Introducing Autochthonous Bacterium and Fungus Composition to Enhance the Phytopathogen-Suppressive Capacity of Composts against Clonostachys rosea, Penicillium solitum and Alternaria alternata In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Compost Mixture
2.2. Obtaining Microbial Inoculums
2.3. Introduction of Microorganisms to the Compost
2.4. Microbial Survival Rate in Compost
2.5. Influence of Pure Cultures of Antagonistic Microorganisms and Composts after the Introduction of Microorganisms on the Seed Germination Index of the Test Plant (Raphanus sativus)
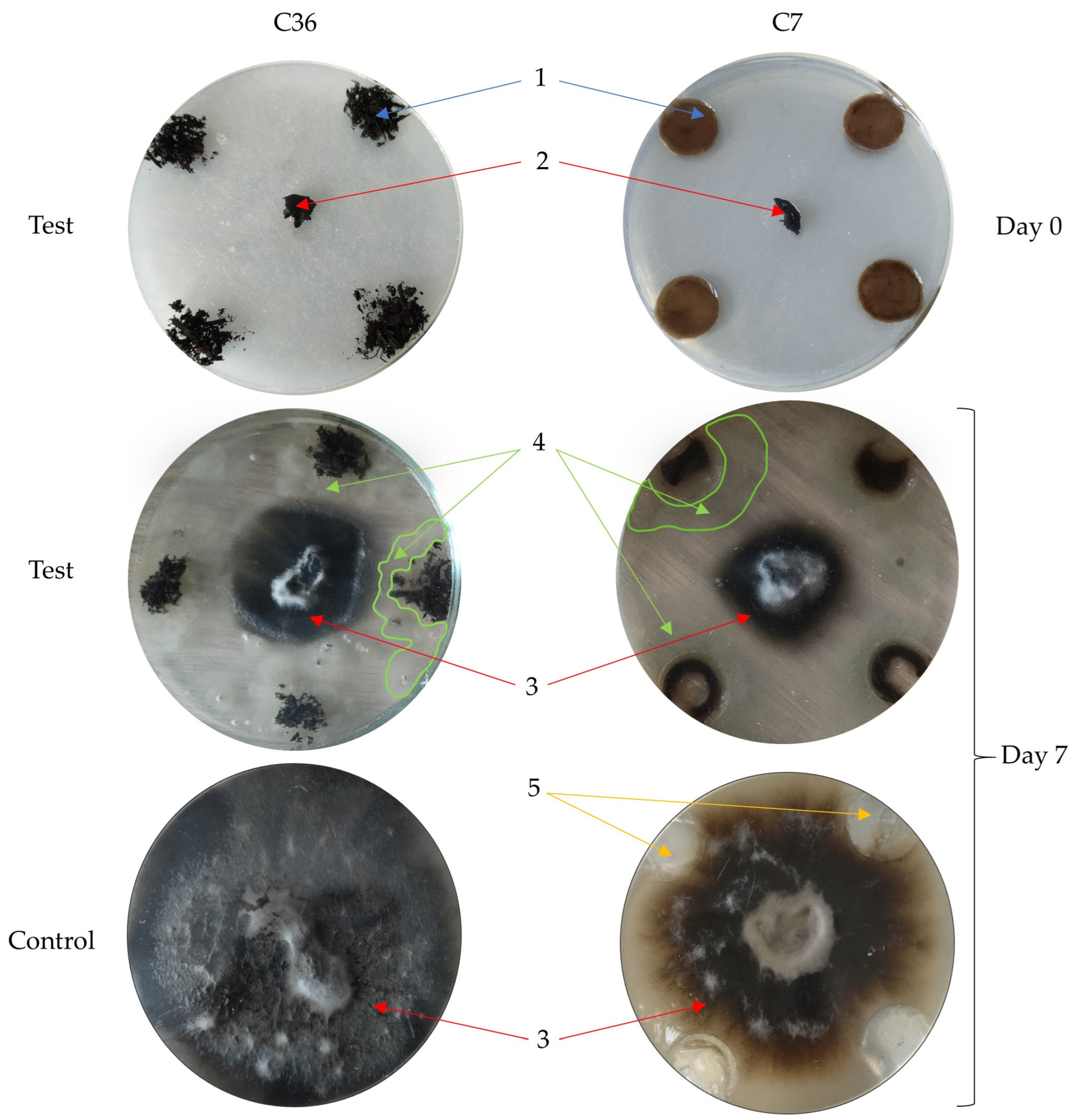
2.6. In Vitro Suppressiveness of Composts
2.7. Analysis of Bacterial Survival Rate in Compost Using Molecular Biological Methods
2.8. Statistical Analysis
3. Results and Discussion
3.1. Microbial Survival Rate in Compost
3.2. Effects of Pure Microbial Cultures and Composts with Those Cultures on the Seed Germination Index of Raphanus sativus
3.2.1. Effect of Pure Cultures on Germination Index Values
3.2.2. Impact of Composts with Antagonists on Germination Index Values
3.3. Changes in the Suppressive Capacity of Composts after Introducing Antagonistic Microorganisms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hoitink, H.A.J. Composted bark, a lightweight growth medium. Plant Dis. 1980, 64, 142–147. [Google Scholar] [CrossRef]
- Nelson, E.B.; Kuter, G.A.; Hoitink, H.A.J. Effects of fungal antagonists and compost age on suppression of Rhizoctonia damping-off in container media amended with composted hardwood bark. J. Ser. Artic. 1983, 6, 83. [Google Scholar] [CrossRef]
- Kwok, O.C.H.; Fahy, P.C.; Hoitink, H.A.J.; Kuter, G.A. Interactions between bacteria and Trichoderma hamatum in suppression of Rhizoctonia damping-off in bark compost media. Phytopathology 1987, 77, 1206–1212. [Google Scholar] [CrossRef]
- Pera, A.; Filippi, C. Controlling of Fusarium wilt in carnation with bark compost. Biol. Wastes 1987, 22, 219–228. [Google Scholar] [CrossRef]
- Noble, R.; Coventry, E. Suppression of soil-borne plant diseases with composts: A review. Biocontrol Sci. Technol. 2005, 15, 3–20. [Google Scholar] [CrossRef]
- Scheuerell, S.J.; Sullivan, D.M.; Mahaffee, W.F. Suppression of Seedling Damping-Off Caused by Pythium ultimum, P. irregulare, and Rhizoctonia solaniin Container Media Amended with a Diverse Range of Pacific Northwest Compost Sources. Phytopathology 2005, 95, 306–315. [Google Scholar] [CrossRef]
- St. Martin, C.C.G. Enhancing Soil Suppressiveness Using Compost and Compost Tea. In Organic Amendments and Soil Suppressiveness in Plant Disease Management. Soil Biology; Meghvansi, M., Varma, A., Eds.; Springer: Cham, Switzerland, 2015; Volume 46. [Google Scholar] [CrossRef]
- Diánez, F.; Marín, F.; Santos, M.; Gea, F.J.; Navarro, M.J.; Piñeiro, M.; González, J.M. Genetic Analysis and In Vitro Enzymatic Determination of Bacterial Community in Compost Teas from Different Sources. Compos. Sci. Util. 2018, 26, 256–270. [Google Scholar] [CrossRef]
