Comparative Assessment of Nutritional Metabolites in Yellow Soybeans at Different Growth Years and Their Antioxidant and α-Glucosidase Inhibition Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Crop Materials and Chemicals
2.2. Equipment
2.3. Evaluation of TPC and TFC
2.4. Evaluation of Fatty Acid Contents
2.5. Evaluation of Amino Acid Contents
2.6. Establishment of Calibration Curves and HPLC Conditions for Isoflavone Quantification
2.7. Antioxidant Properties Based on Radical and FRAP Assays
2.8. Determination of α-Glucosidase Inhibitory Activities
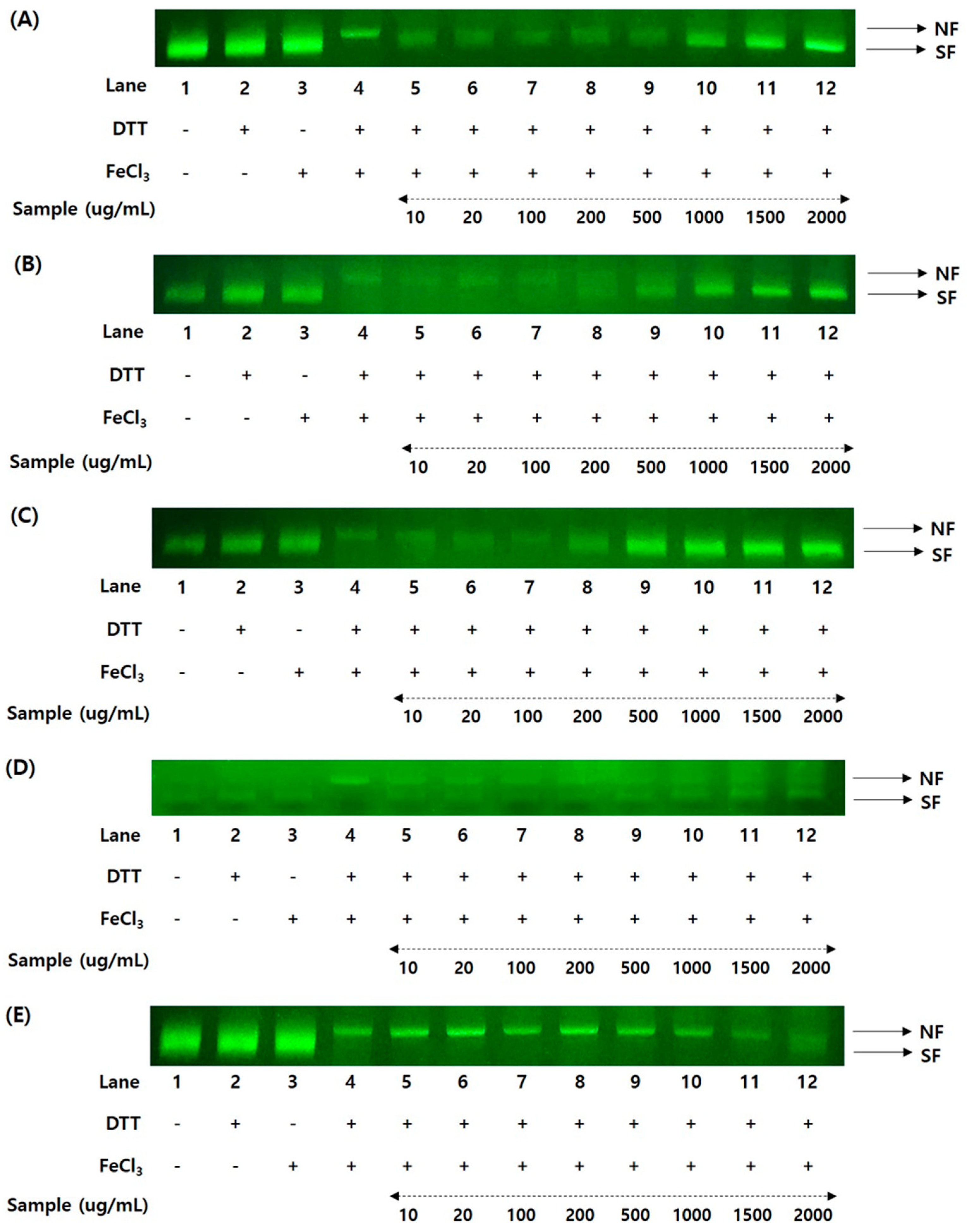
2.9. DNA Damage Protection Rate
2.10. Statistical Analysis
3. Results and Discussion
3.1. Comparisons between TPC and TFC in Yellow Soybeans across Three Different Growth Years
3.2. Comparison of Fatty Acid Contents in Yellow Soybeans across Three Different Growth Years
3.3. Comparison of Amino Acid Contents in Yellow Soybeans across Three Different Growth Years
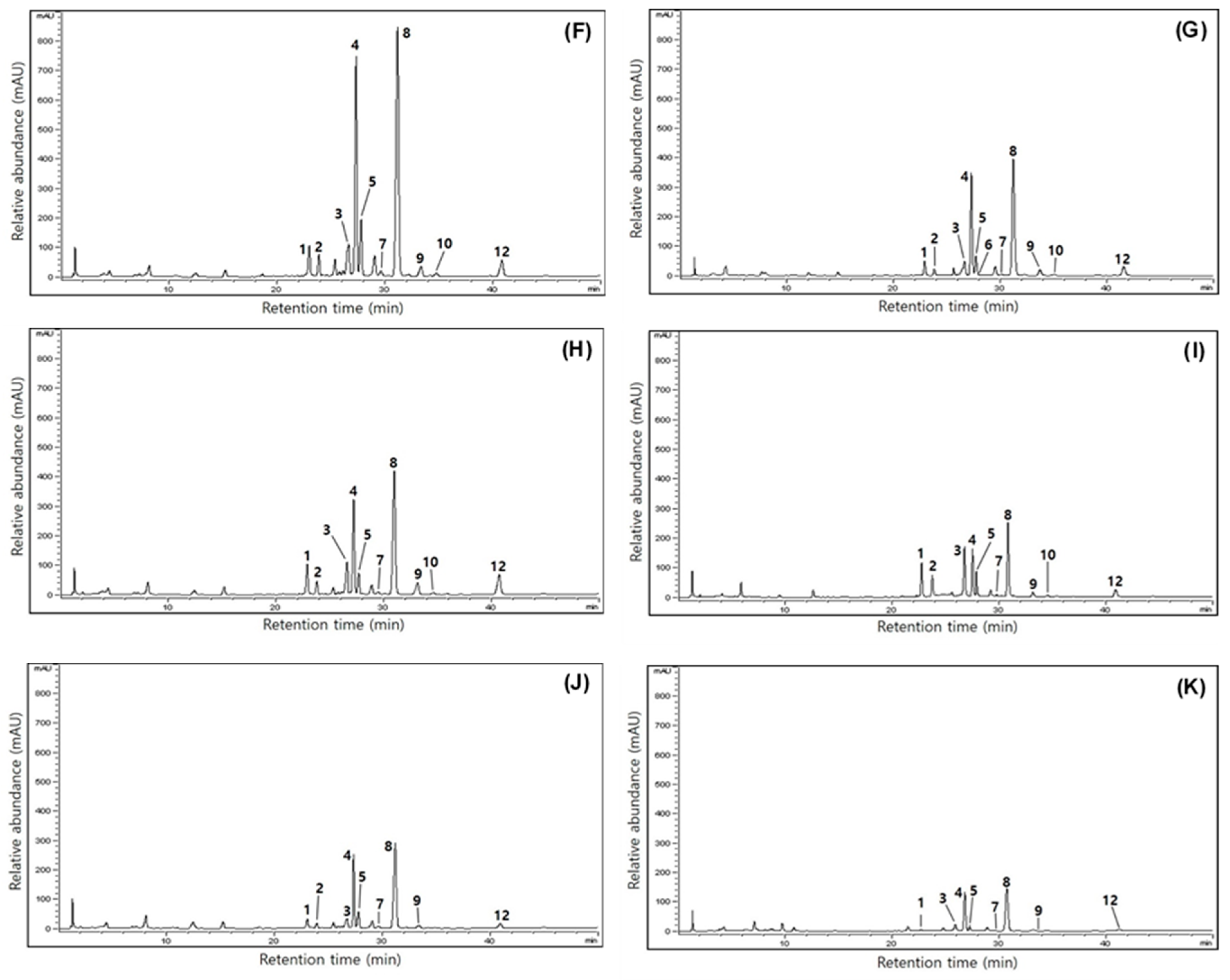
3.4. Comparison of Isoflavone Contents in Yellow Soybeans across Three Different Growth Years
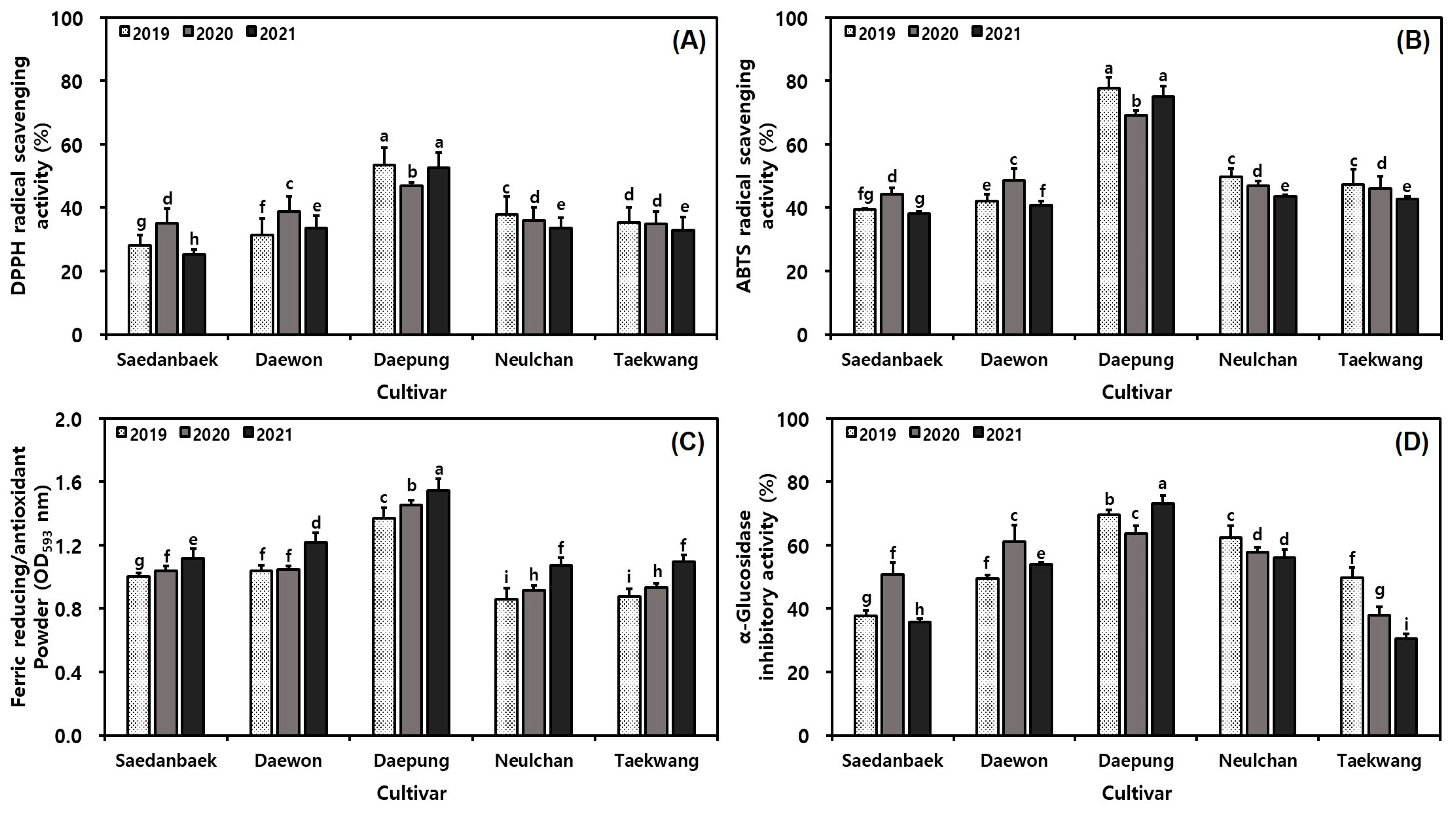
3.5. Variation of Antioxidant Capacities in Yellow Soybeans over Three Different Growth Years
3.6. Variation of α-Glucosidase Inhibitory Abilities in Yellow Soybeans over Three Different Growth Years
3.7. Variation of DNA Protection Rates in Yellow Soybeans during Three Different Growth Years
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lee, K.S.; Woo, S.Y.; Lee, M.J.; Kim, H.Y.; Ham, H.; Lee, D.J.; Choi, S.W.; Seo, W.D. Isoflavones and soyasaponins in the germ of Korean soybean [Glycine max (L.) Merr.] cultivars and their compound-enhanced BMP-2-induced bone formation. Appl. Biol. Chem. 2020, 63, 26. [Google Scholar] [CrossRef]
- Hwang, C.E.; Kim, S.C.; Kim, D.H.; Lee, H.Y.; Suh, H.K.; Cho, K.M.; Lee, J.H. Enhancement of isoflavone aglycone, amino acid, and CLA contents in fermented soybean yogurts using different strains: Screening of antioxidant and digestive enzyme inhibition properties. Food Chem. 2021, 340, 128199. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Hang, J.; Neufeld, J.; Zhao, S.; House, J.D. Estimation of crude protein and amino acid contents in whole, ground and defatted ground soybeans by different types of near-infrared (NIR) reflectance spectroscopy. J. Food Compost. Anal. 2022, 111, 104601. [Google Scholar] [CrossRef]
- Ito, C.; Oki, T.; Yoshida, T.; Nanba, F.; Yamada, K.; Toda, T. Characterisation of proanthocyanidins from black soybeans: Isolation and characterisastion of proanthocyanidin oligomers from black soybean seed coats. Food Chem. 2013, 141, 2507–2512. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Seo, E.Y.; Lee, Y.M. Comparative investigation on variations of nutritional components in whole seeds and seed coats of Korean black soybeans for different crop years and screening of their antioxidant and anti-aging properties. Food Chem. X 2023, 17, 100572. [Google Scholar] [CrossRef] [PubMed]
