Evaluation of the Effect of Water Stress on Clonal Variations of Cv. Monastrell (Vitis vinifera L.) in South-Eastern Spain: Physiology, Nutrition, Yield, Berry, and Wine-Quality Responses
Abstract
:1. Introduction
2. Material and Methods
2.1. Field Conditions, Plant Materials, and Irrigation Treatments
2.2. Vine Water Status, Leaf Gas Exchange and Leaf Hydraulic Conductivity
2.3. Isotope Carbon Composition
2.4. Leaf Mineral Analysis
2.5. Main Shoot Length and Total Leaf Area
2.6. Yield Response, WUE, and Sink/Source Ratios
2.7. Berry Composition and Berry Quality Index
2.8. Microvinifications
2.9. Wine Chemical Composition
2.10. Determination of Volatile Aromatic Compounds in Wines
2.11. Statistical Analysis
3. Results
3.1. Period 2018–2020 All of the Clones Were Irrigated Using the Same Irrigation Strategy and Water Volume
3.1.1. Vine Water Status and Leaf Gas Exchange
3.1.2. Leaf Mineral Nutrition
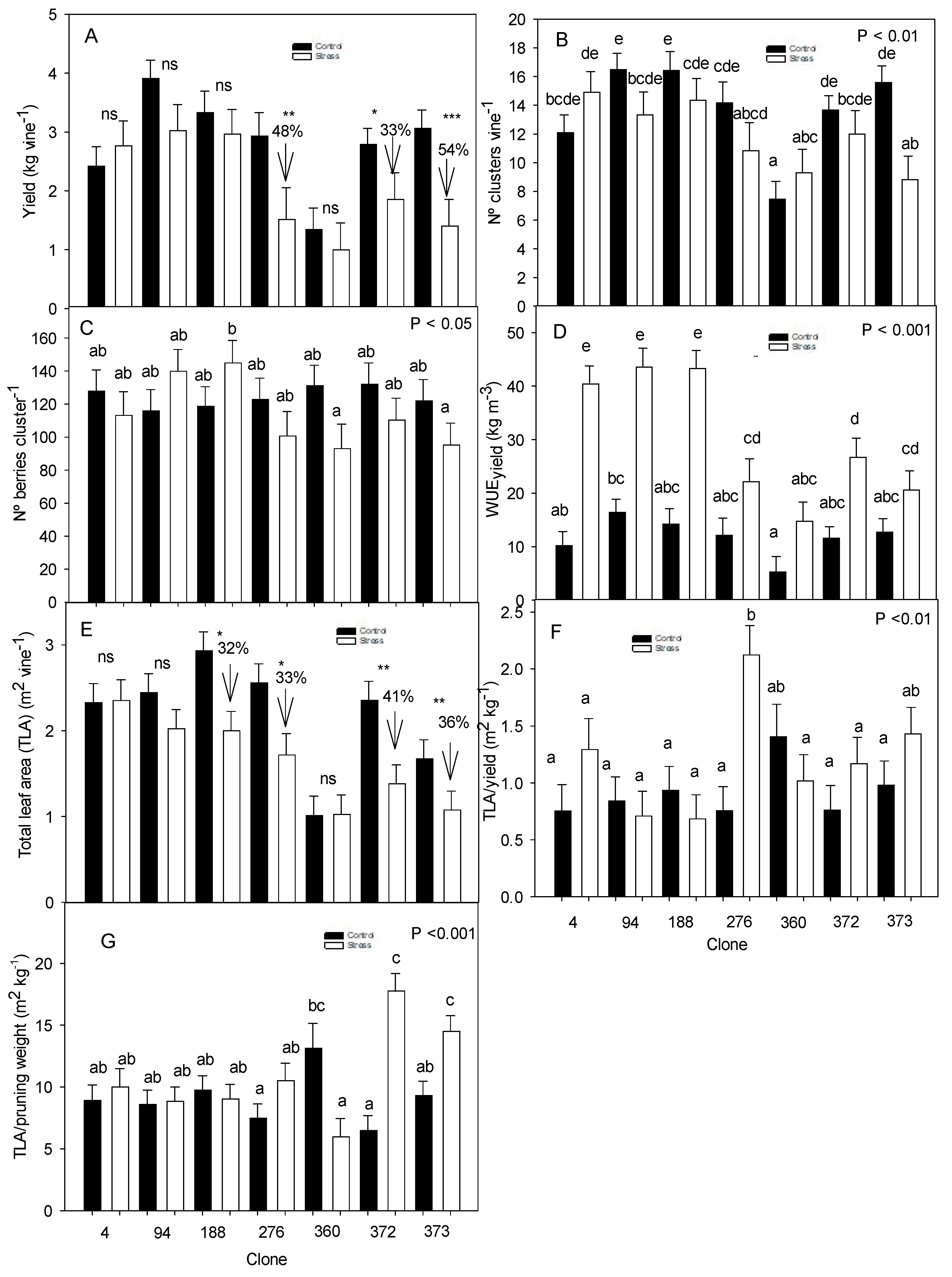
3.1.3. Vegetative Development and Yield Response
3.1.4. Berry and Must Quality Parameters
3.2. Year 2021 Optimization of the Irrigation Water Volume for Each Clone
