Efficacy of Four Insecticides Applied to Fortified Rice with Basil against Major Stored-Product Insect Species
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tested Insects
2.2. Commodity and Insecticide Treatments
2.3. Insecticidal Efficacy
2.4. Statistical Analysis
3. Results
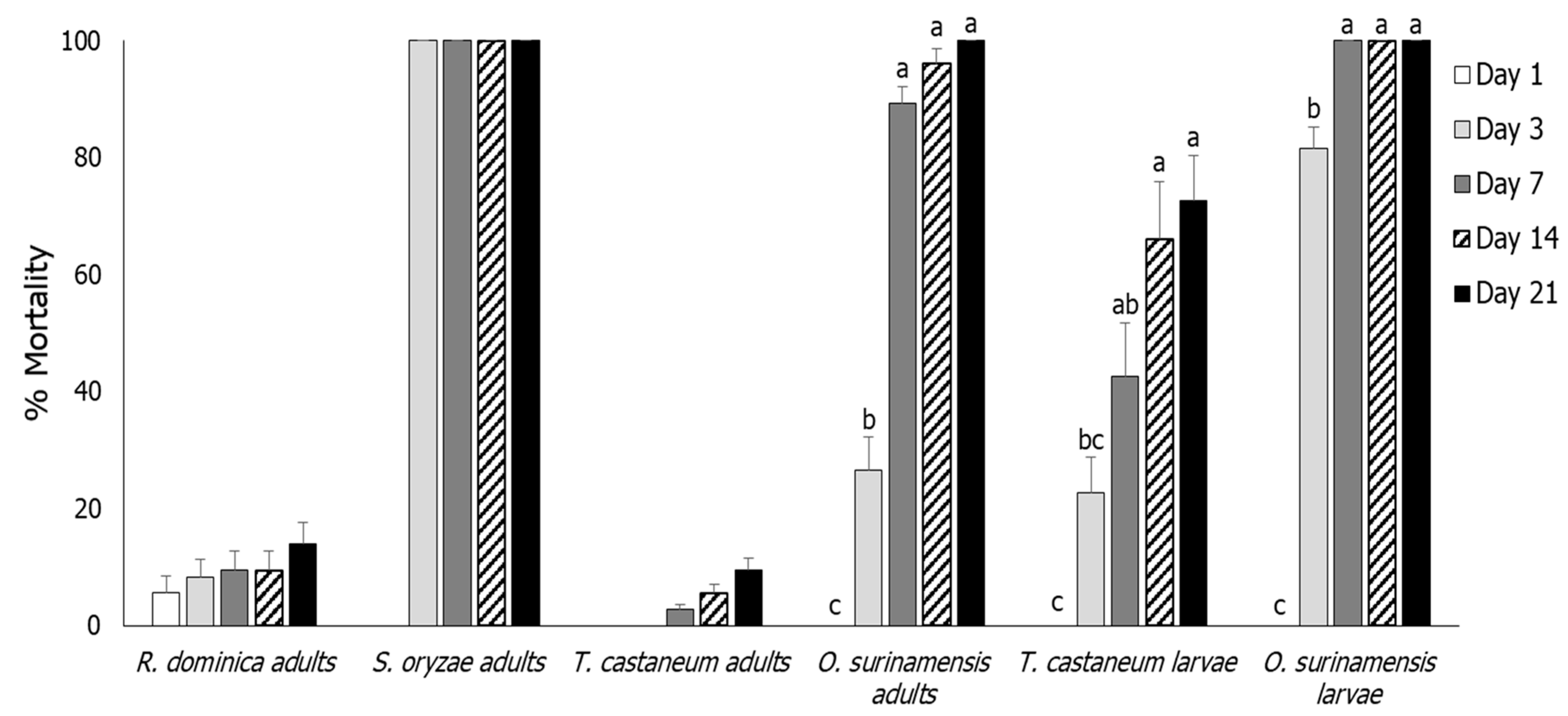
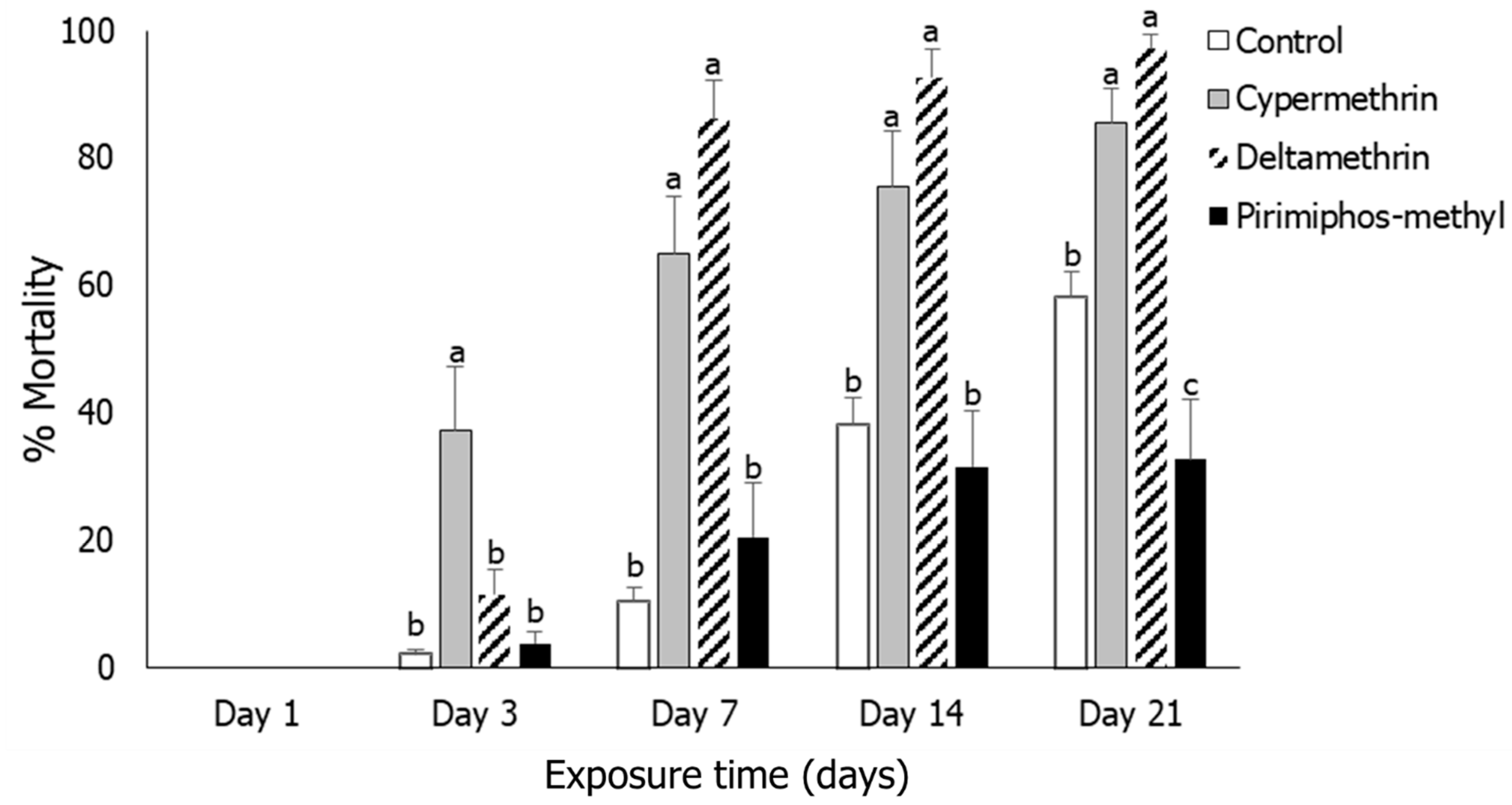
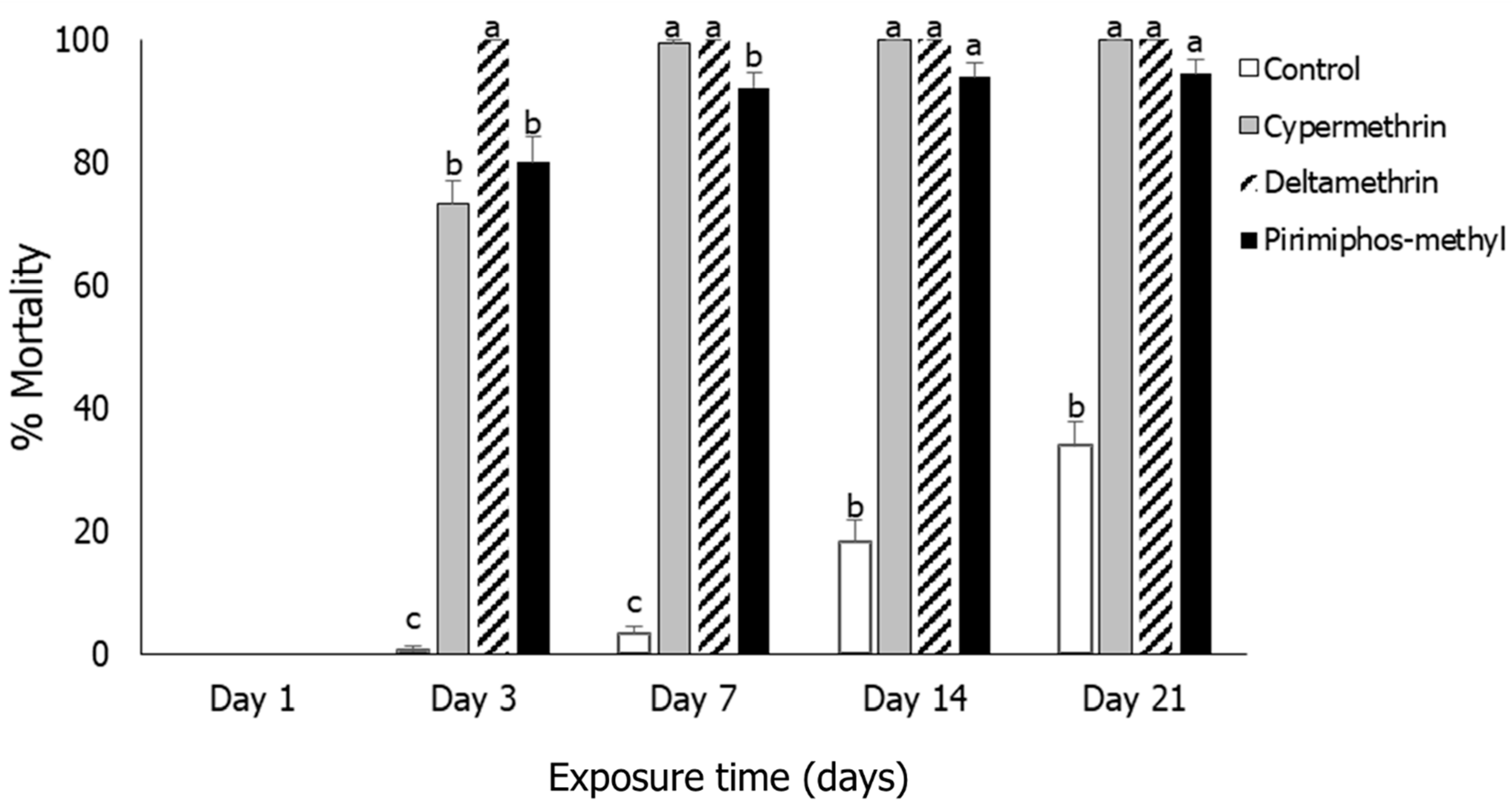
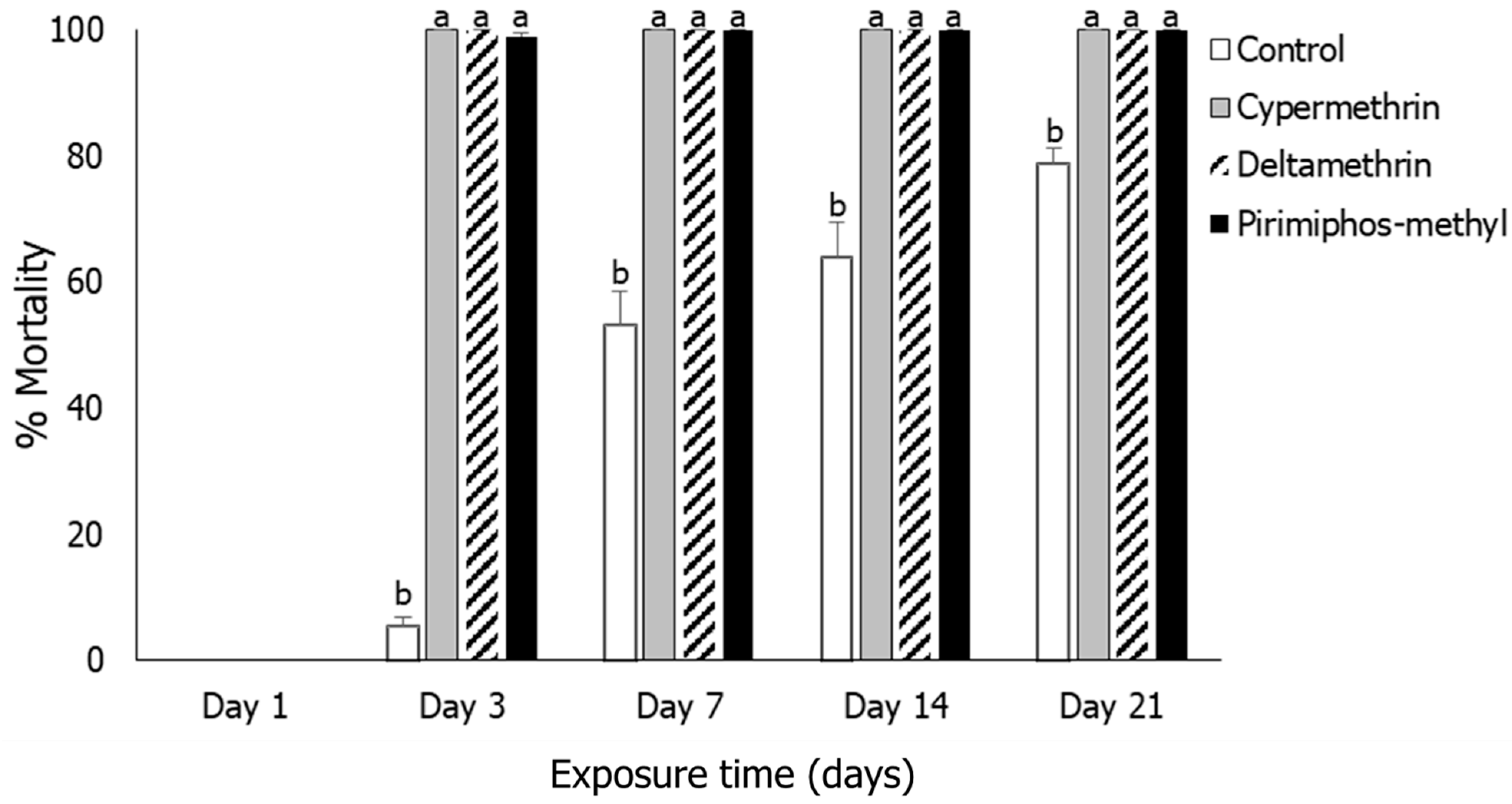
3.1. Insect Mortality
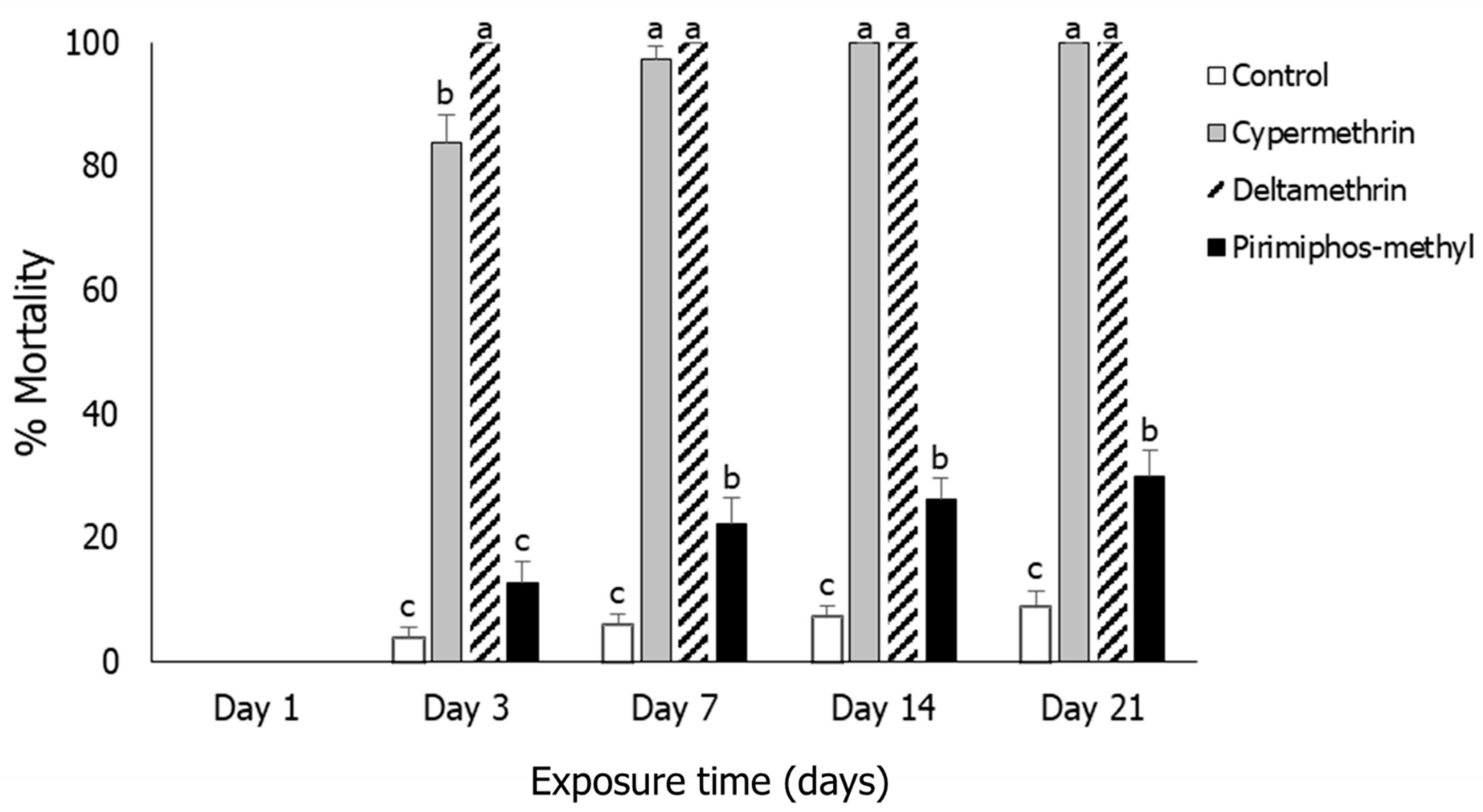
3.2. Rhyzopertha dominica
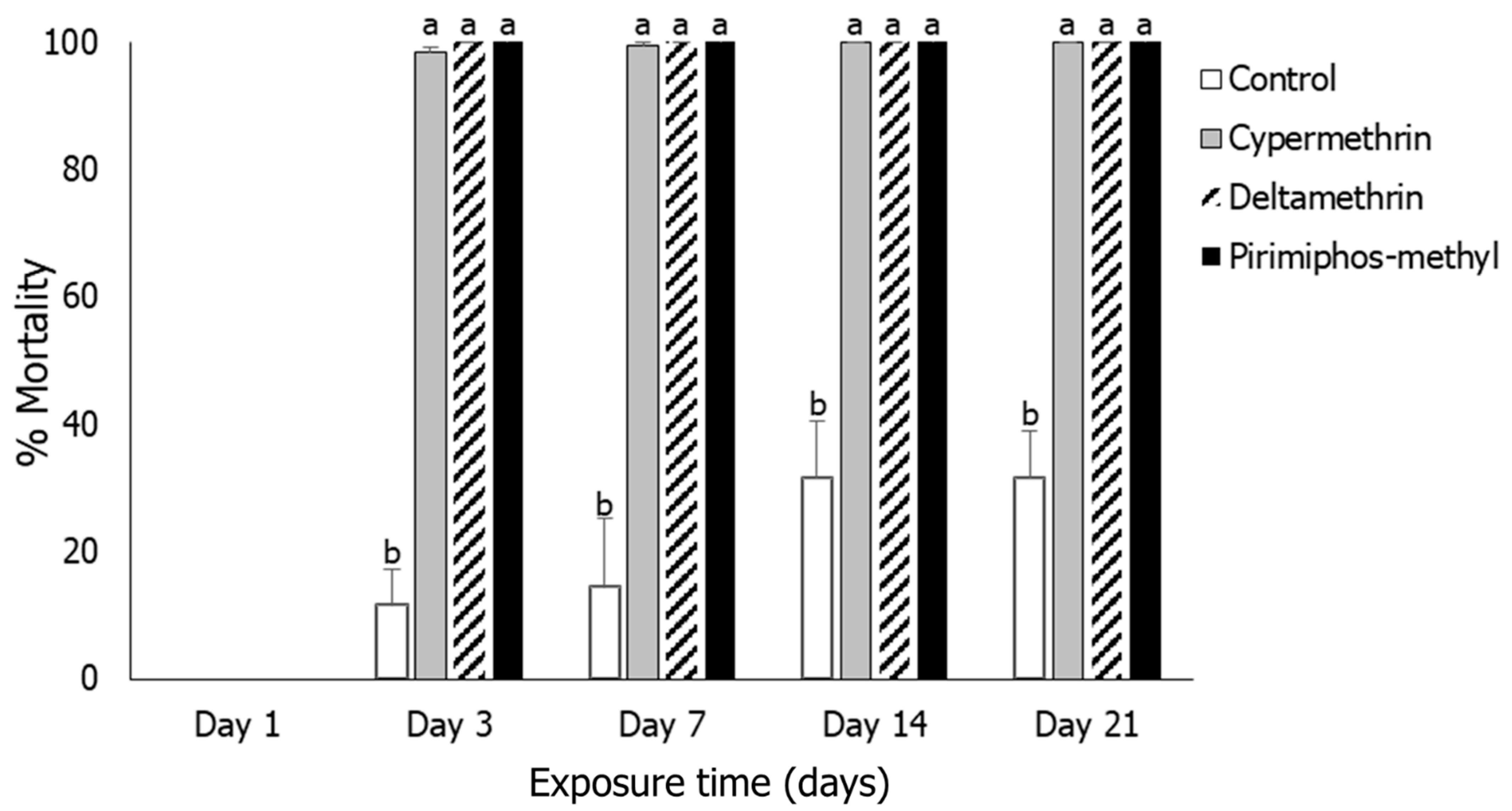
3.3. Sitophilus oryzae
3.4. Tribolium castaneum
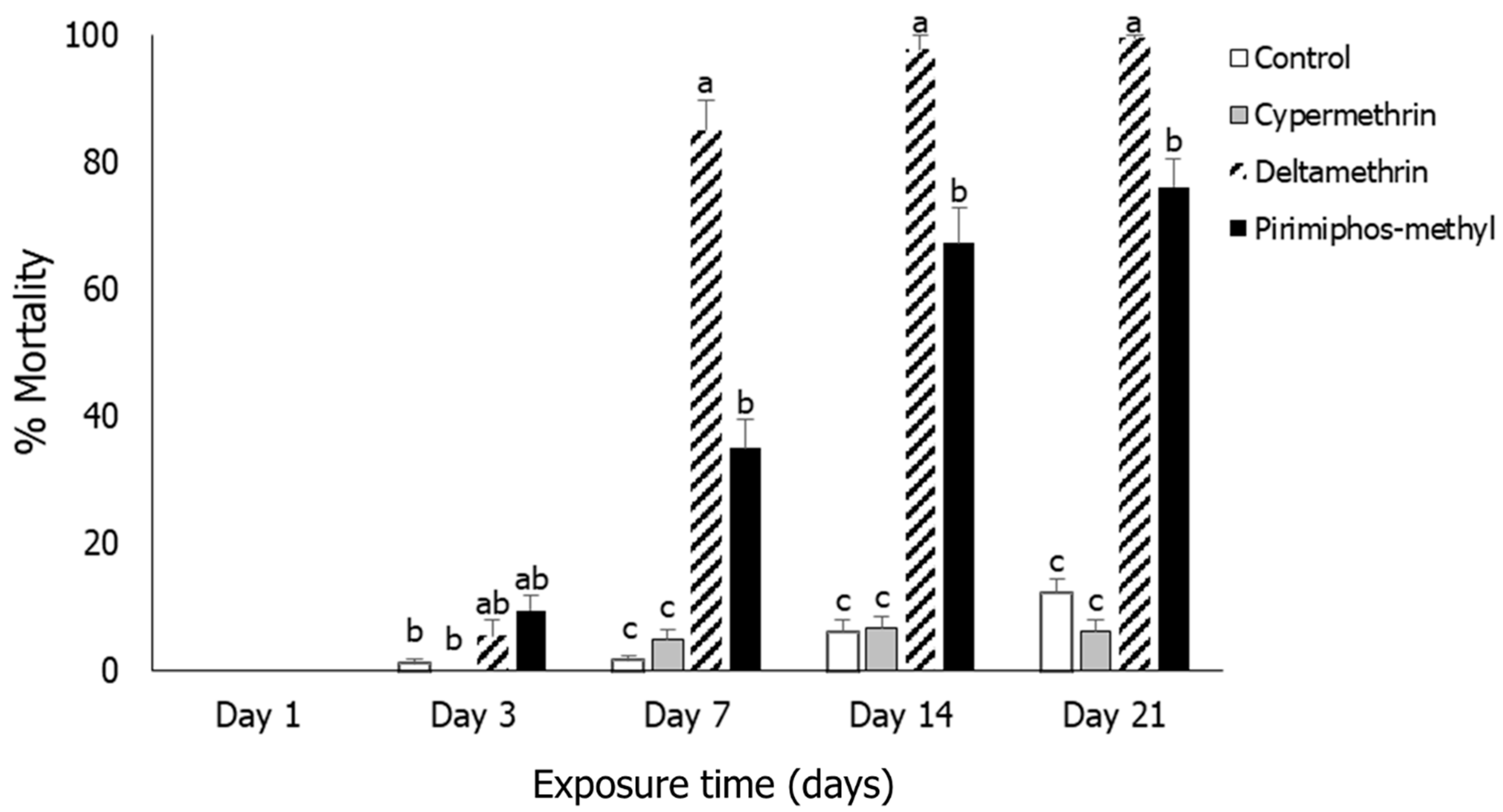
3.5. Oryzaephilus surinamensis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Amini, S.; Rohani, A.; Aghkhani, M.H.; Abbaspour-Fard, M.H.; Asgharipour, M.R. Assessment of land suitability and agricultural production sustainability using a combined approach (Fuzzy-AHP-GIS): A case study of Mazandaran Province. Iran Inf. Process Agric. 2020, 7, 384–402. [Google Scholar] [CrossRef]
- Muthayya, S.; Sugimoto, J.D.; Montgomery, S.; Maberly, G.F. An overview of global rice production, supply, trade, and consumption. Ann. N. Y. Acad. Sci. 2014, 1324, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Juliano, B.O. Rice in Human Nutrition; FAO Food and Nutrition Series, No. 26; International Rice Research Institute: Manila, Philippines, 1993. [Google Scholar]
- FAO; IFAD; UNICEF; WFP; WHO. Repurposing food and agricultural policies to make healthy diets more affordable. In Brief to the State of Food Security and Nutrition in the World; FAO: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- Muthayya, S.; Hall, J.; Bagriansky, J.; Sugimoto, J.; Gundry, D.; Matthias, D.; Prigge, S.; Hindle, P.; Moench-Pfanner, R.; Maberly, G. Rice Fortification—An emerging opportunity to contribute