Root Exudates of Fifteen Common Weed Species: Phytochemical Screening and Allelopathic Effects on T. aestivum L.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection and Preparation of Root Exudate of Weeds
2.2. Seed Germination Assay
2.3. Phytochemical Screening of Root Exudate
2.4. Estimation of Chlorophyll Content
2.5. Determination of CAT
2.6. Determination of SOD
2.7. Determination of LPO
2.8. Statistical Analysis
3. Results
3.1. Germination Percentage
3.2. Mean Germination Time (MGT)
3.3. Shoot Length
3.4. Root Length
3.5. Seedling Dry Weight
3.6. Qualitative Phytochemicals Assay
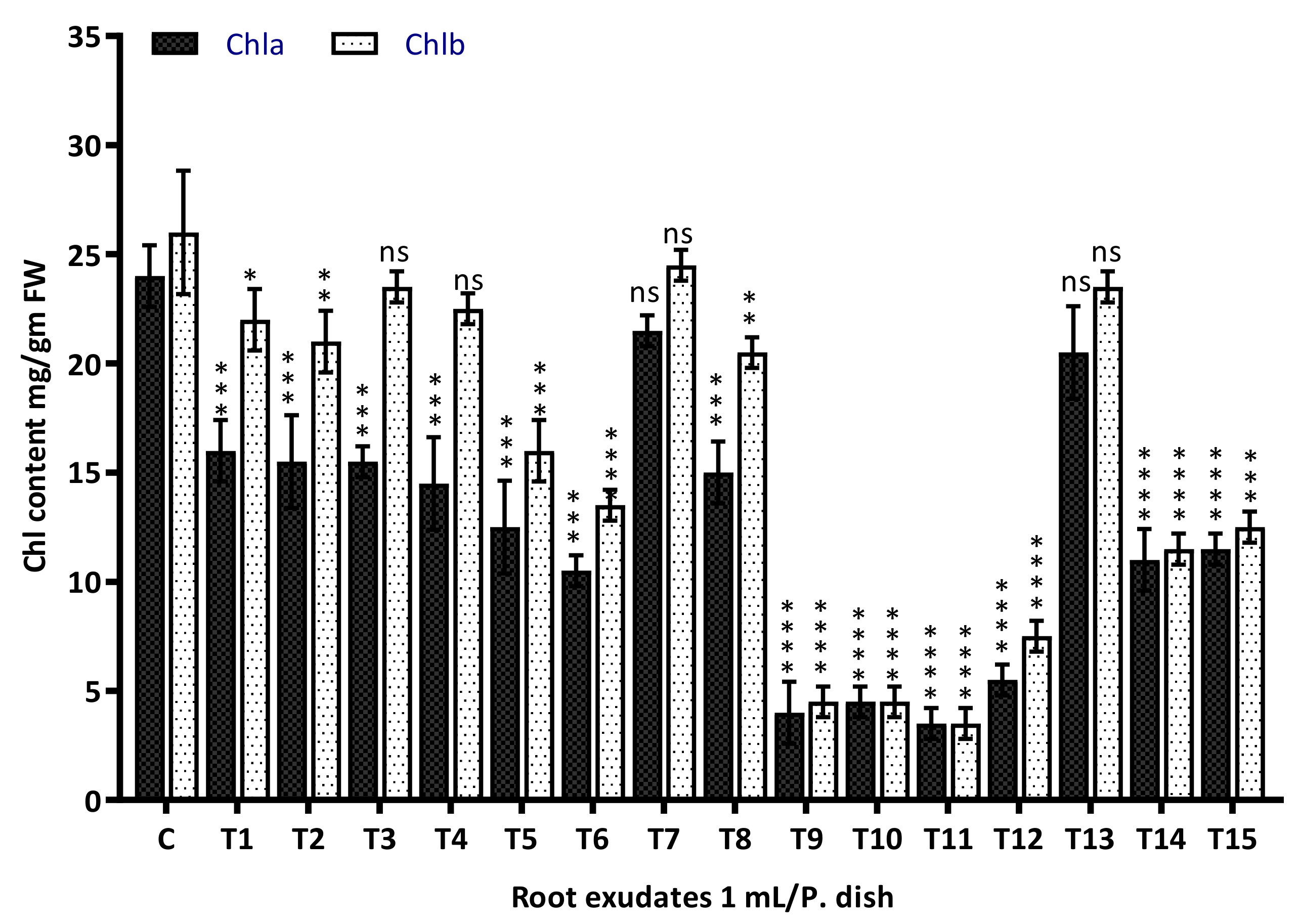
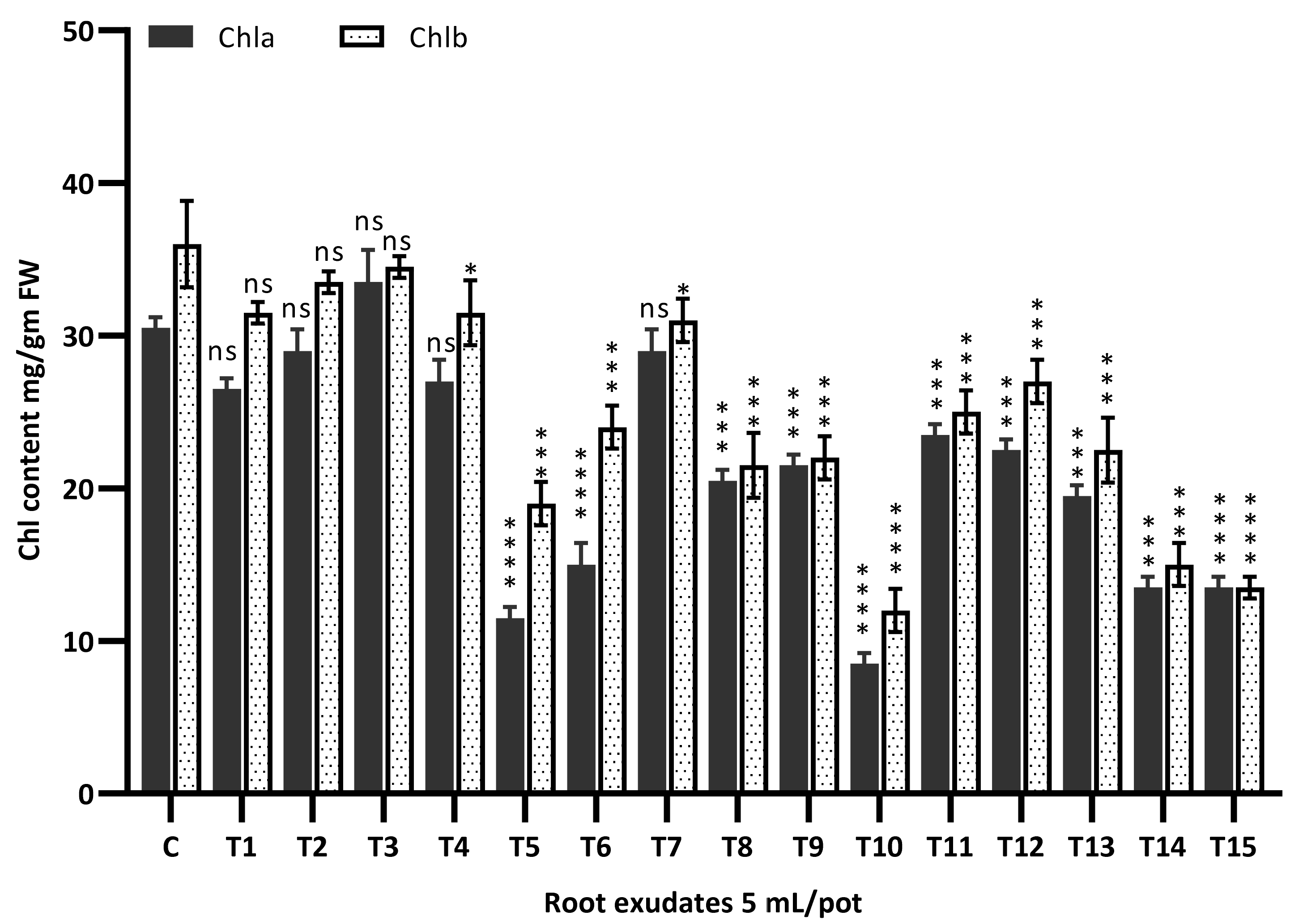
3.7. Chlorophyll Content Assay
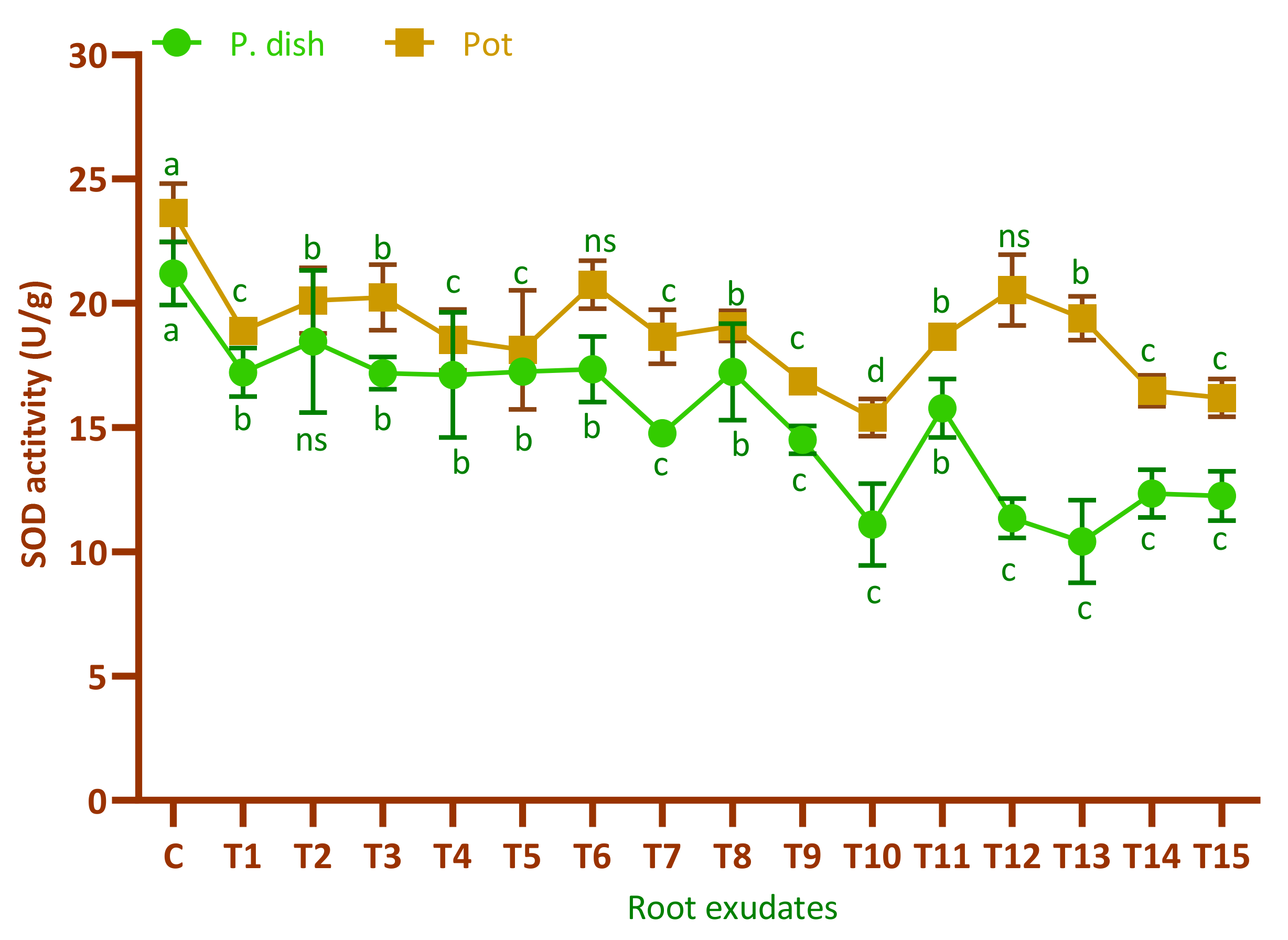
3.8. Antioxidant Enzymes Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scavo, A.; Mauromicale, G. Crop allelopathy for sustainable weed management in agroecosystems: Knowing the present with a view to the future. Agronomy 2021, 11, 2104. [Google Scholar] [CrossRef]
- Farooq, M.; Jabran, K.; Cheema, Z.A.; Wahid, A.; Siddique, K.H.M. Allelopathy for weed management. In Co-Evolution of Secondary Metabolites; Springer: Berlin/Heidelberg, Germany, 2020; pp. 505–519. [Google Scholar]
- Kato-Noguchi, H.; Kurniadie, D. Allelopathy and allelochemicals of Leucaena leucocephala as an invasive plant species. Plants 2022, 11, 1672. [Google Scholar] [CrossRef] [PubMed]
- Tesio, F.; Ferrero, A. Allelopathy, a chance for sustainable weed management. Int. J. Sustain. Dev. World Ecol. 2010, 17, 377–389. [Google Scholar] [CrossRef]
- Sangi, D.P.; Meira, Y.G.; Moreira, N.M.; Lopes, T.A.; Leite, M.P.; Pereira-Flores, M.E.; Alvarenga, E.S. Benzoxazoles as novel herbicidal agents. Pest Manag. Sci. 2019, 75, 262–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jabran, K.; Farooq, M. Implications of potential allelopathic crops in agricultural systems. In Allelopathy; Springer: Berlin/Heidelberg, Germany, 2013; pp. 349–385. [Google Scholar]
- Masum, S.M.; Hossain, M.A.; Akamine, H.; Sakagami, J.I.; Bhowmik, P.C. Allelopathic potential of indigenous Bangladeshi rice varieties. Weed Biol. Manag. 2016, 16, 119–131. [Google Scholar] [CrossRef]
