Tithonia diversifolia Improves In Vitro Rumen Microbial Synthesis of Sheep Diets without Changes in Total Gas and Methane Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Diet and Treatments
| TD0 | TD9 | TD27 | TD45 | SE | p-Value | Regression | |||
|---|---|---|---|---|---|---|---|---|---|
| L | Q | R2 | |||||||
| Ingredients (g kg−1) | |||||||||
| Tithonia diversifolia | 0.0 | 90.0 | 270.0 | 450.0 | |||||
| Tifton 85 | 600.0 | 510.0 | 330.0 | 150.0 | |||||
| Maize grain | 263.0 | 254.7 | 246.2 | 234.9 | |||||
| Soybean meal | 137.0 | 142.6 | 153.8 | 165.1 | |||||
| Chemical composition (g kg−1 DM) | |||||||||
| Dry matter (g kg−1) | 910.6 | 906.9 | 906.4 | 905.7 | 4.31 | 0.8557 | 0.652 | 0.883 | |
| Organic matter | 938.5 | 928.0 | 929.6 | 920.5 | 1.23 | <0.001 | <0.001 | <0.001 | 0.90 |
| Crude protein | 163.0 | 158.6 | 156.5 | 156.3 | 7.02 | 0.0655 | 0.747 | 0.456 | - |
| Neutral detergent fiber a | 409.0 | 396.6 | 393.3 | 392.7 | 24.48 | 0.0593 | 0.226 | 0.118 | |
| Acid detergent fiber b | 299.2 | 300.3 | 312.7 | 345.8 * | 14.51 | 0.0107 | 0.034 | 0.080 | 0.34 |
| Lignin b | 75.6 | 83.92 | 98.48 * | 129.0 * | 5.75 | 0.0032 | <0.001 | <0.001 | 0.71 |
| Gross energy (MJ kg−1 DM) | 15.7 | 15.5 | 15.4 | 15.5 | 0.21 | 0.3520 | 0.664 | 0.907 | |
| Metabolizable energy (MJ kg−1 DM) | 5.27 | 4.73 * | 4.82 * | 4.67 * | 0.068 | 0.0010 | 0.007 | 0.009 | 0.3 |
| Ether extract | 23.7 | 18.0 * | 17.6 * | 17.5 * | 2.62 | 0.0450 | 0.070 | 0.065 | |
| Non-fibrous carbohydrates | 342.7 | 366.1 | 367.0 | 353.8 | 16.48 | 0.0723 | 0.350 | 0.088 | |
| Total phenolic compounds c | 7.67 | 6.73 * | 5.79 * | 4.81 * | 0.24 | <0.001 | <0.001 | 0.576 | 0.74 |
| Total tannins c | 4.30 | 3.66 | 3.02 * | 2.33 * | 0.29 | <0.001 | <0.001 | 0.713 | 0.80 |
| P/E ratio | 29 | 28 | 31 | 38 * | 1.2 | <0.001 | <0.001 | <0.001 | 0.32 |
2.2. In Vitro Trial and Gas Production
2.3. In Vitro Methane (CH4) Measurements
2.4. In Vitro Degradability
2.5. Fermentative Parameters
2.6. Statistical Analysis
3. Results
3.1. Total Phenolics and Total Tannins
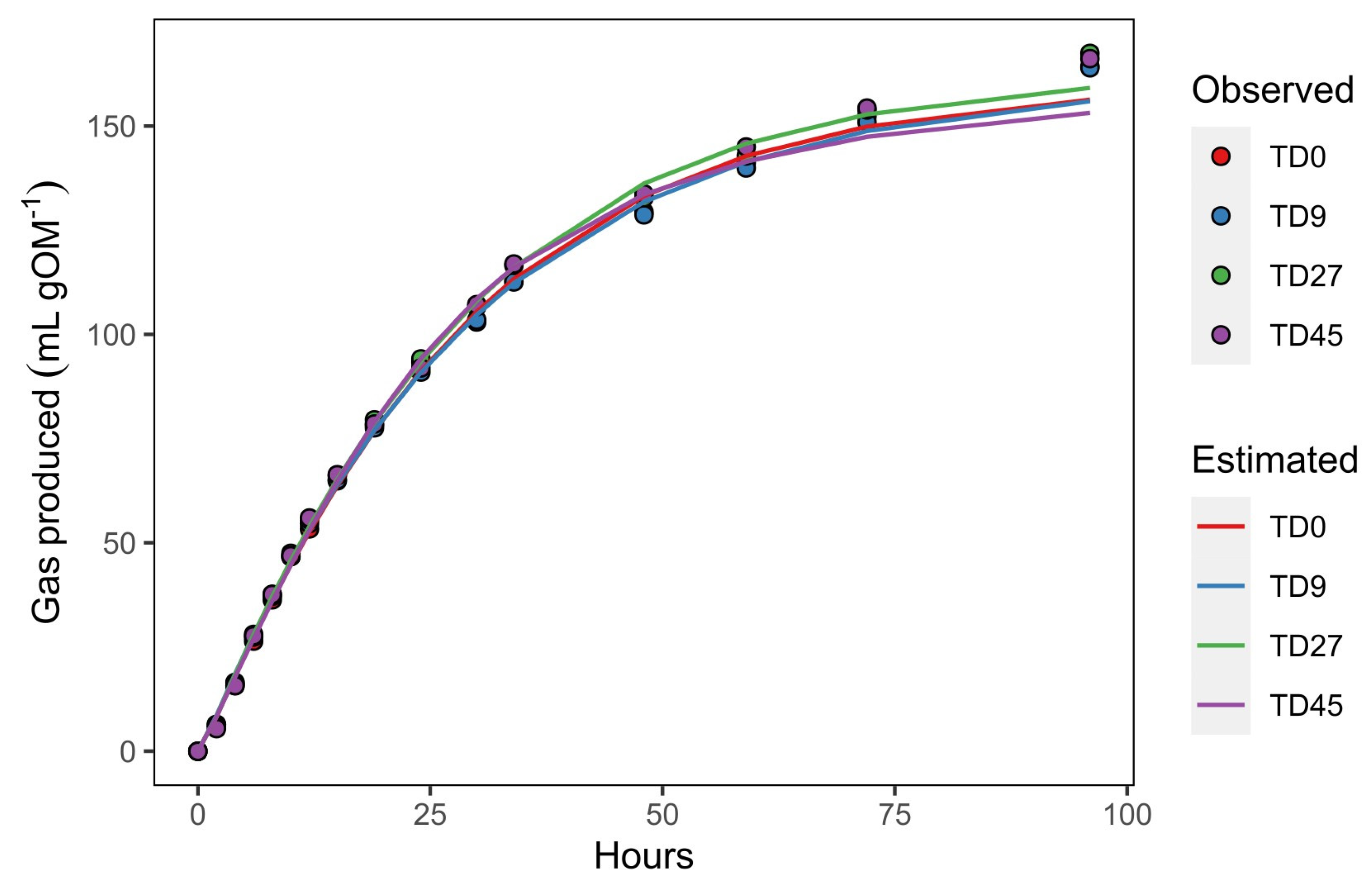
3.2. In Vitro Fermentation Kinetics
3.3. In Vitro Gas Production (IVGP)
3.4. In Vitro Degradability