- Mironov, V.; Vanteeva, A.; Merkel, A. Microbiological Activity during Co-Composting of Food and Agricultural Waste for Soil Amendment. Agronomy 2021, 11, 928. [Google Scholar] [CrossRef]
- Antoniou, A.; Tsolakidou, M.-D.; Stringlis, I.A.; Pantelides, I.S. Rhizosphere Microbiome Recruited from a Suppressive Compost Improves Plant Fitness and Increases Protection against Vascular Wilt Pathogens of Tomato. Front. Plant Sci 2017, 8, 2022. [Google Scholar] [CrossRef]
- Hoitink, H.A.J.; Ramos, L. Impacts of Composts on Soil and Plant Health; Ohio State University: Wooster, OH, USA, 2008. [Google Scholar]
- Mohamed, O.-Z.; Yassine, B.; Hilali Rania, E.; El Hassan, A.; Abdellatif, H.; Rachid, B. Evaluation of compost quality and bioprotection potential against Fusarium wilt of date palm. Waste Manag. 2020, 113, 12–19. [Google Scholar] [CrossRef]
- Pane, C.; Zaccardelli, M. Evaluation of Bacillus strains isolated from solanaceous phylloplane for biocontrol of Alternaria early blight of tomato. Biol. Control 2015, 84, 11–18. [Google Scholar] [CrossRef]
- Ramírez-Cariño, H.F.; Guadarrama-Mendoza, P.C.; Sánchez-López, V.; Cuervo-Parra, J.A.; Ramírez-Reyes, T.; Dunlap, C.A.; Valadez-Blanco, R. Biocontrol of Alternaria alternata and Fusarium oxysporum by Trichoderma asperelloides and Bacillus paralicheniformis in tomato plants. Antonie Van Leeuwenhoek 2020, 113, 1247–1261. [Google Scholar] [CrossRef]
- De Corato, U.; Salimbeni, R.; De Pretis, A. Suppression of soil-borne pathogens in container media amended with on-farm composted agro-bioenergy wastes and residues under glasshouse condition. J. Plant Dis. Prot. 2018, 125, 213–226. [Google Scholar] [CrossRef]
- Chilosi, G.; Esposito, A.; Castellani, F.; Stanzione, V.; Aleandri, M.P.; Dell’Unto, D.; Tomassini, A.; Vannini, A.; Altieri, R. Characterization and Use of Olive Mill Waste Compost as Peat Surrogate in Substrate for Cultivation of Photinia Potted Plants: Assessment of Growth Performance and In Vitro Suppressiveness. Waste Biomass Valor. 2018, 9, 919–928. [Google Scholar] [CrossRef]
- El-Sayed, A.S.A.; Shad Ali, G. Aspergillus flavipes is a novel efficient biocontrol agent of Phytophthora parasitica. Biol. Control. 2020, 140, 104072. [Google Scholar] [CrossRef]
- Hernández-Lara, A.; Ros, M.; Cuartero, J.; Bustamante, M.Á.; Moral, R.; Andreu-Rodríguez, F.J.; Fernández, J.A.; Egea-Gilabert, C.; Pascual, J.A. Bacterial and fungal community dynamics during different stages of agro-industrial waste composting and its relationship with compost suppressiveness. Sci. Total Environ. 2022, 805, 150330. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Estrella, F.; Vargas-García, C.; López, M.J.; Capel, C.; Moreno, J. Antagonistic activity of bacteria and fungi from horticultural compost against Fusarium oxysporum f. sp. melonis. Crop Prot. 2007, 26, 46–53. [Google Scholar] [CrossRef]