to the elimination of vitamin and mineral deficiency worldwide. Food Nutr. Bull. 2012, 33, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Nayak, M.K.; Daglish, G.J. Importance of Stored Product Insects. In Recent Advances in Stored Product Protection; Athanassiou, C., Arthur, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1–17. [Google Scholar]
- Benhalima, H.; Chaudhry, M.Q.; Mills, K.A.; Price, N.R. Phosphine resistance in stored-product insects collected from various grain storage facilities in Morocco. J. Stored Prod. Res. 2004, 40, 241–249. [Google Scholar] [CrossRef]
- Daglish, G.J. Effect of exposure period on degree of dominance of phosphine resistance in adults of Rhyzopertha dominica (Coleoptera: Bostrychidae) and Sitophilus oryzae (Coleoptera: Curculionidae). Pest Manag. Sci. 2004, 60, 822–826. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Collins, P.J.; Gao, X. Optimizing indoor phosphine fumigation of paddy rice bag-stacks under sheeting for control of resistant insects. J. Stored Prod. Res. 2006, 42, 207–217. [Google Scholar] [CrossRef]
- Nayak, M.K.; Collins, P.J.; Holloway, J.K.; Emery, R.N.; Pavic, H.; Bartlet, J. Strong resistance to phosphine in the rusty grain beetle, Cryptolestes ferrugineus (Stephens) (Coleoptera: Laemophloeidae): Its characterization, a rapid assay for diagnosis and its distribution in Australia. Pest Manag. Sci. 2013, 69, 48–53. [Google Scholar] [CrossRef]
- Song, X.; Wang, P.; Zhang, H. Phosphine resistance in Rhyzopertha dominica (Fabricius) (Coleoptera: Bostrichidae) from different geographical populations in China. Afr. J. Biotechnol. 2011, 10, 16367–16373. [Google Scholar]
- Cato, A.J.; Elliot, B.; Nayak, M.K.; Phillips, T.W. Geographic variation in phosphine resistance among North American populations of the red flour beetle (Coleoptera: Tenebrionidae). J. Econ. Entomol. 2017, 110, 1359–1365. [Google Scholar] [CrossRef]
