Effects of the Combined Application of Nitrogen and Selenium on Tea Quality and the Expression of Genes Involved in Nitrogen Uptake and Utilization in Tea Cultivar ‘Chuancha No.2’
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatments
2.2. Determination of Major Tea Quality Components
2.3. Determination of Carbon and Nitrogen Content
2.4. Analysis of the Expression of Key Genes Involved in Nitrogen Uptake and Utilization
2.5. Statistical Analysis
3. Results
3.1. Effects of Nitrogen and Selenium on the Contents of Tea Polyphenols, Free Amino Acids and Caffeine
3.1.1. Contents of Tea Polyphenol in Tea Shoots
3.1.2. Contents of Amino Acids in Tea Shoots
3.1.3. Contents of Caffeine in Tea Shoots
3.2. Content of Tea Polyphenols and Amino Acids in Mature Leaves of Tea Plants
3.3. Total Nitrogen and Carbon Contents of Mature Leaves and Roots
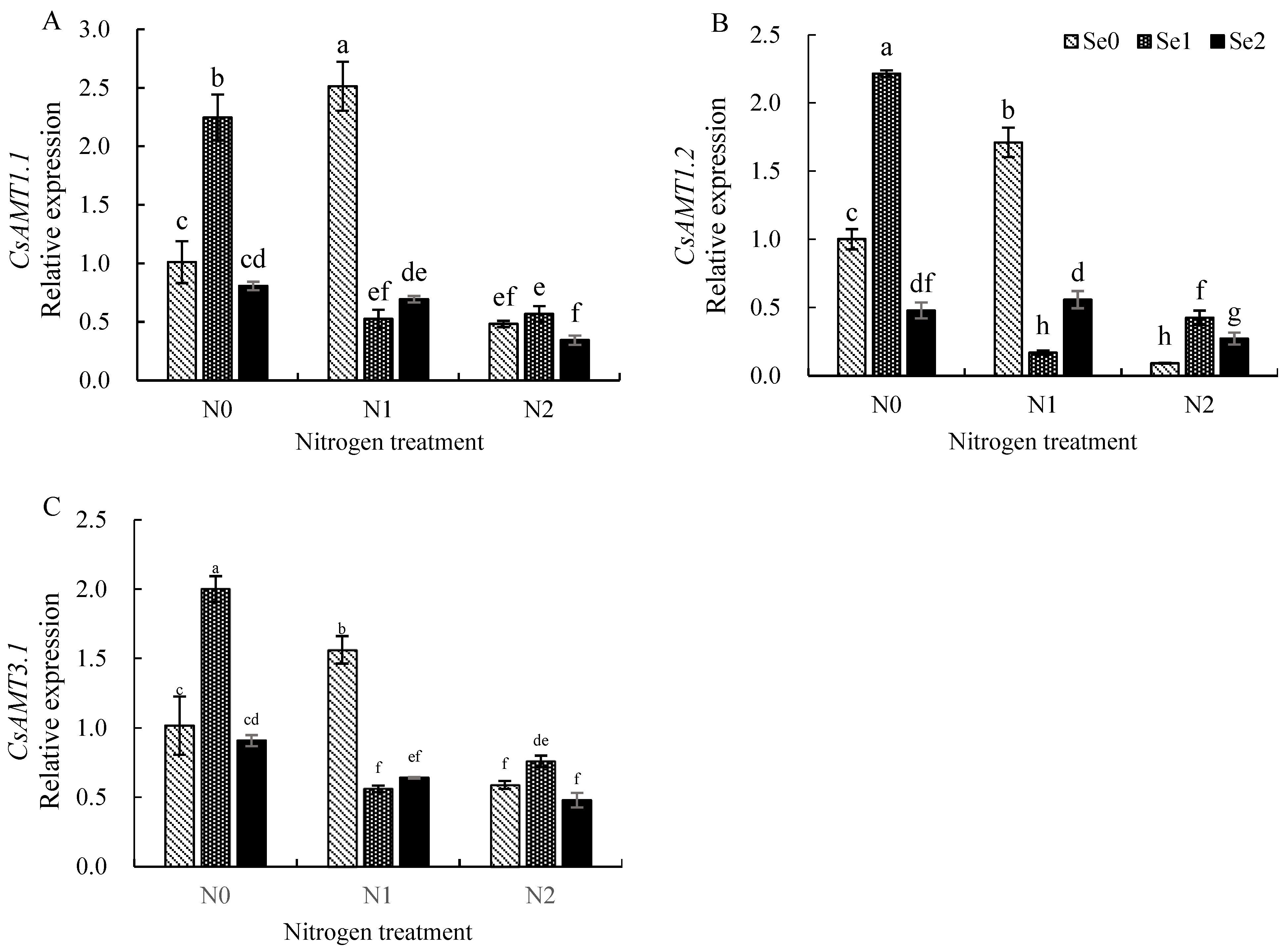
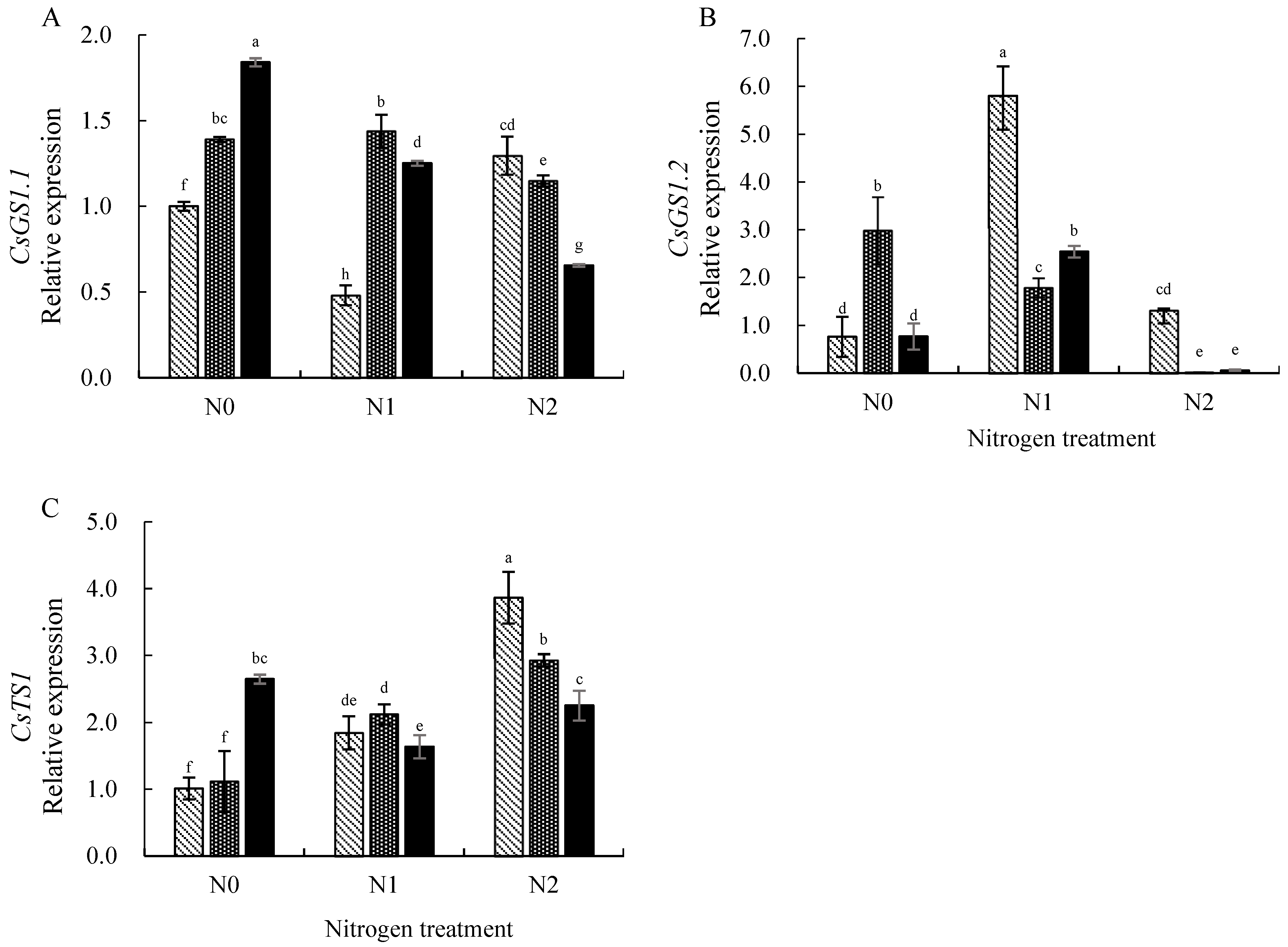
3.4. Expression Levels of Key Genes Involved in Nitrogen Uptake and Utilization in Tea Leaves
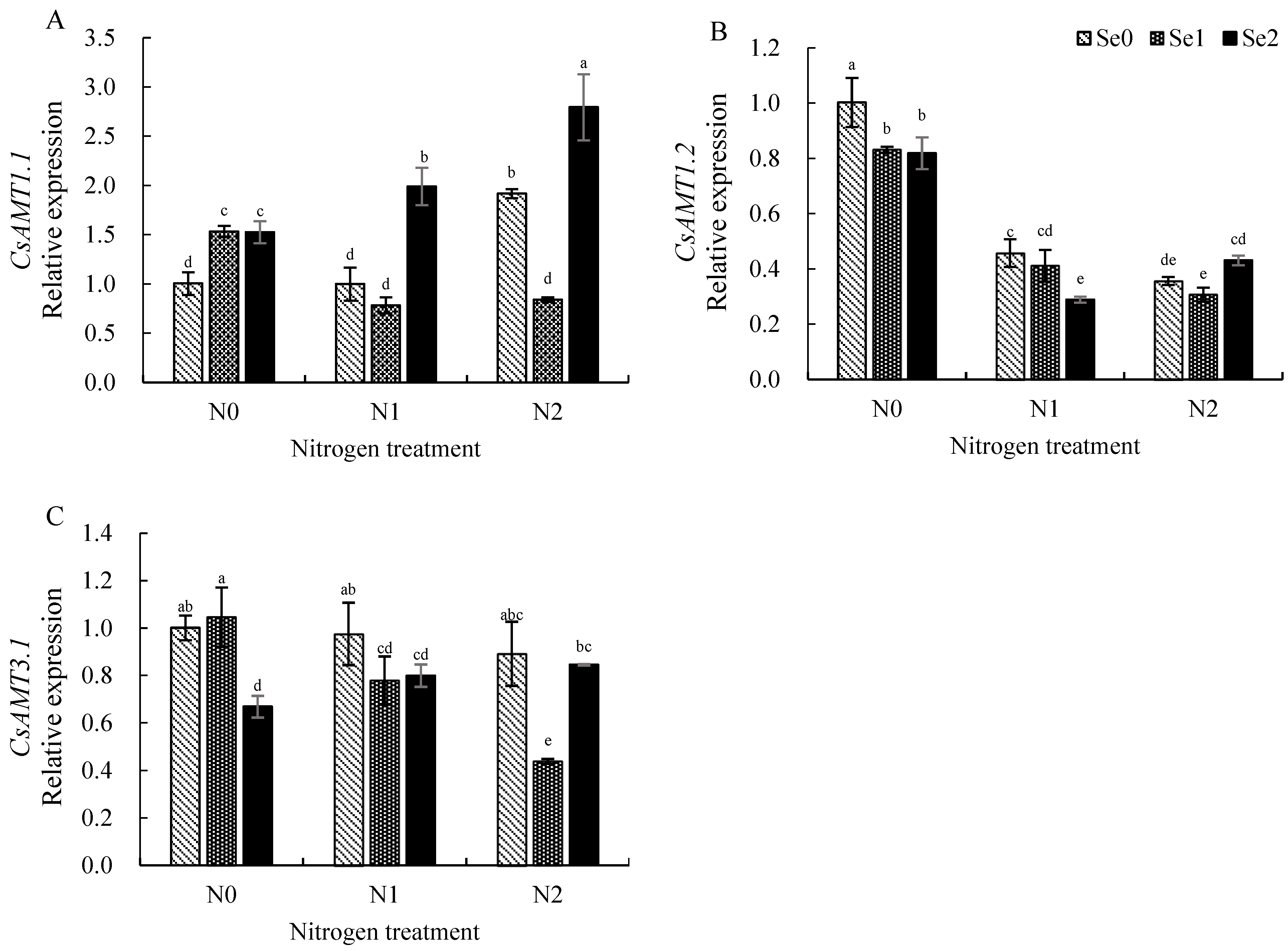
3.5. Expression Levels of Key Genes Involved in Nitrogen Uptake and Utilization in Tea Roots
4. Discussion
4.1. Effect of Nitrogen and Selenium on Tea Quality Components
4.2. Effect of Nitrogen and Selenium Rationing on the Total Nitrogen and Carbon Contents of Mature Leaves and Roots of Tea Plants
4.3. Effects of Nitrogen and Selenium on the Expression of Key Genes in Nitrogen Metabolism in Tea Plants
