Lignocellulosic Composition Not Associated with Stem Borer Resistance in Select Louisiana Sugarcane Cultivars
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Site and Experimental Design
2.2. Growing Season Sample Collection
2.3. Harvest Sample Collection
2.4. Statistical Analyses
3. Results
3.1. Growing Season Lignocellulosic Composition
3.2. Growing Season Brix
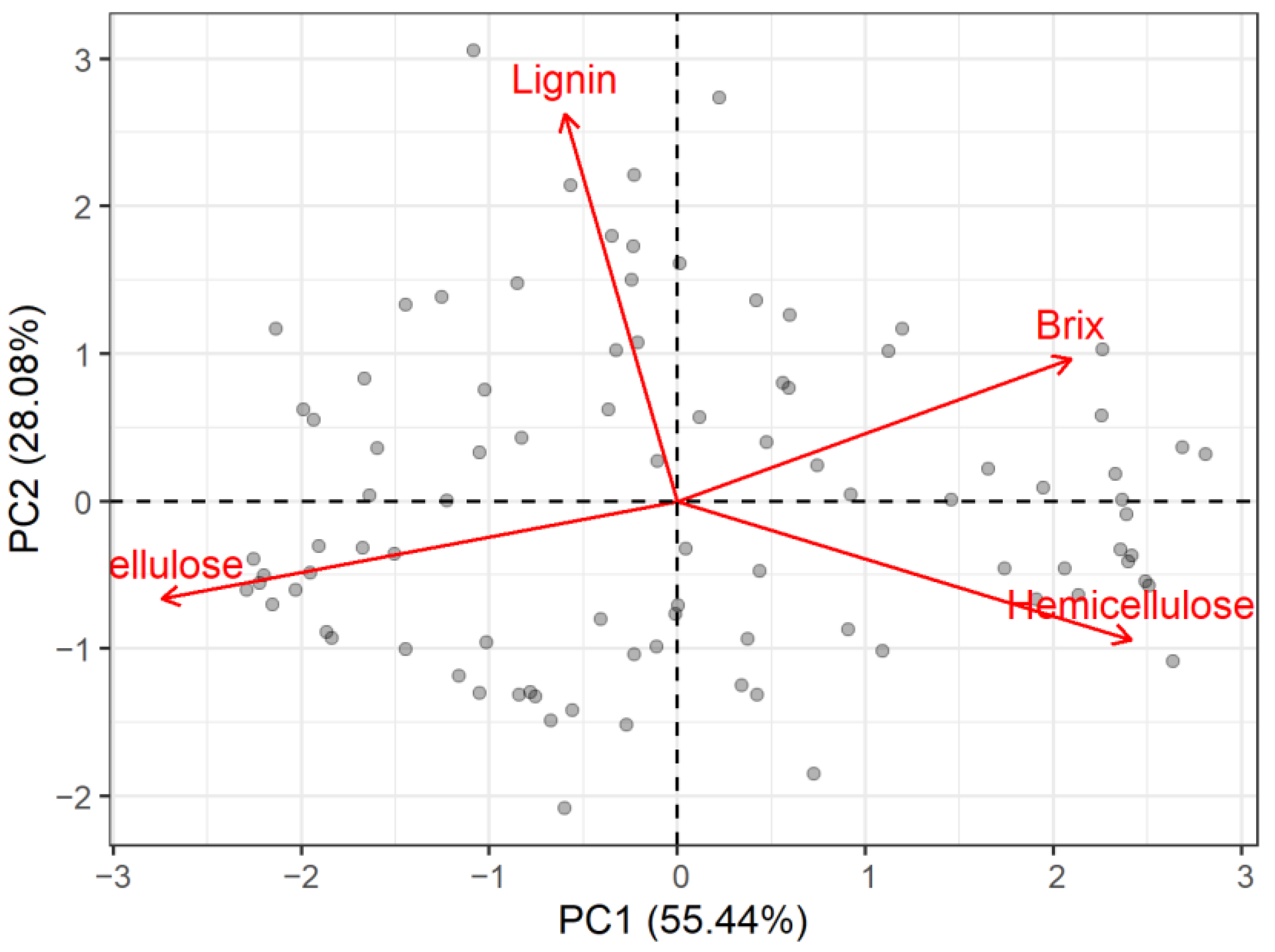
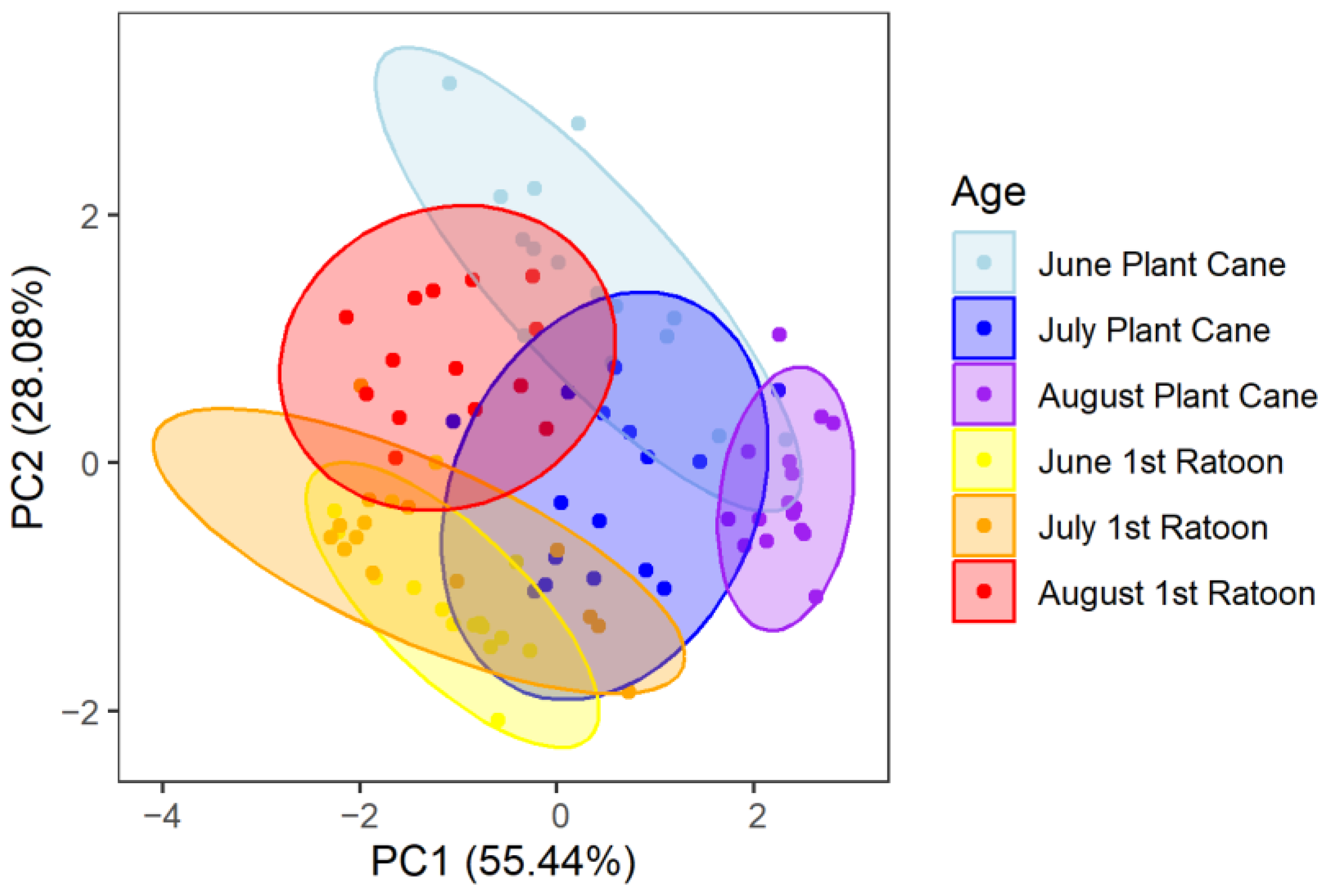
3.3. Growing Season PCA
3.4. Harvest Metrics
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holloway, T.E.; Loftin, U.C. The Sugar-Cane Moth Borer; United State Department of Agriculture: Washington, DC, USA, 1919. [Google Scholar]
- Dyar, H.G.; Heinrich, C. The American Moths of the Genus Diatraea and Allies. Proc. U. S. Natl. Mus. 1927, 71, 1–48. [Google Scholar] [CrossRef]
- Long, W.H.; Hensley, S.D. Insect Pests of Sugar Cane. Annu. Rev. Entomol. 1972, 17, 149–176. [Google Scholar] [CrossRef]
- Reagan, T.E.; Mulcahy, M.M. Interaction of Cultural, Biological, and Varietal Controls for Management of Stalk Borers in Louisiana Sugarcane. Insects 2019, 10, 305. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.E. Successful Integrated Pest Management Minimizes the Economic Impact of Diatraea saccharalis (Lepidoptera: Crambidae) on the Louisiana Sugarcane Industry. J. Econ. Entomol. 2020, 114, 468–471. [Google Scholar] [CrossRef]
- Dyar, H.G. Seven New Crambids from the United States. Insecutor Inscitiae Menstruus 1917, 5, 84–87. [Google Scholar] [CrossRef]
- Showler, A.T. Mexican Rice Borer Control Tactics in United States Sugarcane. Insects 2019, 10, 160. [Google Scholar] [CrossRef]
- Johnson, K.J.R.; Van Leerdam, M.B. Range Extension of Acigona loftini into the Lower Rio Grande Valley of Texas. Sugar Azucar. 1981, 76, 34. [Google Scholar]
- Browning, H.W.; Way, M.O.; Drees, B.M. Managing the Mexican Rice Borer in Texas. Bull. Tex. Agric. Ext. Serv. 1989, B-1620. [Google Scholar]
- Reay-Jones, F.P.F.; Wilson, L.T.; Way, M.O.; Reagan, T.E.; Carlton, C.E. Movement of Mexican Rice Borer (Lepidoptera: Crambidae) through the Texas Rice Belt. J. Econ. Entomol. 2007, 100, 54–60. [Google Scholar] [CrossRef]
- Hummel, N.A.; Hardy, T.; Reagan, T.E.; Pollet, D.; Carlton, C.; Stout, M.J.; Beuzelin, J.M.; Akbar, W.; White, W. Monitoring and First Discovery of the Mexican Rice Borer Eoreuma loftini (Lepidoptera: Crambidae) in Louisiana. Fla. Entomol. 2010, 93, 123–124. [Google Scholar] [CrossRef]