3.2.1. Vine Water Status and Leaf Gas Exchange
3.2.2. Leaf Mineral Nutrition
3.2.3. Vegetative Development and Yield Response
3.2.4. Berry and Must Quality Parameters and Oenological Potential
4. Discussion
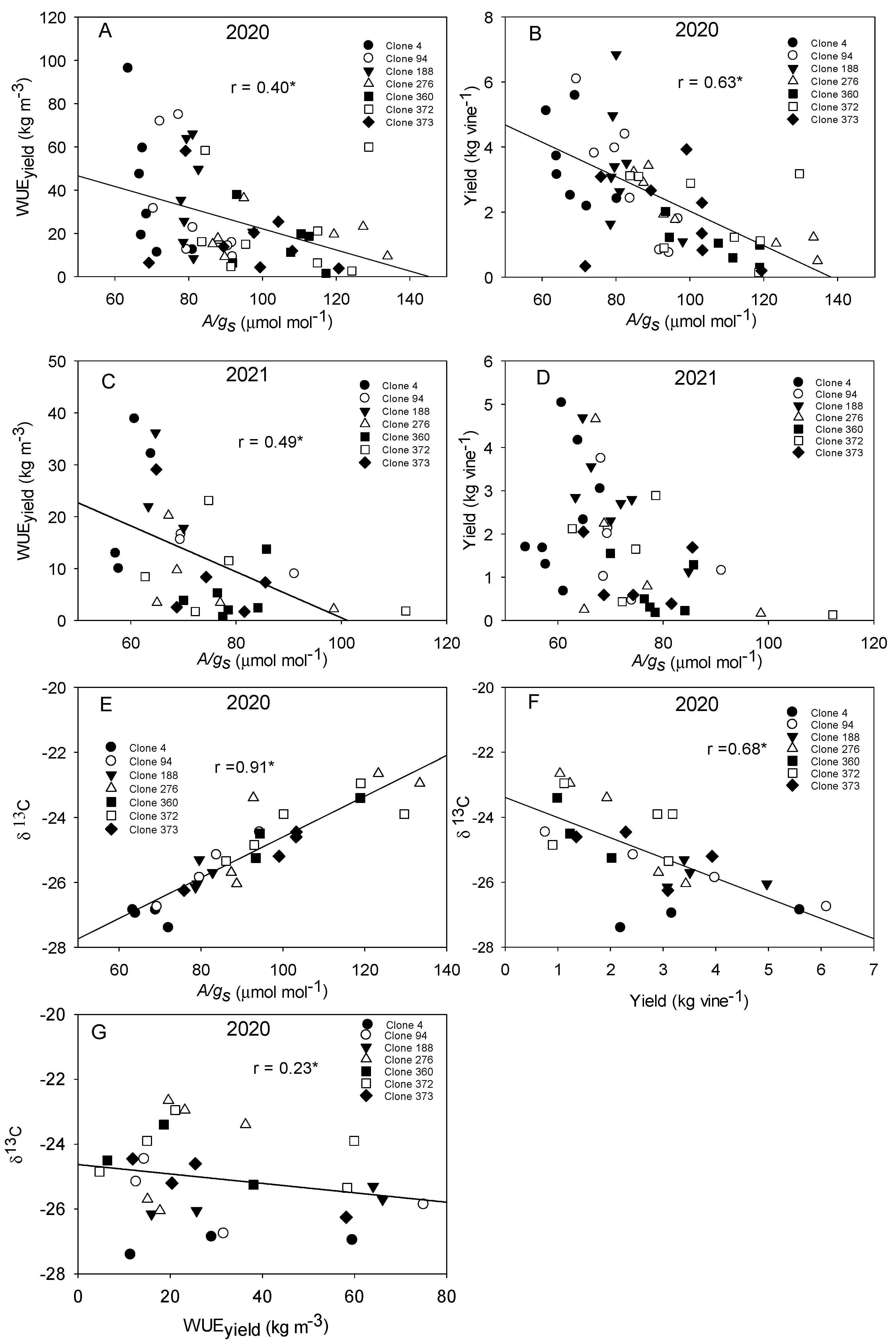
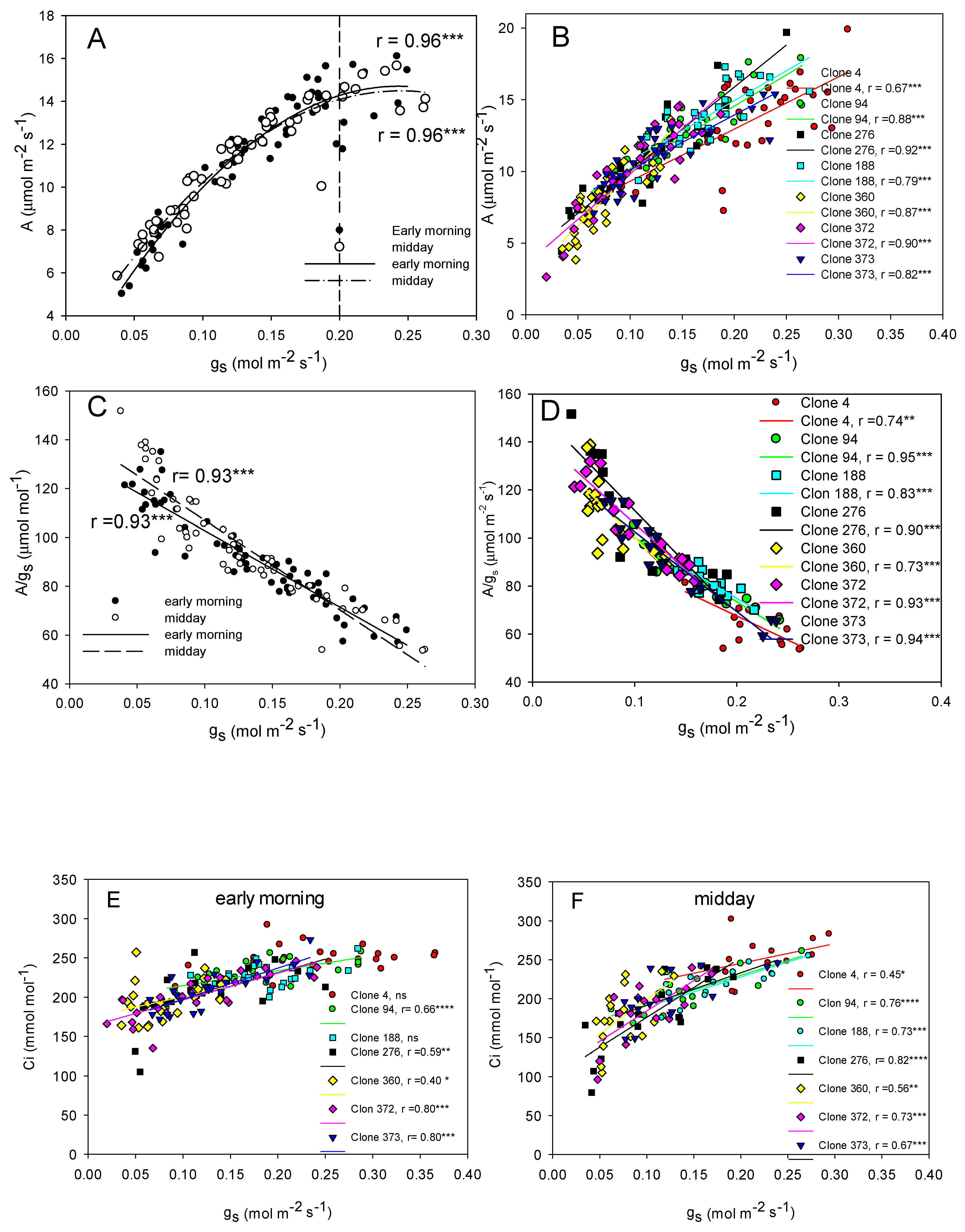
4.1. Traditional Monastrell Clones Show a High Variability in WUE
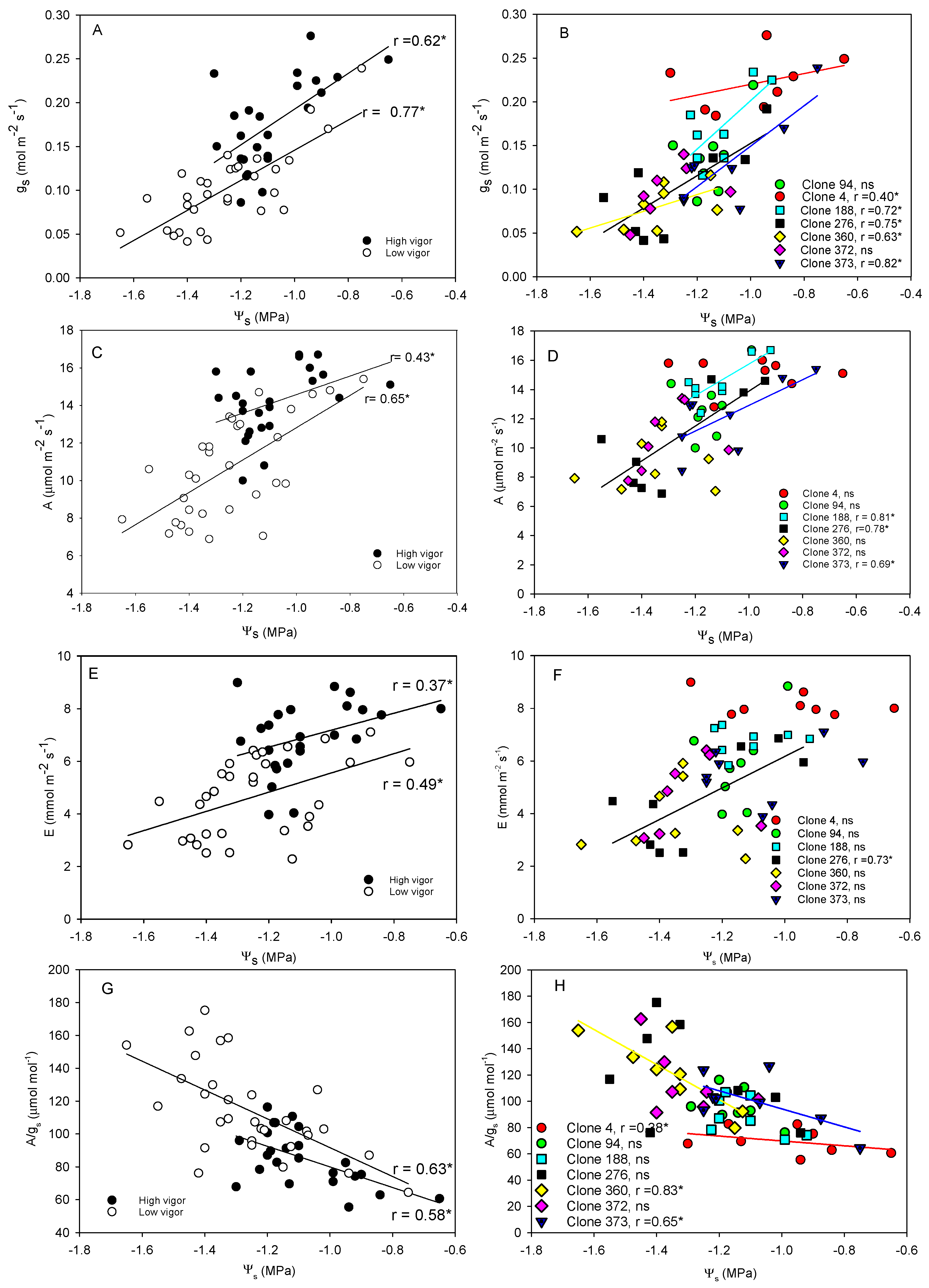
4.2. Clones Show Different Hydraulic Behavior, Stomatal Regulation, and Vine Water Use
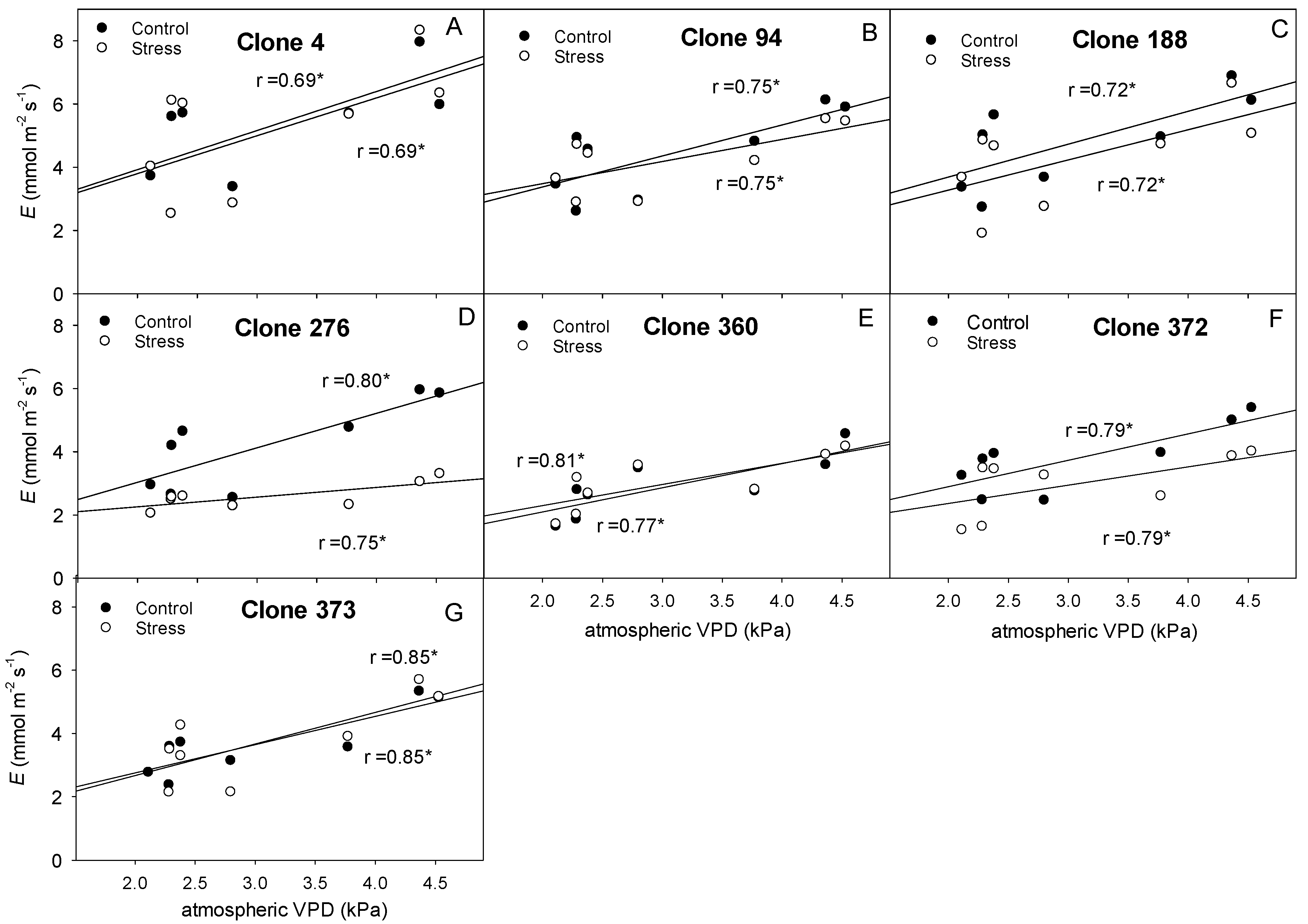
4.3. Clones Differ in Their Photosynthetic Ability and Their Sensibility to Atmospheric VPD and Soil Water Deficit
4.4. Low-Vigor/Productive Clones Enhance Berry and Wine Quality and Aromatic/Nutraceutical Potential Compared to High-Vigor/Productive Clones
4.5. Clone Classification According to Drought Tolerance, Vine Performance, and Oenological Potential
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fraga, H.; García de Cortazar, I.; Malheiro, A.C.; Santos, J.A. Modelling climate change impacts on viticultural yield, phenology and stress conditions in Europe. Glob. Change Biol. 2016, 22, 3774–3788. [Google Scholar] [CrossRef]
- Santillán, D.; Garrote, L.; Iglesias, A.; Sotés, V. Climate change risks and adaptations: New indicators for Mediterranean viticulture. Mitig. Adapt. Strateg. Glob. Change 2020, 25, 881–899. [Google Scholar] [CrossRef]
- Santos, J.A.; Fraga, H.; Malheiro, A.C.; Moutinho-Pereira, J.; Dinis, L.T.; Correia, C.; Moriondo, M.; Leolini, L.; Dibari, C.; Costafreda-Aumedes, S.; et al. A review of the potential climate change impacts and adaptation options for European viticulture. Appl. Sci. 2020, 10, 3092. [Google Scholar] [CrossRef]
- Wolkovich, E.M.; Garcia de Cortazar-Atauri, I.; Morales-Castilla, I.; Nicholas, K.A.; Lacombe, T. From Pinot to Xinomavro in the world’s future wine-growing regions. Nat. Clim. Change 2018, 8, 29–37. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2022 Report. Impacts, Adaptation and Vulnerability. Summary for Policymakers. Working Group II Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; WMO: Geneva, Switzerland; UNEP: Nairobi, Kenya, 2022; p. 35.
