PEG-6000 Priming Improves Aged Soybean Seed Vigor via Carbon Metabolism, ROS Scavenging, Hormone Signaling, and Lignin Synthesis Regulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials, Seed Vigor Testing, Seed Aging, and Seed Priming Treatment
2.2. Morphology and Biomass Assays
2.3. Transcriptome Sequencing and Bioinformatics Analysis
2.4. qRT-PCR Analysis
2.5. Determination of SOD, POD, and GST Activity
2.6. Auxin, Brassinolide, and Lignin Content Determination
2.7. Statistical Analysis of Data
3. Results
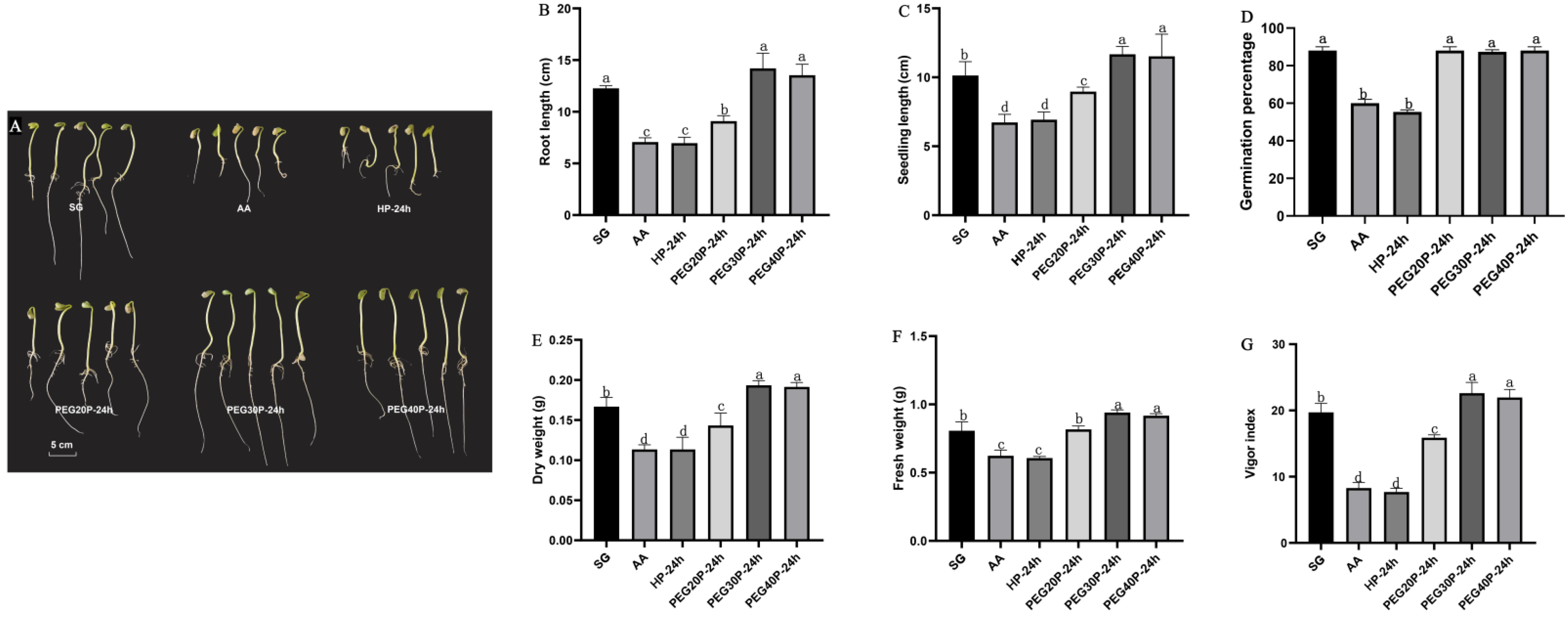
3.1. Effects of Different Concentrations of PEG-6000 Priming on Seed Vigor and Seedling Biomass of Aged Soybean
3.2. Effects of PEG-6000 Priming at Different Duration on Seed Vigor and Seedling Biomass of Aged Soybean
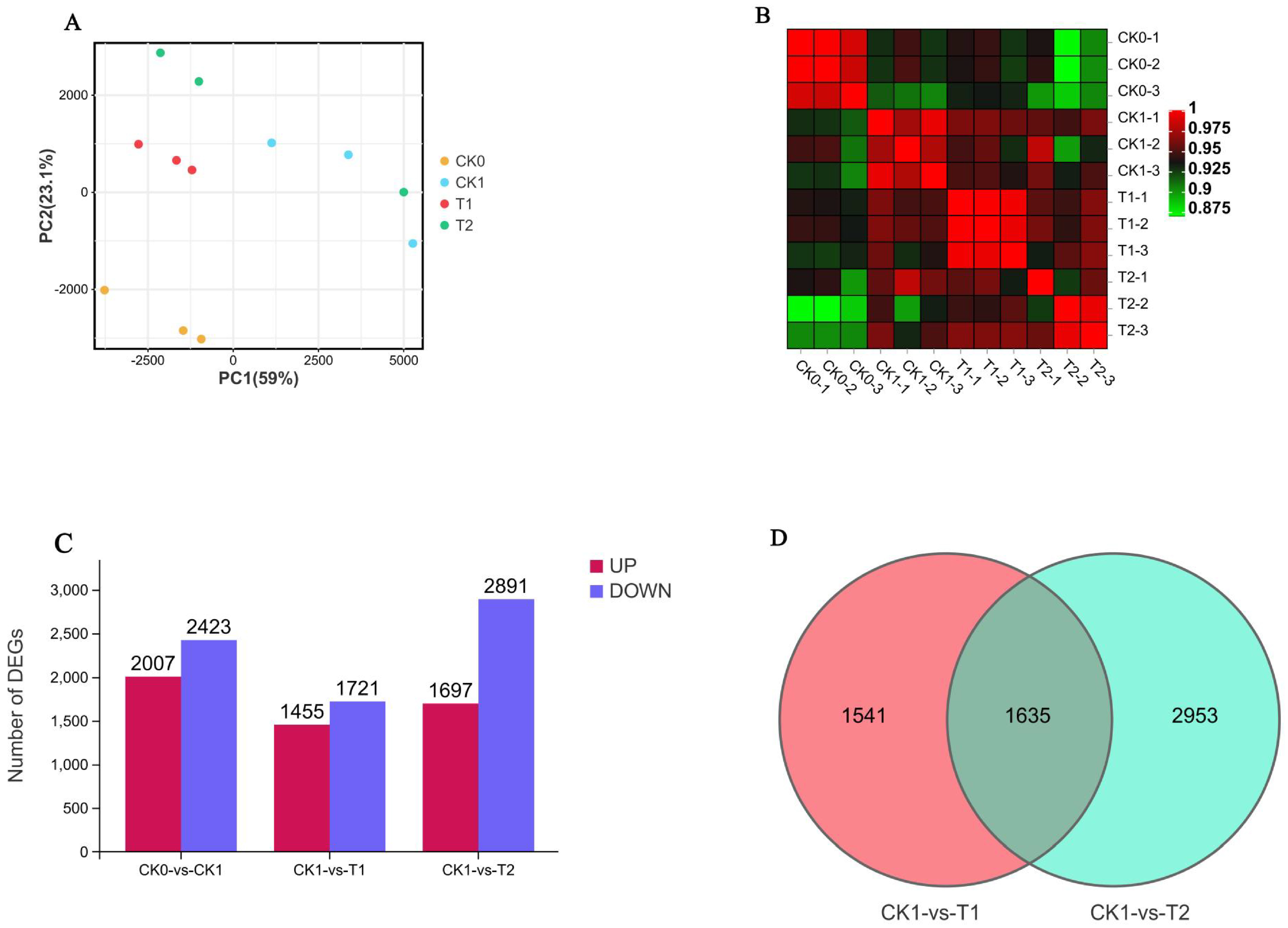
3.3. Transcriptome Sequencing Analysis and Screening of Differentially Expressed Genes (DEGs)
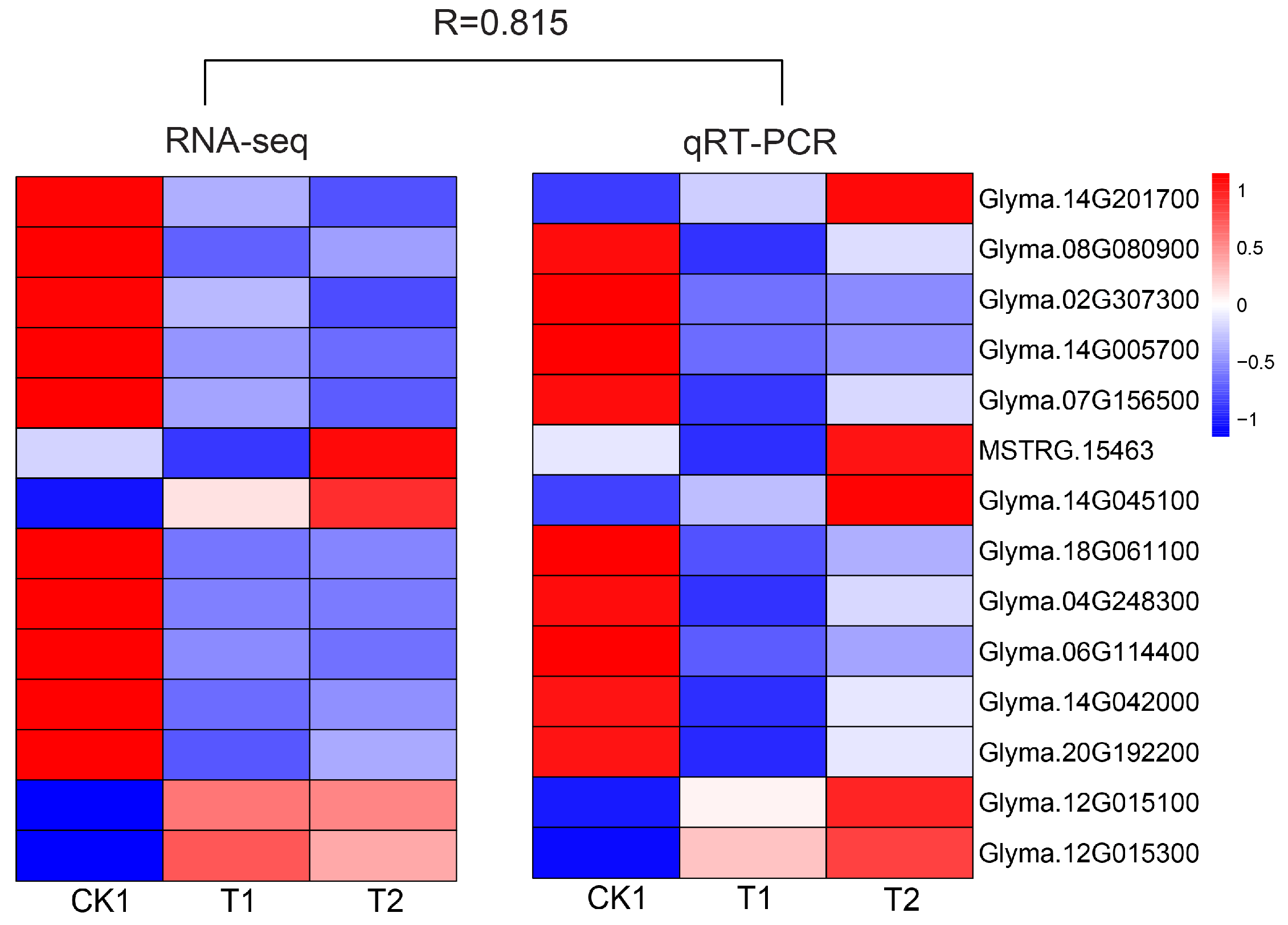
3.4. qRT-PCR Analysis of Some DEG Expression Patterns
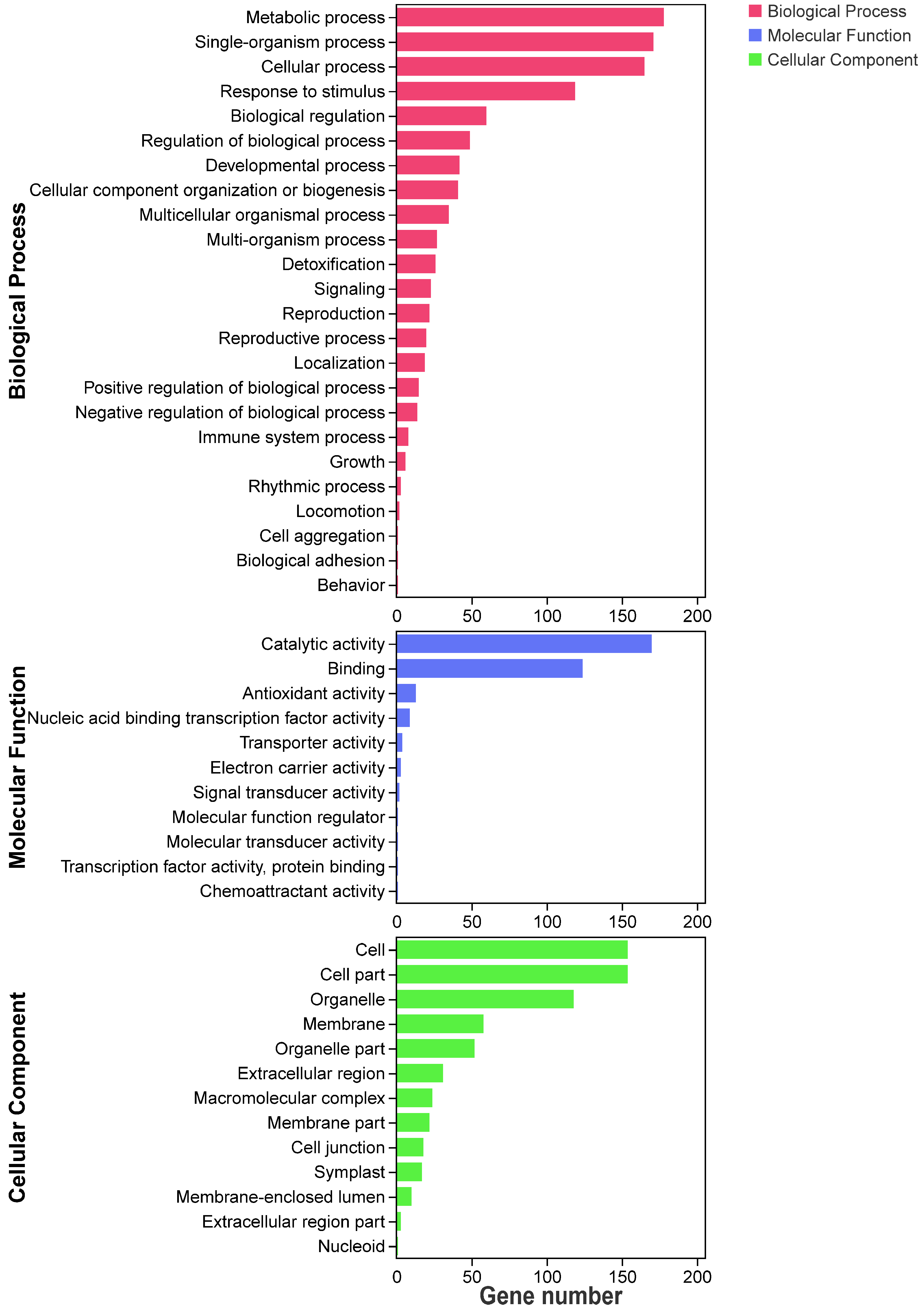
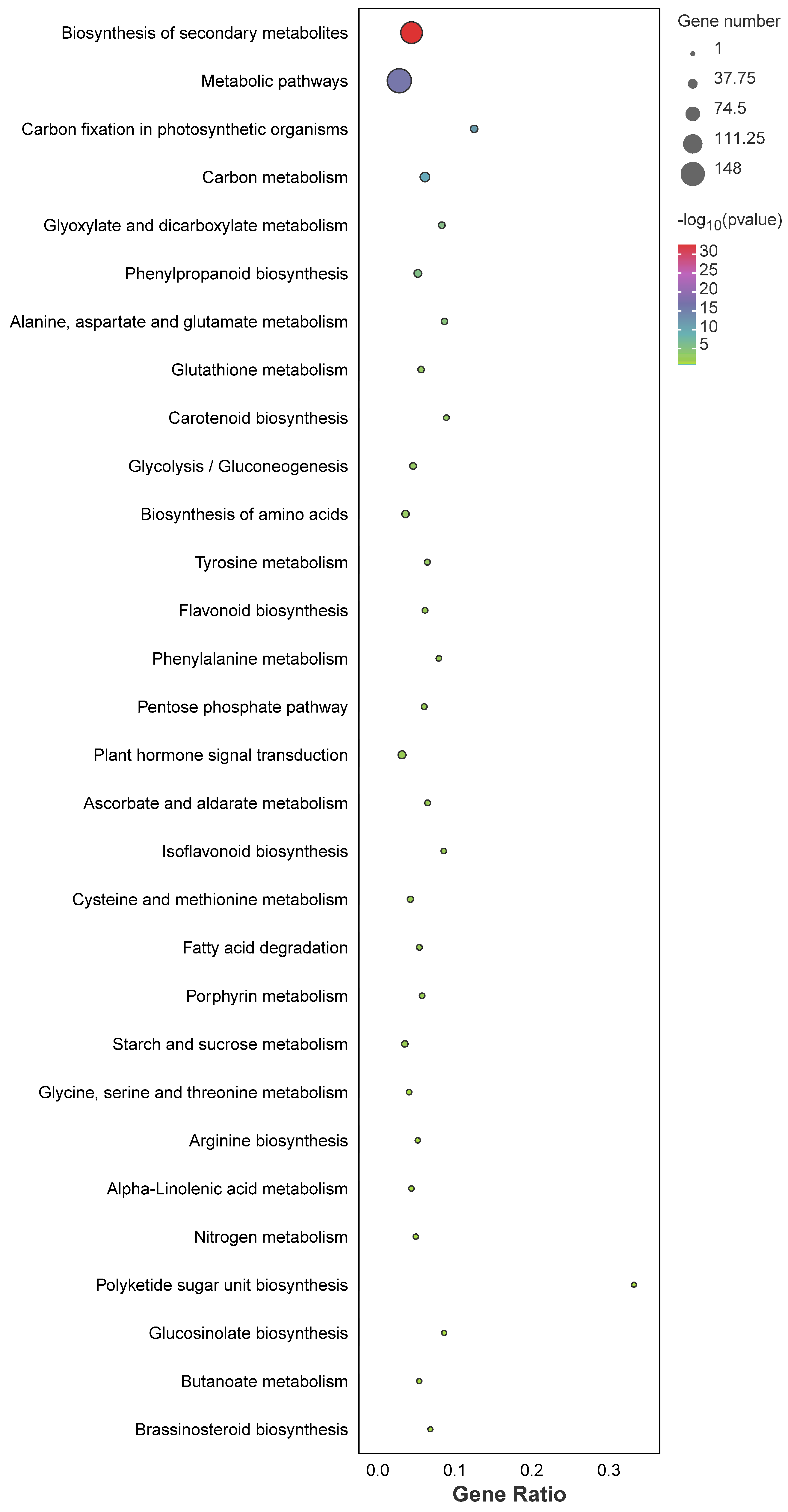
3.5. GO and KEGG Analysis of These DEGs Primed by PEG-6000
3.6. PEG-6000 Priming Induced Gene Expression in Carbon Metabolism, Glyoxylic Acid, and Dicarboxylic Acid Metabolism Pathways