- Luo, M.; Purdy, H.; Avis, T.J. Compost bacteria provide antifungal activity against grey mold and Alternaria rot on bell pepper fruit. Botany 2019, 97, 221–230. [Google Scholar] [CrossRef]
- Al-Ghafri, H.M.; Velazhahan, R.; Shahid, M.S.; Al-Sadi, A.M. Antagonistic activity of Pseudomonas aeruginosa from compost against Pythium aphanidermatum and Fusarium solani. Biocontrol Sci. Technol. 2020, 30, 642–658. [Google Scholar] [CrossRef]
- Dugassa, A.; Alemu, T.; Woldehawariat, Y. In-vitro compatibility assay of indigenous Trichoderma and Pseudomonas species and their antagonistic activities against black root rot disease (Fusarium solani) of faba bean (Vicia faba L.). BMC Microbiol. 2021, 21, 115. [Google Scholar] [CrossRef]
- Hammam, M.M.A.; El-Mohamedy, R.S.R.; Abdel-Kareem, F.; Abd-Elgawad, M.M.M. Evaluation of soil Amended with Bio-Agents and Compost Alone or in Combination for Controlling Citrus nematode Tylenchulus semipenetrans and Fusarium Dry root rot on Volkamer lime under Greenhouse Conditions. Int. J. ChemTech Res. 2016, 9, 88–96. [Google Scholar]
- Johnson, I.; Ramjegathesh, R.; Sheela, J.; Shoba, N.; Maheshwarappa, H.P. Development of microbial consortia for the management of leaf blight disease of coconut. Acta Phytopathol. Entomol. Hung. 2017, 52, 1–14. [Google Scholar] [CrossRef]
- Rajeswari, P. Combination of Trichoderma viride and Pseudomonas fluorescens for the enhanced control of Fusarium wilt disease caused by Fusarium oxysporum infecting Arachis hypogaea L. J. Appl. Nat. Sci. 2019, 11, 138–143. [Google Scholar] [CrossRef]
- Tao, C.; Li, R.; Xiong, W.; Shen, Z.; Liu, S.; Wang, B.; Ruan, Y.; Geisen, S.; Shen, O.; Kowalchuk, G.A. Bio-organic fertilizers stimulate indigenous soil Pseudomonas populations to enhance plant disease suppression. Microbiome 2020, 8, 137. [Google Scholar] [CrossRef]
- Zouari, I.; Masmoudi, F.; Medhioub, K.; Tounsi, S.; Trigui, M. Biocontrol and plant growth-promoting potentiality of bacteria isolated from compost extract. Antonie van Leeuwenhoek 2020, 113, 2107–2122. [Google Scholar] [CrossRef] [PubMed]
- Jambhulkar, P.P.; Sharma, P.; Manokaran, R.; Lakshman, D.K.; Rokadia, P.; Jambhulkar, N. Assessing synergism of combined applications of Trichoderma harzianum and Pseudomonas fluorescens to control blast and bacterial leaf blight of rice. Eur. J. Plant Pathol. 2018, 152, 747–757. [Google Scholar] [CrossRef]
- Hariharan, G.; Rifnas, L.M.; Prasannath, K. Role of Trichoderma spp. in Biocontrol of Plant Diseases. In Microbial Biocontrol: Food Security and Post Harvest Management; Kumar, A., Ed.; Springer: Cham, Switzerland, 2022; pp. 39–78. [Google Scholar] [CrossRef]
- González-González, S.; Astorga-Eló, M.; Campos, M.; Wick, L.Y.; Acuña, J.J.; Jorquera, M.A. Compost Fungi Allow for Effective Dispersal of Putative PGP Bacteria. Agronomy 2021, 11, 1567. [Google Scholar] [CrossRef]