- Widowati, W.; Prahasturis, S.; Hidayat, M.; Hasianna, S.T.; Wahyudianingsih, R.; Eltania, T.F.; Azizah, A.M.; Aviani, J.K.; Subangkit, M.; Handayani, R.A.S.; et al. Detam 1 black soybean against cisptatin-induced acute ren failure on rat model via antioxidant, anti-inflammatory and antiapoptosis potential. J. Tradit. Complement. Med. 2022, 12, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Paucar-Menacho, L.; Amaya-Farfan, J.; Berhow, M.A.; Mandarino, J.M.G.; de Mejia, E.G.; Chang, Y.K. A high-protein soybean cultivar contains lower isoflavones and saponins but higher minerals and bioactive peptides than a low-protein cultivar. Food Chem. 2010, 120, 15–21. [Google Scholar] [CrossRef]
- Woyann, G.; Meira, D.; Zdziarski, A.D.; Matei, G.; Milioli, S.; Rosa, A.C.; Madella, L.A.; Benin, G. Multiple-trait selection of soybean for biodiesel production in Brazil. Ind. Crop Prod. 2019, 140, 111721. [Google Scholar] [CrossRef]
- Benavides, P.T.; Cai, H.; Wang, M.; Bajjalieh, N. Life-cycle analysis of soybean meal, distiller-dried grains with solubles, and synthetic amino acid-based animal feeds for swine and poultry production. Anim. Feed Sci. Technol. 2020, 268, 114607. [Google Scholar] [CrossRef]
- Ademiluyi, A.O.; Oboh, G. Soybean phenolic-rich extracts inhibit key-enzymes linked to type 2 diabetes (α-amylase and α-glucosidase) and hypertension (angiotensin I converting enzyme) in vitro. Exp. Toxicol. Pathol. 2013, 65, 305–309. [Google Scholar] [CrossRef]
- Bai, Y.; Xu, Y.; Wang, B.; Li, S.; Guo, F.; Hua, H.; Zhao, Y.; Yu, Z. Comparison of phenolic compounds, antioxidant and antidiabetic activities between selected edible beans and their different growth periods leaves. J. Funct. Food. 2017, 35, 694–702. [Google Scholar] [CrossRef]
- Nagarajan, S. Mechanisms of anti-atherosclerotic functions of soy-based diets. J. Nutr. Biochem. 2010, 21, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, B.; Hwang, C.E.; Haque, M.A.; Kim, S.C.; Lee, C.S.; Kang, S.S.; Cho, K.M.; Lee, D.H. Changes in conjugated linoleic acid and isoflavone contents from fermented soymilks using Lactobacillus plantarum P1201 and screening for their digestive enzyme inhibition and antioxidant properties. J. Funct. Food. 2018, 43, 17–28. [Google Scholar] [CrossRef]
- Antony, M.S.; Clarkson, T.B.; Hughes, C.L.; Morgan, T.M.; Burke, G.L. Soybean isoflavones improve cardiovascular risk factors without affecting the reproductive system of peripubertal rhesus monkeys. J. Nutr. 1996, 126, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Waheed, S.; Hasmain, A.; Ahmad, A.; Tarar, O.M.; Yaqeen, Z.; Ali, T.M. Effect of botanical extracts on amino acid and fatty acid profile of meat. Braz. J. Poult. Sci. 2018, 20, 507–516. [Google Scholar] [CrossRef]
- Lu, Y.; Wu, Y.; Chen, X.; Yang, X.; Xiao, H. Water extract of shepherd’s purse prevents high-fructose induced-liver injury by regulating glucolipid metabolism and gut microbiota. Food Chem. 2021, 342, 128536. [Google Scholar] [CrossRef] [PubMed]
- Modgil, R.; Tanwar, B.; Goyal, A.; Kumar, V. Soybean (Glycine max). In Oilseeds: Health Attributes and Food Applications; Tanwar, B., Goyal, A., Eds.; Springer: Singapore, 2021. [Google Scholar]
- Hwang, H.S.; Winkler-Moser, J.K. Antioxidant activity of amino acids in soybean oil at frying temperature: Structural effects and synergism with tocopherols. Food Chem. 2017, 221, 1168–1177. [Google Scholar] [CrossRef]