- Wilson, B.E.; Hardy, T.N.; Beuzelin, J.M.; VanWeelden, M.T.; Reagan, T.E.; Miller, R.; Meaux, J.; Stout, M.J.; Carlton, C.E. Expansion of the Mexican Rice Borer (Lepidoptera: Crambidae) into Rice and Sugarcane in Louisiana. Environ. Entomol. 2015, 44, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.E.; Beuzelin, J.M.; Reagan, T.E. Population Distribution and Range Expansion of the Invasive Mexican Rice Borer (Lepidoptera: Crambidae) in Louisiana. Environ. Entomol. 2017, 46, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Legaspi, J.C.; Legaspi, B.C., Jr.; Irvine, J.E.; Johnson, J.; Meagher, R.L., Jr.; Rozeff, N. Stalkborer Damage on Yield and Quality of Sugarcane in Lower Rio Grande Valley of Texas. J. Econ. Entomol. 1999, 92, 228–234. [Google Scholar] [CrossRef]
- Reay-Jones, F.P.F.; Wilson, L.T.; Reagan, T.E.; Legendre, B.L.; Way, M.O. Predicting Economic Losses from the Continued Spread of the Mexican Rice Borer (Lepidoptera: Crambidae). J. Econ. Entomol. 2008, 101, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Kfir, R.; Overholt, W.A.; Khan, Z.R.; Polaszek, A. Biology and Management of Economically Important Lepidopteran Cereal Stem Borers in Africa. Annu. Rev. Entomol. 2002, 47, 701–731. [Google Scholar] [CrossRef]
- Abbasipour, H.; Askarianzadeh, A. Identification of Physical and Biochemical Agents Related to Resistance in Different Sugarcane Cultivars to Stalk Borers, Sesamia spp. (Lep.: Noctuidae). In Crop Plant Resistance to Biotic and Abiotic Factors: Current Potential and Future Demands, Proceedings of the 3rd International Symposium on Plant Protection and Plant Health in Europe, Julius Kühn-Institut, Berlin-Dahlem, Germany, 14–16 May 2009; Julius Kühn-Institut: Berlin-Dahlem, Germany, 2009; Volume 2009, pp. 130–136. [Google Scholar]
- Kouame, D.K.; Pene, C.B.; Zouzou, M. Evaluation of sugarcane varietal resistance to the African tropical stem borer (Eldana saccharina Walker) in Ivory Coast. J. Appl. Biosci. 2010, 26, 1614–1622. [Google Scholar]
- Nikpay, A. Evaluation of Varietal Resistance of Commercial Sugarcane Varieties to Stalk Borers, Sesamia spp. (Lepidoptera: Noctuidae), under Field Conditions. Acta Entomol. Sin. 2016, 59, 785–790. [Google Scholar]
