Germination Strategy of Chenopodium acuminatum Willd. under Fluctuating Salinity Habitats
Abstract
:1. Introduction
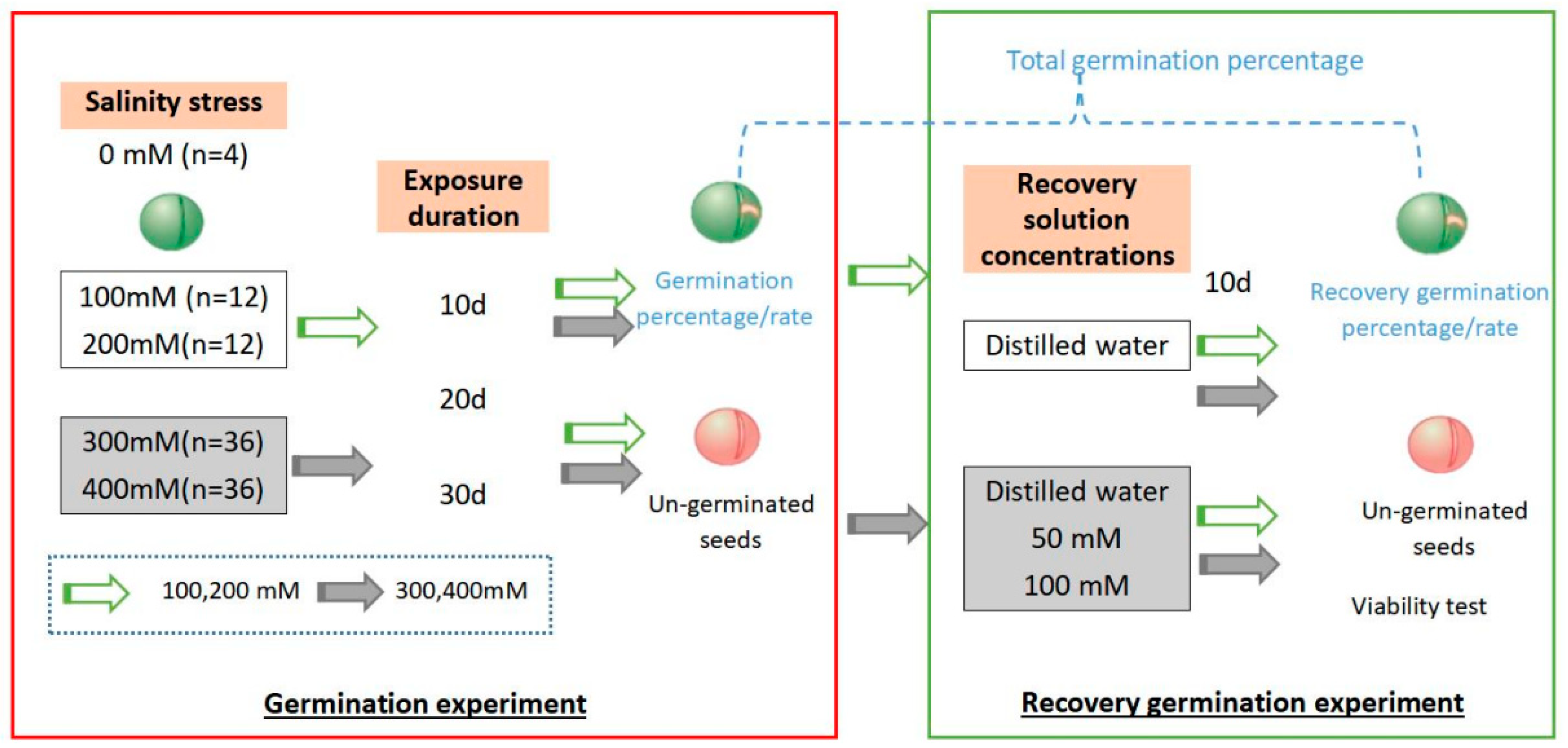
2. Materials and Methods
2.1. Seed Collection and Study Region
2.2. Effects of Salinity and Exposure Duration on Initial Germination
2.3. Recovery Test
2.4. Statistical Analysis
3. Results
3.1. Effects of Salinity and Exposure Duration on Initial Germination
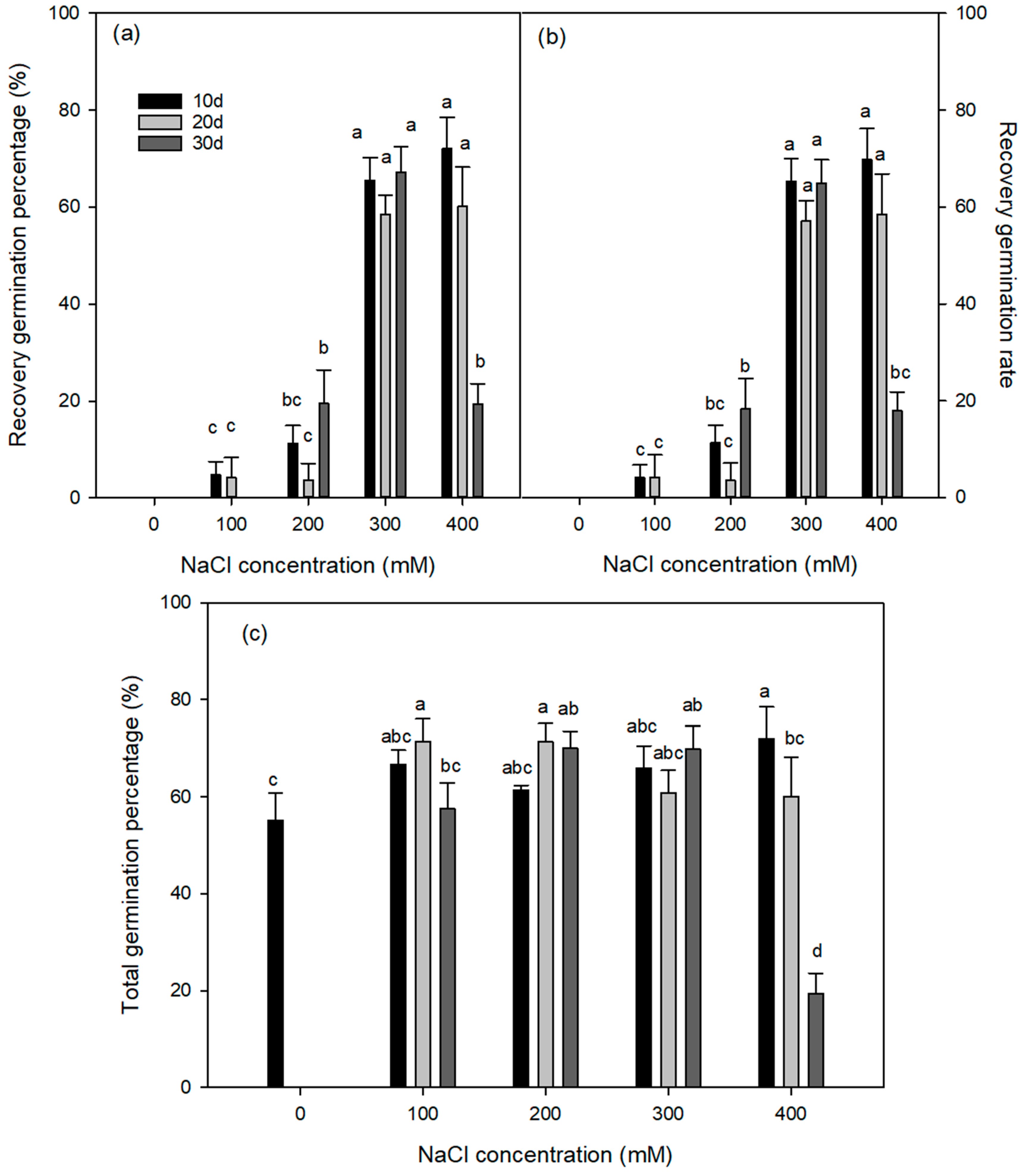
3.2. Effects of Salinity and Exposure Duration on Recovery of Germination in Distilled Water
3.3. Effects of Salinity, Exposure Duration and Recovery Solution Concentrations on Recovery of Germination
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rozema, J.; Flowers, T. Ecology: Crops for a salinized world. Science 2008, 322, 1478–1480. [Google Scholar] [CrossRef]
- Eswar, D.; Karuppusamy, R. Drivers of soil salinity and their correlation with climate change. Curr. Opin. Environ. Sustain. 2021, 50, 310–318. [Google Scholar] [CrossRef]
- Haj-Amor, Z.; Araya, T. Soil salinity and its associated effects on soil microorganisms, greenhouse gas emissions, crop yield, biodiversity and desertification: A review. Sci. Total Environ. 2022, 843, 156946. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Pan, J.A. Bibliometric Review of Plant Growth-Promoting Rhizobacteria in Salt-Affected Soils. Agronomy 2022, 12, 2304. [Google Scholar] [CrossRef]
- Shaddad, S.M.; Buttafuoco, G. Assessment and Mapping of Soil Salinization Risk in an Egyptian Field Using a Probabilistic Approach. Agronomy 2020, 10, 85. [Google Scholar] [CrossRef]
- Lei, C.; Bagavathiannan, M. Osmopriming with Polyethylene Glycol (PEG) for Abiotic Stress Tolerance in Germinating Crop Seeds: A Review. Agronomy 2021, 11, 2149. [Google Scholar] [CrossRef]
- Izadi, Y.; Moosavi, S.A. Salinity affects eco-physiological aspects and biochemical compositions in chia (Salvia hispanica L.) during germination and seedling growth. Sci. Hortic. 2022, 306, 1461. [Google Scholar] [CrossRef]
- Hmissi, M.; Chaieb, M. Differences in the Physiological Indicators of Seed Germination and Seedling Establishment of Durum Wheat (Triticum durum Desf.) Cultivars Subjected to Salinity Stress. Agronomy 2023, 13, 1718. [Google Scholar] [CrossRef]
- Malik, J.A.; Al Qarawi, A.A. Effect of Salinity and Temperature on the Seed Germination and Seedling Growth of Desert Forage Grass Lasiurus scindicus Henr. Sustainability 2022, 14, 8387. [Google Scholar] [CrossRef]
- Walsh, S.K.; Wolki, D. Variability in seed salinity tolerance in an island coastal community. Ann. Bot. 2023, mcad129. [Google Scholar] [CrossRef]