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, L.; Cao, Q.-Q.; Granato, D.; Xu, Y.-Q.; Ho, C.-T. Association between Chemistry and Taste of Tea: A Review. Trends Food Sci. Technol. 2020, 101, 139–149. [Google Scholar] [CrossRef]
- Zhang, W.; Ni, K.; Long, L.; Ruan, J. Nitrogen Transport and Assimilation in Tea Plant (Camellia Sinensis): A Review. Front. Plant Sci. 2023, 14, 1249202. [Google Scholar] [CrossRef]
- Tang, D.; Liu, M.-Y.; Zhang, Q.; Ma, L.; Shi, Y.; Ruan, J. Preferential Assimilation of NH4+ over NO3− in Tea Plant Associated with Genes Involved in Nitrogen Transportation, Utilization and Catechins Biosynthesis. Plant Sci. 2020, 291, 110369. [Google Scholar] [CrossRef]
- Ruan, J.; Ma, L.; Yi, X.; Shi, Y.; Ni, K.; Liu, M.; Zhang, F. Integrated Nutrient Management in Tea Plantation to Reduce Chemical Fertilizer and Increase Nutrient Use Efficiency. J. Tea Sci. 2020, 40, 85–95. [Google Scholar] [CrossRef]
- Ruan, J.; Ma, L.; Shi, Y.; Zhang, F. Effects of Litter Incorporation and Nitrogen Fertilization on the Contents of Extractable Aluminium in the Rhizosphere Soil of Tea Plant (Camallia Sinensis (L.) O. Kuntze). Plant Soil 2004, 263, 283–296. [Google Scholar] [CrossRef]
- Yan, P.; Wu, L.; Wang, D.; Fu, J.; Shen, C.; Li, X.; Zhang, L.; Zhang, L.; Fan, L.; Wenyan, H. Soil Acidification in Chinese Tea Plantations. Sci. Total Environ. 2020, 715, 136963. [Google Scholar] [CrossRef]