- Bessin, R.T.; Moser, E.B.; Reagan, T.E. Integration of Control Tactics for Management of the Sugarcane Borer (Lepidoptera: Pyralidae) in Louisiana Sugarcane. J. Econ. Entomol. 1990, 83, 1563–1569. [Google Scholar] [CrossRef]
- Bessin, R.T.; Reagan, T.E. Cultivar Resistance and Arthropod Predation of Sugarcane Borer (Lepidoptera: Pyralidae) Affects Incidence of Deadhearts in Louisiana Sugarcane. J. Econ. Entomol. 1993, 86, 929–932. [Google Scholar] [CrossRef]
- Mathes, R.; Charpentier, L.J. Some Techniques and Observations in Studying the Resistance of Sugar Cane Varieties to the Sugar Cane Borer in Louisiana. In Proceedings of the International Society of Sugarcane Technologists, 11th Congress, Réduit, Mauritius, 24 September–5 October 1962; International Society of Sugarcane Technologists: Réduit, Mauritius, 1963; Volume 6, pp. 594–603. [Google Scholar]
- Meagher, R.L.; Irvine, J.E.; Breene, R.G.; Pfannenstiel, R.S.; Gallo-Meagher, M. Resistance Mechanisms of Sugarcane to Mexican Rice Borer (Lepidoptera: Pyralidae). J. Econ. Entomol. 1996, 89, 536–543. [Google Scholar] [CrossRef]
- Zhou, M.M.; Kimbeng, C.A.; da Silva, J.A.; White, W.H. Cross-Resistance between the Mexican Rice Borer and the Sugarcane Borer (Lepidoptera: Crambidae): A Case Study Using Sugarcane Breeding Populations. Crop Sci. 2010, 50, 861–869. [Google Scholar] [CrossRef]
- Hale, A.L.; White, W.H.; Dufrene, E.O.; Tew, T.L.; Grisham, M.P.; Pan, Y.B.; Miller, J.D. HoCP 04-838—A New Sugarcane Variety for Louisiana. J. Am. Soc. Sugar Cane Technol. 2012, 32, 84–85. [Google Scholar]
- Keeping, M.G.; Rutherford, R.S. Resistance Mechanisms of South African Sugarcane to the Stalk Borer Eldana Saccharina (Lepidoptera: Pyralidae): A Review. Proc. S. Afr. Sugar Cane Technol. Assoc. 2004, 78, 307–311. [Google Scholar]
- Singh, S.P.; Nigam, A.; Singh, R.K. Influence of Rind Hardness on Sugarcane Quality. Am. J. Plant Sci. 2013, 4, 35951. [Google Scholar] [CrossRef]
- Rutherford, R.S.; Meyer, J.H.; Smith, G.S.; Van Staden, J. Resistance to Eldana Saccharina (Lepidoptera: Pyralidae) in Sugarcane and Some Phytochemical Correlations. Proc. S. Afr. Sugar Cane Technol. Assoc. 1993, 67, 82–87. [Google Scholar]
- Sanghera, G.S.; Kumar, A. A Review on Mechanisms, Screening and Engineering for Pest Resistance in Sugarcane (Saccharum spp.). J. Pharmacogn. Phytochem. 2018, 7, 187–194. [Google Scholar]