3.5. In Vitro CH4 Production
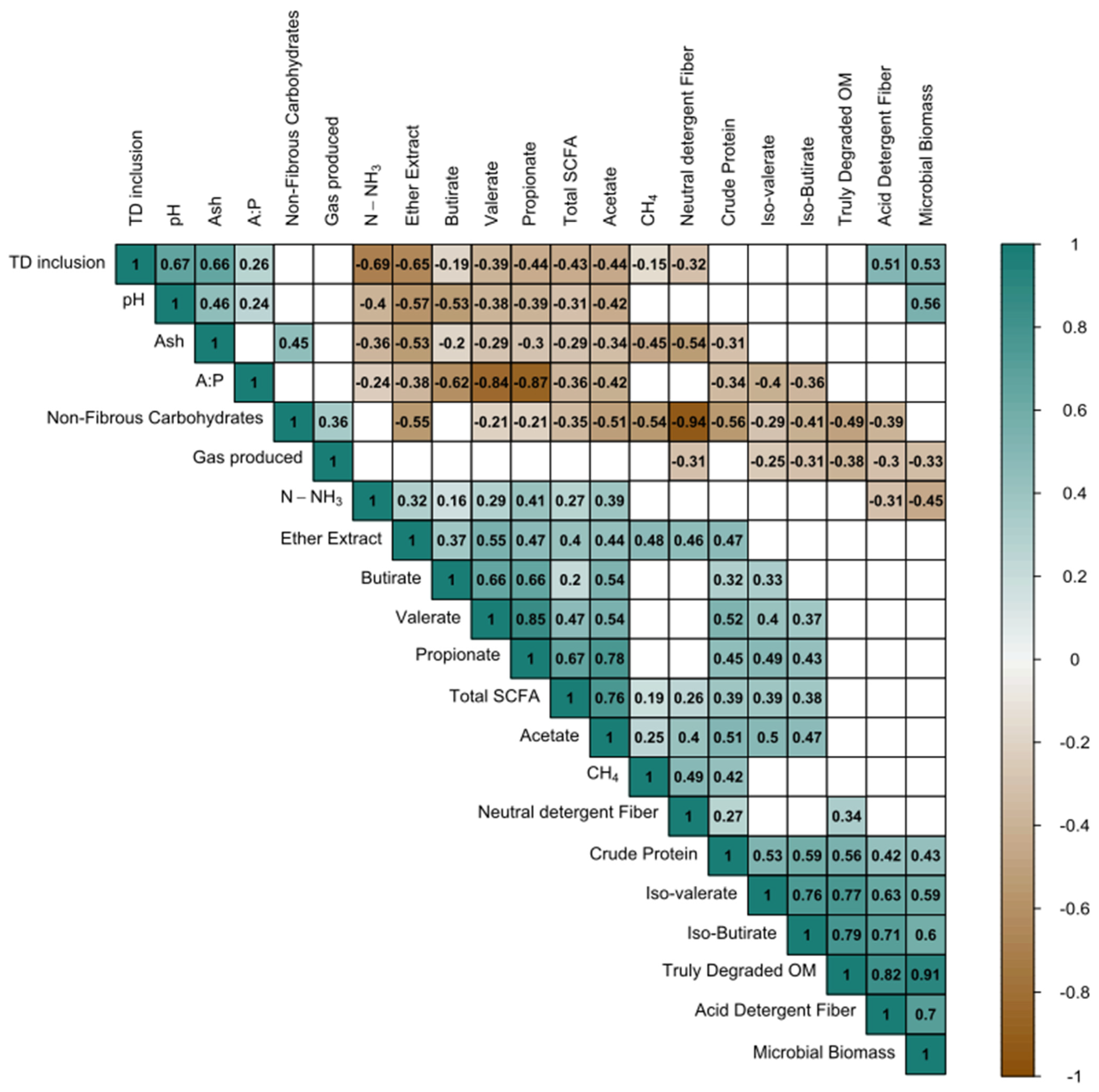
3.6. In Vitro Short-Chain Fatty Acid (SCFA) Concentration
3.7. Ammonia Nitrogen, Partitioning Factor (PF), and Microbial Biomass (MB)
4. Discussion
4.1. In Vitro Fermentation Kinetics
4.2. Presence of Tannins in the Diets
4.3. IVGP, Degradability, and CH4 Production
4.4. Microbial Protein Synthesis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Poore, J.; Nemecek, T. Reducing Food’s Environmental Impacts through Producers and Consumers. Science 2018, 360, 987–992. [Google Scholar] [CrossRef]
- Gerber, P.J.; Steinfeld, H.; Henderson, B.; Mottet, A.; Opio, C.; Dijkman, J.; Falcucci, A.; Tempio, G. Tackling Climate Change through Livestock—A Global Assessment of Emissions and Mitigation Opportunities; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2013; ISBN 9789251079201. [Google Scholar]
- Shukla, P.R.; Skea, J.; Buendia, E.C.; Masson-Delmotte, V.; Pörtner, H.-O.; Roberts, D.C.; Zhai, P.; Slade, R.; Connors, S.; van Diemen, R.; et al. (Eds.) IPCC Climate Change and Land: An IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems; 2019; ISBN 9789291691548. Available online: https://www.ipcc.ch/srccl/ (accessed on 10 October 2023).
- Herrero, M.; Henderson, B.; Havlík, P.; Thornton, P.K.; Conant, R.T.; Smith, P.; Wirsenius, S.; Hristov, A.N.; Gerber, P.; Gill, M.; et al. Greenhouse Gas Mitigation Potentials in the Livestock Sector. Nat. Clim. Chang. 2016, 6, 452–461. [Google Scholar] [CrossRef]
- Zhang, L.; Tian, H.; Shi, H.; Pan, S.; Chang, J.; Dangal, S.R.S.; Qin, X.; Wang, S.; Tubiello, F.N.; Canadell, J.G.; et al. A 130-year Global Inventory of Methane Emissions from Livestock: Trends, Patterns, and Drivers. Glob. Chang. Biol. 2022, 28, 5142–5158. [Google Scholar] [CrossRef] [PubMed]
- Makkar, H. Recent Advances in the in Vitro Gas Method for Evaluation of Nutritional Quality of Feed Resoures. In Assessing Quality and Safety of Animal Feeds; FAO, Animal Production and Health; FAO: Rome, Italy, 2004; Volume 160, p. 155. ISBN 9251050465. Available online: https://www.fao.org/publications/card/en/c/85887fc8-94ee-5bc0-ba37-6e0c30cb9d5b (accessed on 10 October 2023).