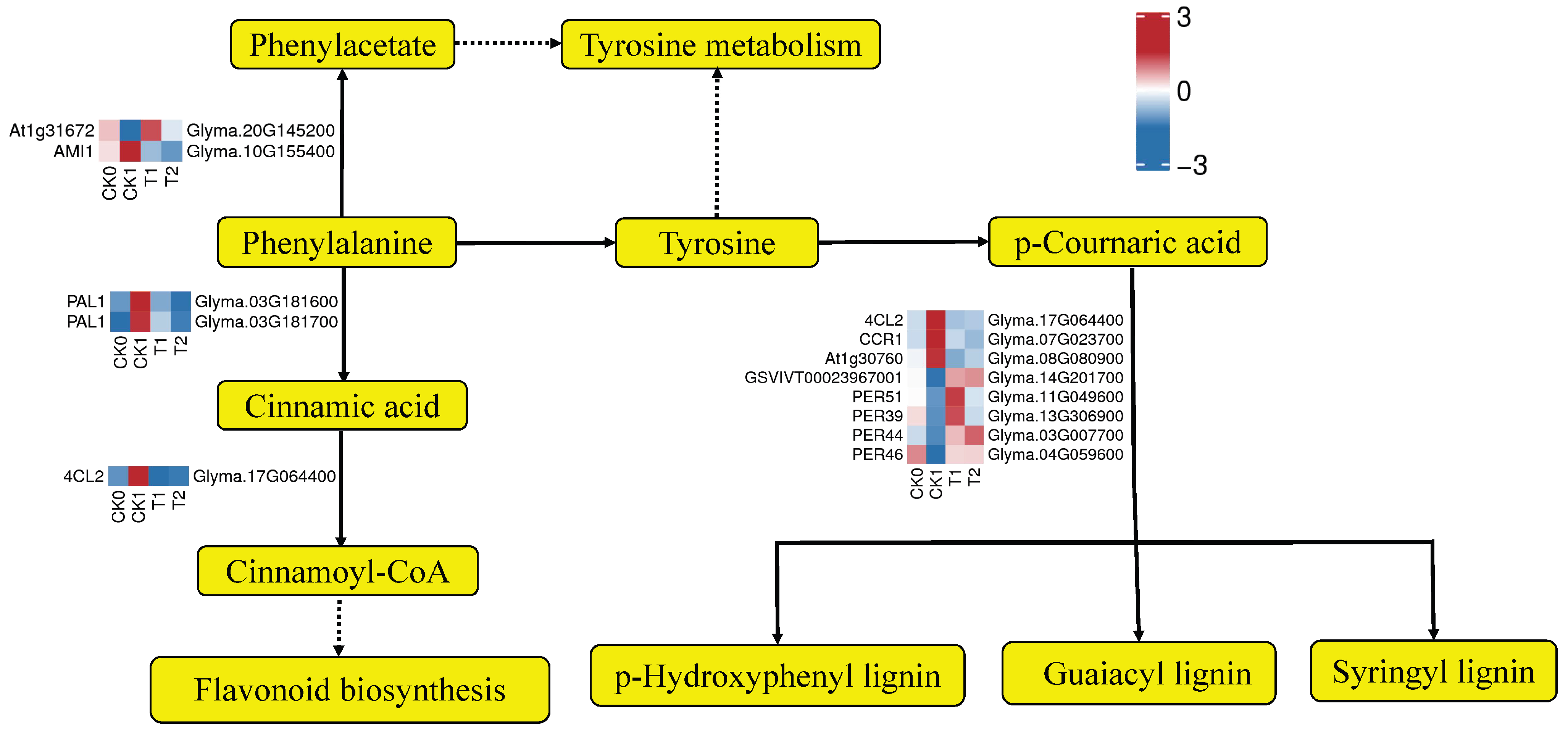
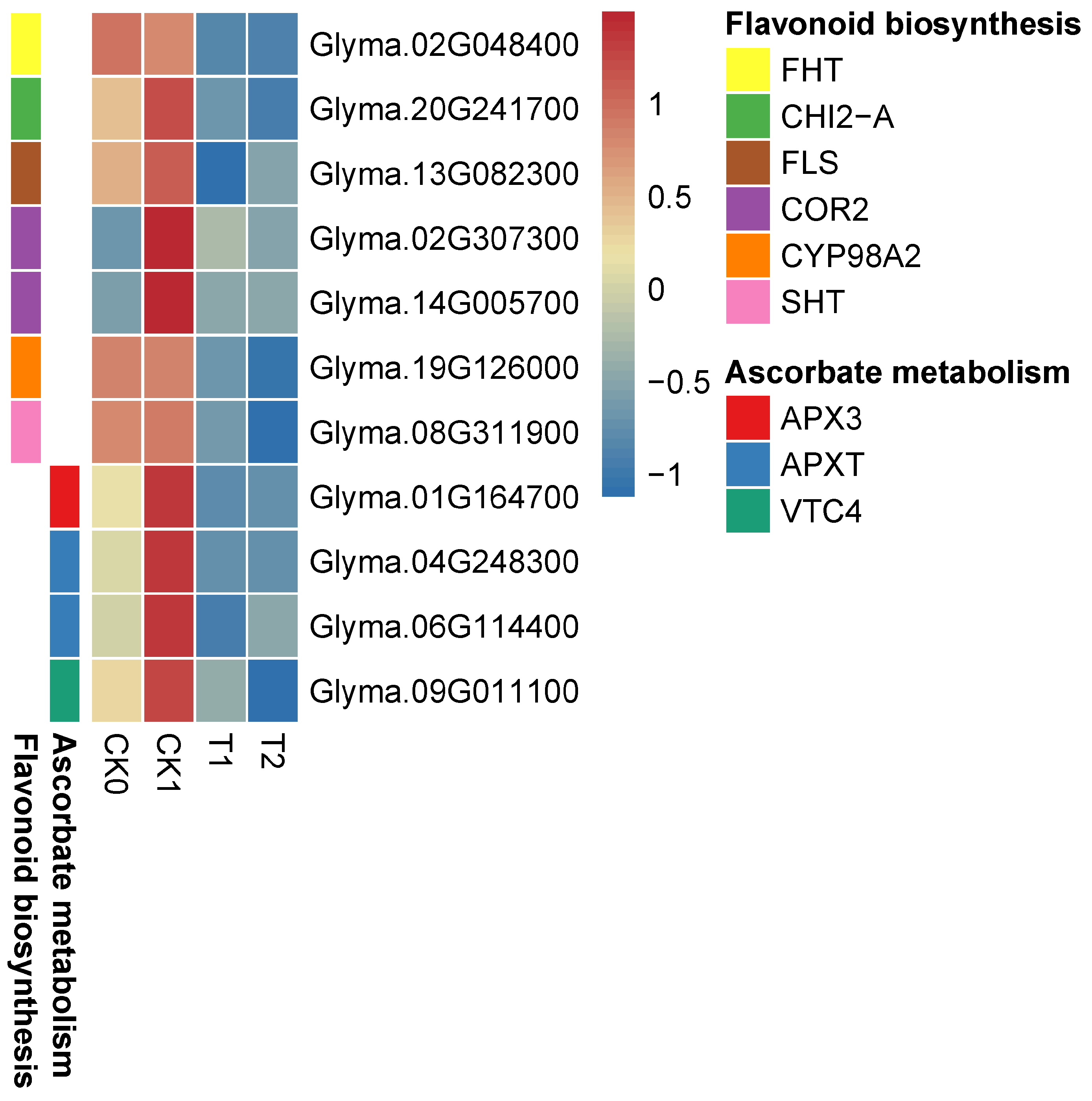
3.7. PEG-6000 Priming Enhanced Gene Expression in the Lignin Biosynthesis Pathway
3.8. PEG-6000 Priming Enhanced the Gene Expression of Antioxidant Enzymes
3.9. PEG-6000 Priming Regulates Gene Expression of Plant Hormone Signal Transduction Pathway
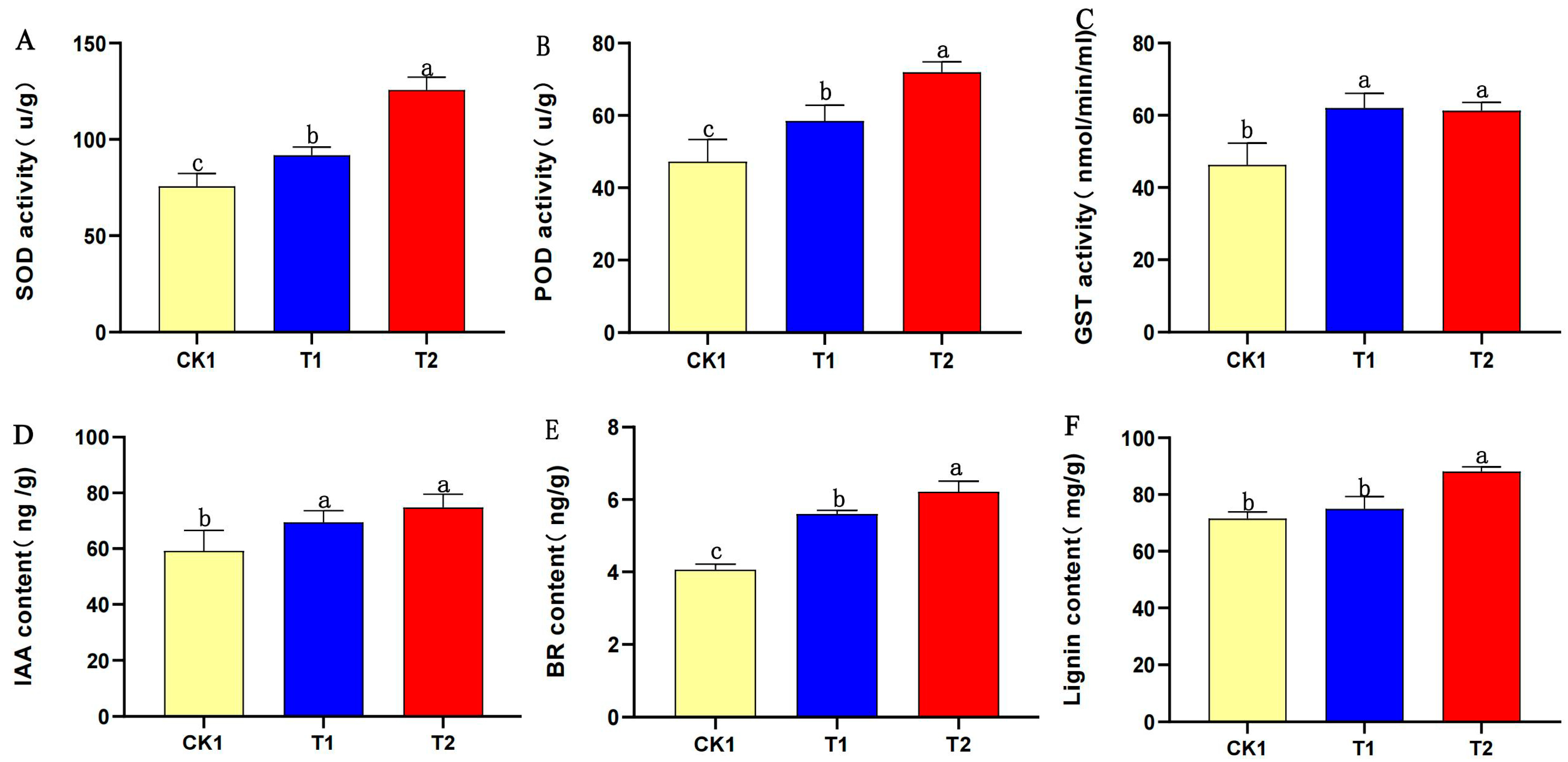
3.10. PEG-6000 Priming Treatment Increased the Enzyme Activity, Hormone Content, and Lignin Content of Aged Soybean Seeds
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lin, Y.X.; Xu, H.J.; Yin, G.K.; Zhou, Y.C.; Lu, X.X.; Xin, X. Dynamic Changes in Membrane Lipid Metabolism and Antioxidant Defense During Soybean (Glycine max L. Merr.) Seed Aging. Front. Plant Sci. 2022, 13, 908949. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.M.; Lu, X.X.; Zhu, L.Y.; Xin, X.; Jiang, X.C. Correlation studies on MDA and 4-HNE contents in soybean seed aging. Seed 2019, 38, 1–9. [Google Scholar] [CrossRef]
- Ziegler, V.; Marini, L.J.; Ferreira, C.D.; Bertinetti, I.A.; Silva, W.S.V.; Goebel, J.T.; Oliveira, M.; Elias, M.C. Effects of temperature and moisture during semi-hermetic storage on the quality e-valuation parameters of soybean grain and oil. Semin-Cienc. 2016, 37, 131–144. [Google Scholar] [CrossRef]