- Castillo, J.M.; Curado, G. Germination syndrome divergence among pairs of sympatric sister species along an estuarine salinity gradient. Environ. Exp. Bot. 2021, 181, 104274. [Google Scholar] [CrossRef]
- Bhatt, A.; Santo, A. Germination and recovery of heteromorphic seeds of Atriplex canescens (Amaranthaceae) under increasing salinity. Plant Ecol. 2016, 217, 1069–1079. [Google Scholar] [CrossRef]
- Ludwiczak, A.; Osiak, M. Osmotic Stress or Ionic Composition: Which Affects the Early Growth of Crop Species More? Agronomy 2021, 11, 435. [Google Scholar] [CrossRef]
- Ungar, I.A. Seed germination and seed-bank ecology in halophytes. In Seed Development and Germination; CRC Press: Bocaraton, FL, USA, 2017; pp. 599–628. [Google Scholar] [CrossRef]
- Castillo, J.M.; Curado, G. Salt tolerance during germination identifies native intertidal plant species at risk under increasing salinity with sea level rise. Mar. Ecol. Prog. Ser. 2022, 684, 57–68. [Google Scholar] [CrossRef]
- Pujol, J.A.; Calvo, J.F. Recovery of germination from different osmotic conditions by four halophytes from southeastern Spain. Ann. Bot. 2000, 85, 279–286. [Google Scholar] [CrossRef]
- Keiffer, C.H.; Ungar, I.A. The effect of extended exposure to hypersaline conditions on the germination of five inland halophyte species. Am. J. Bot. 1997, 84, 104–111. [Google Scholar] [CrossRef]
- Moreno, J.; Terrone, A. Germination patterns along a salinity gradient of closely-related halophytes in sympatry. Estuar. Coast. Shelf Sci. 2022, 264, 107690. [Google Scholar] [CrossRef]
- Zheng, Y.H.; Li, J.D. Halophytes in Songnen Plain and Restoration of Saline-Alkali Grassland; Science Press: Beijing, China, 1999. [Google Scholar]
- Huang, Y.; Zhao, X. Biomass allocation to vegetative and reproductive organs of Chenopodium acuminatum Willd. under soil nutrient and water stress. Bangladesh J. Bot. 2013, 42, 113–121. [Google Scholar] [CrossRef]
- Kinugasa, T.; Hozumi, Y. Germination characteristics of early successional annual species after severe drought in the Mongolian steppe. J. Arid. Environ. 2016, 130, 49–53. [Google Scholar] [CrossRef]
- Kong, X.W. Chenopodium acuminatum Willd. Flora China 1979, 25, 86. [Google Scholar]
- Li, X.; Jiang, D. Soil seed bank characteristics beneath an age sequence of caragana microphylla shrubs in the Horqin sandy land region of northeastern China. Land Degrad. Dev. 2014, 25, 236–243. [Google Scholar] [CrossRef]
