Response of Abscission Zone of Blue Honeysuckle (Lonicera caerulea L.) Fruit to GA3 and 2,4-D Spray Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Experimental Treatments
2.2. Fruit Drop Evaluation
2.3. Preparation of Abscission Zone Paraffin Sections
2.4. Measurement of Abscission-Related Enzyme Activity in the Abscission Zone
2.5. Gene Expression Analysis
2.6. Data Analysis
3. Results
3.1. Effects of 2,4-D and GA3 Treatments on ‘Berel’ Fruit Drop Dynamics
3.2. Effects of 2,4-D and GA3 Treatments on Fruit Abscission Zone Formation
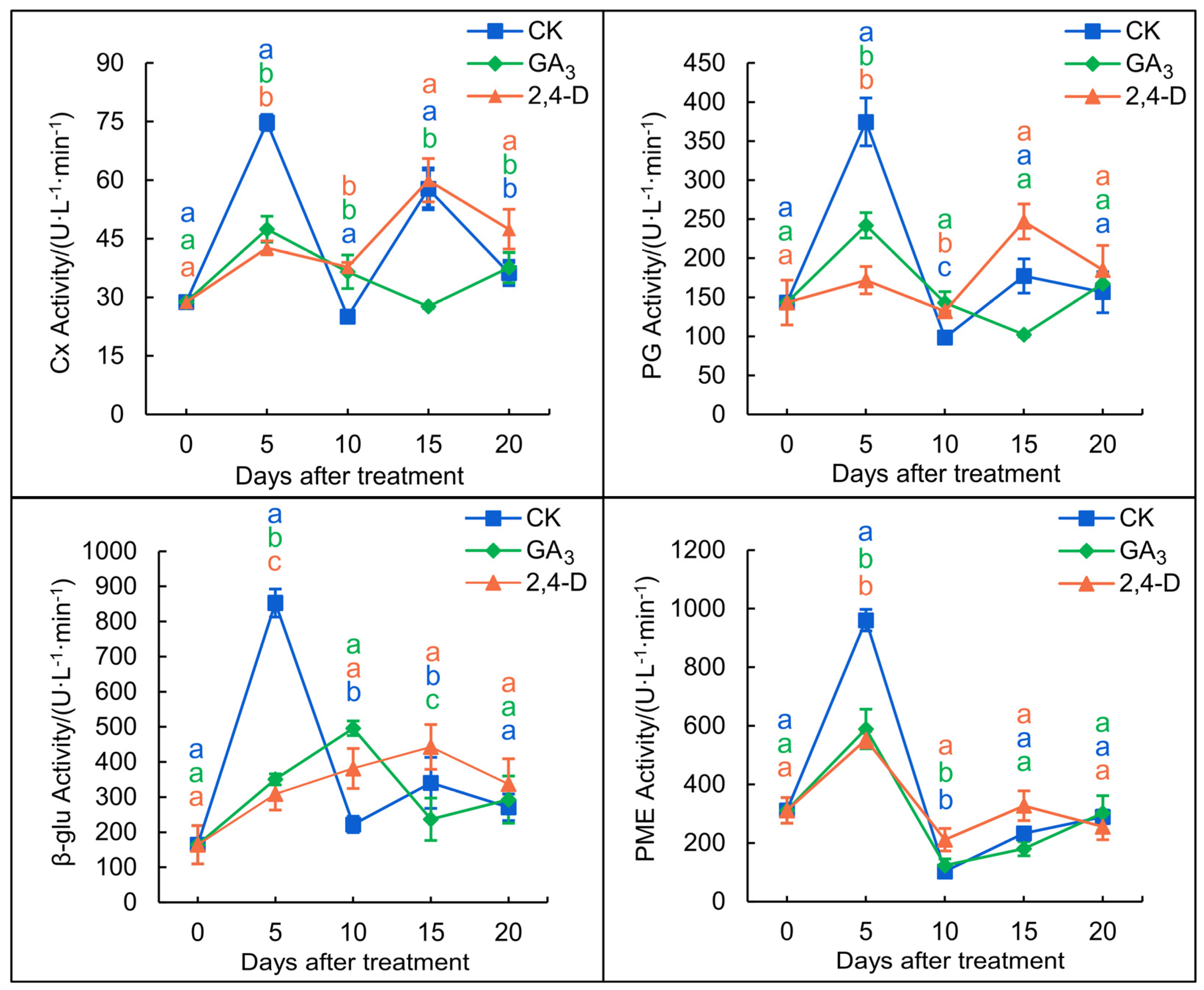
3.3. Effects of 2,4-D and GA3 Treatments on the Activity of Cellulolytic Enzymes in the Fruit Abscission Zone
3.3.1. Cx Activity
3.3.2. β–glu Activity
3.3.3. PG Activity
3.3.4. PME Activity
3.4. Effects of 2,4-D and GA3 Treatments on the Expression Levels of Abscission-Related Genes in the Fruit Abscission Zone
3.4.1. Effects of 2,4-D and GA3 Treatments on the Expression Levels of Cell Wall Degradation-Related Genes in the Fruit Abscission Zone
3.4.2. Effects of 2,4-D and GA3 Treatments on the Expression Levels of Ethylene and Gibberellins Key Synthesis-Related Genes in the Fruit Abscission Zone
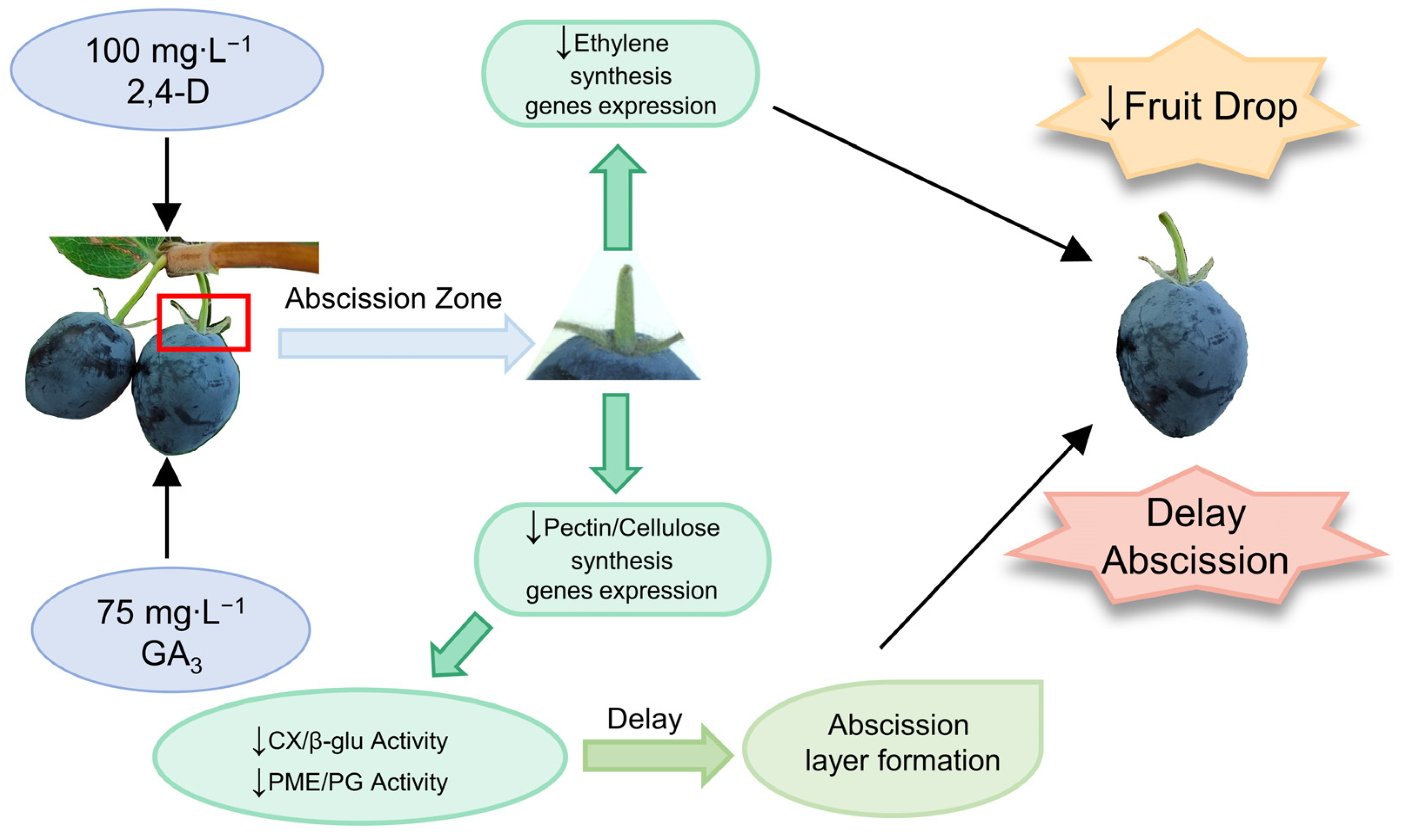
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gang, H.; Sun, F.; Zhu, C.; Huo, J.; Liu, H.; Zhang, L.; Qin, D. Analysis of the genetic diversity of 58 blue honeysuckle (Lonicera caerulea L.) Germplasm resources using SRAP markers. Pak. J. Bot. 2023, 55, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Ma, X.; Xiao, Z.; Sun, A.; Zhao, M.; Wang, Y.; Huang, D.; Sui, X.; Huo, J.; Zhang, Y. Polyphenols in twenty cultivars of blue honeysuckle (Lonicera caerulea L.): Profiling, antioxidant capacity, and α-amylase inhibitory activity. Food Chem. 2023, 421, 136148. [Google Scholar] [CrossRef]
