Field Phenotyping Monitoring Systems for High-Throughput: A Survey of Enabling Technologies, Equipment, and Research Challenges
Abstract
1. Introduction
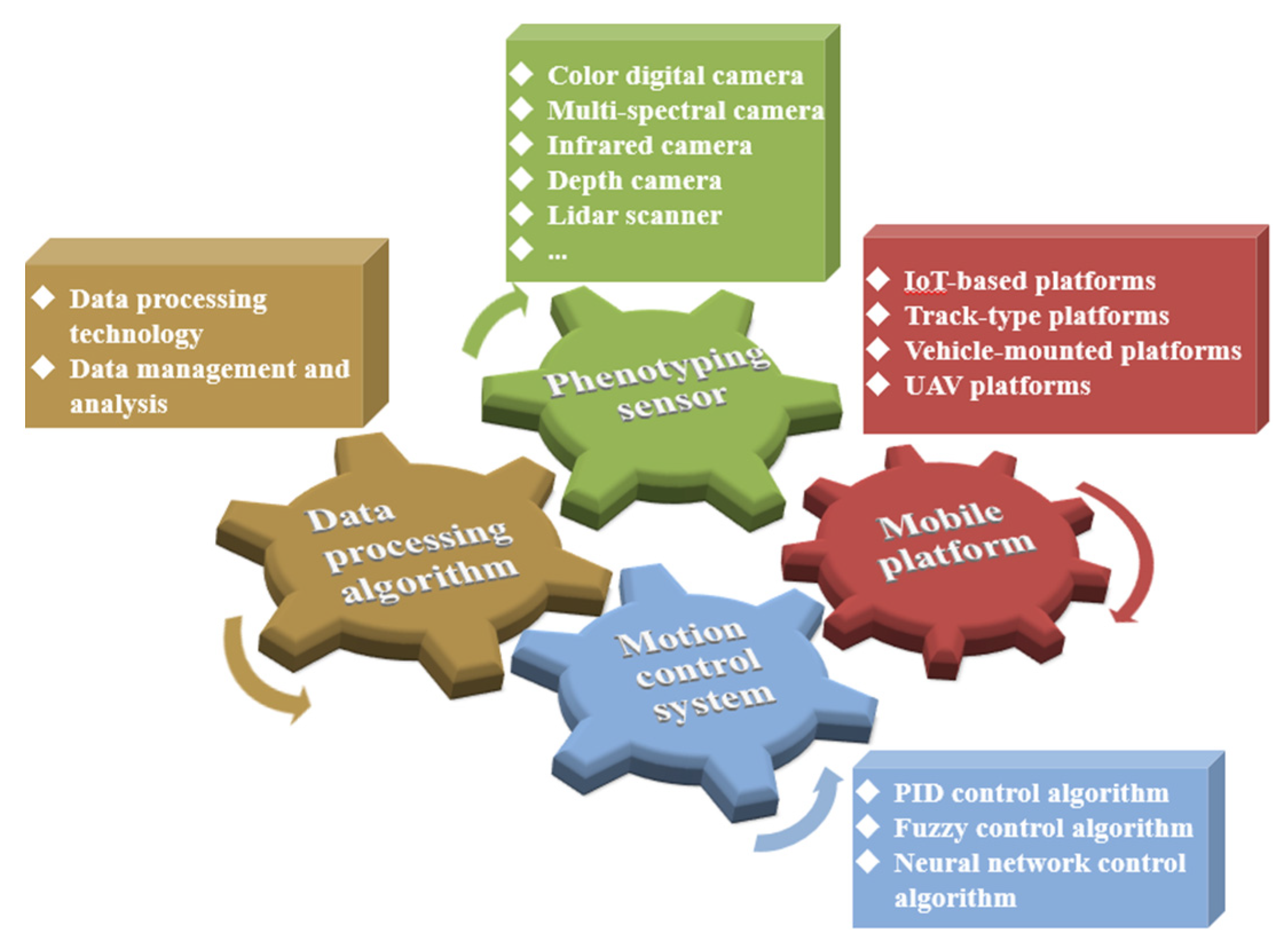
2. Phenotyping Sensors for Field Crops
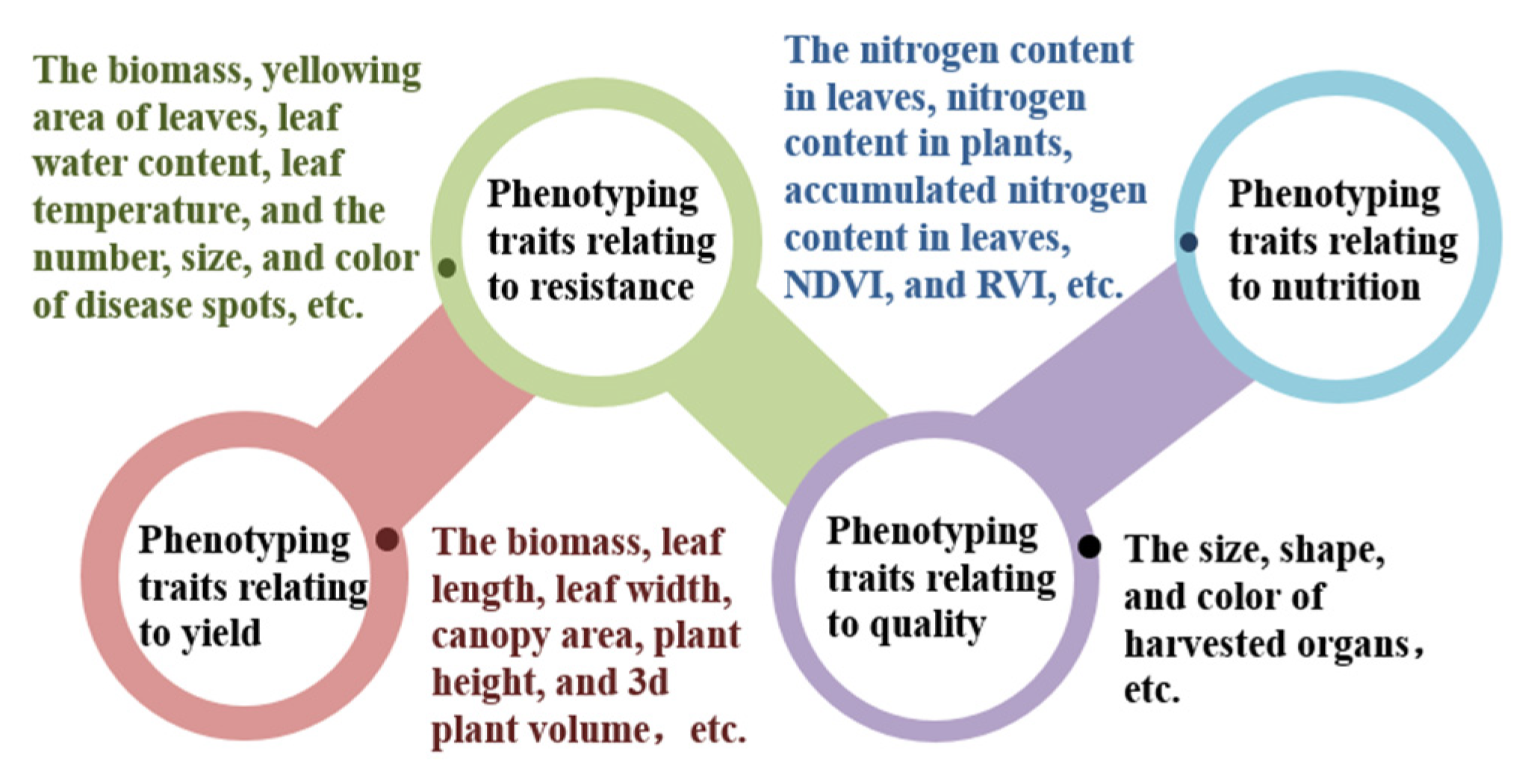
2.1. Classification of Crop Phenotyping Traits
2.2. Common Phenotyping Sensors for Crops
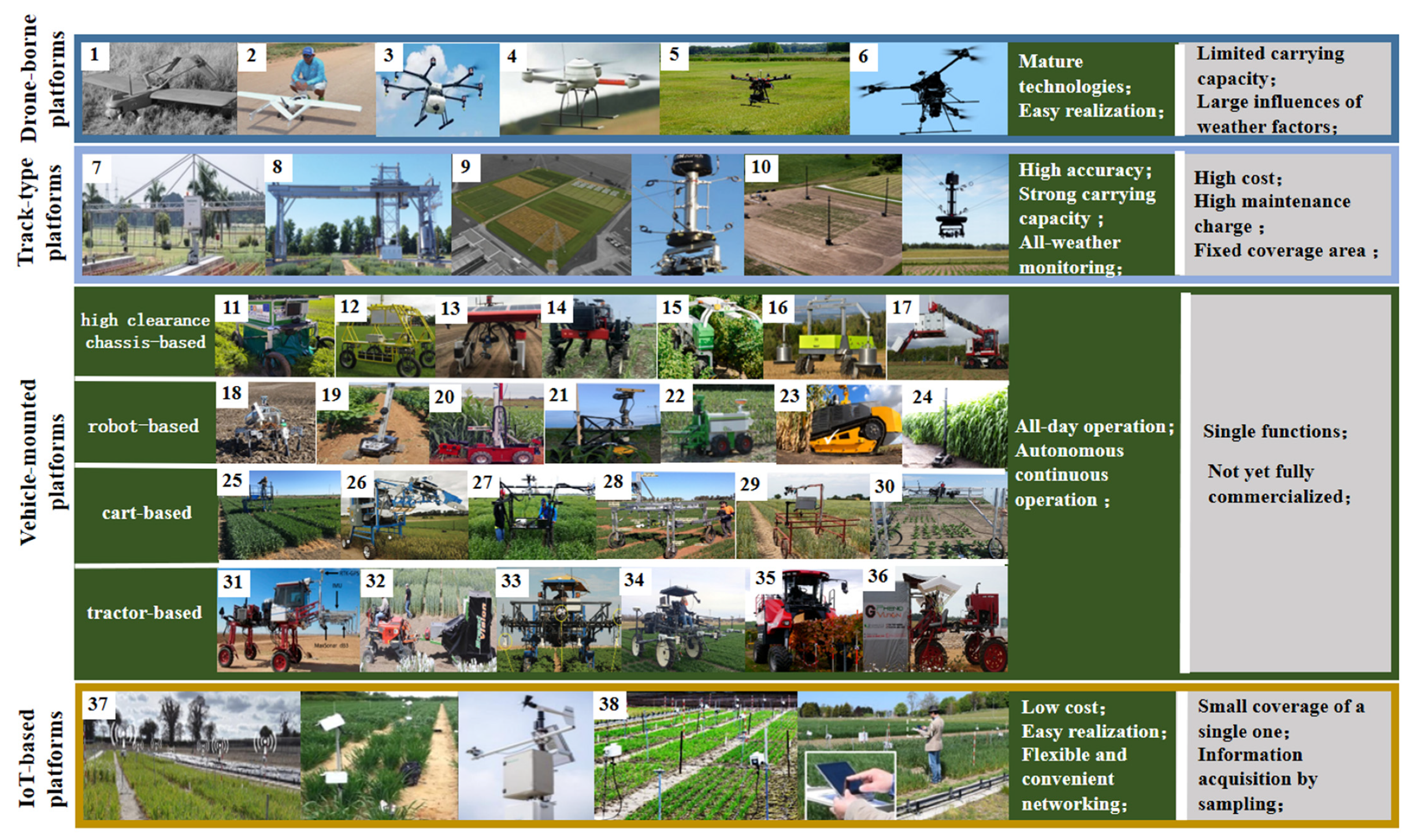
3. Mobile Phenotyping Platforms for Field Crops
3.1. IoT-Based Platforms
3.2. Track-Type Mobile Platforms
3.3. Vehicle-Mounted Mobile Platforms
3.4. Drone-Borne Mobile Platforms
4. Phenotype Monitoring Control System for Field Crops
4.1. Motion Control Algorithms of Phenotyping Platforms for Field Crops
4.1.1. PID Control Algorithm
4.1.2. Fuzzy Control Algorithm
4.1.3. Neural Network Control Algorithm
4.2. Motion Controllers of Phenotyping Platforms for Field Crops
5. Phenotype Data Processing Algorithms for Field Crops
5.1. Phenotype Data Processing Technologies
| Phenotyping Technologies | Phenotyping Methods | Phenotype Parameters | Crops |
|---|---|---|---|
| Machine vision | Convolutional neural network | Plant height, variety classification [143], and wheat spike identification [144,145] | Potato, wheat, and broomcorn |
| Deep learning and machine vision | Deep convolutional neural network (DCNN) | Number of stems, phenotypes of stem width, and yield trait | Broomcorn, sugarcane, cereal, corn, and lettuce |
| Support vector machine (SVM) | Canopy coverage, vegetation index, and flowering phenotype detection | Cotton | |
| Artificial neural network (ANN) | Green area index (GAI) | Wheat | |
| Three-dimensional reconstruction | Structure from motion (SFM) | Plant height [146,147] and crop morphology [148] | Corn and wheat |
5.2. Phenotype Data Processing and Management Software
6. Pending Problems
- Lack of R&D and integration technologies of novel phenotyping sensors. Breakthroughs remain to be made in the R&D and field application of low-cost phenotyping sensors for monitoring traits relating to the resistance and nutrition of crops. Most imaging-type phenotyping sensors are not applicable to the dynamic phenotype monitoring of field crops and cannot overcome sensor shaking due to platform vibration, so the collected images are blurred and distorted. A single sensor can only acquire limited data, while the use of multiple sensors together faces technological problems pertaining to system standards and synchronous calibration. Moreover, technological problems relating to the integration of multi-source phenotype information at different scales in different growth stages also pose a challenge for phenotype research teams.
- Urgent need to develop low-cost and highly applicable phenotyping platforms. Phenotyping platforms for field crops generally use specific commercial software to fulfil hardware control, data management, and trait analysis, to which the investment and maintenance cost are prohibitive. Platforms and sensor systems also cost tens of thousands of dollars. In addition, some phenotyping platforms for field crops are designed to adapt to specific crops and agronomic traits, which limits their utilization in other crops and plots with different agronomic designs. In addition, changes relating to the plant height and size in the crop growth process also limit the utilization of platforms in all the growth stages.
- Incomplete development standards for phenotype monitoring systems. Definite development standards are unavailable for various modules including the sensor acquisition, communication transmission, and data analysis, so that software and hardware systems of many phenotype monitoring systems follow different development and application standards. This limits the secondary development and promotion of the technology.