- Maurício, R.; Ribeiro, R.S.; Silveira, S.R.; Silva, P.L.; Calsavara, L.; Pereira, L.G.R.; Paciullo, D.S. Tithonia diversifolia for Ruminant Nutrition. Trop. Grassl.-Forrajes Trop. 2014, 2, 82–84. [Google Scholar] [CrossRef]
- Calsavara, L.H.F.; Ribeiro, R.S.; Silveira, S.R.; Delarota, G.; Freitas, D.S.; Sacramento, J.P.; Paciullo, D.S.C.; Maurício, R.M. Potencial Forrageiro Da Tithonia diversifolia Para Alimentação de Ruminantes. Livest. Res. Rural Dev. 2016, 28, 17. [Google Scholar]
- Rodríguez García, I. Potencialidades de Tithonia diversifolia (Hemsl.) Gray En La Alimentación Animal. Livest. Res. Rural Dev. 2017, 29, 1–11. [Google Scholar]
- Mahecha, L.; Rosales, M. Valor Nutricional Del Follaje de Botón de Oro (Tithonia diversifolia [Hemsl]. Gray), En La Producción Animal En El Trópico. Livest. Res. For. Rural. Dev. 2005, 17, 1. [Google Scholar]
- Van Sao, N.; Mui, N.T.; van Binh, D. Biomass Production of Tithonia diversifolia (Wild Sunflower), Soil Improvement on Sloping Land and Use as High Protein Foliage for Feeding Goats. Livest. Res. Rural Dev. 2010, 8, 22. [Google Scholar]
- Verdecia, D.M.; Ramirez, J.L.; Leonard, I.; Alvarez, Y.; Bazán, Y.; Bodas, R.; Andrés, S.; Alvarez, J.; Giraldez, F.; Lopez, S. Nutritive Value of the Tithonia diversifolia in a Location of Valle Del Cauto. Rev. Electron. Vet. 2011, 12. Available online: https://digital.csic.es/handle/10261/108935 (accessed on 10 October 2023).
- Porsavatdy, P.; Preston, T.R.; Leng, R.A. Effect on Feed Intake, Digestibility, N Retention and Methane Emissions in Goats of Supplementing Foliages of Cassava (Manihot Esculenta Crantz) and Tithonia diversifolia with Water spinach (Ipomoea aquatica). Livest. Res. Rural Dev. 2016, 28. Available online: http://www.lrrd.org/lrrd28/5/phon28072.html (accessed on 10 October 2023).
- Cardona Iglesias, J.L.; Mahecha Ledesma, L.; Angulo Arizala, J.; Cardona, J.L.; Mahecha, L.; Angulo, J.; Cardona Iglesias, J.L.; Mahecha Ledesma, L.; Angulo Arizala, J. Efecto Sobre La Fermentación in Vitro de Mezclas de Tithonia diversifolia, Cenchrus Clandestinum y Grasas Poliinsaturadas. Agron. Mesoam. 2017, 28, 405. [Google Scholar] [CrossRef]
- Rutunga, V.; Karanja, N.K.; Gachene, C.K.K.; Palm, C. Biomass Production and Nutrient Accumulation by Tephrosia Vogelii (Hemsley) A. Gray and Tithonia diversifolia Hook F. Fallows during the Six-Month Growth Period at Maseno, Western Kenya. Biotechnol. Agron. Social. Environ. 1999, 3, 237–246. [Google Scholar]
- Guatusmal-Gelpud, C.; Escobar-Pachajoa, L.D.; Meneses-Buitrago, D.H.; Cardona-Iglesias, J.L.; Castro-Rincón, E. Producción y Calidad de Tithonia diversifolia y Sambucus Nigra En Trópico Altoandino Colombiano. Agron. Mesoam. 2020, 31, 193–208. [Google Scholar] [CrossRef]
- Mahecha, L.; Escobar, J.P.; Suarez, J.F.; Restrepo, L.F. Evaluación Del Uso de Tithonia diversifolia (Hemsl.) A. Gray ASTERACEAE, Como Suplemento Forrajero de Vacas Cruzadas. In Ganadería del Futuro: Investigación Para el Desarrollo; Murgueitio, E., Cuartas, C.A., Naranjo, J.F., Eds.; CIPAV: Cali, Colombia, 2008; p. 490. ISBN 978-958-9386-55-2. [Google Scholar]
- Rivera, J.E.; Cuartas, C.A.; Naranjo, J.F.; Tafur, O.; Hurtado, E.A.; Arenas, F.A.; Chará, J.; Murgueitio, E. Efecto de La Oferta y El Consumo de Tithonia diversifolia En Un Sistema Silvopastoril Intensivo (SSPi), En La Calidad y Productividad de Leche Bovina En El Piedemonte Amazónico Colombiano. Livest. Res. Rural Dev. 2015, 27. Available online: http://www.lrrd.org/lrrd27/10/rive27189.html (accessed on 10 October 2023).