- Van Leeuwen, C.; Destrac-Irvine, A.; Dubernet, M.; Duchêne, E.; Gowdy, M.; Marguerit, E.; Pieri, P.; Parker, A.; de Rességuier, L.; Ollat, N. An update on the impact of climate change in viticulture and potential adaptations. Agronomy 2019, 9, 514. [Google Scholar] [CrossRef] [Green Version]
- Iglesias, A.; Garrote, L. Adaptation strategies for agricultural water management under climate change in Europe. Agric. Water Manag. 2015, 155, 113–124. [Google Scholar] [CrossRef] [Green Version]
- Resco, P.; Iglesias, A.; Bardají, I.; Sotés, V. Exploring adaptation choices for grapevine regions in Spain. Reg. Environ. Change 2016, 16, 979–993. [Google Scholar] [CrossRef]
- Dos Santos Triches, W.; Pazzini Eckhardt, D.; Nunes da Silva, E.; Gabbardo, M.; Clasen Chaves, F.; Hoffmann, J.F.; Zandoná, G.P.; Valmor Rombaldi, C. Physico-chemical characterization of wines produced by different rootstock and Vitis vinifera cv. Tannat clones in vineyards of subtropical climate region. Aust. J. Crop Sci. 2020, 14, 1506–1518. [Google Scholar] [CrossRef]
- Hébert-Haché, A.; Inglis, D.; Kemp, B.; Willwerth, J.J. Clone and Rootstock Interactions Influence the Cold Hardiness of Vitis vinifera cvs. Riesling and Sauvignon blanc. Am. J. Enol. Vitic. 2021, 72, 126–136. [Google Scholar] [CrossRef]
- Tortosa, I.; Douthe, C.; Pou, A.; Balda, P.; Hernandez-Montes, E.; Toro, G.; Escalona, J.M.; Medrano, H. Variability in water use efficiency of grapevine Tempranillo clones and stability over years at field conditions. Agronomy 2019, 9, 701. [Google Scholar] [CrossRef] [Green Version]
- Tortosa, I.; Escalona, J.M.; Toro, G.; Douthe, C.; Medrano, H. Clonal behavior in response to soil water availability in Tempranillo grapevine cv.: From plant growth to water use efficiency. Agronomy 2020, 10, 862. [Google Scholar] [CrossRef]
- Buesa, I.; Escalona, J.M.; Tortosa, I.; Marín, D.; Loidi, M.; Santesteban, L.G.; Douthe, C.; Medrano, H. Intracultivar genetic diversity in grapevine: Water use efficiency variability within cv. Grenache. Physiol. Plant. 2021, 173, 2226–2237. [Google Scholar] [CrossRef]
- Mairata, A.; Tortosa, I.; Douthe, C.; Escalona, J.M.; Pou, A.; Medrano, H. Comparing Selection Criteria to Select Grapevine Clones by Water Use Efficiency. Agronomy 2022, 12, 1963. [Google Scholar] [CrossRef]
- Buesa, I.; Hernández-Montes, E.; Tortosa, I.; Baraldi, G.; Rosselló, M.; Medrano, H.; Escalona, J.M. Unraveling the Physiological Mechanisms Underlying the Intracultivar Variability of Water Use Efficiency in Vitis vinifera “Grenache”. Plants 2022, 11, 3008. [Google Scholar] [CrossRef] [PubMed]
- Pagay, V.; Furlan, T.S.; Kidman, C.M.; Nagahatenna, D. Long-term drought adaptation of unirrigated grapevines (Vitis vinifera L.). Theor. Exp. Plant Physiol. 2022, 34, 215–225. [Google Scholar] [CrossRef]
- Pagliarani, C.; Boccacci, P.; Chitarra, W.; Cosentino, E.; Sandri, M.; Perrone, I.; Mori, A.; Cuozzo, D.; Nerva, L.; Rossato, M.; et al. Distinct metabolic signals underlie clone by environment interplay in “Nebbiolo” grapes over ripening. Front. Plant Sci. 2019, 10, 1575. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Plaza, E.; Gil-Muñoz, R.; Carreño-Espín, J.; Fernández-López, J.A.; Martínez-Cutillas, A. Investigation on the aroma of wines from seven clones of Monastrell grapes. Eur. Food Res. Technol. 1999, 209, 257–260. [Google Scholar] [CrossRef]
- Gómez-Plaza, E.; Gil-Muñoz, R.; Martínez-Cutillas, A. Multivariate classification of wines from seven clones of Monastrell grapes. J. Sci. Food Agric. 2000, 80, 497–501. [Google Scholar] [CrossRef]
- Romero, P.; Fernández-Fernández, J.I.; Martínez-Cutillas, A. Physiological thresholds for efficient regulated deficit-irrigation management in wine grapes grown under semiarid conditions. Am. J. Enol. Vitic. 2010, 61, 300–312. [Google Scholar] [CrossRef]
- Esteras, C.; López-Lluch, D.; Derdak, S.; Picó, B.; Ruiz, J.J. Genotyping by Sequencing (GBS) to evaluate the genetic diversity of Monastrell, an old grapevine variety in the province of Alicante (Spain). BIO Web Conf. 2017, 9, 1019. [Google Scholar] [CrossRef]
- Yuste, A.; Martínez-Gil, F.; García-Martínez, S.; Lluch, D.L.; Peiró, R.; Jiménez, C.; Ruiz, J.J.; Gisbert, C. Recovering ancient grapevine cultivars in the Spanish provinces of Alicante and Valencia. Acta Hortic. 2019, 1248, 43–46. [Google Scholar] [CrossRef]
- Tsuda, M.; Tyree, M.T. Plant hydraulic conductance measured by the high pressure flow meter in crops plants. J. Exp. Bot. 2000, 51, 823–828. [Google Scholar] [CrossRef] [PubMed]
- del Amor, F.M.; Cuadra-Crespo, P. Alleviation of salinity stress in broccoli using foliar urea or methyl-jasmonate: Analysis of growth, gas exchange, and isotope composition. Plant Growth Regul. 2011, 63, 55–62. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Ehleringer, J.R.; Hubick, K.T. Carbon isotope discrimination and photosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989, 40, 503–537. [Google Scholar] [CrossRef]
- Romero, P.; Botía, P.; Navarro, J.M. Selecting rootstocks to improve vine performance and vineyard sustainability in deficit irrigated Monastrell grapevines under semiarid conditions. Agric. Water Manag. 2018, 209, 73–93. [Google Scholar] [CrossRef]
- Larcher, W. Physiological plant ecology. In Ecophysiology and Stress Physiology of Functional Groups, 4th ed.; Springer: Berlin/Heidelberg, Germany, 2003; p. 511. [Google Scholar]
- Gump, B.H.; Zoecklein, B.W.; Fugelsang, K.C. Food Microbiology Protocols; Spencer, J.F.T., Ragout de Spencer, A.L., Eds.; Methods in Biotechnology; Humana Press: Totowa, NJ, USA, 2001; Volume 14. [Google Scholar]
- Saint-Cricq, N.; Vivas, N.; Glories, Y. Maturité phénolique: Définition et contrôle. Rev. Franç. d’Oenolog. 1998, 173, 22–25. [Google Scholar]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdied, D. Handbook of Enology Volume 2: The Chemistry of Wine and Stabilization and Treatments; John and Wiley and Sons: Chichester, UK, 2006. [Google Scholar]
- Romero, P.; Fernández-Fernández, J.I.; Botía Ordaz, P. Interannual climatic variability effects on yield, berry and wine quality indices in long-term deficit irrigated grapevines, determined by multivariate analysis. Int. J. Wine Res. 2016, 8, 3–17. [Google Scholar] [CrossRef] [Green Version]
- Glories, Y. La couleur des vins rouges. 2eme partie. Mesure, origine et interpretation. Connaiss. Vigne Vin 1984, 18, 253–271. [Google Scholar] [CrossRef]
- Cayla, L.; Cottereau, P.; Renard, R. Estimation de la maturite phenolique des raisins rouges par la methode I.T.V. standard. Rev. Française D’oenologie 2002, 193, 84–87. [Google Scholar]
- Boulton, R.B. The Copigmentation Assay for Red Wines. Department of Viticulture & Enology, University of California, Davis. 2013, p. 3. Available online: http://boulton.ucdavis.edu/copig.htm.
