Anaerobic Digestion Reduces Seed Germination and Viability of Six Plant Species from the Upper Nile Valley, Egypt
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Weed Seed Selection
2.2. Substrate and Inoculum
2.3. Anaerobic Digestion Treatments
2.4. Seed Germination and Viability
2.5. Anaerobic Digestion Culture Conditions
2.6. Statistical Analysis
3. Results
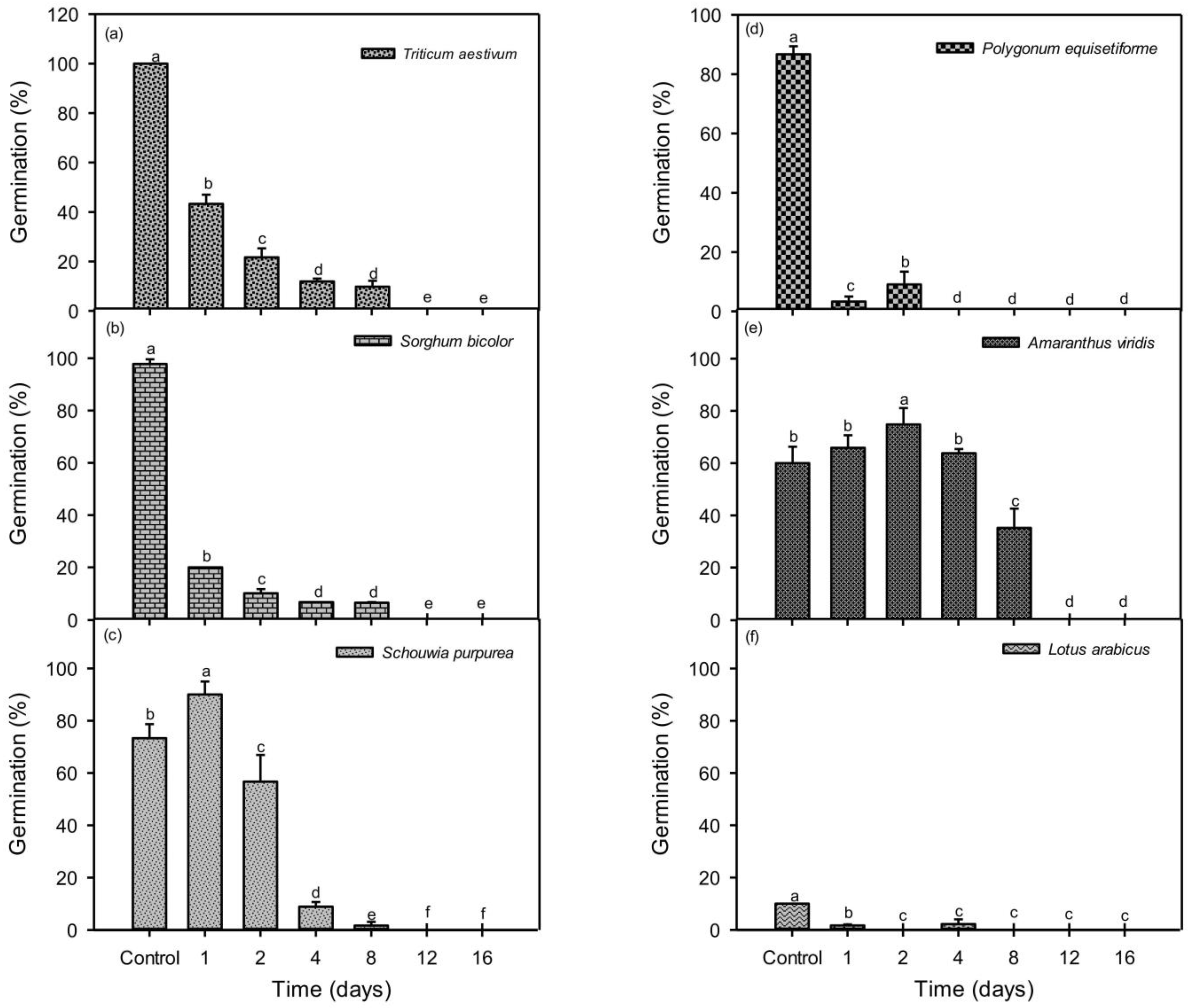
3.1. Seed Germination and Viability
3.2. Anaerobic Digestion Culture Conditions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Westerman, P.R.; Gerowitt, B. Weed seed survival during anaerobic digestion in biogas plants. Bot. Rev. 2013, 79, 281–316. [Google Scholar] [CrossRef]
- Saha, B.; Devi, C.; Khwairakpam, M.; Kalamdhad, A.S. Vermicomposting and anaerobic digestion–viable alternative options for terrestrial weed management–A review. Biotech. Rep. 2018, 17, 70–76. [Google Scholar] [CrossRef]
- Gonzalez-Fernandez, C.; Garcia-Encina, P.A. Impact of substrate to inoculum ratio in anaerobic digestion of swine slurry. Biomass Bioenergy 2009, 33, 1065–1069. [Google Scholar] [CrossRef]
- Eckford, R.E.; Newman, J.C.; Li, X.; Watson, P.R. Thermophilic anaerobic digestion of cattle manure reduces seed viability for four weed species. Int. J. Agric. Biol. Eng. 2012, 5, 71–75. [Google Scholar]
- Westerman, P.R.; Gerowitt, B. The probability of maize biomass contamination with weed seeds. J. Plant Diseases Protec. 2012, 119, 68–73. [Google Scholar] [CrossRef]
- Westerman, P.R.; Hildebrandt, F.; Gerowitt, B. Weed seed survival following ensiling and mesophilic 1069. digestion in batch reactors. Weed Res. 2012, 52, 286–295. [Google Scholar] [CrossRef]
- Johansen, A.; Nielsen, H.B.; Hansen, C.M.; Andreasen, C.; Carlsgart, J.; Hauggard-Nielsen, H.; Roepstorff, A. Survival of weed seeds and animal parasite as affected by anaerobic digestion at meso- and thermophilic conditions. Waste Manag. 2013, 33, 807–812. [Google Scholar] [CrossRef]
- Aragaw, T.; Andargie, M.; Gessesse, A. Co-digestion of cattle manure with organic kitchen waste to increase biogas production using rumen fluid as inoculums. Int. J. Phys. Sci. 2013, 8, 443–450. [Google Scholar]
- Erraji, H.; Afilal, M.E.; Mzabri, I.; Charif, K. The survival of Moroccan invasive weed seeds during anaerobic digestion. Environ. Water Sci. Public Health Territ. Intell. J. 2019, 3, 141–146. [Google Scholar]
- Zhou, L.; Hulsemann, B.; Merkle, W.; Guo, J.; Dong, R.; Piepho, H.-P.; Gerhards, R.; Muller, J.; Oechner, H. Influence of anaerobic digestion processes on the germination of weed seeds. Gesunde Pflanz. 2020, 72, 181–194. [Google Scholar] [CrossRef]
