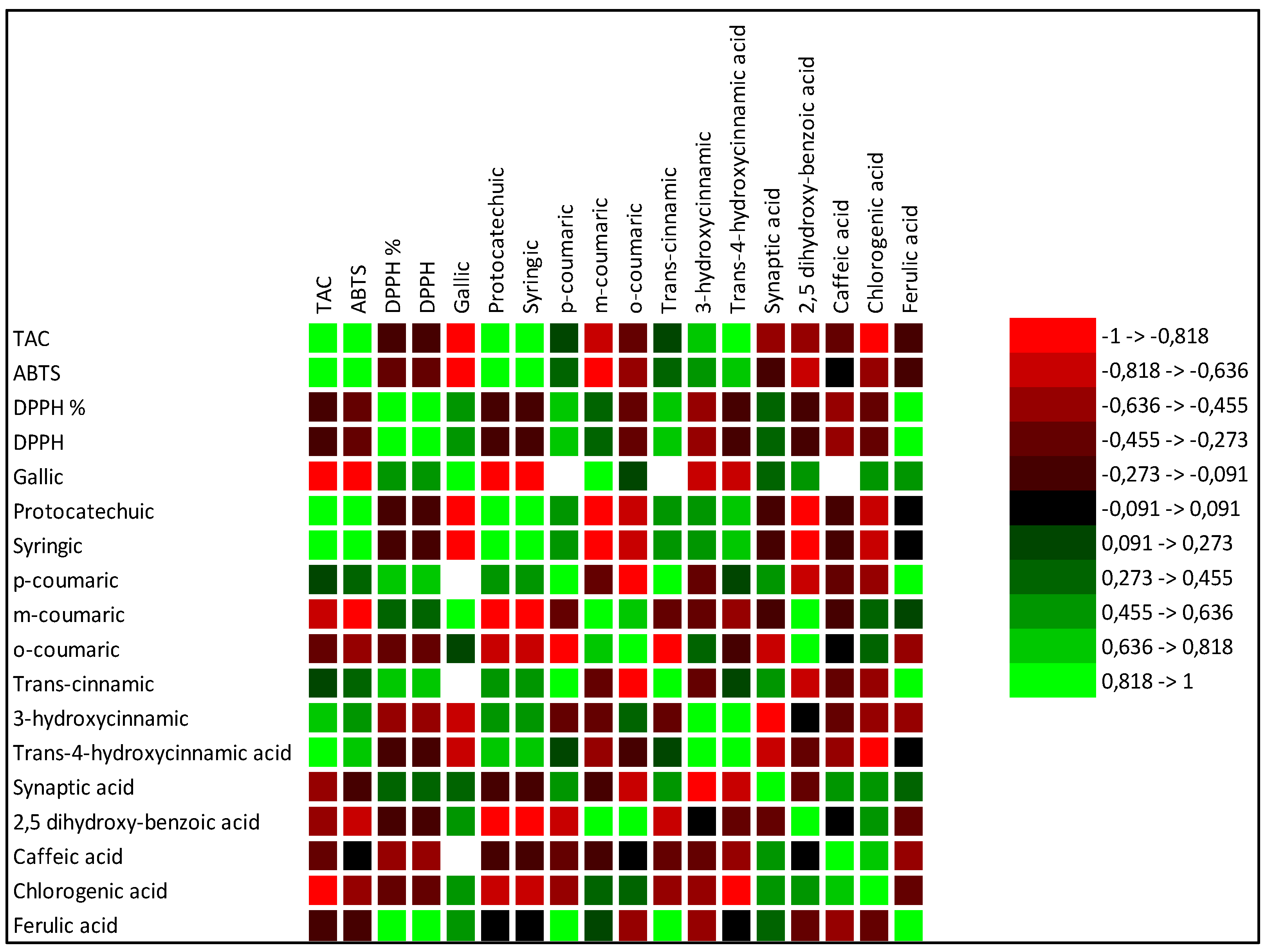
| CTR | NPK | HM | SB | |
|---|---|---|---|---|
| Phenolic acids | mg/g SS | mg/g SS | mg/g SS | mg/g SS |
| Gallic | 0.6 a | 0.3 b | 0.3 b | nd |
| Protocatechuic | nd | 0.01 a | 0.02 a | 0.02 a |
| Syringic | nd | 0.01 a | 0.02 a | 0.02 a |
| p-coumaric | nd | nd | 0.01 | nd |
| m-coumaric | 4 a | 0.6 b | nd | nd |
| o-coumaric | 0.06 a | 0.04 a | 0.01 a | 0.05 a |
| Trans-cinnamic | nd | nd | 2.83 a | nd |
| 3-hydroxycinnamic | nd | nd | nd | nd |
| Trans-4-hydroxycinnamic acid | 0.46 c | 0.3 d | 1.00 b | 1.58 a |
| Synaptic acid | 0.02 a | 0.04 a | 0.04 a | nd |
| 2,5 dihydroxy-benzoic acid | 0.03 a | 0.02 a | 0.01 a | 0.02 a |
| Caffeic acid | 0.01 a | 0.02 a | 0.01 a | 0.01 a |
| Chlorogenic acid | 0.56 b | 0.9 a | 0.1 a | 0.02 c |
| Ferulic acid | 0.2 b | nd | 0.34 a | nd |
| CTR | NPK | HM | SB | |
|---|---|---|---|---|
| mg/g SS | mg/g SS | mg/g SS | mg/g SS | |
| Flavonoids | ||||
| Procyanidin 2 | 0.2 a | 0.03 b | 0.03 b | nd |
| Pelargonidine | 0.05 a | nd | nd | nd |
| Cyanidine 3 O-glucoside | 0.15 a | 0.05 b | 0.03 b | 0.02 b |
| Catechin | 0.08 c | 0.15 b | 0.3 a | 0.3 a |
| Epicatechin | 0.12 a | 0.12 a | 0.03 b | 0.05 b |
| Delphinidin | 0.54 a | 0.52 a | 0.1 b | 0.1 b |
| Myricetin | 1.16 a | 1.42 a | nd | nd |
| Luteolin | 0.04 a | 0.03 a | 0.02 a | 0.03 a |
| Punicalagin | 0.07 a | 0.07 a | nd | nd |
| Naringin | nd | nd | nd | 0.02 a |
| Quercetin | 0.05 a | 0.01 a | nd | 0.02 a |
| Kaempferol | 0.08 c | nd | 2.1 a | 0.16 b |
| Tocopherol | 2.1 b | 1.81 c | nd | 2.99 a |
| Procyanidin 1 | nd | 0.16 a | nd | nd |
| Vicenin 2 | 0.01 b | 0.08 b | 0.3 a | 0.08 b |
