Abstract
In addition to increasing grain yield, improving rice (Oryza sativa L.) quality has received increasing attention recently. The cooking and eating quality (CEQ) is an important indicator of rice quality. Chalkiness and floury endosperm have a significant impact on the CEQ of rice, resulting in noticeable changes. Due to the easily observable phenotype of floury endosperm, cloning single gene mutations that cause floury endosperm and indirectly evaluating changes in CEQs facilitates the exploration of minor genes controlling CEQ. In this study, a stable genetic allele variant of flo4, named flo4-7, was obtained through EMS mutagenesis. The flo4-7 allele variant carries the cyOsPPDKB mutation. flo4-7 showed a significant reduction in compound starch granules and a significant increase in single starch granules in endosperm cells, indicating the involvement of cyOsPPDKB in the synthesis of endosperm starch. Additionally, flo4-7 exhibited a significant decrease in gel consistency (GC) compared to the wild type. Through the analysis of GC data from 166 rice germplasm resources, a C-T variation in the 18th exon of cyOsPPDKB was found to be a crucial site, causing a significant difference in GC between indica and japonica rice. This locus can be used in the future to develop molecular markers for molecular marker-assisted breeding. This study provides a good research foundation for improving the CEQ of rice.
1. Introduction
Rice is the staple food for more than half of the world’s population and is one of the most important crops. Over the past few decades, due to the introduction of semi-dwarf varieties and the utilization of hybrid vigor, rice production has significantly improved. However, compared with rice production, the development of high-quality breeding is lagging behind. Therefore, with the improvement of living conditions, breeders need to improve the quality of rice in order to meet people’s needs. Rice quality involves the milling quality, appearance quality, nutritional quality, and cooking and eating quality (CEQ). The milling quality includes the brown rice rate, milled rice rate, and head rice rate, among which the milled rice rate has received more attention in rice breeding. Appearance quality includes the grain shape, chalky rate, and chalky degree, among which the chalky degree is an important indicator of rice quality. Grain shape is composed of the following three elements: grain length, width, and thickness [1]. Long and slender grain varieties are generally preferred in the international market and most Asian countries, while short and round grains are preferred in Japan and Korea [2]. The nutritional quality of polished rice is determined by proteins stored in the rice endosperm, which mainly comprises starch, with proteins and lipids in small proportions, at 7–10% and less than 1%, respectively [3].
Starch, accounting for more than 70% of the seed weight, has a close relationship with rice quality. Starch properties are quantized by many quality indexes, like CEQ, rapid visco analyzer (RVA) profiles, and starch swelling in the urea solution. The CEQ of rice is usually evaluated via three major indirect indexes reflecting the physical and chemical characteristics of starch; these include amylose content (AC), gel consistency (GC), and gelatinization temperature (GT) [4]. AC is widely demonstrated to be the most crucial factor affecting cooking, and eating quality characteristics and is negatively related to taste palatability, including transparency, viscosity, and rice-milling quality. Varieties of rice are usually classified into waxy (0–2%), very low (3–9%), low (10–19%), intermediate (20–25%), and high (>25%) amylose classes according to the AC [5]. GC is analyzed by measuring the cold paste viscosity of cooked milled rice flour. Rice with a soft gel consistency is highly preferred among consumers. GC can be divided into three categories: soft (>60 mm), adhesive (40–60 mm), and hard GC (<40 mm), and the larger the GC, the softer, stickier, better taste and shinier rice is produced [6]. AC and GC are mainly controlled via the Wx gene, which encodes granule-bound starch synthase I (GBSS I), and GC is negatively correlated with AC [7,8]. So far, at least nine Wx alleles have been identified: Wxa, Wxb, Wxmq, Wxin, Wxmp, Wxop/hp, Wxlv, Wxmw/Wxla, and null Wx. These different allelic variations have different effects on AC [9]. The SNP on exon 10 of the Wx gene from C-T is a significant factor influencing GC [10]. GT is positively correlated with physical characteristics responsible for the cooking time, and the ability to absorb water during cooking. GT can be measured indirectly in terms of an alkali spreading value (ASV) using an alkaline or urea solution. Rice grains with a high GT require more water and cooking time than rice with a low or medium GT. GT was mainly affected by the alk gene, which encodes starch synthase IIa (SSIIa) and is responsible for the differences in the chain-length distribution of amylopectin. The GC/TT SNP could differentiate rice with high or intermediate GT from those with low GT in about 90% of cases [11].
The viscosity of paste has also been used to select rice varieties with desirable eating, cooking, and processing properties [12]. Starch viscosity profiles tested using a rapid visco analyzer can measure the changes in viscosity associated with starch gelatinization [13]. The starch viscosity characteristics used included the following original components: peak viscosity (PV), trough viscosity (TV), and final viscosity (FV). Three secondary parameters, including breakdown (BDV), setback (SBV), and consistency (COV), were calculated based on original data: BDV = PV − TV, SBV = FV − PV, and COV = FV − TV. Good CEQ varieties usually have a higher BDV and lower SBV, COV, and FV [14]. Pasting temperature (PT) denoted the temperature at which the viscosity began to increase during the heating process and the mean peak time (PKT) times reached PV [4]. Selecting both AC, ASV, and GC in conjunction with RVA profiles is significant for improving the accuracy of selection in high-quality breeding programs.
CEQs are quantitative traits regulated by both environmental and genetic factors. However, due to the difficulties in measuring relevant indicators, only Wx and SSIIa have been widely applied in breeding. The research and application of minor effect genes for the regulation of CEQs remains limited. Chalkiness usually results in the potential low eating quality of CEQs [15], and its phenotype is easy to identify, making it possible to indirectly decipher the regulatory effect of genes on CEQs by exploring the genes influencing chalkiness. The chalkiness phenotype in rice is greatly influenced by environmental factors, especially when exposed to high temperatures during the grain-filling period, leading to significant increases in the chalkiness of rice, making it challenging to use materials with a high chalkiness rate or degree to explore regulatory genes for chalkiness. Therefore, in recent years, scientists have utilized physically and chemically induced floury endosperm mutants to uncover factors influencing rice grain quality, as these mutants can bypass the influence of environmental factors, facilitating in-depth investigations of individual genes and their mechanisms of quality regulation. These genes involve starch synthesis enzymes, transcription factors, lipid biosynthesis, storage protein biosynthesis, amyloplast development, mitochondrial metabolism, and other metabolism pathways [9]. The impact of these mutant genes on the CEQs of rice varies. flo6, osgbp, SSIIIa-RNAi, du13, flo11, and flo19 exhibited varying degrees and changes in their amylose content, RVA characteristics, and distribution of the amylopectin chain length [16,17,18,19,20,21]. Compared to the wild type, flo6, osgbp, SSIIIa-RNAi, du13, and flo19 showed a significant decrease in amylose content, while the difference in AC of flo11 was not apparent. The RVA parameters of these mutants were altered to varying degrees, especially in the case of flo6, where RVA parameters reached extremely low levels. The distribution of the amylopectin chain length also showed varying degrees of changes in these mutants. It can be concluded that floury endosperm mutants can serve as genetic materials when exploring factors related to rice CEQs.
Pyruvate orthophosphate dikinase (PPDK, EC 2.7.9.1) catalyzes the interconversion of pyruvate, ATP, and Pi with phosphoenolpyruvate, AMP, and PPi [22,23]. PPDK is an enzyme involved in photosynthesis in C4 plants, specifically in the formation of the CO2 acceptor PEP [24]. In maize, there are two cytosolic PPDK isoforms (cyppdkZm1 and cyppdkZm2) and one chloroplastic PPDK isoform (C4ppdkZm1) [25]. PPDK in C3 plants were at relatively lower protein levels compared to C4 plants [26]. In C3 plants, such as rice, there are two specific genes responsible for encoding PPDK; one gene encodes OsPPDKA, and another gene produces two transcripts as follows: C4-type chloroplastic PPDK (chOsPPDKB) and cytosolic PPDK (cyOsPPDKB, Os05g0405000, LOC_Os05g33570) [23]. The floury endosperm mutant flo4 is caused by the mutation of cyOsPPDKB, which is involved in the synthesis of starch in the rice endosperm [23].
To explore new regulatory factors that regulate CEQ in rice, the flo4-7 mutant was obtained. FLO4-7 is an allelic gene of cyOsPPDKB. The flo4-7 showed a floury endosperm, an increase in single starch granules, and a significant decrease in GC. Our findings demonstrate that cyOsPPDKB influences the CEQ of rice by affecting the GC length.
2. Materials and Methods
2.1. Plant Material and Growth Conditions
The mutant of flo4-7 was generated by the ethyl methanesulfonate (EMS) treatment of the indica variety QJ101. We soaked mature QJ101 seeds in water for 12 h, then transferred them to a 1% EMS solution for 12 h. Then, we rinsed them with clean water for 4 h to complete the mutagenesis. After harvesting the M0 plants individually, M1 seeds with both a floury endosperm and transparent endosperm were isolated through milling and screening. Next, M1 seeds were planted, and after maturity, 10 individual M2 seeds were harvested from each family line. The stable hereditary flo4-7 mutant was obtained by performing phenotype identification again. F2 populations from the crosses of flo4-7×ZXYZ (Oryza sativa L., indica) were used to map the mutant gene. All plants were grown in paddy fields during the normal growing seasons in Hangzhou, Zhejiang province, China (30.3° N, 120.2° E). Mature seeds and other tissues were harvested according to the experimental requirements.
