The Grapevine Transcription Factor VvTGA8 Enhances Resistance to White Rot via the Salicylic Acid Signaling Pathway in Tomato
Abstract
:1. Introduction
2. Materials and Methods
2.1. Grapevine Samples for RNA-seq
2.2. Total RNA Extraction, Library Construction, and Sequencing
2.3. RNA-seq Data Analysis
2.4. Weighted Gene Co-Expression Network and Pathway Enrichment Analysis
2.5. Virus-Induced Gene Silencing Analysis
2.6. Subcellular Localization Analysis of VvTGA8
2.7. Overexpression of VvTGA8 in Tomato Plants
2.8. Salicylic Acid Measurements
2.9. Gene Expression Analysis
3. Results
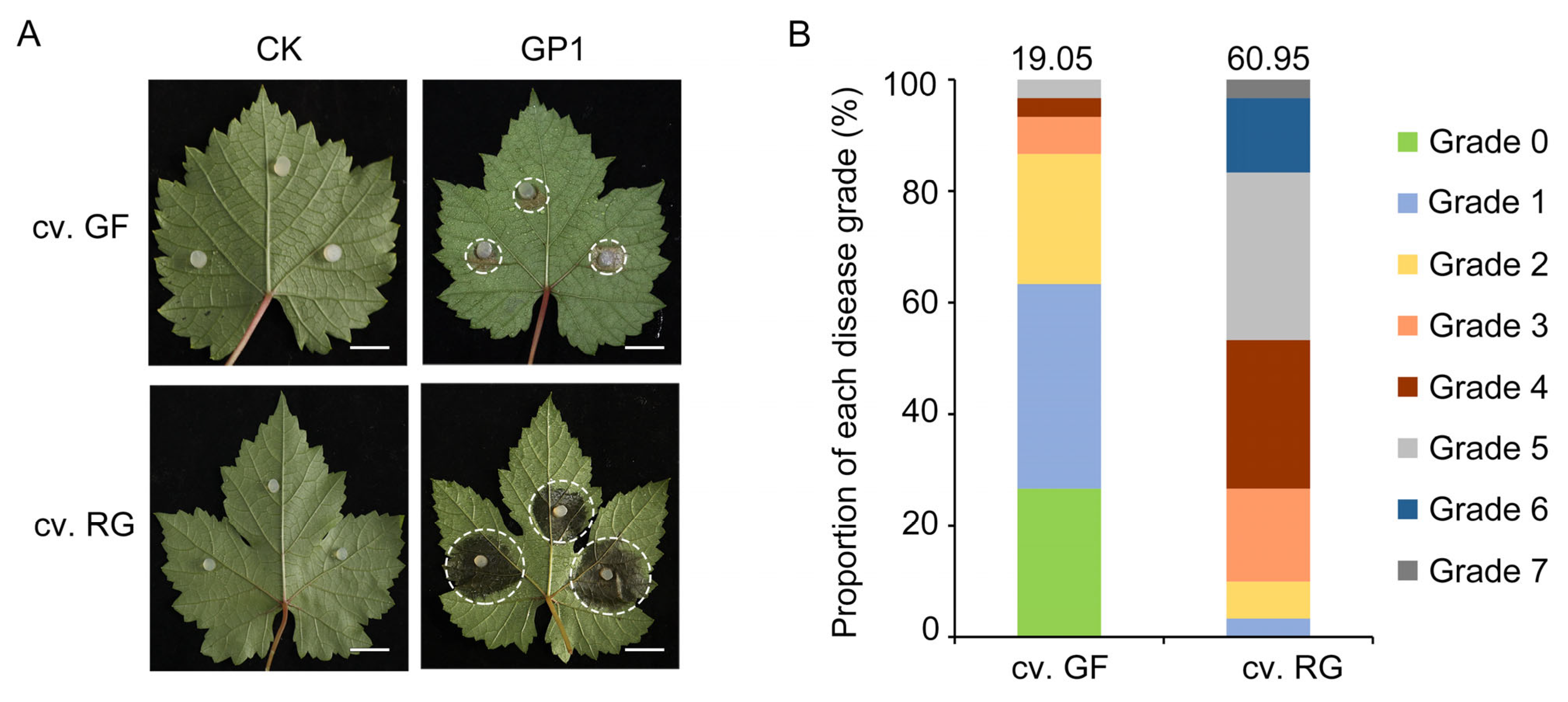
3.1. Differences among Grapevine Varieties in Resistance to C. vitis
3.2. Differential Gene Expression Analysis
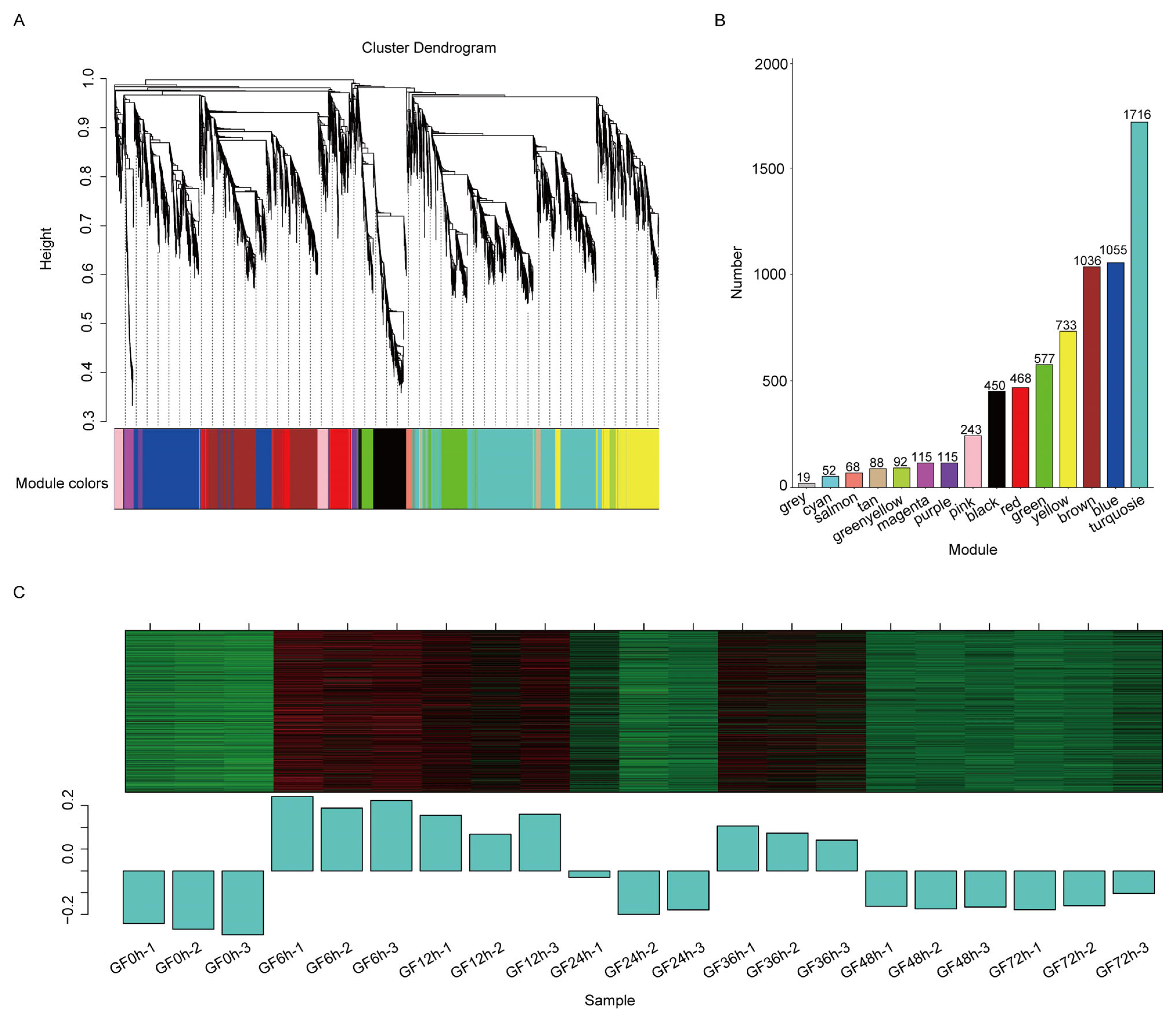
3.3. WGCNA of DEGs
3.4. Analyses of KEGG Pathway Enrichment and VvTGAs
3.5. Role of VvTGA8 in Grapevine Resistance to C. vitis
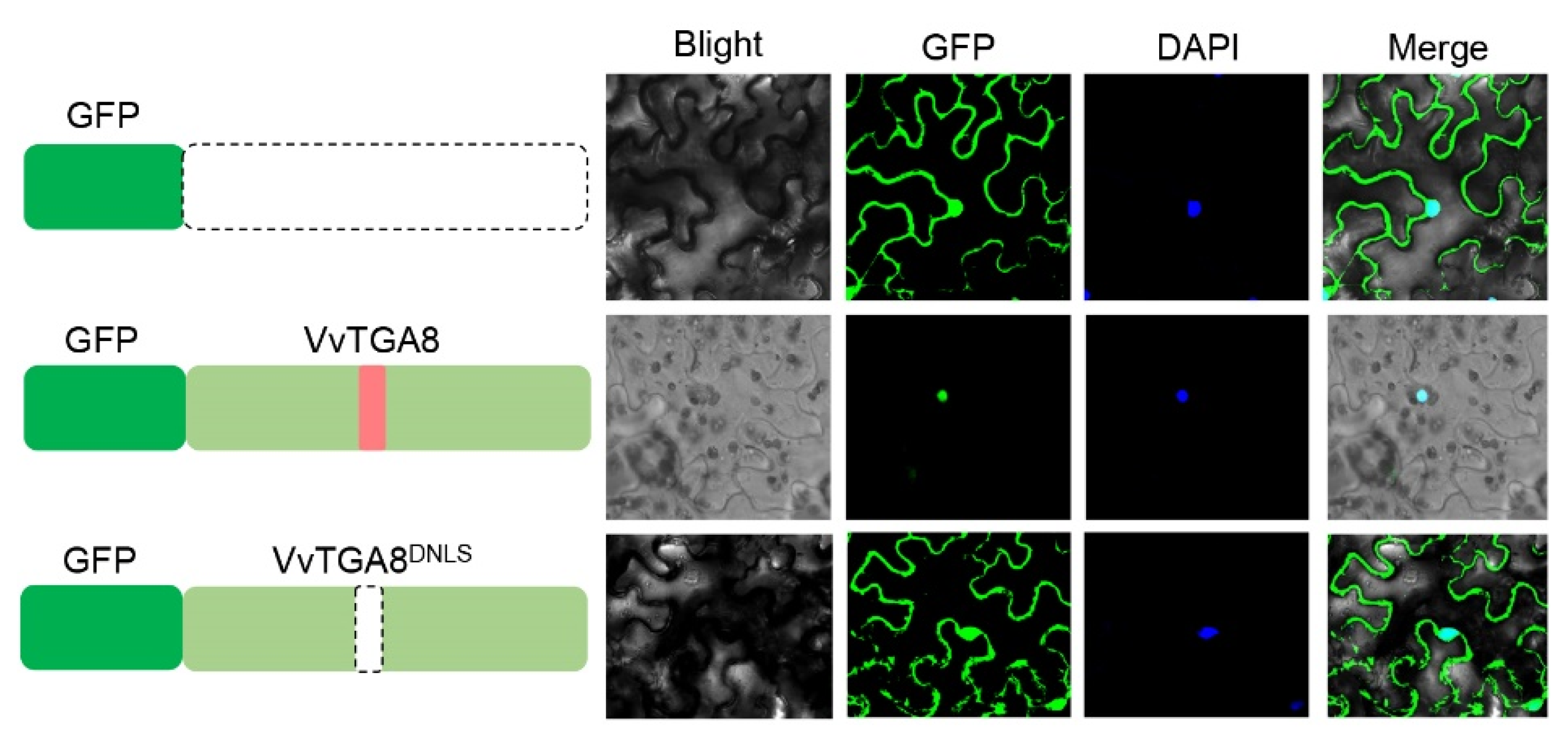
3.6. VvTGA8 Localized in Cell Nuclei
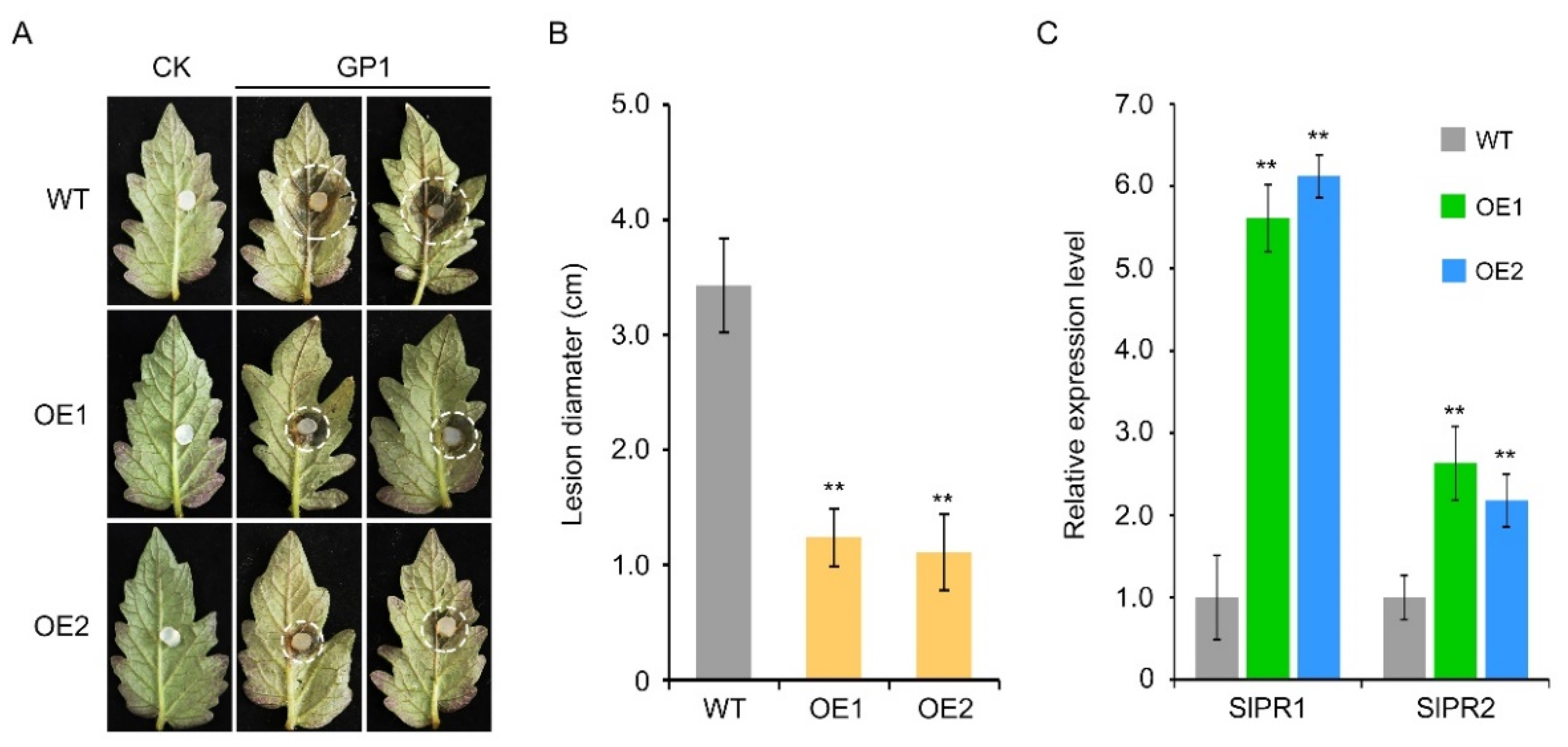
3.7. Heterologous Expression of VvTGA8 in Tomato Improves Resistance to C. vitis
3.8. VvTGA8 Confers Protective Effects via the Salicylic Acid Signaling Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ji, T.; Languasco, L.; Li, M.; Rossi, V. Effects of temperature and wetness duration on infection by Coniella diplodiella, the fungus causing white rot of grape berries. Plants 2021, 10, 1696. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wan, Y.Z.; Wang, Y.J.; He, P.C. Relatedness of resistance to anthracnose and to white rot in Chinese wild grapes. Vitis 2008, 47, 213–215. [Google Scholar] [CrossRef]