- Sangeetha, C.; Baskar, P. Allelopathy in weed management: A critical review. Afr. J. Agric. Res. 2015, 10, 1004–1015. [Google Scholar]
- Masum, S.M.; Hossain, M.A.; Akamine, H.; Sakagami, J.I.; Bhowmik, P.C. Allelopathic effects of sesame extracts on seed germination of moso bamboo and identification of potential allelochemicals. Sci. Rep. 2022, 12, 6661. [Google Scholar]
- Kato-Noguchi, H. Allelopathy and Allelochemicals of Imperata cylindrica as an invasive plant species. Plants 2022, 11, 2551. [Google Scholar] [CrossRef]
- Qasem, J.R. Allelopathy Field Research Methods. 1. Crops-weeds interactions. Allelopath. J. 2012, 30, 159–176. [Google Scholar]
- Lopes, R.W.N.; Marques Morais, E.; Lacerda, J.J.D.J.; Araújo, F.D.D.S. Bioherbicidal potential of plant species with allelopathic effects on the weed Bidens bipinnata L. Sci. Rep. 2022, 12, 13476. [Google Scholar] [CrossRef]
- Ishak, M.; Iberahim, Z.; Mardiana-Jansar, K. Comparative response of Malaysian weedy rice (Oryza sativa) initial growth towards the allelopathic potential of Leucaena leucocephala (Lam.) de Wit and Dicranopteris linearis (Burm. f.) Underw. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2021. [Google Scholar]
- Chon, S.-U.; Nelson, C. Allelopathy in Compositae plants. A review. Agron. Sustain. Dev. 2010, 30, 349–358. [Google Scholar] [CrossRef] [Green Version]
- Tudi, M.; Ruan, H.D.; Wang, L. Agriculture development, pesticide application and its impact on the environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef] [PubMed]
- Hoang Anh, L.; Van Quan, N.; Tuan Nghia, L.; Dang Xuan, T. Phenolic allelochemicals: Achievements, limitations, and prospective approaches in weed management. Weed Biol. Manag. 2021, 21, 37–67. [Google Scholar] [CrossRef]
- Cheng, F.; Cheng, Z. Research progress on the use of plant allelopathy in agriculture and the physiological and ecological mechanisms of allelopathy. Front. Plant Sci. 2015, 6, 1020. [Google Scholar] [CrossRef] [Green Version]