- Michalak, M.; Plitta-Michalak, B.P.; Naskręt-Barciszewska, M.Z.; Barciszewski, J.; Chmielarz, P. DNA methylation as an early indicator of aging in stored seeds of “exceptional” species Populus nigra L. Cells 2022, 11, 2080. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yang, R.C.; Ji, Z.Q.; Zhang, H.X.; Zheng, W.B.; Zhang, H.H.; Feng, F.Q. Evaluation of biochemical and physiological changes in sweet corn seeds under natural aging and artificial accelerated aging. Agronomy 2022, 12, 1028. [Google Scholar] [CrossRef]
- Moncaleano-Escandon, J.; Silva, B.C.F.; Silvia, S.R.S.; Granja, J.A.A.; Alve, M.C.J.L.; Pompelli, M.F. Germination responses of Jatropha curcas L. Seeds to storage and aging. Ind. Crops Prod. 2013, 44, 684–690. [Google Scholar] [CrossRef]
- Zhu, Y.Q.; Yan, H.F.; Xia, F.S.; Chen, Q.Z.; Wang, M.Y.; Mao, P.S. The relationship between mitochondria and seed aging. Pratac. Sci. 2016, 33, 90–298. [Google Scholar] [CrossRef]
- Miquel, J.; Economos, A.C.; Fleming, J.; Johnson, J.E. Mitochondrial role in cell aging. Exp. Gerontol. 1980, 15, 575–591. [Google Scholar] [CrossRef]