- Timeliness of data processing to be improved. It is acknowledged that the interactions between field crops and environments are complex, and the soil shows heterogeneity. This means that relevant external environmental factors can all affect the stability and accuracy of phenotype monitoring systems for field crops in navigation, positioning, target detection, and data transmission in field crop phenotyping monitoring systems. Limited by the computer hardware and due to the influences of algorithms and software, the data processing and phenotyping trait extraction of monitoring systems are mainly performed offline, during which it is challenging to ensure timeliness and online control.
7. Prospects
- Multi-sensor integration and multi-source data fusion. A ground-based automatic acquisition system (e.g., swarm robots) for phenotype information needs to be established, and a multi-dimensional phenotype information acquisition system combining ground-based and aerial platforms is suggested to be deployed. This can realize data acquisition with full spatial coverage and improve the data throughput of multi-scale monitoring systems. A multi-sensor integrated system needs to be developed to achieve high-integration and high-resolution phenotype collection with strong anti-jamming performance and to fully integrate traits recorded by these sensors, so as to realize parallel tests of multiple parameters. Multi-source phenotype data should be further mined, arranged, and visualized. Additionally, multi-source data fusion methods should be explored to acquire the correspondence between genetic characteristics and presentation of phenotyping traits of crops.
- Optimizing platform mechanisms, improving data quality, and enhancing field applicability of platforms. Design of mobile structures of platforms should be innovated to enhance the anti-vibration property and stability of platforms and improve the accuracy of data collected on complex terrains. Automatic regulating devices or modular mechanism design can be used so that platforms are adaptive to different planting systems, including the plant height, row spacing, and field layout, and can be flexibly operated in various environments and can execute tasks to acquire phenotype information about different crops.
- Building a unified, open, standardized technological system. The cooperation between developers of phenotyping platforms and sensors can be enhanced to form the unified and open platform and sensor standards and provide more opportunities of secondary development for more researchers. This can also provide technological support for multi-sensor integration and intelligent acquisition of platforms. Aiming at the acquired multi-source data, normalized and standardized processing standards and data management systems should be established to provide data support for the application of information processing technologies including data storage, sharing, analysis, and decision making.
- Optimizing and upgrading data processing software. Processing software should be developed to meet the demand for efficient data analysis in the context of big data. The application of emerging technologies such as machine learning and AI to the sensing and control of phenotyping platforms should be explored to understand scenarios and extract phenotyping traits more efficiently. Novel data processing algorithms are suggested to be combined to further improve the speed and accuracy of automatic information processing of monitoring systems in practical production environments with varying levels of illumination and backgrounds to achieve high-quality, online, real-time data processing.