- Haggag, W.M.; Saber, M.S.M. Suppression of early blight on tomato and purple blight on onion by foliar sprays of aerated and non-aerated compost teas. J. Food Agric. Env. 2007, 5, 302–309. [Google Scholar]
- Carrascal-Hernández, D.C.; Flórez-López, E.; Peralta-Ruiz, Y.; Chaves-López, C.; Grande-Tovar, C.D. Eco-Friendly Biocontrol Strategies of Alternaria Phytopathogen Fungus: A Focus on Gene-Editing Techniques. Agriculture 2022, 12, 1722. [Google Scholar] [CrossRef]
- Al-Jaradi, A.; Al-Mahmooli, I.; Janke, R.; Maharachchikumbura, S.; Al-Saady, N.; Al-Sadi, A.M. Isolation and identification of pathogenic fungi and oomycetes associated with beans and cowpea root diseases in Oman. PeerJ 2018, 6, e6064. [Google Scholar] [CrossRef]
- Uwaremwe, C.; Yue, L.; Liu, Y.; Tian, Y.; Zhao, X.; Wang, Y.; Xie, Z.; Zhang, Y.; Cui, Z.; Wang, R. Molecular identification and pathogenicity of Fusarium and Alternaria species associated with root rot disease of wolfberry in Gansu and Ningxia provinces, China. Plant Pathol. 2021, 70, 397–406. [Google Scholar] [CrossRef]
- Pianzzola, M.J.; Moscatelli, M.; Vero, S. Characterization of Penicillium isolates associated with blue mold on apple in Uruguay. Plant Dis. 2004, 88, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Yin, G.; Zhang, Y.; Pennerman, K.K.; Wu, G.; Hua, S.S.T.; Yu, J.; Jurick II, W.M.; Guo, A.; Bennett, J.W. Characterization of Blue Mold Penicillium Species Isolated from Stored Fruits Using Multiple Highly Conserved Loci. J. Fungi 2017, 3, 12. [Google Scholar] [CrossRef]
- Pitt, J.I. Penicillium and related genera. In Food Spoilage Microorganisms Woodhead Publishing Series in Food Science, Technology and Nutrition; De, C., Blackburn, W., Eds.; Woodhead Publishing: Sawston, UK, 2006; pp. 437–450. [Google Scholar] [CrossRef]
- Zhang, T.; Huang, C.; Deng, C.; Zhang, Y.; Feng, Y.; Hu, J.; Wang, R.; Zhao, L.; Wang, Y.; Kai, G. First Report of Corm Rot on Saffron Caused by Penicillium solitum in China. Plant Dis. 2020, 104, 579. [Google Scholar] [CrossRef]
- Cota, L.V.; Maffia, L.A.; Mizubuti, E.S.G.; Macedo, P.E.F.; Antunes, R.F. Biological control of strawberry gray mold by Clonostachys rosea under field conditions. Biol. Control 2008, 46, 515–522. [Google Scholar] [CrossRef]
- Hasan, R.; Lv, B.; Uddin, M.J.; Chen, Y.; Fan, L.; Sun, Z.; Sun, M.; Li, S. Monitoring Mycoparasitism of Clonostachys rosea against Botrytis cinerea Using GFP. J. Fungi 2022, 8, 567. [Google Scholar] [CrossRef]
- Bienapfl, J.C.; Floyd, C.M.; Percich, J.A.; Malvick, D.K. First report of Clonostachys rosea causing root rot of soybean in the United States. Plant Dis. 2012, 96, 1700. [Google Scholar] [CrossRef]
- Afshari, N.; Hemmati, R. First report of the occurrence and pathogenicity of Clonostachys rosea on faba bean. Australas. Plant Pathol. 2017, 46, 231–234. [Google Scholar] [CrossRef]