2.2. Measurement of Starch Properties
2.2.1. Determination of CEQs
In total, 250 g of mature wild-type and flo4-7 seeds were milled into polished rice using a rice polisher to remove the hull, embryo, and aleurone layer. The polished rice was then ground into rice flour using a grinder, and the resultant flour was sieved through a 100-mesh sieve to obtain the rice flour required for subsequent measurements of the total starch content, AC, GC, RVA, and urea swelling. Each sample was measured three times for repeatability.
The starch content of rice flour was determined using a starch assay kit (Megazyme, Wicklow, Ireland, http://www.megazyme.com/ (accessed on 10 May 2022) in accordance with the manufacturer’s instructions [16].
AC was evaluated according to the previous report [20].
GT was determined using the alkali digestion test. A duplicate set of six intact milled kernels without any cracks was carefully selected and placed in a plastic box (5 × 5 × 2.5 cm). Afterward, 10 mL of a 1.7% KOH solution was added. Sufficient space was provided between the kernels to ensure even spreading. The boxes were covered and incubated in a 30 °C oven for 23 h. Based on the ASV score, the GT of the rice grains was classified into four groups: high (1–2), high-intermediate (3), intermediate (4–5), and low (6–7).
GC was measured using flour (100 mg) from all samples. The flour was weighed in duplicate and placed into 13 mm × 100 mm tubes. To each tube, 200 μL of ethyl alcohol (95%) containing 0.025% of thymol blue was added, along with 2 mL of 1 M KOH. The tubes were then immersed in a vigorously boiling water bath for 8 min. After removal from the water bath, the tubes were left at room temperature for 5 min, followed by cooling in an ice water bath for 20 min. Subsequently, the tubes were placed horizontally on a lightbox positioned on top of graphing paper. After 1 h, the distance the gel migrated in the tube was measured.
2.2.2. Determination of RVA Profiles
The rice flour samples were tested for their pasting properties using an RVA analyzer from Perten, located in Sydney, Australia. Within a short period of 12.5 min, the analyzer measured the pasting properties. Approximately 3 g of flour (with a moisture content of 12%) from each rice sample was directly weighed onto the aluminum canister. Then, 25 g of distilled water was added and mixed with the flour. The RVA dispersed the samples by rotating the paddle at 960 rpm for the initial 10 s of the test. Following this, viscosity was measured using a constant rotation speed of 160 rpm. The test profiles were conducted with an idle temperature of 50 °C and followed this order: hold at 50 °C for 1.0 min; linearly increase the temperature to 93 °C for 5.5 min; hold at 93 °C for 7 min; linearly reduce the temperature to 50 °C at 11 min; hold at 50 °C for the remaining 12.5 min. All analyses were performed in triplicate, and the viscosity values were recorded in centipose (cp).
2.2.3. Determination of Starch Swelling in Urea
A mixture of 20 mg of WT and eight mutant types of flour was prepared and mixed with a 1 mL solution of urea ranging from 0 to 9 M. The pH of the solution was adjusted to 6.0 using acetic acid in an Eppendorf tube. The mixture was then incubated at 25 °C for 24 h. After the incubation period, the suspension was centrifuged for 20 min at 8000× g and at room temperature. It was then allowed to stand for 1 h. The solubility of the starch granules in the urea solution was determined by measuring the volume of the swollen sediment. This volume was calculated by subtracting the volume of the supernatant from the original 1 mL of urea solution.
2.3. Microscopy Analysis
2.3.1. Scanning Electron Microscopy (SEM)
Transversely cut mature wild-type and flo4-7 seeds were used with a knife at a thickness of approximately 1 mm, and the samples were observed using a Hitachi TM3000 scanning electron microscope [16].
2.3.2. Transmission Electron Microscopy (TEM)
Transverse sections 7 days after flower (DAF) endosperms (approximately 1 mm thickness) for the wild type and flo4-7 were fixed overnight in a 0.1 M phosphate buffer (pH 7.2) with 2% (v/v) glutaraldehyde and 2% (w/v) paraformaldehyde. After dehydration in an ethanol series, samples were embedded in LR white resin (London Resin, Berkshire, UK), sectioned using an ultramicrotome (Leica CM1950), and observed under a microscope (H7650, Hitachi, Tokyo, Japan).
2.3.3. Semi-Thin Sections Microscopy
Semi-thin sections of 7 DAF endosperms from the wild type and flo4-7 were prepared as described before [16]. Sections (1 μm) were stained with I2-KI for 5 s and subsequently examined under a light microscope (Axio Vert.A1, Zeiss, Oberkochen, Germany).
2.4. Molecular Cloning of FLO4-7
The flo4-7 homozygous plant was crossed with an indica variety ZXYZ to create hybrids, and these hybrids were self-pollinated to generate an F2 population. Thirty-five individuals with extreme phenotypes of the wild type and mutant type were selected from the F2 population. These individuals combined with the parent lines ZXYZ and flo4-7 for BSA sequencing. After obtaining preliminary mapping results, KASP markers (Table S1) were designed (http://www.snpway.com/ (accessed on 13 January 2022)) based on SNP differences extracted from the resequencing data of two parent lines for further fine mapping.
2.5. Haplotypes Analysis of cyOsPPDKB
To analyze the haplotypes of cyOsPPDKB when controlling the GC length, the full-length chromosome region of cyOsPPDKB was used for Blast analysis in the RFGB database (https://www.rmbreeding.cn/ (accessed on 5 July 2022)). The resources of 166 cultivars were collected from the RFGB, and GC length data were measured and uploaded to the RFGB for further analysis. The statistical analysis of phenotypic differences between haplotypes was obtained using a methodology based on one-way ANOVA-protected Tukey’s t-tests multiple pairwise comparisons, as indicated at https://www.rmbreeding.cn/Index/manual#haplotype (accessed on 9 July 2022).
2.6. Analysis of Homologous Protein Sequence
Blast analysis in NCBI was used to identify cyOsPPDKB homologs among different species. A phylogenetic tree of neighbor-joining was constructed via MEGA 11.0. The amino acid sequences were aligned using MEGA 11.0.
3. Results
3.1. flo4-7 Exhibits a Floury Endosperm Phenotype
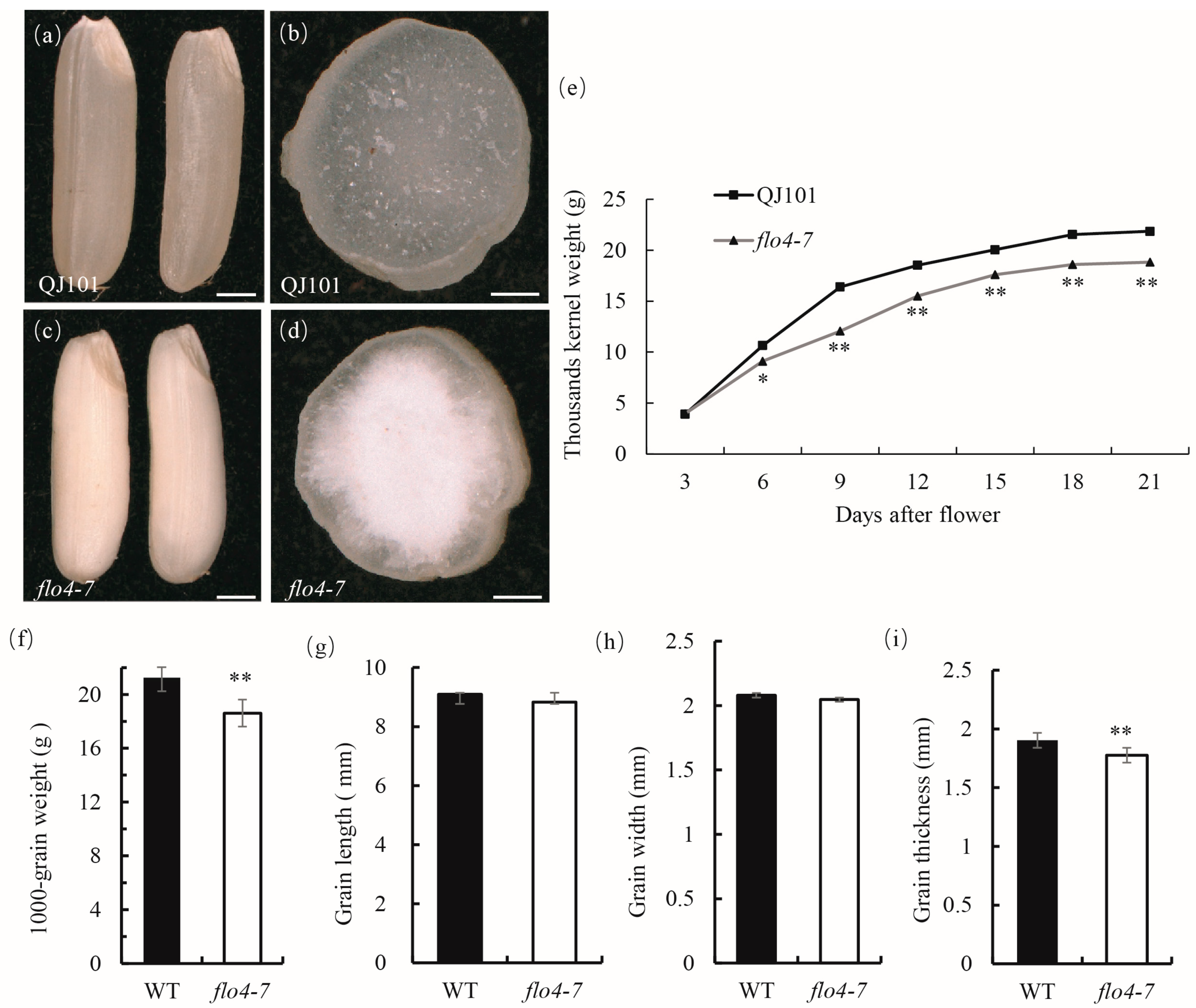
The flo4-7 mutant was derived from EMS mutagenesis of the indica rice variety QJ101 and stabilized as a homozygous line in the M3 generation. Compared with the wild type, the flo4-7 endosperm exhibited a floury phenotype (Figure 1a–d). The filling rate was significantly reduced compared with the wild type (Figure 1e), resulting in a significant decrease in its thousand-grain weight at maturity (Figure 1f). The determination of the grain size of the wild type and mutant revealed that there was no significant change in the length and width of flo4-7 grains, but the thickness of the grains significantly decreased compared to the wild type (Figure 1g–i), resulting in a significant decrease in the final 1000-grain weight. These results suggest that the flo4-7 mutation leads to insufficient endosperm filling.