- Alsharekh, A.; El-Sheikh, M.A.; Alatar, A.A.; Abdel-Salam, E.M. Natural control of weed invasions in hyper-arid arable farms: Allelopathic potential effect of Conocarpus erectus against common weeds and vegetables. Agronomy 2022, 12, 703. [Google Scholar] [CrossRef]
- Cromwell, B. The alkaloids. In Modern Methods of Plant Analysis/Moderne Methoden der Pflanzenanalyse; Springer: Berlin/Heidelberg, Germany, 1955; pp. 367–516. [Google Scholar]
- Boxi, M.; Rajesh, Y.; Kumar, V.R.; Praveen, B.; Mangamma, K. Extraction, phytochemical screening and in-vitro evaluation of anti-oxidant properties of Commicarpuschinesis (aqueous leaf extract). Int. J. Pharm. Biosci. 2010, 1, 537–547. [Google Scholar]
- Sofowora, A. Recent trends in research into African medicinal plants. J. Ethnopharmacol. 1993, 38, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Hiscox, J.; Israelstam, G. A method for the extraction of chlorophyll from leaf tissue without maceration. Can. J. Bot. 1979, 57, 1332–1334. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1. [Google Scholar] [CrossRef] [Green Version]
- Beers, R.F.; Sizer, I.W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 1952, 195, 133–140. [Google Scholar] [CrossRef]
- Kakkar, P.; Das, B.; Viswanathan, P. A modified spectrophotometric assay of superoxide dismutase. Indian J. Biochem. Biophys. 1984, 21, 130–132. [Google Scholar] [PubMed]
- Fridovich, I. Fundamental aspects of reactive oxygen species, or what’s the matter with oxygen? Ann. N. Y. Acad. Sci. 1999, 893, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Högberg, J.; Larson, R.E.; Kristoferson, A.; Orrenius, S. NADPH-dependent reductase solubilized from microsomes by peroxidation and its activity. Biochem. Biophys. Res. Commun. 1974, 56, 836–842. [Google Scholar] [CrossRef]
- Wang, C.; Qi, J.; Liu, Q.; Wang, Y.; Wang, H. Allelopathic Potential of Aqueous Extracts from Fleagrass (Adenosma buchneroides Bonati) against Two Crop and Three Weed Species. Agriculture 2022, 12, 1103. [Google Scholar] [CrossRef]
- Qasem, J. A Survey on the phytotoxicity of common weeds, wild grown species and medicinal plants on wheat. Allelopath. J. 2017, 42, 179–194. [Google Scholar] [CrossRef]