| Erythrocin | nd | 0.05 a | nd | nd |
| Rutin | 0.26 c | 3 a | 0.56 b | 0.02 d |
| Retention Times | Name | Sensory Descriptors |
|---|---|---|
| 13.56-1-A | acetaldehyde | Aldehydic; ethereal; fresh; fruity |
| 49.70-1-A | 3-heptanol | Green; herbaceous |
| 61.40-1-A | ethyl hexanoate | Anise; apple; banana; berry; fruity; fruity(sweet); green; pineapple; strawberry; sweaty; sweet; unripe; waxy |
| 66.63-1-A | (Z)-2-octenal | Earthy; fatty; fruity; green; leafy; walnut |
| 10.68-2-A | unknown | |
| 38.74-2-A | butane-2,3-dione | Butter; caramelized; chlorine; creamy; fruity; pineapple; pungent; spirit; strong; sweet |
| 56.36-2-A | hexanal | Aldehydic; ethereal; fresh; fruity: green; herbaceous |
| 90.80-2-A | 1-nonanol | Dusty; fatty; floral; fresh; fruity; green; oily; orange; rose; wet |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russo, M.; Di Sanzo, R.; Marra, F.; Carabetta, S.; Maffia, A.; Mallamaci, C.; Muscolo, A. Waste-Derived Fertilizer Acts as Biostimulant, Boosting Tomato Quality and Aroma. Agronomy 2023, 13, 2854. https://doi.org/10.3390/agronomy13122854
Russo M, Di Sanzo R, Marra F, Carabetta S, Maffia A, Mallamaci C, Muscolo A. Waste-Derived Fertilizer Acts as Biostimulant, Boosting Tomato Quality and Aroma. Agronomy. 2023; 13(12):2854. https://doi.org/10.3390/agronomy13122854
Chicago/Turabian StyleRusso, Mariateresa, Rosa Di Sanzo, Federica Marra, Sonia Carabetta, Angela Maffia, Carmelo Mallamaci, and Adele Muscolo. 2023. "Waste-Derived Fertilizer Acts as Biostimulant, Boosting Tomato Quality and Aroma" Agronomy 13, no. 12: 2854. https://doi.org/10.3390/agronomy13122854