- Butrón, A.; Malvar, R.A.; Revilla, P.; Soengas, P.; Ordás, A.; Geiger, H.H. Rind Puncture Resistance in Maize: Inheritance and Relationship with Resistance to Pink Stem Borer Attack. Plant Breed. 2002, 121, 378–382. [Google Scholar] [CrossRef]
- Santiago, R.; Souto, X.C.; Sotelo, J.; Butrón, A.; Malvar, R.A. Relationship between Maize Stem Structural Characteristics and Resistance to Pink Stem Borer (Lepidoptera: Noctuidae) Attack. J. Econ. Entomol. 2003, 96, 1563–1570. [Google Scholar] [CrossRef]
- Guo, J.; He, K.; Meng, Y.; Hellmich, R.L.; Chen, S.; Lopez, M.D.; Lauter, N.; Wang, Z. Asian Corn Borer Damage Is Affected by Rind Penetration Strength of Corn Stalks in a Spatiotemporally Dependent Manner. Plant Direct 2022, 6, e381. [Google Scholar] [CrossRef]
- Singh, B.U.; Rana, B.S. Varietal Resistance in Sorghum to Spotted Stem Borer, Chilo partellus (Swinhoe). Int. J. Trop. Insect Sci. 1989, 10, 3–27. [Google Scholar] [CrossRef]
- Besheit, R.; Helmy, S.; Bachoosh, S. Assessment of Resistance to Pink Stem Borer and Aphid Infestation in Sweet Sorghum Cultivars and Their Productivity and Technological Traits under Four Sowing Dates. Egypt. J. Agric. Sci. 2019, 70, 219–228. [Google Scholar] [CrossRef]
- White, W.H.; Tew, T.L.; Richard, E.P. Association of Sugarcane Pith, Rind Hardness, and Fiber with Resistance to the Sugarcane Borer. J. Am. Soc. Sugar Cane Technol. 2006, 26, 87–100. [Google Scholar]
- Posey, F.R.; White, W.H.; Reay-Jones, F.P.F.; Gravois, K.; Salassi, M.E.; Leonard, B.R.; Reagan, T.E. Sugarcane Borer (Lepidoptera: Crambidae) Management Threshold Assessment on Four Sugarcane Cultivars. J. Econ. Entomol. 2006, 99, 966–971. [Google Scholar] [CrossRef] [PubMed]
- Hale, A.L.; Todd, J.; Pan, Y.-B.; Tew, T.L.; Veremis, J.C.; White, W.; Dufrene, E.O.; Grisham, M.P.; Gravois, K.A.; Jackson, W.; et al. Registration of ‘Ho 07-613’ Sugarcane. J. Plant Regist. 2022, 16, 351–362. [Google Scholar] [CrossRef]
- Fuchs, T.W.; Harding, J.A. Oviposition Patterns, Egg Parasitism, and Spring Emergence of the Sugarcane Borer, Diatraea Saccharalis. Environ. Entomol. 1978, 7, 601–604. [Google Scholar] [CrossRef]
- Martin, S.A.; Darrah, L.L.; Hibbard, B.E. Divergent Selection for Rind Penetrometer Resistance and Its Effects on European Corn Borer Damage and Stalk Traits in Corn. Crop Sci. 2004, 44, 711–717. [Google Scholar] [CrossRef]
