Influence of Interannual Climate Conditions on the Composition of Olive Oil from Centenarian Olive Trees
Abstract
:1. Introduction
2. Materials and Methods
2.1. Selection, Harvest, and Extraction
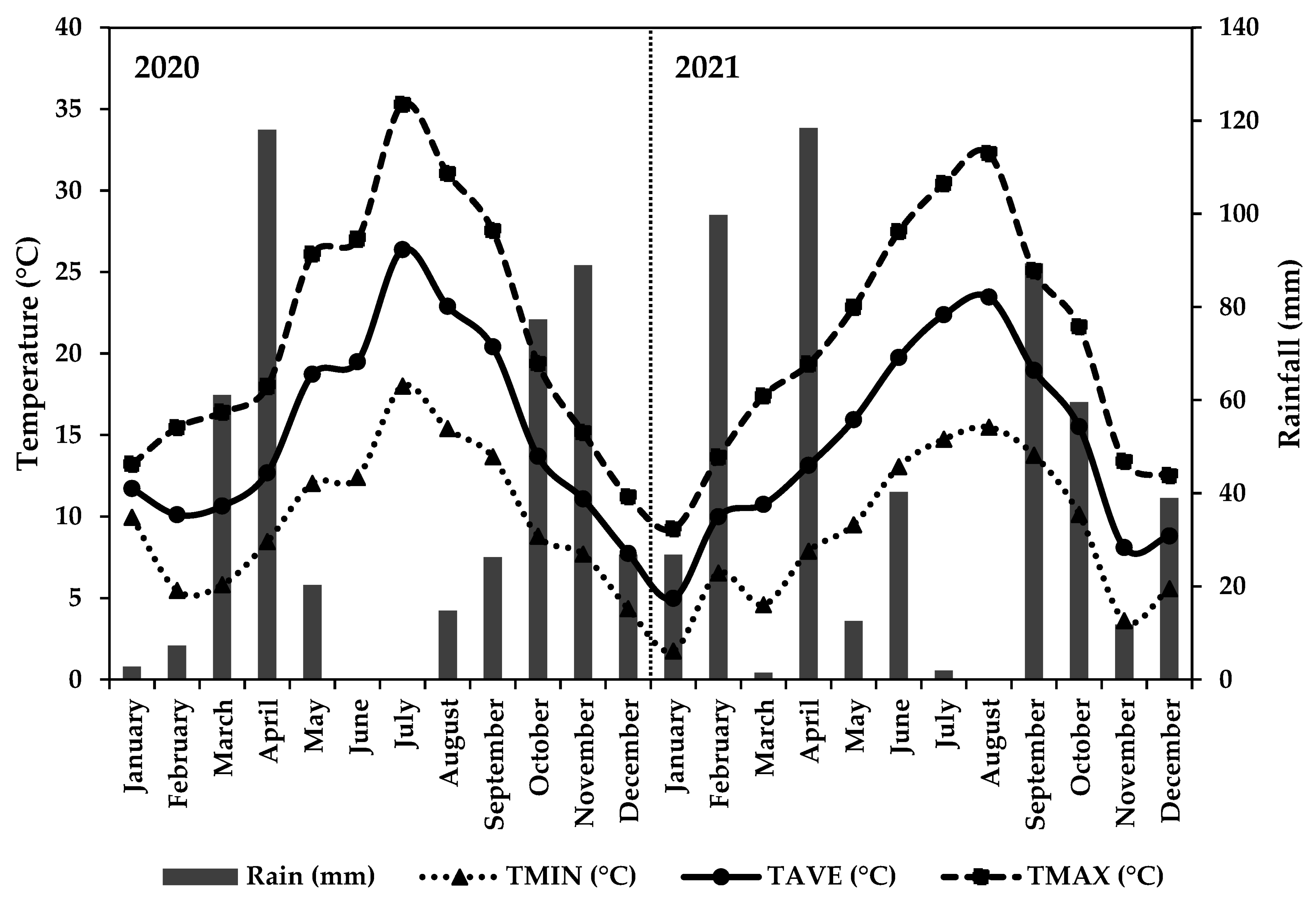
2.2. Meteriological Data
2.3. Fatty Acid Profile
2.4. Tocopherol Contents
2.5. Olive Oils Total Content of Hydroxytyrosol and Tyrosol Derivatives after Acid Hydrolysis of Secoiridoids
2.6. Oxidative Stability, Total Reducing Capacity, and Antioxidant Activity
2.7. Statistical Analysis
3. Results and Discussion
3.1. Meteorological Data
3.2. Fatty Acid Composition
3.3. Tocopherols Content
3.4. Olive Oils Total Content of Hydroxytyrosol and Tyrosol Derivatives
3.5. Oxidative Stability, Antioxidant Activity, and Total Reducing Capacity
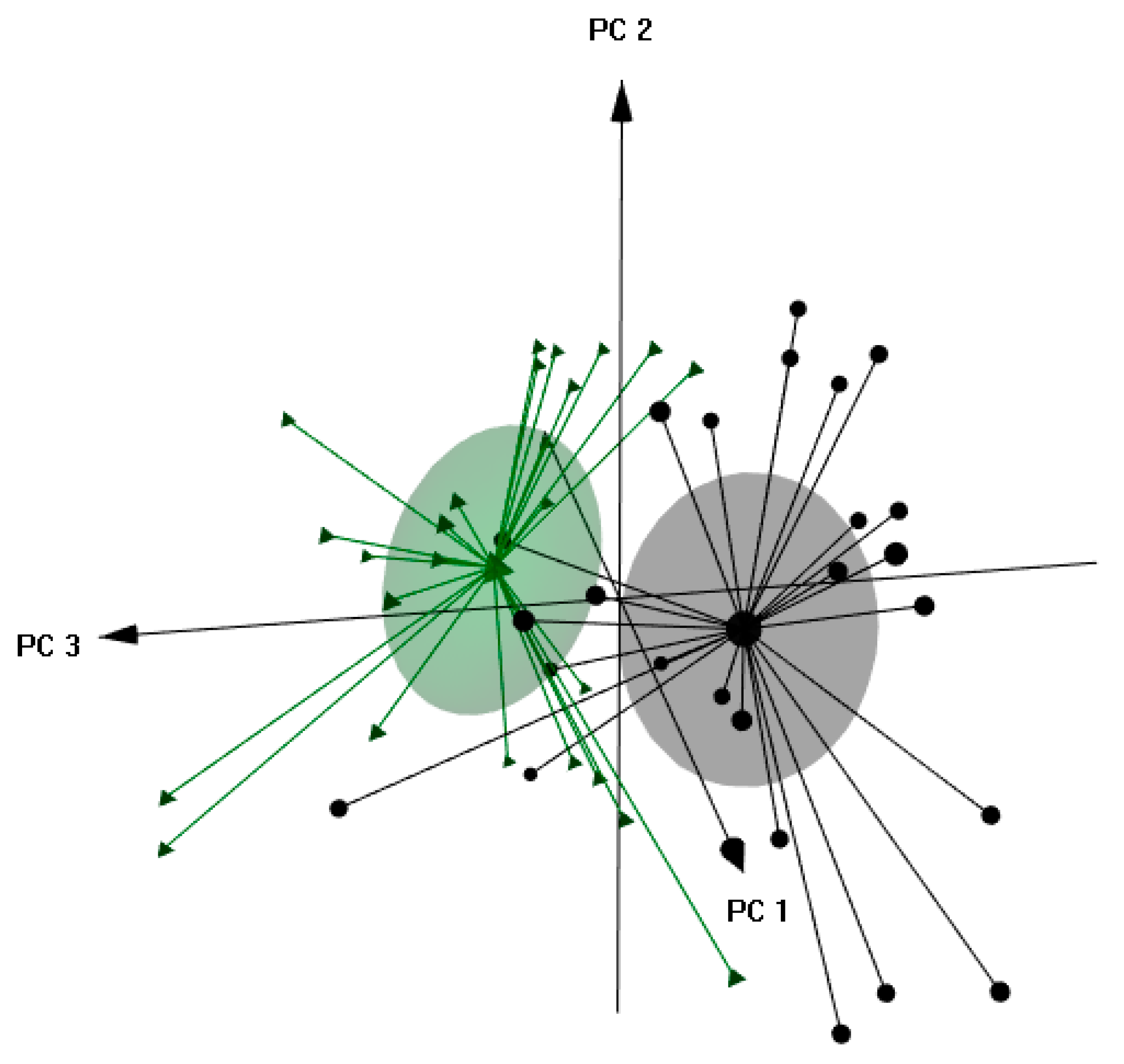
3.6. Discrimination of Olive Oils by Crop Year
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Langsdorf, S.; Löschke, S.; Möller, V.; Okem, A.; Officer, S.; Rama, B.; Belling, D.; Dieck, W.; Götze, S.; Kersher, T.; et al. Terrestrial and Freshwater Ecosystems and Their Services. In Climate Change 2022: Impacts, Adaptation and Vulnerability. In Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Cambridge, UK, 2022; pp. 197–377. [Google Scholar] [CrossRef]
- Rickards, L.; Howden, S.M. Transformational Adaptation: Agriculture and Climate Change. Crop Pasture Sci. 2012, 63, 240–250. [Google Scholar] [CrossRef]
- Campbell, B.M.; Vermeulen, S.J.; Aggarwal, P.K.; Corner-Dolloff, C.; Girvetz, E.; Loboguerrero, A.M.; Ramirez-Villegas, J.; Rosenstock, T.; Sebastian, L.; Thornton, P.K.; et al. Reducing Risks to Food Security from Climate Change. Glob. Food Sec. 2016, 11, 34–43. [Google Scholar] [CrossRef]
- Nugroho, A.D.; Prasada, I.Y.; Lakner, Z. Comparing the Effect of Climate Change on Agricultural Competitiveness in Developing and Developed Countries. J. Clean. Prod. 2023, 406, 137139. [Google Scholar] [CrossRef]