- Gołba, M.; Sokół-Łętowska, A.; Kucharska, A.Z. Health properties and composition of honeysuckle berry Lonicera caerulea L. An update on recent studies. Molecules 2020, 25, 749. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yu, J.; Guo, S.; Fang, H.; Chang, X. Inhibition of pancreatic α-amylase by Lonicera caerulea berry polyphenols in vitro and their potential as hyperglycemic agents. LWT 2020, 126, 109288. [Google Scholar] [CrossRef]
- Becker, R.; Szakiel, A. Phytochemical characteristics and potential therapeutic properties of blue honeysuckle Lonicera caerulea L. (Caprifoliaceae). J. Herb. Med. 2019, 16, 100237. [Google Scholar] [CrossRef]
- Patharkar, O.R.; Walker, J.C. Advances in abscission signaling. J. Exp. Bot. 2018, 69, 733–740. [Google Scholar] [CrossRef]
- Kim, J.; Chun, J.-P.; Tucker, M.L. Transcriptional regulation of abscission zones. Plants 2019, 8, 154. [Google Scholar] [CrossRef]
- Shi, Y.; Song, B.; Liang, Q.; Su, D.; Lu, W.; Liu, Y.; Li, Z. Molecular regulatory events of flower and fruit abscission in horticultural plants. Hortic. Plant J. 2023, 9, 867–883. [Google Scholar] [CrossRef]
- Chao, C.T.; Lovatt, C. Effects of concentration and application time of GA3 and urea on yield, fruit size distribution and crop value of Clementine mandarin in California. Acta Hortic. 2006, 727, 227–238. [Google Scholar] [CrossRef]
- Askarieh, A.; Suleiman, S.; Tawakalna, M. Sweet cherry (Prunus avium L.) fruit drop reduction by plant growth regulators (Naphthalene acetic acid NAA and gibberellic acid GA3). Am. J. Plant Sci. 2021, 12, 1338–1346. [Google Scholar] [CrossRef]
- Lal, D.; Tripathi, D.V.; Kumar, S.; Nayyer, M. Effect of pre-harvest application of gibberellic acid, NAA, and calcium nitrate on fruitdrop, maturity and storage quality of kinnow mandarin. Res. Environ. Life Sci. 2015, 8, 561–564. [Google Scholar]
- Dražeta, L.; Lang, A.; Cappellini, C.; Hall, A.J.; Volz, R.K.; Jameson, P.E. Vessel differentiation in the pedicel of apple and the effects of auxin transport inhibition. Physiol. Plant. 2004, 120, 162–170. [Google Scholar] [CrossRef]
- Reddy, P.; Prasad, D. Effect of plant growth regulators on fruit characters and yield of pomegranate (Punica granatum L.) cv. Ganesh. Int. J. Plant Anim. Environ. Sci. 2012, 2, 91–93. [Google Scholar]