- Berjak, P.; Villiers, T.A. Ageing in plant embryos. New Phytol. 1970, 69, 929–938. [Google Scholar] [CrossRef]
- Zhou, G.Y.; Wu, S.Y.; Wang, L.H.; Wang, Y.Q. Main storage conditions factor of influencing longevity of seed in four crops. PGR 2014, 15, 56–66. [Google Scholar] [CrossRef]
- Liu, J.; Gui, J.; Gao, W.; Ma, J.F.; Wang, Q.Z. Review of the physiological and biochemical reactions and molecular mechanisms of seed aging. Acta Ecol. Sin. 2016, 36, 4997–5006. [Google Scholar] [CrossRef]
- Murthy, U.M.; Kumar, P.P.; Sun, W.Q. Mechanisms of seed ageing under different storage conditions for Vigna radiata (L.) Wilczek: Lipid peroxidation, sugar hydrolysis, Maillard reactions and their relationship to glass state transition. J. Exp. Bot. 2003, 54, 1057–1067. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.F.; Xia, F.S.; Miao, P.S. Research progress of seed aging and vigor repair. Chin. Agric. Sci. Bull. 2014, 30, 20–26. [Google Scholar] [CrossRef]
- Maduo, J.J.; Wang, Y.C. Study Progress of Seed Priming Techniques. Seed 2013, 32, 43–46. [Google Scholar] [CrossRef]
- Jisha, K.C.; Vijayakumari, K.; Puthur, J.T. Seed priming for abiotic stress tolerance: An overview. Acta Physiol. Plant. 2013, 35, 1381–1396. [Google Scholar] [CrossRef]
