Bacterial Inoculation and Extracts of Opuntia Rackets or Marine Algae Trigger Distinct Proline Balances in Tomato Salt Stress Alleviation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biological Materials
2.2. Greenhouse Assay
2.3. High-Performance Liquid Chromatography
2.4. Statistical Analysis
3. Results
3.1. Germination Percentage (GP)
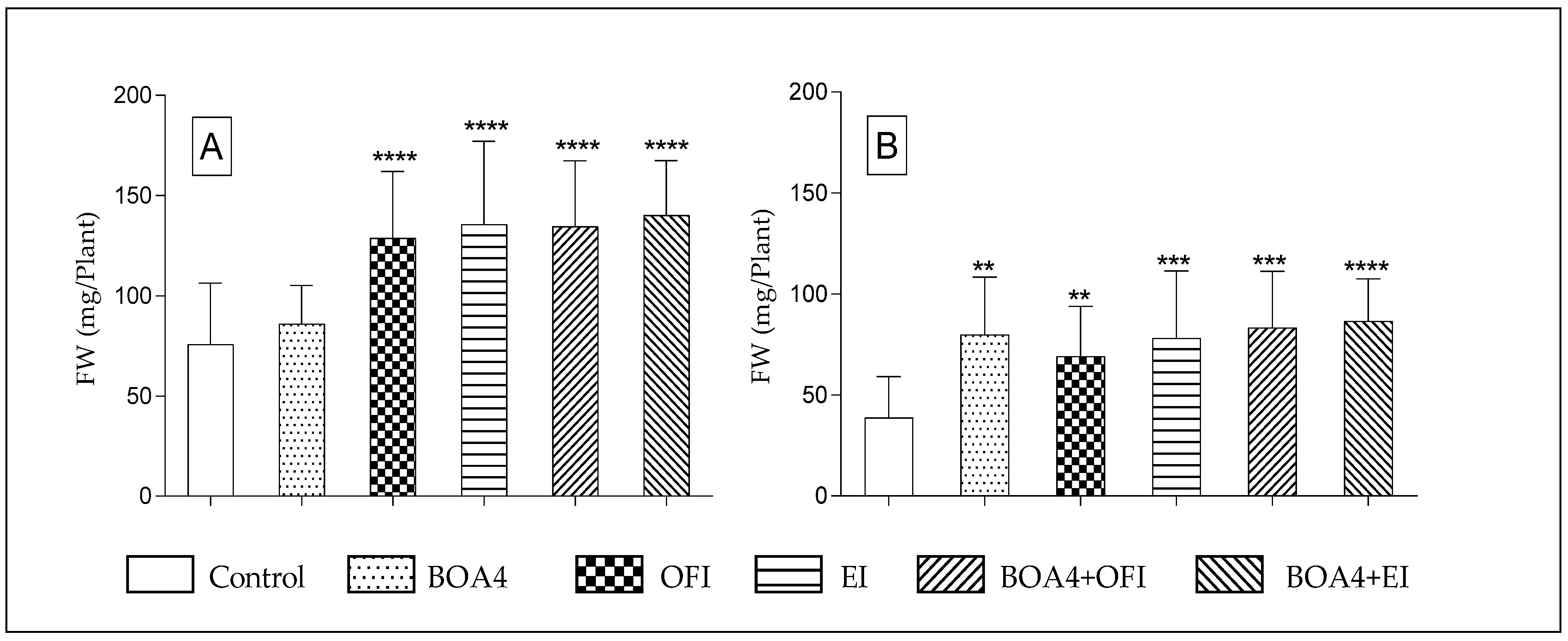
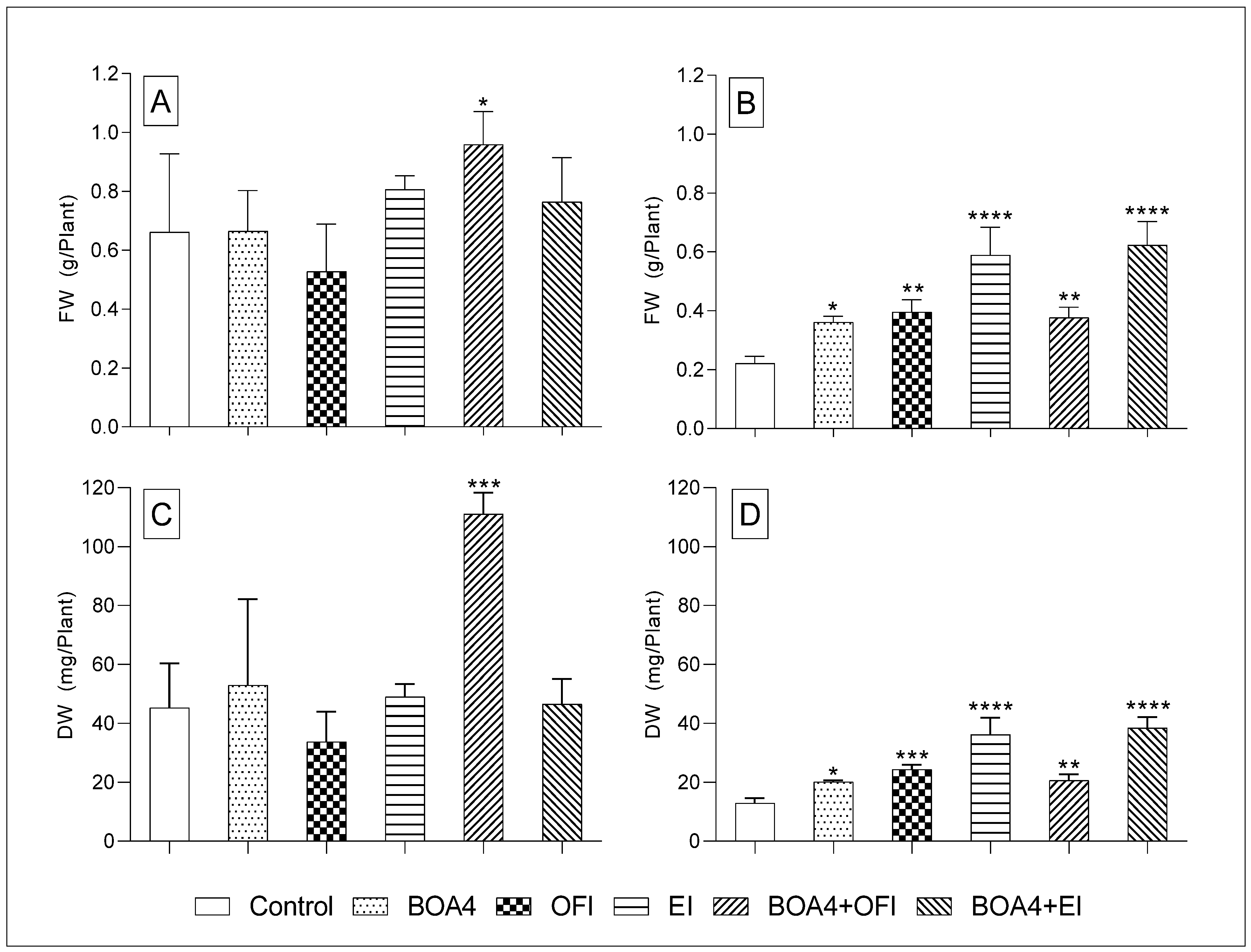
3.2. Plant’s Fresh Weight (FW) and Dry Weight (DW)
3.2.1. Thirty Days after Sowing
3.2.2. Forty-Six Days after Sowing
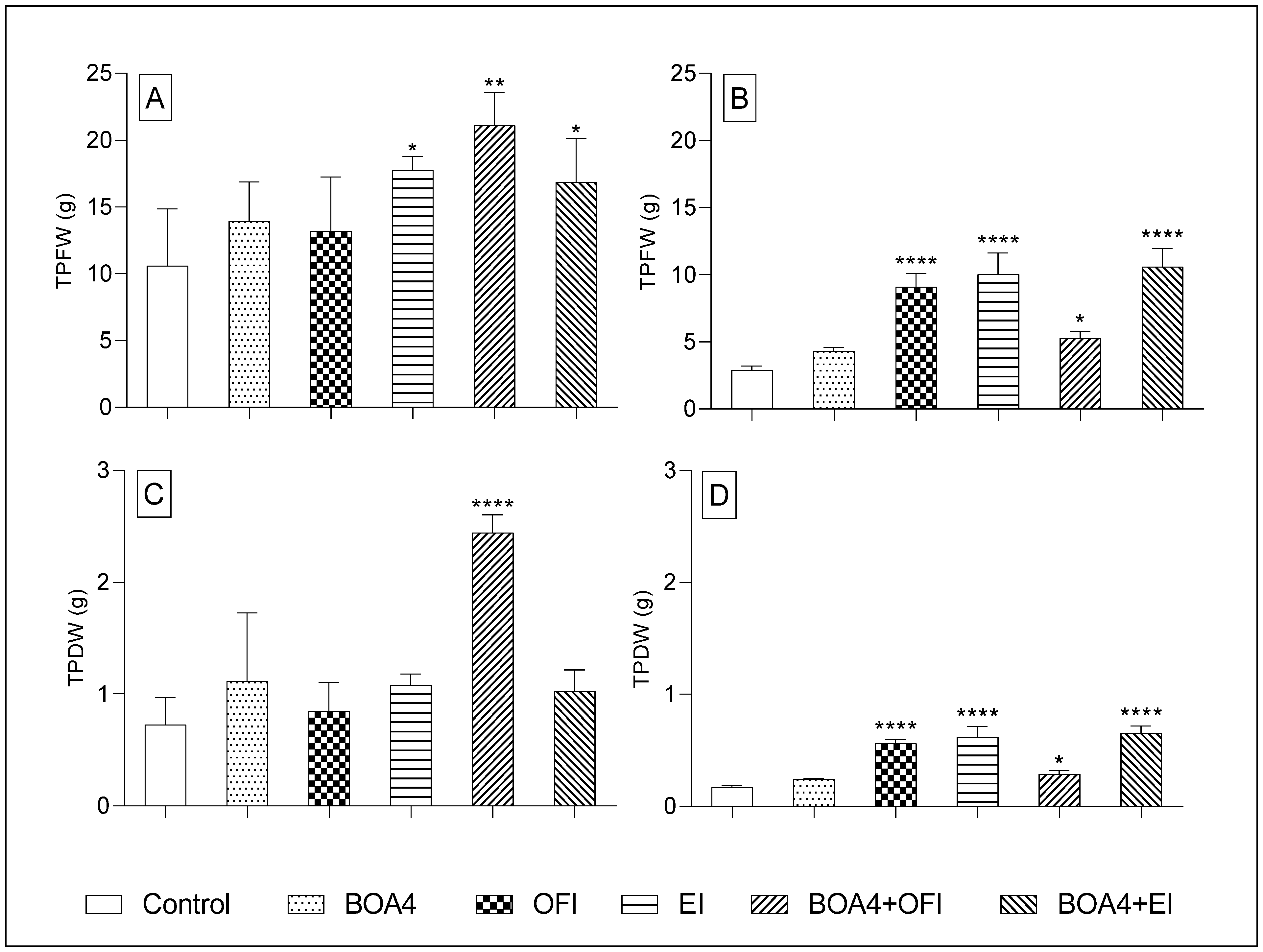
3.3. Estimated Productivity
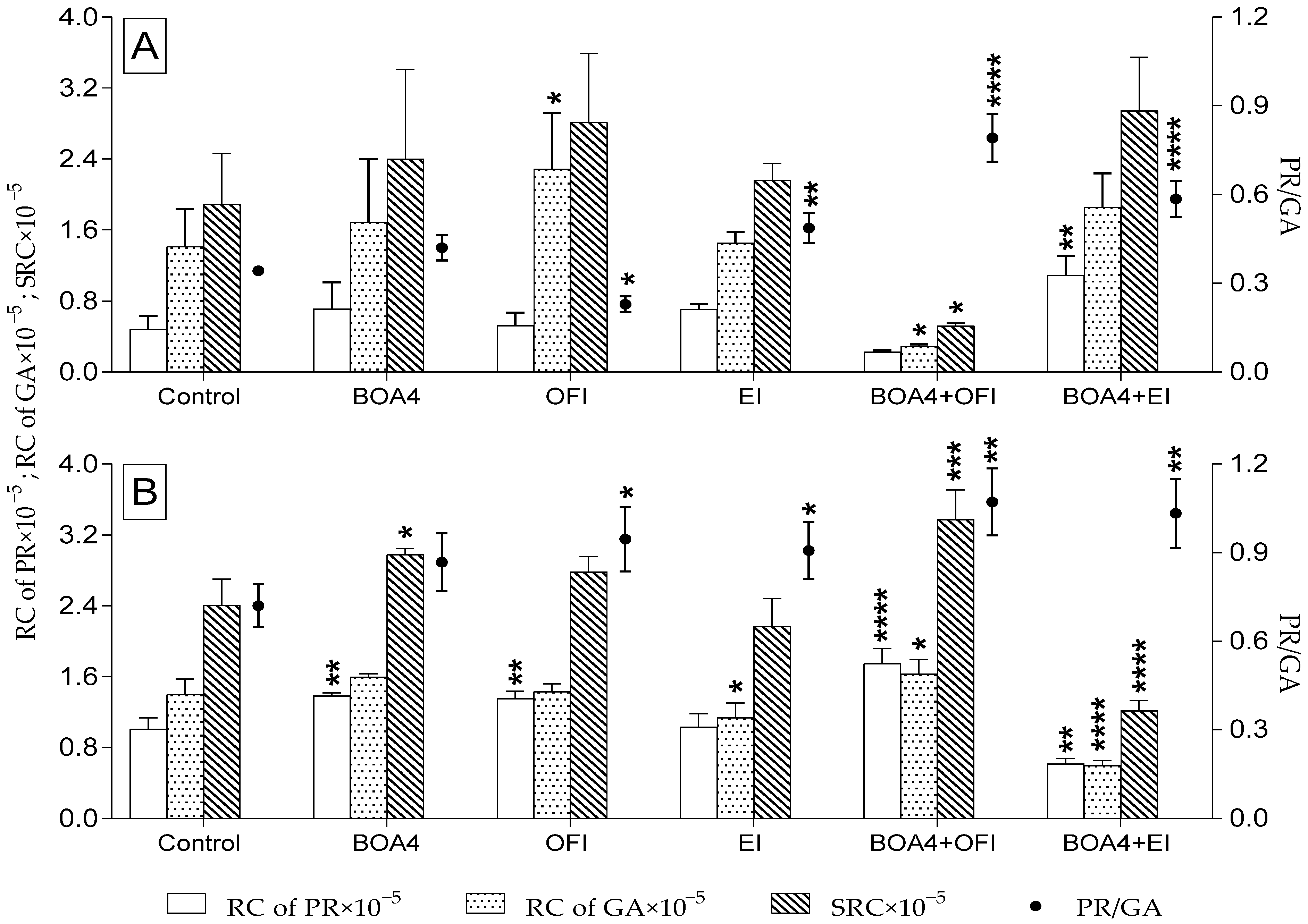
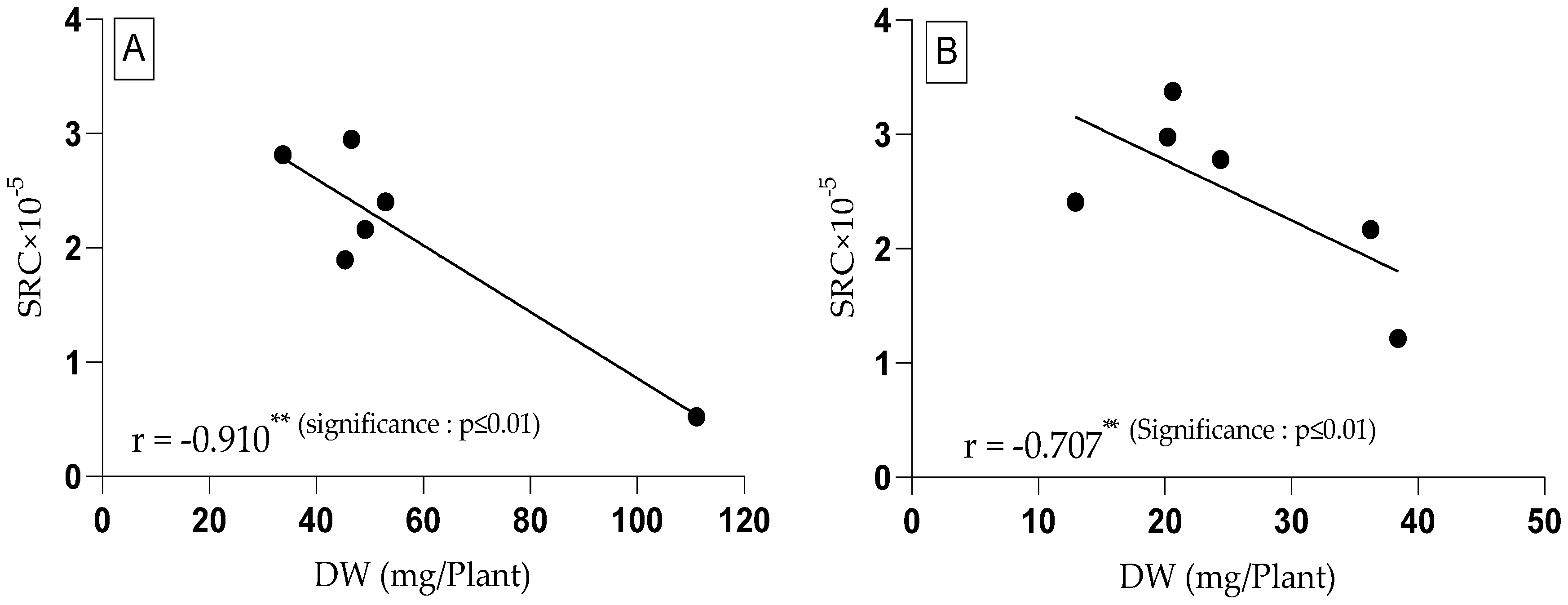
3.4. Relative Proline and Glutamic Acid Content in Tomato Plants
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hussain, S.; Shaukat, M.; Ashraf, M.; Zhu, C.; Jin, Q.; Zhang, J. Salinity Stress in Arid and Semi-Arid Climates: Effects and Management in Field Crops. Climate Change and Agriculture; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Devkota, K.P.; Devkota, M.; Rezaei, M.; Oosterbaan, R. Managing salinity for sustainable agricultural production in salt-affected soils of irrigated drylands. Agric. Syst. 2022, 198, 103390. [Google Scholar] [CrossRef]
- Machado, R.M.A.; Serralheiro, R.P. Soil Salinity: Effect on Vegetable Crop Growth. Management Practices to Prevent and Mitigate Soil Salinization. Horticulturae 2017, 3, 30. [Google Scholar] [CrossRef]
- Kumar, P.; Sharma, P.K. Soil salinity and food security in India. Front. Sustain. Food Syst. 2020, 4, 533781. [Google Scholar] [CrossRef]
- Ashraf, M.; Ahmad, M.S.A.; Öztürk, M.; Aksoy, A. Crop Improvement through different means: Challenges and prospects. In Crop Production for Agricultural Improvement; Ashraf, M., Öztürk, M., Ahmad, M., Aksoy, A., Eds.; Springer Science+Business Media B.V.: Dordrecht, The Netherlands, 2012; pp. 1–15. [Google Scholar]
- Nabti, E.; Sahnoune, M.; Ghoul, M.; Fischer, D.; Hoffmann, A.; Rothballer, M.; Schmid, M.; Hartman, A. Restoration of growth of Durum wheat (Triticum durum var. Waha) under saline conditions due to inoculation with the rhizosphere bacterium Azospirillum brasilense NH and extracts of the marine alga Ulva lactuca. J. Plant Growth Regul. 2010, 29, 6–22. [Google Scholar] [CrossRef]