- Amassaghrou, A.; Barkaoui, K.; Bouaziz, A.; Alaoui, S.B.; Fatemi, Z.E.A.; Daoui, K. Yield and Related Traits of Three Legume Crops Grown in Olive-Based Agroforestry under an Intense Drought in the South Mediterranean. Saudi J. Biol. Sci. 2023, 30, 103597. [Google Scholar] [CrossRef]
- Temani, F.; Bouaziz, A.; Daoui, K.; Wery, J.; Barkaoui, K. Olive Agroforestry Can Improve Land Productivity Even under Low Water Availability in the South Mediterranean. Agric. Ecosyst. Environ. 2021, 307, 107234. [Google Scholar] [CrossRef]
- Arenas-Castro, S.; Gonçalves, J.F.; Moreno, M.; Villar, R. Projected Climate Changes Are Expected to Decrease the Suitability and Production of Olive Varieties in Southern Spain. Sci. Total Environ. 2020, 709, 136161. [Google Scholar] [CrossRef]
- Fraga, H.; Moriondo, M.; Leolini, L.; Santos, J.A. Mediterranean Olive Orchards under Climate Change: A Review of Future Impacts and Adaptation Strategies. Agronomy 2020, 11, 56. [Google Scholar] [CrossRef]
- Arias Montevechio, E.; Crispin Cunya, M.; Fernández Jorquera, F.; Rendon, E.; Vásquez-Lavin, F.; Stehr, A.; Ponce Oliva, R.D. Traditional Crops and Climate Change Adaptation: Insights from the Andean Agricultural Sector. Clim. Dev. 2023, 15, 723–737. [Google Scholar] [CrossRef]
- Deiana, P.; Motroni, A.; Filigheddu, M.R.; Dettori, S.; Nieddu, G.; Mercenaro, L.; Alfei, B.; Culeddu, N.; Santona, M. Effect of Pedoclimatic Variables on Analytical and Organoleptic Characteristics in Olive Fruit and Virgin Olive Oil. Eur. J. Agron. 2023, 148, 126856. [Google Scholar] [CrossRef]
- Fraga, H.; Guimarães, N.; Freitas, T.R.; Malheiro, A.C.; Santos, J.A. Future Scenarios for Olive Tree and Grapevine Potential Yields in the World Heritage Côa Region, Portugal. Agronomy 2022, 12, 350. [Google Scholar] [CrossRef]