- Zhao, M.; Liu, Z.; Zhang, H.; Wang, Y.; Yan, H. Germination Characteristics Is More Associated With Phylogeny-Related Traits of Species in a Salinized Grassland of Northeastern China. Front. Ecol. Evol. 2021, 9, 748038. [Google Scholar] [CrossRef]
- Chen, P.; Jiang, L. Seed Germination Response and Tolerance to Different Abiotic Stresses of Four Salsola Species Growing in an Arid Environment. Front. Plant. Sci. 2022, 13, 892667. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, X.M. Vegetation Change and Its Response to Climate Change between 2000 and 2016 in Marshes of the Songnen Plain, Northeast China. Sustainability 2020, 12, 3569. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Ecologically Meaningful Germination Studies. In Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination, 2nd ed.; Academic Press: San Diego, CA, USA, 2014; pp. 31–32. [Google Scholar]
- de Souza, R.R.; Moraes, M.P. Effects of Trichoderma asperellum on Germination Indexes and Seedling Parameters of Lettuce Cultivars. Curr. Microbiol. 2022, 79, 5. [Google Scholar] [CrossRef] [PubMed]
- Li, X.H.; Jiang, D.M. Effects of salinity and desalination on seed germination of six annual weed species. J. For. Res. 2011, 22, 475−479. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, L. Phylogeny, Seed Trait, and Ecological Correlates of Seed Germination at the Community Level in a Degraded Sandy Grasslan. Front. Plant Sci. 2016, 7, 1532. [Google Scholar] [CrossRef]
- Hourston, J.E.; Steinbrecher, T. Cold-induced secondary dormancy and its regulatory mechanisms in Beta vulgaris. Plant Cell Environ. 2022, 45, 1315–1332. [Google Scholar] [CrossRef]
- Wei, Y.; Dong, M. Factors influencing seed germination of Salsola affinis (Chenopodiaceae), a dominant annual halophyte inhabiting the deserts of Xinjiang, China. Flora 2008, 203, 134–140. [Google Scholar] [CrossRef]
- Cao, D.; Baskin, C.C. Comparison of germination and seed bank dynamics of dimorphic seeds of the cold desert halophyte Suaeda corniculata subsp. Mongolica. Ann. Bot. 2012, 110, 1545–1558. [Google Scholar] [CrossRef]
- Wang, L.; Huang, Z. Germination of dimorphic seeds of the desert annual halophyte Suaeda aralocaspica (Chenopodiaceae), a C4 plant without Kranz anatomy. Ann. Bot. 2008, 102, 757–769. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.Z.; Wang, J.J. Seed Germination and Seed Bank Dynamics of Eruca sativa (Brassicaceae): A Weed on the Northeastern Edge of Tibetan Plateau. Front. Plant. Sci. 2022, 13, 820925. [Google Scholar] [CrossRef] [PubMed]