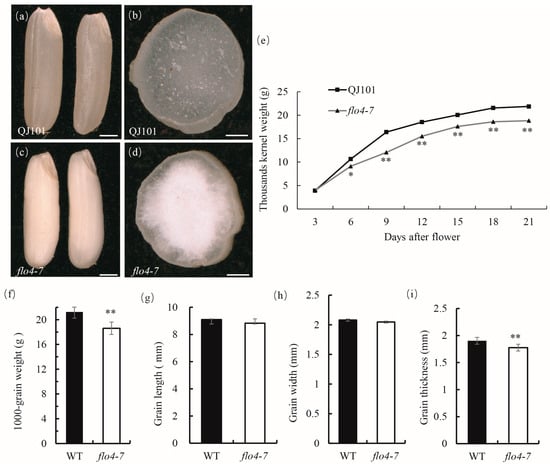
Figure 1.
The appearance of WT and flo4-7 seeds. (a,c) A comparison of WT and flo4-7 seeds; (b,d) Cross sections of WT and flo4-7 seeds; (e) Grain filling of WT and flo4-7 at various developmental stages. Grain weight is the dry weight of 1000 brown rice grains; (f) The thousand kernel weight of mature seeds of the WT and flo4-7 mutant. (g–i) Grain size of the WT and flo4-7. Values are means ± SDs (n = 3). The asterisks indicate statistical significance between the wild type and the mutant, as determined by Student’s t-test (* p < 0.05; ** p < 0.01).
3.2. flo4-7 Shows a Significant Decrease in GC Length
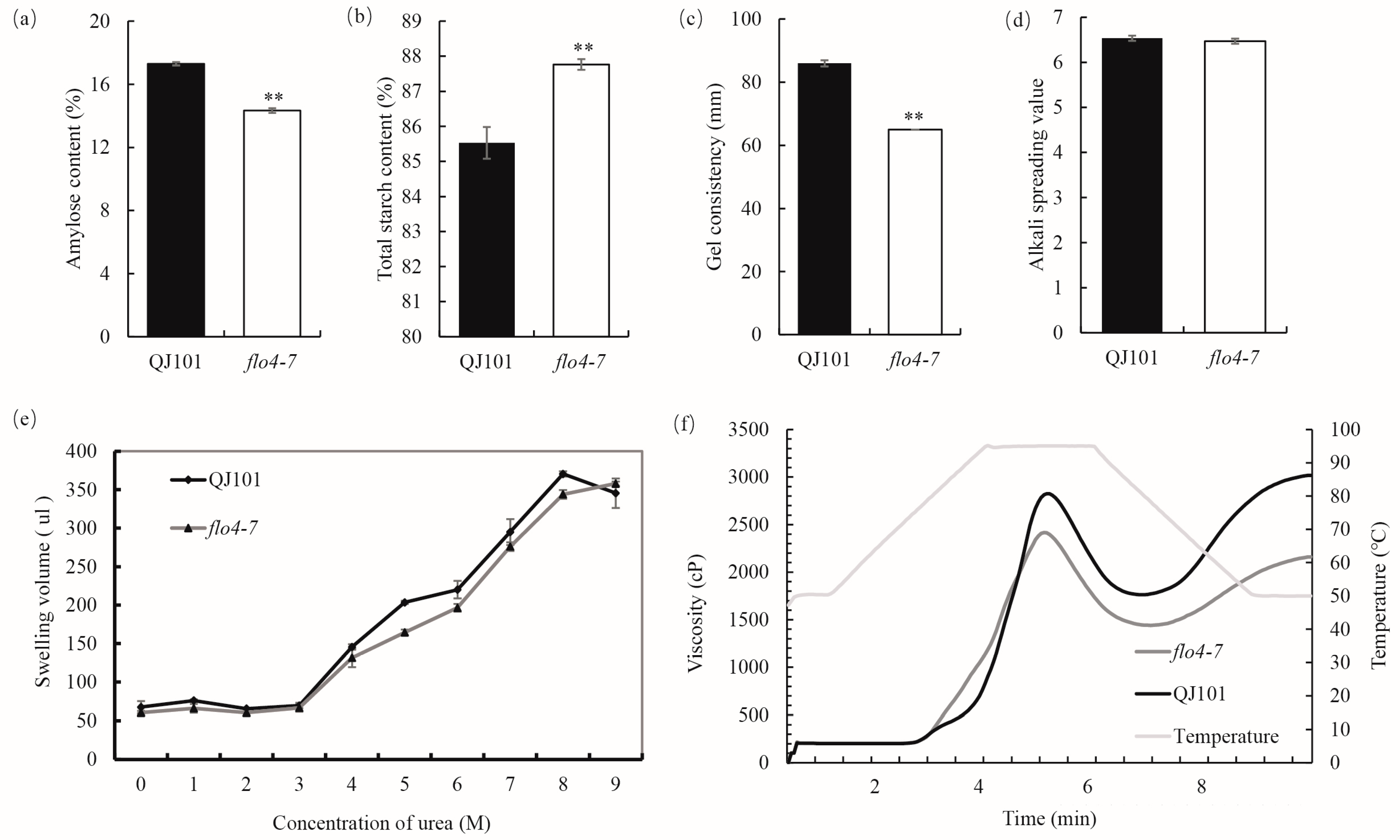
In order to study the effect of FLO4-7 on the CEQ of rice, various relevant indicators were determined. The results showed that compared with the wild type, flo4-7 showed significantly decreased AC, GC, and RVA indicators (Figure 2a,c,f and Table S2) while significantly increased total starch content (Figure 2b). There were no significant changes observed in ASV and starch swelling in the urea (Figure 2d,e).
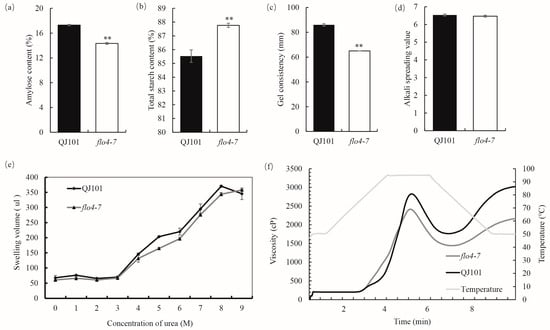
Figure 2.
Properties and physicochemical characteristics of starch in WT and flo4-7. (a–d) CEQs and the total starch content of WT and flo4-7. (e) Volume of WT and flo4-7 endosperm starch swelling in different concentrations of urea. (f) Pasting properties of endosperm starch of WT and flo4-7. Values are means ± SDs (n = 3). The asterisks indicate statistical significance between the wild type and the mutant, as determined by Student’s t-test (** p < 0.01).