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morisse, M.; Wells, D.M.; Millet, E.J.; Lillemo, M.; Fahrner, S.; Cellini, F.; Lootens, P.; Muller, O.; Herrera, J.M.; Bentley, A.R.; et al. A European perspective on opportunities and demands for field-based crop phenotyping. Field Crops Res. 2022, 276, 108371. [Google Scholar] [CrossRef]
- Ying-Hong, P. Analysis of Concepts and Categories of Plant Phenome and Phenomics. Acta Agron. Sin. 2015, 41, 175–186. [Google Scholar] [CrossRef]
- Sheikh, M.; Iqra, F.; Ambreen, H.; Pravin, K.A.; Ikra, M.; Chung, Y.S. Integrating artificial intelligence and high-throughput phenotyping for crop improvement. J. Integr. Agric. 2023. [Google Scholar] [CrossRef]
- Yang, W.; Feng, H.; Zhang, X.; Zhang, J.; Doonan, J.H.; Batchelor, W.D.; Xiong, L.; Yan, J. Crop Phenomics and High-Throughput Phenotyping: Past Decades, Current Challenges, and Future Perspectives. Mol. Plant 2020, 13, 187–214. [Google Scholar] [CrossRef]
- Jin, X.; Yang, W.; Doonan, J.H.; Atzberger, C. Crop phenotyping studies with application to crop monitoring. Crop J. 2022, 10, 1221–1223. [Google Scholar] [CrossRef]
- Lijin to Carry Out Targeted Field Management and Agricultural Gas Services in Wheat Field Greening Period. Available online: http://sd.cma.gov.cn/gslb/dysqxj/xwzx/gzdt/202103/t20210308_2907884.html (accessed on 21 October 2023).
- It is Not Difficult to Wear AR Glasses to Tour the Fields Accurately and Quickly to Identify Pests and Diseases—Financial Headlines. Available online: https://t.cj.sina.com.cn/articles/view/7517400647/1c0126e4705904gq4m (accessed on 24 October 2023).
- Jimenez-Berni, J.A.; Deery, D.M.; Rozas-Larraondo, P.; Condon, A.T.G.; Rebetzke, G.J.; James, R.A.; Bovill, W.D.; Furbank, R.T.; Sirault, X.R.R. High Throughput Determination of Plant Height, Ground Cover, and Above-Ground Biomass in Wheat with LiDAR. Front. Plant Sci. 2018, 9, 237. [Google Scholar] [CrossRef]
- Barker, J.; Zhang, N.; Sharon, J.; Steeves, R.; Wang, X.; Wei, Y.; Poland, J. Development of a field-based high-throughput mobile phenotyping platform. Comput. Electron. Agric. 2016, 122, 74–85. [Google Scholar] [CrossRef]
- Virlet, N.; Sabermanesh, K.; Sadeghi-Tehran, P.; Hawkesford, M.J. Field Scanalyzer: An automated robotic field phenotyping platform for detailed crop monitoring. Funct. Plant Biol. 2017, 44, 143. [Google Scholar] [CrossRef]
- Field Flux Robot—Adigo AS. Available online: https://www.adigo.no/portfolio/field-flux-robot-2-2/?lang=en (accessed on 21 October 2023).
- Caturegli, L.; Corniglia, M.; Gaetani, M.; Grossi, N.; Magni, S.; Migliazzi, M.; Angelini, L.; Mazzoncini, M.; Silvestri, N.; Fontanelli, M.; et al. Unmanned Aerial Vehicle to Estimate Nitrogen Status of Turfgrasses. PLoS ONE 2016, 11, e158268. [Google Scholar] [CrossRef]
- Borges, C.S.; Chakraborty, S.; Weindorf, D.C.; Lopes, G.; Guilherme, L.R.G.; Curi, N.; Li, B.; Ribeiro, B.T. Pocket-sized sensor for controlled, quantitative and instantaneous color acquisition of plant leaves. J. Plant Physiol. 2022, 272, 153686. [Google Scholar] [CrossRef]
- Markwell, J.; Osterman, J.C.; Mitchell, J.L. Calibration of the Minolta SPAD-502 leaf chlorophyll meter. Photosynth. Res. 1995, 46, 467–472. [Google Scholar] [CrossRef]
- Li Zhenhai, W.J.H.P. Modelling of crop chlorophyll content based on Dualex. Trans. Chin. Soc. Agric. Eng. 2015, 31, 191–197. [Google Scholar]
- Danner, M.; Locherer, M.; Hank, T.; Richter, K. Spectral Sampling with the ASD FIELDSPEC 4; GFZ Data Services: Potsdam, Germany, 2015. [Google Scholar]
- Dallon, D. Comparison of the Analytical Spectral Devices FieldSpec Pro JR and the Apogee/StellarNet Model SPEC-PAR/NIR Spectroradiometers; Crop Physiology Laboratory: Logan, UT, USA, 2003. [Google Scholar]
- Kuester, M.; Thome, K.; Krause, K.; Canham, K.; Whittington, E. Comparison of Surface Reflectance Measurements from Three ASD FieldSpec FR Spectroradiometers and One ASD FieldSpec VNIR Spectroradiometer, 2001/1/1, 2001; IEEE: Piscataway, NJ, USA, 2001; pp. 72–74. [Google Scholar]
- Jia, H.; Yan, G.; Lijun, W.; Yan, Z.; Ben, Z.; Laigang, W. Monitor Model of Corn Leaf Area Index Based on CGMD-402. Trans. Chin. Soc. Agric. Mach. 2019, 50, 187–194. [Google Scholar]
- Chen, Q.; Zhang, Z.; Liu, P.; Wang, X.; Jiang, F. Monitoring of Growth Parameters of Sweet Corn Using CGMD302 Spectrometer. Agric. Sci. Technol. 2015, 16, 364. [Google Scholar]
- Jordan, B.S.; Branch, W.D.; Coffin, A.W.; Smith, C.M.; Culbreath, A.K. Comparison of Trimble GreenSeeker and Crop Circle (Model ACS-210) Reflectance Meters for Assessment of Severity of Late Leaf Spot. Peanut Sci. 2019, 46, 110–117. [Google Scholar] [CrossRef]
- Aranguren, M.; Castellón, A.; Aizpurua, A. Crop sensor based non-destructive estimation of nitrogen nutritional status, yield, and grain protein content in wheat. Agriculture 2020, 10, 148. [Google Scholar] [CrossRef]
- Wei, F.; Yonghua, W.; Yingxin, X.; Guozhang, K.; Yunji, Z.; Tiancai, G. Review of Study on Technique of Crop Nitrogen Diagnosis. Chin. Agric. Sci. Bull. 2008, 179–185. Available online: https://kns.cnki.net/kcms2/article/abstract?v=Pk5Eu7LuuI5lpLK-B3loP2_Cov4MdKdf4fwkP4Qmejj0TxnBv_ALCFRazqaCHgL2vD4e5Xq6AdT58g_Byp4YkZEJ-FF6xf4e5Cn-zBOofsCAUPOpU6u-pgNLoXKFRBW1&uniplatform=NZKPT&language=CHS (accessed on 21 October 2023).
- Reuzeau, C.; Pen, J.; Frankard, V.; Wolf, J.D.; Camp, W.V. TraitMill: A Discovery Engine for Identifying Yield-enhancement Genes in Cereals. Mol. Plant Breed. 2005, 3. Available online: https://kns.cnki.net/kcms2/article/abstract?v=Pk5Eu7LuuI54t2gI-sFfh-Qh3lWLI7G9Q2XgvoHDJ0e8E8zDdx6uhXkRHhiSGsG5fIf4LejZJ9uZMy_XEiVjhllHYWiKJhO9MviPmWY6D--dz-2TajovB_Ao84LKbGHR&uniplatform=NZKPT&language=CHS (accessed on 21 October 2023). [CrossRef]
- Reuzeau, C. TraitMill (TM): A high throughput functional genomics platform for the phenotypic analysis of cereals. Vitr. Cell. Dev. Biol. Anim. 2007, 43, S4. [Google Scholar]
- Furbank, R.T.; Tester, M. Phenomics—Technologies to relieve the phenotyping bottleneck. Trends Plant Sci. 2011, 16, 635–644. [Google Scholar] [CrossRef]
- Johansen, K.; Morton, M.J.L.; Malbeteau, Y.M.; Aragon, B.; Al-Mashharawi, S.K.; Ziliani, M.G.; Angel, Y.; Fiene, G.M.; Negrão, S.S.C.; Mousa, M.A.A.; et al. Unmanned Aerial Vehicle-Based Phenotyping Using Morphometric and Spectral Analysis Can Quantify Responses of Wild Tomato Plants to Salinity Stress. Front. Plant Sci. 2019, 10, 370. [Google Scholar] [CrossRef]
- Chen, D.; Neumann, K.; Friedel, S.; Kilian, B.; Chen, M.; Altmann, T.; Klukasa, C. Dissecting the Phenotypic Components of Crop Plant Growth and Drought Responses Based on High-Throughput Image Analysis. Plant Cell 2014, 26, 4636–4655. [Google Scholar] [CrossRef] [PubMed]
- Arvidsson, S.; Perez-Rodriguez, P.; Mueller-Roeber, B. A growth phenotyping pipeline for Arabidopsis thaliana integrating image analysis and rosette area modeling for robust quantification of genotype effects. New Phytol. 2011, 191, 895–907. [Google Scholar] [CrossRef] [PubMed]
- Brief Discussion on Plant Phenotypic Characters. Available online: https://mp.weixin.qq.com/s?__biz=MzU2NzI1NjkzNw==&mid=2247507575&idx=1&sn=1b2849ebee61fcc5d52b89abf8f03996&chksm=fc9d6c71cbeae567c3c25a9255d29db5881c01b646b19122b17d400040dd415215ee6115b69a&scene=27 (accessed on 21 October 2023).