- Podda, L.; Santo, A. Seed germination, salt stress tolerance and seedling growth of Opuntia ficus-indica (Cactaceae), invasive species in the Mediterranean Basin. Flora 2017, 229, 50–57. [Google Scholar] [CrossRef]
- Khan, M.A.; Gul, B.; Weber, D.J. Germination of dimorphic seeds of Suaeda moquinii under high salinity stress. Aus. J. Bot. 2001, 49, 185–192. [Google Scholar] [CrossRef]
- Bajji, M.; Kient, J.M. Osmotic and ionic effects of NaCl on germination, early seedling growth, and ion content of Atriplex halimus (Chenopodiaceae). Can. J. Bot. 2002, 80, 297–304. [Google Scholar] [CrossRef]
- Debez, A.; Belghith, I. High salinity impacts germination of the halophyte Cakile maritima but primes seeds for rapid germination upon stress release. Physiol. Plant. 2018, 164, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Moravcova, L.; Frantik, T. Germination ecology of Puccinellia distans and P-limosa. Biologia 2002, 57, 441–448. [Google Scholar]
- Shah, S.Z.; Rasheed, A. Inter-provenance variation in seed germination response of a cash crop halophyte Suaeda fruticosa to different abiotic factors. Flora 2022, 292, 152079. [Google Scholar] [CrossRef]
- El-Keblawy, A.; Aljasmi, M. Provenance determines salinity tolerance and germination requirements of the multipurpose tree Prosopis juliflora seeds. Arid. Land Res. Manag. 2021, 35, 446–462. [Google Scholar] [CrossRef]
- Gairola, S.; Hameed, A. Seed germination and salinity tolerance of habitat-indifferent halophytes as associated with geographical distribution. Seed Sci. Technol. 2022, 50, 125–140. [Google Scholar] [CrossRef]
- Sharavdorj, K.; Yeongmi, J. Understanding seed germination of forage crops under various salinity and temperature stress. J. Crop. Sci. Biotechnol. 2021, 24, 545–554. [Google Scholar] [CrossRef]
- Wariss, H.M.; Qu, X.J. The complete chloroplast genome of Chenopodium acuminatum Willd. (Amaranthaceae). Mitochondrial DNA B 2021, 6, 174–175. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Baskin, C.C. Dormancy cycling and persistence of seeds in soil of a cold desert halophyte shrub. Ann. Bot. 2014, 113, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Ungar, I.A. Effect of salinity on seed germination, growth, and ion accumulation of Atriplex patula (Chenopodiaceae). Am. J. Bot. 1996, 83, 604–607. [Google Scholar] [CrossRef]
- Woodell, S.R.J. Salinity and seed germination patterns in coastal plants. Vegetatio 1985, 61, 223–229. [Google Scholar] [CrossRef]
- Gul, B.; Hameed, A. Assessing Seed Germination Responses of Great Basin Halophytes to Various Exogenous Chemical Treatments Under Saline Conditions. Sabkha Ecosyst. 2016, 48, 85–104. [Google Scholar] [CrossRef]