- Gómez-Alonso, S.; García-Romero, E.; Hermosín-Gutiérrez, I. HPLC analysis of diverse grape and wine phenolics using direct injection and multidetection by DAD and fluorescence. J. Food Compos. Anal. 2007, 20, 618–626. [Google Scholar] [CrossRef]
- Ribeiro de Lima, M.T.; Waffo-Teguo, P.; Teissedre, P.L.; Pujolas, A.; Vercauteren, J.; Cabanis, J.C.; Merillon, J.M. Determination of stilbenes (trans-astringin, cis and trans-piceid, and cis- and trans-resveratrol) in Portuguese wines. J. Agric. Food Chem. 1999, 47, 2666–2670. [Google Scholar] [CrossRef] [PubMed]
- Sarneckis, C.J.; Dambergs, R.G.; Jones, P.; Mercurio, M.; Herdewrich, M.J.; Smith, P.A. Quantification of condensed tannins by precipitation with methyl cellulose: Development and validation of an optimised tool for grape and wine analysis. Aust. J. Grape Wine Res. 2006, 12, 39–49. [Google Scholar] [CrossRef]
- Romero, P.; Gil Muñoz, R.; Fernández-Fernández, J.; del Amor, F.M.; Martínez-Cutillas, A.; García-García, J. Improvement of yield and grape and wine composition in field-grown Monastrell grapevines by partial root zone irrigation, in comparison with regulated deficit irrigation. Agric. Water Manag. 2015, 149, 55–73. [Google Scholar] [CrossRef]
- Gómez-Plaza, E.; Mestre-Ortuño, L.; Ruiz-García, Y.; Fernández-Fernández, J.I.; López-Roca, J.M. Effect of benzothiadiazole and methyl jasmonate on the volatile compound composition of Vitis vinifera L. Monastrell grapes and wines. Am. J. Enol. Vitic. 2012, 63, 394–401. [Google Scholar] [CrossRef]
- Canuti, V.; Puccioni, S.; Storchi, P.; Zanoni, B.; Picchi, M.; Bertuccioli, M. Enological eligibility of grape clones based on the Simca method: The case of the Sangiovese cultivar from Tuscany. Ital. J. Food Sci. 2018, 30, 184–199. [Google Scholar] [CrossRef]
- Portu, J.; Baroja Hernández, E.; Martínez Garcia, J.; Rivacoba, L.; García-Escudero, E. Preliminary evaluation of agronomic and enological properties of preselected grapevine clones of ‘Tempranillo’ and ‘Graciano’ in DOCa Rioja (Spain). In Proceedings of the 8th International Macrowine Conference on Macromolecules and Secondary Metabolites of Grapevine and Wine, Virtual, 23–30 June 2021. [Google Scholar]
- Chitarra, W.; Perrone, I.; Avanzato, C.G.; Minio, A.; Boccacci, P.; Santini, D.; Gilardi, G.; Siciliano, I.; Gullino, M.L.; Delledonne, M.; et al. Grapevine Grafting: Scion Transcript Profiling and Defense-Related Metabolites Induced by Rootstocks. Front. Plant Sci. 2017, 8, 654. [Google Scholar] [CrossRef]
- Marín, D.; Armengol, J.; Carbonell-Bejerano, P.; Escalona, J.M.; Gramaje, D.; Hernández-Montes, E.; Intrigliolo, D.S.; Martínez-Zapater, J.M.; Medrano, H.; Mirás-Avalos, J.M.; et al. Challenges of viticulture adaptation to global change: Tackling the issue from the roots. Aust. J. Grape Wine Res. 2021, 27, 8–25. [Google Scholar] [CrossRef]
- Cifre, J.; Bota, J.; Escalona, J.M.; Medrano, H.; Flexas, J. Physiological tools for irrigation scheduling in grapevine (Vitis vinifera L.): An open gate to improve water-use efficiency? Agric. Ecosyst. Environ. 2005, 106, 159–170. [Google Scholar] [CrossRef]
- van Leeuwen, C.; Trégoat, O.; Choné, X.; Bois, B.; Pernet, D.; Gaudillére, J.-P. Vine water status is a key factor in grape ripening and vintage quality for red Bordeaux wine. How can it be assessed for vineyard management purposes? J. Int. Sci. Vigne Vin 2009, 43, 121–134. [Google Scholar] [CrossRef]
- Romero, P.; Navarro, J.M.; Botía Ordaz, P. Towards a sustainable viticulture: The combination of deficit irrigation strategies and agroecological practices in Mediterranean vineyards. A review and update. Agric. Water Manag. 2022, 259, 107216. [Google Scholar] [CrossRef]
- Serrano, A.S.; Martínez-Gascueña, J.; Alonso, G.L.; Cebrián-Tarancón, C.; Carmona, M.D.; Mena, A.; Chacón-Vozmediano, J.L. Agronomic Response of 13 Spanish Red Grapevine (Vitis vinifera L.) Cultivars under Drought Conditions in a Semi-Arid Mediterranean Climate. Agronomy 2022, 12, 2399. [Google Scholar] [CrossRef]
- Flexas, J.; Galmés, J.; Gallé, A.; Gulías, J.; Pou, A.; Ribas-Carbo, M.; Tomás, M.; Medrano, H. Improving water use efficiency in grapevines: Potential physiological targets for biotechnological improvement. Aust. J. Grape Wine Res. 2010, 16, 106–121. [Google Scholar] [CrossRef]
- Medrano, H.; Tomás, M.; Martorell, S.; Flexas, J.; Hernández, E.; Rosselló, J.; Pou, A.; Escalona, J.-M.; Bota, J. From leaf to whole plant water use efficiency (WUE) in complex canopies: Limitations of leaf WUE as a selection target. Crop J. 2015, 3, 220–228. [Google Scholar] [CrossRef] [Green Version]
- Romero, P.; Fernández-Fernández, J.I.; Gil-Muñoz, R.; Botía, P. Vigour-yield-quality relationships in long-term deficit irrigated winegrapes grown under semiarid conditions. Theor. Exp. Plant Physiol. 2016, 28, 23–51. [Google Scholar] [CrossRef]
- Padgett-Johnson, M.; Williams, L.E.; Walker, M.A. Vine water relations, gas exchange, and vegetative growth of seventeen Vitis species grown under irrigated and non irrigated conditions in California. J. Am. Soc. Hortic. Sci. 2003, 128, 269–276. [Google Scholar] [CrossRef] [Green Version]
- Bota, J.; Tomás, M.; Flexas, J.; Medrano, H.; Escalona, J.M. Differences among grapevine cultivars in their stomatal behavior andwater use efficiency under progressive water stress. Agric. Water Manag. 2016, 164, 91–99. [Google Scholar] [CrossRef]
- Simonneau, T.; Lebon, E.; Coupel-Ledru, A.; Marguerit, E.; Rossdeutsch, L.; Ollat, N. Adapting plant material to face water stress in vineyards: Which physiological targets for an optimal control of plant water status? OENO One 2017, 51, 167–179. [Google Scholar] [CrossRef]
- Gambetta, G.A.; Herrera, J.C.; Silvina, D.; Feng, S.; Hochberg, Q.; Castellarin, S. The physiology of drought stress in grapevine: Towards an integrative definition of drought tolerance. J. Exp. Bot. 2020, 71, 4658–4676. [Google Scholar] [CrossRef] [PubMed]
- Bandurska, H. Drought Stress Responses: Coping Strategy and Resistance. Plants 2022, 11, 922. [Google Scholar] [CrossRef]
