Halotolerant Rhizobacteria Promote Plant Growth and Decrease Salt Stress in Carya illinoinensis (Wangenh.) K. Koch
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Experimental Design
2.3. Determination of Halotolerance in the Rhizobacteria LBEndo1
2.4. Bacterial Inoculation
2.5. Biostimulation of C. illinoinensis by the Rhizobacteria LBEndo1
2.6. Extraction and Measurement of Proline
2.7. Extraction and Measurement of Chlorophyll
2.8. Statistical Analysis
3. Results
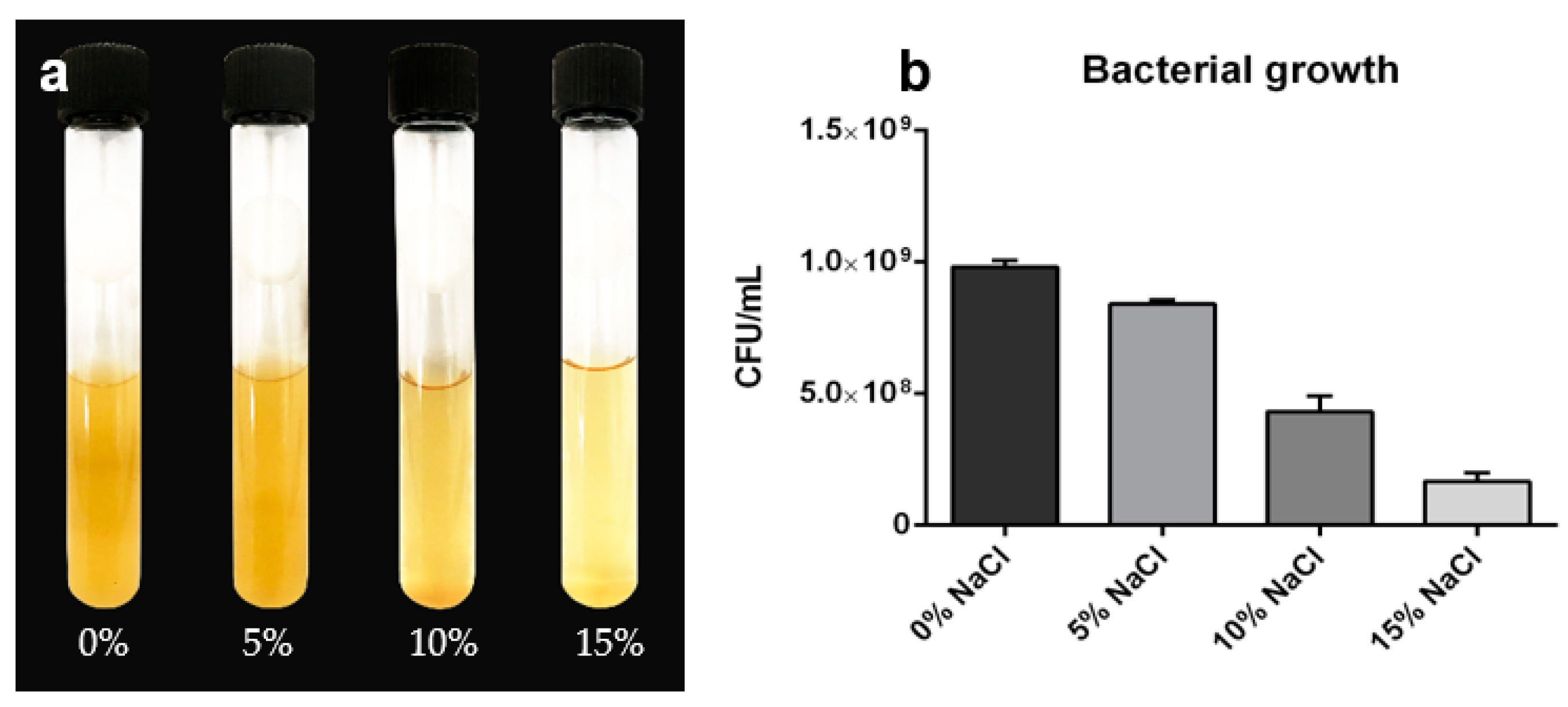
3.1. Halotolerance of the Rhizobacteria LBEndo1
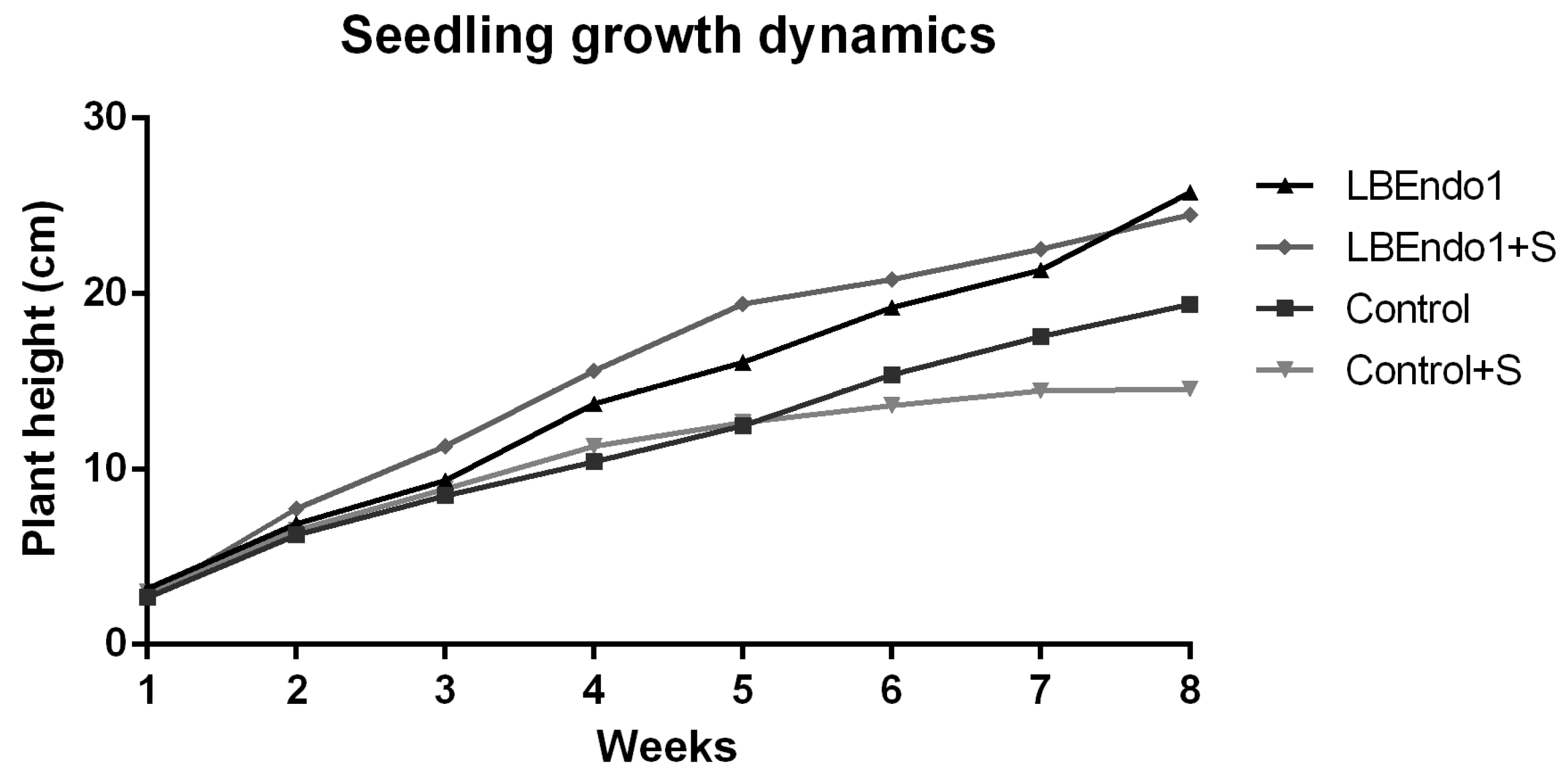
3.2. Seedling Growth Dynamics
3.3. Growth Promotion under Saline and Non-Saline Stress Conditions
3.4. Proline Content
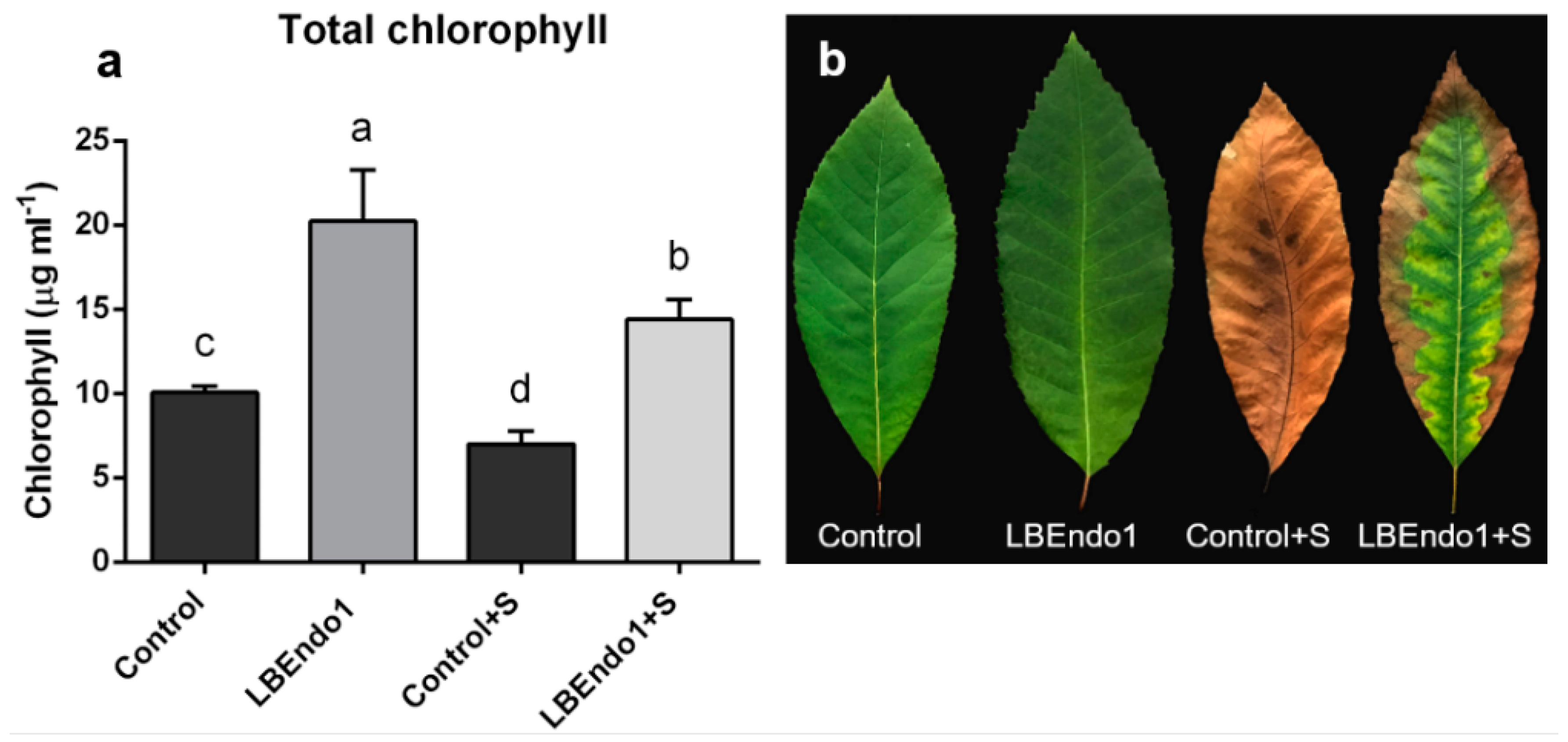
3.5. Chlorophyll Content and Foliar Damage