- Ghosh, S.; Bera, B.; Roy, S. Influence of plant growth regulators on fruit production of sweet orange. J. Crop Weed 2012, 8, 83–85. [Google Scholar]
- Chen, J.; Qin, D.; Yang, X.; Zhang, P.; Chi, R.; Zhang, L.; Gang, H.; Huo, J. Effects of different plant growth regulators on abscission and quality of Lonicera caerulea Berel fruit. J. Fruit Sci. 2022, 39, 2113–2123. [Google Scholar]
- Liu, X.; Pan, Y.; Liu, C.; Ding, Y.; Wang, X.; Cheng, Z.; Meng, H. Cucumber fruit size and shape variations explored from the aspects of morphology, histology, and endogenous hormones. Plants 2020, 9, 772. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ren, B.; Bian, C.; Qin, D.; Zhang, L.; Li, J.; Wei, J.; Wang, A.; Huo, J.; Gang, H. Transcriptomic and metabolomic analyses reveal molecular mechanisms associated with the natural abscission of blue honeysuckle (Lonicera caerulea L.) ripe fruits. Plant Physiol. Biochem. 2023, 199, 107740. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Zhang, L.; Yang, X.; Zhong, G. Profiling gene expression in citrus fruit calyx abscission zone (AZ-C) treated with ethylene. Mol. Genet. Genom. 2015, 290, 1991–2006. [Google Scholar] [CrossRef]
- Zhao, M.; Li, J. Molecular events involved in fruitlet abscission in litchi. Plants 2020, 9, 151. [Google Scholar] [CrossRef]
- Souza, K.O.; Silveira, A.G.; Lopes, M.M.; Moura, C.F.; Silva, E.O.; Ayala-Zavala, J.F.; Soares, L.S.; Miranda, M.R.A. AVG and GA3 prevent preharvest fruit drop and enhance postharvest quality of ‘BRS 189’ cashew. Sci. Hortic. 2019, 257, 108771. [Google Scholar] [CrossRef]
- Cai, N.; Chen, C.; Wan, C.; Chen, J. Effects of pre-harvest gibberellic acid spray on endogenous hormones and fruit quality of kumquat (Citrus japonica) fruits. N. Z. J. Crop Hortic. Sci. 2021, 49, 211–224. [Google Scholar] [CrossRef]
- Siddiqui, M.W.; Dutta, P.; Dhua, R.S.; Dey, A. Changes in biochemical composition of mango in response to pre-harvest gibberellic acid spray. Agric. Conspec. Sci. 2013, 78, 331–335. [Google Scholar]