- Dajanta, K.; Apichartsrangkoon, A.; Chukeatirote, E.; Frazier, R.A. Free-amino acid profiles of thua nao a Thai fermented soybean. Food Chem. 2011, 125, 342–347. [Google Scholar] [CrossRef]
- Desta, K.T.; Hur, O.S.; Lee, S.; Yoon, H.; Shin, M.J.; Yi, J.; Lee, Y.; Ro, N.Y.; Wang, X.; Choi, Y.M. Origin and seed color differently affect the concentrations of metabolites and antioxidant activities in soybean (Glycine max (L.) Merill) seeds. Food Chem. 2020, 381, 132249. [Google Scholar] [CrossRef]
- Kumar, V.; Rani, A.; Dixit, A.K.; Pratap, D.; Bhatnagar, D. A comparative assessment of total phenolic content, ferric reducing-anti-oxidative power, free radical-scavenging activity, vitamin C and isoflavones content in soybean with varying seed coat colour. Food Res. Int. 2010, 43, 323–328. [Google Scholar] [CrossRef]
- Yu, X.; Meenu, M.; Xu, B.; Yu, H. Impact of processing technologies on isoflavones, phenolic acids, and antioxidant-capacities of soymilk prepared from 15 varieties. Food Chem. 2021, 345, 128612. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Jo, H.; Lee, J.D. Combining a Mutant Allele of FAD2-1A with HD improves the ω-6/ω-3 ratio in soybeans. Agronomy 2023, 13, 913. [Google Scholar] [CrossRef]
- Rakotonirina, F.M.; Donno, D.; Razafindrakoto, Z.R.; Tombozara, N.; Rafanomezantsoa, R.M.; Andrianjara, C.; Ramanitrahasimbola, D.; Beccaro, G.L. Quali-quantitative fingerprinting of the fruit extract of Uapaca bojeri Bail. (Euphorbiaceae) and its antioxidant, analgesic, anti-inflammatory, and antihyperglycemic effects: An example of biodiversity conservation and sustainable use of natural resources in Madagascar. Plants 2023, 12, 475. [Google Scholar] [PubMed]
- Song, S.; Kim, D.Y.; Oh, S.M.; Woo, S.Y.; Kim, I.; Kim, M.O.; Park, J.Y.; Kim, N.; Kim, H.Y.; Lee, J.; et al. Assessment of iridoid profles in the growth period of aerial parts of Pseudolysimachion rotundum var. subintegrum and their antioxidant and MUC5AC inhibitory potential. Appl. Biol. Chem. 2023, 66, 43. [Google Scholar] [CrossRef]
- Ng, Z.X.; Koick, Y.T.T.; Yong, P.H. Comparative analyses on radical scavenging and cytotoxic activity of phenolic and flavonoid content from selected medicinal plants. Nat. Prod. Res. 2021, 35, 5271–5276. [Google Scholar] [CrossRef]
- Salar, R.K.; Durewal, S.S.; Sandhu, K.S. Relationships between DNA damage protection activity, total phenolic content, condensed tannin content and antioxidant potential among Indian barley cultivars. Biocatal. Agric. Biotechnol. 2017, 11, 201–206. [Google Scholar] [CrossRef]
- Rodrigues, C.E.; Schäfer, L.; Gregolon, J.G.N.; de Oliveira, J.F.; Baez, O.P.; Deolindo, C.T.P.; de Melo, A.P.Z.; Singer, R.B.; Ledurkist, T.B.; Hoff, R. Determination of amino acid content, fatty acid profiles, and phenolic compounds in non-conventional edible fruits of seven species of palm trees (Arecaceae) native to the southern half of South America. Food Res. Int. 2022, 162, 111995. [Google Scholar] [CrossRef]