- Yancey, P.H. Organic osmolytes as compatible, metabolic, and counteracting cytoprotectants in high osmolarity and other stresses. J. Experim. Biol. 2005, 208, 2819–2830. [Google Scholar] [CrossRef] [PubMed]
- Smiatek, J.; Harishchandra, R.K.; Rubner, O.; Galla, H.J.; Heuer, A. Properties of compatible solutes in aqueous solution. Biophys. Chem. 2012, 160, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Prescott, L.M. Microbiology, 5th ed.; The McGraw−Hill Companies: New York, NY, USA, 2002. [Google Scholar]
- Oren, A. Organic compatible solutes. In Cellular Origin and Life in Extreme Habitats, Volume 5: Halophilic Microorganisms and Their Environments; Oren, A., Ed.; Kluwer Academic: Dordrecht, The Netherlands, 2003; pp. 279–305. [Google Scholar]
- Botsford, J.L.; Lewis, T.A. Osmoregulation in Rhizobium meliloti: Production of glutamic acid in response to osmotic stress. Appl. Environ. Microbiol. 1990, 56, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Courtenay, E.S.; Capp, M.W.; Anderson, C.F.; Record, J.M.T. Vapor pressure osmometry studies of osmolyte-protein interactions: Implications for the action of osmoprotectants in vivo and for the interpretation of “osmotic stress” experiments in vitro. Biochemistry 2000, 39, 4455–4471. [Google Scholar] [CrossRef]
- Nemati, I.; Moradi, F.; Gholizadeh, S.; Esmaeili, M.A.; Bihamta, M.R. The effect of salinity stress on ions and soluble sugars distribution in leaves, leaf sheaths, and roots of rice (Oryza sativa L.) seedlings. Plant Soil Environ. 2011, 57, 26–33. [Google Scholar] [CrossRef]
- Aziz, A.; Martin-Tanguy, J.; Larher, F. Salt stress-induced proline accumulation and changes in tyramine polyamine levels are linked to ionic adjustment in tomato leaf discs. Plant Sci. 1999, 145, 83–91. [Google Scholar] [CrossRef]
- Rai, V.K. Role of amino acids in plant responses to salt stresses. Biol. Plant. 2002, 45, 481–487. [Google Scholar] [CrossRef]