- Ribeiro, R.S.; Terry, S.A.; Sacramento, J.P.; Silveira, S.R.E.; Bento, C.B.P.; da Silva, E.F.; Mantovani, H.C.; da Gama, M.A.S.; Pereira, L.G.R.; Tomich, T.R.; et al. Tithonia diversifolia as a Supplementary Feed for Dairy Cows. PLoS ONE 2016, 11, e0165751. [Google Scholar] [CrossRef]
- Pazla, R.; Jamarun, N.; Elihasridas, E.; Arief, A.; Yanti, G.; Ikhlas, Z. The Impact of Replacement of Concentrates with Fermented Tithonia (Tithonia diversifolia) and Avocado Waste (Persea Americana Miller) in Fermented Sugarcane Shoots (Saccharum Officinarum) Based Rations on Consumption, Digestibility, and Production Perform. Adv. Anim. Vet. Sci. 2023, 11, 394–403. [Google Scholar] [CrossRef]
- Garcia, R.; Guitierrez, D.; Chongo, B.; Gutierrez, O. Feeding of Cattle, Sheep and Goats with Tithonia diversifolia in Latin America and the Caribbean. In Mulberry, Moringa and Tithonia in Animal Feed, and Other Uses. Results in Latin America and the Caribbean; Savon Valdes, L.L., Guttierrez Borroto, O., Febles Perez, G., Eds.; FAO, ICA CUBA: San José de las Lajas, Cuba, 2017; pp. 237–248. [Google Scholar]
- Blümmel, M.; Makkar, H.P.S.; Becker, K. In Vitro Gas Production: A Technique Revisited. J. Anim. Physiol. Anim. Nutr. 1997, 77, 24–34. [Google Scholar] [CrossRef]
- Horwitz, W.; Latimer, G.W. (Eds.) AOAC Official Methods of Analysis of AOAC International, 18th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2011; ISBN 093558482X 9780935584820. [Google Scholar]
- van Soest, P.J.J.; Robertson, J.B.B.; Lewis, B.A.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Mertens, D.R.; Allen, M.; Carmany, J.; Clegg, J.; Davidowicz, A.; Drouches, M.; Frank, K.; Gambin, D.; Garkie, M.; Gildemeister, B.; et al. Gravimetric Determination of Amylase-Treated Neutral Detergent Fiber in Feeds with Refluxing in Beakers or Crucibles: Collaborative Study. J. AOAC Int. 2002, 85, 1217–1240. [Google Scholar]
- Makkar, H. Measurement of Total Phenolics and Tannins Using Folin-Ciocalteu Method. In Quantification of Tannins in Tree and Shrub Foliage; Springer: Dordrecht, The Netherlands, 2003; pp. 49–51. [Google Scholar]
- Lima, P.M.T.; Moreira, G.D.; Sakita, G.Z.; Natel, A.S.; Mattos, W.T.; Gimenes, F.M.A.; Gerdes, L.; McManus, C.; Abdalla, A.L.; Louvandini, H. Nutritional Evaluation of the Legume Macrotyloma Axillare Using in Vitro and in Vivo Bioassays in Sheep. J. Anim. Physiol. Anim. Nutr. 2018, 102, e669–e676. [Google Scholar] [CrossRef]
- Menke, K.H.; Steingass, H. Estimation of the Energetic Feed Value Obtained from Chemical Analysis and in Vitro Gas Production Using Rumen Fluid. Anim. Res. Dev. 1988, 28, 7–55. [Google Scholar]
- Mauricio, R.M.; Mould, F.L.; Dhanoa, M.S.; Owen, E.; Channa, K.S.; Theodorou, M.K. A Semi-Automated in Vitro Gas Production Technique for Ruminant Feedstuff Evaluation. Anim. Feed. Sci. Technol. 1999, 79, 321–330. [Google Scholar] [CrossRef]
- Bueno, I.C.S.; Cabral Filho, S.L.S.; Gobbo, S.P.; Louvandini, H.; Vitti, D.M.S.S.; Abdalla, A.L. Influence of Inoculum Source in a Gas Production Method. Anim. Feed. Sci. Technol. 2005, 123–124, 95–105. [Google Scholar] [CrossRef]
- Longo, C.; Bueno, I.C.S.; Nozella, E.F.; Goddoy, P.B.; Cabral Filho, S.L.S.; Abdalla, A.L. The Influence of Head-Space and Inoculum Dilution on in Vitro Ruminal Methane Measurements. Int. Congr. Ser. 2006, 1293, 62–65. [Google Scholar] [CrossRef]
- Wang, M.; Tang, S.X.; Tan, Z.L. Modeling in Vitro Gas Production Kinetics: Derivation of Logistic-Exponential (LE) Equations and Comparison of Models. Anim. Feed. Sci. Technol. 2011, 165, 137–150. [Google Scholar] [CrossRef]
- Goering, H.K.; van Soest, P.J. Forage Fiber Analyses; U.S. Department of Agriculture: Washington, DC, USA, 1975; pp. 387–598.
- Preston, T.R.; Leng, R.A. Matching Ruminant Production Systems with Available Resources in the Tropics and Sub-Tropics; Penanmbul Books: Armidale, AU, USA, 1987; ISBN 0958829012. [Google Scholar]
- Bergman, E.N.; Reid, R.S.; Murray, M.G.; Brockway, J.M.; Whitelaw, F.G. Interconversions and Production of Volatile Fatty Acids in the Sheep Rumen. Biochem. J. 1965, 97, 53. [Google Scholar] [CrossRef]
- Bergman, E.N. Energy Contributions of Volatile Fatty Acids from the Gastrointestinal Tract in Various Species. Physiol. Rev. 1990, 70, 567–590. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Enviroment for Statistical Computing; R Core Team: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 10 October 2023).
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. LmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Lenth, R. emmeans: Estimated Marginal Means, aka Least-Squares Means. R package version 1.8.8. 2021. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 10 October 2023).
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- Wei, T.; Simko, V. R Package “Corrplot”: Visualization of a Correlation Matrix 2021. Available online: https://github.com/taiyun/corrplot (accessed on 10 October 2023).
- Wickham, H. Ggplot2; Springer International Publishing: Cham, Switzerland, 2016; ISBN 978-3-319-24275-0. [Google Scholar]
- Getachew, G.; Blümmel, M.; Makkar, H.P.S.; Becker, K. In Vitro Gas Measuring Techniques for Assessment of Nutritional Quality of Feeds: A Review. Anim. Feed. Sci. Technol. 1998, 72, 261–281. [Google Scholar] [CrossRef]
- France, J.; Lopez, S.; Kebreab, E.; Bannink, A.; Dhanoa, M.S.; Dijkstra, J. A General Compartmental Model for Interpreting Gas Production Profiles. Anim. Feed. Sci. Technol. 2005, 123–124, 473–485. [Google Scholar] [CrossRef]
- Adesogan, A.T. What Are Feeds Worth? A Critical Evaluation of Selected Nutritive Value Methods. In Proceedings of the 13th Annual Florida Ruminant Nutrition Symposium, Gainesville, FL, USA, 10–11 January 2002; pp. 33–47. Available online: https://animal.ifas.ufl.edu/apps/dairymedia/rns/2002/adesogan.pdf (accessed on 10 October 2023).