- Silva, L.R.; Pereira, M.J.; Azevedo, J.; Mulas, R.; Velazquez, E.; González-Andrés, F.; Valentão, P.; Andrade, P.B. Inoculation with Bradyrhizobium japonicum enhances the organic acid fatty acids content of soybean (Glycine max (L.) Merrill) seeds. Food Chem. 2013, 141, 3636–3648. [Google Scholar] [CrossRef]
- Abdelghany, A.M.; Zhang, S.; Azam, M.; Shaibu, A.S.; Feng, Y.; Li, Y.; Tian, Y.; Hong, H.; Li, B.; Sun, J. Profiling of seed fatty acid composition in 1025 Chinese soybean accessions from diverse ecoregions. Crop J. 2020, 8, 635–644. [Google Scholar] [CrossRef]
- Brechenmacher, L.; Kim, M.; Benitez, M.; Li, M.; Joshi, T.; Calla, B.; Lee, M.P.; Libault, M.; Vodkin, L.O.; Xu, D.; et al. Transcription profiling of soybean nodulation by Bradyrhizobium japonicum. Mol. Plant Microbe Interact. 2008, 21, 631–645. [Google Scholar] [CrossRef]
- Wu, S.Q.; Wang, Y.X.; Beta, T.; Wang, S.Y.; Mendez-Zamora, G.; Laborda, P.; Herrera-Balandrano, D.D. Effect of exogenous melatonin on the isoflavone content and antioxidant properties of soybean sprout. LWT-Food Sci. Technol. 2023, 175, 114498. [Google Scholar] [CrossRef]
- Kim, M.J.; Kwak, H.S.; Kim, S.S. Effects of salinity on bacterial communities, Maillard reactions, isoflavone composition, antioxidation and antiproliferation in Korean fermented soybean paste (doenjang). Food Chem. 2018, 245, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.F.; Zhang, F.X.; Zhang, M.W.; Wei, Z.C.; Yang, C.Y.; Zhang, Y.; Tang, X.J.; Deng, Y.Y.; Chi, J.W. Phenolic composition and antioxidant activity in seed coats of 60 Chinese black soybean (Glycine Max L. Merr.) varieties. J. Agric. Food Chem. 2011, 59, 5935–5944. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.A.; Pacia, N.L. Stress-induced phenylpropanoid metabolism. Plant Cell 1995, 7, 1085–1097. [Google Scholar] [CrossRef] [PubMed]
- Floegel, A.; Kim, D.O.; Chung, S.J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Liu, W.T.; Huang, C.L.; Liu, R.; Yang, T.C.; Lee, C.L.; Tsao, R.; Yang, W.J. Changes in isoflavone profile, antioxidant activity, and phenolic contents in Taiwanese and Canadian soybeans during tempeh processing. LWT—Food Sci. Technol. 2023, 186, 115207. [Google Scholar] [CrossRef]
- Dravie, E.E.; Kortei, N.K.; Essuman, E.K.; Tettey, C.O.; Boakye, A.A.; Hunkpe, G. Antioxidant phytochemical and physicochemical properties of sesame seed (Sesamum indicum L). Sci. Afr. 2020, 8, e00349. [Google Scholar] [CrossRef]
- Farias, T.C.; Abreu, J.P.; Oliveira, J.P.S.; Macedo, A.F.; Rodríguez-Vega, A.; Tonin, A.P.; Cardoso, F.S.N.; Meurer, E.C.; Koblitz, M.G.B. Bioactive properties of peptide fractions from Brazilian soy protein hydrolysates: In silico evaluation and experimental evidence. Food Hydrocoll. Health 2023, 3, 100112. [Google Scholar] [CrossRef]
- Chen, O.; Wang, X.; Yuan, X.; Shi, J.; Zhang, C.; Yan, N.; Jing, C. Comparison of phenolic and flavonoid compound profiles and antioxidant and α-glucosidase inhibition properties of cultivated soybean (Glycine max) and wild soybean (Glycine soja). Plants 2021, 10, 813. [Google Scholar] [CrossRef]
- Sunagar, R.R.; Sreerama, Y.N. Implication of solvent polarities on browntop millet (Urochloa ramose) phenolic antioxidants and their ability to protect oxidative DNA damage and inhibit α-amylase and α-glucosidase enzymes. Food Chem. 2023, 411, 135474. [Google Scholar] [CrossRef]
