Abstract
Elevated temperature and frequent drought events under global climate change may seriously affect soil respiration. However, the underlying mechanism of the effects of warming and drought on soil respiration is not fully understood in the context of the Loess Plateau. This study examined the response of soil respiration (Rs) to multiple factors, including warming (W), drought (P), and their interaction (WP), in the semi-arid grassland of the Loess Plateau in Northwest China. The research period was from May to November 2022, with an open-top heating box used for warming and a rain shelter used for drought. The results showed the following: (1) Rs ranged from 1.67 μmol m−2 s−1 to 4.77 μmol m−2 s−1, with an average of 3.36 ± 0.07 μmol m−2 s−1. The cumulative soil carbon flux ranged from 500.97 g C·m−2 to 566.97 g C·m−2, and the average cumulative soil respiration was 535.28 ± 35.44 g C·m−2. (2) Warming increased Rs by 5.04 ± 3.11%, but drought inhibited Rs by 3.40 ± 3.14%, and the interaction between warming and drought significantly reduced soil respiration by 11.27 ± 3.89%. (3) The content of particulate organic carbon (POC), dissolved organic carbon (DOC), soil organic carbon (SOC), and readily oxidized carbon (ROC) decreased with the increased soil depth. ROC after W and WP treatments was significantly higher than that of the control, and POC after P treatment was significantly higher than CK (p < 0.05). (4) The seasonal variation of soil respiration was positively correlated with soil temperature, soil water content, plant height, and leaf area index (p < 0.05), but the response rules differed during different regeneration periods. Soil water content; soil water content and leaf area index; and soil water content, soil temperature, and leaf area index were the factors that regulated the variation in soil respiration in the first, second, and third regeneration periods, respectively. These results clearly showed the limiting effect of drought stress on the coupling between temperature and soil respiration, especially in semi-arid regions. Collectively, the variations in soil respiration under warming, drought, and their interactions were further regulated by different biotic and abiotic factors. Considering future warming, when coupled with increased drought, our findings indicate the importance of considering the interactive effects of climate change on soil respiration and its components in arid and semi-arid regions over the next decade.
1. Introduction
Global climate change, characterized by temperature elevation and altered rainfall patterns, poses a significant threat to the sustainable development of human society [1]. According to the report of the Intergovernmental Panel on Climate Change (IPCC), global climate change will continue to intensify, global temperatures will continue to rise, arid regions will expand, and extreme drought events caused by intense precipitation will increase in the future [2,3]. These changes have severe implications for the carbon cycle of terrestrial ecosystems. Soil respiration, as the second largest carbon flux between the atmosphere and terrestrial ecosystems, is an important regulator of the global carbon cycle and climate change [4]. Even slight changes in soil respiration can accelerate or decelerate the atmospheric carbon dioxide concentration, thus influencing the global carbon balance. Therefore, it is essential to study the increase in temperature and drought under climate change on soil carbon dynamics.
Soil respiration (Rs) mainly includes two components: autotrophic respiration (root respiration and rhizosphere microbial respiration) and heterotrophic respiration (soil microbial respiration and animal respiration) [5]. Rs is independently or synergistically regulated by several biotic and abiotic factors, including temperature, soil water content, substrate supply, aboveground biomass, enzymatic activity, and microbial community [6,7,8,9,10]. With the continuous intensification of the global climate, variations in temperature and moisture will have an interactive effect on Rs and its components to a certain extent, thus affecting the global carbon cycle [11,12]. Numerous simulation experiments conducted over the past two decades have demonstrated highly variable and complex responses of Rs to warming and drought. Studies have indicated that warming will lead to short-term increases, no effects, or negative effects on Rs in different grassland ecosystems [13,14]. Similarly, drought can significantly inhibit Rs, but it can also stimulate Rs [15,16,17,18,19]. The apparent inconsistency in these responses can be attributed to the interactions between biotic and abiotic factors [8,9,10]. For example, warming can increase microbial activity and soil organic carbon decomposition, releasing more carbon dioxide into the atmosphere [20]. Changes in precipitation can stimulate or inhibit plant photosynthesis and respiration by changing the soil environment and nutrient status [21,22]. In addition to the single factor effects, Rs is also influenced by the interaction of multiple global changes [23]. For instance, increased precipitation can compensate for the reduction in soil moisture caused by warming, while drought exacerbates soil moisture loss under warming [24]. However, to date, few experiments have investigated these two global change factors on carbon flux in the Loess Plateau ecosystem. Clearly, further understanding is needed, especially in relation to the interaction between warming and drought in arid and semi-arid regions.
The Loess Plateau in China represents the largest loess accumulation area globally [25], and it is also an important component of China’s three zones and four belts ecological barrier. Grassland is the most typical vegetation type in this region, accounting for approximately 42% of the total area. The carbon storage of the ecosystem is approximately 1.09–1.46 Pg C [26,27]. However, despite being China’s big carbon (C) pool, the Loess Plateau suffers from severe soil erosion and degradation, making it one of the most critically affected regions worldwide [28]. Therefore, it plays a crucial role in the study of the global soil carbon cycle and climate change. Most of the Loess Plateau constitutes a semi-arid region with low precipitation, low soil water content, low vegetation coverage, and frequent extreme climatic events [29]. In order to restore the regional ecological environment, a large-scale project of converting farmland back to forests and grasslands has been implemented since 1999 [30]. Artificial grassland, the primary vegetation type resulting from the farmland conversion project on the Loess Plateau, possesses certain advantages in accelerating vegetation restoration and enhancing ecological stability [31]. However, because of the influence of a natural environment background, the recovery capacity is limited; the ecosystem in this area is extremely fragile, highly sensitive to changes in the external environment, and particularly vulnerable to global climate change [32]. Under the interactive effects of global changes, such as regional climate warming and frequent extreme droughts, soil carbon emissions from artificial grassland ecosystems on the Loess Plateau have attracted widespread attention. Understanding the trends and driving factors of Rs in artificial grassland ecosystems is of great significance for comprehending the regional carbon cycle and the conversion of ecosystem carbon sources and sinks on the Loess Plateau [30].
We aimed to investigate the response of Rs of artificial grassland in the Loess Plateau to global climate change, especially the response of climate warming and extreme drought events. We selected alfalfa, the dominant grass species in the Loess Plateau, to simulate field experiments of warming and extreme drought. The research period was from May to November 2022, with an open-top heating box used for warming and a rain shelter used for drought. Our objective was to address two questions: (1) How does soil C flux respond to warming, drought, and their interactions, whether independent or interacting? (2) What is the mechanism of regulating this response? We hypothesized that the (1) interaction between warming and drought significantly reduced soil respiration, and (2) the variation in soil respiration under warming, drought, and their interactions is regulated by different biotic and abiotic factors.
2. Materials and Methods
2.1. Study Area
This study was conducted at the National Field Scientific Observation and Research Station of Grassland Agroecosystem in Qingyang, Gansu Province. The experimental station is located in Shishe Township, Xifeng District, Qingyang City, Gansu Province (35°39′ N, 107°51′ E, altitude of 1297 m, Figure 1). The area is affected by a temperate semi-arid continental monsoon climate. The average precipitation for many years (1981–2020) is 537.5 mm, the average annual temperature is 9.4 °C, and the frost-free period is 150 days. In summer, the temperature is high and the rainfall is rainy. The rainfall in July increases, and the rainy season is mostly concentrated between September and October. The annual variation in rainfall is large. The regional soil type is dark loessial soil, and the pH value is between 8.0–8.5.
Figure 1.
Location map of the research area.
2.2. Experimental Design
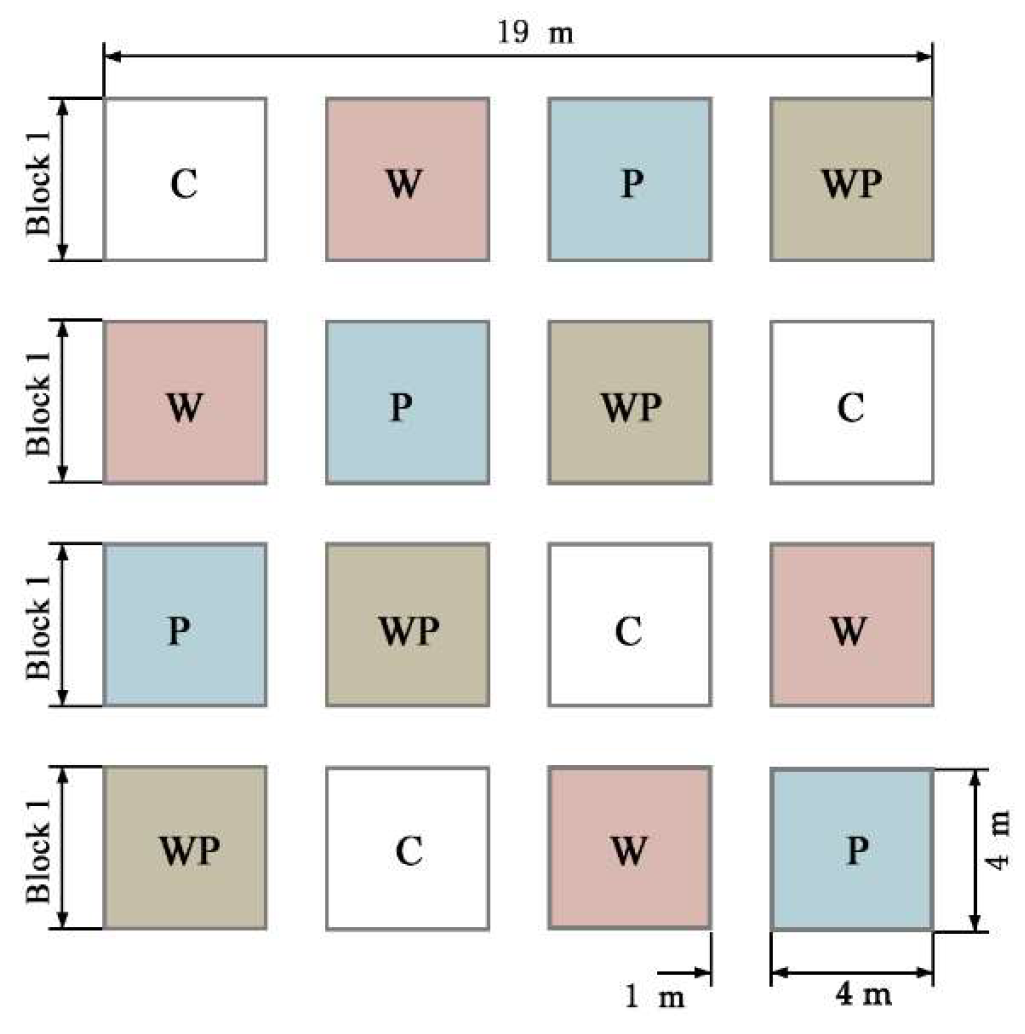
In order to simulate the process of Rs under warming and drought conditions, a completely randomized block design was used in this study. Two factors (warming and drought) and four treatments were set up: control (CK), warming (W), drought (−50% precipitation, P), and warming × drought (warming and −50% precipitation, WP). Each treatment was set up with four replicates, a total of 16 experimental plots, each plot area 4 m × 4 m (16 m2), and 1 m spacing between treatment plots (Figure 2). It is convenient for regular manual monitoring to avoid the experimental error caused by the interference of the test to the adjacent cells. To control the experimental variables, the influencing variables, such as vegetation type and nutrient level in the sample plot, were controlled equally.