- Bianchi, D.; Caramanico, L.; Grossi, D.; Brancadoro, L.; Lorenzis, G.D. How Do novel M-Rootstock (Vitis spp.) genotypes cope with drought? Plants 2020, 9, 1385. [Google Scholar] [CrossRef]
- Tardieu, F.; Simonneau, T. Variability among species of stomatal control under fluctuating soil water status and evaporative demand: Modelling isohydric and anisohydric behaviours. J. Exp. Bot. 1998, 49, 419–432. [Google Scholar] [CrossRef] [Green Version]
- Lavoie-Lamoureux, A.; Sacco, D.; Risse, P.-A.; Lovisolo, C. Factors influencing stomatal conductance in response to water availability in grapevine: A meta-analysis. Physiol. Plant. 2017, 159, 468–482. [Google Scholar] [CrossRef] [PubMed]
- Schultz, H.R. Differences in hydraulic architecture account for near-isohydric and anisohydric behaviour of two field-grown Vitis vinifera L. cultivars during drought. Plant Cell Environ. 2003, 26, 1393–1405. [Google Scholar] [CrossRef]
- Dayer, S.; Herrera, J.C.; Dai, Z.; Burlett, R.; Lamarque, L.J.; Delzon, S.; Bortolami, G.; Cochard, H.; Gambetta, G.A. The sequence and thresholds of leaf hydraulic traits underlying grapevine varietal differences in drought tolerance. J. Exp. Bot. 2020, 71, 4333–4344. [Google Scholar] [CrossRef] [Green Version]
- Villalobos-Soublett, E.; Verdugo-Vásquez, N.; Díaz, I.; Zurita-Silva, A. Adapting Grapevine Productivity and Fitness to Water Deficit by Means of Naturalized Rootstocks. Front. Plant Sci. 2022, 13, 870438. [Google Scholar] [CrossRef]
- Hochberg, U.; Rockwell, F.E.; Holbrook, N.M.; Cochard, H. Iso/Anisohydry: A plant-environment interaction rather than a simple hydraulic trait. Trends Plant Sci. 2018, 23, 112–120. [Google Scholar] [CrossRef]
- Christmann, A.; Grill, E.; Huang, J. Hydraulic signals in long-distance signaling. Curr. Opin. Plant Biol. 2013, 16, 293–300. [Google Scholar] [CrossRef]
- Levin, A.D.; Williams, L.E.; Matthews, M.A. A continuum of stomatal responses to water deficits among 17 wine grape cultivars (Vitis vinifera). Funct. Plant Biol. 2019, 47, 11–25. [Google Scholar] [CrossRef]
- Cochetel, N.; Ghan, R.; Toups, H.S.; Degu, A.; Tillett, R.L.; Schlauch, K.A.; Cramer, G.R. Drought tolerance of the grapevine, Vitis champinii cv. Ramsey, is associatedwith higher photosynthesis and greater transcriptomic responsiveness of abscisic acid biosynthesis and signaling. BMC Plant Biol. 2020, 20, 55. [Google Scholar] [CrossRef] [Green Version]
- Prinsi, B.; Simeoni, F.; Galbiati, M.; Meggio, F.; Tonelli, C.; Scienza, A.; Espen, L. Grapevine Rootstocks Differently affect Physiological and Molecular Responses of the Scion under Water Deficit Condition. Agronomy 2021, 11, 289. [Google Scholar] [CrossRef]
- Flexas, J.; Medrano, H. Drought-inhibition of photosynthesis in C3 plants: Stomatal and non-stomatal limitations revisited. Ann. Bot. 2002, 89, 183–189. [Google Scholar] [CrossRef] [Green Version]
- Gago, J.; Douthe, C.; Florez-Sarasa, I.; Escalona, J.M.; Galmes, J.; Fernie, A.R.; Flexas, J.; Medrano, H. Opportunities for improving leaf water use eficiency under climate change conditions. Plant Sci. 2014, 226, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Zufferey, V.; Verdenal, T.; Dienes, A.; Belcher, S.; Lorenzini, F.; Koestel, C.; Blackford, M.; Bourdin, G.; Gindro, K.; Spangenberg, J.E.; et al. The influence of vine water regime on the leaf gas exchange, berry composition and wine quality of Arvine grapes in Switzerland. OENO One 2020, 54, 553–568. [Google Scholar] [CrossRef]
- Davenport, J.R.; Horneck, D.A. Sampling Guide for Nutrient Assessment of Irrigated Vineyards in the Inland Pacific Northwest; Washington State University: Pullman, WA, USA, 2011; p. 6. [Google Scholar]
- Edwards, E.J.; Betts, A.; Clingeleffer, P.R.; Walker, R.R. Rootstock-conferred traits affect the water use efficiency of fruit production in Shiraz. Aust. J. Grape Wine Res. 2022, 28, 316–327. [Google Scholar] [CrossRef]
- Pou, A.; Flexas, J.; del Mar Alsina, M.; Bota, J.; Carambula, C.; de Herralde, F.; Galmés, J.; Lovisolo, C.; Jimenez, M.; Ribas-Carbó, M.; et al. Adjustments of water use efficiency by stomatal regulation during drought and recovery in the drought-adapted Vitis hybrid Richter-110 (V. berlandieri × V. rupestris). Physiol. Plant. 2008, 134, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Romero, P.; Botía, P.; Keller, M. Hydraulics and gas exchange recover more rapidly from severe drought stress in small pot-grown grapevines than in field-grown plants. J. Plant Physiol. 2017, 216, 58–73. [Google Scholar] [CrossRef] [PubMed]
- Cortell, J.M.; Sivertsen, H.K.; Kennedy, J.A.; Heymann, H. Influence of vine vigor on Pinot noir fruit composition, wine chemical analysis and wine sensory attributes. Am. J. Enol. Vitic. 2008, 59, 1–10. [Google Scholar] [CrossRef]
- Ginestar, C.; Eastham, J.; Gray, S.; Iland, P. Use of sap-flow sensors to schedule vineyard irrigation. II. Effects of post-veraison water deficits on composition of Shiraz grapes. Am. J. Enol. Vitic. 1998, 49, 421–428. [Google Scholar] [CrossRef]
- Genebra, T.; Santos, R.R.; Francisco, R.; Pinto-Marijuan, M.; Brossa, R.; Serra, A.T.; Duarte, C.M.M.; Chaves, M.M.; Zarrouk, O. Proanthocyanidin accumulation and biosynthesis are modulated by the irrigation regime in Tempranillo seeds. Int. J. Mol. Sci. 2014, 15, 11862–11877. [Google Scholar] [CrossRef]
- Intrigliolo, D.S.; Lizama, V.; García-Esparza, M.J.; Abrisqueta, I.; Álvarez, I. Effects of post-veraison irrigation regime on Cabernet Sauvignon grapevines in Valencia, Spain: Yield and grape composition. Agric. Water Manag. 2016, 170, 110–119. [Google Scholar] [CrossRef]
- Cáceres-Mella, A.; Talaverano, M.I.; Villalobos-González, L.; Ribalta-Pizarro, C.; Pastenes, C. Controlled water deficit during ripening affects proanthocyanidin synthesis, concentration and composition in Cabernet Sauvignon grape skins. Plant Physiol. Biochem. 2017, 117, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Lizama, V.; Pérez-Álvarez, E.P.; Intrigliolo, D.S.; Chirivella, C.; Álvarez, I.; García- Esparza, M.J. Effects of the irrigation regimes on grapevine cv. Bobal in a Mediterranean climate: II. Wine, skins, seeds, and grape aromatic composition. Agric. Water Manag. 2021, 256, 107078. [Google Scholar] [CrossRef]