- Amini, F.; Ehsanpour, A.A. Soluble proteins, proline, carbohydrates, and Na+/K+ changes in two tomato (Lycopersicon esculentum Mill.) cultivars under in vitro salt stress. Am. J. Biochem. Biotech. 2005, 1, 212–216. [Google Scholar] [CrossRef]
- Verslues, P.E.; Agarwal, M.; Katiyar-Agarwal, S.; Zhu, J.; Zhu, J.K. Methods and concepts in quantifying resistance to drought, salt, and freezing, abiotic stresses that affect plant water status. Plant J. 2006, 45, 523–539. [Google Scholar] [CrossRef] [PubMed]
- Verslues, P.E.; Sharma, S. Proline metabolism and its implications for plant-environment interaction. Arab. Book 2010, 8, e0140e. [Google Scholar] [CrossRef] [PubMed]
- Smirnoff, N.; Cumbes, Q.J. Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry 1989, 28, 1057–1060. [Google Scholar] [CrossRef]
- Signorelli, S.; Dans, P.D.; Coitiño, E.L.; Borsani, O.; Monza, J. Connecting proline and γ-aminobutyric acid in stressed plants through non-enzymatic reactions. PLoS ONE 2015, 10, e0115349. [Google Scholar] [CrossRef]
- Hare, P.D.; Cress, W.A.; Van Staden, J. Dissecting the roles of osmolyte accumulation during stress. Plant Cell Environ. 1998, 21, 535–553. [Google Scholar] [CrossRef]
- Hare, P.D.; Cress, W.A.; Van Staden, J. Proline synthesis and degradation: A model system for elucidating stress-related signal transduction. J. Experim. Bot. 1999, 50, 413–434. [Google Scholar] [CrossRef]
- Sharma, S.; Verslues, P.E. Mechanisms independent of abscisic acid (ABA) or proline feedback have a predominant role in transcriptional regulation of proline metabolism during low water potential and stress recovery. Plant Cell Environ. 2010, 33, 1838–1851. [Google Scholar] [CrossRef]
- Miller, G.; Stein, H.; Honig, A.; Kapulnik, Y.; Zilberstein, A. Responsive modes of Medicago sativa proline dehydrogenase genes during salt stress and recovery dictate free proline accumulation. Planta 2005, 222, 70–79. [Google Scholar] [CrossRef]
- Skopelitis, D.S.; Paranychianakis, N.V.; Paschalidis, K.A.; Pliakonis, E.D.; Delis, I.D.; Yakoumakis, D.I.; Roubelakis-Angelakis, K.A. Abiotic stress generates ROS that signal expression of anionic glutamate dehydrogenases to form glutamate for proline synthesis in tobacco and grapevine. Plant Cell 2006, 18, 2767–2781. [Google Scholar] [CrossRef]