- Studnicki, M.; Wijata, M.; Sobczyński, G.; Samborski, S.; Gozdowski, D.; Rozbicki, J. Effect of genotype, environment and crop management on yield and quality traits in spring wheat. J. Cereal Sci. 2016, 72, 30–37. [Google Scholar] [CrossRef]
- Ye, J.; Zhong, T.; Zhang, D.; Ma, C.; Wang, L.; Yao, L.; Zhang, Q.; Zhu, M.; Xu, M. The Auxin-Regulated Protein ZmAuxRP1 Coordinates the Balance between Root Growth and Stalk Rot Disease Resistance in Maize. Mol. Plant 2019, 12, 360–373. [Google Scholar] [CrossRef]
- Xiu-ying, H.; Yao-ping, L.; Yong-sheng, C.; Zhao-ming, C.; Yue-han, C. Reviews and prospects for the research of rice grain quality. Rice Res. Inst. Guangdong Acad. Agric. Sci. 2009, 1, 11–16. [Google Scholar] [CrossRef]
- Tripodi, P.; Massa, D.; Venezia, A.; Cardi, T. Sensing technologies for precision phenotyping in vegetable crops: Current status and future challenges. Agronomy 2018, 8, 57. [Google Scholar] [CrossRef]
- Paiao, G.; Fernández, F.; Spackman, J.; Kaiser, D.; Weisberg, S. Ground-based optical canopy sensing technologies for corn nitrogen management in the Upper Midwest. Agron. J. 2020, 112, 2998–3011. [Google Scholar] [CrossRef]
- Jin, X.; Zarco-Tejada, P.J.; Schmidhalter, U.; Reynolds, M.P.; Hawkesford, M.J.; Varshney, R.K.; Yang, T.; Nie, C.; Li, Z.; Ming, B.; et al. High-Throughput Estimation of Crop Traits: A Review of Ground and Aerial Phenotyping Platforms. IEEE Geosci. Rem. Sen. M. 2021, 9, 200–231. [Google Scholar] [CrossRef]
- Yuanqi, Z.; Dunliang, W.; Chen, C.; Rui, L.I.; Dongshuang, L.I.; Tao, L.; Chengming, S.; Xiaochun, Z.; Shengping, L.; Dawei, D. Prediction of wheat yield based on color index and texture feature index of unmanned aerial vehicle RGB image. J. Yangzhou Univ. (Agric. Life Sci. Ed.) 2021, 42, 110–116. [Google Scholar] [CrossRef]
- Bowman, B.C.; Chen, J.; Zhang, J.; Wheeler, J.; Wang, Y.; Zhao, W.; Nayak, S.; Heslot, N.; Bockelman, H.; Bonman, J.M. Evaluating Grain Yield in Spring Wheat with Canopy Spectral Reflectance. Crop Sci. 2015, 55, 1881–1890. [Google Scholar] [CrossRef]
- Yaxiao, N.; Liyuan, Z.; Wenting, H.; Guomin, S. Fractional Vegetation Cover Extraction Method of Winter Wheat Based on UAV Remote Sensing and Vegetation Index. Trans. Chin. Soc. Agric. Mach. 2018, 49, 212–221. [Google Scholar] [CrossRef]
- Qian, W.; Hong, S.; Minzan, L.; Yuanyuan, S.; Yane, Z. Research on precise segmentation and chlorophyll diagnosis of maize multispectral images. Spectrosc. Spect. Anal. 2015, 35, 178–183. [Google Scholar]
- Jun, S.; Xiaming, J.; Hanping, M.; Xiaohong, W.; Wenjing, Z.; Xiaodong, Z.; Hongyan, G. Detection of nitrogen content in lettuce leaves based on spectroscopy and texture using hyperspectral imaging technology. Trans. Chin. Soc. Agric. Eng. 2014, 30, 167–173. [Google Scholar] [CrossRef]
- Choi, S.K.; Lee, S.K.; Jung, S.H.; Choi, J.W.; Choi, D.Y.; Chun, S.J. Estimation of Fractional Vegetation Cover in Sand Dunes Using Multi-spectral Images from Fixed-wing UAV. J. Korean Soc. Surv. Geod. Photogramm. Cartogr. 2016, 34, 431–441. [Google Scholar] [CrossRef]
- Hong, S.; Tao, Z.; Ning, L.; Meng, C.; Minzan, L.; Qin, Z. Vertical distribution of chlorophyll in potato plants based on hyperspectral imaging. Trans. Chin. Soc. Agric. Eng. 2018, 34, 149–156. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, C.; Zhang, X.; Cheng, X.; Feng, G.; Wang, Y.; Gao, Q. Estimating the maize biomass by crop height and narrowband vegetation indices derived from UAV-based hyperspectral images. Ecol. Indic. 2021, 129, 107985. [Google Scholar] [CrossRef]
- Yang, W.; Nigon, T.; Hao, Z.; Dias Paiao, G.; Fernández, F.G.; Mulla, D.; Yang, C. Estimation of corn yield based on hyperspectral imagery and convolutional neural network. Comput. Electron. Agric. 2021, 184, 106092. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, Z.; Ma, Y.; Du, Q.; Williams, P.; Drewry, J.; Luck, B. Alfalfa Yield Prediction Using UAV-Based Hyperspectral Imagery and Ensemble Learning. Remote Sens. 2020, 12, 2028. [Google Scholar] [CrossRef]
- Hang, Y.; Lifu, Z.; Qingxi, T. Identification of corn seed varieties using visible/near infrared imaging spectroscopy. Infrared Laser Eng. 2013, 42, 2437–2441. [Google Scholar]
- Jian, Z.; Jin, M.; BiQuan, Z.; Dongyan, Z.; Jing, X. Prediction of chlorophyll (SPAD) distribution in rice leaves by consumer near-infrared cameras. Spectrosc. Spect. Anal. 2018, 38, 737–744. [Google Scholar]
- Xie, L.; Ying, Y.; Ying, T. Quantification of chlorophyll content and classification of nontransgenic and transgenic tomato leaves using visible/near-infrared diffuse reflectance spectroscopy. J. Agric. Food Chem. 2007, 55, 4645–4650. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, D. The role of near-infrared sensors to measure water relationships in crops and plants. Appl. Spectrosc. Rev. 2017, 52, 837–849. [Google Scholar] [CrossRef]
- Han, M.; Zhang, H.; DeJonge, K.C.; Comas, L.H.; Trout, T.J. Estimating maize water stress by standard deviation of canopy temperature in thermal imagery. Agric. Water Manag. 2016, 177, 400–409. [Google Scholar] [CrossRef]
- Giménez-Gallego, J.; González-Teruel, J.D.; Soto-Valles, F.; Jiménez-Buendía, M.; Navarro-Hellín, H.; Torres-Sánchez, R. Intelligent thermal image-based sensor for affordable measurement of crop canopy temperature. Comput. Electron. Agric. 2021, 188, 106319. [Google Scholar] [CrossRef]
- Biswal, S.; Chatterjee, C.; Mailapalli, D.R. Damage Assessment Due to Wheat Lodging Using UAV-Based Multispectral and Thermal Imageries. J. Indian. Soc. Remote 2023, 51, 935–948. [Google Scholar] [CrossRef]
- Pradawet, C.; Khongdee, N.; Pansak, W.; Spreer, W.; Hilger, T.; Cadisch, G. Thermal imaging for assessment of maize water stress and yield prediction under drought conditions. J. Agron. Crop Sci. 2023, 209, 56–70. [Google Scholar] [CrossRef]
- Guo, J.; Tian, G.; Zhou, Y.; Wang, M.; Ling, N.; Shen, Q.; Guo, S. Evaluation of the grain yield and nitrogen nutrient status of wheat (Triticum aestivum L.) using thermal imaging. Field Crop Res. 2016, 196, 463–472. [Google Scholar] [CrossRef]
- Elsherbiny, O.; Zhou, L.; Feng, L.; Qiu, Z. Integration of Visible and Thermal Imagery with an Artificial Neural Network Approach for Robust Forecasting of Canopy Water Content in Rice. Remote Sens. 2021, 13, 1785. [Google Scholar] [CrossRef]