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rani, S.; Sharma, A.; Wali, V.K.; Kour, K.; Sharma, M.; Sharma, M. Evaluation of genetic diversity of pecan nut [Carya illinoensis (Wang) K. Koch.] in Jammu region. Indian J. Hortic. 2017, 74, 601–603. [Google Scholar] [CrossRef]
- Oliveira de Oliveira, L.; Carlos Beise, D.; Damian Dos Santos, D.; Caroline Nagel, J.; Poletto, T.; Poletto, I.; Stefenon, V.M. Molecular markers in Carya illinoinensis (Juglandaceae): From genetic characterization to molecular breeding. J. Hortic. Sci. Biotechnol. 2021, 96, 560–569. [Google Scholar] [CrossRef]
- Sierra-Zurita, D.; Santana-Espinoza, S.; Rosales-Serna, R.; Ríos-Saucedo, J.C.; Carrillo-Parra, A. Productivity and Characterization of Biomass Obtained from Pruning of Walnut Orchards in México. Energies 2023, 16, 2243. [Google Scholar] [CrossRef]
- Casales, F.G.; van der Watt, E.; Coetzer, G.M. Propagation of Pecan (Carya illinoensis): A review. Afr. J. Biotecnol. 2018, 17, 586–605. [Google Scholar] [CrossRef]
- Wolstenholme, B.N. The ecology of pecan trees. 2. Climatic aspects of growing pecans. Pecan Q. 1979, 13, 14–19. [Google Scholar]
- Miyamoto, S.; Riley, T.; Gobran, G.; Petticrew, J. Effects of saline water irrigation on soil salinity, pecan tree growth and nut production. Irrig. Sci. 1986, 7, 83–95. [Google Scholar] [CrossRef]
- Miyamoto, S. Supplement of Diagnosis and Management of Salinity Problems in Irrigated Pecan Production: Salt Leaching; Technical Report Nº 387A; Texas Water Resources Institute: College Station, TX, USA, 2010; 12p, Available online: https://core.ac.uk/download/pdf/147136462.pdf (accessed on 6 December 2023).