- La, V.H.; Lee, B.-R.; Islam, M.T.; Mamun, M.A.; Park, S.-H.; Bae, D.-W.; Kim, T.-H. Characterization of Glutamate-Mediated Hormonal Regulatory Pathway of the Drought Responses in Relation to Proline Metabolism in Brassica napus L. Plants 2020, 9, 512. [Google Scholar] [CrossRef]
- Kempf, B.; Bremer, E. Uptake and synthesis of compatible solutes as microbial stress responses to high-osmolality environments. Arch. Microbiol. 1998, 170, 319–330. [Google Scholar] [CrossRef]
- Sadok, T.H.; Fatiha, A.; Bellal, M.; Abdul Hussain, M.S. Composition chimique des jeunes cladodes d’Opuntia ficus-indica et possibilités de valorisation alimentaire. JASP 2008, 1, 39–48. [Google Scholar]
- Sáenz, C.; Berger, H.; Félix, A.R.; Galletti, L.; García, J.C.; Sepúlveda, E.; Varnero, M.T.; de Cortázar, V.G.; García, R.C.; Arias, E.; et al. Agro-Industrial Utilization of Cactus Pear; FAO: Rome, Italy, 2013. [Google Scholar]
- Mondragón-Jacobo, C.; Pérez-González, S. Cactus (Opuntia spp.) as Forage. FAO, Plant Production and Protection Paper 169. 2015. Available online: http://www.fao.org/docrep/005/Y2808E/Y2808E00.HTM (accessed on 1 January 2023).
- Edwards, D.M.; Reed, R.H.; Stewart, W.D.P. Osmoacclimation in Enteromorpha intestinalis: Long-Term Effects of Osmotic Stress on organic solute accumulation. Marine Biol. 1988, 98, 467–476. [Google Scholar] [CrossRef]
- Qurashi, A.W.; Sabri, A.N. Osmoadaptation and plant growth promotion by salt-tolerant bacteria under salt stress. Afr. J. Microbiol. Res. 2011, 5, 3546–3554. [Google Scholar]
- Kim, K.; Jang, Y.J.; Lee, S.M.; Oh, B.T.; Chae, J.C.; Lee, K.J. Alleviation of salt stress by Enterobacter sp. EJ01 in Tomato and Arabidopsis is accompanied by up-regulation of conserved salinity responsive factors in plants. Mol. Cells 2014, 37, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Jana, G.A.; Yaish, M.W. Genome analysis of a salinity adapted Achromobacter xylosoxidans rhizobacteria from the date palm. Rhizosphere 2021, 19, 100401. [Google Scholar] [CrossRef]