- He, Z.; Cui, C.Y.; Jiang, J.X. First report of white rot of grape caused by Pilidiella castaneicola in China. Plant Dis. 2017, 101, 1673. [Google Scholar] [CrossRef]
- Chethana, K.W.T.; Zhou, Y.; Zhang, W.; Liu, M.; Xing, Q.K.; Li, X.H.; Yan, J.Y.; Chethana, K.W.T.; Hyde, K.D. Coniella vitis sp. nov. is the common pathogen of white rot in chinese vineyards. Plant Dis. 2017, 101, 2123–2136. [Google Scholar] [CrossRef]
- Yin, X.T.; Li, T.G.; Wei, Y.F.; Liu, Q.B.; Jiang, X.L.; Yuan, L.F. First report of Coniella vitis causing white rot on Virginia creeper (Parthenocissus quinquefolia) in China. Plant Dis. 2023, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Rahman, F.U.; Khan, I.A.; Aslam, A.; Liu, R.T.; Sun, L.; Wu, Y.D.; Aslam, M.M.; Khan, A.U.; Li, P.; Jiang, J.F.; et al. Transcriptome analysis reveals pathogenesis-related gene 1 pathway against salicylic acid treatment in grapevine (Vitis vinifera L.). Front. Genet. 2022, 13, 1033288. [Google Scholar] [CrossRef]
- Yin, W.C.; Wang, X.H.; Liu, H.; Wang, Y.; Nocker, S.; Tu, M.X.; Fang, J.H.; Guo, J.Q.; Li, Z.; Wang, X.P. Overexpression of VqWRKY31 enhances powdery mildew resistance in grapevine by promoting salicylic acid signaling and specific metabolite synthesis. Hortic. Res. 2022, 9, 064. [Google Scholar] [CrossRef]
- Li, T.M.; Cheng, X.; Wang, X.W.; Li, G.G.; Wang, B.B.; Wang, W.Y.; Zhang, N.; Han, Y.L.; Jiao, B.L.; Wang, Y.J.; et al. Glyoxalase I-4 functions downstream of NAC72 to modulate downy mildew resistance in grapevine. Plant J. 2021, 108, 394–410. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.X.; Zhang, X.M.; Zhang, Q.H.; Chai, S.Y.; Yin, W.C.; Gao, M.; Li, Z.; Wang, X.P. The transcription factors VaERF16 and VaMYB306 interact to enhance resistance of grapevine to Botrytis cinerea infection. Mol. Plant Pathol. 2022, 23, 1415–1432. [Google Scholar] [CrossRef]