3.3. flo4-7 Exhibits an Increase in Single Starch Granules in the Endosperm
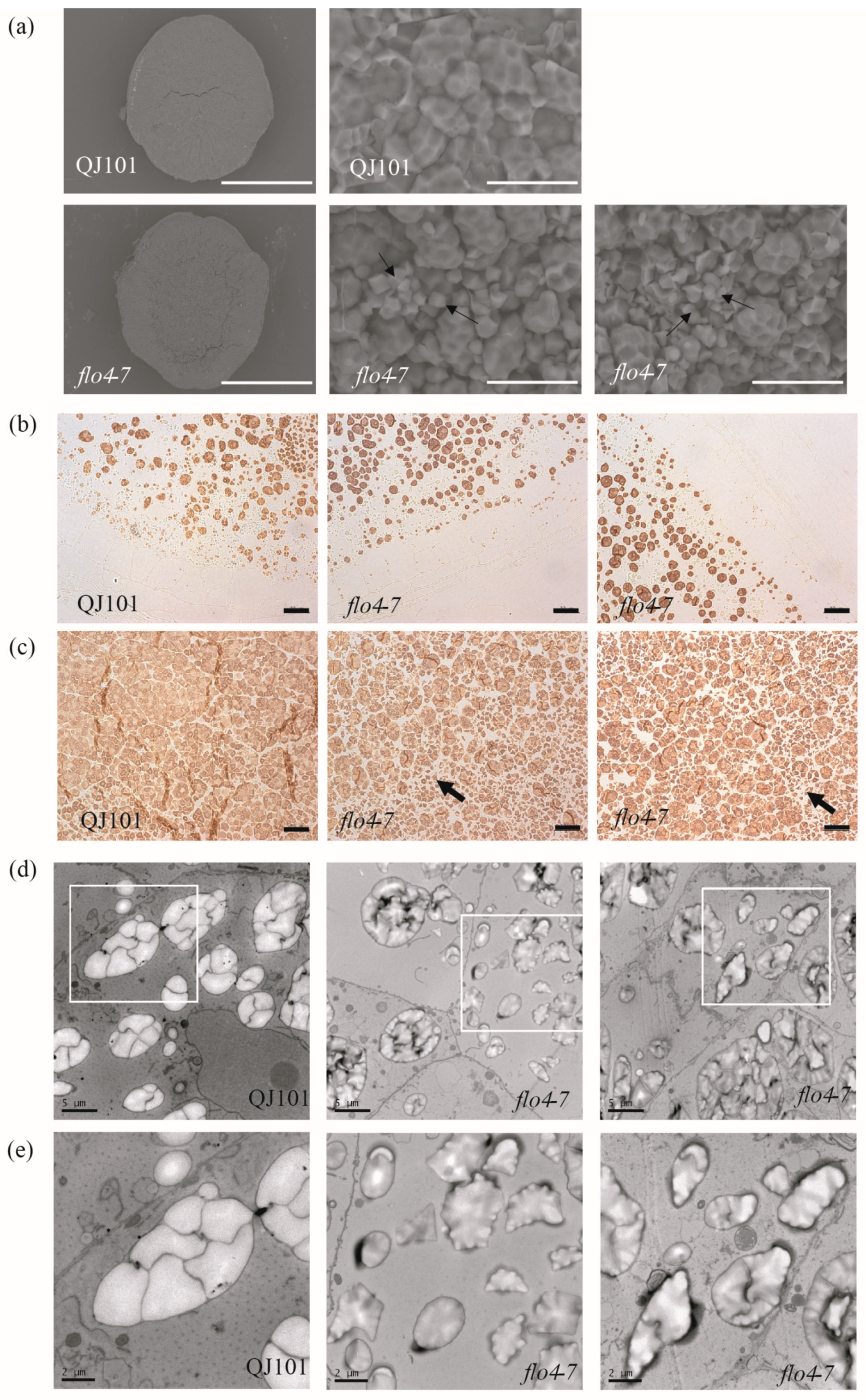
Starch, which accounts for over 70% of seed weight, plays a crucial role in rice quality. The morphology of starch granules reflects the quality of rice to a great extent. In the endosperm, insoluble starch granules (SGs) are formed in amyloplasts, which are organelles involved in starch synthesis and storage. A single amyloplast is assembled from dozens of polyhedral SGs that have sharp edges and are easily separable. SEM, TEM, and semi-thin sections in microscopy were used to observe the SG morphology of the endosperm. SEM revealed that starch granules in the wild type are densely packed, while in flo4-7, the compound starch granules are loosely arranged and become round, indicating incomplete starch filling. The number of single starch granules increases, but their morphology remains unchanged (Figure 3a). Semi-thin section microscopy revealed no significant changes in peripheral endosperm cells between the wild type and flo4-7 (Figure 3b). However, in the central part of the endosperm, the gaps between amyloplasts were significantly increased, which is consistent with the SEM results, and the number of single starch granules increased (Figure 3c). TEM observations were consistent with the SEM and semi-thin sections microscopy observations. In the wild-type endosperm, amyloplasts developed normally, and complete compound starch granules were formed from polyhedral SGs. In the flo4-7 mutant, the number of single starch granules increased, and compound starch granules were significantly reduced (Figure 3d,e). These results indicate that the FLO4-7 mutation leads to abnormal starch development in the endosperm, confirming the involvement of FLO4-7 in the synthesis of rice endosperm starch.
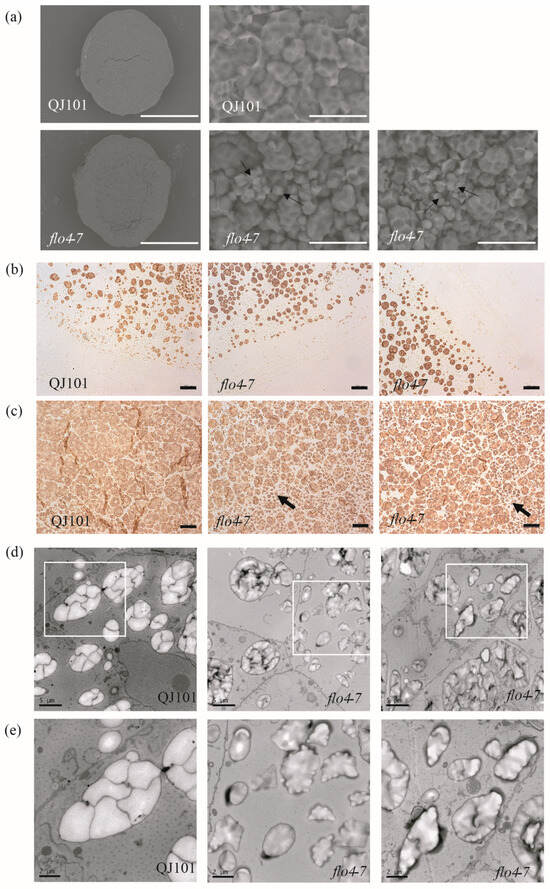
Figure 3.
Microscopy analysis of the endosperm of WT and flo4-7. (a) SEM analysis of the endosperm of WT and flo4-7. Scale bars: 0.5 mm in two pictures on the left 10 μm in three pictures on the right. Arrows represent single starch granules. (b,c) Semi-thin sections of the WT and flo4-7 endosperm at 7 days after flowering (DAF). (b) The periphery of endosperm cells. (c) The center of endosperm cells. Arrows represent single starch granules. Scale bars: 20 μm. (d,e) Transmission electron microscope analysis of the starch grains of WT and flo4-7 at 7 DAF. (e) is an enlarged version of the corresponding place in (d). Scale bars: 5 μm in d, 2 μm in (e).
3.4. The flo4-7 Phenotype is Caused by the Mutation of cyOsPPDKB Gene
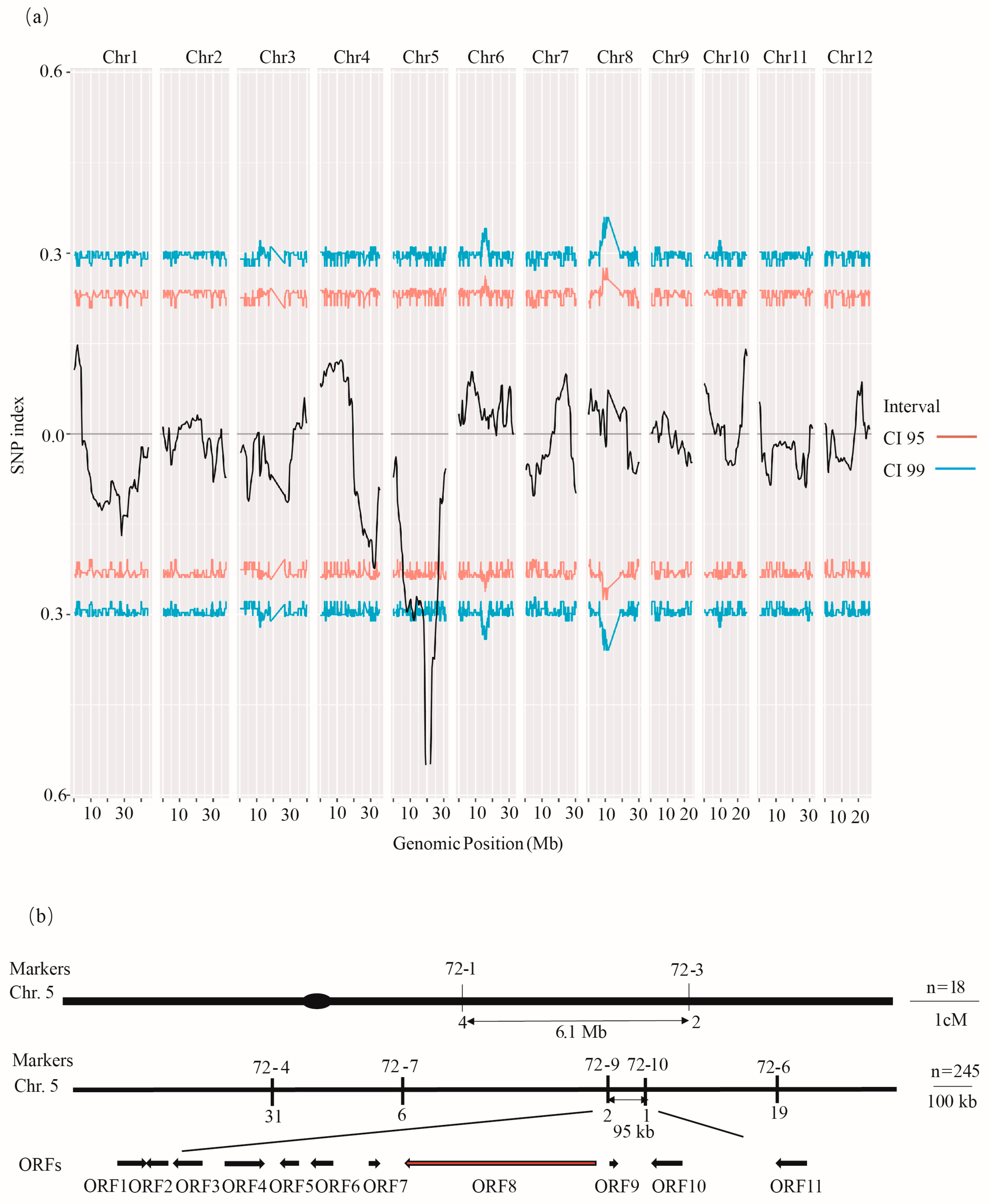
The flo4-7 mutant was crossed with the indica rice variety ZXYZ, and the F2 segregating population showed a 3:1 ratio of transparent individuals to floury endosperm individuals (Table S3), indicating that flo4-7 showed a single-gene recessive mutation. Thirty-five transparent individuals and thirty-five floury endosperm individuals were selected, and BSA sequencing technology was used to initially locate the mutation to chromosome 5, with the highest SNP index reaching 0.55 (Figure 4a). KASP markers were designed in this interval to validate the preliminary mapping of the target gene to a 6.1 Mb region. Further, the KASP marker narrowed down the interval to 95 kb, and the cyOsPPDKB gene was found to be the eighth ORF in this interval (Figure 4b).