- Miyamoto, S.; Nesbitt, M. Effectiveness of soil salinity management practices in basin-irrigated pecan orchards. HortTechnology 2011, 21, 569–576. [Google Scholar] [CrossRef]
- Moreno-Izaguirre, E.; Ojeda-Barrios, D.; Avila-Quezada, G.; Guerrero-Prieto, V.; Parra-Quezada, R.; Ruiz-Anchondo, T. Sodium sulfate exposure slows growth of native pecan seedlings. Phyton 2015, 84, 80. [Google Scholar] [CrossRef]
- Campos-Villarreal, A.G.; Arreola-Ávila, J.G.; Chávez-Simental, J.A.; Calzada, R.T.; de la Rosa, A.B.; Santos, A.L.; Salgado, J.R.H. Respuesta fisiológica, acumulación iónica y peso seco en portainjertos de nogal pecanero (Carya illinoinensis (Wangenh) k. Koch) desarrollados bajo condiciones de estrés salino. Interciencia 2017, 42, 744–749. [Google Scholar]
- Shahid, M.A.; Sarkhosh, A.; Khan, N.; Balal, R.M.; Ali, S.; Rossi, L.; Gómez, C.; Mattson, N.; Nasim, W.; Garcia-Sanchez, F. Insights into the physiological and biochemical impacts of salt stress on plant growth and development. Agronomy 2020, 10, 938. [Google Scholar] [CrossRef]
- Zhu, J.K. Genetic analysis of plant salt tolerance using Arabidopsis. Plant Physiol. 2000, 124, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Angelina, E.; Papatheodorou, E.M.; Demirtzoglou, T.; Monokrousos, N. Effects of Bacillus subtilis and Pseudomonas fluorescens Inoculation on Attributes of the Lettuce (Lactuca sativa L.) Soil Rhizosphere Microbial Community: The Role of the Management System. Agronomy 2020, 10, 1428. [Google Scholar] [CrossRef]
- Bhattacharyya, P.N.; Jha, D.K. Plant growth-promoting rhizobacteria (PGPR): Emergence in agriculture. World J. Microbiol. Biotechnol. 2012, 28, 1327–1350. [Google Scholar] [CrossRef] [PubMed]
- Goswami, M.; Suresh, D.E.K.A. Plant growth-promoting rhizobacteria alleviators of abiotic stresses in soil: A review. Pedosphere 2020, 30, 40–61. [Google Scholar] [CrossRef]
- Dobbelaere, S.; Vanderleyden, J.; Okon, Y. Plant growth-promoting effects of diazotrophs in the rhizosphere. Crit. Rev. Plant Sci. 2003, 22, 107–149. [Google Scholar] [CrossRef]
- Mattioli, R.; Marchese, D.; D’Angeli, S.; Altamura, M.M.; Costantino, P.; Trovato, M. Modulation of intracellular proline levels affects flowering time and inflorescence architecture in Arabidopsis. Plant Mol. Biol. 2008, 66, 277–288. [Google Scholar] [CrossRef]
- Fu, Q.; Liu, C.; Ding, N.; Lin, Y.; Guo, B. Ameliorative effects of inoculation with the plant growth-promoting rhizobacterium Pseudomonas sp. DW1 on growth of eggplant (Solanum melongena L.) seedlings under salt stress. Agric. Water Manag. 2020, 97, 1994–2000. [Google Scholar] [CrossRef]
- Ali, S.; Charles, T.C.; Glick, B.R. Amelioration of high salinity stress damage by plant growth-promoting bacterial endophytes that contain ACC deaminase. Plant Physiol. Biochem. 2014, 80, 160–167. [Google Scholar] [CrossRef]
- Shilev, S.; Sancho, E.D.; Benlloch-González, M. Rhizospheric bacteria alleviate salt-produced stress in sunflower. J. Environ. Manag. 2012, 95, S37–S41. [Google Scholar] [CrossRef]