- Afful, E.; Elliot, B.; Nayak, M.K.; Phillips, T.W. Phosphine resistance in North American of the lesser grain borer, Rhyzopertha dominica (F.) (Coleoptera: Bostrychidae). J. Econ. Entomol. 2018, 111, 463–469. [Google Scholar] [CrossRef]
- Campbell, J.F.; Arthur, F.H.; Mullen, M.A. Insect management in food processing facilities. Adv. Food Nutr. Res. 2004, 48, 267–272. [Google Scholar]
- Kavallieratos, N.G.; Athanassiou, C.G.; Arthur, F.H. Efficacy of detlamethrin against stored-product beetles at short exposure intervals or on a partially treated rice mass. J. Econ. Entomol. 2015, 108, 1416–1421. [Google Scholar] [CrossRef] [PubMed]
- Baliota, G.V.; Lampiri, E.; Batzogianni, E.N.; Athanassiou, C.G. Insecticidal effect of four insecticides for the control of different populations of three stored-product beetles species. Insects 2022, 13, 325. [Google Scholar] [CrossRef] [PubMed]
- Limoee, M.; Davari, B.; Moosa-Kazemi, S.H. Toxicity of pyrethroid and organophosphorous insecticides against two field collected strains of the german cockroach Blattella germanica (Blattaria: Blattellidae). J. Arthr. Dis. 2012, 6, 112–118. [Google Scholar]
- Dusfour, I.; Zorrilla, P.; Guidez, A.; Issaly, J.; Girod, R.; Guillaumot, L.; Robello, C.; Strode, C. Deltamethrin resistance mechanisms in Aedes aegypti populations from three French overseas territories worldwide. PLoS Negl. Trop. Dis. 2015, 9, e0004226. [Google Scholar] [CrossRef] [PubMed]
- Gunning, C.E.; Okamoto, K.W.; Astete, H.; Vasquez, G.M.; Erhardt, E.; del Aguila, C.; Pinedo, R.; Cardenas, R.; Pacheco, C.; Chalco, E.; et al. Efficacy of Aedes aegypti control by indoor Ultra Low Volume (ULV) insecticide spraying in Iquitos, Peru. PLoS Negl. Trop. Dis. 2018, 12, 0006378. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, K.; Matsuo, N.; Kaneko, H.; Khambay, B.P.S. Chirality in Agrochemicals. In Pyrethroids; Kurihara, N., Miyamoto, J., Eds.; John Wiley & Sons: Chichester, UK, 1998; pp. 8–84. [Google Scholar]