- Goldemberg, J.; Johansson, T.B. World Energy Assessment Overview: 2004 Update; United Nations Development Program: New York, NY, USA, 2004. [Google Scholar]
- Gutschick, V.P.; BassiriRad, H. Extreme events as shaping physiology, ecology, and evolution of plants: Toward a unified definition and evaluation of their consequences. New Phytol. 2003, 160, 21–42. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; Luo, Y.; Li, D.; Cao, S.; Xia, J.; Li, J.; Smith, M.D. Plant growth and mortality under climatic extremes: An overview. Environ. Exp. Bot. 2014, 98, 13–19. [Google Scholar] [CrossRef]
- Sanchez, M.; Gomez, X.; Barriocanal, G.; Cuetos, M.; Moran, A. Assessment of the stability of livestock farm waste by anaerobic digestion. Int. Biodeterior. Biodegrad. 2008, 62, 421–426. [Google Scholar] [CrossRef]
- Roopnarain, A.; Adelke, R. Current status, hurdles and future prospects of biogas digestion technology in Africa. Renew. Sustain. Energy Rev. 2017, 67, 1162–1179. [Google Scholar] [CrossRef]
- Moller, K.; Stinner, W. Effects of different manuring systems with and without biogas digestion on soil mineral nitrogen content and on gaseous nitrogen losses (ammonia, nitrous oxides). Eur. J. Agron. 2009, 30, 1–16. [Google Scholar] [CrossRef]
- Insam, H.; Gomez-Brandon, M.; Ascher, J. Manure-based biogas fermentation residues–friend or foe of soil fertility? Soil Biol. Biochem. 2015, 84, 1–14. [Google Scholar] [CrossRef]
- Przygocka-Cyna, K.; Grzebisz, W. Biogas digestate–benefits and risks for soil fertility and crop quality- an evaluation of grain maize response. Open Chem. 2018, 16, 258–271. [Google Scholar] [CrossRef]
- Barzee, T.J.; Edalati, A.; El-Mashad, H.; Wang, D.; Scow, K.; Zhang, R. Digestate biofertilizers support similar or higher tomato yields and quality than mineral fertilizer in subsurface drip fertigation system. Front. Sustain. Food Syst. 2019, 3, 58. [Google Scholar] [CrossRef]
- Doyeni, M.O.; Stulpinaite, U.; Baksinskaite, A.; Suproniene, S.; Tilvikiene, V. The effectiveness of digestate use for fertilization in an agricultural cropping system. Plants 2021, 10, 1734. [Google Scholar] [CrossRef]
- Engeli, H.; Edelmann, W.; Fuchs, J.; Rottermann, K. Survival of plant pathogens and weed seeds during anaerobic digestion. Water Sci. Technol. 1993, 27, 69–76. [Google Scholar] [CrossRef]
- Barnes, J. Pumping possibility: Agricultural expansion through desert reclamation in Egypt. Soc. Stud. Sci. 2012, 42, 517–538. [Google Scholar] [CrossRef]
- Radwan, T.M. Monitoring agricultural expansion in a newly reclaimed area in the western Nile Delta of Egypt using Landsat imageries. Agriculture 2019, 9, 137. [Google Scholar] [CrossRef] [Green Version]