- Yao, L.; Wu, Z.; Zheng, Y.; Kaleem, I.; Li, C. Growth promotion and protection against salt stress by Pseudomonas putida Rs-198 on cotton. Eur. J. Soil Biol. 2010, 46, 49–54. [Google Scholar] [CrossRef]
- Glick, B.R.; Cheng, Z.; Czarny, J.; Duan, J. Promotion of plant growth by ACC deaminase-producing soil bacteria. Eur. J. Plant Pathol. 2007, 119, 329–339. [Google Scholar] [CrossRef]
- Mokrani, S.; Nabti, E.H.; Cruz, C. Current advances in plant growth promoting bacteria alleviating salt stress for sustainable agriculture. Appl. Sci. 2020, 10, 7025. [Google Scholar] [CrossRef]
- Moon, Y.S.; Khan, M.; Khan, M.A.; Ali, S. Ameliorative symbiosis of Serratia fonticola (S1T1) under salt stress condition enhance growth-promoting attributes of Cucumis sativus L. Symbiosis 2023, 89, 283–297. [Google Scholar] [CrossRef]
- Nadeem, S.M.; Zahir, Z.A.; Naveed, M.; Ashraf, M. Microbial ACC-deaminase: Prospects and applications for inducing salt tolerance in plants. Crit. Rev. Plant Sci. 2010, 29, 360–393. [Google Scholar] [CrossRef]
- Mohamed, H.I.; Gomaa, E.Z. Effect of plant growth promoting Bacillus subtilis and Pseudomonas fluorescens on growth and pigment composition of radish plants (Raphanus sativus) under NaCl stress. Photosynthetica 2012, 50, 263–272. [Google Scholar] [CrossRef]
- Ha-Tran, D.M.; Nguyen, T.T.M.; Hung, S.H.; Huang, E.; Huang, C.C. Roles of plant growth-promoting rhizobacteria (PGPR) in stimulating salinity stress defense in plants: A review. Int. J. Mol. Sci. 2021, 22, 3154. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, R. Analysis of malondialdehyde, chlorophyll proline, soluble sugar, and glutathione content in Arabidopsis seedling. Bio-Protocol 2013, 3, 817. [Google Scholar] [CrossRef]
- Zhang, J.; Jiao, Y.; Sharma, A.; Shen, D.; Wei, B.; Hong, C.; Zheng, B.; Pan, C. Transcriptomic analysis reveals potential pathways associated with salt resistance in pecan (Carya illinoensis K. Koch). J. Biotechnol. 2021, 330, 17–26. [Google Scholar] [CrossRef]
- Jiao, Y.; Zhang, J.; Pan, C. Genome-wide analysis of the GDSL genes in pecan (Carya illinoensis k. koch): Phylogeny, structure, promoter cis-elements, co-expression networks, and response to salt stresses. Genes 2022, 13, 1103. [Google Scholar] [CrossRef] [PubMed]
- Etesami, H.; Beattie, G.A. Mining halophytes for plant growth-promoting halotolerant bacteria to enhance the salinity tolerance of non-halophytic crops. Front. Microbiol. 2018, 9, 148. [Google Scholar] [CrossRef]
- Paul, D.; Lade, H. Plant-growth-promoting rhizobacteria to improve crop growth in saline soils: A review. Agron. Sustain. Dev. 2020, 34, 737–752. [Google Scholar] [CrossRef]
- Patani, A.; Prajapati, D.; Ali, D.; Kalasariya, H.; Yadav, V.K.; Tank, J.; Bagatharia, S.; Joshi, M.; Patel, A. Evaluation of the growth-inducing efficacy of various Bacillus species on the salt-stressed tomato (Lycopersicon esculentum Mill.). Front. Plant Sci. 2023, 14, 1168155. [Google Scholar] [CrossRef] [PubMed]
