Efficiency of Vinasse Application on Root-Knot Nematodes in Soybean
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection and Preparation of Nematode Inocula and Acquiring Vinasse
2.2. Location and Experimental Setup
2.3. Evaluation of Agronomic and Parasitism Characteristics
2.4. Statistical Analysis
3. Results
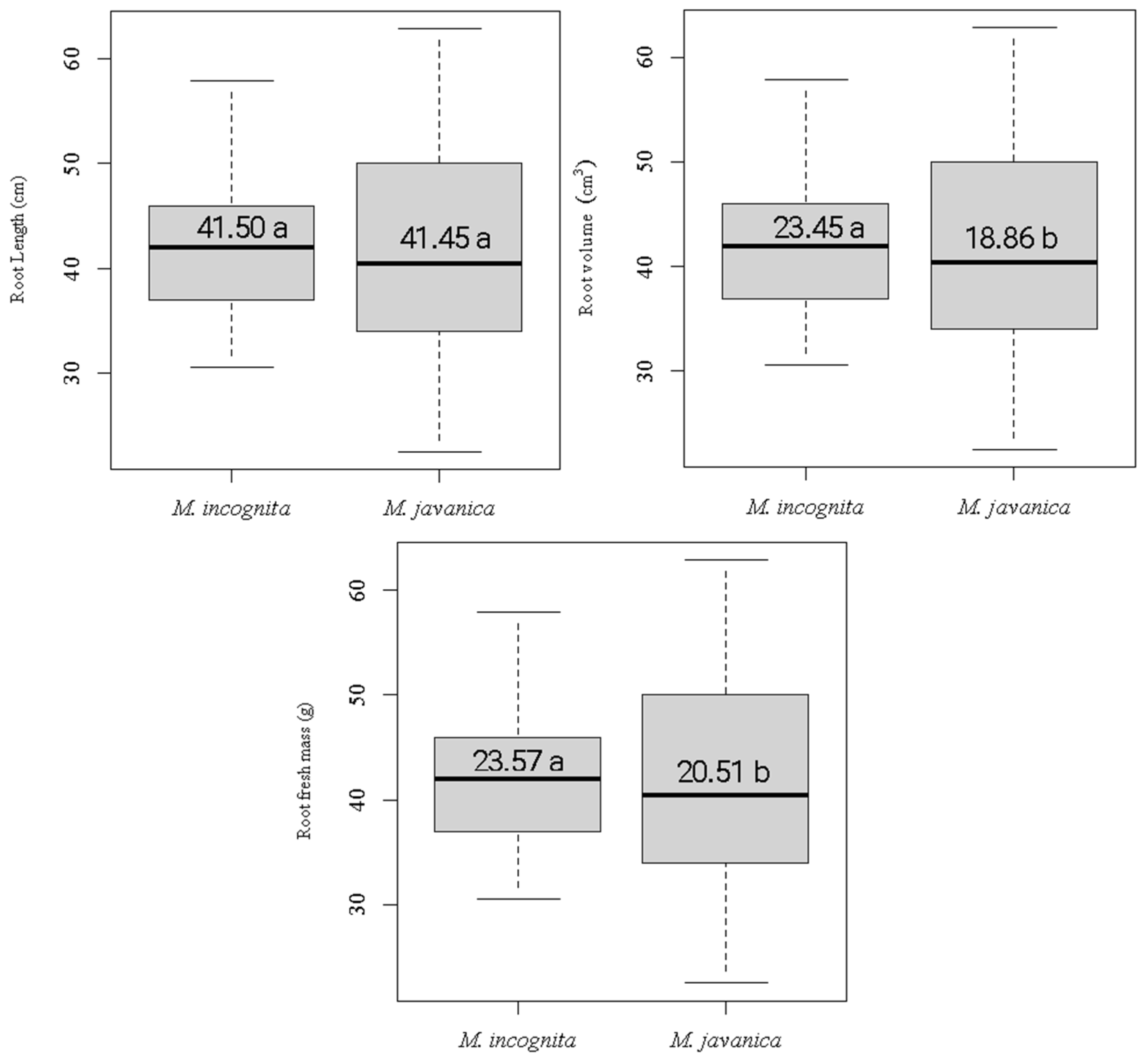
3.1. Agronomic Characteristics
3.2. Characteristics of Parasitism
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Conab (Companhia Nacional Abastecimento). Acompanhamento da Safra Brasileira: Grãos: Safra 2022/23. 11° Levantamento; Conab: Brasília, Brazil, 2023; Volume 10, pp. 1–102. [Google Scholar]
- Avelino, A.C.; Faria, D.A.; Oliveira, L.D.; Terzi, B.G.; Filho, A.S.C.; Afonso, M.F.; Rondon, O.H.S.; Arieira, G.O.; Abreu, J.G.; Peixoto, W.M.; et al. Phytonematodes in integrated crop-livestock systems of tropical regions. J. Exp. Agric. Int. 2019, 37, 1–13. [Google Scholar] [CrossRef]
- Arjoune, Y.; Sugunaraj, N.; Peri, S.; Nair, S.V.; Skurdal, A.; Ranganathan, P.; Johnson, B. Soybean cyst nematode detection and management: A review. Plant Methods 2022, 18, 110. [Google Scholar] [CrossRef] [PubMed]
- Treub, M. Onderzoekingen ever sereh-zik suikkerriet. In Meded. Uit’s Plantentuin, Java; Nabu Press: New York, NY, USA, 1985; Volume 2, pp. 1–39. [Google Scholar]
- Kofoid, C.A.; White, W.A. A new nematode infection in man. J. Am. Med. Assoc. 1919, 72, 567–569. [Google Scholar] [CrossRef]
- Ribeiro, N.R.; Dias, W.P.; Santos, J.M. Distribuição de fitonematoides em regiões produtoras de soja do estado de Mato Grosso. Bol. Pesqui. Soja 2010, 14, 289–296. [Google Scholar]
- Dias, W.P.; Silva, J.F.V.; Carneiro, G.E.S.; Garcia, A.; Arias, C.A.A. Nematoide de cisto da soja: Biologia e manejo pelo uso da resistência genética. Nematol. Bras. 2009, 33, 1–16. [Google Scholar]
- Chinheya, C.C.; Yobo, K.S.; Laing, M.D. Biological control of the rootknot nematode, Meloidogyne javanica (Chitwood) using Bacillus isolates, on soybean. Biol. Control 2017, 109, 37–41. [Google Scholar] [CrossRef]
- Dong, L.Q.; Zhang, K.Q. Microbial control of plant-parasitic nematodes: A five party interaction. Plant Soil 2006, 288, 31–45. [Google Scholar] [CrossRef]