- Ramadan, T.; Amro, A.; Alazazi, S. Comparative allelopathic potential of ten field weeds against seed germination of three economic plants. Biol. Forum. –Int. J. 2018, 10, 168–181. [Google Scholar]
- Bachheti, A.; Sharma, A.; Bachheti, R.K.; Husen, A.; Pandey, D.P. Plant allelochemicals and their various applications. In Co-Evolution of Secondary Metabolites; Springer: Berlin/Heidelberg, Germany, 2020; pp. 441–465. [Google Scholar]
- Shi, S.; Cheng, J.; Ahmad, N.; Zhao, W.; Tian, M.; Yuan, Z.; Zhao, C. Effects of potential allelochemicals in a water extract of Abutilon theophrasti Medik. on germination and growth of Glycine max L., Triticum aestivum L., and Zea mays L. J. Sci. Food Agric. 2022; ahead of print. [Google Scholar] [CrossRef]
- Soltani, E.; Ghaderi-Far, F.; Baskin, C. Problems with using mean germination time to calculate rate of seed germination. Aust. J. Bot. 2015, 63, 631–635. [Google Scholar] [CrossRef]
- Benvenuti, S. Soil texture involvement in germination and emergence of buried weed seeds. Agron. J. 2003, 95, 191–198. [Google Scholar] [CrossRef]
- Zareen, S.; Fawad, M.; Haroon, M.; Ahmad, I.; Zaman, A. Allelopathic potential of summer weeds on germination potential and growth performance of wheat and chickpea. J. Nat. Pestic. Res. 2022, 1, 100002. [Google Scholar] [CrossRef]
- Moreno-Robles, A.; Cala Peralta, A.; Soriano, G.; Zorrilla, J.G.; Masi, M.; Vilariño-Rodríguez, S.; Fernández-Aparicio, M. Identification of Allelochemicals with Differential Modes of Phytotoxicity against Cuscuta campestris. Agriculture 2022, 12, 1746. [Google Scholar] [CrossRef]
- Channappagoudar, B.; Agasimani, C. Physiological studies on weed control effiCiency in garlic. In Proceedings of the National Seminar on New Perspectives in Spices, Medicinal and Aromatic Plants , 27–29 November 2003; Indian Society for Spices: Kozhikode, Kerala, India, 2003; p. 169. [Google Scholar]
- Kapoor, R.T.; Srivastava, A.K.; Meenal, A. Allelotoxicity of aqueous leaf extracts of Parthenium hysterophorus L. on the biomass and physiological-behaviour of Brassica juncea and Zea mays. Electron. J. Environ. Agric. Food Chem. 2012, 11, 53–67. [Google Scholar]