- Yu, Y.H.; Bian, L.; Wan, Y.T.; Jiao, Z.L.; Yu, K.K.; Zhang, G.H.; Guo, D.L. Grape (Vitis vinifera) VvDOF3 functions as a transcription activator and enhances powdery mildew resistance. Plant Physiol. Biochem. 2019, 143, 183–189. [Google Scholar] [CrossRef]
- Vandelle, E.; Ariani, P.; Regaiolo, A.; Danzi, D.; Lovato, A.; Zadra, C.; Vitulo, N.; Gambino, G.; Polverari, A. The grapevine E3 ubiquitin ligase VriATL156 confers resistance against the downy mildew pathogen Plasmopara viticola. Int. J. Mol. Sci. 2021, 22, 940. [Google Scholar] [CrossRef]
- Hu, Y.; Cheng, Y.; Yu, X.N.; Liu, J.; Yang, L.S.; Gao, Y.Y.; Ke, G.H.; Zhou, M.; Mu, B.; Xiao, S.Y.; et al. Overexpression of two CDPKs from wild Chinese grapevine enhances powdery mildew resistance in Vitis vinifera and Arabidopsis. New Phytol. 2021, 230, 2029–2046. [Google Scholar] [CrossRef]
- Qu, J.J.; Dry, I.; Liu, L.L.; Guo, Z.X.; Yin, L. Transcriptional profiling reveals multiple defense responses in downy mildew-resistant transgenic grapevine expressing a TIR-NBS-LRR gene located at the MrRUN1/MrRPV1 locus. Hortic. Res. 2021, 8, 161. [Google Scholar] [CrossRef] [PubMed]
- Su, K.; Guo, Y.S.; Zhao, Y.H.; Gao, H.Y.; Liu, Z.D.; Li, K.; Ma, L.; Guo, X.W. Candidate genes for grape white rot resistance based on SMRT and Illumina sequencing. BMC Plant Biol. 2019, 19, 501. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fan, X.C.; Li, Y.F.; Sun, H.S.; Jiang, J.F.; Liu, C.H. Restriction site-associated DNA sequencing reveals the molecular genetic diversity of grapevine and genes related to white rot disease. Sci. Hortic. 2020, 261, 108907. [Google Scholar] [CrossRef]
