Dynamics of Mineral Uptake and Plant Function during Development of Drug-Type Medical Cannabis Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growing Conditions
2.2. Plant Biomass and Inorganic Mineral Analysis
2.3. Physiological Parameters
2.4. Calculation of Nutrient Uptake, Deposition, and Translocation
2.5. Statistical Analyses
3. Results and Discussion
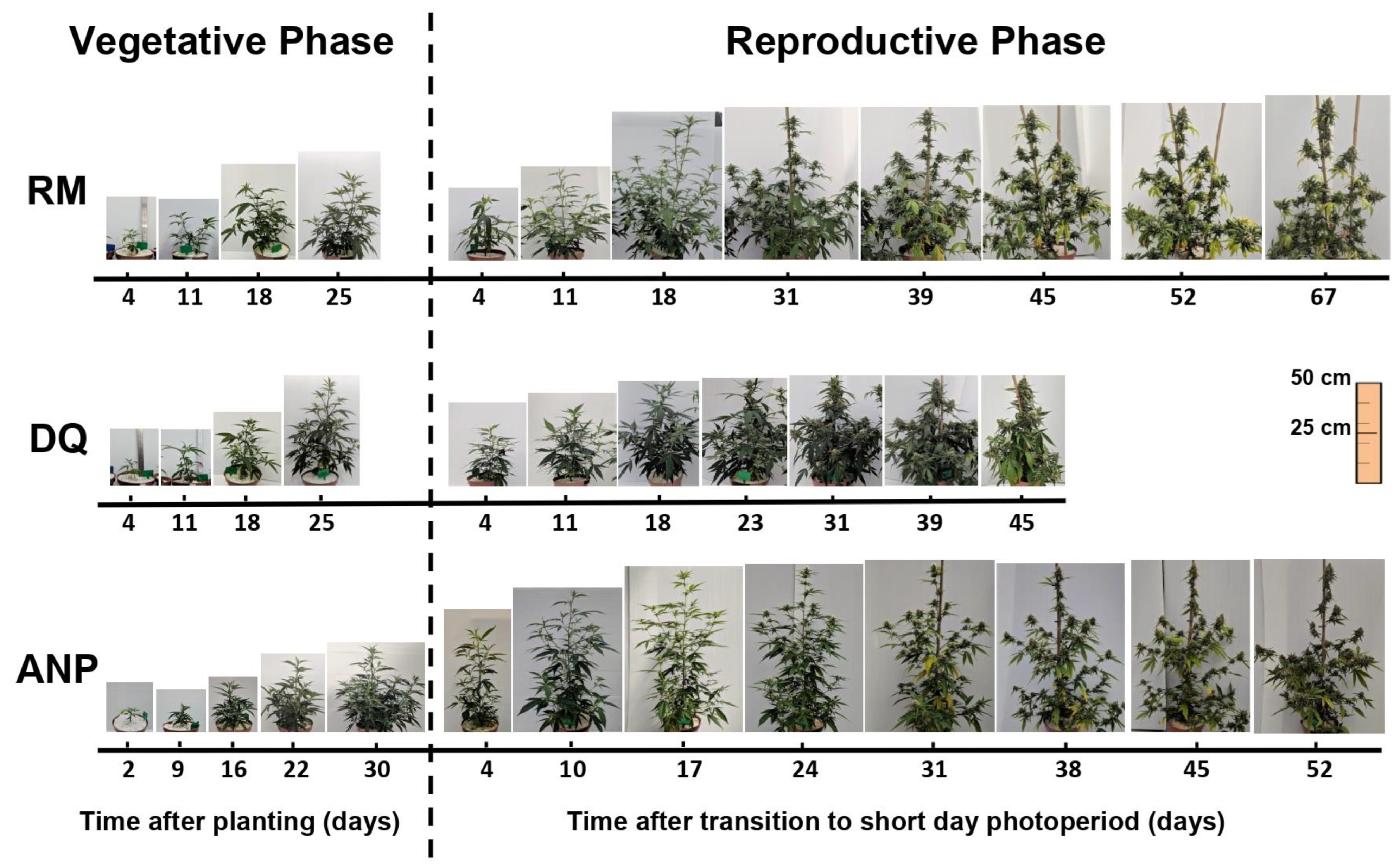
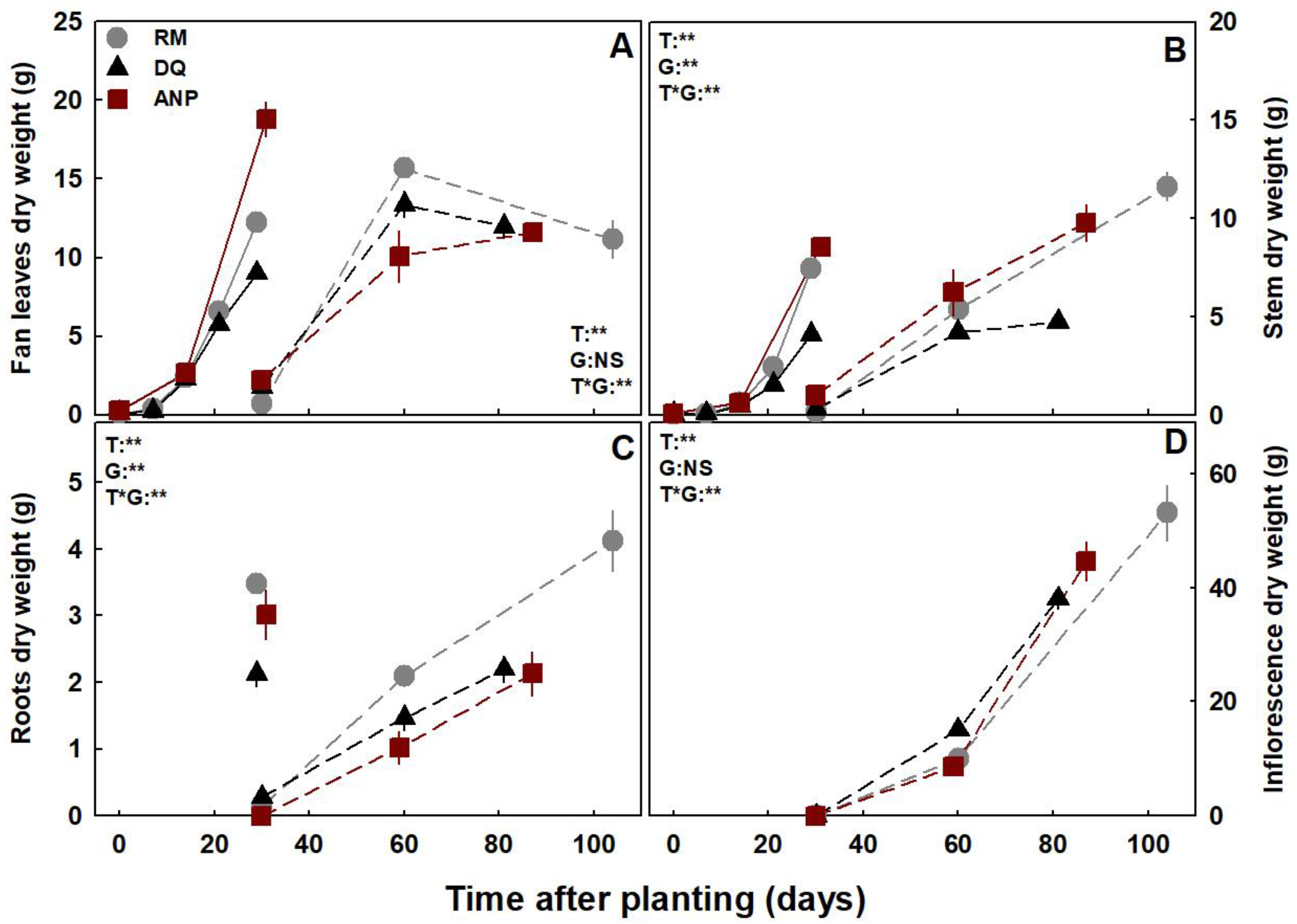
3.1. Plant Development: Biomass Accumulation and Visual Appearance
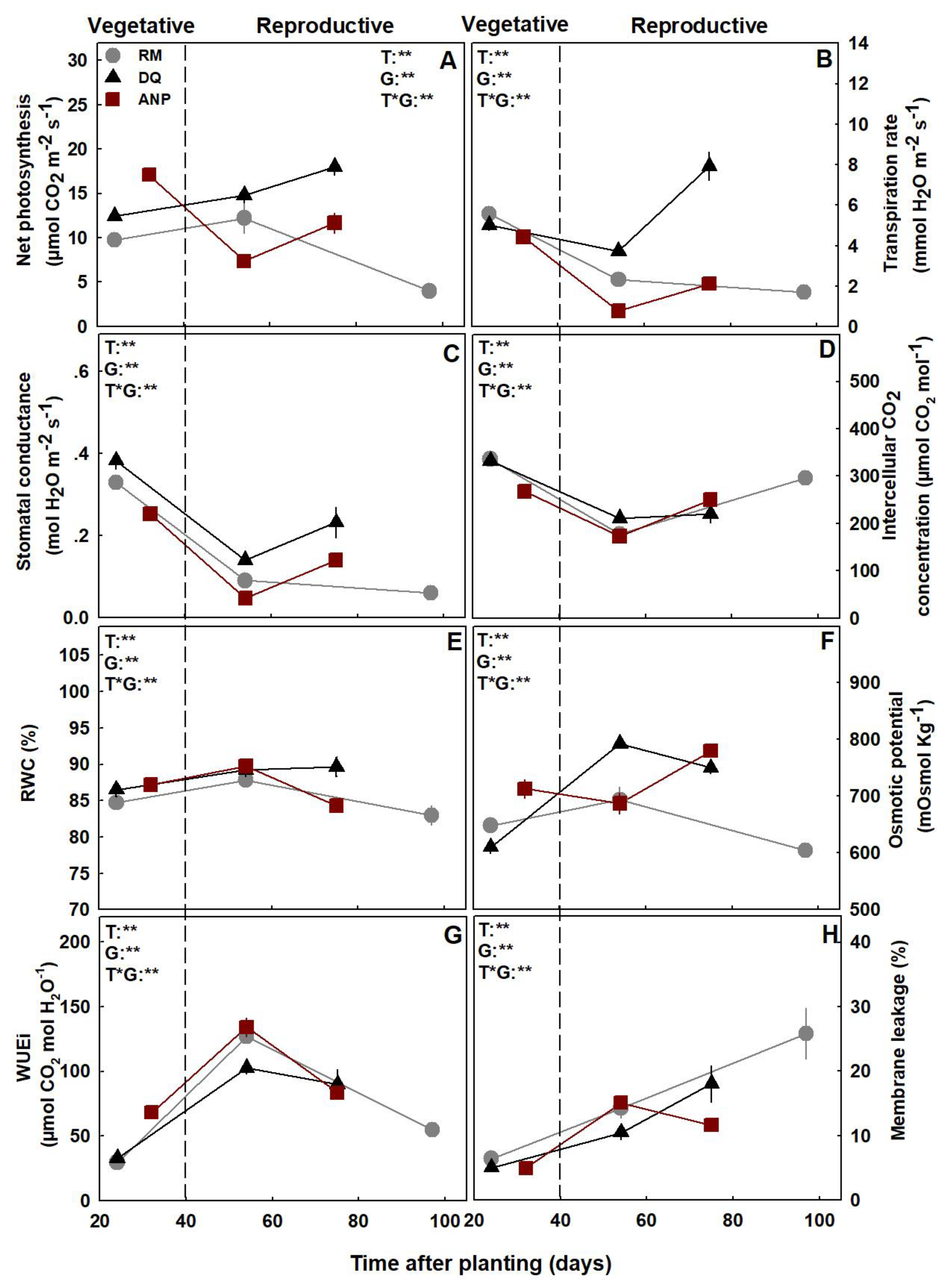
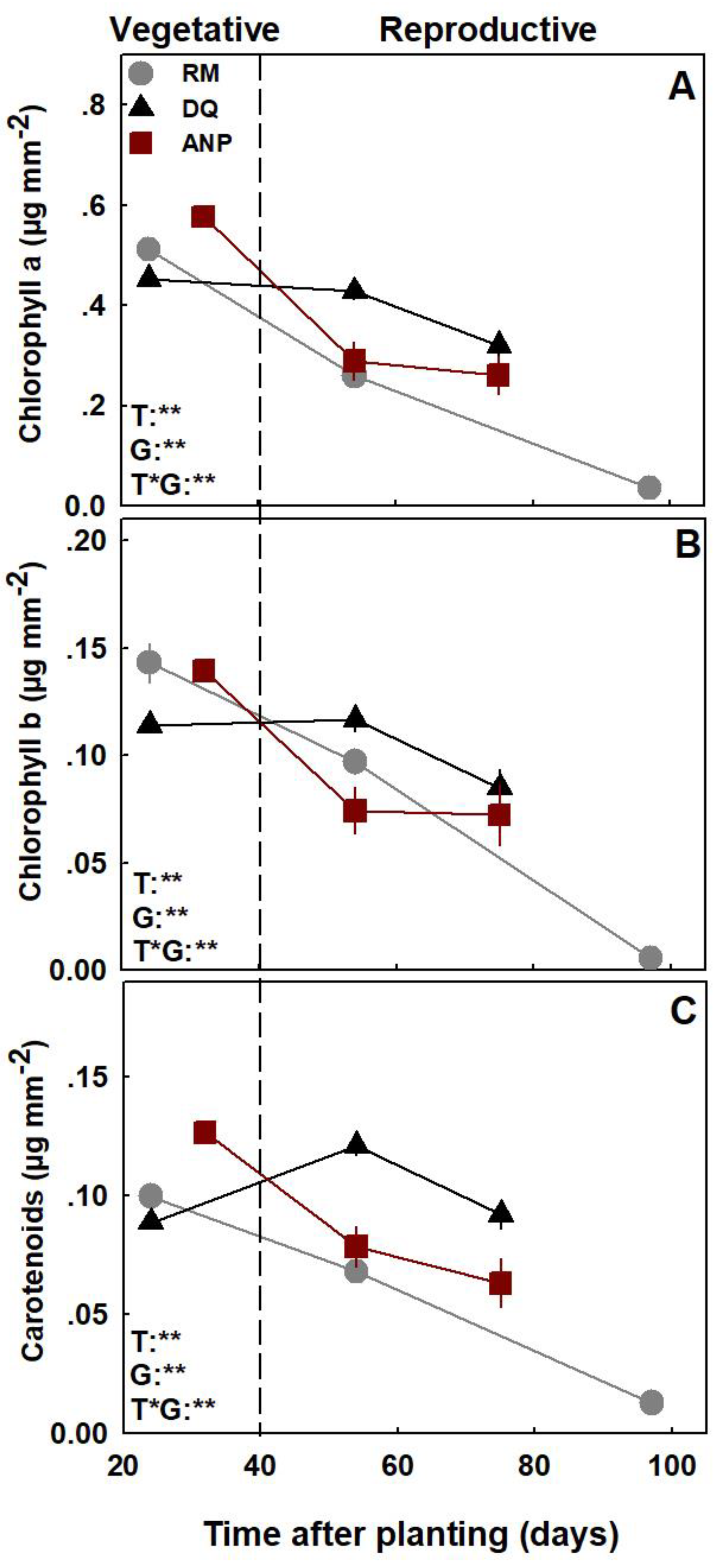
3.2. Gas Exchange, Water Relations, and Photosynthetic Pigments
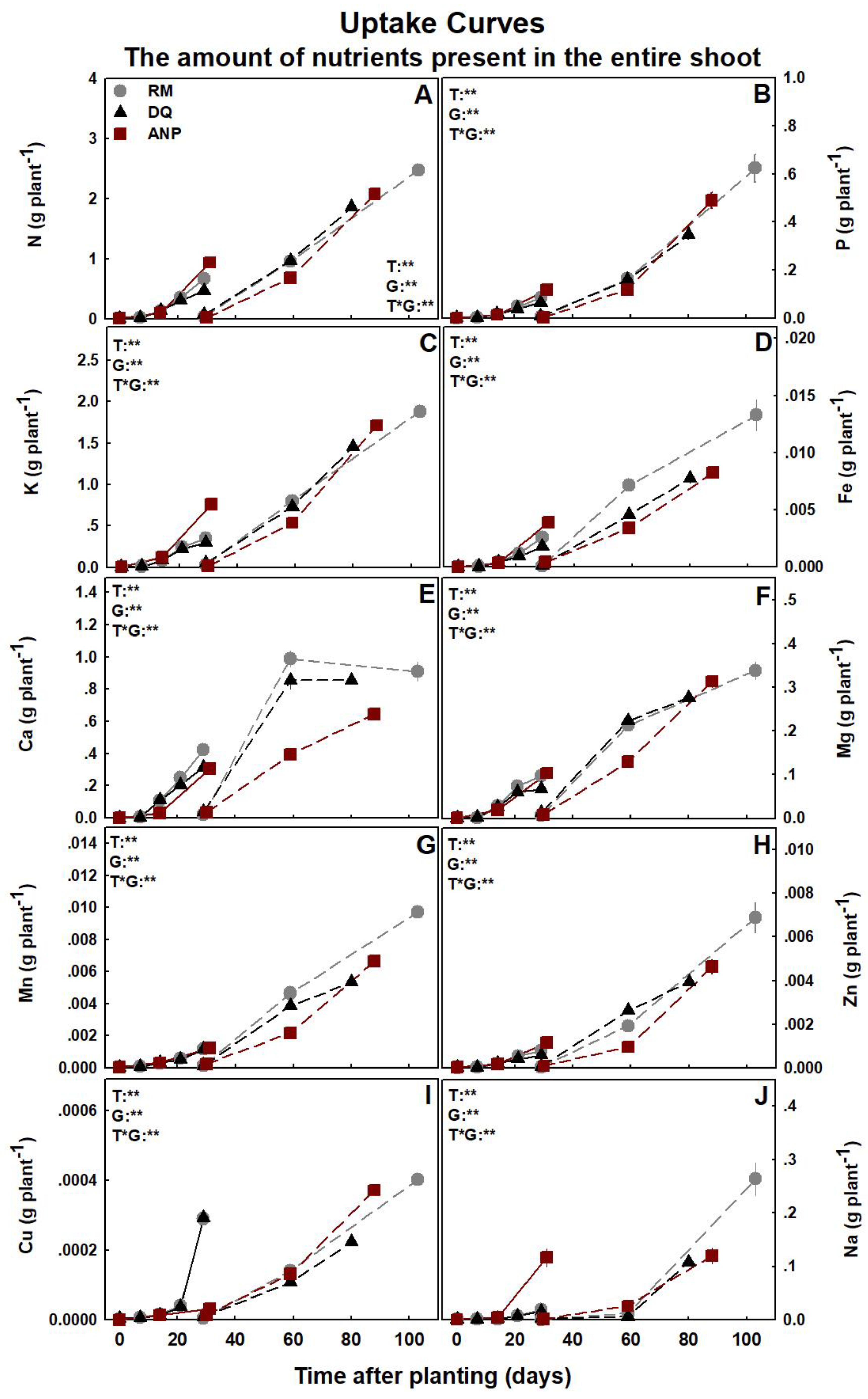
3.3. Nutrient Uptake and Deposition
3.4. Nutrient Translocation and Root:Shoot Ratio
3.5. Agronomic Considerations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saloner, A.; Bernstein, N. Effect of potassium (K) supply on cannabinoids, terpenoids and plant function in medical cannabis. Agronomy 2022, 12, 1242. [Google Scholar] [CrossRef]
- Bevan, L.; Jones, M.; Zheng, Y. Optimisation of nitrogen, phosphorus, and potassium for soilless production of Cannabis sativa in the flowering stage using response surface analysis. Front. Plant Sci. 2021, 12, 764103. [Google Scholar] [CrossRef]
- Shiponi, S.; Bernstein, N. The highs and lows of P supply in medical cannabis: Effects on cannabinoids, the ionome, and morpho-physiology. Front. Plant Sci. 2021, 12, 657323. [Google Scholar] [CrossRef]
- Saloner, A.; Bernstein, N. Nitrogen source matters: High NH4/NO3 ratio reduces cannabinoids, terpenoids, and yield in medical cannabis. Front. Plant Sci. 2022, 13, 830224. [Google Scholar] [CrossRef]
- Saloner, A.; Bernstein, N. Nitrogen supply affects cannabinoid and terpenoid profile in medical cannabis (Cannabis sativa L.). Ind. Crops Prod. 2021, 167, 113516. [Google Scholar] [CrossRef]
- Morad, D.; Bernstein, N. Response of medical cannabis to magnesium (Mg) supply at the vegetative growth phase. Plants 2023, 12, 2676. [Google Scholar] [CrossRef] [PubMed]
- Danziger, N.; Bernstein, N. Light matters: Effect of light spectra on cannabinoid profile and plant development of medical cannabis (Cannabis sativa L.). Ind. Crops Prod. 2021, 164, 113351. [Google Scholar] [CrossRef]
- Eichhorn Bilodeau, S.; Wu, B.S.; Rufyikiri, A.S.; MacPherson, S.; Lefsrud, M. An update on plant photobiology and implications for cannabis production. Front. Plant Sci. 2019, 10, 296. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Morrison, V.; Llewellyn, D.; Zheng, Y. Cannabis inflorescence yield and cannabinoid concentration are not increased with exposure to short-wavelength ultraviolet-B radiation. Front. Plant Sci. 2021, 12, 725078. [Google Scholar] [CrossRef]