- Jayaraj, R.; Megha, P.; Sreedev, P. Organochlorine pesticides, their toxic effects on living organisms and their fate in the environment. Interdiscip. Toxicol. 2016, 9, 90–100. [Google Scholar] [CrossRef]
- Arora, S.; Murmu, G.; Mukherjee, K.; Saha, S.; Maity, D. A comprehensive overview of nanotechnology in sustainable agriculture. J. Biotechnol. 2022, 355, 21–41. [Google Scholar] [CrossRef]
- Rani, N.; Duhan, A.; Pal, A.; Kumari, P.; Beniwal, R.K.; Verma, D.; Goyat, A.; Singh, R. Are nanopesticides truly meant for cleaner production? An overview on recent developments, benefits, environmental hazards and future prospectives. J. Clean. Prod. 2023, 411, 137232. [Google Scholar] [CrossRef]
- Alavi, N.; Daneshpajou, M.; Shirmardi, M.; Goudarzi, G.; Neisi, A.; Babaei, A.A. Investigating the efficiency of cocomposting and vermicomposting of vinasse with the mixture of cow manure wastes, bagasse, and natural zeolite. Waste Manag. 2017, 69, 117–126. [Google Scholar] [CrossRef]
- Garcia, C.F.H.; Souza, R.B.; Souza, C.P.; Christofoletti, C.A.; Fontanetti, C.S. Toxicity of two effluents from agricultural activity: Comparing the genotoxicity of sugarcane and orange vinasse. Ecotoxicol. Environ. Saf. 2017, 142, 216–221. [Google Scholar] [CrossRef]
- Kusumaningtyas, R.D.; Oktafiani, O.; Hartanto, D.; Handayani, P.A. Effects of Solid Vinasse-Based Organic Fertilizer on Some Growth Indices of Tomato Plant. J. Bahan Alam Terbarukan 2018, 6, 190–197. [Google Scholar] [CrossRef]
- Shi, F.; Zhu, Y. Application of statistically based experimental designs in medium optimization for spore production of Bacillus subtilis from distillery effluent. BioControl 2007, 52, 845–853. [Google Scholar] [CrossRef]
- Magrini, F.E.; Almeida, G.M.; Soares, D.M.; Fuentes, L.; Ecthebehere, C.; Beal, L.L.; Silveira, M.M.; Paesi, S. Effect of different heat treatments of inoculum on the production of hydrogen and volatile fatty acids by dark fermentation of sugarcane vinasse. Biomass Convers. Biorefin. 2021, 11, 2443–2456. [Google Scholar] [CrossRef]
- Barros, A.C.B.; Moura, R.M.; Pedrosa, E.M.R. Aplicação de terbufós no controle de Meloidogyne incognita raça 1 e Pratylechus zeae em cinco variedades de cana-de-açúcar no Nordeste. Parte 1—Efeitos na cana planta. Nematol. Bras. 2000, 24, 73–78. [Google Scholar]