- Sevgi, K.; Tepe, B.; Sarikurkcu, C. Antioxidant and DNA damage protection potentials of selected phenolic acids. Food Chem. Toxicol. 2015, 77, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Fiorda, F.A.; de Melo Pereira, G.V.; Thomaz-Soccol, V.; Medeiros, A.P.; Rakshit, S.K.; Soccol, C.R.; Medeiros, A.P.; Rakshit, S.K.; Ricardo, C. Development of kefir-based probiotic beverages with DNA protection and antioxidant activities using soybean hydrolyzed extract, colostrum and honey. LWT—Food Sci. Technol. 2016, 68, 690–697. [Google Scholar] [CrossRef]


| Cultivars | Growth Years | Fatty Acid Contents (mg/g) 1 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Saturated Fatty Acids 2 | Unsaturated Fatty Acids | Total | ||||||||||||||
| C14:0 | C16:0 | C18:0 | C20:0 | C22:0 | Total | C16:1 | C18:1n9c | C18:2n6c | C18:3n3 | C20:1 | C20:2 | C24:1 | Total | |||
| Saedanbaek | 2019 | 0.1 ± 0.0 a | 9.6 ± 0.2 d | 2.7 ± 0.1 d | 0.3 ± 0.0 a | 0.5 ± 0.0 a | 13.2 | 0.1 ± 0.0 a | 19.2 ± 0.4 d | 42.7 ± 0.9 e | 7.5 ± 0.2 b | 0.3 ± 0.0 b | 0.1 ± 0.0 a | tr | 69.9 | 83.1 |
| 2020 | 0.1 ± 0.0 a | 6.7 ± 0.1 h | 2.1 ± 0.0 ef | tr 3 | 0.3 ± 0.0 c | 9.2 | tr | 10.1 ± 0.2 h | 30.1 ± 0.6 f | 5.9 ± 0.1 f | 0.1 ± 0.0 c | tr | tr | 46.2 | 55.4 | |
| 2021 | 0.1 ± 0.0 a | 7.9 ± 0.2 g | 2.1 ± 0.0 ef | 0.2 ± 0.0 b | tr | 10.3 | tr | 10.3 ± 0.2 h | 24.9 ± 0.5 h | 4.2 ± 0.1 h | tr | tr | tr | 39.4 | 49.7 | |
| Daewon | 2019 | 0.1 ± 0.0 a | 10.1 ± 0.2 c | 3.1 ± 0.1 c | 0.3 ± 0.0 a | 0.4 ± 0.0 b | 14.0 | 0.1 ± 0.0 a | 20.4 ± 0.4 c | 54.6 ± 1.1 b | 7.4 ± 0.1 c | 0.3 ± 0.0 b | tr | tr | 82.8 | 96.8 |
| 2020 | 0.1 ± 0.0 a | 8.5 ± 0.2 ef | 2.7 ± 0.1 d | tr | 0.1 ± 0.0 d | 11.4 | 0.1 ± 0.0 a | 14.2 ± 0.3 f | 45.0 ± 0.9 d | 7.3 ± 0.1 c | 0.1 ± 0.0 c | 0.1 ± 0.0 a | tr | 66.8 | 78.2 | |
| 2021 | 0.1 ± 0.0 a | 8.3 ± 0.2 f | 2.2 ± 0.0 e | 0.2 ± 0.0 b | tr | 10.8 | 0.1 ± 0.0 a | 14.3 ± 0.3 f | 25.1 ± 0.5 gh | 4.2 ± 0.1 h | tr | tr | tr | 43.7 | 54.5 | |
| Daepung | 2019 | 0.1 ± 0.0 a | 10.7 ± 0.2 b | 3.6 ± 0.1 b | 0.3 ± 0.0 a | 0.3 ± 0.0 c | 15.0 | 0.1 ± 0.0 a | 16.6 ± 0.3 e | 61.2 ± 1.2 a | 8.2 ± 0.2 a | 0.3 ± 0.0 b | 0.1 ± 0.0 a | tr | 86.5 | 101.5 |
| 2020 | 0.1 ± 0.0 a | 9.5 ± 0.2 d | 3.5 ± 0.1 b | 0.2 ± 0.0 b | 0.1 ± 0.0 d | 13.4 | 0.1 ± 0.0 a | 14.2 ± 0.3 | 49.0 ± 1.0 c | 6.9 ± 0.1 d | 0.1 ± 0.0 c | tr | 0.1 ± 0.0 a | 70.4 | 83.8 | |
| 2021 | 0.1 ± 0.0 a | 9.0 ± 0.2 e | 2.2 ± 0.0 e | 0.2 ± 0.0 b | tr | 11.5 | tr | 17.2 ± 0.3 e | 23.4 ± 0.5 i | 5.0 ± 0.1 g | tr | tr | tr | 45.6 | 57.1 | |
| Neulchan | 2019 | 0.1 ± 0.0 a | 11.2 ± 0.2 a | 3.9 ± 0.1 a | 0.3 ± 0.0 a | 0.4 ± 0.0 b | 15.9 | 0.1 ± 0.0 a | 24.9 ± 0.5 b | 61.9 ± 1.2 a | 7.3 ± 0.1 c | 0.3 ± 0.0 b | 0.1 ± 0.0 a | tr | 94.6 | 110.5 |
| 2020 | 0.1 ± 0.0 a | 8.9 ± 0.2 e | 3.0 ± 0.1 c | tr | 0.4 ± 0.0 b | 12.4 | 0.1 ± 0.0 a | 14.4 ± 0.5 f | 44.7 ± 0.9 de | 6.7 ± 0.1 d | 0.1 ± 0.0 c | 0.1 ± 0.0 a | 0.1 ± 0.0 a | 66.2 | 78.6 | |
| 2021 | 0.1 ± 0.0 a | 8.1 ± 0.2 f | 2.3 ± 0.0 e | 0.2 ± 0.0 b | tr | 10.7 | 0.1 ± 0.0 a | 12.6 ± 0.3 g | 26.9 ± 0.5 g | 3.9 ± 0.1 i | tr | tr | tr | 43.5 | 54.2 | |