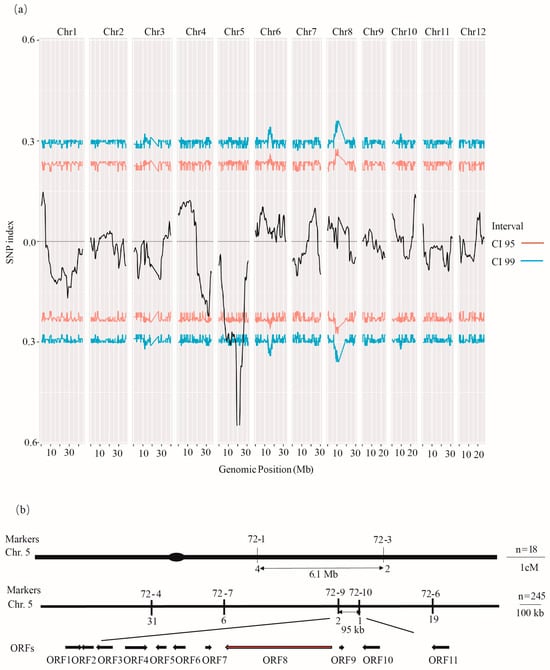
Figure 4.
Map-based cloning of FLO4-7. (a) BSA sequencing identified FLO4-7 to be located on the fifth chromosome. (b) The FLO4-7 locus was mapped to a 95 kb region by markers 72-9 and 72-10. In total, 11 ORFs were predicted (arrows). The numbers of recombinants are indicated below the map.
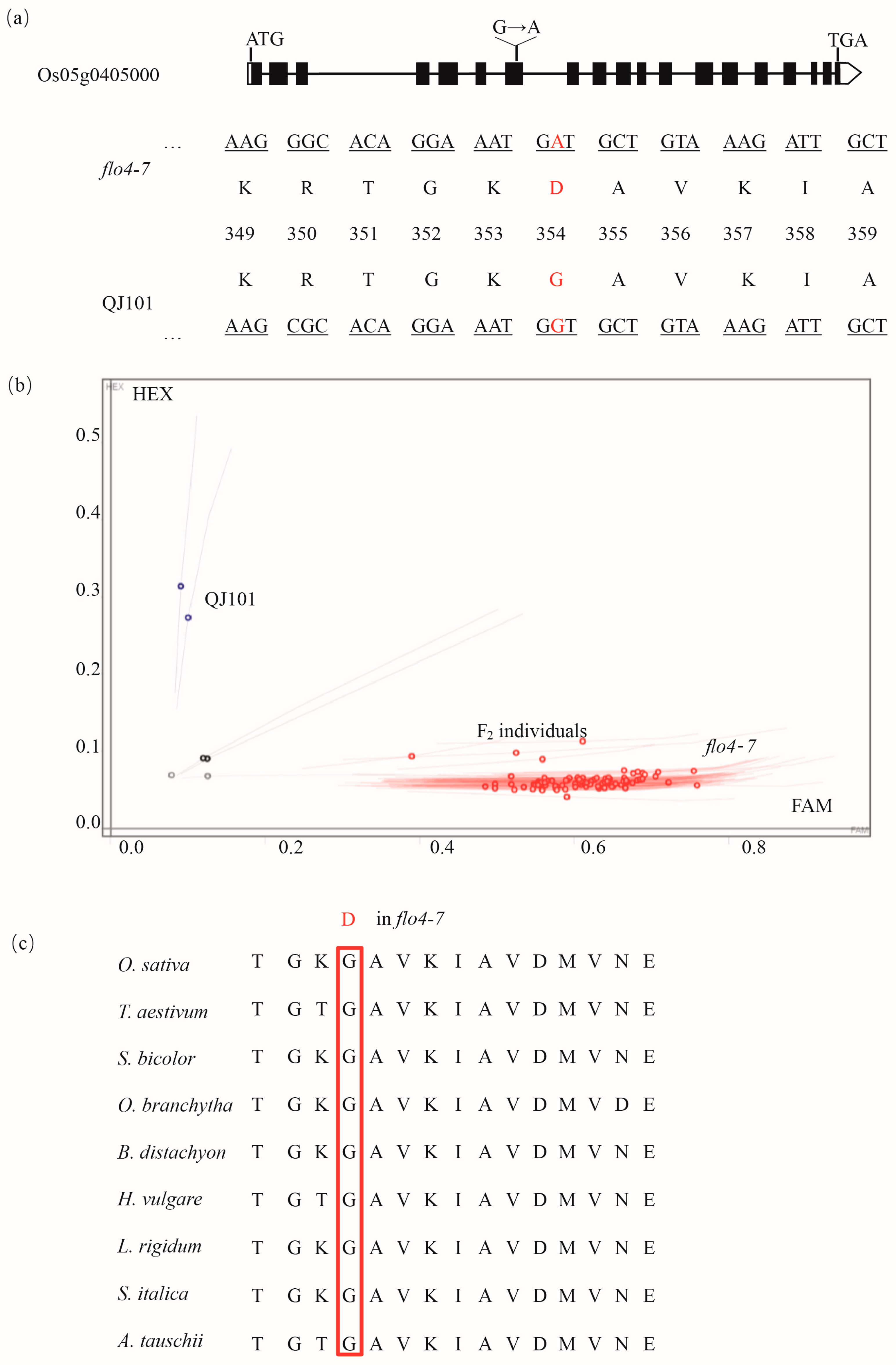
Sequencing revealed that a G-A variation in the seventh exon of cyOsPPDKB resulted in an amino acid change from G to D (Figure 5a). To validate the reliability of these results, the KASP primer is designed based on this SNP to genotype floury endosperm individuals in the F2 population, as well as wild-type and flo4-7. It was found that flo4-7 and all F2 floury endosperm individuals were of the mutant type A, while wild-type QJ101 was of the G type (Figure 5b), confirming that cyOsPPDKB is indeed the mutated gene. A further homology sequence alignment revealed that this site was conserved in different species (Figure 5c).
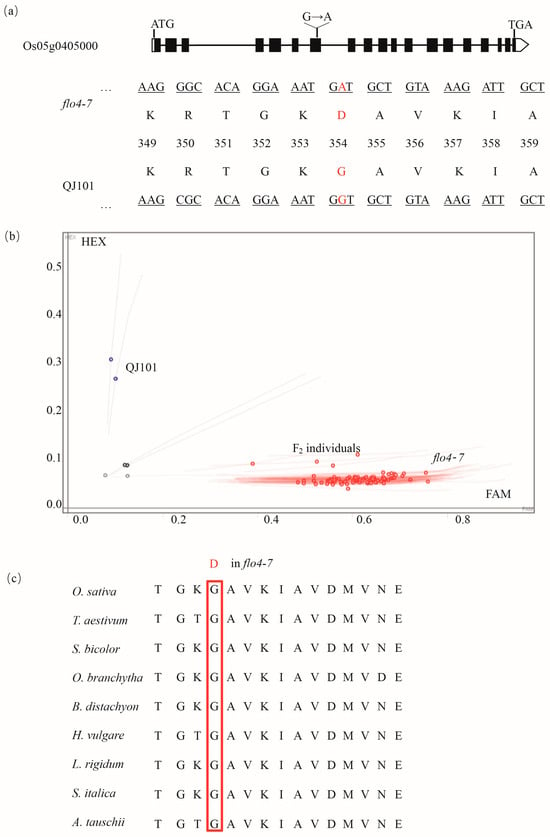
Figure 5.