- Liu, W.; Gan, J.J.; Lee, S.; Werner, I. Isomer selectivity in aquatic toxicity and biodegradation of cypermethrin. J. Agric. Food Chem. 2004, 52, 6233–6238. [Google Scholar] [CrossRef]
- Zahraei-Ramazani, A.R.; Saghafipour, A.; Vatandoost, H. Control of American cockroach (Periplaneta americana) in municipal sewage disposal system, Central Iran. J. Arthropod-Borne Dis. 2018, 12, 172–179. [Google Scholar] [CrossRef]
- Latif, M.A.; Rahman, M.M.; Alam, M.Z. Efficacy of nine insecticides against shoot and fruit borer, Leucinodes orbonalis Guenee (Lepidoptera: Pyralidae) in eggplant. J. Pest Sci. 2010, 83, 391–397. [Google Scholar] [CrossRef]
- Joseph, S.V.; Martin, T.; Steinmann, K.; Kosina, P. Outlook of pyrethroid insecticides for pest management in the Salinas Valley of California. J. Integr. Pest Manag. 2017, 8, 6. [Google Scholar] [CrossRef]
- Kljajic, P.; Peric, I. Susceptibility to contact insecticides of granary weevil Sitophilus granarius (L.) (Coleoptera: Curculionidae) originating from different locations in the former Yugoslavia. J. Stored Prod. Res. 2005, 42, 149–161. [Google Scholar] [CrossRef]
- Chen, C.Y.; Chen, M.E. Susceptibility of field populations of the lesser grain borer, Rhyzopertha dominica (F.), to detlamethrin and spinosad on paddy rice in Taiwan. J. Stored Prod. Res. 2013, 55, 124–127. [Google Scholar] [CrossRef]
- Arthur, F.H. Residual efficacy of a deltamethrin emulsifiable concentrate formulation against Rhyzopertha dominica (F.) and Sitotroga cerealella (Oliver) after partial treatment of brown rice. Insects 2019, 10, 95. [Google Scholar] [CrossRef]