- Li, P.; Tan, X.B.; Liu, R.T.; Rahman, F.U.; Jiang, J.F.; Sun, L.; Fan, X.C.; Liu, J.H.; Liu, C.H.; Zhang, Y. QTL detection and candidate gene analysis of grape white rot resistance by interspecific grape (Vitis vinifera L.× Vitis davidii Foex.) crossing. Hortic. Res. 2023, 10, 063. [Google Scholar] [CrossRef] [PubMed]
- Vlot, A.C.; Dempsey, D.A.; Klessig, D.F. Salicylic acid, a multifaceted hormone to combat disease. Annu. Rev. Phytopathol. 2009, 47, 177–206. [Google Scholar] [CrossRef] [PubMed]
- Kinkema, M.; Fan, W.H.; Dong, X.N. Nuclear localization of NPR1 is required for activation of PR gene expression. Plant Cell 2000, 12, 2339–2350. [Google Scholar] [CrossRef]
- Mou, Z.L.; Fan, W.H.; Dong, X.N. Inducers of plant systemic acquired resistance regulate NPR1 function through redox changes. Cell 2003, 113, 935–944. [Google Scholar] [CrossRef]
- Gatz, C. From pioneers to team players: TGA transcription factors provide a molecular link between different stress pathways. Mol. Plant-Microbe Interact. 2013, 26, 151–159. [Google Scholar] [CrossRef]
- Després, C.; DeLong, C.; Glaze, S.; Liu, E.W.; Fobert, P.R. The Arabidopsis NPR1/NIM1 protein enhances the DNA binding activity of a subgroup of the TGA family of bZIP transcription factors. Plant Cell 2000, 12, 279–290. [Google Scholar] [CrossRef]
- Budimir, J.; Treffon, K.; Nair, A.; Thurow, C.; Gatz, C. Redox-active cysteines in TGACG-BINDING FACTOR 1 (TGA1) do not play a role in salicylic acid or pathogen-induced expression of TGA1-regulated target genes in Arabidopsis thaliana. New Phytol. 2021, 230, 2420–2432. [Google Scholar] [CrossRef]