- Penfield, S. Seed dormancy and germination. Curr. Biol. 2017, 27, R874–R878. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.F.; Mao, P.S. Comparative time-course physiological responses and proteomic analysis of melatonin priming on promoting germination in aged oat (Avena sativa L.) seeds. Int. J. Mol. Sci. 2021, 22, 811. [Google Scholar] [CrossRef]
- Mao, C.L.; Zhu, Y.Q.; Cheng, H.; Yan, H.F.; Zhao, L.Y.; Tang, J.; Ma, X.Q.; Mao, P.S. Nitric oxide regulates seedling growth and mitochondrial responses in aged oat seeds. Int. J. Mol. Sci. 2018, 19, 1502. [Google Scholar] [CrossRef]
- Fang, C.Z.; Wu, X.Y.; Guan, X.; Zheng, C.F.; Zhao, H.Y.; Gu, Z.Z.; Liu, W.C.; Chen, J.; Zheng, Q.S. Concentration effects and its physiological mechanism of soaking seeds with brassinolide on tomato seed germination under salt stress. Acta Ecol. Sin. 2021, 41, 1857–1867. [Google Scholar] [CrossRef]
- Di, C.E.; Boullemant, A.; Courtney, R. Ecotoxicological risk assessment of revegetated bauxite residue: Implications for future rehabilitation programmes. Sci. Total Environ. 2020, 698, 134344. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, X.J.; Dong, Y.T.; Zhang, F.; He, Q.L.; Chen, J.H.; Zhu, S.J.; Zhao, T.L. Seed priming with melatonin improves salt tolerance in cotton through regulating photosynthesis, scavenging reactive oxygen species and coordinating with phytohormone signal pathways. Ind. Crop Prod. 2021, 169, 133671. [Google Scholar] [CrossRef]
- Marthandan, V.; Geetha, R.; Kumutha, K.; Renganathan, V.G.; Karthikeyan, A.; Ramalingam, J. Seed Priming: A Feasible Strategy to Enhance Drought Tolerance in Crop Plants. Int. J. Mol. Sci. 2020, 21, 8258. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Xue, C.; Xiong, Y.; Meng, X.; Li, B.; Shen, R.; Lan, P. Proteomic dissection of the similar and different responses of wheat to drought, salinity and submergence during seed germination. Proteomics 2020, 220, 103756. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Han, Y.C.; Hao, Y.R.; Wang, X.L.; Liu, L.T.; Sun, H.C.; Zhang, Y.J.; Li, C.D. Effects of polyethylene glycol priming on germination and physiological characteristics of cotton seeds under salt stress. Acta Agric. Nucl. Sin. 2021, 35, 202–210. Available online: https://www.hnxb.org.cn/CN/10.11869/j.issn.100-8551.2021.01.0202 (accessed on 7 November 2023).