- Song, X.; Yang, G.; Yang, C.; Wang, J.; Cui, B. Spatial Variability Analysis of Within-Field Winter Wheat Nitrogen and Grain Quality Using Canopy Fluorescence Sensor Measurements. Remote Sens. 2017, 9, 237. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Gu, R. Research Status and Prospects on Plant Canopy Structure Measurement Using Visual Sensors Based on Three-Dimensional Reconstruction. Agriculture 2020, 10, 462. [Google Scholar] [CrossRef]
- Ma, X.; Zhu, K.; Guan, H.; Feng, J.; Yu, S.; Liu, G. High-Throughput Phenotyping Analysis of Potted Soybean Plants Using Colorized Depth Images Based on A Proximal Platform. Remote Sens. 2019, 11, 1085. [Google Scholar] [CrossRef]
- Xiang, L.; Bao, Y.; Tang, L.; Ortiz, D.; Salas-Fernandez, M.G. Automated morphological traits extraction for sorghum plants via 3D point cloud data analysis. Comput. Electron. Agric. 2019, 162, 951–961. [Google Scholar] [CrossRef]
- Gai, J.; Xiang, L.; Tang, L. Using a depth camera for crop row detection and mapping for under-canopy navigation of agricultural robotic vehicle. Comput. Electron. Agric. 2021, 188, 106301. [Google Scholar] [CrossRef]
- Andújar, D.; Fernández-Quintanilla, C.; Dorado, J. Matching the Best Viewing Angle in Depth Cameras for Biomass Estimation Based on Poplar Seedling Geometry. Sensors 2015, 15, 12999–13011. [Google Scholar] [CrossRef]
- Walter, J.D.; Edwards, J.; McDonald, G.; Kuchel, H. Estimating biomass and canopy height with LiDAR for field crop breeding. Front. Plant Sci. 2019, 10, 1145. [Google Scholar] [CrossRef]
- Liu, S.; Baret, F.; Abichou, M.; Boudon, F.; Thomas, S.; Zhao, K.; Fournier, C.; Andrieu, B.; Irfan, K.; Hemmerlé, M. Estimating wheat green area index from ground-based LiDAR measurement using a 3D canopy structure model. Agric. For. Meteorol. 2017, 247, 12–20. [Google Scholar] [CrossRef]
- Wu, L.; Zhu, X.; Lawes, R.; Dunkerley, D.; Zhang, H. Comparison of machine learning algorithms for classification of LiDAR points for characterization of canola canopy structure. Int. J. Remote Sens. 2019, 40, 5973–5991. [Google Scholar] [CrossRef]
- Laliberte, A.S.; Rango, A. Image Processing and Classification Procedures for Analysis of Sub-decimeter Imagery Acquired with an Unmanned Aircraft over Arid Rangelands. GIScience Remote Sens. 2011, 48, 4–23. [Google Scholar] [CrossRef]
- Huichun, Z.; Hongping, Z.; Jiaqiang, Z.; Yufeng, G.; Yangxian, L. Research Progress and Prospect in Plant Phenotyping Platformand Image Analysis Technology. Trans. Chin. Soc. Agric. Mach. 2020, 51, 1–17. [Google Scholar]
- Yihua, L.; Tiemin, Z.; Yubin, L. Design and test of attitude stabilization control system of multi-rotor unmanned aerial vehicle applied in farmland information acquisition. Trans. Chin. Soc. Agric. Eng. 2017, 33, 88–98. [Google Scholar] [CrossRef]
- Schirrmann, M.; Giebel, A.; Gleiniger, F.; Pflanz, M.; Lentschke, J.; Dammer, K. Monitoring Agronomic Parameters of Winter Wheat Crops with Low-Cost UAV Imagery. Remote Sens. 2016, 8, 706. [Google Scholar] [CrossRef]
- Vadez, V.; Kholová, J.; Hummel, G.; Zhokhavets, U.; Gupta, S.K.; Hash, C.T. LeasyScan: A novel concept combining 3D imaging and lysimetry for high-throughput phenotyping of traits controlling plant water budget. J. Exp. Bot. 2015, 66, 5581–5593. [Google Scholar] [CrossRef]
- Kirchgessner, N.; Liebisch, F.; Yu, K.; Pfeifer, J.; Friedli, M.; Hund, A.; Walter, A. The ETH field phenotyping platform FIP: A cable-suspended multi-sensor system. Funct. Plant Biol. 2017, 44, 154. [Google Scholar] [CrossRef]
- Bai, G.; Ge, Y.; Scoby, D.; Leavitt, B.; Stoerger, V.; Kirchgessner, N.; Irmak, S.; Graef, G.; Schnable, J.; Awada, T. NU-Spidercam: A large-scale, cable-driven, integrated sensing and robotic system for advanced phenotyping, remote sensing, and agronomic research. Comput. Electron. Agric. 2019, 160, 71–81. [Google Scholar] [CrossRef]
- Cubero, S.; Marco-Noales, E.; Aleixos, N.; Barbé, S.; Blasco, J. RobHortic: A Field Robot to Detect Pests and Diseases in Horticultural Crops by Proximal Sensing. Agriculture 2020, 10, 276. [Google Scholar] [CrossRef]
- Godoy, E.P.; Tabile, R.A.; Pereira, R.R.; Tangerino, G.T.; Porto, A.J.; Inamasu, R.Y. Design and Implementation of an Electronic Architecture for an Agricultural Mobile Robot; SciELO: São Paulo, Brasil, 2010; Volume 14, pp. 1240–1247. [Google Scholar]
- Underwood, J.; Wendel, A.; Schofield, B.; McMurray, L.; Kimber, R. Efficient in-field plant phenomics for row-crops with an autonomous ground vehicle. J. Field Robot. 2017, 34, 1061–1083. [Google Scholar] [CrossRef]
- Werner, J.P. Flex-Ro: Design, Implementation, and Control of Subassemblies for an Agricultural Robotic Platform. Ph.D. Thesis, University of Nebraska, Lincoln, NE, USA, 2016. Available online: https://digitalcommons.unl.edu/biosysengdiss/60 (accessed on 21 October 2023).
- Oz, Ted, Dino. Available online: https://www.naio-technologies.com/oz/ (accessed on 21 October 2023).
- Baret, F.; Benoit, D.S.; Samuel, T.; Philippe, B.; Shouyang, L.; Comar, A. Phenomobile: A fully automatic robot for high-throughput field phenotyping of a large range of crops with active measurements. In IAMPS-Image Analysis Methods in the Plant Sciences; 2019; Available online: https://hal.inrae.fr/hal-03646863v1/file/IAMPS_Phenomobile.pdf (accessed on 21 October 2023).
- From the Greenhouse to the Fields—Robohub. Available online: https://dorhoutrd.com/ (accessed on 21 October 2023).
- Guzmán, R.; Ariño, J.; Navarro, R.; Lopes, C.M.; Graça, J.; Reyes, M.; Barriguinha, A.; Braga, R. Autonomous hybrid gps/reactive navigation of an unmanned ground vehicle for precision viticulture—VINBOT. In Proceedings of the Intervitis Interfructa Hortitechnica—Technology for Wine, Juice and Special Crops, Stuttgart, Germany, 27–30 November 2016; Available online: https://www.researchgate.net/publication/311264530_Autonomous_hybrid_gpsreactive_navigation_of_an_unmanned_ground_vehicle_for_precision_viticulture_-VINBOT (accessed on 21 October 2023).
- Mueller-Sim, T.; Jenkins, M.; Abel, J.; Kantor, G. The Robotanist: A Ground-Based Agricultural Robot for High-Throughput Crop Phenotyping, 2017/1/1, 2017; IEEE: Piscataway, NJ, USA, 2017; pp. 3634–3639. [Google Scholar]
- Shafiekhani, A.; Kadam, S.; Fritschi, F.; DeSouza, G. Vinobot and Vinoculer: Two Robotic Platforms for High-Throughput Field Phenotyping. Sensors 2017, 17, 214. [Google Scholar] [CrossRef]
- Rowbot. Available online: https://www.rowbot.com/ (accessed on 21 October 2023).