- Leite, M.L.T.; Almeida, F.A.; Fonseca, W.L.; Oliveira, A.M.; Prochnow, J.T.; Pereira, F.F.; Alcântara Neto, F.D. Effect of Vinasse in the Suppressiveness to Pratylenchus brachyurus in Soybean. J. Agric. Sci. 2018, 11, 538–545. [Google Scholar] [CrossRef]
- Coolen, W.A.; D’Herde, C.J. A Method for the Quantitative Extraction of Nematodes from Plant Tissue; State Nematology and Entomology Research Station: Ghent, Belgium, 1972; p. 77. [Google Scholar]
- Hartman, K.M.; Sasser, J.N. Identification of Meloidogyne species on the basis of differencial host test and perineal pattern morphology. In An Advanced Treatise on Meloidogyne; Barker, K.R., Carter, C.C., Sasser, J.N., Eds.; Methodology; North Carolina State University Graphics: Raleigh, NC, USA, 1985; Volume 2, pp. 69–77. [Google Scholar]
- Oliveira, C.M.G.; Tomazini, M.D.; Bessi, R.; Inomoto, M.M. Nematoides. In Técnicas de Diagnóstico de Fitopatógenos; Eiras, M., Galleti, S.R., Eds.; Devir Livraria: São Paulo, Brazil, 2012; pp. 103–136. [Google Scholar]
- Ranzani, G. Consequências da aplicação do restilo ao solo(I). An. Esc. Super. Agric. Luiz Queiroz 1956, 12, 57–67. [Google Scholar] [CrossRef]
- Hussey, R.S.; Barker, K.R.A. A comparison of methods of collecting inocula of Meloidogyne spp. Including a new technique. Plant Dis. Rep. 1973, 57, 1.025–1.028. [Google Scholar]
- Bonetti, J.I.; Ferraz, S. Modificações do metodo de Hussey e Barker para extração de ovos de Meloidogyne exigua em raizes de cafeeiro. In Proceedings of the Congresso da Sociedade Brasileira de Fitopatologia, XIV, Porto Alegre, Brazil, 1981; Volume 6, p. 553. Available online: https://www.scienceopen.com/document?vid=9232a12b-2a57-4b2b-8724-dae6ad63e065 (accessed on 15 January 2022).
- Jenkins, W.R. A rapid centrifugal-flotation technique for extracting nematodes from soil. Plant Dis. Rep. 1964, 48, 692. [Google Scholar]
- Oostenbrink, M. Major characteristic of relation between nematodes and plants. Meded. Landbouwhogesch. 1966, 66, 146. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 15 January 2022).
- Friendly, M.; Fox, J. Candisc: Visualizing Generalized Canonical Discriminant and Canonical Correlation Analysis, R Package Version 0.8-0; R Foundation for Statistical Computing: Vienna, Austria, 2017; Available online: https://CRAN.R-project.org/package=candisc (accessed on 10 January 2022).