- Goswami, A.; Banerjee, R.; Raha, S. Drought resistance in rice seedlings conferred by seed priming: Role of the anti-oxidant defense mechanisms. Protoplasma 2013, 250, 1115–1129. [Google Scholar] [CrossRef] [PubMed]
- Kibinza, S.; Bazin, J.; Bailly, C.; Farrant, J.M.; Corbineau, F.; El-Maarouf-Bouteau, H. Catalase is a key enzyme in seed recovery from ageing during priming. Plant Sci. 2011, 181, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Xia, F.S.; Chen, L.L.; Yan, H.F.; Sun, Y.; Li, M.L.; Mao, P.S. Antioxidant and ultrastructural responses to priming with PEG in aged, ultra-dry oat seed. Seed Sci. Technol. 2016, 44, 556–568. [Google Scholar] [CrossRef]
- Wang, Y.M.; Shen, C.B.; Jiang, Q.C.; Wang, Z.C.; Gao, C.Y.; Wang, W. Seed priming with calcium chloride enhances stress tolerance in rice seedlings. Plant Sci. 2022, 323, 111381. [Google Scholar] [CrossRef]
- Ferreira, R.A.; Volpi, E.S.N.; Dos, S.T.B.; Lima, A.F.; Castilho, C.C.; Barbosa, M.N.N.; Esteves, V.L.G. Regulation of α-expansins genes in Arabidopsis thaliana seeds during post-osmopriming germination. Physiol. Mol. Biol. Plants 2019, 25, 511–522. [Google Scholar] [CrossRef]
- Sheteiwy, M.S.; Fu, Y.Y.; Hu, Q.J.; Nawaz, A.; Guan, Y.J.; Li, Z.; Huang, Y.T.; Hu, J. Seed priming with polyethylene glycol induces antioxidative defense and metabolic regulation of rice under nano-ZnO stress. Environ. Sci. Pollut. Res. Int. 2016, 23, 19989–20002. [Google Scholar] [CrossRef]
- Gao, Y.P.; Young, L.; Bonham-Smith, P.; Gusta, L.V. Characterization and expression of plasma and tonoplast membrane aquaporins in primed seed of Brassica napus during germination under stress conditions. Plant Mol. Biol. 1999, 40, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Amooaghaie, R.; Tabatabaie, F. Osmopriming-induced salt tolerance during seed germination of alfalfa most likely mediates through H2O2 signaling and upregulation of heme oxygenase. Protoplasma 2017, 254, 1791–1803. [Google Scholar] [CrossRef]
- Trapnell, C.; Pacher, L.; Salzberg, S.L. TopHat: Discovering splice junctions with RNA-Seq. Bionformatics 2009, 25, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, C.; Roberts, A.; Goff, L.; Pertea, G.; Kim, D.; Kelley, D.R.; Pimentel, H.; Salzberg, S.L.; Rinn, J.L.; Pachter, L. Differential gene and transcript expression analysis of RNA-Seq experiments with TopHat and Cufflinks. Nat. Protoc. 2012, 7, 562–578. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, P.F.; Xia, H.; Zhao, C.Z.; Hou, L.; Chang, S.; Gao, C.; Wang, X.J.; Zhao, S.Z. Comparative transcriptome analysis of basal and zygote-located tip regions of peanut ovaries provides insight into the mechanism of light regulation in peanut embryo and pod development. BMC Genom. 2016, 17, 606. [Google Scholar] [CrossRef]
- Madhava, R.K.V.; Sresty, T.V. Antioxidant parameters in the seedlings of pigeon pea (Cajanus cajan (L.) Millspaugh) in response to Zn and Ni stresses. Plant Sci. 2000, 157, 113–128. [Google Scholar] [CrossRef] [PubMed]
- Castro, D.; Contreras, L.M.; Kurz, L.; Wilkesman, J. Detection of Guaiacol Peroxidase on Electrophoretic Gels. Methods Mol. Biol. 2017, 1626, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wu, Y.; Fang, C.; Cui, Y.; Jiang, N.; Wang, H. Simultaneous determination of 19 plant growth regulator residues in plant-originated foods by QuEChERS and stable isotope dilution-ultra performance liquid chromatography-mass spectrometry. Anal. Sci. 2017, 33, 1047–1052. [Google Scholar] [CrossRef]
- Hayet, E.; Christophe, B. Oxidative signaling in seed germination and dormancy. Plant Signal Behav. 2008, 3, 332–341. [Google Scholar] [CrossRef]
- Barba-Espin, G.; Diaz-Vivancos, P.; Clemente-Moreno, M.J.; Albacete, A.; Faize, L.; Faize, M.; Pérez-Alfocea, F.; Hernández, J.A. Interaction between hydrogen peroxide and plant hormones during germination and the early growth of pea seedlings. Plant Cell Environ. 2010, 33, 981–994. [Google Scholar] [CrossRef]
- Barba-Espín, G.; Diaz-Vivancos, P.; Job, D.; Belghazi, M.; Job, C.; Hernández, J.A. Understanding the role of H2O2 during pea seed germination: A combined proteomic and hormone profiling approach. Plant Cell Environ. 2011, 34, 1907–1919. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, Y.; Koda, Y.; Zheng, S.H.; Yuasa, T.; Iwaya-Inoue, M. Regulation of soybean seed germination through ethylene production in response to reactive oxygen species. Ann. Bot. 2013, 111, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Vivancos, P.; Barba-Espín, G.; Hernández, J.A. Elucidating hormonal/ROS networks during seed germination: Insights and perspectives. Plant Cell Rep. 2013, 32, 1491–1502. [Google Scholar] [CrossRef] [PubMed]
- Purugganan, M.M.; Braam, J.; Fry, S.C. The Arabidopsis TCH4 xyloglucan endotransglycosylase. Substrate specificity, pH optimum, and cold tolerance. Plant Physiol. 1997, 115, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Yoshida, T.; Yamaguchi-Shinozaki, K. Pivotal role of the AREB/ABF-SnRK2 pathway in ABRE-mediated transcription in response to osmotic stress in plants. Physiol. Plant. 2013, 147, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Nunes, A.; Oliveira, G.; Mexia, T.; Valdecantos, A.; Zucca, C.; Costantini, E.A.C.; Abraham, E.M.; Kyriazopoulos, A.P.; Salah, A.; Prasse, R.; et al. Ecological restoration across the Mediterranean Basin as viewed by practitioners. Sci. Total Environ. 2016, 566, 722–732. [Google Scholar] [CrossRef] [PubMed]
- Pedrini, S.; Dixon, W.K. International principles and standards for native seeds in ecological restoration. Restor. Ecol. 2020, 28, S286–S303. [Google Scholar] [CrossRef]
- Mashela, P.W.; Pofu, K.M.; Bopape-Mabapa, M.P. Efficacy of Seed Priming With Cucurbitacin Phytonematicides Against Meloidogyne incognita on Pea. Front Microbiol. 2022, 13, 863808. [Google Scholar] [CrossRef]
- Catusse, J.; Meinhard, J.; Job, C.; Strub, J.M.; Fischer, U.; Pestsova, E.; Westhoff, P.; Van, D.A.; Job, D. Proteomics reveals potential biomarkers of seed vigor in sugarbeet. Proteomics 2011, 11, 1569–1580. [Google Scholar] [CrossRef]