- Valente, S.; Machado, B.; Pinto, D.C.G.A.; Santos, C.; Silva, A.M.S.; Dias, M.C. Modulation of Phenolic and Lipophilic Compounds of Olive Fruits in Response to Combined Drought and Heat. Food Chem. 2020, 329, 127191. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, N.; Casal, S.; Cruz, R.; Peres, A.M.; Baptista, P.; Pereira, J.A. GxE Effects on Tocopherol Composition of Oils from Very Old and Genetically Diverse Olive Trees. JAOCS J. Am. Oil Chem. Soc. 2020, 97, 497–507. [Google Scholar] [CrossRef]
- Lodolini, E.M.; Ali, S.; Mutawea, M.; Qutub, M.; Arabasi, T.; Pierini, F.; Neri, D. Complementary Irrigation for Sustainable Production in Olive Groves in Palestine. Agric. Water Manag. 2014, 134, 104–109. [Google Scholar] [CrossRef]
- Rey-Giménez, R.; Sánchez-Gimeno, A.C. Crop Year, Harvest Date and Clone Effects on Fruit Characteristics, Chemical Composition and Olive Oil Stability from an Empeltre Clonal Selection Grown in Aragon. J. Sci. Food Agric. 2022, 102, 5778–5786. [Google Scholar] [CrossRef]
- Aparicio, R.; Ferreiro, L.; Alonso, V. Effect of Climate on the Chemical Composition of Virgin Olive Oil. Anal. Chim. Acta 1994, 292, 235–241. [Google Scholar] [CrossRef]
- Beltrán, G.; Jiménez, A.; del Rio, C.; Sánchez, S.; Martínez, L.; Uceda, M.; Aguilera, M.P. Variability of Vitamin E in Virgin Olive Oil by Agronomical and Genetic Factors. J. Food Compos. Anal. 2010, 23, 633–639. [Google Scholar] [CrossRef]
- Bengana, M.; Bakhouche, A.; Lozano-Sánchez, J.; Amir, Y.; Youyou, A.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Influence of Olive Ripeness on Chemical Properties and Phenolic Composition of Chemlal Extra-Virgin Olive Oil. Food Res. Int. 2013, 54, 1868–1875. [Google Scholar] [CrossRef]
- Bongiorno, D.; Di Stefano, V.; Indelicato, S.; Avellone, G.; Ceraulo, L. Bio-Phenols Determination in Olive Oils: Recent Mass Spectrometry Approaches. Mass Spectrom. Rev. 2021, 42, 1462–1502. [Google Scholar] [CrossRef]
- Mansouri, F.; Moumen, A.B.; Belhaj, K.; Richard, G.; Marie-Laure Fauconnier, G.R.; Sindic, M.; Ahmed Elamrani, H.S.C. Effect of Crop Season on the Quality and Composition of Extra Virgin Olive Oils from Greek and Spanish Varieties Grown in the Oriental Region of Morocco. Emir. J. Food Agric. 2018, 30, 549–562. [Google Scholar] [CrossRef]