- Li, H.; Wu, H.; Qi, Q.; Li, H.; Li, Z.; Chen, S.; Ding, Q.; Wang, Q.; Yan, Z.; Gai, Y. Gibberellins play a role in regulating tomato fruit ripening. Plant Cell Physiol. 2019, 60, 1619–1629. [Google Scholar] [CrossRef] [PubMed]
- Adouli, B.; Zamani, Z.; Fattahi-Mohgadam, M.R.; Golein, B.; Rezaei, K. Effects of alternate bearing and 2, 4-D application on fruit growth pattern, abscission enzymes activity, ACC content of calyx and carbohydrates partitioning of fruits in Satsuma mandarin (Citrus unshiu Marc.) cv. Miyagawa. Sci. Hortic. 2018, 238, 58–65. [Google Scholar] [CrossRef]
- Al-Samaraie, O.H.; Al-Falahy, T.H. Effect of bunch covering with different colors of polyethylene and GA3 spray in some fruits characteristics of date palm cv. Braim. Indian J. Ecol. 2020, 47, 132–137. [Google Scholar]
- Patterson, S.E. Cutting loose. Abscission and dehiscence in Arabidopsis. Plant Physiol. 2001, 126, 494–500. [Google Scholar] [CrossRef]
- Stander, O.P.; Theron, K.I.; Cronjé, P.J. Foliar 2, 4-D application after physiological fruit drop reduces fruit splitting of mandarin. HortTechnology 2014, 24, 717–723. [Google Scholar] [CrossRef]
- Kulkarni, S.; Patil, S.; Magar, S. Effect of plant growth regulators on yield and quality of mango (Mangifera indica L.) cv. Kesha. J. Pharmacogn. Phytochem. 2017, 6, 2309–2313. [Google Scholar]
- Castro, R.I.; González-Feliu, A.; Muñoz-Vera, M.; Valenzuela-Riffo, F.; Parra-Palma, C.; Morales-Quintana, L. Effect of exogenous auxin treatment on cell wall polymers of strawberry fruit. Int. J. Mol. Sci. 2021, 22, 6294. [Google Scholar] [CrossRef]
- Wang, G.-L.; An, Y.-H.; Wang, Y.-H.; Liu, J.-X.; Wang, J.-Z.; Sun, M.; Xiong, A.-S. Gibberellin-Induced Alterations to the Expression of Cell Wall-Related Genes in the Xylem of Carrot Root. J. Plant Growth Regul. 2021, 40, 787–797. [Google Scholar] [CrossRef]
- Agustí, J.; Merelo, P.; Cercos, M.; Tadeo, F.R.; Talon, M. Ethylene-induced differential gene expression during abscission of citrus leaves. J. Exp. Bot. 2008, 59, 2717–2733. [Google Scholar] [CrossRef] [PubMed]