- Elbeih, S.F. Evaluation of agricultural expansion areas in the Egyptian deserts: A review using remote sending and GIS. Egypt. J. Remote Sens. Space Sci. 2021, 24, 889–906. [Google Scholar] [CrossRef]
- Kamel, M.; Abu El Ella, E.S.M. Integration of remote sending & GIS to Manage the sustainable development in the Nile Valley desert fringes of Assiut-Sohag governorates, Upper Nile. J. Indian Soc. Remote Sens. 2016, 44, 759–774. [Google Scholar]
- Mohammed, A.M.; Refaee, A.; El-Din, G.K.; Harb, S. Hydrochemical characteristics and quality assessment of shallow groundwater under intensive agriculture practices in arid region, Qena, Egypt. Appl. Water Sci. 2022, 12, 92. [Google Scholar] [CrossRef]
- El-Mashad, H.M.; van Loon, W.K.P.; Zeeman, G.; Bot, G.P.A.; Lettinga, G. Reuse potential of agricultural wastes in semi-arid regions: Egypt as a case study. Rev. Environ. Sci. Biotechnol. 2003, 2, 53–66. [Google Scholar] [CrossRef]
- Gaballah, M.S.; Mansour, H.A.; Hofal, O.A. Balanced fertilization of major crops in Egypt: A review. Plant Arch. 2020, 20, 2453–2458. [Google Scholar]
- El Hadidi, M.N.; Hosny, A.I.; El Husseini, N. Some aspects of the biodiversity of the weed flora in the farmlands of Egypt. In The Biodiversity of African Plants: Proceedings of the XIVth AETFAT Congress, Wageningen, The Netherlands, 22–27 August 1994; Van der Maesen, L.J.G., van der Burgt, X.M., van Mendenbach de Rooy, J.M., Eds.; Kluwer: Amsterdam, The Netherlands, 1996; pp. 788–794. [Google Scholar]
- Abd El-Ghani, M.; Soliman, A.; Hamdy, R.; Bennoba, E. Weed flora in the reclaimed lands along the norther sector of the Nile Valley in Egypt. Turkish J. Bot. 2013, 37, 464–488. [Google Scholar]
- Salama, F.M.; Abd El-Ghani, M.M.; El-Tayeh, N.A.; Amro, A.M.; Abdrabbu, H.S. Weed flora of common crops in desert reclaimed arable lands of southern Egypt. Taeckholmia 2016, 36, 62–85. [Google Scholar] [CrossRef]
- Salama, F.M.; Abd El-Ghani, M.M.; El-Tayeh, N.A.; Amro, A.M.; Abdrabbu, H.S. Correlations between soil variables and weed communities in major crops of the desert reclaimed lands of southern Egypt. Rend. Lincei 2017, 28, 363–378. [Google Scholar] [CrossRef]
- Aurora, S.P. Microbial Digestion in the Ruminant; New Delhi (India) Indian Council of Agricultural Research: New Delhi, India, 1983. [Google Scholar]
- Manimutha, S.; Sathiya Pandi, N.; Asha, G.; Rajendran, S. The biogas production from mixture of agar and rumen wastes. Int. J. Adv. Res. 2015, 3, 362–369. [Google Scholar]
- Jin, W.; Xu, X.; Yang, F.; Li, C.; Zhou, M. Performance enhancement by rumen cultures in anaerobic co-digestion of corn straw with pig manure. Biomass Bioenergy 2018, 115, 120–129. [Google Scholar] [CrossRef]