A G-A substitution in the seventh exon led to a G-R change. (a) Schematic of the mutation in Os05g0405000. (b) Using the G-A mutation, KASP primers were designed for the genotyping of the WT, flo4-7, and F2 floury endosperm individuals. The red circles represent the FAM fluorophore, which binds to the base A during primer design, while the blue circles represent the HEX fluorophore, which binds to the base G. (c) The mutation site was conserved across different species.
3.5. A SNP Variation in the 18th Exon of cyOsPPDK is Associated with GC
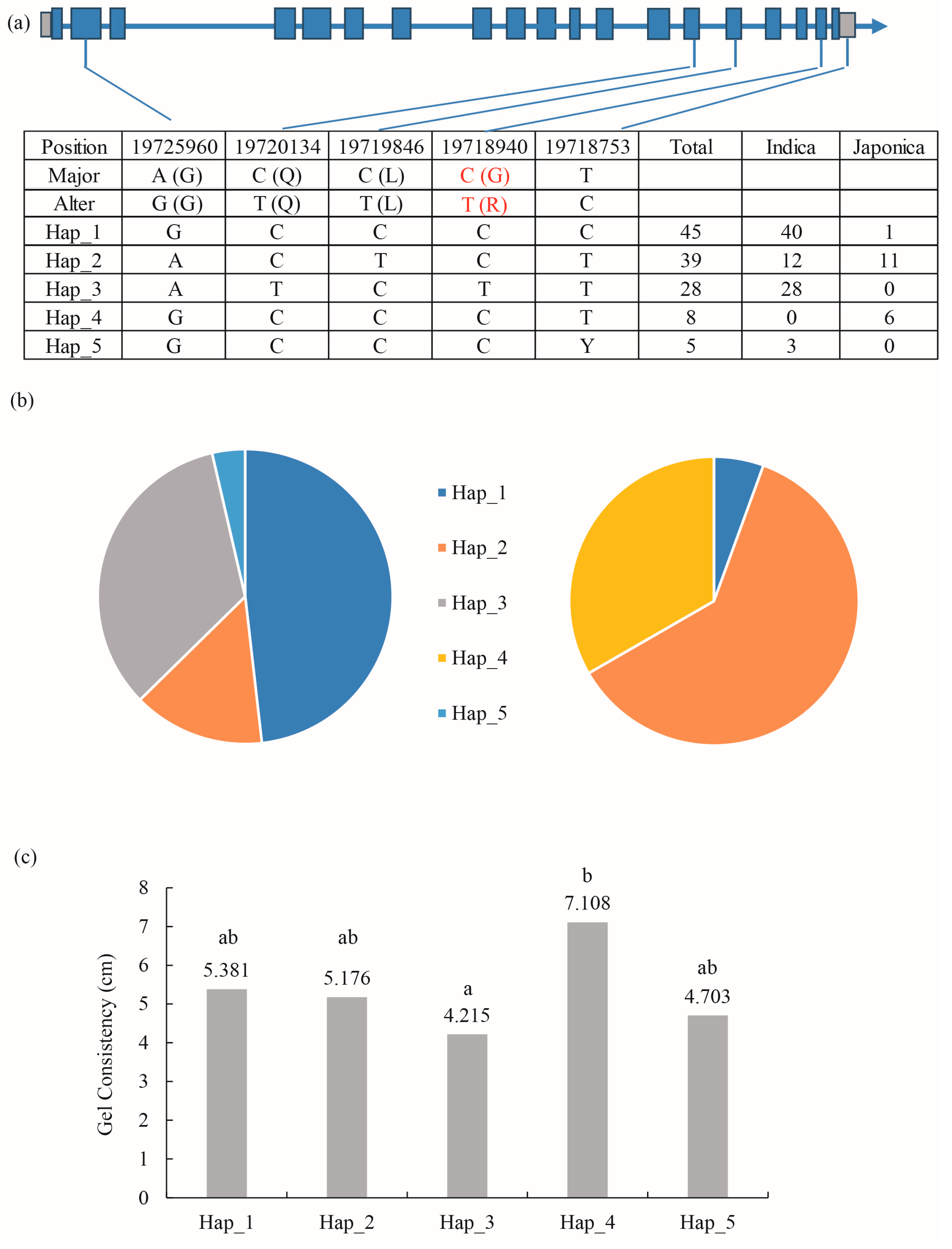
In the preliminary study, GC data for 166 rice resources were measured. Using the genome sequence information already determined on RFGB, the haplotypes of the CDS region of cyOsPPDKB were analyzed in different resources, and a total of five base variations were found. In the CDS region of the cyOsPPDKB gene, a change from C to T occurred at the 2575th base, starting from the start codon ATG and resulting in a corresponding amino acid change from G to R. The location was located in the 18th exon of cyOsPPDKB (Figure 6a). These five variants generated five distinct haplotypes, with haplotypes Hap_1-3 and Hap_5 being predominantly found in indica rice, while Hap_4 was absent in indica rice and mainly distributed in japonica rice (Figure 6b). The significant analysis of GC lengths among these haplotypes revealed a significant difference between Hap_3 and Hap_4 (Figure 6c). This difference can be primarily attributed to the SNP variation in the 18th exon, and these two haplotypes were predominantly present in indica and japonica rice, respectively. Therefore, this locus is considered a crucial determinant for the difference in viscosity between indica and japonica rice.
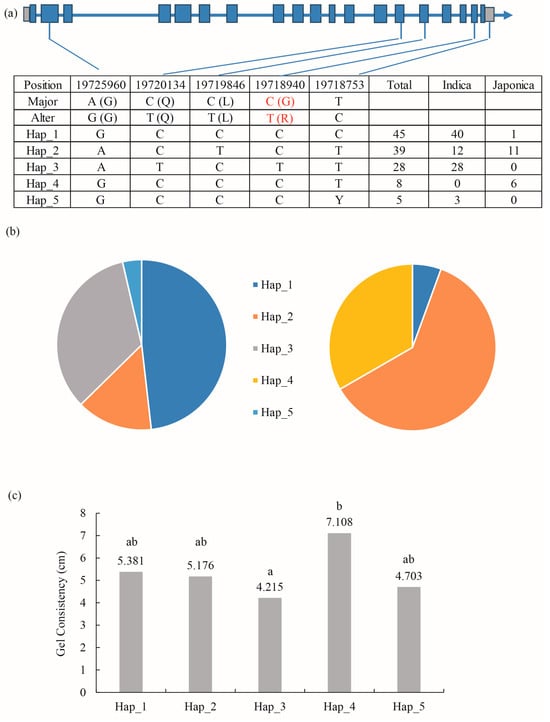
Figure 6.
Haplotype analysis of cyOsPPDKB and haplotype association with rice GC. (a) Polymorphic nucleotides in the cyOsPPDKB CDS region in indica and japonica cultivars. The red-marked C-T base difference and G-R amino acid difference represent the different haplotypes of cyOsPPDKB that cause differences of GC between indica and japonica rice. (b) Distribution of the proportion of five haplotypes (Hap_1 to Hap_5) in indica (left) and japonica (right) cultivars. (c) GC statistics of the five haplotypes. a–b indicate significant differences in Tukey’s t-tests (p < 0.05).
4. Discussion
4.1. flo4-7 Showed White-Core Endosperm Phenotype
According to the position of the opaque part, floury endosperm mutants can be categorized into white-core, white-belly, and white-back. The white-core endosperm is the major type among these mutants, and the opaque part has a different proportion in grains. Previous studies discovered that rice white-core endosperm is regulated by many genes, like FLO8, OsBT1, FLO5, FLO15, GPA5, OsPK2, FLO12, and RSR1 [9]. These genes are involved in multiple metabolic pathways, suggesting that the formation of the rice white-core endosperm is a complex process that requires further exploration of regulatory factors to enhance our understanding of its regulatory mechanisms. To date, previous studies have reported several allelic mutants of the OsPPDKB gene in rice. T-DNA insertion mutants of the OsPPDKB gene, namely flo4-1, flo4-2, and flo4-3, showed a white-core phenotype accompanied by a significant increase in lipid content [23]. Additionally, flo4-4, which carried a missense mutation, also displayed a white-core endosperm. cyOsPPDKB was expressed at considerably higher levels in flo4-4 compared to the wild type during the grain-filling stage [27,28]. In the case of flo4-5, a SNP substitution led to a white-core endosperm phenotype, along with the enhanced expression of AGPS2a, AGPS2b, SSIIb, and SSIIc [29]. As in the M14 mutant, the localization of OsPPDKB was found in both the chloroplast and cytoplasm [25]. Furthermore, the flo4-303 mutant exhibited a floury endosperm phenotype due to a two-bp insertion [30]. flo4-6 showed a floury endosperm phenotype and significantly lower grain hardness, which is suitable for producing dry-milled flour without the soaking and drying processes required in regular wet rice milling [31]. It is worth noting that although the mutant backgrounds and mutagenesis methods of flo4-6 and flo4-7 are different, their mutated bases and positions are exactly the same. The probability of the flo4-6 and flo4-7 mutation at the same position is very low, indicating that the number of different alleles of cyOsPPDKB that can cause the floury endosperm phenotype is limited, and not all sense mutations can cause this phenotype.