- Su, Y.; Wang, Y.; Zhang, Y.; Luo, Y.; Liao, W. Soil Acidification Control and Improvement Technology for Tea Plantations. China Tea 2018, 40, 9–11+15. [Google Scholar]
- Ma, L.; Yang, X.; Shi, Y.; Yi, X.; Ji, L.; Cheng, Y.; Ni, K.; Ruan, J. Response of Tea Yield, Quality and Soil Bacterial Characteristics to Long-Term Nitrogen Fertilization in an Eleven-Year Field Experiment. Appl. Soil Ecol. 2021, 166, 103976. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, F.; Wu, Z.; Jiang, F.; Yu, W.; Yang, J.; Chen, J.; Jian, G.; You, Z.; Zeng, L. Effects of Long-Term Nitrogen Fertilization on the Formation of Metabolites Related to Tea Quality in Subtropical China. Metabolites 2021, 11, 146. [Google Scholar] [CrossRef]
- Liu, C.; Ren, D.; Liu, H.; Zhang, Y.; Wang, L.; Li, Z.; Zhang, M. Optimizing Nitrogen Management Diminished Reactive Nitrogen Loss and Acquired Optimal Net Ecosystem Economic Benefit in a Wheat-Maize Rotation System. J. Clean. Prod. 2022, 331, 129964. [Google Scholar] [CrossRef]
- Ye, R.; Huang, J.; Wang, Z.; Chen, Y.; Dong, Y. Trace Element Selenium Effectively Alleviates Intestinal Diseases. Int. J. Mol. Sci. 2021, 22, 11708. [Google Scholar] [CrossRef]
- Feng, R.; Wang, L.; Yang, J.; Zhao, P.; Zhu, Y.; Li, Y.; Yu, Y.; Liu, H.; Rensing, C.; Wu, Z.; et al. Underlying Mechanisms Responsible for Restriction of Uptake and Translocation of Heavy Metals (Metalloids) by Selenium via Root Application in Plants. J. Hazard. Mater. 2021, 402, 123570. [Google Scholar] [CrossRef]