- Moon, S.J.; Park, H.J.; Kim, T.H.; Kang, J.W.; Lee, J.Y.; Cho, J.H.; Lee, J.H.; Park, D.S.; Byun, M.O.; Kim, B.G.; et al. OsTGA2 confers disease resistance to rice against leaf blight by regulating expression levels of disease related genes via interaction with NH1. PLoS ONE 2018, 13, e0206910. [Google Scholar] [CrossRef]
- Han, Q.; Tan, W.R.; Zhao, Y.Q.; Yang, F.; Yao, X.H.; Lin, H.H.; Zhang, D.W. Salicylic acid-activated BIN2 phosphorylation of TGA3 promotes Arabidopsis PR gene expression and disease resistance. EMBO J. 2022, 41, e110682. [Google Scholar] [CrossRef] [PubMed]
- Noshi, M.; Mori, D.; Tanabe, N.; Maruta, T.; Shigeoka, S. Arabidopsis clade IV TGA transcription factors, TGA10 and TGA9, are involved in ROS-mediated responses to bacterial PAMP Flg22. Plant Sci. 2016, 252, 12–21. [Google Scholar] [CrossRef]
- Venturuzzi, A.L.; Rodriguez, M.C.; Conti, G.; Leone, M.; Caro, M.D.P.; Montecchia, J.F.; Zavallo, D.; Asurmendi, S. Negative Modulation of SA signaling components by the capsid protein of tobacco mosaic virus is required for viral long-distance movement. Plant J. 2021, 106, 896–912. [Google Scholar] [CrossRef]
- Hu, X.C.; Yang, L.Y.; Ren, M.F.; Liu, L.; Fu, J.; Cui, H.C. TGA factors promote plant root growth by modulating redox homeostasis or response. J. Integr. Plant Biol. 2022, 64, 1543–1559. [Google Scholar] [CrossRef] [PubMed]
- Kurth, E.G.; Peremyslov, V.V.; Prokhnevsky, A.I.; Kasschau, K.D.; Miller, M.; Carrington, J.C.; Valerian, V.D. Virus-derived gene expression and RNA interference vector for grapevine. J. Virol. 2012, 86, 6002–6009. [Google Scholar] [CrossRef]