- Martins, V.; Cunha, A.; Gerós, H.; Hanana, M.; Blumwald, E. Mineral compounds in the grape berry. In The Biochemistry of the Grape Berry; Geros, H., Chaves, M., Delrot, S., Eds.; Bentham Science Publishers Ltd.: Sharjah, United Arab Emirates, 2012; pp. 23–43. [Google Scholar]
- De Nisco, M.; Manfra, M.; Bolognese, A.; Sofo, A.; Scopa, A.; Tenore, G.C.; Pagano, F.; Milite, C.; Russo, M.T. Nutraceutical properties and polyphenolic profile of berry skin and wine of Vitis vinifera L. (cv. Aglianico). Food Chem. 2013, 140, 623–629. [Google Scholar] [CrossRef]
- Ouyang, X.; Yuan, G.; Ren, J.; Wang, L.; Wang, M.; Li, Y.; Zhang, B.; Zhu, B. Aromatic compounds and organoleptic features of fermented wolfberry wine: Effects of maceration time. Int. J. Food Prop. 2017, 20, 2234–2248. [Google Scholar] [CrossRef] [Green Version]
- Vilanova, M.; Campo, E.; Escudero, A.; Graña, M.; Masa, A.; Cacho, J. Volatile composition and sensory properties of Vitis vinifera red cultivars from North West Spain: Correlation between sensory and instrumental analysis. Anal. Chim. Acta 2012, 720, 104–111. [Google Scholar] [CrossRef]
- Cortés-Diéguez, S.; Rodriguez-Solana, R.; Domínguez, J.M.; Díaz, E. Impact odorants and sensory profile of young red wines from four Galician (NW of Spain) traditional cultivars. J. Inst. Brew. 2015, 121, 628–635. [Google Scholar] [CrossRef]
- Szikszai, A.R.; Issa Issa, H.; Cano-Lamadrid, M.; Carbonell-Barrachina, A.A. Characteristic volatile compounds of Monastrell wines. Rev. Dr. UMH 2018, 4, 3. [Google Scholar] [CrossRef]
- Hopfer, H.; Nelson, J.; Ebeler, S.E.; Heymann, H. Correlating wine quality indicators to chemical and sensory measurements. Molecules 2015, 20, 8453–8483. [Google Scholar] [CrossRef] [Green Version]
- Welke, J.E.; Zanus, M.; lazzarotto, M.; Alcaraz Zini, C. Quantitative analysis of headspace volatile compounds using comprehensive two-dimensional gas chromatography and their contribution to the aroma of Chardonnay wine. Food Res. Int. 2014, 59, 85–99. [Google Scholar] [CrossRef] [Green Version]
- Roba, K. The Role of Terpene (Secondary Metabolite). Nat. Prod. Chem. Res. 2021, 9, 411. [Google Scholar]
- Varshney, R.K.; Tuberosa, R.; Tardieu, F. Progress in understanding drought tolerance: From alleles to cropping systems. J. Exp. Bot. 2018, 69, 3175–3179. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.-G.; Hasanuzzaman, M.; Jajoo, A.; Lawson, T.; Lin, R.; Liu, C.-M.; Liu, L.-N.; Liu, Z.; Lu, C.; Moustakas, M.; et al. Improving photosynthesis through multidisciplinary efforts: The next frontier of photosynthesis research. Front. Plant Sci. 2022, 13, 967203. [Google Scholar] [CrossRef] [PubMed]
- Passioura, J.B. Drought and drought tolerance. In Drought Tolerance in Higher Plants: Genetical, Physiological and Molecular Biological Analysis; Belhassen, E., Ed.; Springer: Dordrecht, The Netherlands, 1996; pp. 1–5. [Google Scholar]





| Phenological Period | Period of the Year | Eto (mm) | VPD (kPa) | Rain-Fall (mm) | Tamax (°C) | Tamed (°C) | Tamin (°C) | Solar Rad. (W m−2) |
|---|---|---|---|---|---|---|---|---|
| Year 2018 | ||||||||
| Budburst-fruit set | 15 April–14 June | 265 | 0.98 | 78 | 23.7 | 16.7 | 8.8 | 268 |
| Fruit set-veraison | 15 June–31 July | 291 | 2.00 | 0.1 | 32.8 | 24.6 | 14.4 | 328 |
| Veraison-harvest | 1 August–20 September | 219 | 1.39 | 57 | 31.0 | 23.76 | 16.43 | 229 |
| Postharvest | 21 September–31 October | 97 | 0.77 | 26.8 | 23.8 | 16.5 | 9.93 | 165 |
| Dormancy period | 1 November–14 April | 289 | 0.52 | 126.3 | 17.03 | 8.3 | 0.60 | 147 |
| Total/annual average | 1161 | 1.13 | 288.2 | 25.67 | 19.97 | 10.03 | 227 | |
| Year 2019 | ||||||||
| Budburst-fruit set | 15 April–14 June | 280 | 1.06 | 119 | 24.2 | 16.9 | 8.6 | 280 |
| Fruit set-veraison | 15 June–31 July | 280 | 2.04 | 1.4 | 32.9 | 24.5 | 14.2 | 313 |
| Veraison-harvest | 1 August–20 September | 219 | 1.43 | 170 | 30.5 | 23.3 | 15.7 | 234 |
| Postharvest | 21 September–31 October | 95 | 0.79 | 39 | 25.0 | 16.9 | 9.3 | 180 |
| Dormancy period | 1 November–14 April | 261 | 0.45 | 289 | 16.7 | 9.34 | 2.75 | 133 |
| Total/annual average | 1135 | 1.15 | 618 | 25.9 | 18.2 | 10.10 | 228 | |
| Year 2020 | ||||||||
| Budburst-fruit set | 15 April–14 June | 255 | 1.06 | 27 | 25.1 | 17.7 | 9.3 | 264 |
| Fruit set-veraison | 15 June–31 July | 266 | 1.92 | 6 | 33.2 | 24.6 | 14.4 | 316 |
| Veraison-harvest | 1 August–20 September | 231 | 1.71 | 6 | 32.2 | 23.5 | 13.8 | 258 |
| Postharvest | 21 September–31 October | 99 | 0.99 | 9 | 24.5 | 15.2 | 5.8 | 181 |
| Dormancy period | 1 November–14 April | 261 | 0.45 | 233 | 16.6 | 9.0 | 2.2 | 135 |
| Total/annual average | 1112 | 1.23 | 281 | 26.3 | 18.0 | 9.10 | 231 | |
| Year 2021 | ||||||||
| Budburst-fruit set | 15 April–14 June | 253 | 0.94 | 113 | 24.3 | 17.1 | 9.2 | 259 |
| Fruit set-veraison | 15 June–31 July | 247 | 1.75 | 53 | 32.1 | 23.8 | 13.8 | 306 |
| Veraison-harvest | 1 August–20 September | 206 | 1.63 | 3 | 32.3 | 24.1 | 15.5 | 234 |
| Postharvest | 21 September–31 October | 94 | 0.79 | 16 | 24.8 | 17.2 | 10.05 | 162 |
| Dormancy period | 1 November–21 March | 173 | 0.48 | 201 | 16.2 | 8.06 | 0.80 | 120 |
| Total/annual average | 973 | 1.12 | 386 | 25.94 | 18.05 | 9.87 | 216 |
| Period 2018–2020 | Year 2021 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clone | All Clones (avg.) | 4 | 94 | 188 | 276 | 360 | 372 | 373 | ||||||||
| Treatment | C | S | C | S | C | S | C | S | C | S | C | S | C | S | C | S |
| Budburst-fruit set | 25.9 | 23.3 | 11.7 | 0 | 11.7 | 0 | 11.7 | 0 | 20.9 | 24.3 | 36.2 | 31.3 | 22.8 | 23.8 | 20.9 | 23.5 |
| Fruit set-veraison | 27.3 | 0 | 15.2 | 0 | 15.1 | 0 | 15.2 | 0 | 27.0 | 0 | 46.9 | 0 | 29.5 | 0 | 27.0 | 0 |
| Veraison-harvest | 20.6 | 0 | 12.2 | 0 | 12.1 | 0 | 12.2 | 0 | 21.7 | 0 | 37.7 | 0 | 23.7 | 0 | 21.7 | 0 |
| Post-harvest | 10.5 | 0 | 4.1 | 0 | 4.1 | 0 | 4.1 | 0 | 7.3 | 0 | 12.6 | 0 | 7.9 | 0 | 7.3 | 0 |
| Total (mm) | 84.3 | 23.3 | 43.2 | 0 | 42.9 | 0 | 43.2 | 0 | 76.8 | 24.3 | 133.4 | 31.3 | 83.8 | 23.8 | 76.8 | 23.5 |