- Asmus, G.L. Danos causados à cultura da soja por nematoides do gênero Meloidogyne. In Relações Parasito-Hospedeiro nas Meloidoginoses da Soja, 1st ed.; Silva, J.F.V., Ed.; Embrapa Soja-Sociedade Brasileira de Nematologia: Londrina, Brazil, 2001; pp. 39–62. [Google Scholar]
- Guimarães, L.M.P.; Pedrosa, E.M.R.; Coelho, R.S.B.; Chaves, A.; Maranhão, S.R.V.L.; Miranda, T.L. Efeito de metil jasmonato e silicato de potássio no parasitismo de Meloidogyne incognita e Pratylenchus zeae em cana-de-açúcar. Nematol. Bras. 2008, 32, 50–55. [Google Scholar]
- Salgado, S.M.L.; Resende, M.L.V.; Campos, V.P. Efeito de indutores de resistência sobre Meloidogyne exigua do cafeeiro. Cienc. E Agrotecnol. 2007, 31, 1007–1013. [Google Scholar] [CrossRef]
- Silva, A.J.N.; Cabeda, M.S.V.; Carvalho, F.G. Matéria orgânica e propriedades físicas de um argissolo amarelo coeso sob sistemas de manejo com cana-de-açúcar. Rev. Bras. Engen. Agríc. Ambient. 2006, 10, 579–585. [Google Scholar] [CrossRef]
- Oka, Y. Mechanisms of nematode suppression by organic soil amendments—A review. Appl. Soil Ecol. 2010, 44, 101–115. [Google Scholar] [CrossRef]
- Bebé, F.V.; Rolim, M.M.; Pedrosa, E.M.R.; Silva, G.B.; Oliveira, V.S. Avaliação de solo sob diferentes períodos de aplicação com vinhaça. Rev. Bras. Engen. Agríc. Ambient. 2009, 13, 781–787. [Google Scholar] [CrossRef]
- Martins, Y.A.M.; Barbosa, K.P.; Silva, P.C.; Costa, R.A.; Costa, A.R. Aplicação de diferentes doses de vinhaça sob o desenvolvimento vegetativo de plantas de milho. Enciclopédia Biosf. 2013, 9, 277. [Google Scholar]
- Freire, W.J.; Cortez, L.A.B. Vinhaça de Cana-de-Açúcar; Agropecuária: Guaíba, Brazil, 2000. [Google Scholar]
- Meurer, E.J.; Bissani, C.A.; Selbach, P.A. Poluentes do solo e do ambiente. In Fundamentos de Química do Solo; Meurer, E.J., Ed.; Gênesis: Porto Alegre, Brazil, 2000; p. 174. [Google Scholar]
- Lyra, M.R.C.C.; Rolim, M.M.; Silva, J.A.A. Topossequência de solos fertirrigados com vinhaça: Contribuição para a qualidade das águas do lençol freático. Rev. Bras. Engen. Agríc. Ambient. 2003, 7, 523–532. [Google Scholar] [CrossRef]
- Pegard, A.; Brizzard, G.; Fazari, A.; Soucaze, O.; Abad, P.; Djiancaporalino, C. Histological Characterization of resistance to different root-knot nematode species related to phenolics accumulation in Capsicum annuum. Phytopathology 2005, 95, 158–165. [Google Scholar] [CrossRef]
- Caixeta, L.B.; Barbosa, N.M.R.; Barros, P.A.; Leitão, D.A.H.S.; Pedrosa, E.M.R. Efeito da fertirrigação com vinhaça na distribuição espacial de fitonematoides em solo cultivado com cana-de-açúcar. In Jornada de Ensino, Pesquisa e Extensão; UFRPE: Recife, Brazil, 2010; p. 10. [Google Scholar]
- Riegel, C.; Fernandez, F.A.; Noe, J.P. Meloidogyne incognita infested soil amended with chicken litter. J. Nematol. 1996, 28, 369–378. [Google Scholar]
- Sousa, M.J.O.; Almeida, F.A.; Leite, M.L.T.; Fonseca, W.L.; Lopes, K.P.; Gomes, C.D.L.; Sampaio, E.G.; Santos, E.N.; Gondim, A.R.O. Biocidal potential of some organic byproducts on sanitary and physiological quality of red and white fava beans seeds. Aust. J. Crop Sci. 2020, 14, 462–468. [Google Scholar] [CrossRef]