- Teixeira, L.S.; Pimenta, T.M.; Brito, F.A.L.; Malheiros, R.S.P.; Arruda, R.S.; Araújo, W.L.; Ribeiro, D.M. Selenium Uptake and Grain Nutritional Quality Are Affected by Nitrogen Fertilization in Rice (Oryza Sativa L.). Plant Cell Rep. 2021, 40, 871–880. [Google Scholar] [CrossRef]
- Pilon-Smits, E.A.H. On the Ecology of Selenium Accumulation in Plants. Plants 2019, 8, 197. [Google Scholar] [CrossRef]
- Ren, H.; Li, X.; Guo, L.; Wang, L.; Hao, X.; Zeng, J. Integrative Transcriptome and Proteome Analysis Reveals the Absorption and Metabolism of Selenium in Tea Plants [Camellia Sinensis (L.) O. Kuntze]. Front. Plant Sci. 2022, 13, 848349. [Google Scholar] [CrossRef]
- Xiang, J.; Rao, S.; Chen, Q.; Zhang, W.; Cheng, S.; Cong, X.; Zhang, Y.; Yang, X.; Xu, F. Research Progress on the Effects of Selenium on the Growth and Quality of Tea Plants. Plants 2022, 11, 2491. [Google Scholar] [CrossRef]
- Ho, C.-H.; Lin, S.-H.; Hu, H.-C.; Tsay, Y.-F. CHL1 Functions as a Nitrate Sensor in Plants. Cell 2009, 138, 1184–1194. [Google Scholar] [CrossRef]
- Lei, Z.; Li, Q.; Tang, Y.; Zhang, H.; Han, C.; Wang, X.; Zhao, X.; Shi, G. Selenium Enhanced Nitrogen Accumulation in Legumes in Soil with Rhizobia Bacteria. J. Clean. Prod. 2022, 380, 134960. [Google Scholar] [CrossRef]