- Shahrokhi, S.; Darvishzadeh, M.; Mehrpouyan, M.; Farboodi, M.; Akbarzadeh, M. Germination and growth of wheat, Triticum aestivum (cv. azar2) in response to Pigweed, Amaranthus retroflexus L. organs extracts. In Proceedings of the 2nd International Conference on Agricultural and Animal Science, Maldives, 5–27 November 2011. [Google Scholar]
- Kim, L.R.; Adhikari, A.; Kang, Y.; Gam, H.J.; Kang, S.M.; Kim, K.Y.; Lee, I.J. Investigation of Solanum carolinense Dominance and Phytotoxic Effect in Festuca arundinacea with Special Reference to Allelochemical Identification, Analysis of Phytohormones and Antioxidant Mechanisms. Agronomy 2022, 12, 1954. [Google Scholar] [CrossRef]
- Ma, H.; Chen, Y.; Chen, J.; Zhang, Y.; Zhang, T.; He, H. Comparison of allelopathic effects of two typical invasive plants: Mikania micrantha and Ipomoea cairica in Hainan island. Sci. Rep. 2020, 10, 11332. [Google Scholar] [CrossRef]
- Inderjit. Plant phenolics in allelopathy. Bot. Rev. 1996, 62, 186–202. [Google Scholar]
- Talukder, M.R.; Asaduzzaman, M.; Ueno, M.; Tanaka, H.; Asao, T. Alleviation of allelochemical stress-induced growth inhibition and oxidative damage in lettuce under closed hydroponics through electro-degradation. Hortic. Sci. 2020, 47, 53–68. [Google Scholar] [CrossRef]
- Song, L.; Pan, K.W.; Wang, J.C.; Ma, Y.H. Effects of phenolic acids on seed germination and seedling antioxidant enzyme activity of alfalfa. Acta Ecol. Sin. 2006, 26, 3393–3403. [Google Scholar]



| Weeds Species | Code | Family | Life Cycle | Growth form | Status of the Weeds In the Crop Fields |
|---|---|---|---|---|---|
| Centella asiatica (L.) Urb. | T1 | Apiaceae | P | Herb | +++ |
| Rotala indica (Willd.) Koehne | T2 | Lythraceae | P | Climber | ++ |
| Solanum nigrum L. | T3 | Solanaceae | P | Herb | ++ |
| Commelina benghalensis L. | T4 | Commelinaceae | A/P | Creeping Herb | +++ |
| Marsilea quadrifolia L. | T5 | Marsileaceae | P | Herb | ++ |
| Ageratum conyzoides L. | T6 | Asteraceae | A/P | Herb | ++ |
| Cynodon dactylon (L.) pers. | T7 | Poaceae | P | Herb | +++ |
| Spilanthes acmella L. | T8 | Asteraceae | A/P | Herb | ++ |
| Heliotropium indicum L. | T9 | Boraginaceae | A | Herb | +++ |