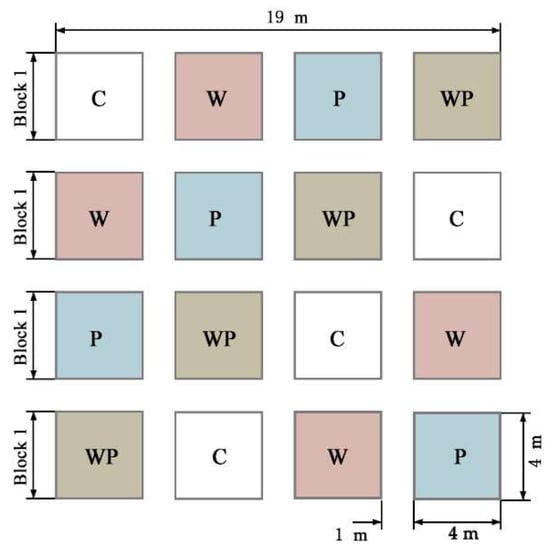
Figure 2.
Layout of the experiment design.C, control treatment; W, warming treatment; P, drought treatment by reducing 50% precipitation; WP, combined warming and drought treatment.
In order to simulate the effect of warming on Rs caused by climate warming, an open top chamber (OTC) was set up in the experiment, which could provide warming to the enclosure throughout the entire year. The material was acrylic organic glass with a transmittance of more than 90%, which was a hexagonal truncated cone. After the warming treatment, the temperature increased by approximately 0.6 °C. According to the semi-arid climate characteristics and change trend in the Loess Plateau, we proposed achieving drought treatment by reducing the precipitation by 50%. The effect of a drought was achieved by installing a canopy to cover 50% of the area of the test area. The height of the canopy from the ground was 1.30–1.60 m. The 20 cm wide tile-shaped transparent groove plate was used to intercept the rainwater and flow it into the rainwater collection device through the PVC sink and catheter. At the same time, 60 cm deep PVC plates were buried around the sample, avoiding the influence of the lateral transport of soil moisture between small areas. In order to eliminate the difference between the treatments caused by the light transmittance of the PVC pipeline in the canopy, the canopy was also placed in the area without drought treatment, and the pipeline was inverted to achieve the effect of natural precipitation. After treatment, the soil moisture significantly decreased and the drought effect was significant.
2.3. Measurement Protocols
Among the Rs measurement plots, a PVC collar (20 cm in diameter and 10 cm in height) was inserted into the soil to a depth of 5 cm at the center of each plot for measuring the soil CO2 efflux. Since May 2022, the CO2 efflux was measured using a LI-6800 (LI-COR, Lincoln, NE, USA). Rs was measured twice a month in the growing season and once a month in the non-growing-season during the study period. All of the soil respiration measurements were carried out between 09:00 and 11:00 a.m. (local time). Each treatment took roughly 1–2 min.
2.4. Soil Temperature and Moisture Measurements
The Ts and VWC at the 10 cm depth were measured simultaneously with the soil respiration rates using the 8150–203 soil temperature probe and GS1 soil moisture sensor (LI-COR, Lincoln, NE, USA), respectively. Meteorological data (Ta, PAR and precipitation) were recorded every half-hour using a PC200W automatic meteorological station (Campbell Scientific, Logan, UT, USA) placed within 50 m of the experimental field.
2.5. Aboveground Biomass and Soil Carbon Fractions
The alfalfa plant height and leaf area index were measured simultaneously using soil respiration. The plant height was measured by taking 10 plants that were randomly selected by tape, and the average values were taken. LAI was measured using LAI2000 (LI-COR, Lincoln, NE, USA). The alfalfa was cut on 29 June and 26 August when the late bud to flowering stage was 10%. Therefore, the beginning of the growing season to 29 June was defined as regeneration period 1, 29 June to 26 August was regeneration period 2, and 26 August to the end of the growing season was regeneration period 3. Moreover, 0–10 cm, 10–20 cm, 20–30 cm, and 30–50 cm soil layers were collected for the soil carbon component determination in September 2022. The soil organic carbon (SOC) and particulate organic carbon (POC) were determined using the potassium dichromate volumetric method and external heating method. Soil microbial biomass carbon (MBC) was extracted by chloroform fumigation using K2SO4, readily oxidized carbon (ROC) using the KMnO4 oxidation method, and dissolved organic carbon (DOC) was determined by ultraviolet spectrophotometry.
2.6. Data Analysis
Repeated-measure ANOVA tests were used to test the effects of warming and drought on Rs and soil carbon fractions. Significant differences were evaluated at the p < 0.05 level. Duncan’s method was used to compare the differences between treatments. Exponential and linear fittings were used to evaluate the relationships between soil respiration and abiotic and biotic factors (soil temperature and moisture, H and LAI). We used (WP − P)/P × 100% and (W − C)/C × 100% to represent the effect of drought on soil warming (relative variation, %). The drought effects with and without soil warming were (WP − W)/W × 100% and (P − C)/C × 100%, respectively [33].
A statistic equation was used to calculate the cumulative repiration:
where X is daily soil respiration, Rs is measured soil respiration (μmol m−2 s−1), 12 is the molar mass of CO2 − C(g mol−1), 3600 and 24 are conversion coefficients of time, i is the first measurement of soil respiration rate, and n is the monitoring number.
X = Rs × 3600 × 24 × 12 × 10−6
The relationships between soil respiration and abiotic and biotic factors (soil temperature, soil moisture, and AGB) were evaluated using exponential and quadratic fitting. All of the statistical analyses were performed using SPSS 21.0 (SPSS Inc., Chicago, IL, USA). Origin (version 21.0) was used for plotting.
3. Results
3.1. Changes in Environmental and Biomass Factors
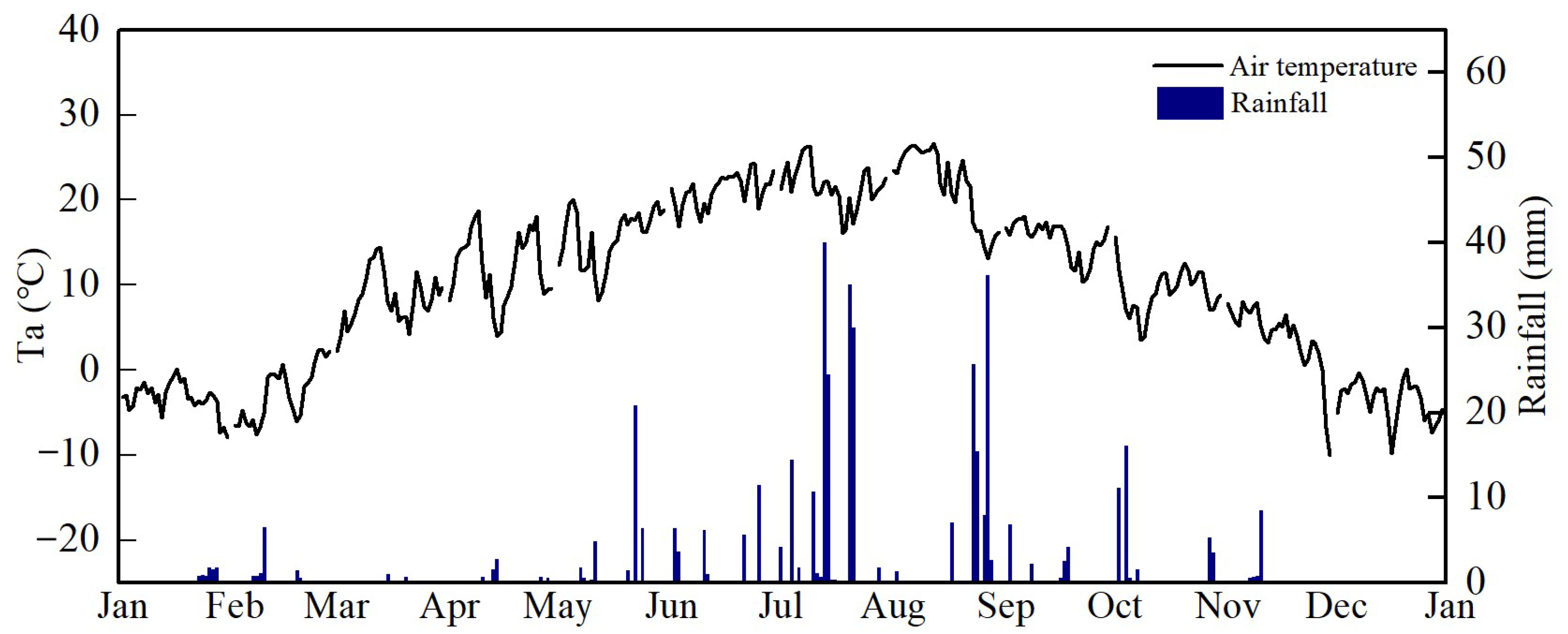
During the experimental period, air temperature (Ta) and rainfall exhibited significant seasonal patterns (Figure 3). Ta gradually increased from winter to summer, followed by a decline after reaching the peak daily maximum temperature of 26.6 °C in August, with an average annual temperature of 10.0 °C, which was about 0.4 °C higher than the long-term average (9.7 °C, from 1970 to 2021). During the study period, the seasonal variation of precipitation was large; the period from June to October received the most annual precipitation (~82.2%), with winter and early spring mostly without precipitation. Abnormal lower precipitation in August led to summer drought. The annual cumulative precipitation was 432.9 mm, which was approximately 20.4% lower than the long-term average (544.0 mm, from 1970 to 2021).
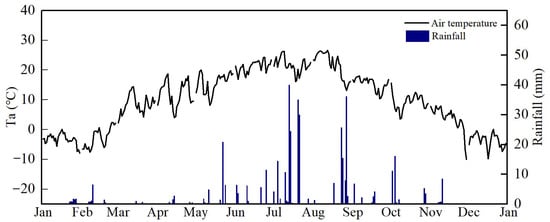
Figure 3.
Seasonal variation of air temperature and rainfall in 2022.