- Budiyono, B.; Widiasa, I.N.; Johari, S.; Sunarso, S. Influence of inoculum content on performance of anaerobic reactors for treating cattle manure using rumen fluid inoculum. Int. J. Eng. Technol. 2009, 1, 109–116. [Google Scholar]
- Rico, C.; Diego, R.; Valcarce, A.; Rico, J.R. Biogas production from various typical organic wastes generated in the region of Cantabria (Spain): Methane yields and co-digestion tests. Smart Grid Renew. Energy 2014, 5, 128–136. [Google Scholar] [CrossRef] [Green Version]
- Kamel, M.; Hammad, S.; Khalaphallah, R.; Elazeem, M.A. Halophytes and salt tolerant wild plants as a feedstock for biogas production. J. BioSci. Biotechnol. 2019, 8, 151–159. [Google Scholar]
- Abbas, A.M.; Mancilla-Leytón, J.M.; Castillo, J.M. Can camels disperse seeds of the invasive tree Prosopis juliflora? Weed Res. 2018, 58, 221–228. [Google Scholar] [CrossRef]
- Manoto, M.M.; Ferreira, M.; Agenbag, G. The effects of temperature on the germination os six selected wed species. S. Afr. J. Plant Soil 2004, 21, 214–219. [Google Scholar] [CrossRef]
- Michael, P.J.; Steadman, K.J.; Plummer, J.A. Climatic regulation of seed dormancy and emergence of diverse Malva parviflora populations from a Mediterranean-type environment. Seed Sci. Res. 2006, 16, 273–281. [Google Scholar] [CrossRef]
- Abbas, A.M.; Rubio-Casal, A.E.; De Cires, A.; Fiqueroa, M.E.; Lambert, A.M.; Castillo, J.M. Effects of flooding on germination and establishment of the invasive cordgrass Spartina densiflora. Weed Res. 2012, 52, 269–276. [Google Scholar] [CrossRef]
- Abbas, A.M.; Al-Kahtani, M.; Mousa, M.A.; Badry, M.O.; Hassaneen, A.S.A.; Ezzat-Ahmed, A.; Mancilla-Leyton, J.M.; Castillo, J.M. Endozoochory by goals of two invasive weeds with contracted propagule traits. Sustainability 2020, 12, 5450. [Google Scholar] [CrossRef]
- Mancilla-Leyton, J.M.; Fernandez-Ales, R.; Vicenta, A.M. Plant-ungulate interaction: Goat gut passage effect on survival and geremination of Mediterranean shrub seeds. J. Veg. Sci. 2011, 22, 1031–1037. [Google Scholar] [CrossRef]
- Awathi, M.K.; Wang, Q.; Huang, H.; Li, R.; Shen, F.; Lahori, A.H.; Wang, P.; Guo, D.; Guo, Z.; Jiang, S.; et al. Efect of biochar amendment on greenhouse gas emission and bio-availability of heavy metals during sewage sludge co-composting. J. Clean. Prod. 2016, 135, 829–835. [Google Scholar] [CrossRef]
- Liu, T.; Wang, M.; Awasthi, M.K.; Chen, H.; Awasthi, S.K.; Duan, Y.; Zhang, Z. Mesurement of cow manure compost toxity and maturity based on weed seed germination. J. Clean. Prod. 2020, 245, 118894. [Google Scholar] [CrossRef]
- Rudydi, A.F. Correlation between conductivity and total dissolved solid in various type of water: A review. IOP Conf. Ser. Earth Environ. Sci. 2017, 188, 012019. [Google Scholar]