| Leucas aspera (Willd.) Link | T10 | Lamiaceae | A | Herb/Shrub | ++ |
| Phyllanthus niruri L. | T11 | Phyllanthaceae | A | Herb | ++ |
| Sida acuta Burm.f | T12 | Malvaceae | A/P | Shrub | +++ |
| Mikania micrantha Kunth | T13 | Asteraceae | P | Climbing | +++ |
| Polygonum hydropiper L. | T14 | Polygonaceae | A | Herb | ++ |
| Physalis heterophylla Nees | T15 | Solanaceae | P | Herb | + |
| Properties | Value |
|---|---|
| Texture | Sandy loam |
| Sand (%) | 58 |
| Silt (%) | 25 |
| Clay (%) | 17 |
| pH | 5.4 |
| Organic carbon (%) | 0.77 |
| Organic matter (%) | 1.33 |
| Cation Exchange Capacity (CEC) (C.mol Kg−1) | 6.48 |
| Electrical conductivity (µS cm−1) | 32.4 |
| Total nitrogen (%) | 0.11 |
| Total phosphorus (%) | 0.04 |
| Total potassium (%) | 0.34 |
| Iron (%) | 0.63 |
| Manganese (mg Kg−1) | 187.58 |
| Zinc (mg Kg−1) | 64.32 |
| Available Bray-1 P (mg Kg−1) | 9.0 |
| Available K (meq/100 g) | 0.19 |
| Available Ca (meq/100 g) | 1.89 |
| Available Mg (meq/100 g) | 1.03 |
| Treatments (Weed Species) | Code | Petri Dish Experiments | Pot Experiments | ||
|---|---|---|---|---|---|
| Germination (%) | Mean Germination Time (MGT) | Germination (%) | Mean Germination Time (MGT) | ||
| Distilled water | C | 86.33 | 4.44 | 87.23 | 3.54 |
| Centella asiatica | T1 | 69.00 * | 6.83 * | 79.00 | 7.00 ** |
| Rotala indica | T2 | 66.67 ** | 7.00 ** | 68.50 ** | 7.00 ** |
| Solanum nigrum | T3 | 71.00 * | 6.66 * | 68.45 ** | 6.00 |
| Commelina benghalensis | T4 | 64.00 *** | 6.00 | 74.00 * | 5.34 |
| Marsilea quadrifolia | T5 | 68.33 ** | 7.00 ** | 70.00 ** | 6.00 |
| Ageratum conyzoides | T6 | 70.00 ** | 8.00 ** | 69.33 ** | 6.00 |
| Cynodon dactylon | T7 | 70.00 ** | 6.00 | 74.00 * | 7.00 ** |
| Spilanthes acmella | T8 | 76.67 | 6.56 * | 73.50 * | 6.33 |
| Heliotropium indicum | T9 | 67.33 ** | 6.50 * | 69.33 ** | 6.00 |
| Leucas aspera | T10 | 67.67 ** | 6.17 | 68.67 ** | 6.50 * |
| Phyllanthus niruri | T11 | 71.67 ns | 6.35 | 76.33 | 7.00 ** |
| Sida acuta | T12 | 68. 67 ** | 6.32 | 69.33 ** | 6.33 |
| Mikania micrantha | T13 | 73.33 | 6.81 * | 84.33 | 5.33 |
| Polygonum hydropiper | T14 | 65.33 ** | 7.00 ** | 70.00 ** | 6.50 |