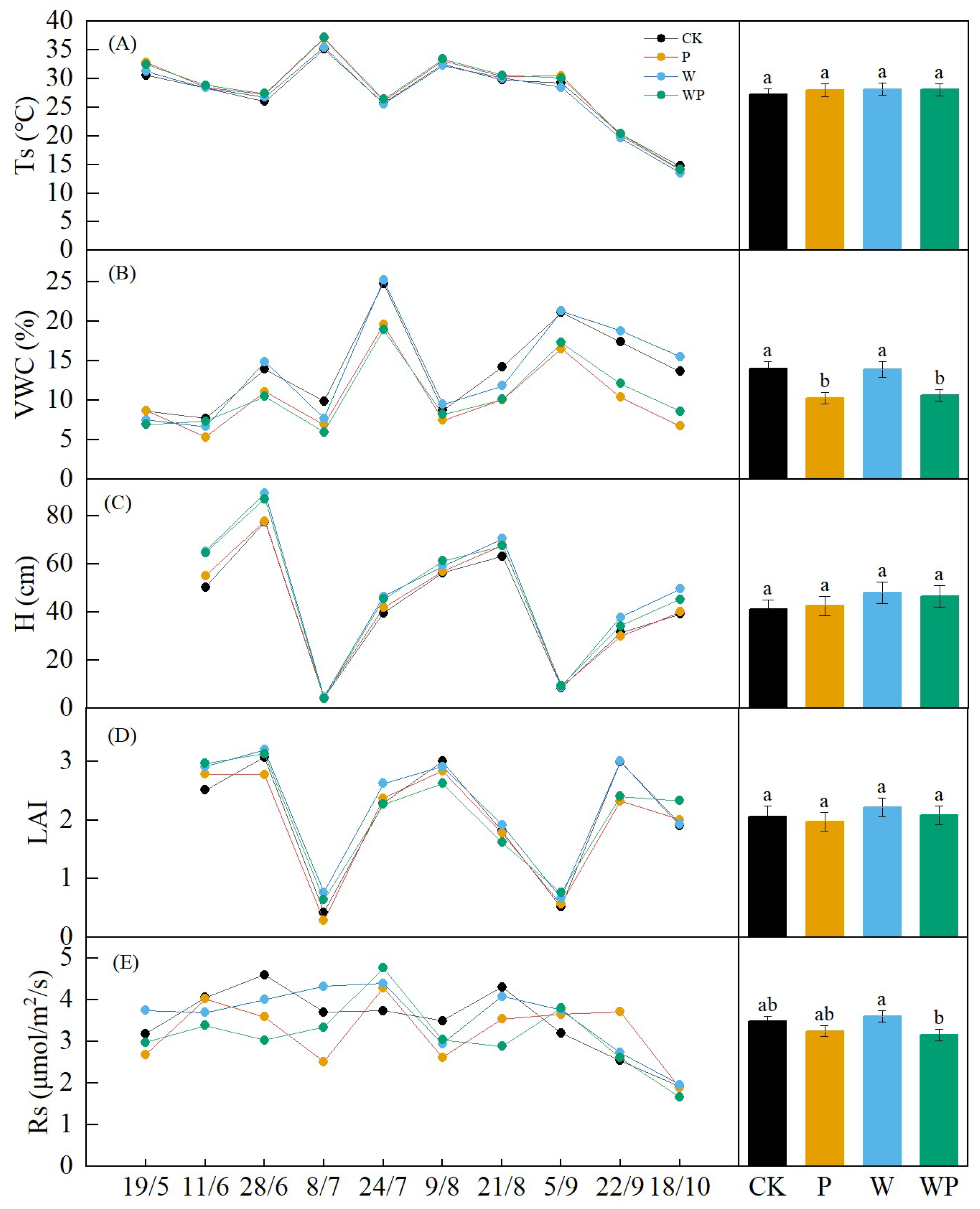
The overall seasonal pattern of soil temperature (Ts) was not significant due to unusual temperature fluctuations caused by abnormal temperature rise in spring and concentrated precipitation in June and late July (Figure 4A). Except for a slight increase in September, the temperature gradually decreased after August. Ts showed no significant difference between treatments (p > 0.05, Figure 4A). The soil volumetric water content (VWC) exhibited three peaks on 28 June, 24 July, and 5 September, with the highest point occurring on 24 July (Figure 4B). Under drought treatment, VWC was significantly lower than in the control and warming treatments (p < 0.05). The seasonal patterns in alfalfa height and leaf area index (LAI) were similar throughout the experimental period, with both significantly decreasing after being mowed twice. Lodging occurred when the alfalfa reached a certain height, leading to a divergence between LAI and alfalfa height on 21 August and 18 October (Figure 4C,D). Although there was no significant difference between treatments (p > 0.05), the growth of alfalfa under warming was higher than that under other treatments in the same period.
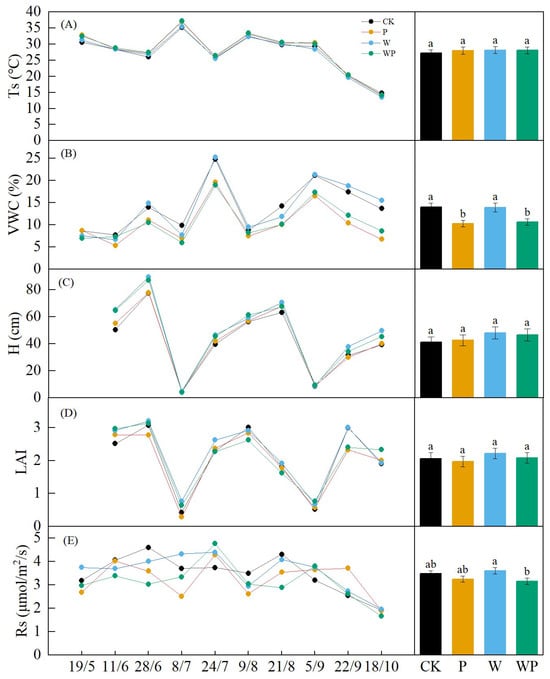
Figure 4.
The seasonal variations and differences among treatments in soil temperature (Ts) (A), soil volumetric water content (VWC) (B), alfalfa height (H) (C), leaf area index (LAI) (D), and soil respiration(Rs) (E). CK, control treatment; P, −50% precipitation treatment; W, warming treatment; WP, combined warming and −50% precipitation. Different letters represent significant difference at p < 0.05.
3.2. Variation in Soil Respiration
Soil respiration (Rs) exhibited distinct seasonal changes, following a single–peak trend. It gradually increased at the beginning of the growing season and decreased after August, with two exceptions on 8 July and 9 August when a declining trend was observed (Figure 4E). Overall, there was a significant difference in Rs between W and WP throughout the growing season (p < 0.05). Warming generally stimulated Rs, while plots subjected to drought treatment displayed lower soil carbon emissions compared with the control.
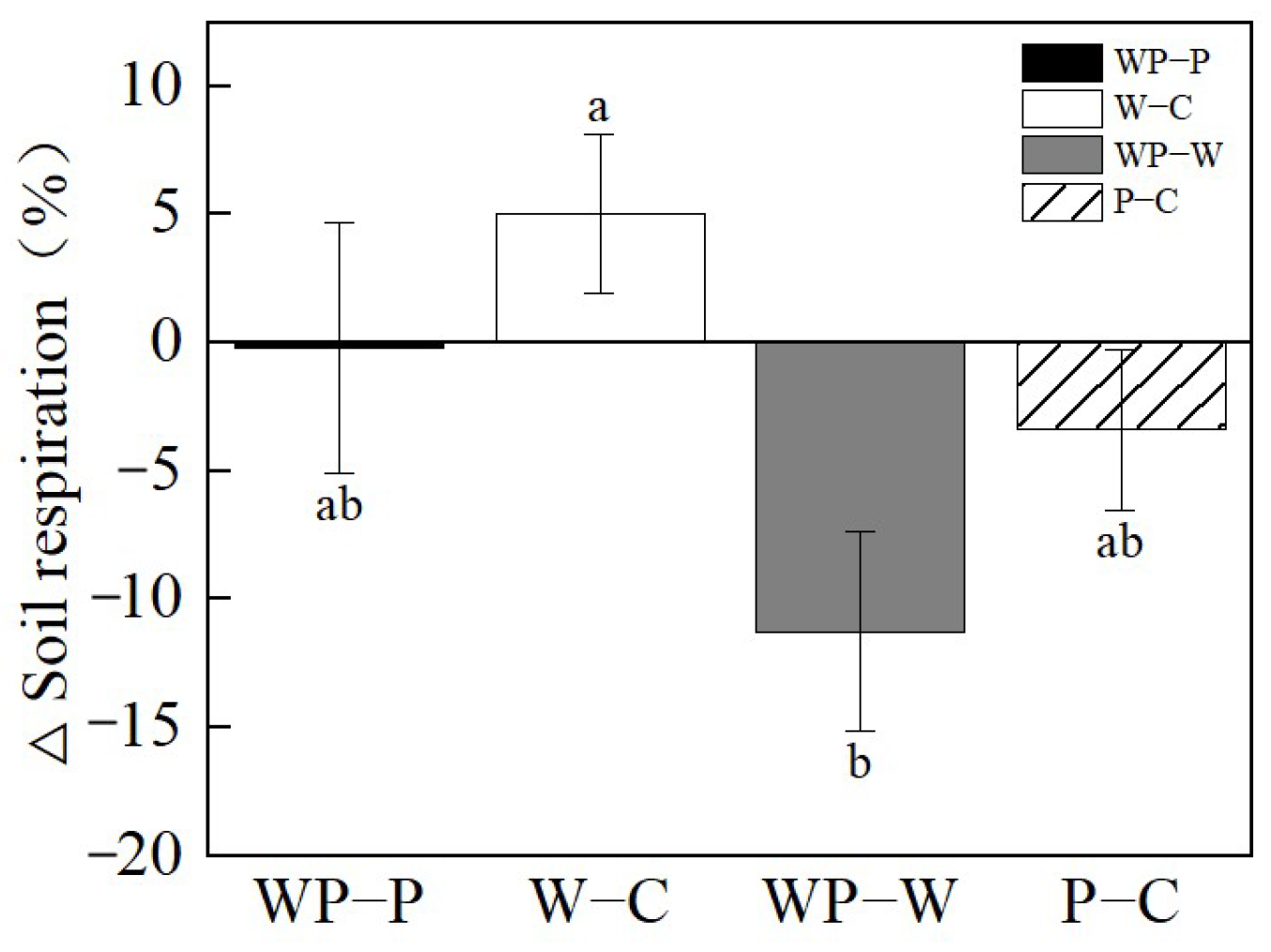
Soil warming substantially stimulated Rs by 5.04% under ambient precipitation (W–C), but suppressed it when drought occurred (WP–P, Figure 5). Drought decreased Rs by 3.40% under ambient temperatures (P–C), and decreased it by 11.27% under warming conditions (WP-W, Figure 5).
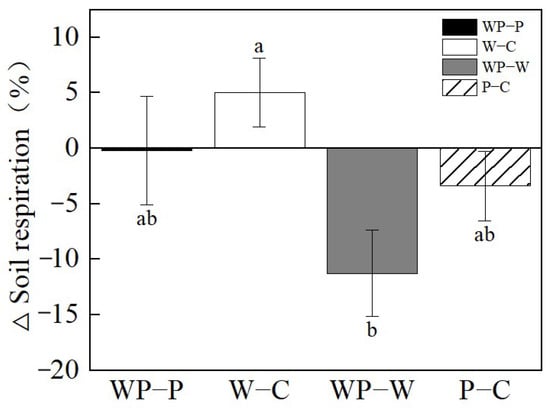
Figure 5.
Relative changes (%) in soil respiration (Rs), induced by drought without soil warming (P–C) and with soil warming (WP–W), and by soil warming without throughfall reduction (W–C) and with throughfall reduction (WP–P) in 2022. Different letters represent significant difference at p < 0.05.