- Zhan, T.; Hu, C.; Kong, Q.; Shi, G.; Tang, Y.; Zhou, Y.; Guo, Z.; Zhai, H.; Xiao, X.; Zhao, X. Chitin Combined with Selenium Reduced Nitrogen Loss in Soil and Improved Nitrogen Uptake Efficiency in Guanxi Pomelo Orchard. Sci. Total Environ. 2021, 799, 149414. [Google Scholar] [CrossRef]
- Li, S.; Chen, H.; Jiang, S.; Hu, F.; Xing, D.; Du, B. Selenium and Nitrogen Fertilizer Management Improves Potato Root Function, Photosynthesis, Yield and Selenium Enrichment. Sustainability 2023, 15, 6060. [Google Scholar] [CrossRef]
- Liu, Q.; Lin, Y.; Hu, X.; Wu, A.; Ye, X.; Chen, F. Influences of N and phosphorus fertilizers on the absorption and accumulation of zinc, selenium, and other trace elements in tea plants. Acta Ecol. Sin. 2021, 41, 637–644. [Google Scholar]
- Li, D.; Zhou, C.; Zou, N.; Wu, Y.; Zhang, J.; An, Q.; Li, J.-Q.; Pan, C. Nanoselenium Foliar Application Enhances Biosynthesis of Tea Leaves in Metabolic Cycles and Associated Responsive Pathways. Environ. Pollut. 2021, 273, 116503. [Google Scholar] [CrossRef]
- Pérez-Millán, R.; Alfosea-Simón, M.; Simón-Grao, S.; Cámara-Zapata, J.M.; Zavala-González, E.A.; Aranda-Martinez, A.; Shahid, M.A.; García-Sánchez, F. Effects of Se Application on Polyamines and Carbon–Nitrogen Metabolism of Pepper Plants Suffering from Cd Toxicity. Agronomy 2021, 11, 2535. [Google Scholar] [CrossRef]
- Rios, J.J.; Blasco, B.; Rosales, M.A.; Sanchez-Rodriguez, E.; Leyva, R.; Cervilla, L.M.; Romero, L.; Ruiz, J.M. Response of Nitrogen Metabolism in Lettuce Plants Subjected to Different Doses and Forms of Selenium: Response of Nitrogen Metabolism to Selenium in Lettuce. J. Sci. Food Agric. 2010, 90, 1914–1919. [Google Scholar] [CrossRef]
