Abstract
Selecting alfalfa germplasm with high stress resistance forms the foundation for breeding new varieties of alfalfa (Medicago sativa L.). This study utilized two alfalfa varieties, WL298HQ and Gongnong No. 1, and subjected them to individual and combined cold and drought stress. By measuring the malondialdehyde (MDA) content among seven physiological and biochemical indices and through transcriptome sequencing of roots and leaves, we compared drought and cold resistance between varieties under various stress treatments. This study aimed to identify the primary regulatory genes and pathways, revealing the molecular mechanisms behind their responses to combined stresses. The results showed that under isolated drought and cold stress, the chlorophyll content of the two types of alfalfa significantly decreased (p < 0.05), while the content of MDA, peroxidase (POD), catalase (CAT), superoxide dismutase (SOD), proline (Pro), and soluble protein (SP) content increased (p < 0.05), quickly returning to normal levels upon rehydration. Compared to the control group, 41,103 and 41,831 differentially expressed genes were identified in WL298HQ and Gongnong No. 1, respectively. Differentially expressed genes in Gongnong No. 1 were significantly enriched in the “response to abiotic stimulus”, “response to abscisic stimulus”, and “response to cold” pathways. WL298HQ was enriched in “response to desiccation”, “gibberellic acid-mediated signaling pathway”, and “cellular response to gibberellin stimulus”. Additionally, nineteen genes were significantly concentrated in the abscisic acid (ABA) signaling pathway, with nine genes significantly upregulated in leaves and ten genes downregulated in roots, suggesting that different parts of the alfalfa respond to stress inconsistently. Our findings provide a theoretical basis for understanding the response mechanisms of alfalfa to combined drought and cold stress.
1. Introduction
Alfalfa (Medicago sativa L.) is a perennial tetraploid legume of high economic value, renowned for its adaptability, nutritional value, and palatability, earning its reputation as the “king of forages”. Biotic stresses, such as cold, drought, and salinization, are significant factors affecting growth and are also crucial constraints on yield and survival. Thus, breeding stress-resistant alfalfa varieties holds both scientific significance and potential application value [1].
Plants activate various mechanisms to cope with adversities such as drought and cold, minimizing the resulting damage [2]. Alfalfa research has transitioned from phenotypic, agronomic traits, and physiological characteristics to deeper molecular-level methodologies such as transcriptomics, metabolomics, proteomics, genomics, and genome-wide association analysis (GWAS) to identify stress-related genes [3,4]. In recent years, scholars worldwide have conducted a series of stress-resistance studies on cold and drought and achieved significant results. Assessing agronomic indicators, morphology, and physiological characteristics of alfalfa seedlings revealed that as the intensity of drought stress increased, the content of chlorophyll and soluble proteins showed a trend of first increasing and then decreasing [5]. Studies have indicated that under drought conditions, the content of MDA and Pro in the leaves and roots of WL363HQ and Juneng7 alfalfa, along with the activities of antioxidative enzymes, increased [6].
Transcription factors (TFs) are proteins that specifically bind to DNA sequences [7]. Plants perceive and respond to various environmental and developmental signals through intricate signaling pathways. These pathways consist of various proteins and non-proteins, with proteins including various enzymes, receptors, and TFs, and non-proteins that include secondary messengers, such as Ca2+, cyclic AMP, and cyclic GMP [8]. When plants counteract various adversities, transcription factors such as NAC (N-acetylcysteine) [9], MYB, WRKY, basic region/leucine zipper (bZIP), and AP2/ERF play pivotal roles [10]. Post drought stress, overexpression of MsNAC2 in tobacco led to reduced MDA content, increased Pro content, and enhanced SOD and POD activities [11]. Overexpression of the MsMYB2L gene in Arabidopsis increased the content of Pro and soluble sugar [12]. Studies found that in Medicago falcata, R2R3-MYB transcription factors, miRNAs, and other factors were related to drought stress regulation [13,14]. As the duration of drought stress increased, MDA and Pro contents increased, and antioxidative enzyme activities such as SOD, POD, and CAT also increased. Through transcriptome sequencing, drought stress-related ERF transcription factor genes such as MfERF053, MfER9, MfER034, and MfRAP2.1 were successfully identified [15]. According to the complete alfalfa genome, the alfalfa-specific gene MsASG166 plays a positive regulatory role in the response to drought stress [16]. Studies suggest that C-repeat binding factors (CBFs) may participate in the cold resistance of alfalfa, and MYB15 can bind to the promoter regions of CBFs, suppressing their expression and weakening their cold resistance [17,18].
Current research advancements in the molecular stress resistance of alfalfa primarily focus on screening for stress-related genes through genomics. Most studies involved transferring related genes to model plants for preliminary functional analysis. Moreover, the identification of resistance genes has become a hot research topic in the field of plant resistance breeding, but more specific regulatory molecular mechanisms remain unclear. Scholars have achieved significant results in studying the molecular mechanisms of drought and cold resistance in model plants and primary crops; there are also numerous reports on alfalfa resistance to abiotic stresses. However, the molecular mechanisms by which alfalfa resists combined stresses, especially drought and cold stresses, remain underexplored.
2. Materials and Methods
2.1. Experimental Materials
According to a preliminary experiment, two alfalfa cultivars were selected for this study [19], namely ‘Gongnong No. 1’ and ‘WL298HQ’, with autumn dormancy ratings of 1.0, and 3.0, respectively. The seeds were provided by the Animal Husbandry Branch of the Jilin Provincial Academy of Agricultural Sciences and Beijing Zhengdao Seed Co., Ltd. (Beijing, China). The experiments were conducted in the Laboratory of Grassland Science at the Beijing Forestry University.
2.2. Alfalfa Seedling Cultivation
Healthy and complete alfalfa seeds were soaked in sodium hypochlorite for 10 min, followed by rinsing with distilled water seven to eight times. Seeds were sown in plastic pots (26 × 29 × 8 cm3) filled with vermiculite. Seedlings were grown in a controlled environment chamber at 25 °C, with a 14 h light/10 h dark cycle and 65% relative humidity for four weeks. The seedlings were watered every three days. All materials were disinfected with 75% ethanol and thoroughly rinsed with distilled water.
2.3. Abiotic Stress Treatments for Alfalfa
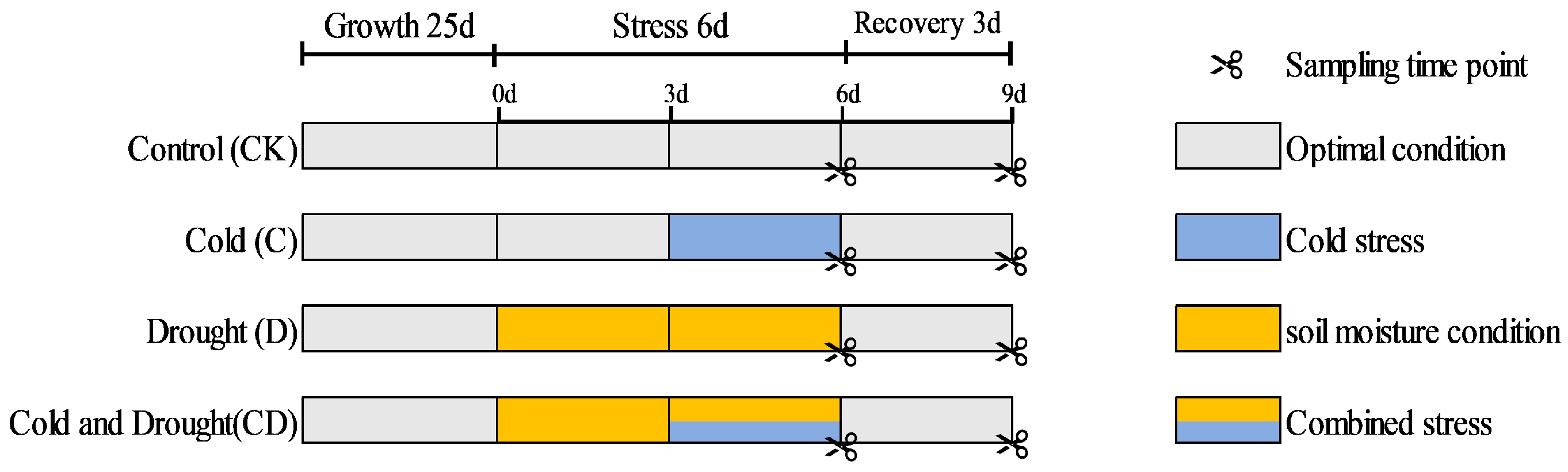
Four-week-old seedlings with consistent growth were selected and divided into four treatment groups, with three biological replications for each treatment. The first group was used as a control, maintaining soil moisture at 75–80% of field capacity. The second group was subjected to drought stress by decreasing soil moisture content and maintaining soil moisture at 15–20% of field capacity for six days. The third group was exposed to cold stress at 4 °C for three days. The fourth group underwent combined cold and soil moisture content; they first experienced soil moisture content for three days, followed by combined cold and soil moisture content for another three days. The preliminary experiments show that drought stress can form by maintaining soil moisture at this level [19]. Following the stress treatments, all seedlings were transferred to a 25 °C chamber for a three-day recovery period.