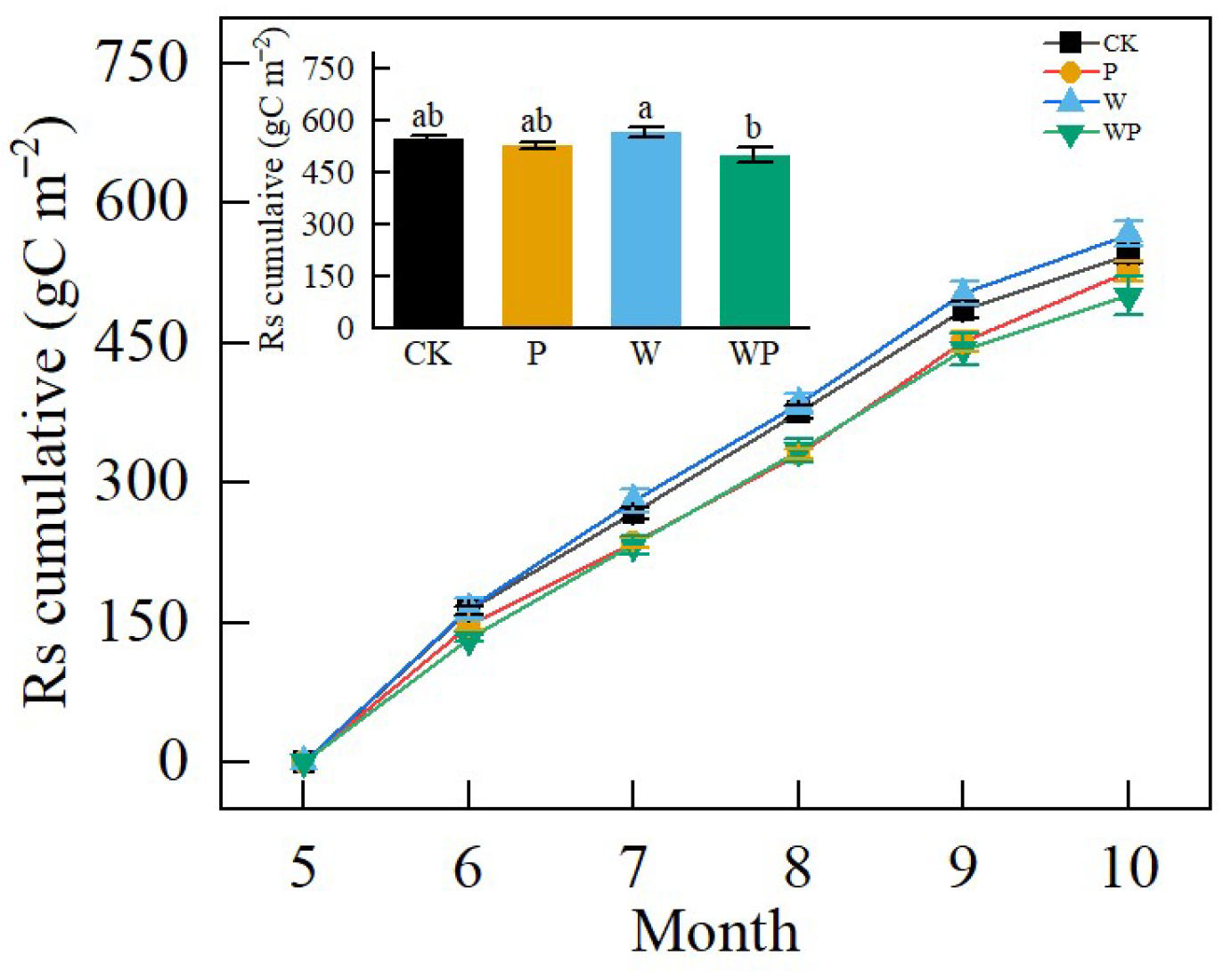
Over the experimental period, the average cumulative soil respiration was 535.28 g C·m−2. Warming enhanced cumulative soil respiration, while P and WP limited cumulative soil respiration (Figure 6). Cumulative soil respiration was 7.48% and 13.17% higher in the W treatment compared with P and WP, respectively (Figure 6). Throughout the entire growing season, cumulative soil respiration was significantly lower in WP compared with W (p < 0.05), while no significant difference was observed between CK and P (p > 0.05).
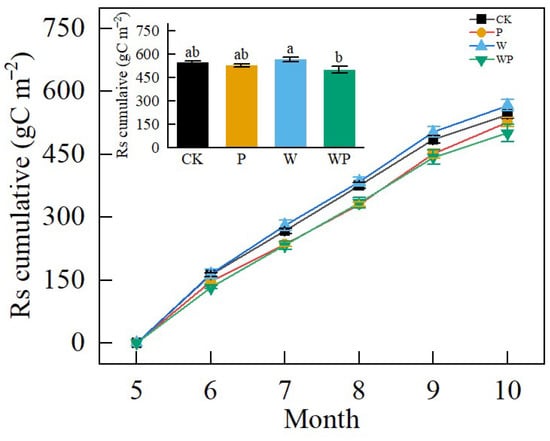
Figure 6.
Cumulative soil CO2 efflux (Rs) from May to October 2022 under the four treatments. Different letters represent significant difference at p < 0.05.
3.3. Variation in Soil Carbon Fractions
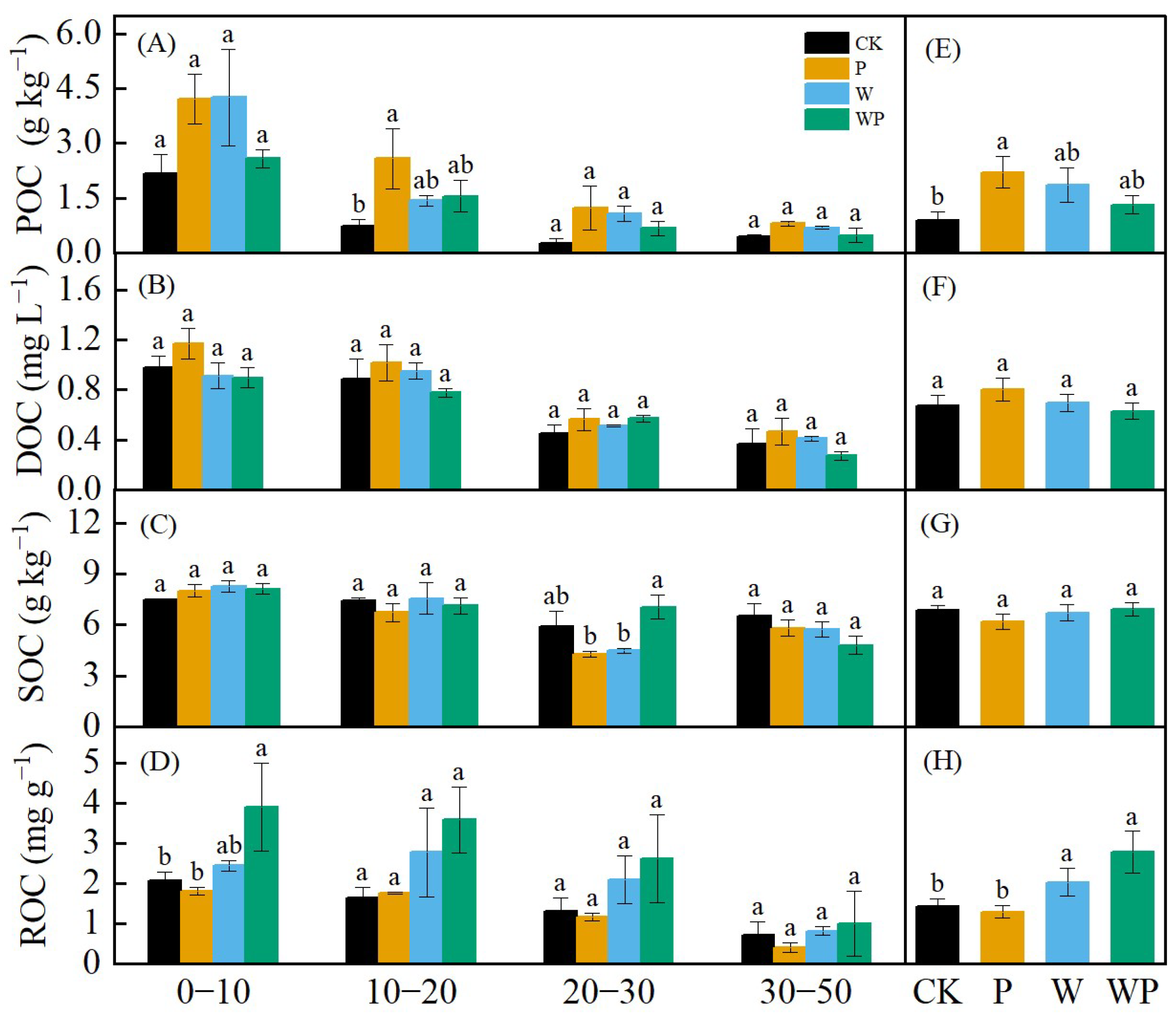
The changes in particulate organic carbon (POC), dissolved organic carbon (DOC), soil organic carbon (SOC), and readily oxidizable carbon (ROC) in different soil depths in 2022 were analyzed (Figure 7). In general, the soil carbon content decreased with the change in soil depth. W, P, and WP increased POC, with P significantly higher than CK (p < 0.05, Figure 7E). No significant differences were observed in DOC between treatments in terms of the depth and total content (p > 0.05), although DOC was higher under drought stress compared with the control (Figure 7B,F). SOC did not vary significantly, except for WP, which exhibited significant differences compared with W and P at the 20–30 cm depth range (p < 0.05). SOC remained relatively stable across different depths (Figure 7C,G). WP had the highest content of ROC, followed by W (Figure 7D,H), and WP and W were significantly higher than that of the CK and P treatments (p < 0.05).
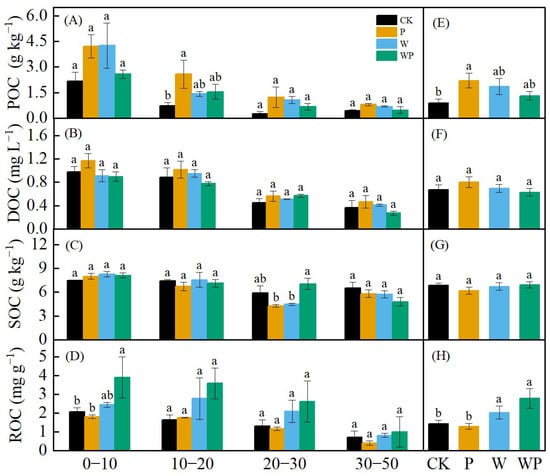
Figure 7.
Layered content of particulate organic carbon (POC) (A), dissolved organic carbon (DOC) (B), soil organic carbon (SOC) (C), and readily oxidized carbon (ROC) (D). Differences in POC (E), DOC (F), SOC (G), ROC (H) among treatments. Different letters represent significant difference at p < 0.05.