- Li, T.G.; Wang, B.L.; Yin, C.M.; Zhang, D.D.; Wang, D.; Song, J.; Zhou, L.; Kong, Z.Q.; Klosterman, S.J.; Li, J.J.; et al. The Gossypium hirsutum TIR-NBS-LRR gene GhDSC1 mediates resistance against Verticillium wilt. Mol. Plant Pathol. 2019, 20, 857–876. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Q.; Jiao, Y.T.; Zhang, C.; Dou, M.R.; Weng, K.; Wang, Y.J.; Xu, Y. VvHDZ28 positively regulate salicylic acid biosynthesis during seed abortion in Thompson seedless. Plant Biotechnol. J. 2021, 19, 1824–1838. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.H.; Jiao, Z.L.; Bian, L.; Wan, Y.T.; Yu, K.K.; Zhang, G.H.; Guo, D.L. Overexpression of Vitis vinifera VvbZIP60 enhances Arabidopsis resistance to powdery mildew via the salicylic acid signaling pathway. Sci. Hortic. 2019, 256, 108640. [Google Scholar] [CrossRef]
- Hu, Y.; Gao, Y.R.; Yang, L.S.; Wang, W.; Wang, Y.J.; Wen, Y.Q. The cytological basis of powdery mildew resistance in wild Chinese Vitis species. Plant Physiol. Biochem. 2019, 144, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fan, X.C.; Sun, H.S.; Jiang, J.F.; Liu, C.H. Identification and evaluation of resistance to white rot in grape resources. J. Fruit Sci. 2017, 34, 1095–1105. [Google Scholar] [CrossRef]
- Bucalossi, G.; Fia, G.; Dinnella, C.; Toffoli, A.D.; Canutia, V.; Zanoni, B.; Servili, M.; Ella Pagliarini, E.; Toschi, T.G.; Monteleone, E. Functional and sensory properties of phenolic compounds from unripe grapes in vegetable food prototypes. Food Chem. 2020, 315, 126291. [Google Scholar] [CrossRef] [PubMed]
- Li, T.G.; Zhang, D.D.; Zhou, L.; Kong, Z.Q.; Hussaini, A.S.; Wang, D.; Li, J.J.; Short, D.P.G.; Dhar, N.; Steven, J.K.; et al. Genome-wide identification and functional analyses of the CRK gene family in cotton reveals GbCRK18 confers Verticillium wilt resistance in Gossypium barbadense. Front. Plant Sci. 2018, 9, 1266. [Google Scholar] [CrossRef]