- Al Hassan, M.; Estrelles, E. Unraveling Salt Tolerance Mechanisms in Halophytes: A Comparative Study on Four Mediterranean Limonium Species with Different Geographic Distribution Patterns. Front. Plant. Sci. 2017, 8, 1438. [Google Scholar] [CrossRef]
- Coops, H.; Vandervelde, G. Seed dispersal, germination and seedling growth of six helophyte species in relation to water-level zonation. Freshw. Biol. 1995, 34, 13–20. [Google Scholar] [CrossRef]
- Rosbakh, S.; Phartyal, S.S. Seed germination traits shape community assembly along a hydroperiod gradient. Ann. Bot. 2020, 125, 67–78. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, D.Y. Factors influencing seed germination of Kalidium caspicum (Chenopodiaceae), a halophytic desert shrub of Xinjiang, China. Seed Sci. Technol. 2009, 37, 281–290. [Google Scholar] [CrossRef]




| Source | Initial Germination Percentage | Initial Germination Rate | Recovery Percentage | Recovery Germination Rate | Total Germination Percentage | |
|---|---|---|---|---|---|---|
| df | F | F | F | F | F | |
| Salinity (S) | 4 | 4.208 * | 1.637 | 9.589 * | 44.858 * | 10.303 * |
| Exposure duration (ED) | 2 | 2.138 | 20.816 * | 1.100 | 35.151 * | 1.588 |
| S × ED | 8 | 0.986 | 5.252 * | 2.624 | 60.065 * | 1.745 |
| Source | Recovery Germination Percentage | Recovery Germination Rate | Total Germination Percentage | |
|---|---|---|---|---|
| df | F | F | F | |
| Salinity (S) | 1 | 9.519 * | 10.116 * | 14.069 * |
| Exposure duration (ED) | 2 | 59.583 * | 64.875 * | 35.931 * |
| Recovery concentration (RC) | 2 | 10.661 * | 10.846 * | 9.042 * |
| S × ED | 2 | 9.907 * | 9.159 * | 6.466 * |
| S × RC | 2 | 0.316 | 0.43 | 0.033 |
| ED × RC | 4 | 3.659 * | 3.929 * | 2.241 |
| S × ED × RC | 4 | 1.826 | 1.945 | 2.489 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Y.; Li, Y.; Zhang, H.; Tennakoon, K.U.; Sun, Z. Germination Strategy of Chenopodium acuminatum Willd. under Fluctuating Salinity Habitats. Agronomy 2023, 13, 2769. https://doi.org/10.3390/agronomy13112769
Tian Y, Li Y, Zhang H, Tennakoon KU, Sun Z. Germination Strategy of Chenopodium acuminatum Willd. under Fluctuating Salinity Habitats. Agronomy. 2023; 13(11):2769. https://doi.org/10.3390/agronomy13112769
Chicago/Turabian StyleTian, Yu, Yang Li, Hongxiang Zhang, Kushan U. Tennakoon, and Zewei Sun. 2023. "Germination Strategy of Chenopodium acuminatum Willd. under Fluctuating Salinity Habitats" Agronomy 13, no. 11: 2769. https://doi.org/10.3390/agronomy13112769