- Ma, H.; Duan, X.; Xu, W.; Ma, G.; Ma, W.; QiQi, H. Root Rot of Angelica sinensis Caused by Clonostachys rosea and Fusarium acuminatum in China. Plant Dis. 2022, 106, 2264. [Google Scholar] [CrossRef]
- Qi, H.; Duan, X.; Xu, W.; Zhou, Y.; Ma, H.; Ma, W.; Ma, G. First Report Disease of Clonostachys rosea Causing Root Rot on Astragalus membranaceus in China. Plant Dis. 2022, 106, 1752. [Google Scholar] [CrossRef]
- Coyotl-Pérez, W.A.; Rubio-Rosas, E.; Morales-Rabanales, Q.N.; Ramírez-García, S.A.; Pacheco-Hernández, Y.; Pérez-España, V.H.; Romero-Arenas, O.; Villa-Ruano, N. Improving the Shelf Life of Avocado Fruit against Clonostachys rosea with Chitosan Hybrid Films Containing Thyme Essential Oil. Polymers 2022, 14, 2050. [Google Scholar] [CrossRef] [PubMed]
- St. Martin, C.C.G.; Brathwaite, R.A.I. Compost and compost tea: Principles and prospects as substrates and soil-borne disease management strategies in soil-less vegetable production. Int. J. Sustain. Prod. Syst. 2012, 28, 1–33. [Google Scholar]
- Dukare, A.S.; Prasanna, R.; Chandra Dubey, S.; Nain, L.; Chaudhary, V.; Singh, R.; Saxena, A.K. Evaluating novel microbe amended composts as biocontrol agents in tomato. Crop Prot. 2011, 30, 436–442. [Google Scholar] [CrossRef]
- Ismael, D.P.; St Martin, C.C.G. Combined Effects And Relationships Of Compost Tea, Fertiliser, And Glomus Intraradices Inoculated-Substrate On Tomato Seedling Quality. In Proceedings of the Caribbean Food Crops Society 49th Annual Meeting, Port of Spain, Trinidad and Tobago, 30 June–6 July 2013; pp. 362–372. [Google Scholar]
- Ivanov, Y.A.; Mironov, V.V. Test results In-vessel composting system at the cattle farm located in the central part of Russia. Agric. Mech. Asia Afr. Lat. Am. 2018, 49, 86–90. [Google Scholar]
- Pane, C.; Celano, G.; Villecco, D.; Zaccardelli, M. Control of Botrytis cinerea, Alternaria alternata and Pyrenochaeta lycopersici on tomato with whey compost-tea applications. Crop Prot. 2012, 38, 80–86. [Google Scholar] [CrossRef]
- Luo, Y.; Liang, J.; Zeng, G.; Chen, M.; Mo, D.; Li, G.; Zhang, D. Seed germination test for toxicity evaluation of compost: Its roles, problems and prospects. Waste Manag. 2018, 71, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Cesaro, A.; Belgiorno, V.; Guida, M. Compost from organic solid waste: Quality assessment and European regulations for its sustainable use. Resour. Conserv. Recycl. 2015, 94, 72–79. [Google Scholar] [CrossRef]
- Singh, J.; Tripathi, N.N. Inhibition of storage fungi of blackgram (Vigna mungo L.) by some essential oils. Flavour Fragr. J. 1999, 14, 1–4. [Google Scholar] [CrossRef]
- Giménez, A.; Fernández, J.A.; Pascual, J.A.; Ros, M.; Saez-Tovar, J.; Martinez-Sabater, E.; Gruda, N.S.; Egea-Gilabert, C. Promising Composts as Growing Media for the Production of Baby Leaf Lettuce in a Floating System. Agronomy 2020, 10, 1540. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Unicycler: Resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput. Biol. 2017, 13, e1005595. [Google Scholar] [CrossRef]