2.4. Measurement of Physiological and Biochemical Indicators in Alfalfa
After each treatment and recovery, alfalfa leaves and roots were weighed separately (Figure 1), quickly wrapped, labeled with tin foil, stored at −80 °C, and physiological and biochemical indices were determined. Chlorophyll, MDA, osmoregulatory factor (soluble protein, free proline) content, and antioxidant enzymes (peroxidase, catalase, and superoxide dismutase) were determined using ELISA kits (Jiangsu Baolei Biotechnology [Enzyme Immunity]). The formulae for calculating the decline percentage and comprehensive evaluation value are as follows:
Decline Percentage = (Control Group − Treatment Group)/Control Group × 100%
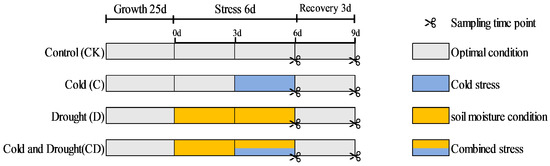
Figure 1.
An illustration of alfalfa developmental stage, stress combination strategy, and sampling time points. Control plants were grown under optimal conditions throughout the experiment. Drought stress was imposed as soil moisture was gradually changed by withholding irrigation. Cold was applied by transferring the plants to a precooled growth chamber at 8 °C day/4 °C night. The combined stress treatment was performed by transferring 3-d-soil moisture plants to a precooled growth chamber.
The formula for calculating the value of the affiliation function is (2) [5], where U (Xij) is the variety of the indicators of the j of the i-th treatment group of the affiliation value and Xmin and Xmax are the minimum and maximum values of the indicators j, respectively.
The comprehensive evaluation was represented by D (3) [20].
U(Xij) = (Xij − Xmin)/(Xmax − Xmin)
D = Σi = 1Xi (i = 1, 2, 3, …, n)
2.5. RNA Sequencing (RNA-Seq)
Leaves and roots from alfalfa seedlings were prepared under both stress treatment and control conditions for RNA-seq analysis using the Novoseq6000 platform. Total RNA was isolated using TRIzol® reagent (Invitrogen) following the manufacturer’s protocol. After total RNA extraction, mRNA was purified, and a library was constructed using the NEBNext® Ultra™ II DNA Library Prep Kit (NEB). Fragmentation buffer was used to fragment the mRNA samples into segments averaging 200 bp in length. Using reverse transcriptase, first-strand cDNA was synthesized via reverse transcription, employing random primers with the extracted mRNA as a template. Thereafter, second-strand synthesis was initiated, resulting in a stable double-stranded cDNA. These cDNA fragments were enriched, purified, and sequenced on the Illumina HiSeq platform.
2.6. RT-PCR Analysis
Leaf and root samples of the two alfalfa cultivars were processed to extract total RNA using the TRIZOL kit (Beijing Solepol Technology Co., Ltd.; Item No. 15596026), according to the kit instructions. SuperScript II reverse transcriptase was used to generate cDNA. Upon synthesis and aptamer ligation, the cDNA fragments were enriched and cleansed. Primers were designed according to the CDS sequences of arbitrarily chosen genes (Supplementary Table S2). The ubiquitin (UBI/UBQ) gene [21] served as an internal control for normalization. Quantitative PCR (qPCR) parameters were set as follows: initial denaturation at 95 °C for 3 min, followed by 40 amplification cycles of denaturation at 95 °C for 10 s and annealing at 60 °C for 30 s. Each sample contained three independent biological replicates. The comparative cycle threshold method (2−ΔΔCt) [22] was used to determine relative levels of gene expression.
2.7. Data Analysis
The Novoseq6000 sequencing platform facilitated transcriptome sequencing for this study. Following the denovo assembly of all quality-filtered data with Trinity (version: trinityrnaseq-r2013-02-25), contigs and singletons were generated. Before annotation, the Open Reading Frame (ORF) prediction tool from Trinity (http://trinityrnaseq.sourceforge.net/analysis/extract_proteins_from_trinity_transcripts.html (accessed on 2 June 2023)) was utilized for all transcript sequences derived from the assembled gene prediction. The assembled nucleotide sequences were aligned with the NR, Swissprot, and eggNOG databases using BlastX (version 2.2.25) to retrieve relevant annotation information. RSEM (http://deweylab.biostat.wisc.edu/rsem/ (accessed on 2 June 2023)) used Bowtie’s alignment results to estimate gene expression by initially acquiring the read counts for each gene per sample, which were then converted to FPKM values, reflecting gene expression levels. The widely accepted Benjamini–Hochberg correction method was applied to rectify the p-values of the initial hypothesis testing, with the adjusted p-value being the primary metric for the screening of differential gene expression. The criteria set for the identification of differentially expressed genes (DEGs) were Pad j < 0.05 and |log2FoldChange| > 2. The predicted DEGs were visualized and analyzed using various online platforms, including http://planttfdb.gaolab.org/prediction.php (accessed on 22 September 2023), Barplot (Origin2022), Veengraph (TBtools), GOEnrich (https://www.omicshare.com/tools/Home/Task/taskdetail (accessed on 22 September 2023)), and Heatmap (https://www.bioinformatics.com.cn (accessed on 7 October 2023)).
3. Results
3.1. Effects of Drought, Cold, and Combined Stress on Alfalfa Leaf Chlorophyll and Malondialdehyde (MDA) Content
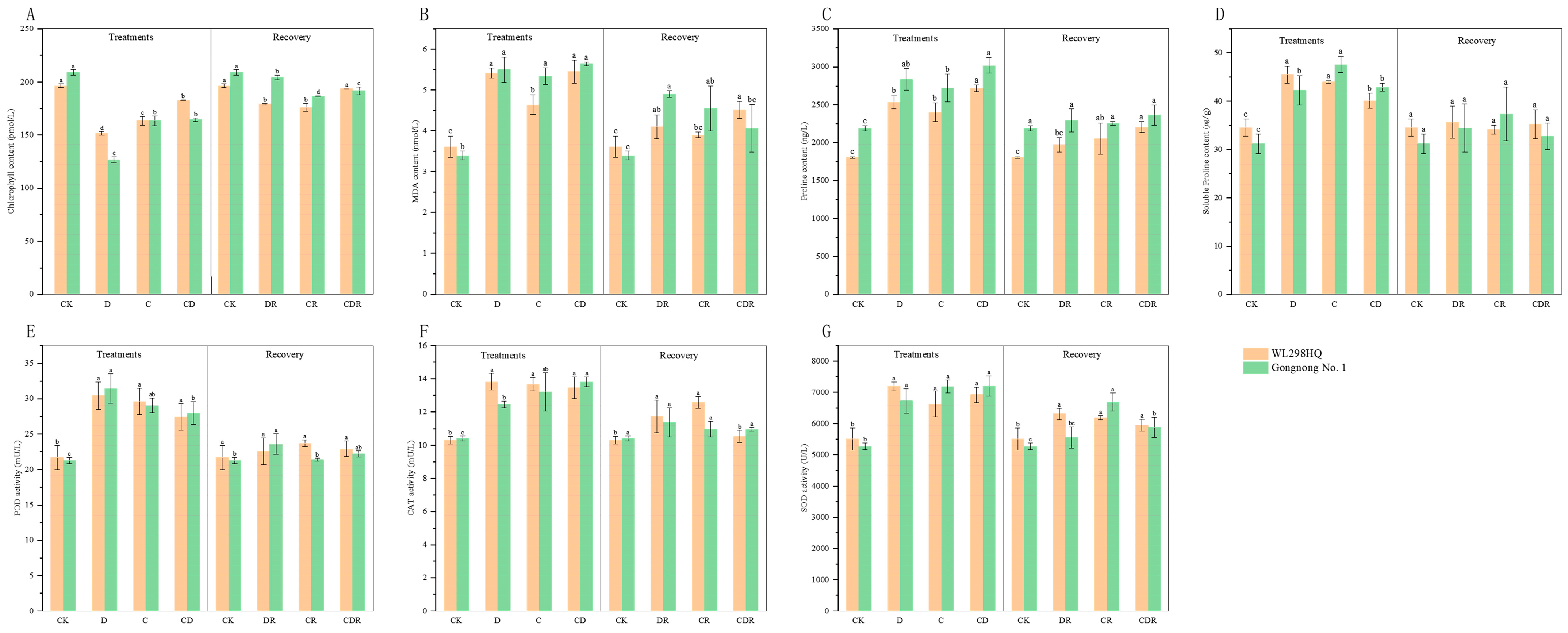
Under the drought (D), cold (C), and cold and drought combined (CD) treatments, the chlorophyll content in the leaves of both varieties showed a decreasing trend compared to the control group (CK). Compared to CK, the chlorophyll content in Gongnong No. 1 under D treatment decreased the most, by 39.33%. Throughout the stress period, the order of chlorophyll content for both Gongnong No. 1 and WL298HQ was CK > CD > C > D. Within each variety, the difference between CK and the other treatments was significant (p < 0.05). After re-watering, the chlorophyll content in the drought recovery (DR), cold recovery (CR), and cold and drought combination recovery (CDR) treatments increased compared to that in D, C, and CD, but remained lower than that in CK (Figure 2A).