- Kljajic, P.; Peric, I. Effectiveness of wheat-applied contact insecticides against Sitophilus granarius (L.) originating from different populations. J. Stored Prod. Res. 2007, 43, 523–529. [Google Scholar] [CrossRef]
- Rumbos, C.I.; Dutton, A.C.; Athanassiou, C.G. Comparison of two pirimiphos-methyl formulations against major stored-product insect species. J. Stored Prod. Res. 2013, 55, 106–115. [Google Scholar] [CrossRef]
- Golob, P. Current status and future perspectives for inert dusts for control of stored product insects. J. Stored Prod. Res. 1997, 33, 69–80. [Google Scholar] [CrossRef]
- Arthur, F.H. Toxicity of diatomaceous earth to red flour beetles and confused flour beetles (Coleoptera: Tenebrionidae): Effects of temperature and relative humidity. J. Econ. Entomol. 2000, 93, 526–532. [Google Scholar] [CrossRef]
- Zeni, V.; Baliota, G.V.; Benelli, G.; Canale, A.; Athanassiou, C.G. Diatomaceous earth for arthropod pest control: Back to the future. Molecules 2021, 26, 7487. [Google Scholar] [CrossRef]
- Vayias, B.J.; Athanassiou, C.G. Factors affecting the insecticidal efficacy of the diatomaceous earth formulation SilicoSec against adults and larvae of the confused flour beetle, Tribolium confusum DuVal (Coleptera: Tenebrionidae). Crop Prot. 2004, 23, 565–573. [Google Scholar] [CrossRef]
- Kyritsi, A.; Tzia, C.; Karathanos, V.T. Vitamin fortified rice grain using spraying and soaking methods. Food Sci. Technol. 2011, 44, 312–320. [Google Scholar] [CrossRef]
- Wei, Y.; Shohag, M.J.I.; Ying, F.; Yang, X.; Wu, C.; Wang, Y. Effect of ferrous sulfate fortification in germinated brown rice on seed iron concentration and bioavailability. Food Chem. 2013, 138, 1952–1958. [Google Scholar] [CrossRef] [PubMed]
- Igoumenidis, P.E.; Lekka, E.G.; Karathanos, V.T. Fortification of white milled rice with phytochemicals during cooking in aqueous extract of Mentha spicata leaves. An adsorption study. LWT Food Sci. Technol. 2016, 65, 589–596. [Google Scholar] [CrossRef]