- Mayak, S.; Tirosh, T.; Glick, B.R. Plant growth-promoting bacteria confer resistance in tomato plants to salt stress. Plant Physiol. Biochem. 2004, 42, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.; Cherif, A.; Cristina, C.; Nabti, E. Extracts from seaweeds and Opuntia ficus-indica cladodes enhance diazotrophic-PGPR halotolerance, their enzymatic potential, and their impact on wheat germination under salt stress. Pedosphere 2018, 28, 241–254. [Google Scholar] [CrossRef]
- Santana, M.M.; Rosa, A.P.; Zamarreño, A.M.; García-Mina, J.M.; Rai, A.; Cruz, C. Achromobacter xylosoxidans and Enteromorpha intestinalis Extract Improve Tomato Growth under Salt Stress. Agronomy 2022, 12, 934. [Google Scholar] [CrossRef]
- Singh, J.; Divakar Sastry, E.V.; Singh, V. Effect of salinity on tomato (Lycopersicon esculentum Mill.) during the seed germination stage. Physiol. Mol. Biol. Plants 2012, 18, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Appenroth, K.J.; Lenk, G.; Goldau, L.; Sharma, R. Tomato seed germination: Regulation of different response modes by phytochrome B2 and phytochrome A. Plant Cell Environ. 2006, 29, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Fabiani, A.; Versari, A.; Parpinello, G.P.; Castellari, M.; Galassi, S. High-performance liquid chromatographic analysis of free amino acids in fruit juices using derivatization with 9-fluorenylmethyl-chloroformate. J. Chromatogr. Sci. 2002, 40, 14–18. [Google Scholar] [CrossRef] [PubMed]
- El-Mostafa, K.; EL Kharrassi, Y.; Badreddine, A.; Andreoletti, P.; Vamecq, J.; El Kebbaj, M.S.; Malki, M.C. Nopal Cactus (Opuntia ficus-indica) as a source of bioactive compounds for nutrition, health, and disease. Molecules 2014, 19, 14879–14901. [Google Scholar] [CrossRef] [PubMed]
- Hayik, I. Cacti and Cacti Parts for Use as Pesticides, Fertilizers, and Regeneration and Growth Enhancers of Plants. WO2017033199A3, 12 March 2017. [Google Scholar]
- Shoukat, R.; Cappai, M.; Pia, G.; Pilia, L. An Updated Review: Opuntia ficus indica (OFI) Chemistry and Its Diverse Applications. Appl. Sci. 2023, 13, 7724. [Google Scholar] [CrossRef]