| Taekwang | 2019 | 0.1 ± 0.0 a | 9.7 ± 0.2 cd | 3.5 ± 0.1 b | 0.3 ± 0.0 a | 0.4 ± 0.0 b | 14.0 | 0.1 ± 0.0 a | 32.7 ± 0.7 a | 43.3 ± 0.9 e | 6.4 ± 0.1 e | 0.4 ± 0.0 a | tr | tr | 82.9 | 96.9 |
| 2020 | tr | 4.7 ± 0.1 i | 2.0 ± 0.0 f | tr | 0.1 ± 0.0 d | 6.8 | tr | 8.8 ± 0.2 i | 26.8 ± 0.5 g | 3.9 ± 0.1 i | 0.1 ± 0.0 c | tr | 0.1 ± 0.0 a | 39.7 | 46.5 | |
| 2021 | 0.1 ± 0.0 a | 7.6 ± 0.2 g | 2.1 ± 0.0 ef | 0.2 ± 0.0 b | tr | 10.0 | 0.1 ± 0.0 a | 18.4 ± 0.4 d | 21.6 ± 0.4 j | 3.4 ± 0.1 j | tr | tr | tr | 43.5 | 53.5 | |
| Average | 0.1 | 8.7 | 2.7 | 0.2 | 0.2 | 11.9 | 0.1 | 16.6 | 38.7 | 5.9 | 0.1 | 0.0 | 0.0 | 61.4 | 73.3 | |
| Cultivars | Growth Years | Amino Acid Contents (mg/100 g) 1 | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-Essential Amino Acids | Essential Amino Acids | Total | |||||||||||||||||||
| Asp | Ser | Glu | Gly | Ala | Cys | Tyr | Arg | Pro | Total | Thr | Val | Met | Iso | Leu | Phe | Lys | His | Total | |||
| Saedanbaek | 2019 | 35.3 ± 0.7 d | 14.4 ± 0.3 e | 60.1 ± 1.2 e | 13.1 ± 0.3 d | 13.3 e ± 0.3 | 4.2 ± 0.1 d | 11.0 ± 0.2 e | 25.3 ± 0.5 d | 15.6 ± 0.3 d | 192.3 | 11.7 ± 0.2 e | 14.1 ± 0.3 d | 4.2 ± 0.1 c | 14.5 ± 0.3 e | 24.9 ± 0.5 e | 16.7 ± 0.3 d | 18.9 ± 0.4 e | 8.6 ± 0.2 f | 113.6 | 305.9 |
| 2020 | 31.0 ± 0.6 e | 11.4 ± 0.2 g | 51.2 ± 1.0 f | 11.7 ± 0.2 f | 12.4 ± 0.2 f | 3.3 ± 0.1 f | 8.1 ± 0.2 h | 23.9 ± 0.5 e | 14.3 ± 0.3 e | 167.3 | 10.2 ± 0.2 f | 14.2 ± 0.3 d | 3.4 ± 0.1 f | 14.0 ± 0.3 e | 22.5 ± 0.5 f | 15.3 ± 0.3 e | 15.3 ± 0.3 f | 7.6 ± 0.2 g | 102.5 | 269.8 | |
| 2021 | 54.1 ± 1.1 b | 24.7 ± 0.5 b | 90.0 ± 1.8 b | 21.0 ± 0.4 b | 22.0 ± 0.4 b | 6.6 ± 0.1 b | 15.9 ± 0.3 b | 34.9 ± 0.7 b | 20.6 ± 0.4 b | 289.8 | 19.3 ± 0.4 h | 22.0 ± 0.4 b | 4.3 ± 0.1 c | 22.8 ± 0.5 d | 39.7 ± 0.8 b | 26.0 ± 0.5 b | 32.0 ± 0.6 b | 13.5 ± 0.3 c | 179.6 | 469.4 | |
| Daewon | 2019 | 26.0 ± 0.5 g | 11.1 ± 0.2 g | 42.8 ± 0.9 i | 10.1 ± 0.2 g | 10.7 ± 0.2 i | 3.5 ± 0.1 ef | 8.1 ± 0.2 h | 17.5 ± 0.4 h | 12.0 ± 0.2 g | 141.8 | 9.4 ± 0.2 g | 10.4 ± 0.2 g | 2.8 ± 0.1 h | 10.9 ± 0.2 i | 18.9 ± 0.4 h | 12.7 ± 0.3 h | 15.0 ± 0.3 f | 6.3 ± 0.1 j | 86.4 | 228.2 |
| 2020 | 29.2 ± 0.6 e | 11.9 ± 0.2 f | 49.2 ± 1.0 g | 11.3 ± 0.2 e | 11.5 ± 0.2 g | 3.6 ± 0.1 e | 7.3 ± 0.1 i | 19.9 ± 0.4 f | 12.9 ± 0.3 f | 156.8 | 10.1 ± 0.2 f | 12.8 ± 0.2 e | 3.7 ± 0.1 e | 12.8 ± 0.3 f | 21.0 ± 0.4 g | 14.2 ± 0.3 f | 14.6 ± 0.3 g | 7.2 ± 0.1 h | 96.4 | 253.2 | |
| 2021 | 50.0 ± 1.0 c | 22.5 ± 0.5 d | 81.8 ± 1.6 d | 19.1 ± 0.4 c | 19.9 ± 0.4 d | 6.5 ± 0.1 b c | 13.6 ± 0.3 d | 32.6 ± 0.7 c | 19.3 ± 0.4 c | 265.3 | 17.7 ± 0.2 d | 20.2 ± 0.4 c | 4.2 ± 0.1 c | 20.7 ± 0.4 d | 35.4 ± 0.7 d | 23.8 ± 0.3 c | 28.5 ± 0.6 d | 12.2 ± 0.2 e | 162.7 | 428.0 | |
| Daepung | 2019 | 27.0 ± 0.5 f | 11.0 ± 0.2 g | 44.1 ± 0.9 h | 10.7 ± 0.2 f | 11.2 ± 0.2 h | 3.7 ± 0.1 e | 9.5 ± 0.2 f | 17.7 ± 0.4 h | 12.6 ± 0.3 fg | 147.5 | 9.7 ± 0.4 g | 11.6 ± 0.2 f | 3.9 ± 0.1 d | 12.1 ± 0.2 g | 20.6 ± 0.4 gh | 13.4 ± 0.5 g | 15.4 ± 0.3 f | 7.1 ± 0.1 h | 93.8 | 241.3 |
| 2020 | 27.2 ± 0.5 f | 10.6 ± 0.2 h | 45.1 ± 0.9 h | 10.6 ± 0.2 f | 11.0 ± 0.2 h | 3.0 ± 0.1 g | 7.4 ± 0.1 i | 18.9 ± 0.4 g | 12.5 ± 0.3 fg | 146.3 | 9.4 ± 0.2 g | 12.5 ± 0.2 e | 3.4 ± 0.1 f | 12.6 ± 0.3 f | 20.5 ± 0.4 gh | 13.5 ± 0.3 g | 14.0 ± 0.3 g | 7.0 ± 0.1 h | 92.9 | 239.2 | |