- Santiago, R.; Butrón, A.; Revilla, P.; Malvar, R.A. Is the Basal Area of Maize Internodes Involved in Borer Resistance? BMC Plant Biol. 2011, 11, 137. [Google Scholar] [CrossRef]
- Bhavani, B.; Reddy, K.D.; Rao, N.V.; Lakshmi, M.B. Biochemical Basis for Antibiosis Mechanism of Resistance in Sugarcane to Early Shoot Borer, Chilo Infuscatellus Snellen. Trop. Agric. Res. 2012, 23, 126–141. [Google Scholar] [CrossRef]
- Rossato, J.A.d.S.; Costa, G.H.G.; Madaleno, L.L.; Mutton, M.J.R.; Higley, L.G.; Fernandes, O.A. Characterization and Impact of the Sugarcane Borer on Sugarcane Yield and Quality. Agron. J. 2013, 105, 643–648. [Google Scholar] [CrossRef]
- Martin, F.A.; Richard, C.A.; Hensley, S.D. Host Resistance to Diatraea saccharalis (F.): Relationship of Sugarcane Internode Hardness to Larval Damage. Environ. Entomol. 1975, 4, 687–688. [Google Scholar] [CrossRef]
- Terrill, T.H.; Wolfe, R.M.; Muir, J.P. Factors Affecting ANKOMTM Fiber Analysis of Forage and Browse Varying in Condensed Tannin Concentration. J. Sci. Food Agric. 2010, 90, 2723–2726. [Google Scholar] [CrossRef] [PubMed]
- Goering, H.K.; Van Soest, P.J. Forage Fiber Analysis (Apparatus, Reagents, Procedures, and Some Applications); USDA ARS: Beltsville, MD, USA, 1970. [Google Scholar]
- Lingle, S.E.; Thomson, J.L. Sugarcane Internode Composition during Crop Development. Bioenerg. Res. 2012, 5, 168–178. [Google Scholar] [CrossRef]
- Legendre, B.L. The Core/Press Method for Predicting the Sugar Yield from Cane for Use in Cane Payment. Sugar J. 1992, 54, 2–7. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing 2023. Available online: https://www.R-project.org/ (accessed on 30 October 2023).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Lenth, R.V.; Buerkner, P.; Herve, M.; Love, J.; Miguez, F.; Riebl, H.; Singmann, H. Emmeans: Estimated Marginal Means, Aka Least-Squares Means, version 1.7. 2; R Core Team: Vienna, Austria, 2022. [Google Scholar]
- Husson, F.; Josse, J.; Le, S. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Wickam, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Williams, P.W.; Davis, F.M.; Buckley, P.M.; Hedin, P.A.; Baker, G.T.; Luthe, D.S. Factors Associated with Resistance to Fall Armyworm (Lepidoptera: Noctuidae) and Southwestern Corn Borer (Lepidoptera: Crambidae) in Corn at Different Vegetative Stages. J. Econ. Entomol. 1998, 91, 1471–1480. [Google Scholar] [CrossRef]