- Ibarra-Villarreal, A.L.; Gándara-Ledezma, A.; Godoy-Flores, A.D.; Herrera-Sepúlveda, A.; Díaz-Rodríguez, A.M.; Parra-Cota, F.I.; de los Santos-Villalobos, S. Salt-tolerant Bacillus species as a promising strategy to mitigate the salinity stress in wheat (Triticum turgidum subsp. durum). J. Arid Environ. 2021, 186, 104399. [Google Scholar] [CrossRef]
- Du, Y.; Ma, J.; Yin, Z.; Liu, K.; Yao, G.; Xu, W.; Fan, L.; Du, B.; Ding, Y.; Wang, C. Comparative genomic analysis of Bacillus paralicheniformis MDJK30 with its closely related species reveals an evolutionary relationship between B. paralicheniformis and B. licheniformis. BMC Genom. 2019, 20, 283. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Fimognari, L.; de Almeida, J.; Jensen, C.N.G.; Compant, S.; Oliveira, T.; Baelum, J.; Pastar, M.; Sessitsch, A.; Moelbak, L.; et al. Effect of Bacillus paralicheniformis on soybean (Glycine max) roots colonization, nutrient uptake and water use efficiency under drought stress. J. Agron. Crop Sci. 2023, 209, 547–565. [Google Scholar] [CrossRef]
- Khalilpour, M.; Mozafari, V.; Abbaszadeh-Dahaji, P. Tolerance to salinity and drought stresses in pistachio (Pistacia vera L.) seedlings inoculated with indigenous stress-tolerant PGPR isolates. Sci. Hortic. 2021, 289, 110440. [Google Scholar] [CrossRef]
- Salimi, F.; Khorshidi, M.; Amirahmadi, F.; Amirahmadi, A. Effectiveness of Phosphate and Zinc Solubilizing Paenarthrobacter nitroguajacolicus P1 as Halotolerant Rhizobacterium with Growth-Promoting Activity on Pistacia vera L. Curr. Microbiol. 2023, 80, 336. [Google Scholar] [CrossRef]
- Shi, J.W.; Lu, L.X.; Shi, H.M.; Ye, J.R. Effects of plant growth promoting rhizobacteria on the growth and soil microbial community of Carya illinoinensis. Curr. Microbiol. 2022, 79, 352. [Google Scholar] [CrossRef]
- Shabaan, M.; Asghar, H.N.; Zahir, Z.A.; Zhang, X.; Sardar, M.F.; Li, H. Salt-tolerant PGPR confer salt tolerance to maize through enhanced soil biological health, enzymatic activities, nutrient uptake and antioxidant defense. Front. Microbiol. 2022, 13, 901865. [Google Scholar] [CrossRef]
- Pérez-Jaramillo, J.E.; Carrión, V.J.; Bosse, M.; Ferrão, L.F.; De Hollander, M.; Garcia, A.A.; Ramírez, C.A.; Mendes, R.; Raaijmakers, J.M. Linking rhizosphere microbiome composition of wild and domesticated Phaseolus vulgaris to genotypic and root phenotypic traits. ISME J. 2017, 11, 2244–2257. [Google Scholar] [CrossRef]
- Hosseinifard, M.; Stefaniak, S.; Ghorbani Javid, M.; Soltani, E.; Wojtyla, Ł.; Garnczarska, M. Contribution of exogenous proline to abiotic stresses tolerance in plants: A review. Int. J. Mol. Sci. 2022, 23, 5186. [Google Scholar] [CrossRef]
- Ji, C.; Tian, H.; Wang, X.; Song, X.; Ju, R.; Li, H.; Liu, X. Bacillus subtilis HG-15, a halotolerant rhizoplane bacterium, promotes growth and salinity tolerance in wheat (Triticum aestivum). BioMed Res. Int. 2022, 2022, 9506227. [Google Scholar] [CrossRef]
- Ashraf, M.F.M.R.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Shahid, S.; Shahbaz, M.; Maqsood, M.F.; Farhat, F.; Zulfiqar, U.; Javed, T.; Fraz Ali, M.; Alhomrani, M.; Alamri, A.S. Proline-induced modifications in morpho-physiological, biochemical and yield attributes of pea (Pisum sativum L.) cultivars under salt stress. Sustainability 2022, 14, 13579. [Google Scholar] [CrossRef]