- Khan, A.M.; Mobli, A.; Werth, J.A.; Chauhan, B.S. Germination and seed persstance og Amaranthus retroflexus and Amaranthu viridis: Two emerging weeds in Australian cotton and sumer crops. PLoS ONE 2022, 17, e0263798. [Google Scholar]
- Mohamed, E.; Kasem, A.; Gobouri, A.A.; El Kelish, A.; Azab, E. Influene of maternal habitat n salt tolerance during germiation and growth in Zygophyllum coccineum. Plants 2010, 9, 1504. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Ayele, B.T. Functional genomics of seed doemancy in wheat: Advances and propects. Front. Plant Sci. 2014, 5, 458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benech-Arnold, R.L.; Enciso, S.; Sanchez, R.A.; Rodriquez, M.V. On the hormonal nature of the stimulatory effect of high incubation temperatures on the germination of dormant sorghum (S. bicolor) caryopses. New Phytol. 2003, 160, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Westerman, P.R.; Heiermann, M.; Pottbeg, U.; Rodeman, B.; Gerowitt, B. Weed seedduring anaerobic digestionin bio-gas plants. Weed Res. 2012, 52, 307–316. [Google Scholar] [CrossRef]
- Smykal, P.; Vrnoud, V.; Blair, M.W.; Soukup, A.; Thompson, R. The role of the test during development and in establishment of dormancy of the legume seed. Front. Plant Sci. 2014, 5, 351. [Google Scholar]
- Gavala, H.N.; Yenal, U.; Skiadas, I.V.; Westerann, P.; Ahrng, B.K. Mesophilic and thermophilic anaerobic and secondary sludge Effect of pre-tretment at elevated temperatures. Water Res. 2003, 37, 4561–4572. [Google Scholar] [CrossRef] [PubMed]
- Vindis, P.; Mursec, B.; Janzekovic, M.; Cus, F. The impact of mesophilic and thermophilic anaerobic digestion onbiogas prodction. J. Achiev. Mater. Manuf. Eng. 2009, 36, 192–198. [Google Scholar]
- Hamzah, M.A.F.; Jahim, J.M.; Abdul, P.M. Comparative start-up beteen mesophilic and thermophilic for acidified palm oil mill effluent treatment. IOP Conf. Ser. Earth Environ. Sci. 2019, 268, 01228. [Google Scholar]


| Species | Time to First Germination (Day) | ||||||
|---|---|---|---|---|---|---|---|
| Control | 1 | 2 | 4 | 8 | 12 | 16 | |
| Triticum aestivum | 1 ± 0 b | 2 ± 0 d | 5 ± 0 c | 5 ± 1 c | 9 ± 0 a | 0 ± 0 | 0 ± 0 |
| Sorghum bicolor | 1 ± 0 b | 4 ± 0 c | 8 ± 1 b | 7 ± 1 b | 9 ± 0 a | 0 ± 0 | 0 ± 0 |
| Schouwia purpurea | 1 ± 0 b | 2 ± 0 d | 3 ± 1 d | 7 ± 0 b | 5 ± 2 b | 0 ± 0 | 0 ± 0 |
| Polygonum equisetiforme | 2 ± 0 a | 17 ± 0 a | 17 ± 3 a | 0 ± 0 d | 0 ± 0 c | 0 ± 0 | 0 ± 0 |
| Amaranthus viridis | 2 ± 0 a | 2 ± 0 d | 4 ± 0 d | 8 ± 1 b | 8 ± 1 a | 0 ± 0 | 0 ± 0 |
| Lotus arabicus | 2 ± 0 a | 10 ± 0 b | 0 ± 0 e | 20 ± 0 a | 0 ± 0 c | 0 ± 0 | 0 ± 0 |