| Physalis heterophylla | T15 | 66.67 ** | 6.36 ns | 69.33 ** | 6.68 * |
| Weed Species | Code | Petri Dish Experiments | Pot Experiments | ||
|---|---|---|---|---|---|
| Shoot Length (cm) | Root Length (cm) | Shoot Length (cm) | Root Length (cm) | ||
| Distilled water (Control) | C | 19.13 | 3.46 | 20.13 | 6.42 |
| Centella asiatica | T1 | 13.68 * | 3.42 | 11.72 * | 5.55 ** |
| Rotala indica | T2 | 14.00 | 2.33 * | 10.43 ** | 5.68 * |
| Solanum nigrum | T3 | 13.70 * | 3.26 | 10.33 ** | 5.86 |
| Commelina benghalensis | T4 | 12.63 ** | 2.31 * | 10.87 ** | 5.32 ** |
| Marsilea quadrifolia | T5 | 18.04 | 3.36 | 14.80 | 5.92 |
| Ageratum conyzoides | T6 | 17.02 | 3.32 | 11.98 * | 5.58 ** |
| Cynodon dactylon | T7 | 12.70 ** | 2.19 ** | 10.83 ** | 5.62 * |
| Spilanthes acmella | T8 | 12.68 ** | 2.35 * | 10.36 ** | 4.34 ** |
| Heliotropium indicum | T9 | 18.02 | 3.22 | 13.67 * | 4.92 ** |
| Leucas aspera | T10 | 13.02 * | 2.17 ** | 10.02 ** | 5.92 |
| Phyllanthus niruri | T11 | 12.08 ** | 3.34 | 9.82 ** | 5.50 ** |
| Sida acuta | T12 | 13.02 * | 2.40 * | 10.58 ** | 5.40 ** |
| Mikania micrantha | T13 | 12.01 ** | 2.30 * | 9.72 *** | 4.64 ** |
| Polygonum hydropiper | T14 | 17.39 | 3.24 | 14.53 | 5.38 ** |
| Physalis heterophylla | T15 | 17.03 | 3.34 | 14.02 * | 5.51 ** |
| Treatments (Weed Species) | (Code) | Petri Dish Experiments | Pot Experiments |
|---|---|---|---|
| Seedling Dry Wt (mg Plant−1) | Seedling Dry Wt (mg Plant−1) | ||
| Distilled water (Control) | C | 30.23 | 33.33 |
| Centella asiatica | T1 | 27.32 * | 27.00 |
| Rotala indica | T2 | 28.44 | 29.78 |
| Solanum nigrum | T3 | 19.55 | 25.89 |
| Commelina benghalensis | T4 | 20.38 ** | 20.78 ** |
| Marsilea quadrifolia | T5 | 22.34 ** | 21.78 ** |
| Ageratum conyzoides | T6 | 25.33 ** | 25.33 * |
| Cynodon dactylon | T7 | 18.52 ** | 27.78 |
| Spilanthes acmella | T8 | 23.43 ** | 19.71 ** |
| Heliotropium indicum | T9 | 27.67 ** | 22.11 ** |
| Leucas aspera | T10 | 23.51 | 26.89 |
| Phyllanthus niruri | T11 | 27.33 ** | 23.78 ** |
| Sida acuta | T12 | 27.67 | 26.56 |
| Mikania micrantha | T13 | 24.33 ** | 25.89 |
| Polygonum hydropiper | T14 | 19.00 ** | 20.78 ** |