- Rey-Giménez, R.; Sánchez-Gimeno, A.C. Authenticity in Olive Oils from an Empeltre Clonal Selection in Aragon (Spain): How Environmental, Agronomic, and Genetic Factors Affect Sterol Composition. Foods 2022, 11, 2587. [Google Scholar] [CrossRef]
- Romero, M.P.; Tovar, M.J.; Ramo, T.; Motilva, M.J. Effect of Crop Season on the Composition of Virgin Olive Oil with Protected Designation of Origin “Les Garrigues”. J. Am. Oil Chem. Soc. 2003, 80, 423–430. [Google Scholar] [CrossRef]
- Giuffrè, A.M. The Effects of Cultivar and Harvest Year on the Fatty Alcohol Composition of Olive Oils from Southwest Calabria (Italy). Grasas Aceites 2014, 65, e011. [Google Scholar] [CrossRef]
- Mele, M.A.; Islam, M.Z.; Kang, H.M.; Giuffre, A.M. Pre-and Post-Harvest Factors and Their Impact on Oil Composition and Quality of Olive Fruit. Emir. J. Food Agric. 2018, 30, 592. [Google Scholar] [CrossRef]
- Lechhab, T.; Lechhab, W.; Cacciola, F.; Salmoun, F. Sets of Internal and External Factors Influencing Olive Oil (Olea Europaea L.) Composition: A Review. Eur. Food Res. Technol. 2022, 248, 1069–1088. [Google Scholar] [CrossRef]
- Rouina, Y.B.; Zouari, M.; Zouari, N.; Rouina, B.B.; Bouaziz, M. Olive Tree (Olea Europaea L. Cv. Zelmati) Grown in Hot Desert Climate: Physio-Biochemical Responses and Olive Oil Quality. Sci. Hortic. 2020, 261, 108915. [Google Scholar] [CrossRef]
- Oğraş, Ş.Ş.; Kaban, G.; Kaya, M. The Effects of Geographic Region, Cultivar and Harvest Year on Fatty Acid Composition of Olive Oil. J. Oleo Sci. 2016, 65, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Beltrán, G.; Del Rio, C.; Sánchez, S.; Martínez, L. Influence of Harvest Date and Crop Yield on the Fatty Acid Composition of Virgin Olive Oils from Cv. Picual. J. Agric. Food Chem. 2004, 52, 3434–3440. [Google Scholar] [CrossRef]
- Squeo, G.; Silletti, R.; Mangini, G.; Summo, C.; Caponio, F. The Potential of Apulian Olive Biodiversity: The Case of Oliva Rossa Virgin Olive Oil. Foods 2021, 10, 369. [Google Scholar] [CrossRef]