- Buendgen, M.R.; Coors, J.G.; Grombacher, A.W.; Russell, W.A. European Corn Borer Resistance and Cell Wall Composition of Three Maize Populations. Crop Sci. 1990, 30, 505–510. [Google Scholar] [CrossRef]
- López-Malvar, A.; Ordás, B.; Souto, C.; Encina, A.; Malvar, R.A.; Santiago, R. Chemical Changes during Maize Tissue Aging and Its Relationship with Mediterranean Corn Borer Resistance. J. Agric. Food Chem. 2017, 65, 9180–9185. [Google Scholar] [CrossRef]
- Hall, M.B.; Mertens, D.R. Comparison of Alternative Neutral Detergent Fiber Methods to the AOAC Definitive Method. J. Dairy Sci. 2023, 106, 5364–5378. [Google Scholar] [CrossRef]
- White, P.M.; Rice, C.W.; Baldock, J.A.; Tuinstra, M.R. Soil Biological Properties Following Additions of Bmr Mutant Grain Sorghum. Soil Biol. Biochem. 2007, 39, 1518–1532. [Google Scholar] [CrossRef]
- Chaudhary, J.P.; Yadav, S.R. Effect of Different Levels of Cellulose, Lignin, Silica and Ash Contents Available in Mid-Ribs, Growing Points and Leaf Blades of Sugarcane Genotypes on Incidence of Top Borer, Scirpophaga excerptalis Walker (Lepidoptera: Pyralidae). Coop. Sugar 1998, 29, 337. [Google Scholar]
- Sandoya, G.; Santiago, R.; Malvar, R.A.; Butrón, A. Evaluation of Structural and Antibiosis Resistance Mechanisms during Selection against Mediterranean Corn Borer (Sesamia nonagrioides Lef) in the Maize Synthetic EPS12. Crop Prot. 2010, 29, 7–10. [Google Scholar] [CrossRef]
- Spurgeon, D.W.; Raulston, J.R.; Lingren, P.D.; Shaver, T.N. Vertical Distribution of Mexican Rice Borer (Lepidoptera: Pyralidae) Larvae and Tunnels in Lower Rio Grande Valley Sugarcane. J. Econ. Entomol. 1999, 92, 870–874. [Google Scholar] [CrossRef]
- Azevedo, R.A.; Carvalho, R.F.; Cia, M.C.; Gratão, P.L. Sugarcane under Pressure: An Overview of Biochemical and Physiological Studies of Abiotic Stress. Trop. Plant Biol. 2011, 4, 42–51. [Google Scholar] [CrossRef]
- Lakshmanan, P.; Robinson, N. Stress Physiology: Abiotic Stresses. In Sugarcane: Physiology, Biochemistry, and Functional Biology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013; pp. 411–434. ISBN 978-1-118-77128-0. [Google Scholar]