- Firsoni, F.; Sasongko, W.T.; Wahyono, T. Nutritive Value and Fermentation Characteristics of Tithonia diversifolia and Moringa Oleifera Evaluated by Gas Production Technique in Vitro. In Proceedings of the International Conference on Improving Tropical Animal Production for Food Security (ITAPS 2021), Kendari, Indonesia, 20–21 November 2021; Atlantis Press: Amsterdam, The Netherlands, 2022. [Google Scholar] [CrossRef]
- Rivera, J.; Chará, J.; Arango, J.; Barahona, R. Effect of Different Genotypes of Tithonia diversifolia on Fermentation of Feed Mixtures with Urochloa Brizantha Cv. Marandú. Crop Pasture Sci. 2021, 72, 850. [Google Scholar] [CrossRef]
- Makkar, H. Effects and Fate of Tannins in Ruminant Animals, Adaptation to Tannins, and Strategies to Overcome Detrimental Effects of Feeding Tannin-Rich Feeds. Small Rumin. Res. 2003, 49, 241–256. [Google Scholar] [CrossRef]
- Naumann, H.D.; Tedeschi, L.O.; Zeller, W.E.; Huntley, N.F. The Role of Condensed Tannins in Ruminant Animal Production: Advances, Limitations and Future Directions. Rev. Bras. De Zootec. 2017, 46, 929–949. [Google Scholar] [CrossRef]
- Frutos, P.; Hervás, G.; Ramos, G.; Giráldez, F.J.; Mantecón, A.R. Condensed Tannin Content of Several Shrub Species from a Mountain Area in Northern Spain, and Its Relationship to Various Indicators of Nutritive Value. Anim. Feed. Sci. Technol. 2002, 95, 215–226. [Google Scholar] [CrossRef]
- Siwaporn, P.; Anan, P.; Rayudika Aprilia Patindra, P.; Pramote, P. Protein-Binding Affinity of Various Condensed Tannin Molecular Weights from Tropical Leaf Peel. J. Appl. Pharm. Sci. 2021, 11, 114–120. [Google Scholar] [CrossRef]
- Mueller-Harvey, I. Unravelling the Conundrum of Tannins in Animal Nutrition and Health. J. Sci. Food Agric. 2006, 86, 2010–2037. [Google Scholar] [CrossRef]
- Ban, C.; Paengkoum, S.; Yang, S.; Tian, X.; Thongpea, S.; Purba, R.A.P.; Paengkoum, P. Feeding Meat Goats Mangosteen (Garcinia mangostana L.) Peel Rich in Condensed Tannins, Flavonoids, and Cinnamic Acid Improves Growth Performance and Plasma Antioxidant Activity under Tropical Conditions. J. Appl. Anim. Res. 2022, 50, 307–315. [Google Scholar] [CrossRef]
- Getachew, G.; Makkar, H.P.S.; Becker, K. Tropical Browses: Contents of Phenolic Compounds, in Vitro Gas Production and Stoichiometric Relationship between Short Chain Fatty Acid and in Vitro Gas Production. J. Agric. Sci. 2002, 139, 341–352. [Google Scholar] [CrossRef]
- Delgado, D.C.; Galindo, J.; González, R.; González, N.; Scull, I.; Dihigo, L.; Cairo, J.; Aldama, A.I.; Moreira, O. Feeding of Tropical Trees and Shrub Foliages as a Strategy to Reduce Ruminal Methanogenesis: Studies Conducted in Cuba. Trop. Anim. Health Prod. 2012, 44, 1097–1104. [Google Scholar] [CrossRef]
- Galindo, J.; Gonzales, N.; Marrero, Y.; Sosa, A.; Ruiz, T.; Febles, G.; Torres, V.; Aldana, A.I.; Achang, G.; Moreira, O.; et al. Effect of Tropical Plant Foliage on the Control of Methane Production and in Vitro Ruminal Protozoa Population. Cuba. J. Agric. Sci. 2014, 48, 359–364. [Google Scholar]
- Smith, A.H.; Zoetendal, E.; Mackie, R.I. Bacterial Mechanisms to Overcome Inhibitory Effects of Dietary Tannins. Microb. Ecol. 2005, 50, 197–205. [Google Scholar] [CrossRef]
- Asres, K.; Bucar, F.; Knauder, E.; Yardley, V.; Kendrick, H.; Croft, S.L. In Vitro Antiprotozoal Activity of Extract and Compounds from the Stem Bark of Combretum Molle. Phytother. Res. 2001, 15, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Makkar, H. Treatment of Plant Material, Extraction of Tannins, and an Overview of Tannin Assays Presented in the Manual. In Quantification of Tannins in Tree and Shrub Foliage; Springer: Dordrecht, The Netherlands, 2003; pp. 43–48. [Google Scholar]
- Terry, S.A.; Ribeiro, R.S.; Freitas, D.S.; Delarota, G.D.; Pereira, L.G.R.R.; Tomich, T.R.; Maurício, R.M.; Chaves, A.V. Effects of Tithonia diversifolia on in Vitro Methane Production and Ruminal Fermentation Characteristics. Anim. Prod. Sci. 2016, 56, 437–441. [Google Scholar] [CrossRef]
- Hill, J.; McSweeney, C.; Wright, A.D.G.; Bishop-Hurley, G.; Kalantar-zadeh, K. Measuring Methane Production from Ruminants. Trends Biotechnol. 2016, 34, 26–35. [Google Scholar] [CrossRef]
- Greening, C.; Geier, R.; Wang, C.; Woods, L.C.; Morales, S.E.; McDonald, M.J.; Rushton-Green, R.; Morgan, X.C.; Koike, S.; Leahy, S.C.; et al. Diverse Hydrogen Production and Consumption Pathways Influence Methane Production in Ruminants. ISME J. 2019, 13, 2617–2632. [Google Scholar] [CrossRef]
- Morgavi, D.P.; Forano, E.; Martin, C.; Newbold, C.J. Microbial Ecosystem and Methanogenesis in Ruminants. Animal 2010, 4, 1024–1036. [Google Scholar] [CrossRef]
- Dijkstra, J. Production and Absorption of Volatile Fatty Acids in the Rumen. Livest. Prod. Sci. 1994, 39, 61–69. [Google Scholar] [CrossRef]
- Pazla, R.; Jamarun, N.; Zain, M. Arief Microbial Protein Synthesis and in Vitro Fermentability of Fermented Oil Palm Fronds by Phanerochaete chrysosporium in Combination with Tithonia (Tithonia diversifolia) and Elephant grass (Pennisetum purpureum). Pak. J. Nutr. 2018, 17, 462–470. [Google Scholar] [CrossRef]
- Jamarun, N.; Pazla, R.; Zain, M. Arief Comparison of in Vitro Digestibility and Rumen Fluid Characteristics between the Tithonia (Tithonia diversifolia) with Elephant grass (Pennisetum purpureum). IOP Conf. Ser. Earth Environ. Sci. 2019, 287, 012019. [Google Scholar] [CrossRef]
- Rivera, J.E.; Naranjo, J.F.; Cuartas, C.A.; Arenas, F.A. Fermentación in Vitro y Composición Química de Algunos Forrajes y Dietas Ofrecidas Bajo Un Sistema Silvopastoril En El Trópico de Altura. Livest. Res. for Rural Dev. 2013, 25. Available online: http://www.lrrd.org/lrrd25/10/rive25174.htm (accessed on 10 October 2023).