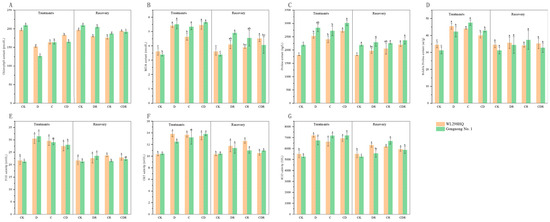
Figure 2.
Physiological index of two materials tested under drought, cold, and combined stress. (A) Chlorophyll content. (B) Malondialdehyde (MDA) content. (C) Proline (Pro) content. (D) Soluble Proline (SP) content. (E) Peroxidase (POD) activity. (F) Catalase (CAT) activity. (G) Superoxide dismutase (SOD) activity. Different letters indicate significant differences between treatments and control plants from Student’s unpaired two-tailed t test (p < 0.05). CK, Optimal condition; D, Drought stress; C, Cold stress; CD, Cold and Drought combined stress; DR, Drought Recovery; CR, Cold Recovery; CDR, Cold and Drought combined recovery.
Compared to CK, the MDA content in the leaves of both varieties increased to varying extents under all treatments. During the stress period, the MDA content for both varieties followed the order CD > D > C > CK, with Gongnong No. 1 having a higher MDA content in treatments D, C, and CD than in WL298HQ. After re-watering, the MDA content in the DR, CR, and CDR treatments of both varieties decreased, but remained higher than that in the CK (Figure 2B).
3.2. Effects of Soil Drought, Cold, and Combined Stress on Alfalfa Osmotic Regulation Factors
Compared to CK, the Pro content in both varieties increased under all treatments, with the increase being significant (p < 0.05). During the stress period, the Pro content in Gongnong No. 1 and WL298HQ followed the order CD > D > C > CK, with Gongnong No. 1 exhibiting a higher Pro content than WL298HQ. After re-watering, the differences in Pro content between CK and DR, CR, and CDR in Gongnong No. 1 were not significant (p > 0.05), whereas in WL298HQ the differences were significant (p < 0.05) (Figure 2C).
Similarly, the SP content in both varieties increased under all stress treatments. During the stress period, the SP content increased the most in the Gongnong No. 1 C treatment by 52.77% (p < 0.05), whereas in the WL298HQ D treatment, it increased the most by 31.74% (p < 0.05). After re-watering, the SP content in both varieties returned to the control levels when compared with DR, CR, and CDR, with no significant differences observed (Figure 2D).
3.3. Effects of Drought, Cold, and Combined Stress on Alfalfa Leaf Antioxidant Enzyme Content
Compared to CK, under treatments with D, C, and CD, both varieties showed varying increases in POD, CAT, and SOD content. During the stress period, the POD content in Gongnong No. 1 followed the order D > C > CD > CK, whereas the CAT and SOD contents followed the order CD > C > D > CK, all with significant differences (p < 0.05). For WL298HQ, the POD and CAT contents followed the order D > C > CD > CK, and the SOD content followed the order D > CD > C > CK, with significant differences (p < 0.05). After re-watering, POD, CAT, and SOD in the DR, CR, and CDR treatments of both varieties decreased to some extent compared to D, C, and CD, but remained higher than those in CK (Figure 2E–G).
3.4. Comprehensive Assessment of Different Alfalfa Varieties under Drought, Cold, and Combined Stress
Under drought stress, the drought resistance ranking of the two varieties was WL298HQ > Gongnong No. 1 (Table 1), where the SOD, CAT, and Pro activities were higher than those of Gongnong No. 1 and its MDA content was lower. Under cold stress, the cold resistance ranking was Gongnong No. 1 > WL298HQ (Table 1), with WL298HQ having higher Chlorophyll (CHL), CAT, POD, and SP contents than Gongnong No. 1, while the MDA and SOD activities of Gongnong No. 1 were higher than those of WL298HQ. Under combined cold and drought stress, the overall stress resistance ranking was Gongnong No. 1 > WL298HQ (Table 1), with the CHL, MDA, SOD, CAT, POD, Pro, and SP of Gongnong No. 1 being higher or equal to WL298HQ. Combining all stress treatments, the overall resistance ranking remained Gongnong No. 1 > WL298HQ (Table 1).

Table 1.
Comparative evaluation of WL298HQ and Gongnong No. 1 across different stress conditions.
3.5. Sequencing the Transcriptome of Alfalfa under Drought, Cold, and Combined Stresses
In this study, transcriptome sequencing was performed on the aboveground and belowground parts of two purple-flowered alfalfa varieties, WL298HQ and Gongnong No. 1, in eight treatment groups, for a total of 96 samples. Thus, 3,731,603,228 raw reads were obtained. After quality control, 3,469,831,420 clean reads were obtained, with an average length of 1121 bp and an N50 value of 1291 bp (Table 2). Based on NCBI nucleotide BLAST searches, 7178 unique genes were identified as transcription factors (TFs), covering 57 TF families (Supplementary Table S1). The top ten TF families, ranked by gene count, included C2H2 (955 genes), bHLH (637 genes), bZIP (470 genes), ERF (436 genes), MYB_related (357 genes), WRKY (345 genes), NAC (301 genes), C3H (287 genes), MYB (277 genes), and FAR1 (264 genes) (Supplementary Table S1).

Table 2.
Transcriptome sequencing overview for WL298HQ and Gongnong No. 1.
3.6. Identification of Differentially Expressed Genes under Drought, Cold, and Combined Stresses
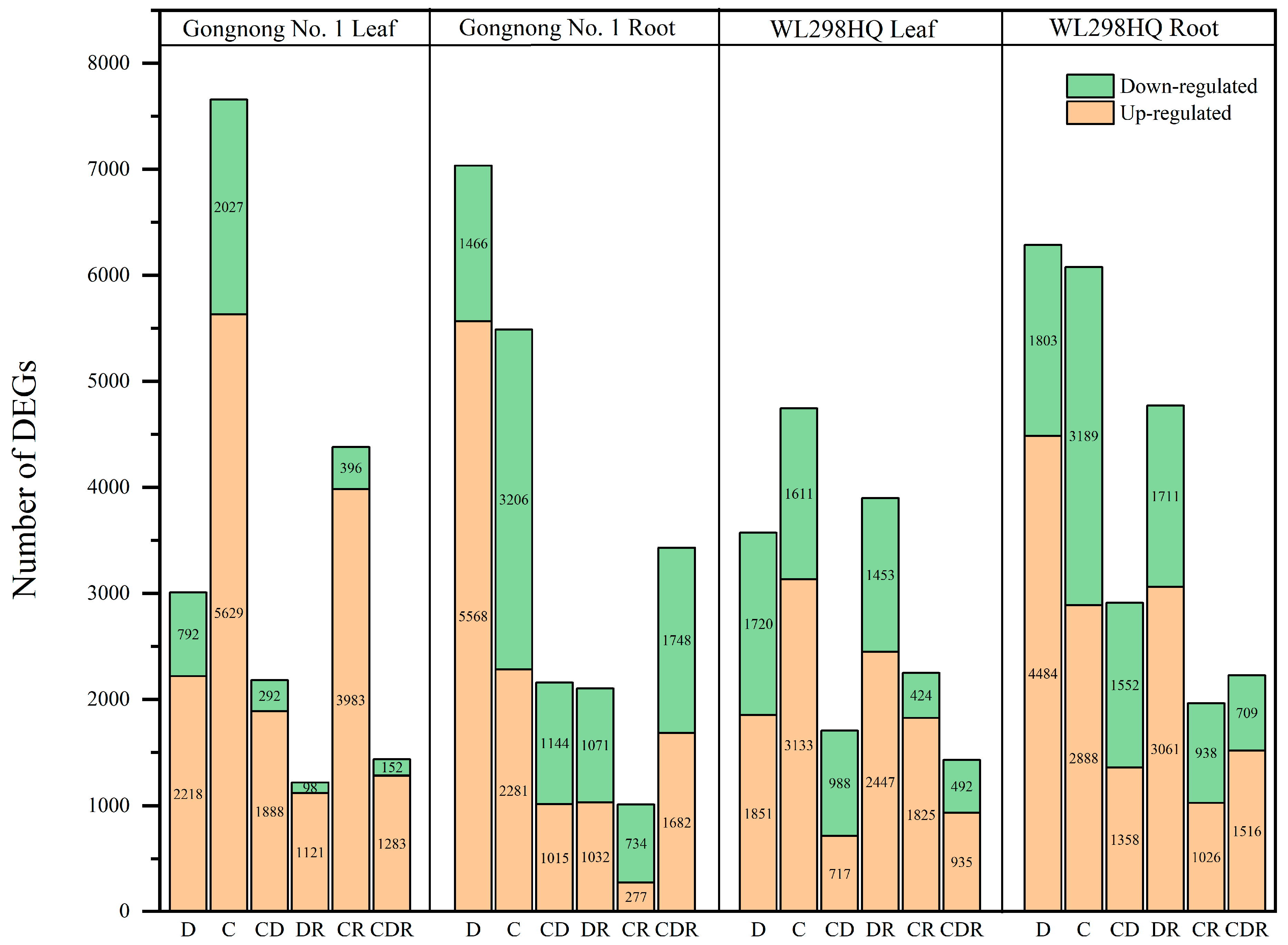
Using a threshold of log2FoldChange ≥ 2 and p ≤ 0.05, differentially expressed genes (DEGs) were screened between each treatment group and the control group (Figure 3). For the Gongnong No. 1 variety in the six treatment combinations (D, C, CD, DR, CR, and CDR), the number of DEGs was 10,044, 13,143, 4339, 3322, 5390, and 4865, respectively. For WL298HQ, the counts were 9858, 10,821, 4615, 8672, 4213, and 3652 (Figure 3). Among these, Gongnong No. 1 had 16,122 up-regulated and 3757 downregulated DEGs in the leaves, whereas 11,855 DEGs were up-regulated and 9369 downregulated in the roots. For WL298HQ, 10,908 DEGs were up-regulated, 6688 were downregulated in the leaves, whereas 14,333 DEGs were up-regulated, and 9902 were downregulated in the roots (Figure 3).