- Gomathi, R.; Vasantha, S.; Venkataramana, S.; Rao, P.G.; Rakkiyappan, P. Response of Sugarcane to Abiotic Stresses and Management. In Current Status of Sugarcane Research in India; Nova Publishers: Hauppauge, NY, USA, 2015; pp. 55–87. ISBN 978-1-63463-458-8. [Google Scholar]
- Vasantha, S.; Gomathi, R.; Brindha, C. Growth and Nutrient Composition of Sugarcane Genotypes Subjected to Salinity and Drought Stresses. Commun. Soil Sci. Plant Anal. 2017, 48, 989–998. [Google Scholar] [CrossRef]
- Leanasawat, N.; Kosittrakun, M.; Lontom, W.; Songsri, P. Physiological and Agronomic Traits of Certain Sugarcane Genotypes Grown under Field Conditions as Influenced by Early Drought Stress. Agronomy 2021, 11, 2319. [Google Scholar] [CrossRef]
- Perrier, L.; Rouan, L.; Jaffuel, S.; Clément-Vidal, A.; Roques, S.; Soutiras, A.; Baptiste, C.; Bastianelli, D.; Fabre, D.; Dubois, C.; et al. Plasticity of Sorghum Stem Biomass Accumulation in Response to Water Deficit: A Multiscale Analysis from Internode Tissue to Plant Level. Front. Plant Sci. 2017, 8, 1516. [Google Scholar] [CrossRef]
- Nuss, K.J.; Bond, R.S.; Atkinson, P.R. Susceptibility of Sugarcane to the Borer Eldana Saccharina Walker and Selection for Resistance. In Proceedings of the Annual Congress-South African Sugar Technologists’ Association, Durban, South Africa, 16–19 June 1986; pp. 153–155. [Google Scholar]
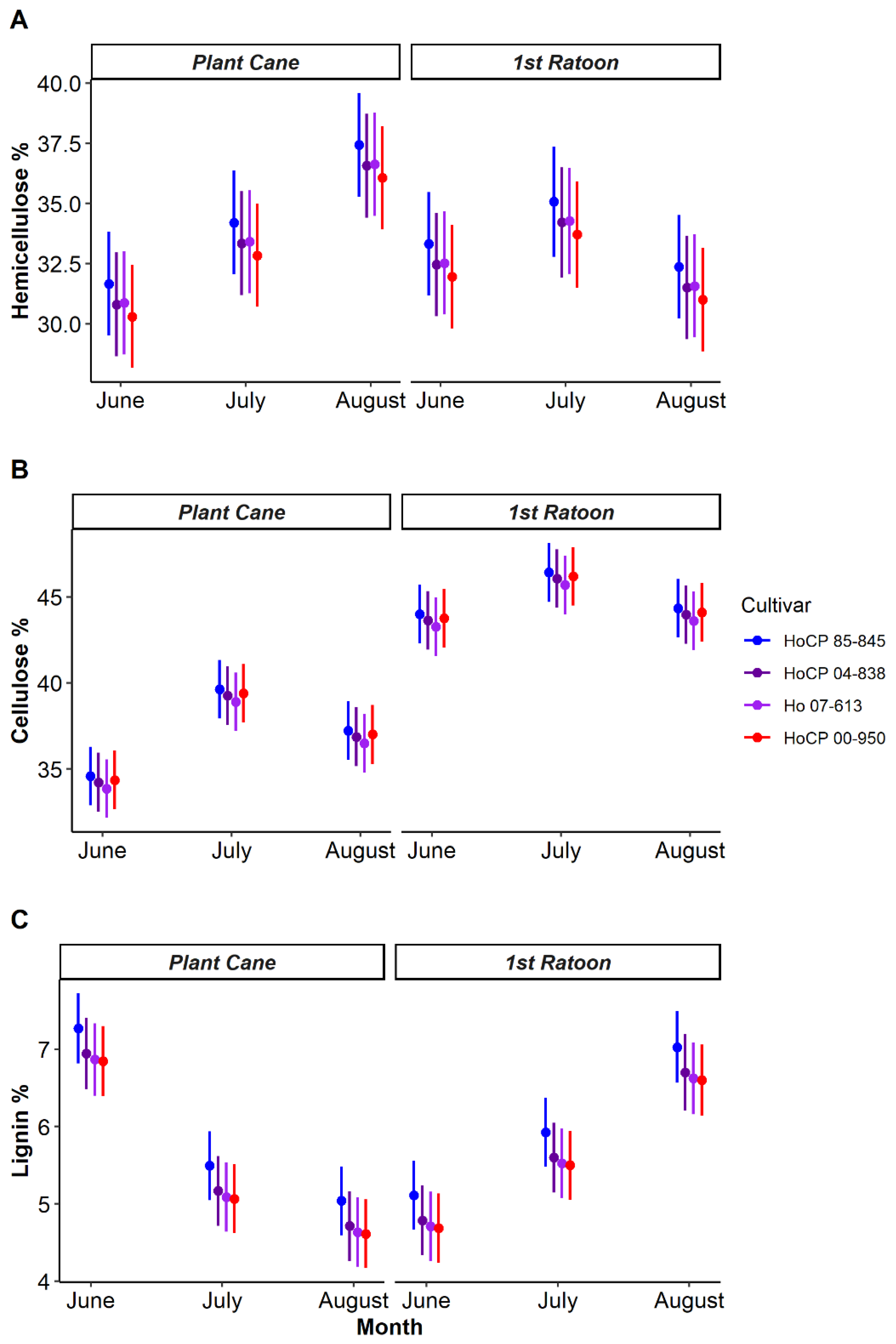

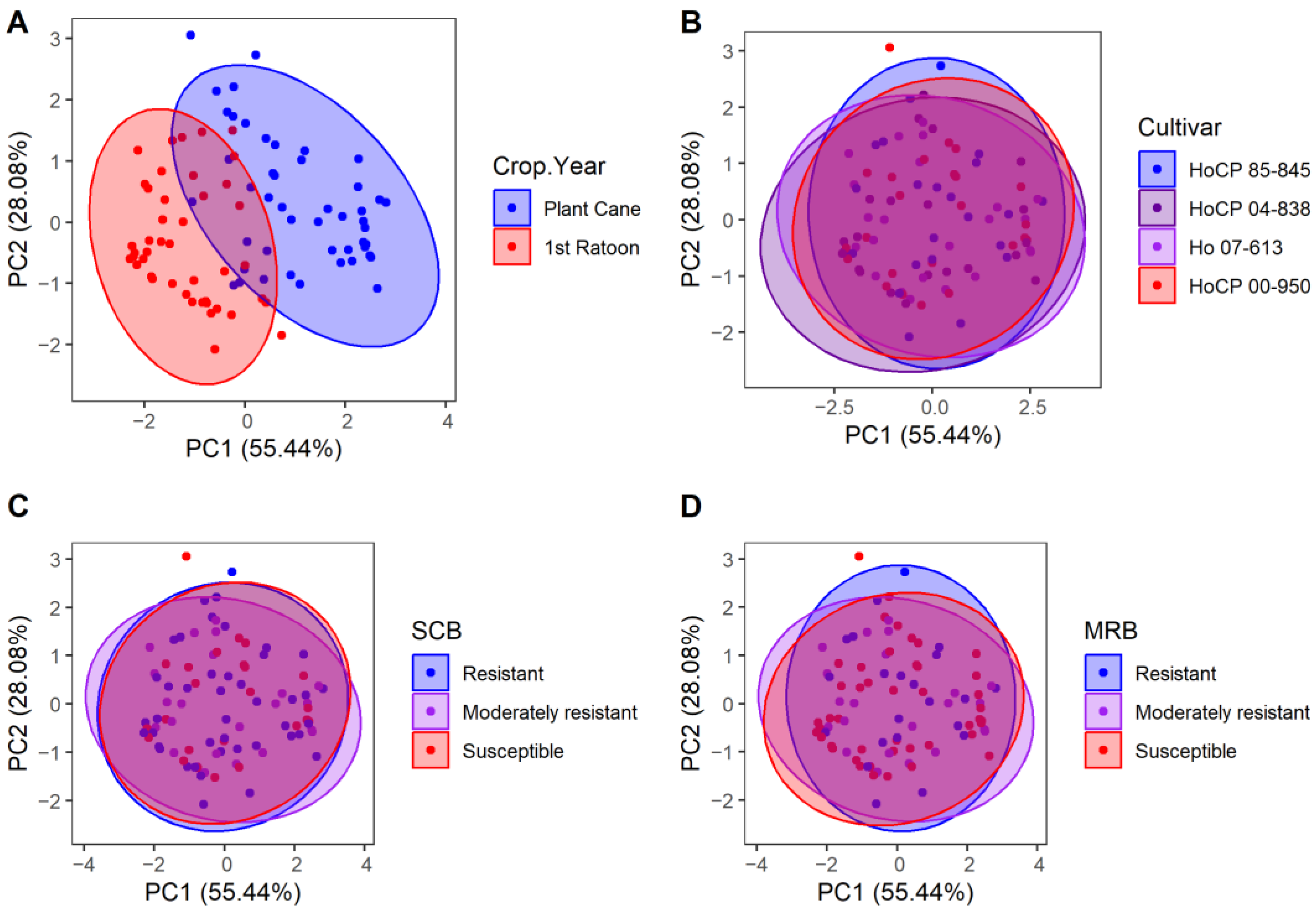

| Cultivar | Fiber Content | SCB Resistance | MRB Resistance |
|---|---|---|---|
| HoCP 85-845 | High (13.2%) | Resistant | Resistant |
| HoCP 04-838 | High (13.3%) | Resistant | Susceptible |
| Ho 07-613 | Low (10.2%) | Moderately resistant | Moderately resistant |
| HoCP 00-950 | Moderate (11.5%) | Susceptible | Susceptible |
| Component | Source | DFN | DFD | F Ratio | p-Value |
|---|---|---|---|---|---|
| Hemicellulose | Cultivar | 3 | 70 | 0.831 | 0.481 |
| Month | 2 | 70 | 4.535 | 0.014 | |
| Crop year | 1 | 70 | 1.459 | 0.231 | |
| Cultivar × Month | 6 | 70 | 1.296 | 0.271 | |
| Cultivar × Crop year | 3 | 70 | 1.729 | 0.169 | |
| Month × Crop year | 2 | 70 | 8.782 | <0.001 | |
| Cultivar × Month × Crop year | 6 | 70 | 0.979 | 0.446 | |
| Cellulose | Cultivar | 3 | 69 | 0.372 | 0.774 |
| Month | 2 | 69 | 18.583 | <0.001 | |
| Crop year | 1 | 69 | 238.073 | <0.001 | |
| Cultivar × Month | 6 | 69 | 1.270 | 0.283 | |