- Young, S.N.; Kayacan, E.; Peschel, J.M. Design and field evaluation of a ground robot for high-throughput phenotyping of energy sorghum. Precis. Agric. 2019, 20, 697–722. [Google Scholar] [CrossRef]
- Pérez-Ruiz, M.; Prior, A.; Martinez-Guanter, J.; Apolo-Apolo, O.E.; Andrade-Sanchez, P.; Egea, G. Development and evaluation of a self-propelled electric platform for high-throughput field phenotyping in wheat breeding trials. Comput. Electron. Agric. 2020, 169, 105237. [Google Scholar] [CrossRef]
- Deery, D.; Jimenez-Berni, J.; Jones, H.; Sirault, X.; Furbank, R. Proximal Remote Sensing Buggies and Potential Applications for Field-Based Phenotyping. Agronomy 2014, 4, 349–379. [Google Scholar] [CrossRef]
- Bai, G.; Ge, Y.; Hussain, W.; Baenziger, P.S.; Graef, G. A multi-sensor system for high throughput field phenotyping in soybean and wheat breeding. Comput. Electron. Agric. 2016, 128, 181–192. [Google Scholar] [CrossRef]
- Kumar, D.; Kushwaha, S.; Delvento, C.; Liatukas, Ž.; Vivekanand, V.; Svensson, J.T.; Henriksson, T.; Brazauskas, G.; Chawade, A. Affordable Phenotyping of Winter Wheat under Field and Controlled Conditions for Drought Tolerance. Agronomy 2020, 10, 882. [Google Scholar] [CrossRef]
- Meacham-Hensold, K.; Fu, P.; Wu, J.; Serbin, S.; Montes, C.M.; Ainsworth, E.; Guan, K.; Dracup, E.; Pederson, T.; Driever, S.; et al. Plot-level rapid screening for photosynthetic parameters using proximal hyperspectral imaging. J. Exp. Bot. 2020, 71, 2312–2328. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.; Thorp, K.; Conley, M.; Elshikha, D.; French, A.; Andrade-Sanchez, P.; Pauli, D. Comparing Nadir and Multi-Angle View Sensor Technologies for Measuring in-Field Plant Height of Upland Cotton. Remote Sens. 2019, 11, 700. [Google Scholar] [CrossRef]
- Busemeyer, L.; Mentrup, D.; Möller, K.; Wunder, E.; Alheit, K.; Hahn, V.; Maurer, H.; Reif, J.; Würschum, T.; Müller, J.; et al. BreedVision—A Multi-Sensor Platform for Non-Destructive Field-Based Phenotyping in Plant Breeding. Sensors 2013, 13, 2830–2847. [Google Scholar] [CrossRef]
- Andrade-Sanchez, P.; Gore, M.A.; Heun, J.T.; Thorp, K.R.; Carmo-Silva, A.E.; French, A.N.; Salvucci, M.E.; White, J.W. Development and evaluation of a field-based high-throughput phenotyping platform. Funct. Plant Biol. 2014, 41, 68. [Google Scholar] [CrossRef]
- Kicherer, A.; Herzog, K.; Bendel, N.; Klück, H.; Backhaus, A.; Wieland, M.; Rose, J.; Klingbeil, L.; Läbe, T.; Hohl, C.; et al. Phenoliner: A New Field Phenotyping Platform for Grapevine Research. Sensors 2017, 17, 1625. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, C.; Robertson, J.S.; Sun, S.; Xu, R.; Paterson, A.H. GPhenoVision: A Ground Mobile System with Multi-modal Imaging for Field-Based High Throughput Phenotyping of Cotton. Sci. Rep. 2018, 8, 1213. [Google Scholar] [CrossRef]
- Zhou, J.; Reynolds, D.; Websdale, D.; Cornu, T.L.; Gonzaleznavarro, O.; Lister, C.; Orford, S.; Laycock, S.; Finlayson, G.; Stitt, T. CropQuant: An automated and scalable field phenotyping platform for crop monitoring and trait measurements to facilitate breeding and digital agriculture. BioRxiv 2017, 161547. [Google Scholar] [CrossRef]
- Reynolds, D.; Ball, J.; Bauer, A.; Davey, R.; Griffiths, S.; Zhou, J. CropSight: A scalable and open-source information management system for distributed plant phenotyping and IoT-based crop management. Gigascience 2019, 8, giz009. [Google Scholar] [CrossRef]
- Villarrubia, G.; Paz, J.F.D.; Iglesia, D.H.D.L.; Bajo, J. Combining Multi-Agent Systems and Wireless Sensor Networks for Monitoring Crop Irrigation. Sensors 2017, 17, 1775. [Google Scholar] [CrossRef]
- Millet, E.; Welcker, C.; Kruijer, W.; Negro, S.; Nicolas, S.; Praud, S.; Ranc, N.; Presterl, T.; Tuberosa, R.; Bedo, Z.; et al. Genome-wide analysis of yield in Europe: Allelic effects as functions of drought and heat scenarios. Plant Physiol. 2016, 172, 621–2016. [Google Scholar] [CrossRef]
- Alkhudaydi, T.; Reynolds, D.; Griffiths, S.; Zhou, J.; de la Iglesia, B. An Exploration of Deep-Learning Based Phenotypic Analysis to Detect Spike Regions in Field Conditions for UK Bread Wheat. Plant Phenomics 2019, 2019, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Hirafuji, M.; Yoichi, H.; Kiura, T.; Matsumoto, K.; Fukatsu, T.; Tanaka, K.; Shibuya, Y.; Itoh, A.; Nesumi, H.; Hoshi, N. Creating High-Performance/Low-Cost Ambient Sensor Cloud System Using OpenFS (Open Field Server) for High-Throughput Phenotyping, 2011/1/1, 2011; IEEE: Piscataway, NJ, USA, 2011; pp. 2090–2092. [Google Scholar]
- Beauchêne, K.; Leroy, F.; Fournier, A.; Huet, C.; Bonnefoy, M.; Lorgeou, J.; de Solan, B.; Piquemal, B.; Thomas, S.; Cohan, J. Management and Characterization of Abiotic Stress via PhénoField®, a High-Throughput Field Phenotyping Platform. Front. Plant Sci. 2019, 10, 904. [Google Scholar] [CrossRef]
- White, J.W.; Bostelman, R.V. Large-area overhead manipulator for access of fields. In Proceedings of the 4th International Multi-Conference on Engineering and Technological Innovation (IMETI), Orlando, FL, USA, 19–22 July 2011. [Google Scholar]
- Ji, Z.; Francois, T.; Tony, P.; John, D.; Daniel, R.; Neil, H.; Simon, G.; Tao, C.; Yan, Z.; Xiue, W.; et al. Plant phenoomics: Developments, current status, and challenges. J. Nanjing Agric. Univ. 2018, 41, 580–588. [Google Scholar]
- Higgs, N.; Leyeza, B.; Ubbens, J.; Kocur, J.; van der Kamp, W.; Cory, T.; Eynck, C.; Vail, S.; Eramian, M.; Stavness, I. ProTractor: A Lightweight Ground Imaging and Analysis System for Early-Season Field Phenotyping, 2019/1/1, 2019; IEEE: Piscataway, NJ, USA, 2019; pp. 2629–2638. [Google Scholar]
- Crain, J.L.; Wei, Y.; Barker, J.; Thompson, S.M.; Alderman, P.D.; Reynolds, M.; Zhang, N.; Poland, J. Development and Deployment of a Portable Field Phenotyping Platform. Crop Sci. 2016, 56, 965–975. [Google Scholar] [CrossRef]
- Thompson, A.L.; Thorp, K.R.; Conley, M.; Andrade-Sanchez, P.; Heun, J.T.; Dyer, J.M.; White, J.W. Deploying a Proximal Sensing Cart to Identify Drought-Adaptive Traits in Upland Cotton for High-Throughput Phenotyping. Front. Plant Sci. 2018, 9, 507. [Google Scholar] [CrossRef]
- Bao, Y.; Nakami, A.D.; Tang, L. Development of a Field Robotic Phenotyping System for Sorghum Biomass Yield Component Traits Characterization. In Proceedings of the Annual International Meeting of the American Society of Agricultural and Biological Engineers, Montreal, QC, Canada, 13–16 July 2014. [Google Scholar]
- Bao, Y.; Tang, L. Field-based Robotic Phenotyping for Sorghum Biomass Yield Component Traits Characterization Using Stereo Vision. IFAC-PapersOnLine 2016, 49, 265–270. [Google Scholar] [CrossRef]
- Sudduth, K.A.; Kitchen, N.R.; Drummond, S.T. Comparison of Three Canopy Reflectance Sensors for Variable-Rate Nitrogen Application in Corn, 2010/1/1, 2010; IEEE: Piscataway, NJ, USA, 2010; pp. 18–21. [Google Scholar]
- Weiss, U.; Biber, P. Plant detection and mapping for agricultural robots using a 3D LIDAR sensor. Robot. Auton. Syst. 2011, 59, 265–273. [Google Scholar] [CrossRef]
- Murman, J.N. Flex-Ro: A Robotic High Throughput Field Phenotyping System. Ph.D. Thesis, University of Nebraska, Lincoln, NE, USA, 2019. [Google Scholar]
- Fan, Z.; Sun, N.; Qiu, Q.; Li, T.; Zhao, C. A High-Throughput Phenotyping Robot for Measuring Stalk Diameters of Maize Crops; IEEE: Piscataway, NJ, USA, 2021. [Google Scholar] [CrossRef]
- Tuel, T.L. A Robotic Proximal Sensing Platform for In-Field High-Throughput Crop Phenotyping. Ph.D. Thesis, Iowa State University, Ames, IA, USA, 2019. [Google Scholar]
- MYCE_Agriculture. Available online: http://www.wall-ye.com/ (accessed on 21 October 2023).