4.2. cyOsPPDKB Provides Energy during Endosperm Development
In addition to catalyzing the interconversion of ATP, Pi, and pyruvate to AMP, PPi, and PEP, cyOsPPDKB also converts AMP to ATP in hypoxic developing seeds, providing energy for seed development [23]. Previous studies have suggested that assimilated transport occurs through two pathways: sucrose moves via the symplastic pathway in the nucellar epidermis and via the symplastic pathway and aleurone cells into the endosperm [32,33]. It is possible that the mutation of cyOsPPDKB could lead to an insufficient energy supply, resulting in the inadequate accumulation of carbohydrates from the dorsal to the belly portion of grains during grain filling, thereby causing a significant decrease in grain thickness and weak starch filling. Furthermore, in the flo4-7 mutant, there was a significant increase in single starch granules and a decrease in compound starch granules, similar to other reported floury endosperm mutants that affect starch synthesis through energy metabolism, such as OsPK2, OsPK3, and RL1 [34,35,36]. These mutants did not alter the morphology of single starch granules but rather had an insufficient energy supply during endosperm development, leading to insufficient filling and impeding the formation of compound starch granules.
4.3. cyOsPPDKB can be Applied for Improving the GC Quality of Rice Grains
Since it is challenging to directly measure the CEQ traits, currently, the only cloned major genes are Wx and SSIIa through QTL mapping. However, the phenotypes of the chalkiness and floury endosperm are easily identifiable, and these traits usually affect CEQs with potentially low eating qualities [15]. Therefore, by screening for mutants with floury endosperm, it is possible to clone minor-effect genes that influence the CEQs, thus overcoming the bottleneck of relying solely on the Wx and SSIIa genes to improve the rice eating quality in molecular breeding.
Compared to previous cyOsPPDK allelic mutants, flo4-7 also exhibited a decrease in AC, which is a phenotype observed in multiple reported floury endosperm mutants [9]. Although the Wx gene is the major regulator of amylose synthesis, different rice varieties still display varying AC levels in the presence of the same Wx genotype, indicating the significant role of minor genes in the synthesis of linear starch. From the functional characterization of these cloned genes, it is evident that the synthesis of linear starch involves multiple pathways.
Compared to the wild type, flo4-7 showed only slight changes in urea solubility but significantly decreased the RVA indicators. The urea-induced swelling of starch granules depends on the structure of branched starch rather than the extent or structure of linear starch [37]. RVA indicators are mainly influenced by the Wx gene, followed by PUL, which plays a role in determining the fine structure of amylopectin [38]. Both of these indicators are related to the synthesis of branched starch. Therefore, it has been speculated that cyOsPPDK not only affects the AC but also has an impact on the synthesis and structure of branched starch. By measuring the distribution of branch-chain lengths in M14, it has also been confirmed that cyOsPPDKB has an effect on the formation of the short-chain structure of amylopectin [25].
In addition, flo4-7 significantly affects the GC length, with a decrease from 86 mm to 65 mm after mutation. Previous studies on floury mutants have reported limited effects on the GC. This study is the first to discover that cyOsPPDKB can affect the GC of rice flour. GC is a quantitative trait, and exploring and analyzing the effects of minor genes other than Wx on GC can provide a research basis for improving rice quality through breeding applications. A C-T base difference in the 10th exon of the Wx gene is a major site that regulates the GC length [10]. However, even among different varieties with the same Wx genotype, there are still variations in GC length, indicating the importance of minor genes in GC. This project measured the GC length of 166 rice germplasm resources and, in combination with whole-genome sequencing data from the RFGB website, conducted the haplotype analysis of cyOsPPDKB. It was found that an amino acid variation in the 18th exon of cyOsPPDKB could be an important regulatory site for the difference in the GC length between indica and japonica rice. Although the quantity of rice resources used in this study, especially japonica rice, was insufficient, the effect of cyOsPPDKB on the differentiation of the GC length in both indica and japonica rice was still evident. In the future, the size of the resource population should be expanded to further validate the reliability of these results. Additionally, this SNP could be designed as a KASP marker and combined with the C-T SNP difference in the 10th exon of Wx for breeding applications aimed at improving GC quality.
5. Conclusions
In this study, we characterized the rice flo4-7 mutant, which exhibited a floury phenotype in the endosperm. The substitution of G to A in cyOsPPDKB led to defects in the starch phenotype. An analysis of SEM, TEM, and semi-thin sections showed that there was a significant increase in single starch granules and a decrease in compound starch granules in flo4-7. In addition, a significant decrease in GC followed the mutation of FLO4-7. The haplotype analysis of GC data from 166 rice resources identified a significant difference in the GC length between Hap_3 and Hap_4. A base change from C to T at position 19718940 in the 18th exon resulted in an amino acid substitution from G to R, which could potentially serve as the main regulatory site for GC variation between indica and japonica rice. In conclusion, our findings demonstrate that cyOsPPDKB influences the CEQ of rice by affecting the GC length.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy13112759/s1, Table S1: Primers used in this study; Table S2: Pasting properties for endosperm starch of WT and flo4-7. Table S3: χ2 test of flo4-7.
Author Contributions
Z.T. conceived the study and designed the experiments. Z.G., C.L., F.H., J.X., Y.H. (Yong He) and Y.D. performed the experiments. Y.H. (Yuanyuan Hao) and Z.G. analysed the data and wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number 32001524; the Key Laboratory of Digital Upland Crops of Zhejiang Province, grant number 2022E10012; the State Key Laboratory for Conservation and Utilization of Subtropical Agro-bioresources, grant number SKLCUSA-b202305; and the Major Projects of Rice Breeding in the 14th Five Year Plan of Zhejiang Province, grant number 2021C02063-1.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. The data we used are publicly available, and the sources have been noted in the article.
References
- Xing, Z.; Tan, F.; Hua, P.; Sun, L.; Xu, G.; Zhang, Q. Characterization of the main effects, epistatic effects and their environmental interactions of QTLs on the genetic basis of yield traits in rice. Theor. Appl. Genet. 2002, 105, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.J.; Gong, R.; Tan, Y.B.; Yu, S.B. Mapping and characterization of the major quantitative trait locus qSS7 associated with increased length and decreased width of rice seeds. Theor. Appl. Genet. 2012, 125, 1717–1726. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.C.; Lin, Y.J.; Chen, H. Improving nutritional quality of rice for human health. Theor. Appl. Genet. 2020, 133, 1397–1413. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Q.; Liu, W.J.; Xu, Y.; He, Y.Q.; Luo, L.J.; Xing, Y.Z.; Xu, C.G.; Zhang, Q.F. Genetic basis of 17 traits and viscosity parameters characterizing the eating and cooking quality of rice grain. Theor. Appl. Genet. 2007, 115, 463–476. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Lin, Q.P.; Li, X.F.; Wang, F.Q.; Chen, Z.H.; Wang, J.; Li, W.Q.; Fan, F.J.; Tao, Y.J.; Jiang, Y.J.; et al. Fine-tuning the amylose content of rice by precise base editing of the Wx gene. Plant Biotechnol. J. 2021, 19, 11–13. [Google Scholar] [CrossRef]
- Zhang, A.P.; Gao, Y.; Li, Y.Y.; Ruan, B.P.; Yang, S.L.; Liu, C.L.; Zhang, B.; Jiang, H.Z.; Fang, G.N.; Ding, S.L.; et al. Genetic analysis for cooking and eating quality of super rice and fine mapping of a novel locus qgc10 for gel consistency. Front. Plant Sci. 2020, 11, 342. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Zheng, F.Q.; Shen, G.Z.; Gao, J.P.; Snustad, D.P.; Li, M.G.; Zhang, J.L.; Hong, M. The amylose content in rice endosperm is related to the post-transcriptional regulation of the waxy gene. Plant J. 1995, 7, 613–622. [Google Scholar] [CrossRef]