- Kirst, G.O. Osmotic adjustment in phytoplankton and macroalgae. The Use of Dimethylsulfoniopropionate (DMSP). In Biological and Environmental Chemistry of DMSP and Related Sulfonium Compounds; Kiene, R.P., Visscher, P.T., Keller, M.D., Kirst, G.O., Eds.; Plenum Press: New York, NY, USA, 1996; pp. 121–129. [Google Scholar]
- Haroon, A.M.; Szaniawska, A.; Normant, M.; Janas, U. The biochemical composition of Enteromorpha spp. from the Gulf of Gdańsk coast on the southern Baltic Sea. Oceanologia 2000, 42, 19–28. [Google Scholar]
- Ghoul, M.; Minet, J.; Bernard, T.; Dupray, E.; Cormier, M. Marine Macroalgae as a Source for Osmoprotection for Escherichia coli. Microb. Ecol. 1995, 30, 171–181. [Google Scholar] [CrossRef]
- Lewis, E.J.; Gonzalves, E.A. Amino acid contents of some marine algae from Bombay. New Phytol. 1960, 59, 109–115. [Google Scholar] [CrossRef]
- Schwachtje, J.; Karojet, S.; Thormählen, I.; Bernholz, C.; Kunz, S.; Brouwer, S.; Schwochow, M.; Köhl, K.; van Dongen, J.T. A naturally associated rhizobacterium of Arabidopsis thaliana induces a starvation-like transcriptional response while promoting growth. PLoS ONE 2011, 6, e29382. [Google Scholar] [CrossRef]
- Poupin, M.J.; Timmermann, T.; Vega, A.; Zuñiga, A.; González, B. Effects of the plant growth-promoting bacterium Burkholderia phytofirmans PsJN throughout the life cycle of Arabidopsis thaliana. PLoS ONE 2013, 8, e69435. [Google Scholar] [CrossRef]
- Mattioli, R.; Costantino, P.; Trovato, M. Proline accumulation in plants: Not only stress. Plant Signal. Behav. 2009, 4, 1016–1018. [Google Scholar] [CrossRef] [PubMed]
- Trovato, M.; Maras, B.; Linhares, F.; Costantino, P. The plant oncogene rolD encodes a functional ornithine cyclodeaminase. Proc. Nat. Acad. Sci. USA 2001, 298, 13449–13453. [Google Scholar] [CrossRef]
- Schwacke, R.; Grallath, S.; Breitkreuz, K.E.; Stransky, H.; Frommer, W.B.; Rentsch, D. LeProT1, a transporter for proline, glycine betaine, and γ-amino butyric acid in tomato pollen. Plant Cell 1999, 11, 377–391. [Google Scholar] [PubMed]
- Snowalter, A.M. Structure and function of plant cell wall proteins. Plant Cell 1993, 5, 9–23. [Google Scholar]