- Carvalho, R.F.; Piotto, F.A.; Schmidt, D.; Peters, L.P.; Monteiro, C.C.; Azevedo, R.A. Seed priming with hormones does not alleviate induced oxidative stress in maize seedlings subjected to salt stress. Sci. Agr. 2011, 68, 598–602. [Google Scholar] [CrossRef]
- Gao, Y.; Bonham-Smith, C.P.; Gusta, V.L. The role of peroxiredoxin antioxidant and calmodulin in ABA-primed seeds of Brassica napus exposed to abiotic stresses during germination. J Plant Physiol. 2002, 159, 951–958. [Google Scholar] [CrossRef]
- Aydinoğlu, B.; Shabani, A.; Safavi, S.M. Impact of Priming on Seed Germination, Seedling Growth and Gene Expression in Common Vetch under Salinity Stress. Cell Mol. Biol. 2019, 65, 18–24. Available online: https://pubmed.ncbi.nlm.nih.gov/30942152/ (accessed on 7 November 2023). [CrossRef] [PubMed]
- Bourioug, M.; Ezzaza, K.; Bouabid, R.; Alaoui-Mhamdi, M.; Bungau, S.; Bourgeade, P.; Alaoui-Sossé, L.; Alaoui-Sossé, B.; Aleya, L. Influence of hydro- and osmo-priming on sunflower seeds to break dormancy and improve crop performance under water stress. Environ. Sci. Pollut. Res. Int. 2020, 27, 13215–13226. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.; Gaivão, I.; Lima-Brito, J. Seed osmopriming with PEG solutions in seeds of three infraspecific taxa of Pinus nigra: Impacts on germination, mitosis and nuclear DNA. Forest Ecol. Manag. 2020, 456, 117739. [Google Scholar] [CrossRef]
- Wang, S.G.; Zhao, J.G.; Ning, X.L. Effect of PEG-6000 priming on seed membrane permeability and the activity of protection enzyme of aging soybean. Acta Agric. Boreali-Sin. 2012, 27, 113–117. [Google Scholar] [CrossRef]
- Kucera, B.; Cohn, A.M.; Leubner-Metzger, G. Plant hormone interactions during seed dormancy release and germination. Seed Sci. Res. 2005, 15, 281–307. [Google Scholar] [CrossRef]
- Linkies, A.; Müller, K.; Morris, K.; Turecková, V.; Wenk, M.; Cadman, C.S.; Corbineau, F.; Strnad, M.; Lynn, J.R.; Finch-Savage, W.E.; et al. Ethylene interacts with abscisic acid to regulate endosperm rupture during germination: A comparative approach using Lepidium sativum and Arabidopsis thaliana. Plant Cell. 2009, 21, 3803–3822. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, V.; Reddy, K.J. Interactions between ethylene, abscisic acid and cytokinin during germination and seedling establishment in Arabidopsis. J. Biosci. 2010, 35, 451–458. [Google Scholar] [CrossRef]
- Chiwocha, S.D.; Cutler, A.J.; Abrams, S.R.; Ambrose, S.J.; Yang, J.; Ross, A.R.; Kermode, A.R. The etr1-2 mutation in Arabidopsis thaliana affects the abscisic acid, auxin, cytokinin and gibberellin metabolic pathways during maintenance of seed dormancy, moist-chilling and germination. Plant J. 2005, 42, 35–48. [Google Scholar] [CrossRef]
- Jia, S.Q. Cloning and Preliminary Functional Identification of SAUR71 Gene in Creeping Bentgrass. BFU. 2020. Available online: https://kns.cnki.net/KCMS/detail/detail.aspx?dbname=CMFD202101&filename=1020338257.nh (accessed on 7 November 2023).
- Hou, K.; Wu, W.; Gan, S.S. SAUR36, a small auxin up RNA gene, is involved in the promotion of leaf senescence in Arabidopsis. Plant Physiol. 2013, 161, 1002–1009. [Google Scholar] [CrossRef]
- Barba-Espin, G.; Nicolas, E.; Almansa, M.S.; Cantero-Navarro, E.; Albacete, A.; Hernández, J.A.; Díaz-Vivancos, P. Role of thioproline on seed germination: Interaction ROS-ABA and effects on antioxidative metabolism. Plant Physiol. Bioch. 2012, 59, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Bahin, E.; Bailly, C.; Sotta, B.; Kranner, I.; Corbineau, F.; Leymarie, J. Crosstalk between reactive oxygen species and hormonal signaling pathway regulates grain dormancy in barley. Plant Cell Environ. 2011, 34, 980–993. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.H.R.; Weng, C.C.; Lo, H.F.; Chen, J.T. Study of the root antioxidative system of tomatoes and eggplants under waterlogged conditions. Plant Sci. 2004, 167, 159–388. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Van, B.F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Tewari, R.K.; Praveen, K.; Sharma, P.N. Magnesium deficiency induced oxidative stress and antioxidant responses in mulberry plants. Sci. Hortic-Amst. 2006, 108, 7–14. [Google Scholar] [CrossRef]
- Yang, R.S. The Regulation Mechanism of MSCA1 and Paralogous Genes in Maize Ear Development. HZAU. 2021. Available online: https://kns.cnki.net/KCMS/detail/detail.aspx?dbname=CDFDLAST2023&filename=1022802522.nh (accessed on 7 November 2023).
- Wood, Z.A.; Schröder, E.; Harris, J.R.; Poole, L.B. Structure, mechanism and regulation of peroxiredoxins. Trends Biochem. Sci. 2003, 28, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Li, W.; Zhang, Y.; Xia, C.; Liu, Y.; Wang, C.; Xu, R.; Zhang, L. Identification of genes/proteins related to submergence tolerance by transcriptome and proteome analyses in soybean. Sci. Rep. 2019, 9, 14688. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhou, E.; Yao, M.; Xue, D.; Zhao, N.; Zhou, Y.; Li, B.; Wang, K.; Miao, Y.; Gu, C.; et al. PEG-6000 Priming Improves Aged Soybean Seed Vigor via Carbon Metabolism, ROS Scavenging, Hormone Signaling, and Lignin Synthesis Regulation. Agronomy 2023, 13, 3021. https://doi.org/10.3390/agronomy13123021
Wang Y, Zhou E, Yao M, Xue D, Zhao N, Zhou Y, Li B, Wang K, Miao Y, Gu C, et al. PEG-6000 Priming Improves Aged Soybean Seed Vigor via Carbon Metabolism, ROS Scavenging, Hormone Signaling, and Lignin Synthesis Regulation. Agronomy. 2023; 13(12):3021. https://doi.org/10.3390/agronomy13123021
Chicago/Turabian StyleWang, Yongqiang, Enqiang Zhou, Mengnan Yao, Dong Xue, Na Zhao, Yao Zhou, Bo Li, Kaihua Wang, Yamei Miao, Chunyan Gu, and et al. 2023. "PEG-6000 Priming Improves Aged Soybean Seed Vigor via Carbon Metabolism, ROS Scavenging, Hormone Signaling, and Lignin Synthesis Regulation" Agronomy 13, no. 12: 3021. https://doi.org/10.3390/agronomy13123021