- Rodriguez-Morrison, V.; Llewellyn, D.; Zheng, Y. Cannabis yield, potency, and leaf photosynthesis respond differently to increasing light levels in an indoor environment. Front. Plant Sci. 2021, 12, 456. [Google Scholar] [CrossRef] [PubMed]
- Westmoreland, M.; Kusuma, P.; Bugbee, B. Cannabis lighting: Decreasing blue photon fraction increases yield but efficacy is more important for cost effective production of cannabinoids. PLoS ONE 2021, 16, e0248988. [Google Scholar] [CrossRef]
- Yep, B.; Gale, N.V.; Zheng, Y. Aquaponic and hydroponic solutions modulate NaCl-induced stress in drug-type Cannabis sativa L. Front. Plant Sci. 2020, 11, 1169. [Google Scholar] [CrossRef] [PubMed]
- Yep, B.; Gale, N.V.; Zheng, Y. Comparing hydroponic and aquaponic rootzones on the growth of two drug-type Cannabis sativa L. cultivars during the flowering stage. Ind. Crops Prod. 2020, 157, 112881. [Google Scholar] [CrossRef]
- Danziger, N.; Bernstein, N. Too dense or not too dense: Higher planting density reduces cannabinoid uniformity but increases yield/area in drug-type medical cannabis. Front. Plant Sci. 2022, 13, 713481. [Google Scholar] [CrossRef]
- Danziger, N.; Bernstein, N. Plant architecture manipulation increases cannabinoid standardization in ‘drug-type’ medical cannabis. Ind. Crops Prod. 2021, 167, 113528. [Google Scholar] [CrossRef]
- Danziger, N.; Bernstein, N. Shape matters: Plant architecture affects chemical uniformity in large-size medical cannabis plants. Plants 2021, 10, 1834. [Google Scholar] [CrossRef]
- Clarke, R.C.; Merlin, M.D. Cannabis domestication, breeding history, present-day genetic diversity, and future prospects. CRC. Crit. Rev. Plant Sci. 2017, 35, 293–327. [Google Scholar] [CrossRef]
- Moher, M.; Jones, M.; Zheng, Y. Photoperiodic response of in vitro Cannabis sativa plants. HortScience 2021, 56, 108–113. [Google Scholar] [CrossRef]
- Weller, J.L.; Kendrick, R.E. Photomorphogenesis and photoperiodism in plants. In Photobiology: The Science of Life and Light, 2nd ed.; Björn, L.O., Ed.; Springer: New York, NY, USA, 2008; pp. 417–463. ISBN 9780387726540. [Google Scholar]
- Vitale, L.; Arena, C.; Carillo, P.; di Tommasi, P.; Mesolella, B.; Nacca, F.; de Santo, A.V.; Fuggi, A.; Magliulo, V. Gas exchange and leaf metabolism of irrigated maize at different growth stages. Plant Biosyst. Int. J. Deal. Asp. Plant Biol. 2011, 145, 485–494. [Google Scholar] [CrossRef]