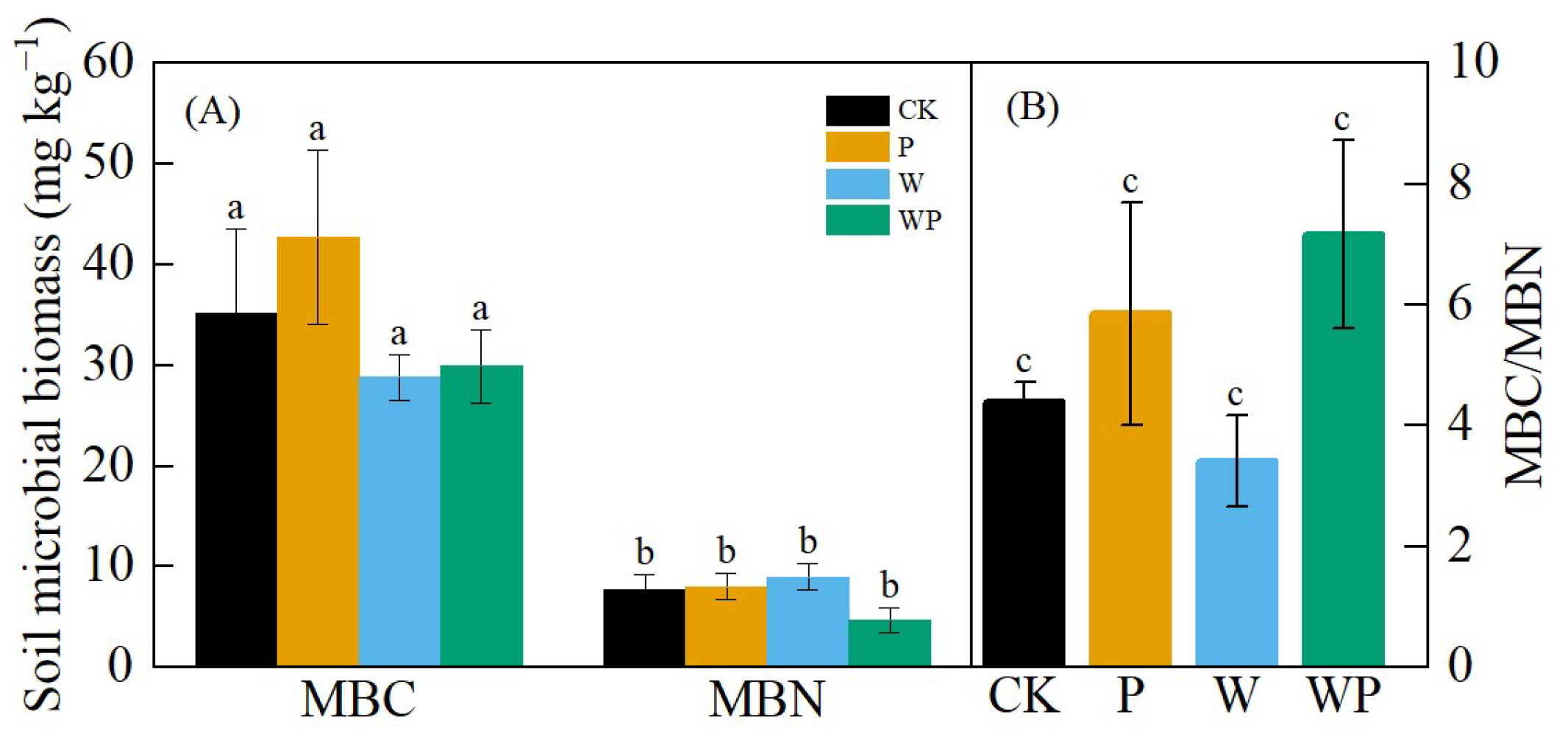
While there were no significant differences in microbial biomass carbon (MBC) and microbial biomass nitrogen (MBN) among treatments (p > 0.05), W and WP decreased MBC compared with CK, P increased MBC but had no significant effect on MBN, and the WP treatment moderately decreased MBN (Figure 8A). The soil microbial biomass carbon-to-nitrogen ratio (MBC/MBN) did not vary significantly among the different treatments (p > 0.05), but the MBC/MBN ratios under P and WP were higher than that of CK, while W showed a lower ratio compared with CK (Figure 8B).
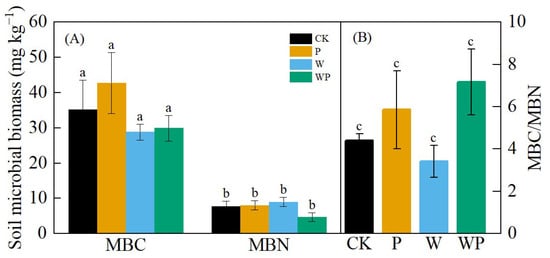
Figure 8.
Soil microbial biomass carbon (MBC) and soil microbial biomass carbon nitrogen (MBN) (A) and carbon nitrogen ratio value (B). Different letters represent significant difference at p < 0.05.
3.4. Relationships between Soil Respiration and Environmental Factors
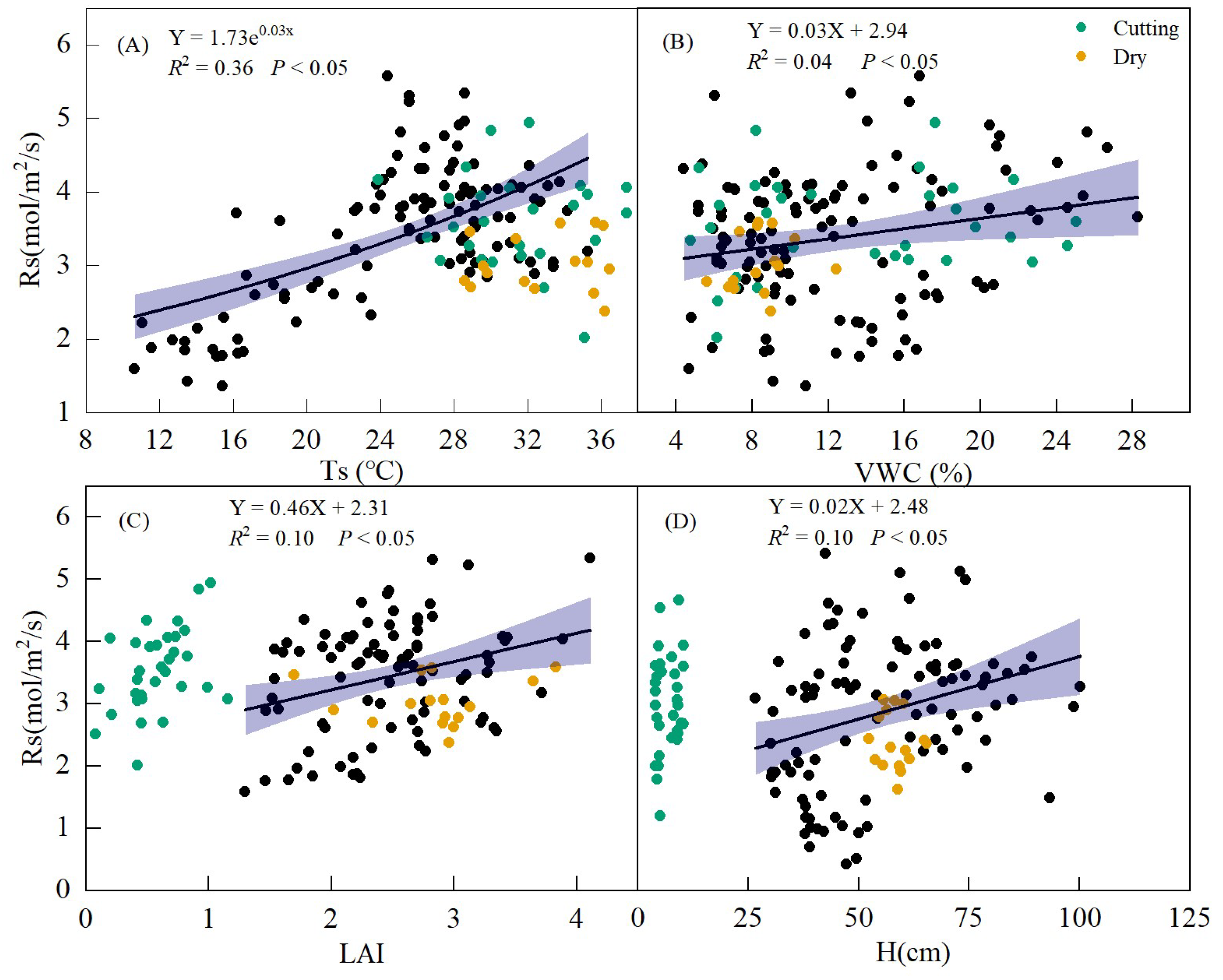
Based on the observed values across all of the treatments, Rs exhibited an exponential increase with Ts (R2 = 0.36, p < 0.05, Figure 9A). Rs also showed a linear positive correlation with VWC, H, and LAI (p < 0.05, Figure 9B–D).
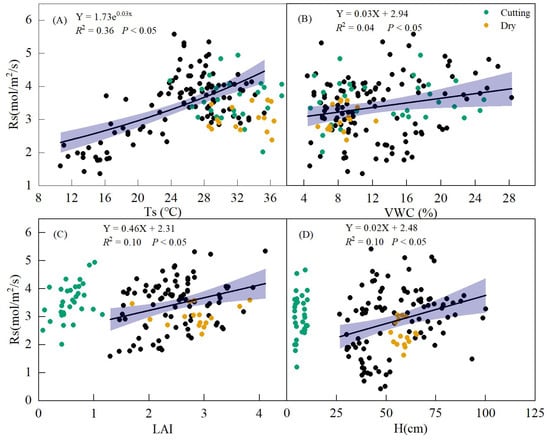
Figure 9.
Relationships between soil respiration (Rs) and soil temperature (Ts) (A), soil volumetric water content (VWC) (B), leaf area index (LAI) (C), and alfalfa height (H) (D) during the whole growing season. The yellow and green points represent the values of the dry period and the mowing period, respectively. Black dots represent values during normal periods.
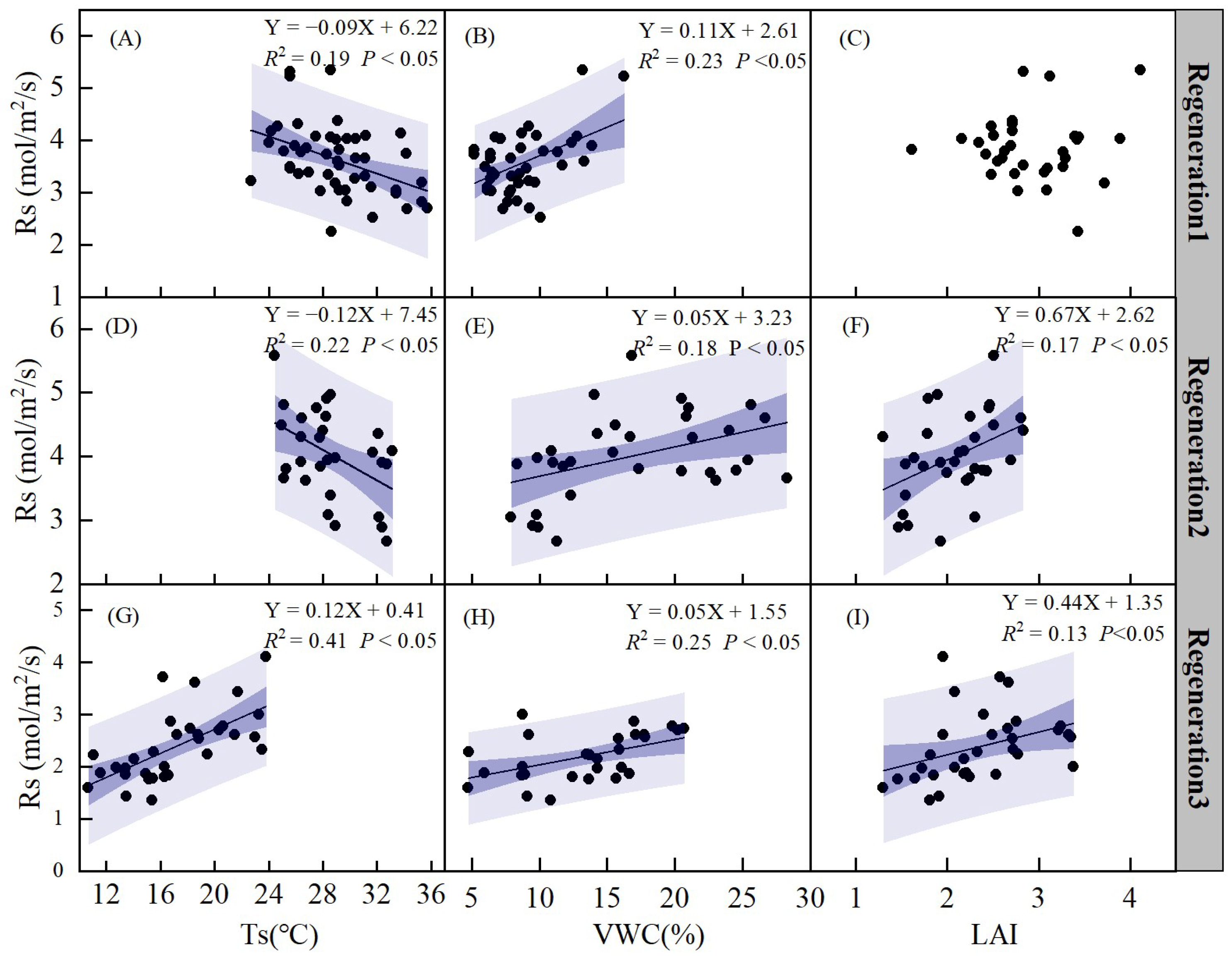
The relationship between soil respiration (Rs) and Ts, VWC, and LAI was analyzed using linear models at different regeneration stages (Figure 10). There was a significant negative correlation between Rs and Ts, except during regeneration period 3 (p < 0.05). Rs exhibited a linear increase with increasing VWC (p < 0.05, Figure 10B,E,H). Rs also showed a linear increase with increasing LAI during regeneration periods 2 and 3 (p < 0.05, Figure 8F,I), but no correlation was found during regeneration period 1 (Figure 10C).