- Tabile, R.A.; Godoy, E.P.; Pereira, R.R.D.; Tangerino, G.T.; Porto, A.J.V.; Inamasu, R.Y. Design of the mechatronic architecture of an agricultural mobile robot. IFAC Proc. Vol. 2010, 43, 717–724. [Google Scholar] [CrossRef]
- Xu, R.; Li, C. A modular agricultural robotic system (MARS) for precision farming: Concept and implementation. J. Field Robot. 2022, 39, 387–409. [Google Scholar] [CrossRef]
- Ruckelshausen, A.B.P.D. BoniRob–an autonomous field robot platform for individual plant phenotyping. Precis. Agric. 2009, 841, 1. [Google Scholar]
- Bangert, W.; Kielhorn, A.; Rahe, F.; Dreyer, A.; Trautz, D. Field-Robot-Based Agriculture: “RemoteFarming. 1” and “BoniRob-Apps”. VDI-Berichte 2013, 2193, 2-1. [Google Scholar]
- Peter, B.; Weiss, U.; Dorna, M.; Albert, A. Navigation system of the autonomous agricultural robot “BoniRob”. In Proceedings of the Workshop on Agricultural Robotics: Enabling Safe, Efficient, and Affordable Robots for Food Production, Vilamoura, Portugal, 11 October 2012. [Google Scholar]
- Burud, I.; Lange, G.; Lillemo, M.; Bleken, E.; Grimstad, L.; Johan From, P. Exploring Robots and UAVs as Phenotyping Tools in Plant Breeding. IFAC-PapersOnLine 2017, 50, 11479–11484. [Google Scholar] [CrossRef]
- Freeman, P.K.; Freeland, R.S. Agricultural UAVs in the U.S.: Potential, policy, and hype. Remote Sens. Appl. Soc. Environ. 2015, 2, 35–43. [Google Scholar] [CrossRef]
- Jiangang, L.; Chunjiang, Z.; Guijun, Y.; Haiyang, Y.; Xiaoqing, Z.; Bo, X.; Qinglin, N. Review of field-based phenotyping by unmanned aerial vehicle remote sensing platform. Trans. Chin. Soc. Agric. Eng. 2016, 32, 98–106. [Google Scholar]
- Shafian, S.; Rajan, N.; Schnell, R.; Bagavathiannan, M.; Valasek, J.; Shi, Y.; Olsenholler, J. Unmanned aerial systems-based remote sensing for monitoring sorghum growth and development. PLoS ONE 2018, 13, e196605. [Google Scholar] [CrossRef]
- Araus, J.L.; Cairns, J.E. Field high-throughput phenotyping: The new crop breeding frontier. Trends Plant Sci. 2014, 19, 52–61. [Google Scholar] [CrossRef]
- Sugiura, R.; Noguchi, N.; Ishii, K. Remote-sensing technology for vegetation monitoring using an unmanned helicopter. Biosyst. Eng. 2005, 90, 369–379. [Google Scholar] [CrossRef]
- Zongnan, L.; Zhongxin, C.; Limin, W.; Jia, L.; Qingbo, Z. Area extraction of maize lodging based on remote sensing by small unmanned aerial vehicle. Trans. Chin. Soc. Agric. Eng. 2014, 30, 207–213. [Google Scholar] [CrossRef]
- Binglin, Z.; Min, L.; Fusang, L.; Aobo, J.; Xiu, M.; Yan, G. Modeling of Canopy Structure of Field-grown Maize Based on UAV Images. Trans. Chin. Soc. Agric. Mach. 2021, 52, 170–177. [Google Scholar]
- Kai, K. Design of self-propelled field phenotyping platform. Master’s Thesis, Hebei Agricultural University, Baoding, China, 2020. [Google Scholar]
- Bakker, T.; van Asselt, K.; Bontsema, J.; Müller, J.; van Straten, G. Autonomous navigation using a robot platform in a sugar beet field. Biosyst. Eng. 2011, 109, 357–368. [Google Scholar] [CrossRef]
- Jing, Z.; Du, C.; Shumao, W.; Xiaoan, H.; Dong, W. Design and experiment of four-wheel independent steering driving and control system for agricultural wheeled robot. Trans. Chin. Soc. Agric. Eng. 2015, 31, 63–70. [Google Scholar] [CrossRef]
- Youchun, D.; Peng, Z.; Yawen, Z.; Junqiang, Y.; Wenyu, Z.; Kai, Z. Design and experiment of motion controller for information collection platform in field with Beidou positioning. Trans. Chin. Soc. Agric. Eng. 2017, 33, 178–185. [Google Scholar] [CrossRef]
- Kannan, P.; Natarajan, S.K.; Dash, S.S. Design and Implementation of Fuzzy Logic Controller for Online Computer Controlled Steering System for Navigation of a Teleoperated Agricultural Vehicle. Math. Probl. Eng. 2013, 2013, 590861. [Google Scholar] [CrossRef]
- Bengochea-Guevara, J.M.; Conesa-Muñoz, J.; Andújar, D.; Ribeiro, A. Merge fuzzy visual servoing and GPS-based planning to obtain a proper navigation behavior for a small crop-inspection robot. Sensors 2016, 16, 276. [Google Scholar] [CrossRef]
- Jodas, D.S.; Marranghello, N.; Pereira, A.S.; Guido, R.C. Comparing Support Vector Machines and Artificial Neural Networks in the Recognition of Steering Angle for Driving of Mobile Robots Through Paths in Plantations. Procedia Comput. Sci. 2013, 18, 240–249. [Google Scholar] [CrossRef][Green Version]
- Eski, O.; Kuş, Z.A. Control of unmanned agricultural vehicles using neural network-based control system. Neural Comput. Appl. 2019, 31, 583–595. [Google Scholar] [CrossRef]
- Jun, C.; Zhongxiang, Z.; Ryo, T.; Jun-ichi, T. Automatic On-tracking Control of Farm Vehicle Based on Neural Network. Trans. Chin. Soc. Agric. Mach. 2007, 38, 121, 131–133. [Google Scholar] [CrossRef]
- Xiwen, L.; Yinggang, Q. Development of agricultural intelligent mobile work platform model. Trans. Chin. Soc. Agric. Eng. 2005, 83–85. [Google Scholar]
- Bak, T.; Jakobsen, H. Agricultural Robotic Platform with Four Wheel Steering for Weed Detection. Biosyst. Eng. 2004, 87, 125–136. [Google Scholar] [CrossRef]
- Shaozhi, L. Design and Experiment of Field Crop Phenotype Detection Platform. Jorunal Huazhong Agric. Univ. 2021, 40, 209–218. [Google Scholar]
- Dean, Z.; Weikuan, J.; Yun, Z.; Yuyan, Z.; Wei, J.; Yun, L. Design of Agricultural Robot Autonomous Navigation Control Based on Improved Self-adaptive Filter. Trans. Chin. Soc. Agric. Mach. 2015, 46, 1–6. [Google Scholar] [CrossRef]
- Sabanci, K.; Aydin, C. Smart Robotic Weed Control System for Sugar Beet. J. Agric Sci. Tech. 2017, 19, 73–83. [Google Scholar]
- Yang, W.; Rui, Z.; Chenming, W.; Meng, W.; Xiujie, W.; Yongjin, L. A survey on deep-learning-based plant phenotype research in agriculture. Sci. Sin. Vitae 2019, 49, 698–716. (In Chinese) [Google Scholar] [CrossRef]
- Shengmei, H.; Zhonglai, L.; Zhonghu, H. Classification of Wheat Cultivar by Digital Image Analysis. Sci. Agric. Sin. 2005, 38, 1869–1875. [Google Scholar]
- Mengyang, F.; Qin, M.; Junming, L.; Qing, W.; Yue, W.; Xiongchun, D. Counting Method of Wheatear in Field Based on Machine Vision Technology. Trans. Chin. Soc. Agric. Mach. 2015, 46, 234–239. [Google Scholar]
- Wenchao, L.; Bin, L.; Dayu, P.; Yong, Z.; Chunhua, Y.; Cheng, W. Synchronous measurement of wheat ear length and spikelets number based on image processing. J. Chin. Agric. Mech. 2016, 37, 210–215. [Google Scholar] [CrossRef]
- Hongming, Z.; Ziwei, T.; Wenting, H.; Shanna, Z.; Shuyin, Z.; Chenyu, G. Extraction Method of Maize Height Based on UAV Remote Sensing. Trans. Chin. Soc. Agric. Mach. 2019, 50, 241–250. [Google Scholar] [CrossRef]
- Weiss, M.; Baret, F. Using 3D point clouds derived from UAV RGB imagery to describe vineyard 3D macro-structure. Remote Sens. 2017, 9, 111. [Google Scholar] [CrossRef]
- Zhikai, L.; Yaoxiao, N.; Yi, W.; Wenting, H. Estimation of Plant Height of Winter Wheat Based on UAV Visible Image. J. Triticeae Crops 2019, 39, 859–866. [Google Scholar]
- Klukas, C.; Pape, J.; Entzian, A. Analysis of high-throughput plant image data with the information system IAP. J. Integr. Bioinform. 2012, 9, 16–18. [Google Scholar] [CrossRef][Green Version]
- Fabre, J.; Dauzat, M.; Nègre, V.; Wuyts, N.; Tireau, A.; Gennari, E.; Neveu, P.; Tisné, S.; Massonnet, C.; Hummel, I. PHENOPSIS DB: An Information System for Arabidopsis thalianaphenotypic data in an environmental context. BMC Plant Biol. 2011, 11, 77. [Google Scholar]
- Tessmer, O.L.; Jiao, Y.; Cruz, J.A.; Kramer, D.M.; Chen, J. Functional approach to high-throughput plant growth analysis. BMC Syst. Biol. 2013, 7 (Suppl. S6), S17. [Google Scholar] [CrossRef] [PubMed]