- Kamran, M.; Imran, Q.M.; Ahmed, M.B.; Falak, N.; Khatoon, A.; Yun, B.W. Endophyte-mediated stress tolerance in plants: A sustainable strategy to enhance resilience and assist crop improvement. Cells 2022, 11, 3292. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Zhang, M.; Du, L.; Zhang, L.; Li, B. Effects of Bacillus amyloliquefaciens QST713 on photosynthesis and antioxidant characteristics of Alfalfa (Medicago sativa L.) under drought stress. Agronomy 2022, 12, 2177. [Google Scholar] [CrossRef]
- Altuntaş, C.; Demiralay, M.; Sezgin Muslu, A.; Terzi, R. Proline-stimulated signaling primarily targets the chlorophyll degradation pathway and photosynthesis associated processes to cope with short-term water deficit in maize. Photosynth. Res. 2020, 144, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Asaf, S.; Khan, A.L.; Adhikari, A.; Jan, R.; Ali, S.; Imran, M.; Kim, K.-M.; Lee, I.J. Halotolerant rhizobacterial strains mitigate the adverse effects of NaCl stress in soybean seedlings. BioMed Res. Int. 2019, 2019, 9530963. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]


| Leaf Dry Weight (g) | Stem Dry Weight (g) | Root Dry Weight (g) | Total Dry Weight (g) | Seedling Height (cm) | Stem Diameter (cm) | |
|---|---|---|---|---|---|---|
| Control | 1.3 ± 0.19 c | 1.6 ± 0.19 c | 10.1 ± 0.94 b | 13.0 ± 0.89 b | 19.3 ± 0.67 b | 3.7 ± 0.39 a |
| LBEndo1 | 2.4 ± 0.13 a | 2.5 ± 0.10 a | 16.0 ± 2.02 a | 21.0 ± 2.18 a | 25.7 ± 1.22 a | 4.1 ± 0.34 a |
| Control+S | 0.7 ± 0.13 d | 1.1 ± 0.10 d | 6.9 ± 0.65 c | 8.7 ± 0.67 c | 14.5 ± 1.37 c | 2.9 ± 0.35 b |
| LBEndo1+S | 1.9 ± 0.22 b | 2.2 ± 0.16 b | 14.6 ± 1.06 a | 18.9 ± 0.75 a | 24.4 ± 1.37 a | 3.9 ± 0.23 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palacio-Rodríguez, R.; Sáenz-Mata, J.; Trejo-Calzada, R.; Ochoa-García, P.P.; Arreola-Ávila, J.G. Halotolerant Rhizobacteria Promote Plant Growth and Decrease Salt Stress in Carya illinoinensis (Wangenh.) K. Koch. Agronomy 2023, 13, 3045. https://doi.org/10.3390/agronomy13123045
Palacio-Rodríguez R, Sáenz-Mata J, Trejo-Calzada R, Ochoa-García PP, Arreola-Ávila JG. Halotolerant Rhizobacteria Promote Plant Growth and Decrease Salt Stress in Carya illinoinensis (Wangenh.) K. Koch. Agronomy. 2023; 13(12):3045. https://doi.org/10.3390/agronomy13123045
Chicago/Turabian StylePalacio-Rodríguez, Rubén, Jorge Sáenz-Mata, Ricardo Trejo-Calzada, Perla Patricia Ochoa-García, and Jesús G. Arreola-Ávila. 2023. "Halotolerant Rhizobacteria Promote Plant Growth and Decrease Salt Stress in Carya illinoinensis (Wangenh.) K. Koch" Agronomy 13, no. 12: 3045. https://doi.org/10.3390/agronomy13123045
APA StylePalacio-Rodríguez, R., Sáenz-Mata, J., Trejo-Calzada, R., Ochoa-García, P. P., & Arreola-Ávila, J. G. (2023). Halotolerant Rhizobacteria Promote Plant Growth and Decrease Salt Stress in Carya illinoinensis (Wangenh.) K. Koch. Agronomy, 13(12), 3045. https://doi.org/10.3390/agronomy13123045