- Tatusova, T.; DiCuccio, M.; Badretdin, A.; Chetvernin, V.; Nawrocki, E.P.; Zaslavsky, L.; Lomsadze, A.; Pruitt, K.D.; Borodovsky, M.; Ostell, J. NCBI prokaryotic genome annotation pipeline. Nucleic Acids Res. 2016, 44, 6614–6624. [Google Scholar] [CrossRef] [PubMed]
- Merkel, A.Y.; Chernyh, N.A.; Pimenov, N.V.; Bonch-Osmolovskaya, E.A.; Slobodkin, A.I. Diversity and metabolic potential of the terrestrial mud volcano microbial community with a high abundance of archaea mediating the anaerobic oxidation of methane. Life 2021, 11, 953. [Google Scholar] [CrossRef] [PubMed]
- Khattak, S.U.; Iqbal, Z.; Lutfullah, G.; Bacha, N.; Khan, A.A.; Saeed, M.; Ali, M. Phytotoxic and herbicidal activities of Aspergillus and Penicillium species isolated from rhizosphere and soil. Pak. J. Weed Sci. Res. 2014, 20, 293–303. [Google Scholar]
- Blom, D.; Fabbri, C.; Eberl, L.; Weisskopf, L. Volatile-Mediated Killing of Arabidopsis thalianaby Bacteria Is Mainly Due to Hydrogen Cyanide. Appl. Environ. Microbiol. 2010, 77, 1000–1008. [Google Scholar] [CrossRef]
- Kremer, R.; Souissi, T. Cyanide Production by Rhizobacteria and Potential for Suppression of Weed Seedling Growth. Curr. Microbiol. 2001, 43, 182–186. [Google Scholar] [CrossRef]
- Martínez-Gallardo, M.R.; López, M.J.; Jurado, M.M.; Suárez-Estrella, F.; López-González, J.A.; Sáez, J.A.; Moral, R.; Moreno, J. Bioremediation of Olive Mill Wastewater sediments in evaporation ponds through in situ composting assisted by bioaugmentation. Sci. Total Environ. 2019, 703, 135537. [Google Scholar] [CrossRef]
- Naidu, Y.; Meon, S.; Kadir, J.; Siddiqui, Y. Microbial starter for the enhancement of biological activity of compost tea. Int. J. Agric. Biol. 2010, 12, 51–56. [Google Scholar]
- Gur, A.; Luzzati, J.; Katan, J. Alternatives for soil fumigation in combating apple replant disease. Acta Hortic. 1998, 477, 107–113. [Google Scholar] [CrossRef]
- Asghar, W.; Kataoka, R. Effect of co-application of Trichoderma spp. with organic composts on plant growth enhancement, soil enzymes and fungal community in soil. Arch. Microbiol. 2021, 203, 4281–4291. [Google Scholar] [CrossRef]
- Wolna-Maruwka, A.; Niewiadomska, A.; Piechota, T.; Karwatka, K.; Pilarska, A.A. The Handling of Composted Onion Waste in the Form of Substrates for the Proliferation of the Trichoderma sp. Rocz. Ochr. Sr. 2019, 21, 629–645. [Google Scholar]
- Scheuerell, S.J.; Mahaffee, W.F. Compost Tea as a Container Medium Drench for Suppressing Seedling Damping-Off Caused by Pythium ultimum. Phytopathology 2004, 94, 1156–1163. [Google Scholar] [CrossRef] [PubMed]
- Deepthi, P.K.; Reddy, P.N. Compost Teas: A Potential Source Of Antagonistic Microflora Against Plant Diseases. J. Cell Life Sci. 2013, 1, 6–19. [Google Scholar]
- Daami-Remadi, M.; Dkhili, I.; Jabnoun-Khiareddine, H.; El Mahjoub, M. Biological control of potato leak with antagonistic fungi isolated from compost teas and solarized and non-solarized soils. Pest Technol. 2012, 6, 32–40. [Google Scholar]