- Weight, C.; Parnham, D.; Waites, R. Technical advance: LeafAnalyser: A computational method for rapid and large-scale analyses of leaf shape variation. Plant J. 2008, 53, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Applegate, C.; Alonso, A.D.; Reynolds, D.; Orford, S.; Mackiewicz, M.; Griffiths, S.; Penfield, S.; Pullen, N. Leaf-GP: An open and automated software application for measuring growth phenotypes for arabidopsis and wheat. Plant Methods 2017, 13, 117. [Google Scholar] [CrossRef] [PubMed]
- Minervini, M.; Giuffrida, M.V.; Perata, P.; Tsaftaris, S.A. Phenotiki: An open software and hardware platform for affordable and easy image-based phenotyping of rosette-shaped plants. Plant J. 2017, 90, 204–216. [Google Scholar] [CrossRef]
- Islam ElManawy, A.; Sun, D.; Abdalla, A.; Zhu, Y.; Cen, H. HSI-PP: A flexible open-source software for hyperspectral imaging-based plant phenotyping. Comput. Electron. Agric. 2022, 200, 107248. [Google Scholar] [CrossRef]


| Phenotyping Traits | Phenotyping Sensors | |||||||
|---|---|---|---|---|---|---|---|---|
| RGB Camera | Imaging Spectrometer | Thermal Camera | Fluorescent Imager | Depth-Sensing Camera | Lidar Scanner | Spectral Sensor | ||
| Phenotyping traits relating to yield | Plant density | ■ | ■ | ■ | ||||
| Canopy coverage | ■ | ■ | ■ | ■ | ■ | ■ | ||
| Canopy height | ■ | ■ | ■ | ■ | ||||
| Cover fraction | ■ | ■ | ■ | |||||
| Grain number and size | ■ | |||||||
| Biomass | ■ | ■ | ■ | ■ | ■ | |||
| Chlorophyll content | ■ | ■ | ■ | |||||
| Phenotyping traits relating to quality | Fruit/inflorescence size | ■ | ■ | |||||
| Grain quality | ■ | ■ | ||||||
| Water content | ■ | ■ | ■ | |||||
| Phenotyping traits relating to resistance | Canopy temperature | ■ | ■ | |||||
| Leaf rolling | ■ | ■ | ■ | ■ | ■ | |||
| Leaf wilting | ■ | ■ | ||||||
| Lodging | ■ | ■ | ■ | ■ | ■ | ■ | ||
| GNDVI (green normalized difference vegetation index) | ■ | |||||||
| Phenotyping traits relating to nutrition | Nitrogen content | ■ | ■ | ■ | ||||
| LAI (leaf area index) | ■ | ■ | ||||||
| PNA (plant nitrogen accumulation) | ■ | |||||||
| Commercialized or not | Y | Y | Y | Y | Y | Y | Y | |
| Models of sensors | Canon; Nikon; and Sony | MS3100 Duncan Camera; SOC710E; and Hyper Spec VNIR | FLIR T series | Multiples 2, 3 | RealSense series; CamCube 3.0; SR4000; and Kinect 2.0 | LMS series; VLP-16; and HDL-32E | GreenSeeker RT 100, 200; CropCircle ACS 210, 430, 470; and N-sensor | |
| Whether supporting secondary development or not | Y | N | Y | N | Y | N | N | |
| Control Algorithms or Controllers | Advantages | Limitations |
|---|---|---|
| PID control algorithm | Easy-to-use, flexibility, and convenient adjustment | Low regulation precision |
| Fuzzy control algorithm | Easy realization, high robustness, and strong fault-tolerant ability | Low dynamic quality and lack of systematicity |
| Neural network control algorithm | Non-linearity, high fault-tolerant ability, and strong expansibility | Proneness to overfitting |
| Programmable logic controller (PLC) | High reliability, high protection class, and good stability | High hardware cost and difficulties in programming and maintenance |
| Single-board computer | High integrity, low cost, high flexibility, and good portability | Long response time and narrow application range |
| Industrial personal computer (IPC) | High applicability, good expansibility, and powerful functions | Poor compatibility and high price |
| Software | R&D Institutions (Year) | Types of Analyzed Data | Obtained Phenotype Information | Characteristics |
|---|---|---|---|---|
| ImageJ version 1.8.0 | National Institutes of Health (2007) | Visible images | Leaf area, leaf perimeter, leaf length, leaf width, and plant height | Public image processing software |
| IAP (Integrated Analysis Platform) [149] | Leibniz Institute of Plant Genetics and Crop Plant Research (2012) | Visible, fluorescence, near-infrared, and infrared images | Morphological and structural traits including plant height, leaf area, biomass, and leaf inclination, color traits, fluorescence intensity, and near-infrared reflectivity | Image data management and analysis platform |
| HTPheno [150] | Leibniz Institute of Plant Genetics and Crop Plant Research (2011) | Visible images | Width, height, and projected shoot area | ImageJ plug-in and open-source image data analysis software system |
| HPGA (High-throughput Plant Growth Analysis) [151] | Michigan State University (2016) | Three digital images | Plant area, leaf shape | High-throughput phenotyping platforms for growth modeling and function analysis of plants |
| Leaf Analyzer [152] | University of York (2007) | 2D or 3D images | Leaf shape and size | Software for rapid, large-scale, automatic analysis of variation in leaf shape |
| Leasyscan [70] | ICRISAT—Crop Physiology Laboratory (2015) | 3D point cloud images | 3D leaf area, projected leaf area, leaf area index, leaf inclination, leaf angle, plant height, maximum plant height, optical penetration depth, biomass | Commercial integrated analysis software based on multispectral laser 3D scanning and measuring instrument PlantEye |
| LemnaGrid [29] | LemnaTec, Germany | Visible images | Morphological and structural traits including leaf area and compactness | Commercial integrated analysis software based on Scanalyzer 3D platform |
| Leaf-GP [153] | Earlham Institute, Norwich Research Park | Visible images | Number of leaves, morphological and structural traits including projected leaf area and perimeter, and color traits | Open source, extensibility, easy-to-use, and ability to simply resolve images of Arabidopsis thaliana and wheat taken by low-cost imaging devices such as smart phones and digital cameras |
| Phenotiki [154] | IMT School for Advanced Studies, Piazza S. | Visible images | Morphological and structural traits, color traits, number of leaves, dynamic growth curves of plants | Economy and ease of deployment |
| HSI-PP [155] | State Key Laboratory of Modern Optical Instrumentation, Zhejiang University | Hyperspectral images | Projected leaf area, leaf perimeter, plant diameter, leaf convex hull, stockiness, and compactness | Machine learning and deep learning models that can preprocess hyperspectral images so that they are more applicable to training classification and regression |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, H.; Song, M.; Liu, Y.; Xie, Q.; Cao, W.; Zhu, Y.; Ni, J. Field Phenotyping Monitoring Systems for High-Throughput: A Survey of Enabling Technologies, Equipment, and Research Challenges. Agronomy 2023, 13, 2832. https://doi.org/10.3390/agronomy13112832
Yuan H, Song M, Liu Y, Xie Q, Cao W, Zhu Y, Ni J. Field Phenotyping Monitoring Systems for High-Throughput: A Survey of Enabling Technologies, Equipment, and Research Challenges. Agronomy. 2023; 13(11):2832. https://doi.org/10.3390/agronomy13112832
Chicago/Turabian StyleYuan, Huali, Minghan Song, Yiming Liu, Qi Xie, Weixing Cao, Yan Zhu, and Jun Ni. 2023. "Field Phenotyping Monitoring Systems for High-Throughput: A Survey of Enabling Technologies, Equipment, and Research Challenges" Agronomy 13, no. 11: 2832. https://doi.org/10.3390/agronomy13112832
APA StyleYuan, H., Song, M., Liu, Y., Xie, Q., Cao, W., Zhu, Y., & Ni, J. (2023). Field Phenotyping Monitoring Systems for High-Throughput: A Survey of Enabling Technologies, Equipment, and Research Challenges. Agronomy, 13(11), 2832. https://doi.org/10.3390/agronomy13112832