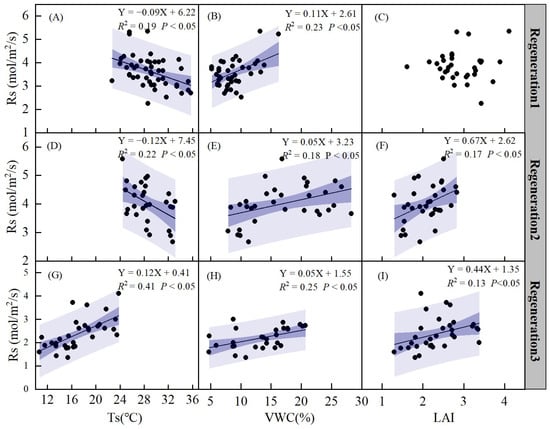
Figure 10.
Relationships between soil respiration (Rs) and soil temperature (Ts) in regeneration period 1 (A), regeneration period 2 (D), regeneration period 3 (G). Relationships between soil respiration (Rs) and soil volumetric water content (VWC) in regeneration period 1 (B), regeneration period 2 (E), regeneration period 3 (H). Relationships between soil respiration (Rs) and leaf area index (LAI) in regeneration period 1 (C), regeneration period 2 (F), regeneration period 3 (I). Regeneration period 1 is from May to June. Regeneration period 2 is from July to August. Regeneration period 3 is from September to October. The blue regions are the confidence band and prediction band, respectively.
4. Discussion
4.1. Variation Characteristics of Soil Respiration
Seasonal dynamics of Rs are widespread in different ecosystems; however, the range in numerical variation may vary depending on the ecosystem type. In our study, Rs were ranged from 1.67 μmol m−2 s−1 to 4.77 μmol m−2 s−1 during the growing season, with an average of 3.36 μmol m−2 s−1, which was within the range of global grassland soil respiration (0.13 μmol m−2 s−1~7.27 μmol m−2 s−1) [34]. However, the values obtained in our study were higher than the annual average soil respiration reported in grassland ecosystems (2.05 μmol m−2 s−1) and farmland ecosystems on the Loess Plateau (1.7 μmol m−2 s−1) [35,36]. This discrepancy could be attributed to the high density of artificial grassland. It is known that root biomass has a significant impact on Rs [13,37]. Therefore, developed dense roots can promote autotrophic respiration and increase Rs. A large amount of aboveground biomass not only meets the substrate supply required for Rs, but also serves as the main source of litter formation, which has a positive effect on the soil organic matter content and the formation of buffering interfaces to regulate the surface microenvironment, thereby stimulating Rs. The study of Moyes et al. (2013) [38] and Mottee et al. (2018) [39] supported these findings. Another factor that may lead to a higher average Rs is a high soil organic carbon content, which is different from long-term cultivated farmland.
4.2. Effect of Warming on Soil Respiration
The response of Rs to warming and its driving factors have been widely studied in various ecosystems. Studies found that warming stimulated Rs in the boreal, temperate, arid, and Mediterranean [14,40,41,42,43]. Our results show that warming relatively increased Rs by 5.04%, which was similar to previous studies. Under the warming treatment, the plant height exceeded that of CK, and the increased aboveground biomass contributed to the input of organic carbon from plants to the soil. This increase in organic carbon input, as reflected by the higher content of POC and ROC (Figure 7), suggests that elevated temperatures could accelerate the migration rate of substrate particulate organic carbon and readily oxidizable carbon in the soil [44], thereby promoting soil mineralization and root development to increase the root biomass [13,45]. Additionally, warming led to a slight reduction in the carbon–nitrogen ratio of soil microbial biomass (Figure 8), indicating less nitrogen limitation in the soil. This reduction in nitrogen limitation was manifested by the promotion of underground carbohydrate distribution and increased growth of aboveground and underground plant parts [46]. All of the above are beneficial for increasing the contribution rate of autotrophic respiration to Rs and stimulating Rs. The study by Lu et al. [47] also found that warming significantly enhanced the plant carbon pool in both aboveground and underground parts in a grassland experiment. However, compared with other studies, the increase in Rs under W was not statistically significant (p > 0.05, Figure 4E). This lack of significance could be attributed to the fact that warming did not significantly increase Ts (p > 0.05; Figure 4A), which was due to high vegetation coverage. In our study station, the density and height of vegetation reduced the duration of solar radiation received by the ground, leading to reduced energy absorption and limited temperature rise.
4.3. Effect of Drought on Soil Respiration
In the context of future climate change, extreme drought and rainfall events are expected to occur more frequently, and their impact on ecosystem processes is often greater than the effects of warming and nitrogen deposition [48,49]. Most studies have shown that drought reduces Rs [46,50]. However, the response of Rs to drought varies across different ecosystems. Studies have shown that drought stress can reduce Rs in mesic and xeric ecosystems [15,16], while stimulating it in aquatic ecosystems [17,18,19]. In our experiment, drought relatively reduced Rs by 3.4%. This was consistent with previous studies on the semi-arid grassland ecosystem in Inner Mongolia and the Mediterranean ecosystem [14,49,51,52,53]. In arid and semi-arid ecosystems, water availability is the primary factor influencing the productivity and carbon source for Rs. Therefore, the lack of water causes many microorganisms to enter a dormant state, and restrict root and microbial growth, impede substrate and enzyme diffusion in the soil, limit soil biological activities, and consequently reduce autotrophic and heterotrophic respiration [54,55].
4.4. Interactive Effect of Warming and Drought on Soil Respiration
In most ecosystems (forest, farmland, grassland, wetland, and desert), most climatic factors have a positive effect on Rs, but the average respiration rate of single-factor treatments is lower than that of double-factor treatments [46]. Nonetheless, in our experiment, Rs decreased by 11.27% under WP, aligning with the findings of Zhou et al. (2016) [46] who integrated the effects of 150 global climate factors on Rs. When warming and drought scenarios coexist, water-limited soils generally exhibit reduced sensitivity to temperature changes, with water becoming a limiting factor, particularly in arid and semi-arid regions [56]. Rs becomes more responsive to changes in water availability [57]. Drought stress disrupts the temperature–soil respiration coupling by impeding the diffusion of soluble carbon substrates and extracellular enzymes, thus limiting microbial activity [58]. This will limit heterotrophic respiration. On the other hand, under drought conditions, soil warming further intensifies soil water stress, which may exceed the threshold that is suitable for the metabolism of plant roots, resulting in a decrease in C allocation to belowground [42]. This will reduce autotrophic respiration. Additionally, soil water deficit significantly reduces the temperature sensitivity of Rs [59,60,61]. In cases where a small degree of warming does not substantially impact the soil moisture’s physical and chemical properties, the dominant influence of soil moisture overwhelms the effect of temperature, as observed in several field experiments [49,62,63]. Overall, in future climate change scenarios, not only are changes in the magnitude of climate variables expected, but so as changes in their interannual and interannual variability [1]. The net carbon dioxide emissions from soil to the atmosphere will ultimately depend on the specific balance between warm and drought [64].
4.5. Responses of Soil Respiration to Environmental and Biotic Factors
Numerous studies have emphasized the crucial role of temperature in regulating Rs, with changes in Ts generally accounting for most of the seasonal variation in Rs [65,66]. In our study, a significant exponential relationship between Ts and Rs was observed throughout the growing season (p < 0.05), indicating that temperature was the main regulating factor of Rs dynamics. However, positive effects of Ts and Rs were observed during periods of sufficient VWC (Figure 10G), and negative effects were observed during periods of higher temperature (Figure 10A,D). The main reason was that high Ts resulted in increased evapotranspiration and reduced VWC. The negative impact of water limitation offset the positive effect of high temperature and vigorous growth, leading to different responses of Rs to temperature during different periods of regeneration.
In arid and semi-arid ecosystems, VWC is considered one of the most important factors affecting Rs [54,67,68]. Our research results in the semi-arid grassland ecosystem of the Loess Plateau support this conclusion, indicating a positive correlation between VWC and Rs (Figure 9B). The results of different regeneration periods also reinforce this conclusion (Figure 10B,E,H). Similar conclusions were reached in Inner Mongolia grasslands by Dong et al. [69]. Water directly impacts root and microbial processes, thereby influencing soil CO2 emissions [70,71]. Water limitation in grassland growth can lead to reduced litter input, diminished supply of photosynthate and other residues to roots [72,73], and decreased autotrophic respiration and Rs. Moreover, soil water limitation can impede the diffusion of organic solutes near microorganisms or reduce the microbial population size, resulting in reduced heterotrophic respiration and Rs [55,74,75].
Aboveground and belowground biomass are major biological factors influencing the Rs, as the material source for Rs is derived from photosynthesis, with aboveground biomass representing the primary product of photosynthesis [76]. In our study, Rs significantly increased with the increase in LAI and plant height (p < 0.05, Figure 7C,D). There was also a linear positive correlation between Rs and aboveground biomass at regeneration periods 2 and 3 (p < 0.05, Figure 10F,I). This is mainly because the increase in aboveground biomass enhanced the photosynthetic capacity, leading to a greater distribution of photosynthetic products to the root system and subsequent increases in belowground biomass. Root respiration is a component of Rs, and the quantity of root biomass directly determines the strength of Rs [77]. Additionally, the input of aboveground litter is crucial for carbon accumulation in the soil. Aboveground biomass facilitates litter accumulation, which promotes microbial growth, decomposition rates, and the synthesis and secretion of extracellular enzymes, all contributing to soil carbon mineralization and subsequent carbon emissions [78]. This conclusion is consistent with findings in the semi-arid grasslands of Hungary, the arid grasslands of Inner Mongolia, and the semi-arid grasslands of the Loess Plateau described in this study [79,80].
5. Conclusions
The conducted operational experiments provide valuable insights into the response of soil respiration to warming and drought. Our findings indicate that warming enhances soil respiration, while drought inhibits it. The interaction between warming and drought results in a significant reduction in soil respiration, emphasizing the limiting effect of drought stress on the temperature-soil respiration coupling, particularly in semi-arid regions. Furthermore, our results highlight the significance of soil temperature, soil water content, plant height, and leaf area index in regulating soil respiration. The exponential model successfully explains the relationship between soil temperature and respiration, while soil respiration demonstrates a linear positive correlation with soil water content, plant height, and leaf area index. These findings hold great importance for vegetation restoration efforts in degraded land within the context of global climate change. The research will provide scientific support for optimizing the carbon sequestration/emission reduction management plan of artificial grassland ecosystems in the Loess Plateau under the interaction of global change in the future. However, the duration of the experiment may be crucial for evaluating the response of the C process to environmental changes, as the effect of global change drivers on soil respiration will significantly change over time. Therefore, in future field experiments, the temporal variation in soil respiration and its components should be considered to prolong our understanding of the feedback of terrestrial carbon cycling on global change.