| 2021 | 70.1 ± 1.4 a | 29.7 ± 0.6 a | 113.3 ± 2.3 a | 24.9 ± 0.5 a | 25.4 ± 0.4 a | 7.5 ± 0.2 a | 18.6 ± 0.4 a | 54.9 ± 1.1 a | 25.3 ± 0.5 a | 369.7 | 22.3 ± 0.2 a | 25.9 ± 0.4 a | 5.5 ± 0.1 a | 26.7 ± 0.5 a | 46.1 ± 0.9 a | 31.4 ± 0.6 a | 36.7 ± 0.7 a | 16.7 ± 0.3 b | 211.3 | 581.0 | |
| Neulchan | 2019 | 22.0 ± 0.4 h | 8.6 ± 0.2 k | 35.5 ± 0.7 jk | 8.6 ± 0.2 h | 9.7 ± 0.2 j | 3.0 ± 0.1 g | 8.5 ± 0.2 g | 14.8 ± 0.3 i | 10.9 ± 0.2 h | 121.6 | 8.0 ± 0.4 i | 10.0 ± 0.2 h | 3.1 ± 0.1 g | 10.4 ± 0.2 j | 17.6 ± 0.4 i | 11.6 ± 0.2 i | 13.1 ± 0.3 h | 6.0 ± 1.2 a | 79.8 | 201.4 |
| 2020 | 25.4 ± 0.5 g | 9.8 ± 0.2 i | 41.5 ± 0.8 | 10.0 ± 0.2 g | 11.1 ± 0.2 h | 2.6 ± 0.1 h | 7.1 ± 0.1 j | 18.3 ± 0.4 g | 12.3 ± 0.2 g | 138.1 | 8.9 ± 0.2 h | 12.2 ± 0.2 f | 3.4 ± 0.1 f | 12.2 ± 0.2 g | 19.8 ± 0.4 h | 13.2 ± 0.3 g | 13.5 ± 0.3 h | 6.6 ± 0.1 i | 89.8 | 227.9 | |
| 2021 | 51.5 ± 1.0 c | 23.5 ± 0.5 c | 85.4 ± 1.7 c | 19.5 ± 0.4 c | 20.2 ± 0.4 cd | 6.6 ± 0.1 b | 14.3 ± 0.3 c | 33.4 ± 0.7 bc | 19.8 ± 0.4 bc | 274.2 | 17.9 ± 0.4 d | 20.8 ± 0.4 c | 4.3 ± 0.1 c | 21.7 ± 0.4 c | 37.5 ± 0.8 c | 24.6 ± 0.5 c | 29.3 ± 0.6 d | 12.7 ± 0.3 d | 168.8 | 443.0 | |
| Taekwang | 2019 | 23.8 ± 0.5 g | 9.1 ± 0.2 j | 37.0 ± 0.7 j | 9.9 ± 0.2 g | 11.7 ± 0.2 g | 3.5 ± 0.1 ef | 9.4 ± 0.2 f | 17.8 ± 0.4 h | 12.5 ± 0.3 fg | 134.7 | 9.0 ± 0.2 h | 11.7 ± 0.2 f | 4.0 ± 0.1 d | 11.9 ± 0.2 h | 19.6 ± 0.4 h | 13.3 ± 0.3 g | 15.0 ± 0.3 fg | 6.8 ± 0.1 | 91.3 | 226.0 |
| 2020 | 21.4 ± 0.4 i | 8.5 ± 0.2 k | 34.3 ± 0.7 k | 8.9 ± 0.2 h | 9.7 ± 0.2 j | 2.3 ± 0.1 i | 5.7 ± 0.1 k | 15.3 ± 0.3 i | 10.7 ± 0.2 h | 116.8 | 7.8 ± 0.1 i | 10.8 ± 0.2 g | 2.8 ± 0.1 h | 10.7 ± 0.2 i | 16.9 ± 0.3 i | 11.6 ± 0.2 i | 12.0 ± 0.2 i | 5.9 ± 0.1 k | 78.5 | 195.3 | |
| 2021 | 51.3 ± 1.0 c | 23.7 ± 0.5 c | 87.8 ± 1.8 bc | 20.2 ± 0.4 b | 21.0 ± 0.4 c | 6.3 ± 0.1 c | 14.5 ± 0.3 c | 34.2 ± 0.7 b | 20.1 ± 0.4 b | 279.1 | 18.4 ± 0.2 c | 21.4 ± 0.4 b | 4.7 ± 0.1 b | 22.0 ± 0.4 b | 37.5 ± 0.8 c | 25.2 ± 0.5 b | 30.3 ± 0.6 b | 12.9 ± 0.3 d | 172.4 | 451.5 | |
| Average | 36.4 | 15.4 | 59.9 | 14.0 | 14.7 | 4.4 | 10.6 | 25.3 | 15.4 | 196.1 | 12.7 | 15.4 | 3.8 | 15.7 | 26.6 | 17.8 | 20.2 | 12.7 | 124.9 | 321.0 | |
| Cultivars | Growth Years | Isoflavone Contents (μg/g) 1 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glucoside | Malonylglucoside | Acetylglucoside | Aglycone | Total | ||||||||||
| Din (1) | Gly (2) | Gin (3) | MDin (4) | MGly (5) | MGin (8) | AcDin (6) | AcGly (7) | AcGin (11) | Dein (9) | Glein (10) | Gein (12) | |||
| Saedanbaek | 2019 | 145.6 ± 2.9 f | 136.6 ± 2.7 f | 119.1 ± 2.3 f | 332.0 ± 6.6 m | 105.0 ± 2.1 j | 455.8 ± 9.1 l | 9.6 ± 0.1 a | 2.2 ±0.0 k | nd 2 | 15.1 ± 0.3 j | 2.9 ± 0.1 h | 18.7 ± 0.3 i | 1342.6 |
| 2020 | 70.4 ± 1.4 l | 87.5 ± 1.7 i | 22.6 ± 0.45 n | 703.1 ± 14.0 g | 112.8 ± 2.2 i | 930.2 ± 20.6 h | 1.9 ± 0.0 b | 3.2 ± 0.1 f | nd | 20.2 ± 0.3 h | nd | 20.9 ± 0.4 h | 1972.8 | |
| 2021 | 89.6 ± 1.7 i | 89.9 ± 1.8 i | 43.7 ± 0.8 m | 431.5 ± 8.6 k | 89.5 ± 1.7 e | 554.1± 11.0 k | tr 3 | nd | nd | 14.1 ± 0.2 k | 5.0 ± 0.1 g | 15.5 ± 0.3 j | 1332.9 | |