- Reekie, E.G.; Bazzaz, F.A. Reproductive effort in plants. 3. Effect of reproduction on vegetative activity. Am. Nat. 1987, 129, 907–919. [Google Scholar] [CrossRef]
- Bielczynski, L.W.; Łaçki, M.K.; Hoefnagels, I.; Gambin, A.; Croce, R. Leaf and plant age affects photosynthetic performance and photoprotective capacity. Plant Physiol. 2017, 175, 1634–1648. [Google Scholar] [CrossRef] [PubMed]
- Murray, G.A. The relationship of light quality, duration, and intensity to vegetative and reproductive growth in alfalfa (Medicago sativa L.). PhD Thesis, The University of Arizona, Tucson, AZ, USA, 1967. [Google Scholar]
- Nadalini, S.; Zucchi, P.; Andreotti, C. Effects of blue and red LED lights on soilless cultivated strawberry growth performances and fruit quality. Eur. J. Hortic. Sci. 2017, 82, 12–20. [Google Scholar] [CrossRef]
- Schwarz, D.; Thompson, A.J.; Kläring, H.P. Guidelines to use tomato in experiments with a controlled environment. Front. Plant Sci. 2014, 5, 625. [Google Scholar] [CrossRef] [PubMed]
- Saloner, A.; Sacks, M.M.; Bernstein, N. Response of medical cannabis (Cannabis sativa L.) genotypes to K supply under long photoperiod. Front. Plant Sci. 2019, 10, 1369. [Google Scholar] [CrossRef]
- Muntendam, R.; Happyana, N.; Erkelens, T.; Bruining, F.; Kayser, O. Time dependent metabolomics and transcriptional analysis of cannabinoid biosynthesis in Cannabis sativa var. Bedrobinol and Bediol grown under standardized condition and with genetic homogeneity. Online Int. J. Med. Plant Res 2012, 1, 31–40. [Google Scholar] [CrossRef]
- Apicella, P.V.; Sands, L.B.; Ma, Y.; Berkowitz, G.A. Delineating genetic regulation of cannabinoid biosynthesis during female flower development in Cannabis sativa. Plant Direct 2022, 6, e412. [Google Scholar] [CrossRef] [PubMed]
- Aizpurua-Olaizola, O.; Soydaner, U.; Öztürk, E.; Schibano, D.; Simsir, Y.; Navarro, P.; Etxebarria, N.; Usobiaga, A. Evolution of the cannabinoid and terpene content during the growth of Cannabis sativa plants from different chemotypes. J. Nat. Prod. 2016, 79, 324–331. [Google Scholar] [CrossRef]
- Bauerle, W.L.; McCullough, C.; Iversen, M.; Hazlett, M. Leaf age and position effects on quantum yield and photosynthetic capacity in hemp crowns. Plants 2020, 9, 271. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.F. Mineral analysis of plant tissues. Annu. Rev. Plant Physiol. 1962, 13, 81–108. [Google Scholar] [CrossRef]