Author Contributions
Material preparation and data collection, J.L., J.Z. (Jingui Zhang), R.W., Q.X., L.L. and J.Z. (Jianjun Zhang); data analysis, J.L., T.M., W.L., Q.Y. and X.W.; data curation, J.L. and J.M.; writing—original draft preparation, J.L.; writing—review, J.M. and Y.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by grants from the National Natural Science Foundation of China (NSFC, 32371969), the Science and Technology Planning Project of Gansu Province (Basic Research Program, grant number 23JRRA1039, and Technology Innovation Guidance Program, grant number 20CX9NA105), and the Soil and Water Conservation Compensation Fee Project of Gansu Province in 2023 (Gan Shui Bao Fa [2022] No. 467).
Data Availability Statement
Data are contained within the article.
Acknowledgments
We are grateful to Juncheng Li for his assistance with the field measurements and instrumentation maintenance. We would also like to thank the editors and anonymous reviewers for their valuable comments and suggestions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Alexander, L.; Allen, S.; Bindoff, N. Climate Change 2013: The Physical Science Basis, in Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Ren, J.; Allison, I.; Carrasco, J.F. Synthesis Report. Contribution of Working Groups I, II & III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2007. [Google Scholar]
- Field, C.; Barros, V.; Stocker, T. Managing the Risks of Extreme Events and Disasters to Advance Climate Change Adaptation; Cambridge University Press: Cambridge, UK, 2012. [Google Scholar]
- Meng, C.; Niu, S.; Chang, W.; Quan, Q.; Zeng, H. Effects of warming and clipping on soil respiration and its components in alpine meadow. Acta Ecol. Sin. 2020, 40, 6405–6415. [Google Scholar]
- Guo, W.; Jing, C.; Wang, G.; Hou, Z.; Zhao, W. Responses of soil respiration and ecosystem respiration to precipitation in desert steppe on the northern slope of Tianshan Mountains. Acta Agrestia Sin. 2021, 29, 2031–2039. [Google Scholar]
- Luo, Y.; Zhou, X. Soil Respiration and the Environment; Elsevier Inc.: San Diego, CA, USA, 2006; pp. 64–68. [Google Scholar]
- Melillo, J.; Steudler, P.; Aber, J.; Newkirk, K.; Lux, H.; Bowles, F.; Catricala, C.; Magill, A.; Ahrens, T.; Morrisseau, S. Soil warming and carbon-cycle feedbacks to the climate system. Science 2002, 298, 2173–2176. [Google Scholar] [CrossRef] [PubMed]
- Kuzyakov, Y. Sources of CO2 efflux from soil and review of partitioning methods. Soil Biol. Biochem. 2006, 38, 425–448. [Google Scholar] [CrossRef]
- Phillips, C.; Nickerson, N.; Risk, D.; Bond, B. Interpreting diel hysteresis between soil respiration and temperature. Glob. Change Biol. 2011, 17, 515–527. [Google Scholar] [CrossRef]
- Guan, C.; Chen, N.; Qiao, L.; Zhao, C. Photosynthesis regulates the diel hysteresis pattern between soil respiration and soil temperature in a steppe grassland. Geoderma 2022, 408, 115561. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, L.; Xu, W.; Wang, H.; Wang, J.; Wang, W.; He, J. Several important issues of soil respiration under the background of climate warming. Acta Pratacult. Sin. 2019, 28, 164–173. [Google Scholar]
- Yang, Q.; Tian, D.; Zeng, H.; Niu, S. The main influencing factors and regulation process of soil respiration change under the background of precipitation pattern change. Chin. J. Plant Ecol. 2017, 41, 1239–1250. [Google Scholar]
- Xu, X.; Shi, Z.; Li, D.; Zhou, X.; Sherry, R.; Luo, Y. Plant community structure regulates responses of prairie soil respiration to decadal experimental warming. Glob. Change Biol. 2015, 21, 3846–3853. [Google Scholar] [CrossRef]
- Reynolds, L.; Johnson, B.; Pfeifer-Meister, L.; Bridgham, S. Soil respiration response to climate change in Pacific Northwest prairies is mediated by a regional Mediterranean climate gradient. Glob. Change Biol. 2015, 21, 487–500. [Google Scholar] [CrossRef]
- Borken, W.; Savage, K.; Davidson, E. Effects of experimental drought on soil respiration and radiocarbon efflux from a temperate forest soil. Glob. Change Biol. 2006, 12, 177–193. [Google Scholar] [CrossRef]
- Joos, O.; Heim, H.; Gilgen, A.; Schmid, M.; Siegolf, R.; Buchmann, N. Summer drought reduces total and litter-derived soil CO2 effluxes in temperate grassland-clues from a 13C litter addition experiment. Biogeosciences 2009, 6, 11005–11034. [Google Scholar]
- Savage, K.; Davidson, E. Interannual variation of soil respiration in two New England forests. Glob. Biogeochem. Cycles 2001, 15, 337–350. [Google Scholar] [CrossRef]
- Knorr, W.; Gobron, N.; Scholze, M.; Kaminski, T.; Schnur, R.; Pinty, B. Impact of terrestrial biosphere carbon exchanges on the anomalous CO2 increase in 2002–2003. Geophys. Res. Lett. 2007, 34, L09703. [Google Scholar] [CrossRef]
- Sowerby, A.; Emmett, B.; Tietema, A.; Claus, B. Contrasting effects of repeated summer drought on soil carbon efflux in hydric and mesic heathland soils. Glob. Change Biol. 2008, 14, 2388–2404. [Google Scholar] [CrossRef]
- Illeris, L.; Christensen, T.; Mastepanov, M. Moisture effects on temperature sensitivity of CO2 exchange in a subarctic heath ecosystem. Biogeochemistry 2004, 70, 317–330. [Google Scholar] [CrossRef]
- Patrick, L.; Cable, J.; Potts, D.; Ignace, D.; Barron-Gafford, G.; Griffith, A.; Alpert, H.; Van, G.; Robertson, T.; Huxman, T.; et al. Effects of an increase in summer precipitation on leaf, soil, and ecosystem fluxes of CO2 and H2O in a sotol grassland in Big Bend National Park, Texas. Oecologia 2007, 151, 704–778. [Google Scholar] [CrossRef]
- Zhang, B.; Tan, X.; Wang, S.; Chen, M.; Chen, S.; Ren, T.; Xia, J.; Bai, Y.; Han, X. Asymmetric sensitivity of ecosystem carbon and water processes in response to precipitation change in a semi-arid steppe. Funct. Ecol. 2017, 31, 1301–1311. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Y. Seasonal pattern of soil respiration and gradual changing effects of nitrogen addition in a soil of the Gurbantunggut Desert, northwestern China. Atmos. Environ. 2014, 85, 187–194. [Google Scholar] [CrossRef]
- Niu, S.; Wu, M.; Han, Y.; Xia, J.; Li, L.; Wan, S. Water-mediated responses of ecosystem carbon fluxes to climatic change in a temperate steppe. New Phytol. 2008, 177, 209–219. [Google Scholar] [CrossRef]
- Gao, X.; Zhao, Q.; Zhao, X.; Wu, P.; Pan, W.; Gao, X.; Sun, M. Temporal and spatial evolution of the standardized precipitation evapotranspiration index (SPEI) in the Loess Plateau under climate change from 2001 to 2050. Sci. Total Environ. 2017, 595, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Li, M. Assessment of Carbon Storage Status and Carbon Sequestration Potential of Ecosystem in Loess Plateau. Master’s Thesis, University of Chinese Academy of Sciences, Beijing, China, 2021. [Google Scholar]
- Wang, Y.; Deng, L.; Wu, G.; Wang, K.; Shangguan, Z. Estimates of carbon storage in grassland ecosystems on the loess plateau. Catena 2018, 164, 23–31. [Google Scholar] [CrossRef]
- Li, Z.; Yang, L.; Wang, G.; Hou, J.; Xin, B.; Liu, G.; Fu, B. The management of soil and water conservation the Loess Plateau of China: Present situations, problems, and counter-solutions. Acta Ecol. Sin. 2019, 39, 7398–7409. [Google Scholar]
- Fang, C.; Ye, J.; Gong, Y.; Pei, J.; Yuan, Z.; Xie, C.; Zhu, Y.; Yu, Y. Seasonal responses of soil respiration to warming and nitrogen addition in a semi-arid alfalfa-pasture of the Loess Plateau, China. Sci. Total Environ. 2017, 590–591, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhong, Z.; Zhu, Y.; Wang, J.; Yang, G.; Ren, C.; Han, X. Effects of Warming and Precipitation Enhancement on Soil Respiration of Abandoned Grassland in Loess Hilly Region. Environ. Sci. 2021, 43, 1657–1667. [Google Scholar]
- Deng, L. Response Mechanism of Ecosystem Carbon Sequestration to Vegetation Restoration in the Loess Plateau. Master’s Thesis, Northwest A&F University, Xianyang, China, 2014. [Google Scholar]
- Huang, Q.; Jiao, F.; Huang, Y.; Li, N.; Wang, B.; Gao, H.; An, S. Response of soil fungal community composition and functions on the alteration of precipitation in the grassland of Loess Plateau. Sci. Total Environ. 2021, 751, 142273. [Google Scholar] [CrossRef] [PubMed]
- Klein, J.; Harte, J.; Zhao, X. Experimental warming causes large and rapidspecies loss, dampened by simulated grazing, on the Tibetan Plateau. Ecol. Lett. 2004, 7, 1170–1179. [Google Scholar] [CrossRef]
- Chen, S.; Huang, Y.; Zou, J.; Shen, Q.; Hu, Z.; Qin, Y.; Chen, H.; Pan, G. Modeling interannual variability of global soil respiration from climate and soil properties. Agric. For. Meteorol. 2010, 150, 590–605. [Google Scholar] [CrossRef]
- Yu, H.; Li, Y.; Oshunsanya, S.; Are, K.; Geng, Y.; Saggar, S.; Liu, W.; Geng, Y.; Saggar, S.; Liu, W. Re-introduction of light grazing reduces soil erosion and soil respiration in a converted grassland on the Loess Plateau, China. Agric. Ecosyst. Environ. 2019, 280, 43–52. [Google Scholar] [CrossRef]
- Wang, R.; Wang, Z.; Sun, Q.; Zhao, M.; Du, L.; Wu, D.; Li, R.; Gao, X.; Guo, S. Effects of crop types and nitrogen fertilization on temperature sensitivity of soil respiration in the semi-arid Loess Plateau. Soil Tillage Res. 2016, 163, 1–9. [Google Scholar] [CrossRef]
- Verlinden, M.; Broeckx, L.; Wei, H.; Ceulemans, R. Soil CO2 efflux in a bioenergy plantation with fast-growing Populus trees—Influence of former land use, inter-row spacing and genotype. Plant Soil 2013, 369, 631–644. [Google Scholar] [CrossRef][Green Version]
- Moyes, A.; Bowling, D. Interannual variation in seasonal drivers of soil respiration in a semi-arid Rocky Mountain meadow. Biogeochemistry 2013, 113, 683–697. [Google Scholar] [CrossRef]
- Motte, L.; Mamadou, O.; Beckers, Y.; Bodson, B.; Heinesch, B.; Aubinet, M. Rotational and continuous grazing does not affect the total net ecosystem exchange of a pasture grazed by cattle but modifies CO2 exchange dynamics. Agric. Ecosyst. Environ. 2018, 253, 157–165. [Google Scholar] [CrossRef]