- Albuquerque, P.H.S.; Pedrosa, E.M.R.; Moura, R.M. Relações nematóides-hospedeiro em solo infestado por Meloidogyne spp. e tratado com torta de filtro e vinhaça. Nematol. Bras. 2002, 26, 27–34. [Google Scholar]
- Matos, D.S.S.; Pedrosa, E.M.R.; Guimarães, L.M.P.; Rodrigues, C.V.M.A.; Barbosa, N.M.R. Relações entre a nematofauna e atributos químicos de solo com vinhaça. Nematropica 2011, 41, 23–38. [Google Scholar]
- Pedrosa, E.M.R.; Rolim, M.M.; Albuquerque, P.H.S.; Cunha, A.C. Supressividade de nematoides em cana-de-açúcar por adição de vinhaça ao solo. Rev. Bras. Engen. Agríc. Ambient. 2005, 9, 197–201. [Google Scholar] [CrossRef]






| Source of Variation | Agronomic Variables | ||
|---|---|---|---|
| Root Length | Root Volume | Root Fresh Mass | |
| (NS) | 0.03 ns | 315.10 ** | 140.11 * |
| (CCv) | 406.04 ** | 129.81 ** | 274.71 * |
| NS x CCv | 73.42 ns | 15.81 ns | 9.73 ns |
| C.V. (%) | 16.06 | 26.27 | 20.74 |
| Variation Source | Parasitism Variables | ||||||
|---|---|---|---|---|---|---|---|
| JR | NGR | JS | NG | ER | ES | RF | |
| (NS) | 17,957.40 ** | 0.57 ns | 25,833.75 ** | 728.01 ** | 7216.06 ** | 58.01 ** | 0.0026 ** |
| (CCv) | 5296.22 ** | 246.13 ** | 11,773.78 ** | 1711.27 ** | 7242.70 ** | 28.49 ** | 0.0043 ** |
| NS x CCV | 833.56 ** | 19.61 ** | 3135.91 ** | 385.61 ** | 4775.78 ** | 3.37 ns | 0.0004 ** |
| C.V. (%) | 25.5 | 29.01 | 11.58 | 13.51 | 17.06 | 23.71 | 18.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leite, M.L.T.; Almeida, F.A.d.; Fonseca, W.L.; Oliveira, A.M.d.; Zuffo, A.M.; Pereira, F.F.; Alcântara Neto, F.d.; Barreto, A.F.; Al-Askar, A.A.; Carvalho, R.M.; et al. Efficiency of Vinasse Application on Root-Knot Nematodes in Soybean. Agronomy 2023, 13, 2719. https://doi.org/10.3390/agronomy13112719
Leite MLT, Almeida FAd, Fonseca WL, Oliveira AMd, Zuffo AM, Pereira FF, Alcântara Neto Fd, Barreto AF, Al-Askar AA, Carvalho RM, et al. Efficiency of Vinasse Application on Root-Knot Nematodes in Soybean. Agronomy. 2023; 13(11):2719. https://doi.org/10.3390/agronomy13112719
Chicago/Turabian StyleLeite, Maria Lúcia Tiburtino, Fernandes Antonio de Almeida, Wéverson Lima Fonseca, Augusto Matias de Oliveira, Alan Mario Zuffo, Francisco Fernandes Pereira, Francisco de Alcântara Neto, Artur Franco Barreto, Abdulaziz A. Al-Askar, Rezanio Martins Carvalho, and et al. 2023. "Efficiency of Vinasse Application on Root-Knot Nematodes in Soybean" Agronomy 13, no. 11: 2719. https://doi.org/10.3390/agronomy13112719
APA StyleLeite, M. L. T., Almeida, F. A. d., Fonseca, W. L., Oliveira, A. M. d., Zuffo, A. M., Pereira, F. F., Alcântara Neto, F. d., Barreto, A. F., Al-Askar, A. A., Carvalho, R. M., Marey, S. A., Gondim, A. R. d. O., Hashem, A. H., Leite, M. R. L., & AbdElgawad, H. (2023). Efficiency of Vinasse Application on Root-Knot Nematodes in Soybean. Agronomy, 13(11), 2719. https://doi.org/10.3390/agronomy13112719