| Species | ||||||
|---|---|---|---|---|---|---|
| Incubation Time (day) | Triticum aestivum | Sorghum bicolor | Schouwia purpurea | |||
| Viable (%) | Non-viable (%) | Viable (%) | Non-viable (%) | Viable (%) | Non-viable (%) | |
| Control | 0 ± 0 d | 0 ± 0 f | 2.2 ± 1.8 d | 0 ± 0 f | 28.9 ± 2.1 d | 2.2 ± 1.8 e |
| 1 | 50.0 ± 3.7 a | 6.7 ± 0.1 e | 66.7 ± 2.6 a | 13.3 ± 2.4 e | 1.7 ± 1.4 e | 8.3 ± 4.3 d |
| 2 | 48.3 ± 3.6 a | 30 ± 3.7 d | 64 ± 1.4 a | 27 ± 2.8 d | 36.6 ± 7.7 b | 6.7 ± 1.1 d |
| 4 | 41.6 ± 2.7 b | 46.5 ± 2.4 c | 53.3 ± 2.3 b | 40 ± 2.1 c | 51.2 ± 1.8 a | 39.9 ± 1.7 c |
| 8 | 42.7 ± 3.4 b | 47.5 ± 1.8 c | 19.3 ± 1.3 c | 74.3 ± 2.7 b | 37.1 ± 4.6 b | 61.2 ± 4.4 b |
| 12 | 10.8 ± 1.4 c | 89.2 ± 6.3 b | 4.3 ± 1.8 d | 95.7 ± 3.8 a | 39.8 ± 2.7 b | 60.2 ± 3.6 b |
| 16 | 0 ± 0 d | 100 ± 0 a | 0 ± 0 e | 100 ± 0 a | 32.5 ± 0.1 c | 67.5 ± 0.6 a |
| Species | ||||||
| Incubation Time (day) | Polygonum equisetiforme | Amaranthus viridis | Lotus arabicus | |||
| Viable (%) | Non-viable (%) | Viable (%) | Non-viable (%) | Viable (%) | Non-viable (%) | |
| Control | 6.7 ± 0.5 e | 6.6 ± 1.2 e | 22.2 ± 1.6 a,b | 17.8 ± 1.2 e | 84.4 ± 1.8 a | 5.6 ± 0.3 f |
| 1 | 93.3 ± 3.1 a | 3.4 ± 0.02 f | 25.4 ± 1.4 a | 7.5 ± 0.3 f | 74.5 ± 2.8 a,b | 23.8 ± 1.3 e |
| 2 | 76.5 ± 2.2 b | 14.4 ± 2.4 d | 5 ± 1.4 d | 21.2 ± 5.4 d | 38.3 ± 4.9 d | 61.7 ± 3.2 b |
| 4 | 24.1 ± 0.3 c | 75.9 ± 1.4 c | 8.4 ± 0.3 c | 27.8 ± 2.3 d | 60 ± 1.8 c | 37.8 ± 1.3 c |
| 8 | 18.2 ± 3.9 d | 82.8 ± 3.3 b | 18.5 ± 1.3 b | 46.3 ± 1.1 c | 70 ± 5.4 b | 30 ± 2.1 d |
| 12 | 1.45 ± 0.1 f | 98.5 ± 1.5 a | 17.5 ± 0.4 b | 82.5 ± 1.3 b | 15.9 ± 1.3 e | 84.1 ± 3.6 a |
| 16 | 6.7 ± 0.8 e | 93.3 ± 0.7 a | 8.8 ± 2.19 c | 89.2 ± 1.7 a | 9.7 ± 4.1 f | 90.3 ± 5.2 a |
| Incubation Time (Day) | Temperature (°C) |
|---|---|
| Control | -- |
| 1 | 28.6 ± 0.1 d |
| 2 | 29.8 ± 0.2 d |
| 4 | 32.5 ± 1.2 c |
| 8 | 35.6 ± 1.4 b |
| 12 | 37.2 ± 0.6 a |
| 16 | 39.8 ± 1.3 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbas, A.M.; Abdelazeem, M.; Novak, S.J. Anaerobic Digestion Reduces Seed Germination and Viability of Six Plant Species from the Upper Nile Valley, Egypt. Agronomy 2023, 13, 396. https://doi.org/10.3390/agronomy13020396
Abbas AM, Abdelazeem M, Novak SJ. Anaerobic Digestion Reduces Seed Germination and Viability of Six Plant Species from the Upper Nile Valley, Egypt. Agronomy. 2023; 13(2):396. https://doi.org/10.3390/agronomy13020396
Chicago/Turabian StyleAbbas, Ahmed M., Mohamed Abdelazeem, and Stephen J. Novak. 2023. "Anaerobic Digestion Reduces Seed Germination and Viability of Six Plant Species from the Upper Nile Valley, Egypt" Agronomy 13, no. 2: 396. https://doi.org/10.3390/agronomy13020396