- White, P.J. Ion uptake mechanisms of individual cells and roots: Short-distance transport. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marchner, P., Ed.; Academic Press: London, UK, 2012; pp. 7–47. ISBN 9780123849052. [Google Scholar]
- Wang, H.; Inukai, Y.; Yamauchi, A. Root development and nutrient uptake. CRC Crit. Rev. Plant Sci. 2006, 25, 279–301. [Google Scholar] [CrossRef]
- Nestby, R.; Lieten, F.; Pivot, D.; Raynal Lacroix, C.; Tagliavini, M. Influence of mineral nutrients on strawberry fruit quality and their accumulation in plant organs: A review. Int. J. fruit Sci. 2008, 5, 139–156. [Google Scholar] [CrossRef]
- Garcia, C.B.; Grusak, M.A. Mineral accumulation in vegetative and reproductive tissues during seed development in Medicago truncatula. Front. Plant Sci. 2015, 6, 622. [Google Scholar] [CrossRef] [PubMed]
- Hawkesford, M.; Horst, W.; Kichey, T.; Lambers, H.; Schjoerring, J.; Skrumsager Møller, I.; White, P. Functions of macronutrients. In Marschner’s Mineral Nutrition of Higher Plants; Marschner, P., Ed.; Academic Press: London, UK, 2012; pp. 135–190. ISBN 9780123849052. [Google Scholar]
- Vittori Antisari, L.; Carbone, S.; Gatti, A.; Vianello, G.; Nannipieri, P. Uptake and translocation of metals and nutrients in tomato grown in soil polluted with metal oxide (CeO2, Fe3O4, SnO2, TiO2) or metallic (Ag, Co, Ni) engineered nanoparticles. Environ. Sci. Pollut. Res. 2015, 22, 1841–1853. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Y.; Lu, L.L.; Yang, X.E.; Feng, Y.; Wei, Y.Y.; Hao, H.L.; Stoffella, P.J.; He, Z.L. Uptake, translocation, and remobilization of zinc absorbed at different growth stages by rice genotypes of different Zn densities. J. Agric. Food Chem. 2010, 58, 6767–6773. [Google Scholar] [CrossRef] [PubMed]
- Akhter, M.F.; Macfie, S. Species-specific relationship between transpiration and cadmium translocation in lettuce, barley and radish. J. Plant Stud. 2012, 1, 2. [Google Scholar] [CrossRef]
- Clarkson, D.T.; Hanson, J.B. The mineral nutrition of higher plants. Annu. Rev. Plant Physiol. 1980, 31, 239–298. [Google Scholar] [CrossRef]
- Engels, C.; Marschner, H. Root to shoot translocation of macronutrients in relation to shoot demand in maize (Zea mays L.) grown at different root zone temperatures. Z. Pflanzenernähr. Bodenkd. 1992, 155, 121–128. [Google Scholar] [CrossRef]
- Saloner, A.; Bernstein, N. Response of medical cannabis (Cannabis sativa L.) to nitrogen supply under long photoperiod. Front. Plant Sci. 2020, 11, 1517. [Google Scholar] [CrossRef]