- Grallath, S.; Weimar, T.; Meyer, A.; Gumy, C.; Suter-Grotemeyer, M.; Neuhaus, J.M.; Rentsch, D. The AtProT family. Compatible solute transporters with similar substrate specificity but differential expression patterns. Plant Physiol. 2005, 137, 117–126. [Google Scholar] [PubMed]
- Tegeder, M. Transporters for amino acids in plant cells: Some functions and many unknowns. Curr. Opi. Plant Biol. 2012, 15, 315–321. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef]
- Waditee, R.; Hibino, T.; Tanaka, Y.; Nakamura, T.; Incharoensakdi, A.; Hayakawa, S.; Suzuki, S.; Futsuhara, Y.; Kawamitsu, Y.; Takabe, T. Functional characterization of betaine/proline transporters in betaine-accumulating mangrove. J. Biol. Chem. 2002, 277, 18373–18382. [Google Scholar] [CrossRef]
- Ueda, A.; Shi, W.M.; Sanmiya, K.; Shono, M.; Takabe, T. Functional analysis of salt-inducible proline transporter of barley roots. Plant Cell Physiol. 2001, 42, 1282–1289. [Google Scholar] [CrossRef]
- Widodo Patterson, J.H.; Newbigin, E.; Tester, M.; Bacic, A.; Roessner, U. Metabolic responses to salt stress of barley (Hordeum vulgare L.) cultivars, Sahara and Clipper, which differ in salinity tolerance. J. Experim. Bot. 2009, 60, 4089–4103. [Google Scholar] [CrossRef] [PubMed]
- Hayano-Kanashiro, C.; Calderon-Vazquez, C.; Ibarra-Laclette, E.; Herrera-Estrella, L.; Simpson, J. Analysis of gene expression and physiological responses in three Mexican maize landraces under drought stress and recovery irrigation. PLoS ONE 2009, 4, e7531. [Google Scholar] [CrossRef] [PubMed]
- Angus, S.; Dargie, T. The UK Machair habitat action plan: Progress and problems. Bot. J. Scotl. 2002, 54, 63–74. [Google Scholar] [CrossRef]
- MacArtain, P.; Gill, C.I.; Brooks, M.; Campbell, R.; Rowland, I.R. Nutritional value of edible seaweeds. Nutr. Rev. 2007, 65, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Escrig, A.; Sánchez-Muniz, F.J. Dietary fibre from edible seaweeds: Chemical structure, physicochemical properties and effects on cholesterol metabolism. Nutr. Res. 2000, 20, 585–598. [Google Scholar] [CrossRef]