- Andries, J.I.; Buysse, F.X.; de Brabander, D.L.; Cottyn, B.G. Isoacids in Ruminant Nutrition: Their Role in Ruminal and Intermediary Metabolism and Possible Influences on Performances—A Review. Anim. Feed. Sci. Technol. 1987, 18, 169–180. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, C.; Huang, Y.X.; Dong, K.H.; Yang, W.Z.; Zhang, S.L.; Wang, H. Effects of Isovalerate on Ruminal Fermentation, Urinary Excretion of Purine Derivatives and Digestibility in Steers. J. Anim. Physiol. Anim. Nutr. 2009, 93, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, C.; Yang, W.Z.; Zhang, B.; Yang, X.M.; He, D.C.; Zhang, P.; Dong, K.H.; Huang, Y.X. Effects of Isobutyrate on Rumen Fermentation, Lactation Performance and Plasma Characteristics in Dairy Cows. Anim. Feed. Sci. Technol. 2009, 154, 58–67. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, C.; Pei, C.X.; Li, H.Y.; Wang, Y.X.; Zhang, S.L.; Zhang, Y.L.; He, J.P.; Wang, H.; Yang, W.Z.; et al. Effects of Isovalerate Supplementation on Microbial Status and Rumen Enzyme Profile in Steers Fed on Corn Stover Based Diet. Livest. Sci. 2014, 161, 60–68. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, C.; Huang, Y.; Dong, K.; Wang, H.; Yang, W. Effects of Isobutyrate on Rumen Fermentation, Urinary Excretion of Purine Derivatives and Digestibility in Steers. Arch. Anim. Nutr. 2008, 62, 377–388. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, C.; Zhang, Y.L.; Pei, C.X.; Zhang, S.L.; Wang, Y.X.; Zhang, Z.W.; Yang, W.Z.; Wang, H.; Guo, G.; et al. Effects of Isovalerate Supplementation on Growth Performance and Ruminal Fermentation in Pre- and Post-Weaning Dairy Calves. J. Agric. Sci. 2016, 154, 1499–1508. [Google Scholar] [CrossRef]
- Roman-Garcia, Y.; Mitchell, K.E.; Lee, C.; Socha, M.T.; Park, T.; Wenner, B.A.; Firkins, J.L. Conditions Stimulating Neutral Detergent Fiber Degradation by Dosing Branched-Chain Volatile Fatty Acids. III: Relation with Solid Passage Rate and PH on Prokaryotic Fatty Acid Profile and Community in Continuous Culture. J. Dairy Sci. 2021, 104, 9868–9885. [Google Scholar] [CrossRef]
- Roman-Garcia, Y.; Mitchell, K.E.; Denton, B.L.; Lee, C.; Socha, M.T.; Wenner, B.A.; Firkins, J.L. Conditions Stimulating Neutral Detergent Fiber Degradation by Dosing Branched-Chain Volatile Fatty Acids. II: Relation with Solid Passage Rate and PH on Neutral Detergent Fiber Degradation and Microbial Function in Continuous Culture. J. Dairy Sci. 2021, 104, 9853–9867. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Q.; Zhang, Y.L.; Pei, C.X.; Zhang, S.L.; Wang, Y.X.; Yang, W.Z.; Bai, Y.S.; Shi, Z.G.; Liu, X.N. Effects of Isobutyrate Supplementation on Ruminal Microflora, Rumen Enzyme Activities and Methane Emissions in Simmental Steers. J. Anim. Physiol. Anim. Nutr. 2015, 99, 123–131. [Google Scholar] [CrossRef]
- Oluwasola, T.A.; Dairo, F.A.S. Proximate Composition, Amino Acid Profile and Some Anti-Nutrients of Tithonia diversifolia Cut at Two Different Times. Afr. J. Agric. Res. 2016, 11, 3659–3663. [Google Scholar] [CrossRef]
- Fasuyi, O.A.; Ibitayo, F.J. Preliminary Analyses and Amino Acid Profile of Wild Sunflower (Tithonia diversifolia) Leaves. Int. J. Biol. Chem. Sci. 2011, 5, 164–170. [Google Scholar] [CrossRef]
- Akanmu, A.M.; Hassen, A.; Adejoro, F.A. Gas Production, Digestibility and Efficacy of Stored or Fresh Plant Extracts to Reduce Methane Production on Different Substrates. Animals 2020, 10, 146. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.B.; O’Connor, J.D.; Fox, D.G.; Van Soest, P.J.; Sniffen, C.J. A Net Carbohydrate and Protein System for Evaluating Cattle Diets: I. Ruminal Fermentation. J. Anim. Sci. 1992, 70, 3551–3561. [Google Scholar] [CrossRef]
- Slyter, L.L.; Satter, L.D.; Dinius, D.A. Effect of Ruminal Ammonia Concentration on Nitrogen Utilization by Steers. J. Anim. Sci. 1979, 48, 906–912. [Google Scholar] [CrossRef]
- Hristov, A.N.; Ropp, J.K. Effect of Dietary Carbohydrate Composition and Availability on Utilization of Ruminal Ammonia Nitrogen for Milk Protein Synthesis in Dairy Cows. J. Dairy Sci. 2003, 86, 2416–2427. [Google Scholar] [CrossRef] [PubMed]
- Galindo, J.; Gonzales, N.; Sosa, A.; Ruiz, T.; Torres, V.; Aldana, A.I.; Diaz, H.; Moreira, O.; Sarduy, L.; Noda, A.C. Efecto de Tithonia diversifolia (Hemsl.) A. Gray (Botón de Oro) En La Poblacion de Protozoos y Metanogénos Ruminales En Condiciones in Vitro. Rev. Cuba. Cienc. Agrícola 2011, 45, 33–37. [Google Scholar]