| Cultivar × Crop year | 3 | 69 | 1.958 | 0.128 | |
| Month × Crop year | 2 | 69 | 2.673 | 0.076 | |
| Cultivar × Month × Crop year | 6 | 69 | 1.643 | 0.149 | |
| Lignin | Cultivar | 3 | 69 | 1.751 | 0.165 |
| Month | 2 | 69 | 3.043 | 0.054 | |
| Crop year | 1 | 69 | 0.370 | 0.545 | |
| Cultivar × Month | 6 | 69 | 0.659 | 0.683 | |
| Cultivar × Crop year | 3 | 69 | 1.538 | 0.212 | |
| Month × Crop year | 2 | 69 | 55.603 | <0.001 | |
| Cultivar × Month × Crop year | 6 | 69 | 0.306 | 0.932 | |
| Brix | Cultivar | 3 | 69 | 20.131 | <0.001 |
| Month | 2 | 69 | 866.908 | <0.001 | |
| Crop year | 1 | 69 | 750.462 | <0.001 | |
| Cultivar × Month | 6 | 69 | 4.439 | 0.001 | |
| Cultivar × Crop year | 3 | 69 | 1.408 | 0.248 | |
| Month × Crop year | 2 | 69 | 24.719 | <0.001 | |
| Cultivar × Month × Crop year | 6 | 69 | 1.176 | 0.329 |
| Variable | Source | DFN | DFD | F Ratio | p-Value |
|---|---|---|---|---|---|
| Fiber (%) | Cultivar | 3 | 21 | 8.901 | 0.001 |
| Crop year | 1 | 21 | 47.684 | <0.001 | |
| Cultivar × Crop year | 3 | 21 | 0.590 | 0.629 | |
| Sucrose content (kg·Mg−1) | Cultivar | 3 | 21 | 19.136 | <0.001 |
| Crop year | 1 | 21 | 59.012 | <0.001 | |
| Cultivar × Crop year | 3 | 21 | 2.646 | 0.076 | |
| Cane (Mg·ha−1) | Cultivar | 3 | 21 | 3.558 | 0.032 |
| Crop year | 1 | 21 | 265.533 | <0.001 | |
| Cultivar × Crop year | 3 | 21 | 1.690 | 0.200 | |
| Sugar (Mg·ha−1) | Cultivar | 3 | 21 | 2.553 | 0.083 |
| Crop year | 1 | 21 | 119.881 | <0.001 | |
| Cultivar × Crop year | 3 | 21 | 1.118 | 0.364 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Penn, H.J.; Johnson, R.M.; Richard, K.A.; Richard, R.T.; White, W.H. Lignocellulosic Composition Not Associated with Stem Borer Resistance in Select Louisiana Sugarcane Cultivars. Agronomy 2023, 13, 2764. https://doi.org/10.3390/agronomy13112764
Penn HJ, Johnson RM, Richard KA, Richard RT, White WH. Lignocellulosic Composition Not Associated with Stem Borer Resistance in Select Louisiana Sugarcane Cultivars. Agronomy. 2023; 13(11):2764. https://doi.org/10.3390/agronomy13112764
Chicago/Turabian StylePenn, Hannah J., Richard M. Johnson, Katie A. Richard, Randy T. Richard, and William H. White. 2023. "Lignocellulosic Composition Not Associated with Stem Borer Resistance in Select Louisiana Sugarcane Cultivars" Agronomy 13, no. 11: 2764. https://doi.org/10.3390/agronomy13112764