- Rodrigues, N.; Casal, S.; Peres, A.M.; Baptista, P.; Pereira, J.A. Seeking for Sensory Differentiated Olive Oils? The Urge to Preserve Old Autochthonous Olive Cultivars. Food Res. Int. 2020, 128, 108759. [Google Scholar] [CrossRef]
- Alowaiesh, B.; Singh, Z.; Fang, Z.; Kailis, S.G. Harvest Time Impacts the Fatty Acid Compositions, Phenolic Compounds and Sensory Attributes of Frantoio and Manzanilla Olive Oil. Sci. Hortic. 2018, 234, 74–80. [Google Scholar] [CrossRef]
- Petruccelli, R.; Giordano, C.; Salvatici, M.C.; Beghè, D.; Rodolfi, M.; Fabbri, A.; Benelli, C. Characterization and Conservation of “Olivo Della Strega”: An Ancient Olive Tree, Precious Resource for Natural and Cultural Heritage. Rend. Lincei 2021, 32, 311–324. [Google Scholar] [CrossRef]
- Rodrigues, N.; Casal, S.; Pinho, T.; Cruz, R.; Peres, A.M.; Baptista, P.; Pereira, J.A. Fatty Acid Composition from Olive Oils of Portuguese Centenarian Trees Is Highly Dependent on Olive Cultivar and Crop Year. Foods 2021, 10, 496. [Google Scholar] [CrossRef] [PubMed]
- Sabetta, W.; Mascio, I.; Squeo, G.; Gadaleta, S.; Flamminii, F.; Conte, P.; Di Mattia, C.D.; Piga, A.; Caponio, F.; Montemurro, C. Bioactive Potential of Minor Italian Olive Genotypes from Apulia, Sardinia and Abruzzo. Foods 2021, 10, 1371. [Google Scholar] [CrossRef] [PubMed]
- International Olive Council. Guide for the Determination of the Characteristics of Oil-Olives; COI/OH/Doc. No 1; International Olive Council: Madrid, Spain, 2011; p. 39. [Google Scholar]
- International Olive Council. Determination of the Content of Waxes and Fatty Acid Ethyl Esters by Capillary Gas Chromatography; COI/T.20/Doc. No 28/Rev. 3 November 2022; International Olive Council: Madrid, Spain, 2022; pp. 1–21. [Google Scholar]
- ISO 9936:2016; Animal and Vegetable Fats and Oils—Determination of Tocopherol and Tocotrienol Contents by High-Performance Liquid Chromatography. ISO: Geneva, Switzerland, 2016.