- Shiponi, S.; Bernstein, N. Response of medical cannabis (Cannabis sativa L.) genotypes to P supply under long photoperiod: Functional phenotyping and the ionome. Ind. Crops Prod. 2021, 161, 113154. [Google Scholar] [CrossRef]
- Hall, J.; Bhattarai, S.P.; Midmore, D.J. Review of flowering control in industrial hemp. J. Nat. Fibers 2012, 9, 23–36. [Google Scholar] [CrossRef]
- Clarke, R. Botany of the genus Cannabis. In Advances in Hemp Research; Haworth Press: Binghamton, NY, USA, 1999; pp. 1–19. [Google Scholar]
- Bernstein, N.; Gorelick, J.; Zerahia, R.; Koch, S. Impact of N, P, K, and humic acid supplementation on the chemical profile of medical cannabis (Cannabis sativa L.). Front. Plant Sci. 2019, 10, 736. [Google Scholar] [CrossRef]
- Angelini, L.; Tavarini, S.; Cestone, B.; Beni, C. Variation in mineral composition in three different plant organs of five fibre hemp (Cannabis sativa L.) cultivars. Agrochimica 2014, 58, 1–18. [Google Scholar]
- Small, E. Cannabis: A Complete Guide; CRC Press: Boca Raton, FL, USA, 2016; ISBN 9781315367583. [Google Scholar]
- Bernstein, N.; Ioffe, M.; Bruner, M.; Nishri, Y.; Luria, G.; Dori, I.; Matan, E.; Philosoph-Hadas, S.; Umiel, N.; Hagiladi, A. Effects of supplied nitrogen form and quantity on growth and postharvest quality of Ranunculus asiaticus flowers. HortScience 2005, 40, 1879–1886. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Bernstein, N.; Shoresh, M.; Xu, Y.; Huang, B. Involvement of the plant antioxidative response in the differential growth sensitivity to salinity of leaves vs roots during cell development. Free Radic. Biol. Med. 2010, 49, 1161–1171. [Google Scholar] [CrossRef]
- Chouvy, P.A. Cannabis cultivation in the world: Heritages, trends and challenges. In EchoGéo; OpenEdition Press: Marseille, France, 2019. [Google Scholar] [CrossRef]
- Small, E. Botanical Classification and Nomenclatural Issues. In Cannabis: A Complete Guide; CRC Press: Boca Raton, FL, USA, 2016; pp. 477–504. [Google Scholar]
- Munné-Bosch, S.; Alegre, L. Die and let live: Leaf senescence contributes to plant survival under drought stress. Funct. Plant Biol. 2004, 31, 203–216. [Google Scholar] [CrossRef]
- Wingler, A.; Purdy, S.; MacLean, J.A.; Pourtau, N. The role of sugars in integrating environmental signals during the regulation of leaf senescence. J. Exp. Bot. 2006, 57, 391–399. [Google Scholar] [CrossRef]
- Wolstenholme, B.N. Root, shoot or fruit. South Afr. Avocado Grow. Assoc. Yearb. 1981, 4, 27–29. [Google Scholar]