- Galindo, J.; González, N.; Scull, I.; Marrero, Y.; Sosa, A.; Aldana, A.I.; Moreira, O.; Delgado, D.; Ruiz, T.; Febles, G.; et al. Efecto de Samanea Saman (Jacq.) Merr., Albizia lebbeck (L.) Benth y Tithonia diversifolia (Hemsl.) Gray (Material Vegetal 23) En La Población de Metanógenos y En La Ecología Microbiana Ruminal. Rev. Cuba. Cienc. Agric. 2012, 46, 273–278. [Google Scholar]

| Nutrient Content | Soybean Meal | Maize Grain | Tifton 85 Hay | T. diversifolia Hay |
|---|---|---|---|---|
| Dry matter | 897.89 | 887.00 | 903.04 | 887.07 |
| Crude protein | 479.23 | 127.14 | 140.45 | 78.84 |
| Neutral detergent fiber a | 150.00 | 111.48 | 735.81 | 677.71 |
| Acid detergent fiber b | 110.96 | 34.20 | 360.23 | 566.62 |
| Total digestible nutrients | 832.25 | 830.32 | 550.15 | 630.21 |
| Ash | 69.19 | 12.79 | 98.96 | 111.35 |
| Total phenolic compounds c | - | - | 7.14 | 3.94 |
| Total tannins c | - | - | 3.84 | 1.92 |
| Condensed tannins d | - | - | 0.18 | 0.19 |
| Parameter | TD0 | TD9 | TD27 | TD45 | SE | p-Value | Regression | |
|---|---|---|---|---|---|---|---|---|
| L | R2 | |||||||
| Vf (mL g−1 OM) | 168.8 | 167.7 | 169.3 | 167.8 | 6.18 | 0.9074 | 0.9754 | <0.01 |
| k (h−1) | 0.032 | 0.033 | 0.036 | 0.038 | 0.0017 | 0.0532 | 0.0958 | 0.19 |
| L (h) | 0.41 | 0.32 | 0.42 | 0.44 | 0.041 | 0.1843 | 0.7652 | <0.01 |
| T0.5 (h) | 21.7 | 21.7 | 21.1 | 20.8 | 0.49 | 0.2483 | 0.1824 | 0.12 |
| V0.5 (mL g−1 OM) | 84.4 | 83.8 | 84.6 | 83.9 | 3.09 | 0.9074 | 0.9754 | <0.01 |
| μ0.5 (h−1) | 0.032 | 0.032 | 0.034 | 0.035 * | 0.0007 | 0.0366 | 0.1110 | 0.17 |
| V96 (mL g−1 OM) | 161.3 | 160.2 | 163.0 | 162.5 | 6.01 | 0.8465 | 0.8603 | <0.01 |
| Incubation Time (h) | TD0 | TD9 | TD27 | TD45 | SE | p-Value | Regression | ||
|---|---|---|---|---|---|---|---|---|---|
| L | Q | R2 | |||||||
| in vitro gas production (mL g −1 DOM) | |||||||||
| 2 | 2.19 | 2.41 | 1.64 | 1.83 | 2.823 | 0.1537 | 0.133 | 0.290 | - |
| 6 | 9.24 | 9.51 | 10.54 | 10.80 | 0.1819 | 0.006 | 0.123 | 0.19 | |
| 10 | 20.79 | 17.94 | 18.49 | 18.53 | 0.1136 | 0.165 | 0.198 | - | |
| 24 | 48.33 | 41.71 | 43.81 | 44.18 | 0.5125 | 0.555 | 0.468 | - | |
| 96 | 104.46 | 101.05 | 106.93 | 91.52 | 0.1054 | 0.459 | 0.525 | - | |
| in vitro degradability of organic matter (g kg −1) | |||||||||
| 2 | 332.34 | 323.08 | 329.00 | 368.49 | 16.412 | 0.0749 | 0.028 | 0.139 | 0.16 |
| 6 | 370.20 | 380.89 | 398.13 | 426.49 * | 0.0140 | 0.009 | 0.084 | 0.20 | |
| 10 | 448.67 | 417.56 | 437.03 | 470.25 | 0.1068 | 0.077 | 0.033 | 0.21 | |
| 24 | 529.39 | 508.12 | 516.16 | 547.76 | 0.2501 | 0.327 | 0.146 | - | |
| 96 | 686.77 | 687.56 | 703.20 | 669.55 | 0.3344 | 0.452 | 0.218 | - | |
| in vitro methane production (mL g −1 DOM) | |||||||||
| 2 | 0.054 | 0.029 | 0.056 | 0.049 | 0.3155 | 0.9385 | 0.571 | 0.768 | - |
| 6 | 0.387 | 0.297 | 0.335 | 0.363 | 0.7855 | 0.983 | 0.767 | - | |
| 10 | 1.572 | 0.942 | 1.273 | 1.251 | 0.0928 | 0.662 | 0.435 | - | |
| 24 | 4.162 | 2.980 * | 3.649 | 3.575 | 0.0096 | 0.683 | 0.429 | - | |
| 96 | 5.549 | 4.670 * | 4.958 | 4.837 | 0.0317 | 0.340 | 0.428 | - | |
| Incubation Time (h) | TD0 | TD9 | TD27 | TD45 | SE | p-Value | Regression | ||
|---|---|---|---|---|---|---|---|---|---|
| L | Q | R2 | |||||||
| Total SCFA (μmol mL−1) | |||||||||
| 2 | 73.69 | 75.20 | 71.90 | 67.92 * | 4.321 | 0.0087 | 0.049 | 0.111 | 0.12 |
| 6 | 85.99 | 85.69 | 84.78 | 77.72 * | 0.0015 | 0.068 | 0.128 | - | |
| 10 | 100.09 | 98.53 | 95.06 | 89.50 * | 0.0003 | 0.008 | 0.031 | 0.21 | |
| 24 | 125.53 | 125.38 | 122.42 | 114.75 * | 0.0030 | 0.047 | 0.112 | 0.13 | |
| 96 | 169.64 | 171.59 | 167.91 | 162.67 | 0.1919 | 0.098 | 0.198 | - | |
| Acetate (mol 100 mol−1) | |||||||||
| 2 | 69.06 | 68.21 | 68.17 | 68.03 * | 0.373 | 0.0370 | 0.321 | 0.525 | - |
| 6 | 64.88 | 63.85 | 63.31 * | 64.23 | 0.0127 | 0.509 | 0.215 | - | |
| 10 | 60.21 | 59.47 | 59.82 | 60.50 | 0.2197 | 0.570 | 0.556 | - | |
| 24 | 54.46 | 53.96 | 53.95 | 54.24 | 0.4635 | 0.894 | 0.859 | - | |
| 96 | 49.67 | 49.57 | 49.84 | 50.87 | 0.1052 | 0.165 | 0.299 | - | |
| Propionate (mol 100 mol−1) | |||||||||
| 2 | 16.00 | 16.47 | 15.97 | 15.12 | 0.74 | 0.209 | 0.159 | 0.241 | - |
| 6 | 18.47 | 19.00 | 18.98 | 17.41 | 0.167 | 0.125 | 0.052 | 0.18 | |
| 10 | 21.62 | 22.11 | 21.31 | 19.91 | 0.054 | 0.006 | 0.008 | 0.29 | |
| 24 | 24.84 | 25.81 | 25.01 | 23.64 | 0.168 | 0.027 | 0.013 | 0.26 | |
| 96 | 26.44 | 27.40 | 26.57 | 25.06 | 0.129 | 0.023 | 0.013 | 0.26 | |
| Butyrate (mol 100 mol−1) | |||||||||
| 2 | 11.53 | 11.57 | 11.77 | 11.91 | 0.494 | 0.532 | 0.434 | 0.741 | - |
| 6 | 12.91 | 12.96 | 13.31 | 13.04 | 0.486 | 0.723 | 0.813 | - | |
| 10 | 14.09 | 13.87 | 14.28 | 14.06 | 0.656 | 0.823 | 0.957 | - | |
| 24 | 15.75 | 14.96 | 15.72 | 16.37 | 0.153 | 0.133 | 0.165 | - | |
| 96 | 16.00 | 15.06 | 16.05 | 16.15 | 0.090 | 0.268 | 0.400 | - | |