- Marx, Í.M.G.; Casal, S.; Rodrigues, N.; Veloso, A.C.A.; Pereira, J.A.; Peres, A.M. Estimating Hydroxytyrosol-Tyrosol Derivatives Amounts in Cv. Cobrançosa Olive Oils Based on the Electronic Tongue Analysis of Olive Paste Extracts. LWT 2021, 147, 111542. [Google Scholar] [CrossRef]
- Cherif, M.; Rodrigues, N.; Veloso, A.C.A.; Pereira, J.A.; Peres, A.M. Kinetic Study of the Microwave-induced Thermal Degradation of Cv. Arbequina Olive Oils Flavored with Lemon Verbena Essential Oil. J. Am. Oil Chem. Soc. 2021, 98, 1021–1032. [Google Scholar] [CrossRef]
- Samaniego Sánchez, C.; Troncoso González, A.M.; García-Parrilla, M.C.; Quesada Granados, J.J.; López García de la Serrana, H.; López Martínez, M.C. Different Radical Scavenging Tests in Virgin Olive Oil and Their Relation to the Total Phenol Content. Anal. Chim. Acta 2007, 593, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Hijawi, T. Characterizing of Oil Quality and Fatty Acid Profiles of Old Olive Trees in Palestine. J. Oleo Sci. 2021, 70, 1585–1606. [Google Scholar] [CrossRef]
- Portarena, S.; Farinelli, D.; Lauteri, M.; Famiani, F.; Esti, M.; Brugnoli, E. Stable Isotope and Fatty Acid Compositions of Monovarietal Olive Oils: Implications of Ripening Stage and Climate Effects as Determinants in Traceability Studies. Food Control 2015, 57, 129–135. [Google Scholar] [CrossRef]
- Ângelo, M.; Carlos, R.; Correia, M. Manual da Safra e Contra Safra Olival; Instituto Politécnico: Bragança, Portugal, 2009. [Google Scholar]
- Borges, T.H.; López, L.C.; Pereira, J.A.; Cabrera-Vique, C.; Seiquer, I. Comparative Analysis of Minor Bioactive Constituents (CoQ10, Tocopherols and Phenolic Compounds) in Arbequina Extra Virgin Olive Oils from Brazil and Spain. J. Food Compos. Anal. 2017, 63, 47–54. [Google Scholar] [CrossRef]
- García-Inza, G.P.; Castro, D.N.; Hall, A.J.; Rousseaux, M.C. Responses to Temperature of Fruit Dry Weight, Oil Concentration, and Oil Fatty Acid Composition in Olive (Olea europaea L. Var. ‘Arauco’). Eur. J. Agron. 2014, 54, 107–115. [Google Scholar] [CrossRef]
- Ayton, J.; Mailer, R.J.; Haigh, A.; Tronson, D.; Conlan, D. Quality and oxidative stability of australian olive oil according to harvest date and irrigation. J. Food Lipids 2007, 14, 138–156. [Google Scholar] [CrossRef]
- Ben Hmida, R.; Gargouri, B.; Chtourou, F.; Abichou, M.; Sevim, D.; Bouaziz, M. Study on the Effect of Climate Changes on the Composition and Quality Parameters of Virgin Olive Oil “Zalmati” Harvested at Three Consecutive Crop Seasons: Chemometric Discrimination. ACS Omega 2022, 7, 40078–40090. [Google Scholar] [CrossRef] [PubMed]
- Giuffrè, A.M. The Evolution of Free Acidity and Oxidation Related Parameters in Olive Oil during Olive Ripening from Cultivars Grown in the Region of Calabria, South Italy. Emir. J. Food Agric. 2018, 30, 539–548. [Google Scholar]
- Yu, L.; Wang, Y.; Wu, G.; Jin, J.; Jin, Q.; Wang, X. Chemical and Volatile Characteristics of Olive Oils Extracted from Four Varieties Grown in Southwest of China. Food Res. Int. 2021, 140, 109987. [Google Scholar] [CrossRef] [PubMed]
- Benincasa, C.; Russo, A.; Romano, E.; Elsorady, M.E.; Perri, E.; Muzzalupo, I. Chemical and sensory analysis of some Egyptian virgin olive oils. J. Nutr. Food Sci. 2011, 5, 118. [Google Scholar] [CrossRef]