- Igoumenidis, P.E.; Karathanos, V.T. Diffusion and thermal stability of phenolic compounds during fortified rice rehydration process. J. Food Eng. 2016, 174, 1–7. [Google Scholar] [CrossRef]
- Agrafioti, P.; Lampiri, E.; Igoumenidis, P.I.; Karathanos, V.T.; Perdikaris, A.; Athanassiou, C.G. Population growth changes in major stored product insects on rice fortified with spearmint and basil. Agronomy 2022, 12, 2088. [Google Scholar] [CrossRef]
- Zar, H.J. Biostatistical Analysis; Prentice-Hall Inc.: Upper Saddle River, NJ, USA, 1999. [Google Scholar]
- Athanassiou, C.G.; Kavallieratos, N.G.; Tsaganou, F.C.; Vayias, B.J.; Dimizas, C.B.; Buchelos, C.T. Effect of grain type on the insecticidal efficacy of SilicoSec against Sitophilus oryzae (L.) (Coleoptera: Curculionidae). Crop Prot. 2003, 22, 1141–1147. [Google Scholar] [CrossRef]
- Korunic, Z. Review Diatomaceous earths, a group of natural insecticides. J. Stored Prod. Res. 1998, 34, 87–97. [Google Scholar] [CrossRef]
- Subramanyam, B.; Roesli, R. Inert dusts. In Alternatives to Pesticides in Stored-Product IPM; Subramanyam, B., Hagstrum, D.W., Eds.; Kluwer Academic Publishers: Dordreecht, The Netherlands, 2000; pp. 321–380. [Google Scholar]
- Aldryhim, Y.N. Combination of classes of wheat and environmental factors affecting the efficacy of amorphous silica dust, dryacide, against Rhyzopertha dominica (F.). J. Stored Prod. Res. 1993, 29, 271–275. [Google Scholar] [CrossRef]
- Fields, P.; Korunic, Z. The effect of grain moisture content and temperature on the efficacy of diatomaceous earths from different geographical locations against stored-product beetles. J. Stored Prod. Res. 2000, 36, 1–13. [Google Scholar] [CrossRef]