- Sun, M.; Wang, J.; Liu, W.; Yin, P.; Guo, G.; Tong, C.; Chang, Y. Effect and Mechanism of Exogenous Selenium on Selenium Content and Quality of Fresh Tea Leaves. Not. Bot. Horti Agrobot. 2022, 50, 12814. [Google Scholar] [CrossRef]
- Ruan, J.; Gerendás, J.; Härdter, R.; Sattelmacher, B. Effect of Nitrogen Form and Root-Zone pH on Growth and Nitrogen Uptake of Tea (Camellia Sinensis) Plants. Ann. Bot. 2007, 99, 301–310. [Google Scholar] [CrossRef]
- Ruan, J.; Gerendás, J.; Härdter, R.; Sattelmacher, B. Effect of Root Zone pH and Form and Concentration of Nitrogen on Accumulation of Quality-related Components in Green Tea. J. Sci. Food Agric. 2007, 87, 1505–1516. [Google Scholar] [CrossRef]
- Wang, D.; Huang, Y.; Zhou, Z.; Li, T.; Wu, F.; Yao, Q. Effects of selenate at different concentrations on growth and physiological indexes of tea tree. Guihaia 2021, 41, 183–194. [Google Scholar]
- GB/T 8313-2018[S]; Determination of Total Polyphenols and Catechins Content in Tea. State Administration for Market Regulation: Beijing, China, 2018.
- GB/T 8314-2013[S]; Tea-Determination of Free Amino Acids Conten. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2013.
- GB/T 8312-2013[S]; Tea-Determination of Caffeine Content. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2013.