- Cin, V.D.; Danesin, M.; Boschetti, A.; Dorigoni, A.; Ramina, A. Ethylene biosynthesis and perception in apple fruitlet abscission (Malus domestica L. Borck). J. Exp. Bot. 2005, 56, 2995–3005. [Google Scholar] [CrossRef] [PubMed]
- Goldental-Cohen, S.; Burstein, C.; Biton, I.; Ben Sasson, S.; Sadeh, A.; Many, Y.; Doron-Faigenboim, A.; Zemach, H.; Mugira, Y.; Schneider, D. Ethephon induced oxidative stress in the olive leaf abscission zone enables development of a selective abscission compound. BMC Plant Biol. 2017, 17, 87. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.K.; Gurung, G.; Yadav, A.; Laha, R.; Mishra, V.K. Factors associated with citrus fruit abscission and management strategies developed so far: A review. N. Z. J. Crop Hortic. Sci. 2022, 51, 467–488. [Google Scholar] [CrossRef]
- Wang, P.; Yu, A.; Ji, X.; Mu, Q.; Haider, M.S.; Wei, R.; Leng, X.; Fang, J. Transcriptome and metabolite integrated analysis reveals that exogenous ethylene controls berry ripening processes in grapevine. Food Res. Int. 2022, 155, 111084. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Hu, Y.; Shen, J.; Meng, X.; Suo, J.; Zhang, Z.; Song, L.; Wu, J. Acceleration of aril cracking by ethylene in Torreya grandis during nut maturation. Front. Plant Sci. 2021, 12, 761139. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wu, T.; Liu, J.; Cong, L.; Zhu, Y.; Zhai, R.; Yang, C.; Wang, Z.; Ma, F.; Xu, L. PbGA20ox2 regulates fruit set and induces parthenocarpy by enhancing GA4 content. Front. Plant Sci. 2020, 11, 113. [Google Scholar] [CrossRef]
- Jiang, S.; Luo, J.; Xu, F.; Zhang, X. Transcriptome analysis reveals candidate genes involved in gibberellin-induced fruit setting in triploid loquat (Eriobotrya japonica). Front. Plant Sci. 2016, 7, 1924. [Google Scholar] [CrossRef]