- Bonifas, K.D.; Walters, D.T.; Cassman, K.G.; Lindquist, J.L. Nitrogen supply affects root:shoot ratio in corn and velvetleaf (Abutilon theophrasti). Weed Sci. 2005, 53, 670–675. [Google Scholar] [CrossRef]
- Iwasa, Y.; Roughgarden, J. Shoot/root balance of plants: Optimal growth of a system with many vegetative organs. Theor. Popul. Biol. 1984, 25, 78–105. [Google Scholar] [CrossRef]
- Tezara, W.; Domínguez, T.S.T.; Loyaga, D.W.; Ortiz, R.N.; Chila, V.H.R.; Ortega, M.J.B. Photosynthetic activity of oil palm (Elaeis guineensis) and interspecific hybrid genotypes (Elaeis oleifera × Elaeis guineensis), and response of hybrids to water deficit. Sci. Hortic. 2021, 287, 110263. [Google Scholar] [CrossRef]
- Haldimann, P. Low growth temperature-induced changes to pigment composition and photosynthesis in Zea mays genotypes differing in chilling sensitivity. Plant. Cell Environ. 1998, 21, 200–208. [Google Scholar] [CrossRef]
- Bacelar, E.A.; Moutinho-Pereira, J.M.; Gonçalves, B.C.; Lopes, J.I.; Correia, C.M. Physiological responses of different olive genotypes to drought conditions. Acta Physiol. Plant. 2009, 31, 611–621. [Google Scholar] [CrossRef]
- Bond, B.J. Age-related changes in photosynthesis of woody plants. Trends Plant Sci. 2000, 5, 349–353. [Google Scholar] [CrossRef]
- Niinemets, Ü. Stomatal conductance alone does not explain the decline in foliar photosynthetic rates with increasing tree age and size in Picea abies and Pinus sylvestris. Tree Physiol. 2002, 22, 515–535. [Google Scholar] [CrossRef]
- Kennedy, R.A.; Johnson, D. Changes in photosynthetic characteristics during leaf development in apple. Photosynth. Res. 1981, 2, 213–223. [Google Scholar] [CrossRef]
- Munné-Bosch, S.; Alegre, L. Plant aging increases oxidative stress in chloroplasts. Planta 2002, 214, 608–615. [Google Scholar] [CrossRef]
- Bonini, S.A.; Premoli, M.; Tambaro, S.; Kumar, A.; Maccarinelli, G.; Memo, M.; Mastinu, A. Cannabis sativa: A comprehensive ethnopharmacological review of a medicinal plant with a long history. J. Ethnopharmacol. 2018, 227, 300–315. [Google Scholar] [CrossRef]
- Sklensky, D.E.; Davies, P.J. Resource partitioning to male and female flowers of Spinacia oleracea L. in relation to whole-plant monocarpic senescence. J. Exp. Bot. 2011, 62, 4323–4336. [Google Scholar] [CrossRef]
- Fan, K.; Zhang, Q.; Liu, M.; Ma, L.; Shi, Y.; Ruan, J. Metabolomic and transcriptional analyses reveal the mechanism of C, N allocation from source leaf to flower in tea plant (Camellia sinensis L.). J. Plant Physiol. 2019, 232, 200–208. [Google Scholar] [CrossRef]