| Daewon | 2019 | 117.4 ± 2.3 h | 297.2 ± 5.9 d | 227.8 ± 4.5 d | 379.9 ± 7.6 l | 166.1 ± 3.3 g | 724.0 ± 14.4 i | nd | 3.2 ± 0.1 f | nd | 16.9 ± 0.3 i | 1.6 ± 0.0 i | 35.6 ± 0.7 f | 1969.8 |
| 2020 | 208.6 ± 4.1 d | 109.6 ± 2.1 g | 75.3± 1.51 i | 953.2 ± 19.0 c | 246.6 ± 4.9 c | 1366.2± 27.3 d | nd | 3.5 ± 0.1 e | nd | 31.9 ± 0.6 g | 17.8 ± 0.4 d | 65.1 ± 1.3 d | 3077.8 | |
| 2021 | 82.9 ± 1.6 j | 112.2 ± 2.2 g | 44.6 ± 0.8 k | 736.9 ± 14.7 f | 150.0 ± 3.0 h | 1142.9 ± 22.8 f | tr | 3.7 ± 0.1 b | nd | 19.4 ± 0.3 h | 9.7 ± 0.2 f | 26.6 ± 1.1 g | 2328.9 | |
| Daepung | 2019 | 470.0 ± 9.4 a | 449.7 ± 8.9 b | 500.1 ± 10.0 a | 892.1 ± 17.8 d | 272.6 ± 5.4 b | 1408.9 ± 28.1 c | tr | 8.0 ± 0.2 d | nd | 55.5 ± 1.1 c | 25.0 ± 0.5 b | 99.3 ± 1.9 b | 4181.2 |
| 2020 | 172.1 ± 3.4 e | 181.6 ± 3.6 e | 123.3± 2.47 f | 1115.8 ± 22.3 b | 204.9 ± 4.1 e | 1572.7 ± 31.4 b | tr | 4.7 ± 0.1 a | nd | 42.5 ± 0.8 d | 19.9± 0.4 c | 78.1 ± 1.5 c | 3515.6 | |
| 2021 | 285.0 ± 5.7 c | 319.3 ± 6.3 c | 214.1± 4.2 e | 1303.3 ± 26.0 a | 349.1 ± 6.9 a | 1778.9 ± 35.5 a | nd | 11.4 ± 0.2 a | nd | 62.2 ± 1.2 b | 30.6 ± 0.6 a | 94.1 ± 2.8 b | 4448.0 | |
| Neulchan | 2019 | 317.2± 6.3 b | 310.9 ± 6.2 c | 299.4 ± 5.9 c | 848.7 ± 16.9 e | 264.6 ± 5.2 b | 1022.8 ± 20.4 g | nd | 5.7 ± 0.1 c | nd | 68.5 ± 1.3 a | 20.5 ± 0.4 c | 111.5 ± 2.2 a | 3269.8 |
| 2020 | 133.0 ± 2.6 g | 97.7 ± 1.9 h | 89.3 ± 1.7 g | 965.9 ± 19.3 c | 141.4 ± 4.8 h | 1233.3 ± 24.6 e | nd | 2.6 ± 0.1 i | nd | 34.7 ± 0.6 f | nd | 34.0 ± 0.7 f | 2731.9 | |
| 2021 | 285.4 ± 5.7 c | 492.1 ± 9.8 a | 374.4 ± 7.4 b | 550.9 ± 11.0 i | 231.7 ± 4.6 d | 461.3 ± 9.2 l | nd | 2.7 ± 0.1 i | nd | 37.4 ± 0.7 e | 15.6 ± 0.3 e | 61.7 ± 1.2 e | 2513.2 | |
| Taekwang | 2019 | 73.6 ± 1.4 k | 88.7 ± 1.7 i | 78.8 ± 1.5 h | 676.3± 13.5 h | 177.9 ± 3.5 f | 985.8 ± 25.7 h | nd | 4.3 ± 0.1 d | nd | 12.7 ± 0.2 l | nd | 24.2 ± 0.9 g | 2122.3 |
| 2020 | 90.0 ± 1.8 i | 80.5± 1.6 j | 66.9± 1.3 j | 462.3 ± 9.2 j | 80.9 ± 1.6 m | 632.1 ± 12.6 j | tr | 2.3 ± 0.1 j | nd | 12.4 ± 0.2 l | nd | 12.9 ± 0.2 k | 1440.3 | |
| 2021 | 19.4 ± 0.3 m | 63.5 ± 1.2 k | 35.9 ± 0.7 l | 334.0 ± 6.6 m | 99.2 ± 1.9 k | 619.5 ± 12.3 j | nd | 2.9 ± 0.1h | nd | 7.8 ± 0.2 m | nd | 10.2 ± 0.2 l | 1192.4 | |
| Average | 170.7 | 194.5 | 154.4 | 712.4 | 179.5 | 992.6 | 0.8 | 4.0 | nd | 30.1 | 9.9 | 47.2 | 2496.0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, K.M.; Lee, Y.M.; Lee, H.Y.; Lee, J.H. Comparative Assessment of Nutritional Metabolites in Yellow Soybeans at Different Growth Years and Their Antioxidant and α-Glucosidase Inhibition Properties. Agronomy 2023, 13, 2914. https://doi.org/10.3390/agronomy13122914
Cho KM, Lee YM, Lee HY, Lee JH. Comparative Assessment of Nutritional Metabolites in Yellow Soybeans at Different Growth Years and Their Antioxidant and α-Glucosidase Inhibition Properties. Agronomy. 2023; 13(12):2914. https://doi.org/10.3390/agronomy13122914
Chicago/Turabian StyleCho, Kye Man, Young Min Lee, Hee Yul Lee, and Jin Hwan Lee. 2023. "Comparative Assessment of Nutritional Metabolites in Yellow Soybeans at Different Growth Years and Their Antioxidant and α-Glucosidase Inhibition Properties" Agronomy 13, no. 12: 2914. https://doi.org/10.3390/agronomy13122914