- Su, Y.; Rao, Y.C.; Hu, S.K.; Yang, Y.L.; Gao, Z.Y.; Zhang, G.H.; Liu, J.; Hu, J.; Yan, M.X.; Dong, G.J.; et al. Map-based cloning proves qGC-6, a major QTL for gel consistency of japonica/indica cross, responds by Waxy in rice (Oryza sativa L.). Theor. Appl. Genet. 2011, 123, 859–867. [Google Scholar] [CrossRef]
- Zhao, D.S.; Zhang, C.Q.; Li, Q.F.; Liu, Q.Q. Genetic control of grain appearance quality in rice. Biotechnol. Adv. 2022, 60, 108014. [Google Scholar] [CrossRef]
- Tran, N.A.; Daygon, V.D.; Resurreccion, A.P.; Cuevas, R.P.; Corpuz, H.M.; Fitzgerald, M.A. A single nucleotide polymorphism in the Waxy gene explains a significant component of gel consistency. Theor. Appl. Genet. 2011, 123, 519–525. [Google Scholar] [CrossRef]
- Bao, J.S.; Corke, H.; Sun, M. Nucleotide diversity in starch synthase IIa and validation of single nucleotide polymorphisms in relation to starch gelatinization temperature and other physicochemical properties in rice (Oryza sativa L.). Theor. Appl. Genet. 2006, 113, 1171–1183. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, M.A.; Martin, M.; Ward, R.M.; Park, W.D.; Shead, H.J. Viscosity of rice flour: A rheological and biological study. J. Agric. Food Chem. 2003, 51, 2295–2299. [Google Scholar] [CrossRef] [PubMed]
- Buenafe, R.; Kumanduri, V.; Sreenivasulu, N. Deploying viscosity and starch polymer properties to predict cooking and eating quality models: A novel breeding tool to predict texture. Carbohydr. Polym. 2021, 260, 117766. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.L.; Ali, J.; Wang, X.Q.; Franje, N.J.; Revilleza, J.E.; Xu, J.L.; Li, Z.K. Relationship of rice grain amylose, gelatinization temperature and pasting properties for breeding better eating and cooking quality of rice varieties. PLoS ONE 2016, 11, e168483. [Google Scholar] [CrossRef] [PubMed]
- Chun, A.; Song, J.; Kim, K.; Lee, H. Quality of head and chalky rice and deterioration of eating quality by chalky rice. J. Crop Sci. Biotechnol. 2009, 12, 239–244. [Google Scholar] [CrossRef]
- Peng, C.; Wang, Y.H.; Liu, F.; Ren, Y.L.; Zhou, K.N.; Lv, J.; Zheng, M.; Zhao, S.L.; Zhang, L.; Wang, C.; et al. FLOURY ENDOSPERM6 encodes a CBM48 domain-containing protein involved in compound granule formation and starch synthesis in rice endosperm. Plant J. 2014, 77, 917–930. [Google Scholar] [CrossRef]
- Zhao, Q.; Ye, Y.; Han, Z.Y.; Zhou, L.J.; Guan, X.Y.; Pan, G.; Asad, M.A.U.; Cheng, F.M. SSIIIa-RNAi suppression associated changes in rice grain quality and starch biosynthesis metabolism in response to high temperature. Plant Sci. 2020, 294, 110443. [Google Scholar] [CrossRef]
- Cai, Y.; Zhang, W.W.; Fu, Y.S.; Shan, Z.Z.; Xu, J.H.; Wang, P.; Kong, F.; Jin, J.; Yan, H.G.; Ge, X.Y.; et al. Du13 encodes a C2H2 zinc-finger protein that regulates Wxb pre-mRNA splicing and microRNA biogenesis in rice endosperm. Plant Biotechnol. J. 2022, 20, 1387–1401. [Google Scholar] [CrossRef]
- Wang, W.; Wei, X.J.; Jiao, G.A.; Chen, W.Q.; Wu, Y.W.; Sheng, Z.H.; Hu, S.K.; Xie, L.H.; Wang, J.Y.; Tang, S.Q.; et al. GBSS-BINDING PROTEIN, encoding a CBM48 domain-containing protein, affects rice quality and yield. J. Integr. Plant Biol. 2020, 62, 948–966. [Google Scholar] [CrossRef]
- Lei, J.; Teng, X.; Wang, Y.L.; Jiang, X.K.; Zhao, H.H.; Zheng, X.M.; Ren, Y.L.; Dong, H.H.; Wang, Y.L.; Duan, E.C.; et al. Plastidic pyruvate dehydrogenase complex E1 component subunit Alpha1 is involved in galactolipid biosynthesis required for amyloplast development in rice. Plant Biotechnol. J. 2022, 20, 437–453. [Google Scholar] [CrossRef]
- Zhu, X.P.; Teng, X.; Wang, Y.L.; Hao, Y.Y.; Jing, R.N.; Wang, Y.F.; Liu, Y.; Zhu, J.P.; Wu, M.M.; Zhong, M.S.; et al. FLOURY ENDOSPERM11 encoding a plastid heat shock protein 70 is essential for amyloplast development in rice. Plant Sci. 2018, 277, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Swain, A.; Behera, D.; Karmakar, S.; Dash, M.; Dash, B.; Swain, P.; Molla, K.; Baig, M. Morphophysiological alterations in transgenic rice lines expressing PPDK and ME genes from the C4 model Setaria italica. J. Plant Physiol. 2021, 264, 153482. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Park, S.; Makoto, M.; An, G. White-core endosperm floury endosperm-4 in rice is generated by knockout mutations in the C4-type pyruvate orthophosphate dikinase gene (OsPPDKB). Plant J. 2005, 42, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Chastain, C.J.; Failing, C.J.; Manandhar, L.; Zimmerman, M.A.; Lakner, M.M.; Nguyen, T.H. Functional evolution C4 pyruvate, orthophosphate dikinase. J. Exp. Bot. 2011, 62, 3083–3091. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, L.L.; Lin, L.S.; Zhao, L.X.; Liu, Q.Q.; Wei, C.X. A novel mutation of OsPPDKB, encoding pyruvate orthophosphate dikinase, affects metabolism and structure of starch in the rice endosperm. Int. J. Mol. Sci. 2018, 19, 2268. [Google Scholar] [CrossRef]
- Wang, Z.M.; Li, H.X.; Liu, X.F.; He, Y.; Zeng, H.L. Reduction of pyruvate orthophosphate dikinase activity is associated with high temperature-induced chalkiness in rice grains. Plant Physiol. Biochem. 2015, 89, 76–84. [Google Scholar] [CrossRef]
- Wang, H.; Mo, Y.; Im, D.; Jang, S.; Ham, T.; Lee, J.; Jeung, J.; Kwon, S. A new SNP in cyOsPPDK gene is associated with floury endosperm in Suweon 542. Mol. Genet. Genomics 2018, 293, 1151–1158. [Google Scholar] [CrossRef]
- Mo, Y.; Jeung, J.; Shin, Y.; Park, C.; Kang, K.; Kim, B. Agronomic and genetic analysis of Suweon 542, a rice floury mutant line suitable for dry milling. Rice 2013, 6, 37. [Google Scholar] [CrossRef]
- Wang, H.; Ham, T.; Im, D.; Lar, S.; Jang, S.; Lee, J.; Mo, Y.; Jeung, J.; Kim, S.; Kwon, S. A new SNP in rice gene encoding pyruvate phosphate dikinase (PPDK) associated with floury endosperm. Genes 2020, 11, 465. [Google Scholar] [CrossRef]
- Matsuba, S.; Maruyama-Funatsuki, W.; Umemoto, T.; Kato, H.; Kuroki, M.; Yokogami, N.; Ikegaya, T.; Shimizu, H.; Iriki, N. The induced mutant allele flo4-303 confers floury characteristics on the japonica rice cultivar ‘Hoshinoko’. Breed. Sci. 2022, 72, 383–388. [Google Scholar] [CrossRef]
- Ha, S.-K.; Lee, H.-S.; Lee, S.Y.; Lee, C.-M.; Mo, Y.; Jeung, J.-U. Characterization of flo4-6, a novel cyOsPPDKB allele conferring floury endosperm characteristics suitable for dry-milled rice flour production. Agronomy 2023, 13, 1306. [Google Scholar] [CrossRef]
- Wu, M.M.; Cai, M.H.; Zhai, R.R.; Ye, J.; Zhu, G.F.; Yu, F.M.; Ye, S.H.; Zhang, X.M. A mitochondrion-associated PPR protein, WBG1, regulates grain chalkiness in rice. Front. Plant Sci. 2023, 14, 1136849. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Luo, D.; Yang, B.; Frommer, W.B.; Eom, J.S. SWEET11 and 15 as key players in seed filling in rice. New Phytol. 2018, 218, 604–615. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.C.; Li, S.F.; Jiao, G.A.; Sheng, Z.H.; Wu, Y.W.; Shao, G.N.; Xie, L.H.; Peng, C.; Xu, J.F.; Tang, S.Q.; et al. OsPK2 encodes a plastidic pyruvate kinase involved in rice endosperm starch synthesis, compound granule formation and grain filling. Plant Biotechnol. J. 2018, 16, 1878–1891. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Tu, B.; Yang, W.; Yuan, H.; Li, J.L.; Guo, L.N.; Zheng, L.; Chen, W.L.; Zhu, X.B.; Wang, Y.P.; et al. Mitochondria-associated pyruvate kinase complexes regulate grain filling in rice. Plant Physiol. 2020, 183, 1073–1087. [Google Scholar] [CrossRef]
- Wu, M.W.; Zhao, H.; Zhang, J.D.; Guo, L.; Liu, C.M. RADICLELESS 1 (RL1)-mediated nad4 intron 1 splicing is crucial for embryo and endosperm development in rice (Oryza sativa L.). Biochem. Biophys. Res. Commun. 2020, 523, 220–225. [Google Scholar] [CrossRef]
- Nishi, A.; Nakamura, Y.; Tanaka, N.; Satoh, H. Biochemical and genetic analysis of the effects of amylose-extender mutation in rice endosperm. Plant Physiol. 2001, 127, 459–472. [Google Scholar] [CrossRef]
- Yan, C.J.; Tian, Z.X.; Fang, Y.W.; Yang, Y.C.; Li, J.; Zeng, S.Y.; Gu, S.L.; Xu, C.W.; Tang, S.Z.; Gu, M.H. Genetic analysis of starch paste viscosity parameters in glutinous rice (Oryza sativa L.). Theor. Appl. Genet. 2011, 122, 63–76. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).