- Jiménez-Herrera, R.; Pacheco-López, B.; Peragón, J. Water Stress, Irrigation and Concentrations of Pentacyclic Triterpenes and Phenols in Olea Europaea L. Cv. Picual Olive Trees. Antioxidants 2019, 8, 294. [Google Scholar] [CrossRef] [PubMed]
- Faghim, J.; Mohamed, M.B.; Bagues, M.; Nagaz, K.; Triki, T.; Guasmi, F. Irrigation Effects on Phenolic Profile and Extra Virgin Olive Oil Quality of “Chemlali” Variety Grown in South Tunisia. S. Afr. J. Bot. 2021, 141, 322–329. [Google Scholar] [CrossRef]
- Uslu, N.; Özcan, M.M. The Effect of Irrigation and Harvest Time on Bioactive Properties of Olive Fruits Issued from Some Olive Varieties Grown in Mediterranean Region. Eur. Food Res. Technol. 2020, 246, 2587–2599. [Google Scholar] [CrossRef]
- Wani, T.A.; Masoodi, F.A.; Gani, A.; Baba, W.N.; Rahmanian, N.; Akhter, R.; Wani, I.A.; Ahmad, M. Olive Oil and Its Principal Bioactive Compound: Hydroxytyrosol—A Review of the Recent Literature. Trends Food Sci. Technol. 2018, 77, 77–90. [Google Scholar] [CrossRef]
- Famiani, F.; Farinelli, D.; Rollo, S.; Camposeo, S.; Di Vaio, C.; Inglese, P. Evaluation of Different Mechanical Fruit Harvesting Systems and Oil Quality in Very Large Size Olive Trees. Span. J. Agric. Res. 2014, 12, 960–972. [Google Scholar] [CrossRef]
- Baccouri, B.; Sieren, T.; Mohamed, S.N.; Willenberg, I. Fingerprinting of Tocopherol, Phenolic Compounds and Oxidative Properties of Unstudied Minor and Rare Tunisian Olive Oils. S. Afr. J. Bot. 2023, 156, 54–64. [Google Scholar] [CrossRef]
- García-Garví, J.M.; Sánchez-Bravo, P.; Hernández, F.; Sendra, E.; Corell, M.; Moriana, A.; Burgos-Hernández, A.; Carbonell-Barrachina, Á.A. Effect of Regulated Deficit Irrigation on the Quality of “Arbequina”; Extra Virgin Olive Oil Produced on a Super-High-Intensive Orchard. Agronomy 2022, 12, 1892. [Google Scholar] [CrossRef]
- Ben Brahim, S.; Kelebek, H.; Ammar, S.; Abichou, M.; Bouaziz, M. LC–MS Phenolic Profiling Combined with Multivariate Analysis as an Approach for the Characterization of Extra Virgin Olive Oils of Four Rare Tunisian Cultivars during Ripening. Food Chem. 2017, 229, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Reboredo-Rodríguez, P.; González-Barreiro, C.; Cancho-Grande, B.; Valli, E.; Bendini, A.; Gallina Toschi, T.; Simal-Gandara, J. Characterization of Virgin Olive Oils Produced with Autochthonous Galician Varieties. Food Chem. 2016, 212, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Nenadis, N.; Mastralexi, A.; Tsimidou, M.Z. Physicochemical Characteristics and Antioxidant Potential of the Greek PDO and PGI Virgin Olive Oils (VOOs). Eur. J. Lipid Sci. Technol. 2019, 121, 1800172. [Google Scholar] [CrossRef]
| Fatty Acid Relative Abundance (%) | Crop Year | p-Value | Δ (%) | |
|---|---|---|---|---|
| 2020 | 2021 | |||
| Palmitic acid (C16:0) | 12.9 ± 1.6 b (9.7–17.3) | 13.6 ± 1.2 a (10.7–16.4) | 0.0114 * | +5.4 |
| Palmitoleic acid (C16:1) | 0.9 ± 0.3 a (0.5–2.0) | 0.9 ± 0.3 a (0.6–1.4) | 0.9339 ** | +1.1 |
| Stearic acid (C18:0) | 2.7 ± 0.4 a (1.7–3.6) | 2.3 ± 0.3 b (1.6–2.9) | <0.0001 * | −14.8 |