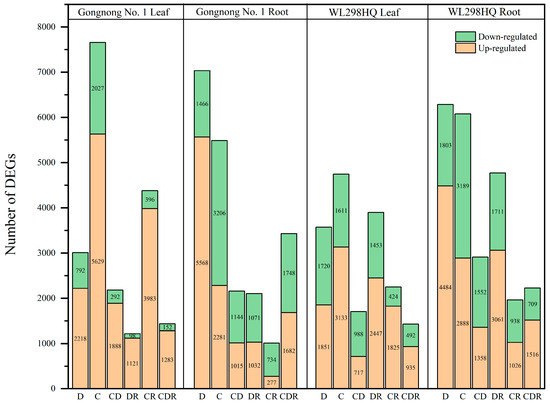
Figure 3.
Statistical chart of number of DEGs. D, Drought stress; C, Cold stress; CD, Cold and Drought combined stress; DR, Drought Recovery; CR, Cold Recovery; CDR, Cold and Drought combined Recovery.
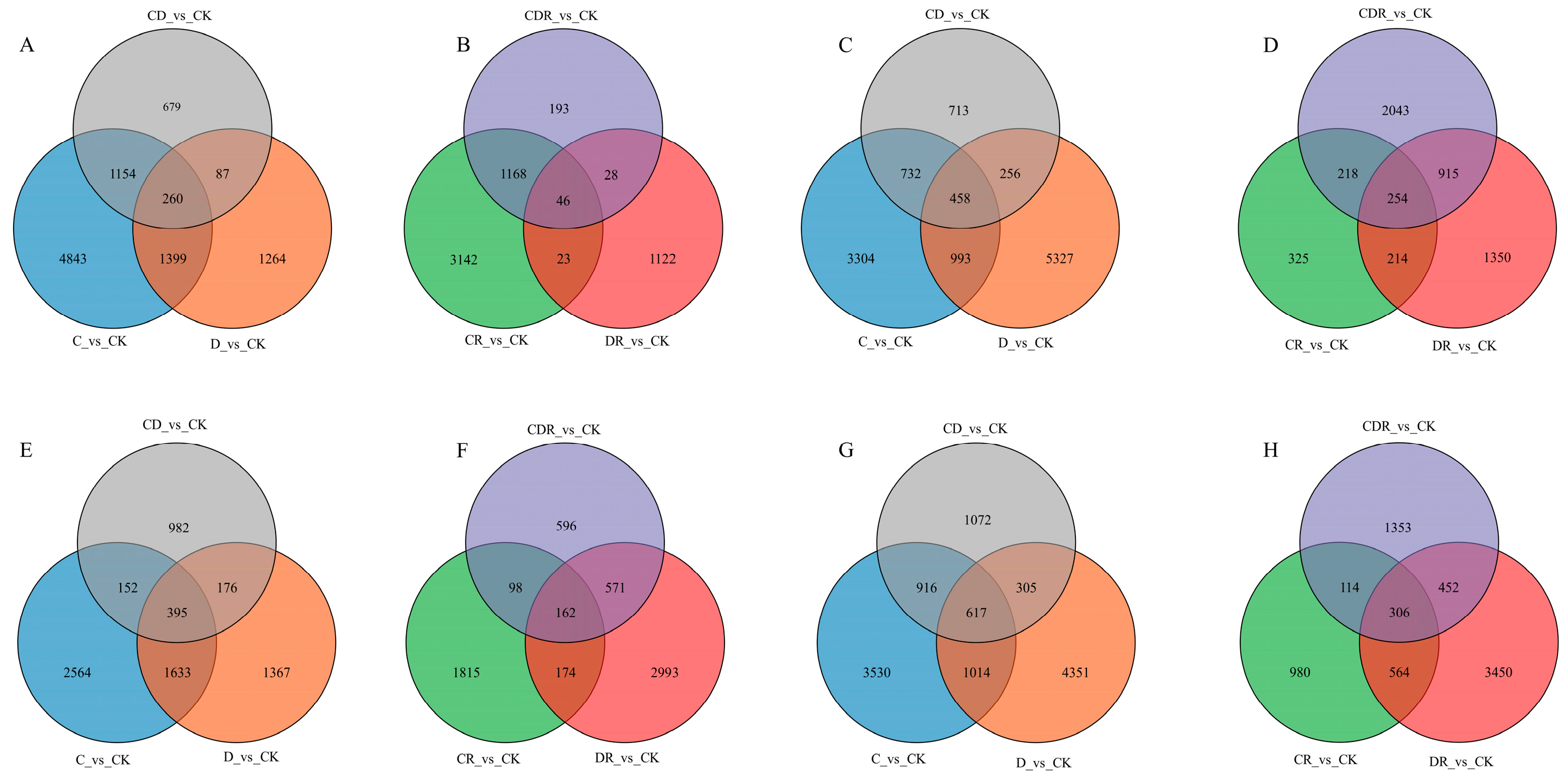
Comparing the D_vs_CK, C_vs_CK, and CD_vs_CK groups during the stress periods, Gongnong No. 1 and WL298HQ exhibited 260 (Figure 4A), 458 (Figure 4C), 395 (Figure 4E), and 617 (Figure 4G) DEGs in the leaves and roots, respectively. In contrast, comparing the DR_vs_CK, CR_vs_CK, and CDR_vs_CK groups during the recovery period, there were 46 (Figure 4B), 254 (Figure 4D), 162 (Figure 4F), and 306 (Figure 4H) DEGs, respectively (Figure 4B,D). Both varieties showed a higher number of common DEGs during the stress periods than during the recovery periods (Figure 4).
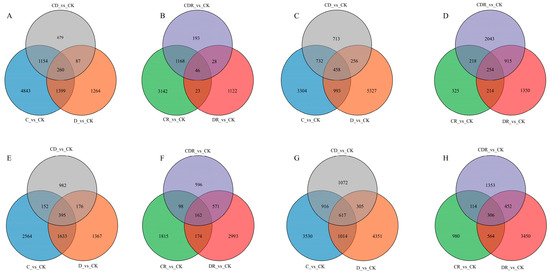
Figure 4.