- Li, C.H.; Wang, K.T.; Huang, Y.X.; Lei, C.Y.; Cao, S.F.; Qiu, L.L.; Xu, F.; Jiang, Y.B.; Zou, Y.Y.; Zheng, Y.H. Activation of the BABA-induced priming defence through redox homeostasis and the modules of TGA1 and MAPKK5 in postharvest peach fruit. Mol. Plant Pathol. 2021, 22, 1624–1640. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Dong, T.; Chen, W.L.; Zou, N.X.; Chen, Y.L.; Li, Y.Q.; Chen, K.L.; Wang, M.Y.; Liu, J.F. Expression analysis of MaTGA8 transcription factor in banana and its defence functional analysis by overexpression in Arabidopsis. Int. J. Mol. Sci. 2021, 22, 9344. [Google Scholar] [CrossRef]
- Xv, X.D.; Chen, Y.; Li, B.Q.; Zhang, Z.Q.; Qin, G.Z.; Chen, T.; Tian, S.P. Molecular mechanisms underlying multi-level defense responses of horticultural crops to fungal pathogens. Hortic. Res. 2022, 9, uhac066. [Google Scholar] [CrossRef]
- Henanff, G.L.; Farine, S.; Kieffer-Mazet, F.; Miclot, A.; Heitz, T.; Mestre, P.; Bertsch, C.; Chong, J. Vitis vinifera VvNPR1.1 is the functional ortholog of AtNPR1 and its overexpression in grapevine triggers constitutive activation of PR genes and enhanced resistance to powdery mildew. Planta 2011, 234, 405–417. [Google Scholar] [CrossRef]
- Fu, Z.Q.; Yan, S.P.; Saleh, A.; Wang, W.; Ruble, J.; Oka, N.; Mohan, R.; Spoel, S.H.; Tada, Y.; Zheng, N.; et al. NPR3 and NPR4 are receptors for the immune signal salicylic acid in plants. Nature 2012, 486, 228–232. [Google Scholar] [CrossRef]
- Johnson, C.; Boden, E.; Arias, J. Salicylic acid and NPR1 induce the recruitment of trans-activating TGA factors to a defense gene promoter in Arabidopsis. Plant Cell 2003, 15, 1846–1858. [Google Scholar] [CrossRef] [PubMed]