| Oleic acid (C18:1) | 71.6 ± 6.1 a (59.4–79.1) | 72.3 ± 5.3 a (64.3–79.8) | 0.4910 ** | +1.0 |
| Linoleic acid (C18:2) | 9.8 ± 5.1 a (3.4–18.6) | 8.8 ± 4.6 a (2.9–16.4) | 0.3221 ** | −10.2 |
| Linolenic acid (C18:3) | 0.9 ± 0.2 a (0.7–1.2) | 0.9 ± 0.2 a (0.6–1.2) | 0.9250 ** | 0.0 |
| Arachidic acid (C20:0) | 0.4 ± 0.0 a (0.3–0.5) | 0.4 ± 0.0 b (0.3–0.5) | 0.0138 * | −5.4 |
| Eicosenoic acid (C20:1) | 0.3 ± 0.0 a (0.2–0.4) | 0.3 ± 0.0 a (0.2–0.4) | 0.3072 ** | +3.6 |
| SFA | 16.3 ± 4.0 a (13.0–20.5) | 16.4 ± 4.0 a (13.3–19.5) | 0.3958 ** | +0.6 |
| MUFA | 73.0 ± 6.1 a (61.1–80.6) | 74.4 ± 5.5 a (67.2–81.4) | 0.2195 ** | +2.0 |
| PUFA | 10.7 ± 5.2 a (4.1–19.5) | 9.4 ± 4.6 a (3.7–16.0) | 0.2041 ** | −11.9 |
| Tocopherol Concentration (mg/kg) | Crop Year | p-Value | Δ (%) | |
|---|---|---|---|---|
| 2020 | 2021 | |||
| α-tocopherol | 254.3 ± 70.1 a (121.0–362.3) | 252.3 ± 63.1 a (134.0–323.3) | 0.8820 ** | −0.8 |
| β-tocopherol | 1.3 ± 0.6 b (0.1–2.1) | 2.0 ± 0.6 a (1.0–2.9) | <0.0001 * | +54.2 |
| γ-tocopherol | 4.5 ± 3.3 a (0.4–14.6) | 5.0 ± 2.2 a (2.0–9.6) | 0.3650 ** | +11.4 |
| Vitamin E | 260.1 ± 72.5 a (122.7–369.3) | 259.3 ± 64.8 a (138.0–342.8) | 0.9552 ** | −0.3 |
| Phenolic Acids Concentration (mg/kg) | Crop Year | p-Value | Δ (%) | |
|---|---|---|---|---|
| 2020 | 2021 | |||
| Hydroxytyrosol | 290.2 ± 68.0 a (130.5–400.1) | 220.50 ± 62.22 b (130.80–380.30) | <0.0001 * | −24.0 |
| Tyrosol | 170.3 ± 92.0 a (36.5–427.3) | 135.93 ± 59.19 b (41.80–338.40) | 0.0169 * | −20.2 |
| Hydroxytyrosol + Tyrosol | 460.6 ± 142.4 a (167.4–772.7) | 356.4 ± 98.7 b (179.5–532.3) | <0.0001 * | −3.3 |
| Antioxidant Activity | Crop Year | p-Value | Δ (%) | |
|---|---|---|---|---|
| 2020 | 2021 | |||
| Oxidative stability | 18.2 ± 8.3 a (6.6–31.6) | 19.0 ± 9.1 a (8.0–44.5) | 0.6472 ** | +4.7 |
| Total reduction capacity | 375.2 ± 160.6 b (61.3–710.7) | 569.5 ± 189.5 a (234.1–965.7) | 2.32 × 10−7 * | +51.8 |
| DPPH | 57.1 ± 17.7 a (12.6–84.1) | 53.2 ± 18.0 a (17.1–88.6) | 0.2922 ** | −6.8 |
| ABTS | 40.8 ± 8.5 a (21.1–54.7) | 35.0 ± 5.6 b (23.9–45.8) | 9.07 × 10−5 * | −14.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bortoluzzi, L.; Casal, S.; Cruz, R.; Peres, A.M.; Baptista, P.; Rodrigues, N. Influence of Interannual Climate Conditions on the Composition of Olive Oil from Centenarian Olive Trees. Agronomy 2023, 13, 2884. https://doi.org/10.3390/agronomy13122884
Bortoluzzi L, Casal S, Cruz R, Peres AM, Baptista P, Rodrigues N. Influence of Interannual Climate Conditions on the Composition of Olive Oil from Centenarian Olive Trees. Agronomy. 2023; 13(12):2884. https://doi.org/10.3390/agronomy13122884
Chicago/Turabian StyleBortoluzzi, Leticia, Susana Casal, Rebeca Cruz, António M. Peres, Paula Baptista, and Nuno Rodrigues. 2023. "Influence of Interannual Climate Conditions on the Composition of Olive Oil from Centenarian Olive Trees" Agronomy 13, no. 12: 2884. https://doi.org/10.3390/agronomy13122884
APA StyleBortoluzzi, L., Casal, S., Cruz, R., Peres, A. M., Baptista, P., & Rodrigues, N. (2023). Influence of Interannual Climate Conditions on the Composition of Olive Oil from Centenarian Olive Trees. Agronomy, 13(12), 2884. https://doi.org/10.3390/agronomy13122884