Venn diagrams showing the overlap among DEGs in each of the different treatments and recovery. (A) Gongnong No. 1 leaf treatment; (B) Gongnong No. 1 leaf recovery; (C) Gongnong No. 1 root treatment; (D) Gongnong No. 1 root recover; (E) WL298HQ leaf treatment; (F) WL298HQ leaf recovery; (G) WL298HQ root treatment; (H) WL298HQ root recovery. CK, Optimal condition; D, Drought stress; C, Cold stress; CD, Cold and Drought combined stress; DR, Drought Recovery; CR, Cold Recovery; CDR, Cold and Drought combined recovery.
3.7. Functional Classification and Expression of Differentially Expressed Genes
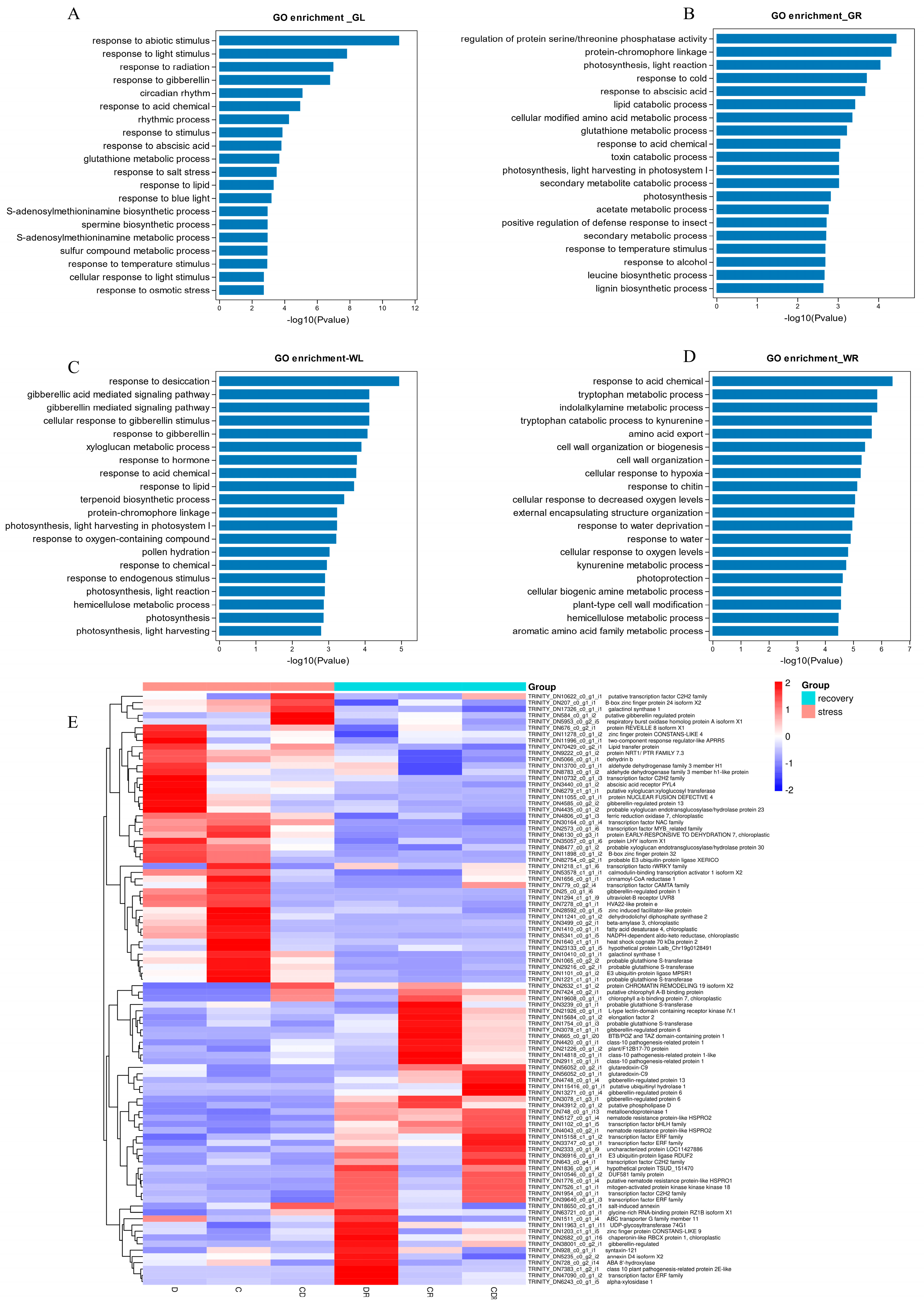
In Gongnong No. 1 leaves, 668 DEGs were significantly enriched in 38 Gene Ontology (GO) pathways (Supplementary Table S3): biological processes (19 pathways, 377 genes), molecular functions (8 pathways, 98 genes), and cellular components (11 pathways, 193 genes). In particular, the enriched pathways included “response to abiotic stimulus (GO:0009628)”, “response to light stimulus (GO:0009416)”, and “response to abscisic stimulus (GO:0009737)” (Figure 5A). In roots, 1248 DEGs were mainly enriched in 53 GO pathways: biological processes (23 pathways, 523 genes), molecular functions (12 pathways, 215 genes), and cellular components (18 pathways, 510 genes). Enriched pathways encompassed “response to abscisic acid (GO:0009737)”, “gibberellic acid-mediated signaling pathway (GO:0009740)”, “regulation of protein serine/threonine phosphatase activity (GO:0080163)”, and “response to cold (GO:0009409)” (Figure 5B).
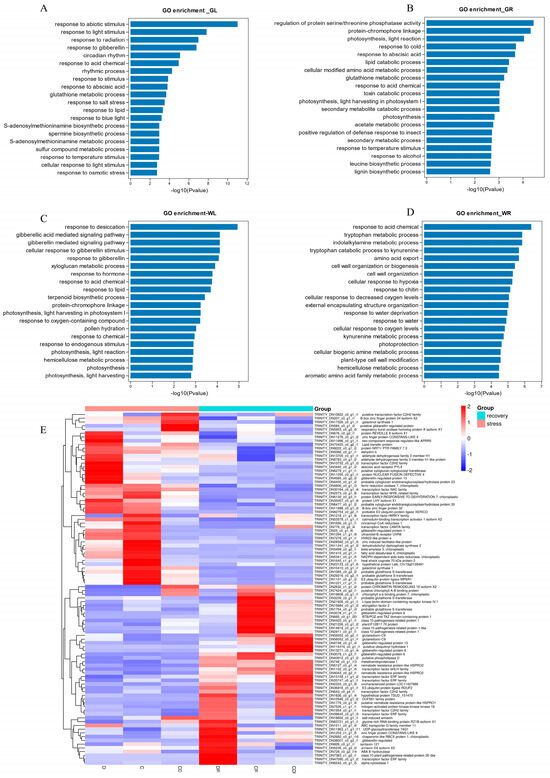
Figure 5.
The DEGs in each of the different treatments and recovery. (A) GO annotation of common DEGs in Gongnong No. 1 leaf. (B) GO annotation of common DEGs in Gongnong No. 1 root. (C) GO annotation of common DEGs in WL298HQ leaf. (D) GO annotation of common DEGs in WL298HQ root. (E) The expression profile of common DEGs. D, Drought stress; C, Cold stress; CD, Cold and Drought combined stress; DR, Drought Recovery; CR, Cold Recovery; CDR, Cold and Drought combined Recovery.
In the leaves of WL298HQ, 969 DEGs were significantly enriched in 46 GO pathways (Supplementary Table S4), including biological processes (22 pathways and 457 genes), molecular functions (9 pathways and 153 genes), and cellular components (15 pathways and 359 genes). The highlighted pathways included: “response to desiccation (GO:0009269)”, “gibberellic acid-mediated signaling pathway (GO:0009740)”, “cellular response to gibberellin stimulus (GO:0071370)”, “response to gibberellin (GO:0009739)”, and “xyloglucan metabolic process (GO:0010411)” (Figure 5C). In roots, 1374 DEGs were primarily enriched in 40 GO pathways: biological processes (20 pathways, 723 genes), molecular functions (8 pathways, 195 genes), and cellular components (12 pathways, 456 genes). The most significantly enriched pathways were “response to acid chemical (GO:0001101)”, “tryptophan metabolic process (GO:0006568)”, and “amino acid export (GO:0032973)” (Figure 5D).
Further investigation of DEGs significantly enriched in GO pathways revealed eleven transcription factors in seven families, including three ERF genes, three C2H2 genes, one NAC gene, one MYB_related gene, one CAMTA gene, one WRKY gene, and one bHLH gene (Figure 5E). These were represented by specific gene IDs, such as TRINITY_DN47090_c0_g1_i2 and TRINITY_DN15158_c1_g1_i2.