- Zhang, F.; Liu, Y.; Wang, L.; Bai, P.; Ruan, L.; Zhang, C.; Wei, K.; Cheng, H. Molecular Cloning and Expression Analysis of Ammonium Transporters in Tea Plants (Camellia Sinensis (L.) O. Kuntze) under Different Nitrogen Treatments. Gene 2018, 658, 136–145. [Google Scholar] [CrossRef]
- Tang, D.; Liu, M.; Zhang, Q.; Fan, K.; Shi, Y.; Ma, L.; Yi, X.; Ni, K.; Ruan, J. Isolation and expression profiles of cytosolic glutamine synthetase genes CsGS1s in tea plant (Camellia sinensis). Plant Physiol. J. 2018, 54, 71–80. [Google Scholar] [CrossRef]
- Wei, C.; Yang, H.; Wang, S.; Zhao, J.; Liu, C.; Gao, L.; Xia, E.; Lu, Y.; Tai, Y.; She, G.; et al. Draft Genome Sequence of Camellia Sinensis Var. Sinensis Provides Insights into the Evolution of the Tea Genome and Tea Quality. Proc. Natl. Acad. Sci. USA 2018, 115, E4151–E4158. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Chandran, A.K.N.; Priatama, R.A.; Kumar, V.; Xuan, Y.; Je, B.I.; Kim, C.M.; Jung, K.-H.; Han, C. Genome-Wide Transcriptome Analysis of Expression in Rice Seedling Roots in Response to Supplemental Nitrogen. J. Plant Physiol. 2016, 200, 62–75. [Google Scholar] [CrossRef]
- Kant, S.; Bi, Y.-M.; Rothstein, S.J. Understanding Plant Response to Nitrogen Limitation for the Improvement of Crop Nitrogen Use Efficiency. J. Exp. Bot. 2011, 62, 1499–1509. [Google Scholar] [CrossRef]
- Li, W.; Xiang, F.; Zhong, M.; Zhou, L.; Liu, H.; Li, S.; Wang, X. Transcriptome and Metabolite Analysis Identifies Nitrogen Utilization Genes in Tea Plant (Camellia Sinensis). Sci. Rep. 2017, 7, 1693. [Google Scholar] [CrossRef]
- Wang, Y.; Xuan, Y.; Wang, S.; Fan, D.; Wang, X.; Zheng, X. Genome-wide Identification, Characterization, and Expression Analysis of the Ammonium Transporter Gene Family in Tea Plants (Camellia Sinensis L.). Physiol. Plant. 2022, 174, e13646. [Google Scholar] [CrossRef]



| Treatment | Tea Polyphenols Content (%) | Amino Acid Content (%) |
|---|---|---|
| N0Se0 | 15.06 ± 0.053 b | 2.21 ± 0.028 bc |
| N0Se1 | 13.92 ± 0.070 e | 2.06 ± 0.029 e |
| N0Se2 | 14.50 ± 0.026 d | 2.20 ± 0.008 c |
| N1Se0 | 14.79 ± 0.070 c | 2.06 ± 0.023 e |
| N1Se1 | 12.98 ± 0.026 f | 2.05 ± 0.034 e |
| N1Se2 | 16.49 ± 0.388 a | 2.94 ± 0.004 a |
| N2Se0 | 12.55 ± 0.046 g | 2.13 ± 0.067 d |
| N2Se1 | 13.86 ± 0.070 e | 2.25 ± 0.017 b |
| N2Se2 | 12.36 ± 0.050 h | 2.08 ± 0.023 e |
| ANOVA (F-value) | ||
| N | 6918.26 ** | 175.31 ** |
| Se | 1340.83 ** | 349.36 ** |
| N × Se | 4130.62 ** | 444.21 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, S.; Bai, Y.; Jin, Z.; Long, L.; Diao, W.; Chen, W.; Tan, L.; Tang, Q.; Tang, D. Effects of the Combined Application of Nitrogen and Selenium on Tea Quality and the Expression of Genes Involved in Nitrogen Uptake and Utilization in Tea Cultivar ‘Chuancha No.2’. Agronomy 2023, 13, 2997. https://doi.org/10.3390/agronomy13122997
Zhao S, Bai Y, Jin Z, Long L, Diao W, Chen W, Tan L, Tang Q, Tang D. Effects of the Combined Application of Nitrogen and Selenium on Tea Quality and the Expression of Genes Involved in Nitrogen Uptake and Utilization in Tea Cultivar ‘Chuancha No.2’. Agronomy. 2023; 13(12):2997. https://doi.org/10.3390/agronomy13122997
Chicago/Turabian StyleZhao, Shenxin, Yanling Bai, Zhen Jin, Lu Long, Wantian Diao, Wei Chen, Liqiang Tan, Qian Tang, and Dandan Tang. 2023. "Effects of the Combined Application of Nitrogen and Selenium on Tea Quality and the Expression of Genes Involved in Nitrogen Uptake and Utilization in Tea Cultivar ‘Chuancha No.2’" Agronomy 13, no. 12: 2997. https://doi.org/10.3390/agronomy13122997