- Cheng, C.; Jiao, C.; Singer, S.D.; Gao, M.; Xu, X.; Zhou, Y.; Li, Z.; Fei, Z.; Wang, Y.; Wang, X. Gibberellin-induced changes in the transcriptome of grapevine (Vitis labrusca × V. vinifera) cv. Kyoho flowers. BMC Genom. 2015, 16, 128. [Google Scholar] [CrossRef]
- Bound, S.A. Managing crop load in european pear (Pyrus communis L.)—A review. Agriculture 2021, 11, 637. [Google Scholar] [CrossRef]




| Gene Name | Forward Primer Sequence (5′–3′) | Reverse Primer Sequence (5′–3′) |
|---|---|---|
| β-Actin | ACCTGCTGACGAGTGCCGATAC | TCACCCTTGAAACATCAGGAGACCA |
| CX1 | TCGTTCGTCCTTTCTTCC | TAGCCATCAGCAGTCCAGTC |
| CX2 | CTCCTATTCCCATACACTCC | CATCGTCGTATCCACTAACA |
| PE | AAAGTTTGATTGCTGGAGG | GGGTGGTGTTGGCTTATT |
| PL20 | ATAGCACCGAAAGGAGAAA | TATGGAACAGTCCGCAAGG |
| GA2OX1 | AACTGTATGGGCTTCTCA | ACCCTATCGCTATGTATGT |
| GA1 | GCAAAGGTAGTTCCCGTCTA | TCATCCTCCACAATCTCG |
| ACO | GTACGAAGAGGGTGGTGT | CTGGATTGGGAGGATTAG |
| ACO3 | GAGCCATCATAAGGAGGT | TCTCGCCCAATACACTAA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, B.; Zhang, L.; Chen, J.; Wang, H.; Bian, C.; Shi, Y.; Qin, D.; Huo, J.; Gang, H. Response of Abscission Zone of Blue Honeysuckle (Lonicera caerulea L.) Fruit to GA3 and 2,4-D Spray Application. Agronomy 2023, 13, 2937. https://doi.org/10.3390/agronomy13122937
Ren B, Zhang L, Chen J, Wang H, Bian C, Shi Y, Qin D, Huo J, Gang H. Response of Abscission Zone of Blue Honeysuckle (Lonicera caerulea L.) Fruit to GA3 and 2,4-D Spray Application. Agronomy. 2023; 13(12):2937. https://doi.org/10.3390/agronomy13122937
Chicago/Turabian StyleRen, Bingbing, Lijun Zhang, Jing Chen, Haoyu Wang, Chunyang Bian, Yuying Shi, Dong Qin, Junwei Huo, and Huixin Gang. 2023. "Response of Abscission Zone of Blue Honeysuckle (Lonicera caerulea L.) Fruit to GA3 and 2,4-D Spray Application" Agronomy 13, no. 12: 2937. https://doi.org/10.3390/agronomy13122937
APA StyleRen, B., Zhang, L., Chen, J., Wang, H., Bian, C., Shi, Y., Qin, D., Huo, J., & Gang, H. (2023). Response of Abscission Zone of Blue Honeysuckle (Lonicera caerulea L.) Fruit to GA3 and 2,4-D Spray Application. Agronomy, 13(12), 2937. https://doi.org/10.3390/agronomy13122937