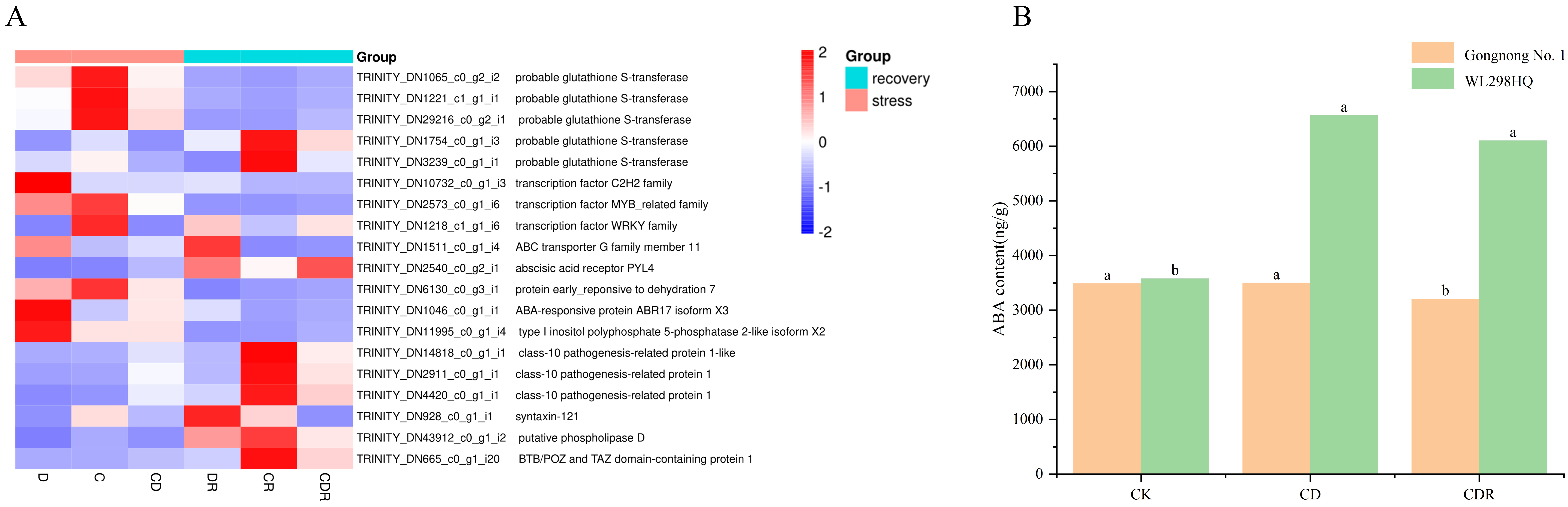
Based on the GO enrichment results, 19 genes involved in the ABA signaling pathway were further examined. During stress treatments, nine genes were significantly up-regulated in leaves compared to the control, while ten were significantly down-regulated in roots. After rehydration, their expression returned to control levels. Five genes annotated with probable glutathione S-transferase were mainly up-regulated during treatment. Other annotations included transcription factors (C2H2, MYB, WRKY), one for the ABC transporter G family, one for the abscisic acid receptor PYL4, one for the ABA-responsive protein ABR17 isoform X3, one for the DUF581 protein family, and three for the pathogenesis-related protein, all of which were negatively regulated during treatments (Figure 6A).
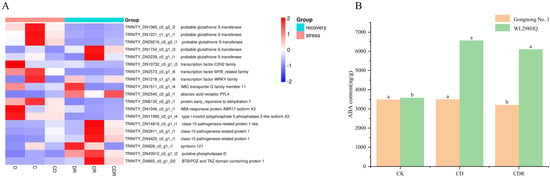
Figure 6.
Relative expression of significantly enriched genes on the ABA pathway (A) and ABA content (B) in leaves under different treatments and recovery. D, Drought stress; C, Cold stress; CD, Cold and Drought combined stress; DR, Drought Recovery; CR, Cold Recovery; CDR, Cold and Drought combined Recovery. The different letters represent significant differences (p < 0.05).
Furthermore, the ABA content in Gongnong No. 1 and WL298HQ alfalfa under normal conditions and combined cold and drought stress was measured. Stress treatments induced significant changes in ABA levels in alfalfa leaves. Compared to the control, both Gongnong No. 1 and WL298HQ under combined cold and drought stress were elevated, where WL298HQ was significantly increased by stress and Gongnong No. 1 was also increased but not significantly, respectively (Figure 6A,B).
3.8. Validation through Quantitative Real-Time PCR (qRT-PCR)
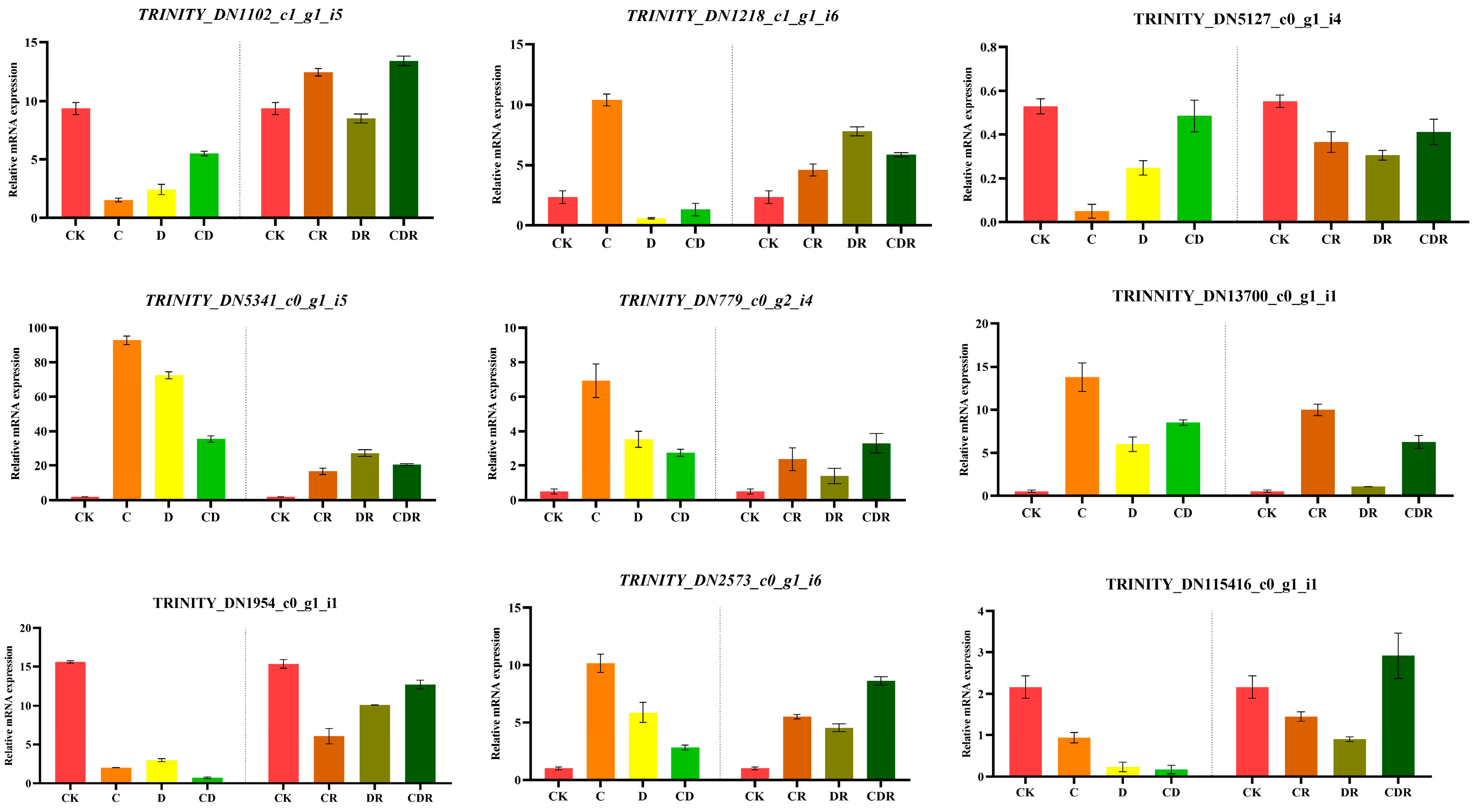
To validate the reliability of the RNA-seq data, nine genes were randomly selected for validation using qRT-PCR. The expression levels of these genes were calculated using the 2−ΔΔCt method. The genes selected for verification included transcription factors (bHLH, C2H2, CAMTA, and MYB), stress resistance proteins (HSPRO2), and NADPH-dependent aldo-keto reductase. The results indicated that the RNA-seq data (SUB13967522) (Supplementary Table S5) were consistent with the findings obtained from qRT-PCR analysis. This suggests that the experimental data generated in this study were of considerable precision (Figure 7).