- Guedes, R.N.C.; Dover, B.A.; Kambhampati, S. Resistance to chlorpyrifos-methyl, pirimiphos-methyl, and malathion in Brazilian and U.S. populations of Rhyzopertha dominica (Coleoptera: Bostrichidae). J. Econ. Entomol. 1996, 89, 27–32. [Google Scholar] [CrossRef]
- Rumbos, C.I.; Dutton, A.C.; Tsiropoulos, N.G.; Athanassiou, C.G. Persistence and residual toxicity of two pirimiphos-methyl formulations on wheat against three stored-product pests. J. Stored Prod. Res. 2018, 76, 14–21. [Google Scholar] [CrossRef]
- Daglish, G.J.; Nayak, M.K. Prevalence of resistance to deltamethrin in Rhyzopertha dominica (F.) in eastern Australia. J. Stored Prod. Res. 2018, 78, 45–49. [Google Scholar] [CrossRef]
- Ortega, D.S.; Bacca, T.; Sliva, A.O.N.; Canal, N.A.; Haddi, K. Control failure and insecticides resistance in populations of Rhyzopertha dominica (Coleptera: Bostrichidae) from Colombia. J. Stored Prod. Res. 2023, 92, 101802. [Google Scholar] [CrossRef]
- Arthur, F.H. Differential effectiveness of deltamethrin dust on plywood, concrete, and tile surfaces against three stored-product beetles. J. Stored Prod. Res. 1997, 33, 167–173. [Google Scholar] [CrossRef]
- Trostanetsky, A.; Quinn, E.; Rapaport, A.; Harush, A.; Gottlieb, D. Efficacy of deltamethrin emulsifiable concentrate against stored-product insects. J. Stored Prod. Res. 2023, 101, 102072. [Google Scholar] [CrossRef]
| Adults | Larvae | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Source | Rhyzopertha dominica | Sitophilus oryzae | Tribolium castaneum | Oryzaephilus surinamensis | Tribolium castaneum | Oryzaephilus surinamensis | |||||||
| df | F | p | F | p | F | p | F | p | F | p | F | p | |
| Intercept | 1 | 8.49 | 0.019 | 0 | 1.0 | 19.69 | 0.002 | 947.76 | <0.001 | 42.91 | <0.001 | 10,488.2 | <0.001 |
| Time | 4 | 3.07 | 0.124 | 0 | 1.0 | 3.11 | 0.122 | 410.25 | <0.001 | 45.97 | <0.001 | 75.03 | <0.001 |