| Physalis heterophylla | T15 | 17.49 ** | 22.44 ** |
| Weed Species | (Code) | Alkaloids | C. | Fla. | Phe. | P. | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| D | W | M | T | H | Fe | ||||||
| Centella asiatica. | T1 | + | +++ | + | +++ | +++ | + | +++ | ,, | ,, | - |
| Rotala indica | T2 | ++ | ++ | ++ | +++ | +++ | ++ | +++ | ,, | ,, | - |
| Solanum nigrum | T3 | + | ++ | ++ | +++ | ++ | + | + | ,, | ,, | - |
| Commelina benghalensis | T4 | + | + | + | ++ | + | + | ++ | ,, | ,, | - |
| Marsilea quadrifolia | T5 | + | + | + | ++ | + | + | + | ,, | ,, | |
| Ageratum conyzoides | T6 | + | + | + | +++ | ++ | + | ++ | ,, | ,, | - |
| Cynodon dactylon | T7 | ++ | ++ | +++ | +++ | ++ | ++ | +++ | ,, | ,, | - |
| Spilanthes acmella | T8 | +++ | ++ | ++ | +++ | +++ | ++ | ++ | ,, | ,, | - |
| Heliotropium indicum | T9 | +++ | ++ | ++ | +++ | +++ | ++ | +++ | ,, | ,, | |
| Leucas aspera | T10 | ++ | ++ | ++ | +++ | +++ | + | + | ,, | ,, | - |
| Phyllanthus niruri | T11 | + | + | + | +++ | + | + | +++ | ,, | ,, | - |
| Sida acuta | T12 | +++ | +++ | +++ | ++ | +++ | ++ | ++ | ,, | ,, | - |
| Mikania micrantha | T13 | +++ | +++ | +++ | ++ | ++ | +++ | ,, | ,, | - | |
| Polygonum hydropiper | T14 | ++ | ++ | ++ | +++ | +++ | ++ | ++ | ,, | ,, | - |
| Physalis heterophylla | T15 | ++ | + | ++ | +++ | ++ | + | +++ | ,, | ,, | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akter, P.; Ahmed, A.M.A.; Promie, F.K.; Haque, M.E. Root Exudates of Fifteen Common Weed Species: Phytochemical Screening and Allelopathic Effects on T. aestivum L. Agronomy 2023, 13, 381. https://doi.org/10.3390/agronomy13020381
Akter P, Ahmed AMA, Promie FK, Haque ME. Root Exudates of Fifteen Common Weed Species: Phytochemical Screening and Allelopathic Effects on T. aestivum L. Agronomy. 2023; 13(2):381. https://doi.org/10.3390/agronomy13020381
Chicago/Turabian StyleAkter, Pervin, A. M. Abu Ahmed, Fahmida Khanam Promie, and Md. Enamul Haque. 2023. "Root Exudates of Fifteen Common Weed Species: Phytochemical Screening and Allelopathic Effects on T. aestivum L." Agronomy 13, no. 2: 381. https://doi.org/10.3390/agronomy13020381