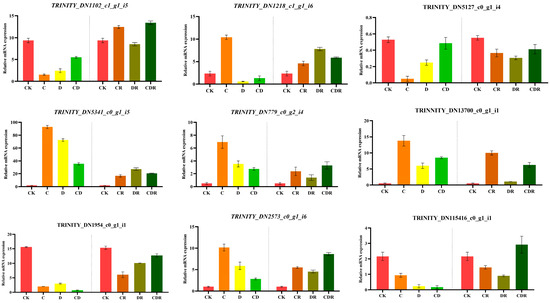
Figure 7.
Real-time fluorescence quantitative PCR validation RNA_seq data. CK, Optimal condition; D, Drought stress; C, Cold stress; CD, Cold and Drought combined stress; DR, Drought Recovery; CR, Cold Recovery; CDR, Cold and Drought combined recovery.
4. Discussion
Drought and cold are the most extensive and prolonged natural disasters, having significant impacts on the cultivation, natural distribution, and growth of forage crops, especially alfalfa [23]. Such adversities have gradually become crucial factors limiting the cultivation of alfalfa. Addressing these non-biological stresses by exploring drought- and cold-resistant germplasm, breeding resilient varieties, and subsequently promoting the cultivation of these resistant types is an essential strategy.
Photosynthesis is fundamental for the maintenance of normal plant physiological activities, and chlorophyll plays a pivotal role in this process. Studies have indicated that chlorophyll content can serve as an indicator of drought tolerance [24]. In our study, under stress conditions, both varieties of alfalfa showed a significant decline in chlorophyll content, which is in agreement with the findings of Han [25] and Wang [26]. Li [27] observed that wheat plants, after an initial preconditioning for drought, exhibited enhanced cold tolerance. Compared to the control group (CK), in our study, groups C, D, and CD displayed varying rates of chlorophyll degradation. Specifically, group D showed the fastest decline, followed by CD, with group C being the least affected. This suggests that cold stress has a relatively lesser deteriorative effect on chlorophyll and that moderate cold stress can slow the rate of chlorophyll degradation during soil moisture conditions.
MDA serves as an oxidative product of cell membranes under stress, reflects the degree of oxidative damage, and is thus a vital indicator of plant stress. Higher MDA levels indicate increased cellular damage [28]. In our study, compared to the control group (CK), the MDA content in groups D, C, and CD was significantly higher (p < 0.05). This suggests a certain degree of membrane damage due to stress, which is consistent with the findings for small rye by Liu [29]. Furthermore, compared to WL298HQ, Gongnong No. 1 exhibited a higher increase in MDA content under drought, cold, and combined stresses, indicating that Gongnong No. 1 might be more susceptible to soil moisture-induced damage.
When exposed to stresses such as cold and drought, the internal dynamic equilibrium of plants is disturbed, leading to the overproduction of reactive oxygen species (ROS). Excessive ROS generation can result in lipid peroxidation, DNA damage, protein inactivation, and even cell dysfunction and death, causing numerous detrimental effects in plants [30]. SOD acts as the first line of defense against ROS in the antioxidant system, whereas POD and CAT catalyze the decomposition of H2O2 [29], aiding in protecting plants from ROS-induced damage. In our study, the content of SOD, POD, and CAT increased to varying extents. Under combined stresses, the activities of POD and CAT enzymes in WL298HQ leaves were lower than those under drought stress but also lower than those under cold stress, indicating that combined stress caused less oxidative stress to WL298HQ compared to individual cold or drought stresses. Proline and soluble proteins, as osmotic regulatory factors, adjust cell osmotic pressure and potential. Under drought, cold, and combined stresses, both Gongnong No. 1 and WL298HQ showed increasing trends in SP and Pro content throughout the treatment period, indicating rapid activation of the internal osmotic regulators of alfalfa in response to stress. Among the two varieties, combined stress resulted in the highest accumulation of Pro, followed by drought stress, and cold stress resulted in the lowest Pro activity. This corroborates the research by Yang [31] which showed that MsVDAC modulates ROS clearance, osmotic balance, and stress response gene expression in alfalfa to improve cold resistance. In our study, both alfalfa varieties, under drought, cold, and combined stresses, maintained osmotic balance by increasing the content of SOD, POD, CAT, Pro, and SP, which is consistent with previous studies [32,33,34,35].
To discern the molecular mechanisms underlying the responses of the two alfalfa varieties to individual (drought, cold) and combined stresses, we analyzed the expression of DEGs in the leaves and roots of Gongnong No. 1 and WL298HQ. Our results revealed that the number of DEGs under combined stress was markedly lower than that under individual stresses, suggesting that alfalfa, when responding to single stresses, mobilizes a broader array of genes and biological processes. In the Gongnong No. 1 variety, the number of DEGs was higher than that of DEGs under drought and combined stresses, whereas under cold stress, the number of leaf DEGs surpassed that of the leaves and exceeded that of the roots. However, for WL298HQ, the number of DEG in the roots and leaves was relatively consistent between all stress treatments.
To summarize, our study emphasizes the intricate mechanisms involved in the response of alfalfa to individual and combined cold and drought stresses. Our findings revealed that both varieties of alfalfa undergo adaptive physiological and molecular changes under stress conditions, thus safeguarding cellular structures and maintaining normal physiological processes. A comprehensive analysis of these adaptive responses provides invaluable information for the development of alfalfa varieties that are resistant to cold, drought, and their combined stresses.
5. Conclusions
In this study, two alfalfa cultivars, WL298HQ and Gongnong No. 1, were subjected to drought (soil moisture condition), cold, and combined stress treatments. Changes in chlorophyll, MDA, and Pro content under different stress conditions were consistent for both cultivars. The chlorophyll content decreased the most in group D and the least in group CD. During the stress treatments, MDA, Pro, SP, POD, CAT, and SOD levels increased in both cultivars. Upon recovery from rehydration, these levels decreased compared to the stress period but remained higher than those in the control group.
Transcriptome sequencing revealed that WL298HQ and Gongnong No. 1 had 41,103 and 41,831 DEGs, respectively, compared to the control group. WL298HQ had a higher number of shared DEGs (1480) under stress conditions than Gongnong No. 1 (1018) (Figure 4), indicating a more substantial response to stress in WL298HQ. The DEGs in Gongnong No. 1 were notably enriched in pathways related to “response to abiotic stimulus”, “response to abscisic stimulus”, and “response to cold”. In contrast, WL298HQ DEGs were significantly enriched in the “response to desiccation”, “gibberellic acid-mediated signaling pathway”, and “cellular response to gibberellin stimulus” pathways.
Additionally, under cold and drought stress conditions, the ABA content in both cultivars increased. After rehydration recovery, the ABA content decreased but was still higher than that in the control group. Nineteen genes were found to be significantly enriched in the ABA signaling pathway. Among them, nine genes were consistently upregulated in the leaves, whereas ten genes were downregulated in the roots, suggesting that different alfalfa tissues have different responses to stress. Overall, these findings offer valuable theoretical insights for future studies on the mechanisms by which alfalfa copes with abiotic stress.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy13123002/s1. Table S1. Details of Transcription Factor (TF) Predictions; Table S2: Primer Sequences for PCR Analysis; Table S3. Gene Ontology (GO) pathways in Gongnong No. 1 leaves and roots; Table S4. Gene Ontology (GO) pathways in WL298HQ leaves and roots; Table S5 RNA-seq results for random selected genes. Raw and processed RNA-Seq data files were deposited in SRA (https://www.ncbi.nlm.nih.gov/sra/ (accessed on 18 October 2023)) under the following accession numbers SUB13967522.
Author Contributions
T.Z. designed the experiments; R.A., Y.D., R.Y., X.Z., Y.H. and H.L. performed the experiments; R.A. and S.J. analyzed the data; L.Y. revised the paper; R.A. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Breeding and Industrialization Demonstration of New High-quality Alfalfa Varieties (No. 2022JBGS0020), Breeding of New Alfalfa Varieties (SJCZFY2022-3 to T.Z.), and Project of Ecological Restoration of Degraded Grassland in High-altitude Frozen Soil Areas (Qing2021TG07).