- Jaulneau, V.; Lafitte, C.; Jacquet, C.; Fournier, S.; Salamagne, S.; Briand, X.; Esquerré-Tugayé, T.; Dumas, B. Ulvan, a sulfated polysaccharide from green algae, activates plant immunity through the jasmonic acid signaling pathway. BioMed Res. Int. 2010, 2010, 525291. [Google Scholar] [CrossRef] [PubMed]
- Dadolahi-Sohrab, A.; Nikvarz, A.; Nabavi, S.M.B.; Safahyeh, A.; Ketal-Mohseni, M. Environmental monitoring of heavy metals in seaweed and associated sediment from the Strait of Hormuz, IR Iran. WJFMS 2011, 3, 576–589. [Google Scholar]
- Brady, N.C.; Weil, R.R.; Weil, R.R. The Nature and Properties of Soils; Prentice Hall: Upper Saddle River, NJ, USA, 2008; Volume 13, pp. 662–710. [Google Scholar]
| Treatment * | In Absence of Salt Stress (%) | In Presence of Salt Stress (%) |
|---|---|---|
| Control | 64 | 52 |
| OFI | 100 | 92 |
| EI | 88 | 68 |
| BOA4 | 84 | 48 |
| BOA4 + OFI | 88 | 56 |
| BOA4 + EI | 88 | 68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rai, A.; Santana, M.M.; Maia, R.N.; Tavares, J.; Nabti, E.; Cruz, C. Bacterial Inoculation and Extracts of Opuntia Rackets or Marine Algae Trigger Distinct Proline Balances in Tomato Salt Stress Alleviation. Agronomy 2023, 13, 2921. https://doi.org/10.3390/agronomy13122921
Rai A, Santana MM, Maia RN, Tavares J, Nabti E, Cruz C. Bacterial Inoculation and Extracts of Opuntia Rackets or Marine Algae Trigger Distinct Proline Balances in Tomato Salt Stress Alleviation. Agronomy. 2023; 13(12):2921. https://doi.org/10.3390/agronomy13122921
Chicago/Turabian StyleRai, Abdelwahab, Margarida M. Santana, Rodrigo Nascimento Maia, João Tavares, Elhafid Nabti, and Cristina Cruz. 2023. "Bacterial Inoculation and Extracts of Opuntia Rackets or Marine Algae Trigger Distinct Proline Balances in Tomato Salt Stress Alleviation" Agronomy 13, no. 12: 2921. https://doi.org/10.3390/agronomy13122921
APA StyleRai, A., Santana, M. M., Maia, R. N., Tavares, J., Nabti, E., & Cruz, C. (2023). Bacterial Inoculation and Extracts of Opuntia Rackets or Marine Algae Trigger Distinct Proline Balances in Tomato Salt Stress Alleviation. Agronomy, 13(12), 2921. https://doi.org/10.3390/agronomy13122921