- Clifford, P.E.; Neo, H.H.; Hew, C.S. Regulation of assimilate partitioning in flowering plants of the monopodial orchid Aranda Noorah Alsagoff. New Phytol. 1995, 130, 381–389. [Google Scholar] [CrossRef]
- Poulson, M.E.; Thai, T. Effect of high light intensity on photoinhibition, oxyradicals and artemisinin content in Artemisia annua L. Photosynthetica 2015, 53, 403–409. [Google Scholar] [CrossRef]
- Allen, R.D.; Webb, R.P.; Schake, S.A. Use of transgenic plants to study antioxidant defenses. Free Radic. Biol. Med. 1997, 23, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Faisal, M.; Anis, M. Changes in photosynthetic activity, pigment composition, electrolyte leakage, lipid peroxidation, and antioxidant enzymes during ex vitro establishment of micropropagated Rauvolfia tetraphylla plantlets. Plant Cell. Tissue Organ Cult. 2009, 99, 125–132. [Google Scholar] [CrossRef]
- Cakmak, I.; Kurz, H.; Marschner, H. Short-term effects of boron, germanium and high light intensity on membrane permeability in boron deficient leaves of sunflower. Physiol. Plant. 1995, 95, 11–18. [Google Scholar] [CrossRef]
- Maathuis, F.J. Physiological functions of mineral macronutrients. Curr. Opin. Plant Biol. 2009, 12, 250–258. [Google Scholar] [CrossRef]
- Mitra, G. Essential plant nutrients and recent concepts about their uptake. In Essential Plant Nutrients; Naeem, M., Ansari, A., Gill, S., Eds.; Springer: Cham, Switzerland, 2017; pp. 3–36. ISBN 9783319588414. [Google Scholar]
- Bassirirad, H. Kinetics of nutrient uptake by roots: Responses to global change. New Phytol. 2000, 147, 155–169. [Google Scholar] [CrossRef]
- Cockson, P.; Schroeder-Moreno, M.; Veazie, P.; Barajas, G.; Logan, D.; Davis, M.; Whipker, B.E. Impact of phosphorus on Cannabis sativa reproduction, cannabinoids, and terpenes. Appl. Sci. 2020, 10, 7875. [Google Scholar] [CrossRef]
- Pardo, J.M.; Quintero, F.J. Plants and sodium ions: Keeping company with the enemy. Genome Biol. 2002, 3, 1–4. [Google Scholar] [CrossRef]
- Monaci, F.; Leidi, E.O.; Mingorance, M.D.; Valdés, B.; Oliva, S.R.; Bargagli, R. Selective uptake of major and trace elements in Erica andevalensis, an endemic species to extreme habitats in the Iberian Pyrite Belt. J. Environ. Sci. 2011, 23, 444–452. [Google Scholar] [CrossRef]
- Qin, S.; Liu, H.; Nie, Z.; Rengel, Z.; Gao, W.; Li, C.; Zhao, P. Toxicity of cadmium and its competition with mineral nutrients for uptake by plants: A review. Pedosphere 2020, 30, 168–180. [Google Scholar] [CrossRef]