- Tremblay, S.; D’Orangeville, L.; Lambert, M.; Houle, D. Transplanting boreal soils to a warmer region increases soil heterotrophic respiration as well as its temperature sensitivity. Soil Biol. Biochem. 2018, 116, 203–212. [Google Scholar] [CrossRef]
- Liu, T.; Xu, Z.; Hou, Y.; Zhou, G. Effects of warming and changing precipitation rates on soil respiration over two years in a desert steppe of northern China. Plant Soil 2016, 400, 15–27. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, S.; Wan, S.; Wang, J.; Luan, J.; Wang, H. Differential responses of soil respiration to soil warming and experimental throughfall reduction in a transitional oak forest in central China. Agric. For. Meteorol. 2016, 226–227, 186–198. [Google Scholar] [CrossRef]
- Escolar, C.; Maestre, F.; Rey, A. Biocrusts modulate warming and rainfall exclusion effects on soil respiration in a semi-arid grassland. Soil Biol. Biochem. 2015, 80, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Sing, H. Soil carbon sequestration and rhizospheric microbial population in apricot orchards following plastic film mulching under cold arid region. Int. J. Hortic. 2013, 3, 8. [Google Scholar] [CrossRef]
- Cuello, J.; Hwang, H.; Gutierrez, J.; Kim, S.; Kim, P. Impact of plastic film mulching on increasing greenhouse gas emissions in temperate upland soil during maize cultivation. Appl. Soil Ecol. 2015, 91, 48–57. [Google Scholar] [CrossRef]
- Zhou, L.; Zhou, X.; Shao, J.; Nie, Y.; He, Y.; Jiang, L.; Wu, Z.; Bai, S. Interactive effects of global change factors on soil respiration and its components: A meta-analysis. Glob. Change Biol. 2016, 22, 3157–3169. [Google Scholar] [CrossRef]
- Lu, M.; Zhou, X.; Yang, Q.; Li, H.; Luo, Y.; Fang, C.; Chen, J.; Yang, X.; Li, B. Responses of ecosystem carbon cycle to experimental warming: A meta-analysis. Ecology 2013, 94, 726–738. [Google Scholar] [CrossRef]
- Kardol, P.; Cregger, M.; Campany, C.; Classen, A. Soil ecosystem functioning under climate change: Plant species and community effects. Ecology 2010, 91, 767–781. [Google Scholar] [CrossRef]
- Matias, L.; Castro, J.; Zamora, R. Effect of simulated climate-change on soil respiration in a Mediterranean-type ecosystem: Rainfall and habitat-type are more important than temperature or the soil carbon pool. Ecosystems 2012, 15, 299–310. [Google Scholar] [CrossRef]
- Selsted, M.; Linden, L.; Ibrom, A.; Michelsen, A.; Larsen, K.; Pedersen, J.; Mikkelsen, T.; Pilegaard, K.; Beier, C.; Ambus, P. Soil respiration is stimulated by elevated CO2 and reduced by summer drought: Three years of measurements in a multifactor ecosystem manipulation experiment in a temperate heathland (CLIMAITE). Glob. Change Biol. 2012, 18, 1216–1230. [Google Scholar] [CrossRef]
- Qian, R.; Hao, Y.; Li, L.; Zheng, Z.; Wen, F.; Cui, X.; Wang, Y.; Zhao, T.; Tang, Z.; Du, J.; et al. Joint control of seasonal timing and plant function types on drought responses of soil respiration in a semiarid grassland. Front. Plant Sci. 2022, 13, 974418. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Calcerrada, J.; Salomon, R.; Barba, J.; Gordaliza, G.; Curiel Yuste, J.; Magro, C.; Gil, L. Regeneration in the understory of declining overstory trees contributes to soil respiration homeostasis along succession in a sub-Mediterranean Beech forest. Forests 2019, 10, 727. [Google Scholar] [CrossRef]
- Talmon, Y.; Sternberg, M.; Grünzweig, J. Impact of rainfall manipulations and biotic controls on soil respiration in Mediterranean and desert ecosystems along an aridity gradient. Glob. Change Biol. 2011, 17, 1108–1118. [Google Scholar] [CrossRef]
- Schimel, D.; House, J.; Hibbard, K.; Bousquet, P.; Ciais, P.; Peylin, P.; Braswell, B.; Apps, M.; Baker, D.; Bondeau, A.; et al. Recent patterns and mechanisms of carbon exchange by terrestrial ecosystems. Nature 2001, 414, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Davidson, E.; Janssens, I. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 2006, 440, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Suseela, V.; Conant, R.; Wallenstein, M.; Dukes, J. Effects of soi moisture on the temperature sensitivity of heterotrophic respiration vary seasonally in an oldfield climate change experiment. Glob. Change Biol. 2012, 18, 336–348. [Google Scholar] [CrossRef]
- Liu, L.; Wang, X.; Lajeunesse, M.; Miao, G.; Piao, S.; Wan, S.; Wu, Y.; Wang, Z.; Yang, S.; Li, P.; et al. A cross-biome synthesis of soil respiration and its determinants under simulated precipitation changes. Glob. Change Biol. 2016, 22, 1394–1405. [Google Scholar] [CrossRef]
- Manzoni, S.; Schimel, J.; Porporato, A. Responses of soil microbial communities to water stress: Results from a meta-analysis. Ecology 2012, 93, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Sampson, D.; Janssens, I.; Curiel Yuste, J.; Ceulemans, R. Basal rates of soil respiration are correlated with photosynthesis in a mixed temperate forest. Glob. Change Biol. 2007, 13, 2008–2017. [Google Scholar] [CrossRef]
- Jassal, R.; Black, T.; Novak, M.; Gaumont-Guay, D.; Nesic, Z. Effect of soil water stress on soil respiration and its temperature sensitivity in an 18-year-old temperate Douglas-fir stand. Glob. Change Biol. 2008, 14, 1305–1318. [Google Scholar] [CrossRef]
- Rey, A.; Pegoraro, E.; Tedeschi, V.; De Parri, I. Annual variation in soil respiration and its components in a coppice oak forest in central Italy. Glob. Change Biol. 2002, 8, 851–866. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Z.; Wan, S. Predominant role of water in regulating soil and microbial respiration and their responses to climate change in a semiarid grassland. Glob. Change Biol. 2009, 15, 184–195. [Google Scholar] [CrossRef]
- Suseela, V.; Dukes, J. The responses of soil and rhizosphere respiration to simulated climatic changes vary by season. Ecology 2013, 94, 403–413. [Google Scholar] [CrossRef]
- Matías, L.; Hidalgo-Galvez, M.; Cambrollé, J.; Domínguez, M.; Pérez-Ramos, I. How will forecasted warming and drought affect soil respiration in savannah ecosystems? The role of tree canopy and grazing legacy. Agric. For. Meteorol. 2021, 304–305, 108425. [Google Scholar] [CrossRef]
- Fang, C.; Moncrieff, J. The dependence of soil CO2 efflux on temperature. Soil Biol. Biochem. 2001, 33, 155–165. [Google Scholar] [CrossRef]
- Liu, X.; Wan, S.; Su, B.; Hui, D.; Luo, Y. Response of soil CO2 efflux to water manipulation in a tall grass prairie ecosystem. Plant Soil 2002, 240, 213–223. [Google Scholar] [CrossRef]
- Shi, G.; Geng, H.; Wang, Y.; Wnag, Y.; Qi, X. Daily and seasonal dynamics of soil respiration and their environmental controlling factors in Stipa krylovii steppe. Acta Ecol. Sin. 2008, 28, 3408–3416. [Google Scholar]
- Ru, J.; Zhou, Y.; Hui, D.; Zheng, M.; Wan, S. Shifts of growingseason precipitation peaks decrease soil respiration in a semiarid grassland. Glob. Change Biol. 2018, 24, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Zhang, S.; Qi, Y.; Chen, Z.; Geng, Y. Simultaneous observation and diurnal variation of CO2, N2O, CH4 fluxes in typical grasslands of Inner Mongolia. Chin. Sci. Bull. 2000, 3, 318–322. [Google Scholar]
- Linn, D.; Doran, J. Effect of water-filled pore-space on carbondioxide and nitrous-oxide production in tilled and nontilled soils. Soil Sci. Soc. Am. J. 1984, 8, 1267–1272. [Google Scholar] [CrossRef]
- Zhang, C.; Niu, D.; Hall, S.; Wen, H.; Lia, X.; Fu, H.; Wan, C.; Elser, J. Effects of simulated nitrogen deposition on soil respiration components and their temperature sensitivities in a semiarid grassland. Soil Biol. Biochem. 2014, 75, 113–123. [Google Scholar] [CrossRef]
- Carbone, M.; Still, C.; Ambrose, A.; Dawson, T.; Williams, A.; Boot, C.; Schaeffer, S.; Schimel, J. Seasonal and episodic moisture controls on plant and microbial contributions to soil respiration. Oecologia 2011, 167, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Casanovas, N.; Matamala, R.; Cook, D.; Gonzalez-Meler, M. Net ecosystem exchange modifies the relationship between the autotrophic and heterotrophic components of soil respiration. Biol. Fertil. Soils 2012, 55, 275–283. [Google Scholar]
- Sowerby, A.; Emmett, B.; Beier, C.; Tietema, A.; Peñuelas, J.; Estiarte, M.; VanMeeteren, M.; Hughes, S.; Freeman, C. Microbial community changes in heathland soil communities along a geographical gradient: Interaction with climate change manipulations. Soil Biol. Biochem. 2005, 37, 1805–1813. [Google Scholar] [CrossRef]
- Zhang, Q.; Zak, J. Effects of water and nitrogen amendment on soil microbial biomass and fine root production in a semi-arid environment in West Texas. Soil Biol. Biochem. 1998, 30, 39–45. [Google Scholar] [CrossRef]
- Rochette, P.; Flanagan, L. Quantifying rhizosphere respiration in a corn crop under field conditions. Soil Sci. Soc. Am. J. 1997, 61, 466–474. [Google Scholar] [CrossRef]
- Hanson, P.; Edwards, N.; Garten, C.; Andrews, J. Separating root and soil microbial contributions to soil respiration: A review of methods and observations. Biogeochemistry 2000, 48, 115–146. [Google Scholar] [CrossRef]
- Domínguez, M.; Gutiérrez, E.; González-Domínguez, B.; Román, M.; Ávila, J.; Ramo, C.; Gonzalez, J.; García, L. Impacts of protected colonial birds on soil microbial communities: When protection leads to degradation. Soil Biol. Biochem. 2017, 105, 59–70. [Google Scholar] [CrossRef]
- Koncz, P.; Balogh, J.; Papp, M.; Hidy, D.; Pinter, K.; Foti, S.; Klumpp, K.; Nagy, Z. Higher soil respiration under mowing than under grazing explained by biomass differences. Nutr. Cycl. Agroecosyst. 2015, 103, 201–215. [Google Scholar] [CrossRef]
- Hao, Y.; Zhou, C.; Liu, W.; Li, L.; Kang, X.; Jiang, L.; Cui, X.; Wang, Y.; Zhou, X.; Xu, C. Aboveground net primary productivity and carbon balance remain stable under extreme precipitation events in a semiarid steppe ecosystem. Agric. For. Meteorol. 2017, 240–241, 1–9. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).