| A:P | |||||||||
| 2 | 4.34 | 4.20 | 4.32 | 4.52 | 0.133 | 0.241 | 0.365 | 0.503 | - |
| 6 | 3.52 | 3.4 | 3.36 | 3.69 | 0.257 | 0.318 | 0.116 | - | |
| 10 | 2.78 | 2.72 | 2.81 | 3.04 | 0.100 | 0.022 | 0.031 | 0.21 | |
| 24 | 2.19 | 2.09 | 2.17 | 2.30 | 0.454 | 0.131 | 0.124 | - | |
| 96 | 1.88 | 1.81 | 1.87 | 1.97 | 0.509 | 0.024 | 0.018 | 0.24 | |
| Valerate (mol 100 mol−1) | |||||||||
| 2 | 0.88 | 0.92 | 0.94 | 1.06 * | 0.083 | 0.007 | 0.004 | 0.014 | 0.24 |
| 6 | 1.23 | 1.26 | 1.26 | 1.34 | 0.111 | 0.102 | 0.230 | - | |
| 10 | 1.46 | 1.51 | 1.44 | 1.49 | 0.497 | 0.875 | 0.961 | - | |
| 24 | 1.92 | 1.94 | 1.86 | 1.87 | 0.461 | 0.446 | 0.747 | - | |
| 96 | 3.25 | 3.01 | 2.85 * | 2.74 * | 0.014 | 0.007 | 0.019 | 0.22 | |
| Isovalerate (mol 100 mol−1) | |||||||||
| 2 | 2.12 | 2.29 | 2.43 | 2.76 * | 0.231 | 0.019 | <0.001 | <0.001 | 0.43 |
| 6 | 2.22 | 2.45 | 2.50 | 2.95 * | 0.016 | <0.001 | <0.001 | 0.45 | |
| 10 | 2.26 | 2.54 | 2.52 | 3.08 * | 0.012 | <0.001 | <0.001 | 0.38 | |
| 24 | 2.65 | 2.92 | 2.94 | 3.19 | 0.075 | <0.001 | 0.004 | 0.32 | |
| 96 | 3.81 | 4.03 | 3.90 | 4.03 | 0.561 | 0.273 | 0.542 | - | |
| Isobutyrate (mol 100 mol−1) | |||||||||
| 2 | 0.42 | 0.54 | 0.71 | 1.80 * | 0.430 | 0.016 | <0.001 | <0.001 | 0.52 |
| 6 | 0.29 | 0.48 | 0.64 * | 1.62 * | 0.029 | <0.001 | <0.001 | 0.56 | |
| 10 | 0.38 | 0.50 | 0.62 | 1.34 * | 0.014 | 0.002 | 0.006 | 0.29 | |
| 24 | 0.37 | 0.42 | 0.52 | 1.02 * | 0.034 | <0.001 | 0.005 | 0.30 | |
| 96 | 0.81 | 0.93 | 0.80 | 1.81 | 0.241 | 0.047 | 0.057 | 0.12 | |
| pH | |||||||||
| 2 | 6.8 | 6.8 | 6.9 * | 7.0 * | 0.04 | 0.005 | <0.001 | <0.001 | 0.39 |
| 6 | 6.7 | 6.8 | 6.9 * | 7.0 * | 0.005 | <0.001 | <0.001 | 0.53 | |
| 10 | 6.7 | 6.8 | 6.7 | 6.9 * | 0.008 | <0.001 | 0.007 | 0.26 | |
| 24 | 6.3 | 6.4 | 6.4 | 6.5 | 0.064 | 0.345 | 0.345 | - | |
| 96 | 6.3 | 6.4 | 6.4 | 6.5 * | 0.008 | 0.004 | 0.018 | 0.21 | |
| Incubation Time (h) | TD0 | TD9 | TD27 | TD45 | SE | p-Value | Regression | ||
|---|---|---|---|---|---|---|---|---|---|
| L | Q | R2 | |||||||
| Ammonia nitrogen (mg dL−1) | |||||||||
| 2 | 21.61 | 22.14 | 22.57 | 19.02 | 2.045 | 0.252 | 0.285 | 0.284 | - |
| 6 | 23.27 | 23.94 | 23.50 | 22.73 | 0.753 | 0.590 | 0.739 | - | |
| 10 | 29.24 | 28.61 | 25.06 | 23.55 * | 0.032 | <0.001 | <0.001 | 0.45 | |
| 24 | 34.80 | 36.99 | 31.67 | 31.81 | 0.093 | 0.014 | 0.053 | 0.18 | |
| 96 | 54.70 | 54.98 | 53.88 | 50.11 | 0.065 | 0.018 | 0.022 | 0.23 | |
| Partitioning factor | |||||||||
| 2 | 11.84 | 12.28 | 13.32 | 16.90 * | 0.441 | <0.001 | <0.001 | <0.001 | 0.50 |
| 6 | 5.69 | 6.06 | 6.16 | 7.58 * | 0.001 | <0.001 | <0.001 | 0.39 | |
| 10 | 4.57 | 4.59 | 4.82 | 5.68 * | 0.043 | <0.001 | <0.001 | 0.41 | |
| 24 | 3.21 | 3.68 | 3.45 | 3.89 * | 0.008 | 0.006 | 0.021 | 0.23 | |
| 96 | 2.78 | 2.73 | 2.81 | 2.84 | 0.671 | 0.570 | 0.842 | - | |
| Microbial biomass (mg) | |||||||||
| 2 | 134.03 | 132.26 | 133.26 | 180.02 * | 15.195 | 0.023 | 0.003 | 0.001 | 0.37 |
| 6 | 151.82 | 171.15 | 179.38 | 234.45 * | 0.001 | <0.001 | <0.001 | 0.55 | |
| 10 | 207.12 | 188.17 | 205.19 | 247.35 * | 0.038 | 0.002 | <0.001 | 0.42 | |
| 24 | 236.87 | 226.96 | 245.92 | 279.62 * | 0.038 | <0.001 | <0.001 | 0.40 | |
| 96 | 349.24 | 317.01 | 334.85 | 319.26 | 0.078 | 0.271 | 0.510 | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Márquez, S.; Ovani, V.S.; Lima, P.d.M.T.; Lana, Â.M.Q.; Louvandini, H.; Abdalla, A.L.; Maurício, R.M. Tithonia diversifolia Improves In Vitro Rumen Microbial Synthesis of Sheep Diets without Changes in Total Gas and Methane Production. Agronomy 2023, 13, 2768. https://doi.org/10.3390/agronomy13112768
Pérez-Márquez S, Ovani VS, Lima PdMT, Lana ÂMQ, Louvandini H, Abdalla AL, Maurício RM. Tithonia diversifolia Improves In Vitro Rumen Microbial Synthesis of Sheep Diets without Changes in Total Gas and Methane Production. Agronomy. 2023; 13(11):2768. https://doi.org/10.3390/agronomy13112768
Chicago/Turabian StylePérez-Márquez, Simón, Vagner S. Ovani, Paulo de Mello Tavares Lima, Ângela Maria Quintão Lana, Helder Louvandini, Adibe Luiz Abdalla, and Rogério Martins Maurício. 2023. "Tithonia diversifolia Improves In Vitro Rumen Microbial Synthesis of Sheep Diets without Changes in Total Gas and Methane Production" Agronomy 13, no. 11: 2768. https://doi.org/10.3390/agronomy13112768
APA StylePérez-Márquez, S., Ovani, V. S., Lima, P. d. M. T., Lana, Â. M. Q., Louvandini, H., Abdalla, A. L., & Maurício, R. M. (2023). Tithonia diversifolia Improves In Vitro Rumen Microbial Synthesis of Sheep Diets without Changes in Total Gas and Methane Production. Agronomy, 13(11), 2768. https://doi.org/10.3390/agronomy13112768