| Compost Variant Abbreviation | Inoculum | Variant Name |
|---|---|---|
| C36 | B. subtilis | C36B1 |
| B. amyloliquefaciens | C36B2 | |
| P. aeruginosa | C36P | |
| A. corrugatus | C36A | |
| composition | C36C | |
| C7 | B. subtilis | C7B |
| B. amyloliquefaciens | C7B2 | |
| P. aeruginosa | C7P | |
| A. corrugatus | C7A | |
| composition | C7C | |
| Control variants | ||
| C36 | water | C36W |
| LB medium | C36L | |
| C7 | water | C7W |
| LB medium | C7L | |
| Variant Abbreviation | Description |
|---|---|
| C36L0 | Control variant: compost with 36% dry matter content with added LB nutrient medium and without introduced microorganisms, 0 days (without incubation). |
| C7L0 | Control variant: compost with 7% dry matter content with added LB nutrient medium and without introduced microorganisms, 0 days (without incubation). |
| C36C0 | Test variant: compost with 36% dry matter content with introduced antagonist microorganism composition, 0 days (without incubation). |
| C7C0 | Test variant: compost with 7% dry matter content, with the introduced antagonist microorganism composition, 0 days (without incubation). |
| C36L14 | Control variant: compost with 36% dry matter content with added LB nutrient medium and without introduced microorganisms, 14 days of incubation. |
| C7L14 | Control variant: compost with 7% dry matter content with added LB nutrient medium and without introduced microorganisms, 14 days of incubation. |
| C36C14 | Test variant: compost with 36% dry matter content with introduced antagonist microorganism composition, 14 days of incubation. |
| C7C14 | Test variant: compost with 36% dry matter content with introduced antagonist microorganism composition, 14 days of incubation. |
| Culture | Initial Titer, CFU per g (mL) of C36 and C7 | Bacterial And Fungal Titers on Day 14 of Incubation, CFU per g of C36 | Bacterial and Fungal Titers on Day 14 of Incubation, CFU per mL of C7 |
|---|---|---|---|
| B. subtilis | 4.5 × 108 | 1.7 × 107 | 1.6 × 109 |
| B. amyloliquefaciens | 3.5 × 108 | 1.8 × 107 | 1.2 × 1010 |
| P. aeruginosa | 6.0 × 108 | 2.1 × 108 | 8.0 × 108 |
| A. corrugatus | 5.5 × 105 | 3.0 × 104 | 1.0 × 104 |
| Sample | Number of 16S rRNA Prokaryote Gene Copies per g of Compost | Number of holA Gene Copies per g of Compost |
|---|---|---|
| C36L0 | 1.12 (±0.13) × 108 | n.p.p. 1 |
| C7L0 | 3.51 (±0.67) × 108 | n.p.p. |
| C36C0 | 1.23 (±0.19) × 108 | 2.40 (±0.35) × 105 |
| C7C0 | 4.24 (±1.22) × 107 | 2.45 (±0.63) × 104 |
| C36L14 | 1.84 (±0.53) × 105 | n.p.p. |
| C7L14 | 1.28 (±0.30) × 107 | n.p.p |
| C36C14 | 1.89 (±0.42) × 108 | 8.54 (±1.43) × 103 |
| C7C14 | 3.94 (±0.50) × 107 | 5.24 (±0.76) × 102 |
| Culture | GI 1, % | |
|---|---|---|
| CS | CS 1:1000 | |
| B. subtilis | 0.0 ± 0.0 a | 63.9 ± 6.9 c |
| B. amyloliquefaciens | 0.0 ± 0.0 a | 82.9 ± 12.1 d |