Data Availability Statement
Upon reasonable request, the datasets used and/or analyzed in this study are available from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, Y.; Liu, G.; Gao, H.; Wang, Z.; Zhao, H.; Xie, N.; Wang, Y. Study on Comprehensive Evaluation of Drought Resistance of Medicago sativa L. Germplasm at Seedling Stage. Acta Agrestia Sin. 2009, 1, 807–812. [Google Scholar]
- Zhang, M.; Sun, S.; Sun, J.; Chen, J.; Yan, H. Molecular Mechanism of Abiotic Stress Response in Medicago sativa. Mol. Plant Breed. 2023, 21, 2642–2654. [Google Scholar]
- Zhang, J.Y.; Cruz, D.E.; Carvalho, M.H.; Torres-Jerez, I.; Kang, Y.; Allen, S.N.; Huhman, D.V.; Tang, Y.; Murray, J.; Sumner, L.W.; et al. Global Reprogramming of Transcription and Metabolism in Medicago truncatula during Progressive Drought and after Rewatering. Plant Cell Environ. 2014, 37, 2553–2576. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Jiang, W.; Wang, Y.; Zhang, B.; Pang, Y. Research Progresses on the Drought Resistance of Medicago at Molecular Level. Biotechnol. Bull. 2021, 37, 243–252. [Google Scholar]
- Wang, Y.; Zhao, M.; Zhang, H.; Lu, Z.; Mu, C. Effects of Drought Stress on Morphological and Physiological Characteristics of Alfalfa Seedlings. Chin. J. Grassl. 2021, 43, 78–87. [Google Scholar]
- Zang, Z.F.; Bai, J.; Liu, C.; Zan, K.Z.; Long, M.X.; He, S.B. Variety Specificity of Alfalfa Morphological and Physiological Characteristics in Response to Drought Stress. Acta Pratacult. Sin. 2021, 30, 73–81. [Google Scholar]
- Feng, K.; Hou, X.L.; Xing, G.M.; Liu, J.X.; Duan, A.Q.; Xu, Z.S.; Li, M.Y.; Zhuang, J.; Xiong, A.S. Advances in AP2/ERF super-family transcription factors in plant. Crit. Rev. Biotechnol. 2020, 40, 750–776. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, Z.; Memon, A.G.; Ahmad, A.; Iqbal, M.S. Calcium Mediated Cold Acclimation in Plants: Underlying Signaling and Molecular Mechanisms. Front. Plant Sci. 2022, 13, 855559. [Google Scholar] [CrossRef]
- He, F.; Zhang, L.; Zhao, G.; Kang, J.; Long, R.; Li, M.; Yang, Q.; Chen, L. Genome-Wide Identification and Expression Analysis of the NAC Gene Family in Alfalfa Revealed Its Potential Roles in Response to Multiple Abiotic Stresses. Int. J. Mol. Sci. 2022, 23, 10015. [Google Scholar] [CrossRef]
- Li, T.; Sun, J.; Liu, J. Role of Different Transcription Factor Families in the Regulatory Networks of Drought and Salinity Tolerance in Plants. Chin. Bull. Life Sci. 2015, 27, 217–227. [Google Scholar]
- Shen, Y.; Xu, Z.; Tang, X.; Yang, X.; Huang, W.; Wu, X.; Zhang, W. Cloning and Function Analysis of the MsNAC2 Gene with NAC Transcription Factor from Alfalfa. Sci. Agric. Sin. 2015, 48, 2925–2938. [Google Scholar]
- Song, Y.; Lv, J.; Qiu, N.; Bai, Y.; Yang, N.; Dong, W. The constitutive expression of alfalfa MsMYB2L enhances salinity and drought tolerance of Arabidopsis thaliana. Plant Physiol. Biochem. 2019, 141, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y. Discovery of R2R3-MYB Transcription Factors in Tribulus Terrestris Alfalfa and Their Response to Abiotic Stress. Master’s Thesis, Harbin Normal University, Harbin, China, May 2017. [Google Scholar]
- Wang, T.; Chen, L.; Zhao, M.; Tian, Q.; Zhang, W.H. Identification of drought-responsive microRNAs in Medicago truncatula by genome-wide high-throughput sequencing. BMC Genom. 2011, 12, 367. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Jiang, W.; Jiang, Z.; Du, W.; Song, J.; Qiang, Z.; Zhang, B.; Pang, Y.; Wang, Y. Transcriptome and functional analyses reveal ERF053 from Medicago falcata as key regulator in drought resistances. Front. Plant Sci. 2022, 13, 995754. [Google Scholar]
- Yitong, M. Identification and Functional Analysis of Alfalfa-Specific Gene MsASG166 in Response to Drought Stress. Master’s Thesis, Lanzhou University, Lanzhou, China, May 2022. [Google Scholar]
- Fu, J. Mechanism of Low Temperature Adaptation of Wild Pitcher Plant in Tibet. Ph.D. Thesis, Northwest Agriculture and Forestry University, Xianyang, China, March 2017. [Google Scholar]
- Liu, H.; Li, D.; Deng, Z. Advances in Research of Transcriptional Regulatory Network in Response to Cold Stress in Plants. Sci. Agric. Sin. 2014, 47, 3523–3533. [Google Scholar]
- Fang, M.; Wang, Y.; Zhao, Y. Morphological and Physiological Responses of Eight Alfalfa Cultivars to Combined Stress of Cold and Drought. J. Grassl. Sci. 2022, 30, 2967–2974. [Google Scholar]
- Han, R.; Lu, X.; Gao, G.; Yang, X. Analysis of the Principal Components and the Subordinate Function of Alfalfa Drought Resistance. J. Acta Agrestia Sin. 2006, 2, 142–146. [Google Scholar]
- Zhang, D.; Sun, Y.; Zhao, L.; Chou, M. Reference Gene Selection for Quantitative Real-time PCR Normalization in Medicago Lupulina Under Zinc Stress. Screening of real-time quantitative PCR internal reference genes under zinc stress in sky blue alfalfa. China Environ. Sci. 2015, 3, 833–838. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J. Abiotic stress responses in plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef]
- Deng, X.; Qiao, D.; Li, L.; Zhang, N.; Lei, G.; Cao, Y. The Effects of Chilling Stress on Physiological Characters of Medicago Sativa. J. Sichuan Univ. 2005, 01, 190–194. [Google Scholar]
- Han, R.; Lu, X.; Gao, G.; Yang, X. Photosynthetic physiological response of alfalfa (Medicago sativa) to drought stress. J. Acta Ecol. Sinca. 2007, 12, 5229–5237. [Google Scholar]
- Chen, L.; Wang, J.; Qiu, X.; Sun, H.; Zhang, W.; Wang, T. Differences of Physiological Responses and Transcriptional Regulation of Alfalfa with Different Drought Tolerances Under Drought Stresses. Acta Agron. Sin. 2023, 49, 2122–2132. [Google Scholar]
- Li, X.; Topbjerg, H.B.; Jiang, D.; Liu, F. Drought priming at vegetative stage improves the antioxidant capacity and photosynthesis performance of wheat exposed to a short-term low temperature stress at jointing stage. Plant Soil 2015, 393, 307–318. [Google Scholar] [CrossRef]
- Liu, Y.; Geng, J.; Song, J.; Sha, X.; Hu, T.; Yang, P. Effect of Root Nodules on Cold Resistance of Alfalfa (Medicago sativa). J. Acta Agrestia Sin. 2018, 26, 631–638. [Google Scholar]
- Liu, J.; Wu, J.; Laduo; Luo, L.; Wang, Y. Effects of Combined Cold and Drought Stress on the Physiological and Biochemical Characteristics of Triticale wittmack. Tibet J. Agric. Sci. 2022, 44, 53–61. [Google Scholar]
- Wei, J.; Xu, C.; Li, K.; He, H.; Xu, Q. Progress on Superoxide Dismutase and Plant Stress Resistance. J. Plant Physiol. J. 2020, 56, 2571–2584. [Google Scholar]
- Yang, M.; Duan, X.; Wang, Z.; Yin, H.; Zang, J.; Zhu, K.; Wang, Y.; Zhang, P. Overexpression of a Voltage-Dependent Anion-Selective Channel (VDAC) Protein-Encoding Gene MsVDAC from Medicago sativa Confers Cold and Drought Tolerance to Transgenic Tobacco. Genes 2021, 12, 1706. [Google Scholar] [CrossRef]
- Ma, Y.; Yang, G.; Tong, Y.; Liu, Z. Effects of Low Temperature Stress on the Physiological Characteristics of Medicago sativa Seedlings. J. Qinghai Univ. 2022, 40, 16–19. [Google Scholar]
- Sun, Y.; Li, J.; Sun, B.; Wang, G.; ShenN, X.; Ran, N.Z. Responses of Cold Resistance Physiological Indices of Different Alfalfa Varieties to Cold Stress. J. Shenyang Agric. Univ. 2017, 48, 591–596. [Google Scholar]
- Han, Z.; Zheng, M.; Liang, X.; Kang, J.; Chen, Y. Effects of Drought Stress on Morphological and Physiological Characteristics of Different Alfalfa Cultivars. Chin. J. Grassl. 2020, 42, 37–43. [Google Scholar]
- Zhou, Q.; Luo, D.; Chai, X.; Wu, Y.; Wang, Y.; Nan, Z.; Yang, Q.; Liu, W.; Liu, Z. Multiple Regulatory Networks Are Activated during Cold Stress in Medicago sativa L. Int. J. Mol. Sci. 2018, 19, 3169. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).