- Drechsler, N.; Zheng, Y.; Bohner, A.; Nobmann, B.; von Wirén, N.; Kunze, R.; Rausch, C. Nitrate-dependent control of shoot K homeostasis by the nitrate transporter1/peptide transporter family member NPF7.3/NRT1.5 and the Stelar K+ outward rectifier SKOR in arabidopsis. Plant Physiol. 2015, 169, 2832–2847. [Google Scholar] [CrossRef] [PubMed]
- White, P.J. Long-distance Transport in the Xylem and Phloem. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marchner, P., Ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2012; pp. 49–70. ISBN 9780123849052. [Google Scholar]
- Broadley, M.; Brown, P.; Cakmak, I.; Rengel, Z.; Zhao, F. Function of nutrients: Micronutrients. In Marschner’s Mineral Nutrition of Higher Plants; Marschner, P., Ed.; Academic Press: Cambridge, MA, USA, 2012; pp. 191–248. ISBN 9780123849052. [Google Scholar]
- Rahman, H.; Sabreen, S.; Alam, S.; Kawai, S. Effects of nickel on growth and composition of metal micronutrients in barley plants grown in nutrient solution. J. Plant Nutr. 2007, 28, 393–404. [Google Scholar] [CrossRef]
- Siebrecht, S.; Herdel, K.; Schurr, U.; Tischner, R. Nutrient translocation in the xylem of poplar—Diurnal variations and spatial distribution along the shoot axis. Planta 2003, 217, 783–793. [Google Scholar] [CrossRef]
- Mclaughlin, S.B.; Wimmer, R. Calcium physiology and terrestrialecosystem processes. New Phytol. 1999, 142, 373–417. [Google Scholar] [CrossRef]
- Kobayashi, T.; Nishizawa, N.K. Iron uptake, translocation, and regulation in higher plants. Annu. Rev. Plant Biol. 2012, 63, 131–152. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saloner, A.; Bernstein, N. Dynamics of Mineral Uptake and Plant Function during Development of Drug-Type Medical Cannabis Plants. Agronomy 2023, 13, 2865. https://doi.org/10.3390/agronomy13122865
Saloner A, Bernstein N. Dynamics of Mineral Uptake and Plant Function during Development of Drug-Type Medical Cannabis Plants. Agronomy. 2023; 13(12):2865. https://doi.org/10.3390/agronomy13122865
Chicago/Turabian StyleSaloner, Avia, and Nirit Bernstein. 2023. "Dynamics of Mineral Uptake and Plant Function during Development of Drug-Type Medical Cannabis Plants" Agronomy 13, no. 12: 2865. https://doi.org/10.3390/agronomy13122865
APA StyleSaloner, A., & Bernstein, N. (2023). Dynamics of Mineral Uptake and Plant Function during Development of Drug-Type Medical Cannabis Plants. Agronomy, 13(12), 2865. https://doi.org/10.3390/agronomy13122865




_Qin.png)