| Adults | Larvae | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Source | Rhyzopertha dominica | Sitophilus oryzae | Tribolium castaneum | Oryzaephilus surinamensis | Tribolium castaneum | Oryzaephilus surinamensis | |||||||
| df | F | p | F | p | F | p | F | p | F | p | F | p | |
| Intercept | 1 | 2484.79 | <0.001 | 52,153.60 | <0.001 | 913.66 | <0.001 | 8102.71 | <0.001 | 287.59 | <0.001 | 11,634.67 | <0.001 |
| Treatment a | 3 | 461.30 | <0.001 | 3578.14 | <0.001 | 242.46 | <0.001 | 598.79 | <0.001 | 21.58 | <0.001 | 232.45 | <0.001 |
| Time | 4 | 747.87 | <0.001 | 24,063.47 | <0.001 | 316.07 | <0.001 | 3143.82 | <0.001 | 135.21 | <0.001 | 28,732.96 | <0.001 |
| Time × Treatment | 12 | 31.73 * | <0.001 | 84.50 * | <0.001 | 32.38 * | <0.001 | 54.41 * | <0.001 | 11.33 * | <0.001 | 64.12 * | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agrafioti, P.; Vrontaki, M.; Lampiri, E.; Rumbos, C.I.; Athanassiou, C.G. Efficacy of Four Insecticides Applied to Fortified Rice with Basil against Major Stored-Product Insect Species. Agronomy 2023, 13, 3055. https://doi.org/10.3390/agronomy13123055
Agrafioti P, Vrontaki M, Lampiri E, Rumbos CI, Athanassiou CG. Efficacy of Four Insecticides Applied to Fortified Rice with Basil against Major Stored-Product Insect Species. Agronomy. 2023; 13(12):3055. https://doi.org/10.3390/agronomy13123055
Chicago/Turabian StyleAgrafioti, Paraskevi, Mariastela Vrontaki, Evagelia Lampiri, Christos I. Rumbos, and Christos G. Athanassiou. 2023. "Efficacy of Four Insecticides Applied to Fortified Rice with Basil against Major Stored-Product Insect Species" Agronomy 13, no. 12: 3055. https://doi.org/10.3390/agronomy13123055
APA StyleAgrafioti, P., Vrontaki, M., Lampiri, E., Rumbos, C. I., & Athanassiou, C. G. (2023). Efficacy of Four Insecticides Applied to Fortified Rice with Basil against Major Stored-Product Insect Species. Agronomy, 13(12), 3055. https://doi.org/10.3390/agronomy13123055