| P. aeruginosa | 0.0 ± 0.0 a | 38.1 ± 1.3 b |
| A. corrugatus | 0.0 ± 0.0 a | 48.2 ± 1.6 b |
| Variant Name | GI 1, % |
|---|---|
| C36B2 | 80.6 ± 8.7 a |
| C36W | 84.9 ± 15.4 ab |
| C36B1 | 87.8 ± 2.0 abcd |
| C7B1 | 89.7 ± 4.7 abcd |
| C7B | 90.3 ± 4.1 abcd |
| C7B2 | 91.8 ± 1.0 abcd |
| C36B | 96.9 ± 0.5 abcde |
| C36B | 98.5 ± 15.2 abcde |
| C7W | 103.8 ± 4.0 cdef |
| C7B | 108.5 ± 7.4 def |
| C36L | 109.4 ± 3.7 def |
| C7L | 110.6 ± 11.2 def |
| C36C | 114.8 ± 1.9 ef |
| C7C | 120.9 ± 9.4 f |
| Variant Name | MGI (%) | ||
|---|---|---|---|
| C. rosea | P. solitum | A. alternata | |
| C36W | 86.3 ± 1.0 bc | 11.1 ± 0.0 a | 76.6 ± 3.7 c |
| C36L | 86.0 ± 1.5 bc | 26.0 ± 6.7 b | 73.1 ± 2.4 c |
| C36B1 | 84.0 ± 4.8 bc | 83.4 ± 3.2 fg | 71.1 ± 4.9 c |
| C36B2 | 89.5 ± 3.5 bc | 70.6 ± 0.6 de | 74.9 ± 4.5 c |
| C36P | 87.1 ± 1.8 bc | 59.0 ± 12.7 cd | 77.0 ± 1.5 c |
| C36A | 91.4 ± 1.7 c | 77.2 ± 8.9 ef | 76.7 ± 4.1 c |
| C36C | 91.7 ± 0.7 c | 84.0 ± 3.5 fg | 81.9 ± 1.1 c |
| C7W | 58.4 ± 5.8 a | 0.0 ± 0.0a | 41.4 ± 15.3 b |
| C7L | 54.4 ± 2.7 a | 0.0 ± 0.0 a | 26.5 ± 13.9 a |
| C7B1 | 56.6 ± 10.6 a | 0.0 ± 0.0 a | 75.1 ± 5.4 c |
| C7B2 | 90.5 ± 4.7 c | 58.3 ± 4.8 c | 78.1 ± 2.5 c |
| C7P | 53.3 ± 4.7 a | 0.0 ± 0.0 a | 77.6 ± 5.9 c |
| C7A | 79.9 ± 3.1 a | 67.1 ± 5.2 cde | 81.0 ± 5.5 c |
| C7C | 81.9 ± 1.0 bc | 89.9 ± 2.5 g | 81.9 ± 6.8 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mironov, V.; Shchelushkina, A.; Selitskaya, O.; Nikolaev, Y.; Merkel, A.; Zhang, S. Introducing Autochthonous Bacterium and Fungus Composition to Enhance the Phytopathogen-Suppressive Capacity of Composts against Clonostachys rosea, Penicillium solitum and Alternaria alternata In Vitro. Agronomy 2023, 13, 2841. https://doi.org/10.3390/agronomy13112841
Mironov V, Shchelushkina A, Selitskaya O, Nikolaev Y, Merkel A, Zhang S. Introducing Autochthonous Bacterium and Fungus Composition to Enhance the Phytopathogen-Suppressive Capacity of Composts against Clonostachys rosea, Penicillium solitum and Alternaria alternata In Vitro. Agronomy. 2023; 13(11):2841. https://doi.org/10.3390/agronomy13112841
Chicago/Turabian StyleMironov, Vladimir, Anna Shchelushkina, Olga Selitskaya, Yury Nikolaev, Alexander Merkel, and Shenghua Zhang. 2023. "Introducing Autochthonous Bacterium and Fungus Composition to Enhance the Phytopathogen-Suppressive Capacity of Composts against Clonostachys rosea, Penicillium solitum and Alternaria alternata In Vitro" Agronomy 13, no. 11: 2841. https://doi.org/10.3390/agronomy13112841
APA StyleMironov, V., Shchelushkina, A., Selitskaya, O., Nikolaev, Y., Merkel, A., & Zhang, S. (2023). Introducing Autochthonous Bacterium and Fungus Composition to Enhance the Phytopathogen-Suppressive Capacity of Composts against Clonostachys rosea, Penicillium solitum and Alternaria alternata In Vitro. Agronomy, 13(11), 2841. https://doi.org/10.3390/agronomy13112841