- Ekengren, S.K.; Liu, Y.; Schiff, M.; Dinesh-Kumar, S.P.; Martin, G.B. Two MAPK cascades, NPR1, and TGA transcription factors play a role in Pto-mediated disease resistance in tomato. Plant J. 2003, 36, 905–917. [Google Scholar] [CrossRef] [PubMed]
- Luján-Soto, E.; Aguirre de la Cruz, P.I.; Juárez-González, V.T.; Reyes, J.L.; Sanchez, M.d.l.P.; Dinkova, T.D. Transcriptional regulation of zma-MIR528a by action of nitrate and auxin in maize. Int. J. Mol. Sci. 2022, 23, 15718. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.T.; Li, C.H.; Lei, C.Y.; Jiang, Y.B.; Qiu, L.L.; Zou, X.Y.; Zheng, Y.H. β-aminobutyric acid induces priming defence against Botrytis cinerea in grapefruit by reducing intercellular redox status that modifies posttranslation of VvNPR1 and its interaction with VvTGA1. Plant Physiol. Biochem. 2020, 156, 552–565. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Yuan, L.; Yin, X.; Jiang, X.; Wei, Y.; Tang, X.; Li, N.; Liu, Q. The Grapevine Transcription Factor VvTGA8 Enhances Resistance to White Rot via the Salicylic Acid Signaling Pathway in Tomato. Agronomy 2023, 13, 3054. https://doi.org/10.3390/agronomy13123054
Li T, Yuan L, Yin X, Jiang X, Wei Y, Tang X, Li N, Liu Q. The Grapevine Transcription Factor VvTGA8 Enhances Resistance to White Rot via the Salicylic Acid Signaling Pathway in Tomato. Agronomy. 2023; 13(12):3054. https://doi.org/10.3390/agronomy13123054
Chicago/Turabian StyleLi, Tinggang, Lifang Yuan, Xiangtian Yin, Xilong Jiang, Yanfeng Wei, Xiaoning Tang, Nanyang Li, and Qibao Liu. 2023. "The Grapevine Transcription Factor VvTGA8 Enhances Resistance to White Rot via the Salicylic Acid Signaling Pathway in Tomato" Agronomy 13, no. 12: 3054. https://doi.org/10.3390/agronomy13123054