| Year 2020 | |||||||||||||||||||
| Pre-veraison period (June-July) | Post-veraison period (August-September) | (2019) | (2020) | (2020) | |||||||||||||||
| Clone | Ψs | A | gs | Ci | E | A/gs | A/E | Ψl | Ψs | ΔΨ | A | gs | Ci | E | A/gs | A/E | δC13 (berry) | δC13 (berry) | NUEph |
| 4 | −0.92c | 17.1b | 0.185b | 182b | 4.35 | 105a | 5.04 | −1.41 | −0.99d | 0.50b | 14.84c | 0.236d | 249c | 5.14d | 65a | 2.94a | −27.1a | −27.0a | 10.51c |
| 94 | −0.99bc | 17.9b | 0.181b | 179b | 4.22 | 106a | 5.06 | −1.55 | −1.15bcd | 0.36a | 13.62c | 0.181c | 230b | 4.28c | 78b | 3.31b | −26.4ab | −25.9b | 9.18bc |
| 188 | −1.01b | 16.8ab | 0.158ab | 165ab | 3.79 | 116ab | 5.37 | −1.62 | −1.11cd | 0.53b | 14.33c | 0.185c | 228b | 4.41c | 79b | 3.31b | −25.9b | −25.7b | 9.02bc |
| 276 | −1.05b | 16.7ab | 0.148ab | 157ab | 3.58 | 121ab | 5.29 | −1.58 | −1.28abc | 0.29a | 11.04b | 0.121b | 204a | 3.18ab | 98cd | 3.62bc | −25.7bc | −24.1c | 8.21ab |
| 360 | −1.18a | 14.2a | 0.116a | 137a | 3.11 | 137b | 5.49 | −1.57 | −1.35a | 0.29a | 8.84a | 0.087a | 197a | 2.50a | 105d | 3.67c | −24.7d | −24.7c | 6.45a |
| 372 | −1.07b | 15.6ab | 0.129a | 143a | 3.38 | 132b | 5.54 | −1.59 | −1.30ab | 0.28a | 10.50ab | 0.117b | 202a | 3.12ab | 100cd | 3.61bc | −24.9cd | −24.2c | 7.87ab |
| 373 | −1.03b | 15.9ab | 0.143ab | 160ab | 3.70 | 120ab | 5.16 | −1.41 | −1.08d | 0.31a | 11.41b | 0.129b | 209a | 3.44b | 94c | 3.54bc | −25.8bc | −25.0bc | 7.84ab |
| Treatment | |||||||||||||||||||
| Control | −1.02 | 16.92 | 0.162 | 168 | 3.95 | 114 | 5.16 | −1.52 | −1.16 | 0.37 | 12.73 | 0.159 | 219 | 3.90 | 87 | 3.43 | −25.9 | −25.5 | 8.72 |
| Stress | −1.05 | 15.68 | 0.141 | 153 | 3.51 | 125 | 5.40 | −1.55 | −1.20 | 0.37 | 11.43 | 0.143 | 215 | 3.55 | 90 | 3.42 | −25.7 | −24.9 | 8.16 |
| ANOVA | |||||||||||||||||||
| Clone | **** | * | *** | *** | ns | ** | ns | ns | **** | **** | **** | **** | **** | **** | **** | **** | **** | **** | **** |
| Treatment | ns | * | * | ** | ns | ** | ns | ns | ns | ns | *** | * | ns | * | ns | ns | ns | ** | ns |
| Interaction | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | *** | ** | ** | ** | *** | ns | ns | ns | ns |
| Year 2021 | |||||||||||||||||||
| Pre-veraison period (June-July) | Post-veraison period (August-September) | ||||||||||||||||||
| Clone | Ψs (June) | Ψs (July) | A | gs | Ci | E | A/gs | A/E | Ψs | A | gs | Ci | E | A/gs | A/E | ||||
| 4 | −0.59a | −0.98 | 20.2b | 0.33c | 241b | 7.83c | 61a | 2.61 | −0.96b | 14.24d | 0.142c | 190 | 3.47c | 103a | 4.16 | ||||
| 94 | −0.67ab | −1.00 | 18.3ab | 0.25ab | 224a | 6.69b | 74b | 2.80 | −1.05ab | 10.45bc | 0.090b | 157 | 2.43b | 129b | 4.62 | ||||
| 188 | −0.71b | −1.03 | 18.7ab | 0.27b | 230ab | 6.95bc | 69ab | 2.73 | −1.01ab | 11.20c | 0.096b | 170 | 2.61b | 120ab | 4.40 | ||||
| 276 | −0.74b | −1.11 | 17.0a | 0.23ab | 219a | 6.06ab | 79b | 2.85 | −1.10a | 7.04a | 0.055a | 143 | 1.59a | 142b | 4.78 | ||||
| 360 | −0.76b | −0.99 | 16.3a | 0.21a | 219a | 5.59a | 80b | 3.06 | −0.98b | 8.56ab | 0.081ab | 175 | 2.19ab | 120ab | 4.25 | ||||
| 372 | −0.72b | −1.06 | 17.1a | 0.23ab | 217a | 5.98ab | 80b | 2.91 | −1.03ab | 9.26abc | 0.075ab | 153 | 2.08ab | 133b | 4.57 | ||||
| 373 | −0.69b | −1.04 | 18.0ab | 0.25ab | 224a | 6.29ab | 75b | 2.91 | −1.00b | 9.63bc | 0.081ab | 168 | 2.21ab | 123ab | 4.47 | ||||
| Treatment | |||||||||||||||||||
| Control | −0.68 | −0.94 | 18.1 | 0.26 | 227 | 6.65 | 72 | 2.80 | −0.96 | 10.22 | 0.092 | 171 | 2.45 | 121 | 4.36 | ||||
| Stress | −0.72 | −1.12 | 17.8 | 0.25 | 222 | 6.32 | 76 | 2.88 | −1.08 | 9.89 | 0.085 | 159 | 2.29 | 128 | 4.56 | ||||
| ANOVA | |||||||||||||||||||
| Clone | ** | ns | ** | **** | ** | **** | *** | ns | ** | **** | **** | ns | **** | ** | ns | ||||
| Treatment | ns | **** | ns | ns | ns | ns | ns | ns | **** | ns | ns | ns | ns | ns | ns | ||||
| Interaction | ns | ns | ns | ns | ** | ns | * | ns | *** | ** | ns | ns | ns | ns | ns | ||||
| 3-Year Period (2018–2020) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Clone | N | P | K | Ca | Mg | B | Fe | Cu | Mn | Zn | |
| 4 | 2.31 | 0.107 | 0.62a | 2.03 | 0.31b | 56.0c | 71.1a | 11.4a | 103.9ab | 21.3 | |
| 94 | 2.30 | 0.111 | 0.65a | 2.06 | 0.34b | 44.8b | 82.9ab | 8.9a | 94.5abc | 20.7 | |
| 188 | 2.35 | 0.112 | 0.63a | 2.12 | 0.34b | 41.8ab | 87.1ab | 8.5a | 86.2a | 20.8 | |
| 276 | 2.14 | 0.114 | 0.73b | 1.93 | 0.28a | 44.1ab | 98.9b | 8.5a | 100.6abc | 23.3 | |
| 360 | 2.19 | 0.112 | 0.60a | 2.09 | 0.38c | 51.9c | 79.2ab | 19.4b | 111.5bcd | 24.9 | |
| 372 | 2.19 | 0.107 | 0.62a | 1.99 | 0.33b | 37.1a | 73.9a | 10.8a | 126.6d | 24.5 | |
| 373 | 2.18 | 0.106 | 0.63a | 1.97 | 0.32b | 39.9ab | 69.2a | 8.0a | 117.0cd | 21.1 | |
| Treat. | |||||||||||
| Control | 2.26 | 0.111 | 0.64 | 2.03 | 0.33 | 43.2 | 81.5 | 10.9 | 103.5 | 22.0 | |
| Stress | 2.21 | 0.109 | 0.63 | 2.02 | 0.33 | 47.0 | 79.1 | 10.6 | 108.0 | 22.7 | |
| Clone × Treat. | |||||||||||
| 4 | Control | 2.30b | 0.106 | 0.61abc | 1.86 | 0.30b | 48.3 | 64.8 | 11.4 | 102.8 | 20.1 |
| Stress | 2.31b | 0.108 | 0.63abcd | 2.19 | 0.32bc | 63.7 | 77.4 | 11.3 | 105.1 | 22.5 | |
| 94 | Control | 2.25b | 0.106 | 0.62abc | 2.11 | 0.32bc | 44.5 | 89.2 | 9.3 | 105.3 | 20.3 |
| Stress | 2.36b | 0.116 | 0.67bcd | 2.02 | 0.36cde | 45.2 | 76.7 | 8.6 | 83.7 | 21.0 | |
| 188 | Control | 2.35b | 0.113 | 0.69cd | 2.02 | 0.32bc | 39.3 | 95.4 | 7.8 | 88.2 | 23.2 |
| Stress | 2.34b | 0.112 | 0.57ab | 2.22 | 0.36cde | 44.3 | 78.9 | 9.2 | 84.2 | 18.4 | |
| 276 | Control | 2.35b | 0.119 | 0.74d | 1.95 | 0.33bc | 44.3 | 90.4 | 9.0 | 87.6 | 22.0 |
| Stress | 1.93a | 0.109 | 0.72cd | 1.90 | 0.24a | 43.9 | 107.4 | 7.9 | 113.7 | 24.6 | |
| 360 | Control | 2.22b | 0.116 | 0.53a | 2.19 | 0.39e | 50.5 | 82.9 | 19.6 | 114.7 | 24.6 |
| Stress | 2.16ab | 0.108 | 0.67bcd | 1.99 | 0.38de | 53.4 | 75.5 | 19.2 | 108.3 | 25.1 | |
| 372 | Control | 2.20ab | 0.109 | 0.63abc | 2.14 | 0.31bc | 35.8 | 75.4 | 10.6 | 116.8 | 22.6 |
| Stress | 2.18ab | 0.104 | 0.61abc | 1.85 | 0.35bcde | 38.4 | 72.3 | 10.9 | 136.3 | 26.4 | |
| 373 | Control | 2.19ab | 0.107 | 0.69cd | 1.97 | 0.32bc | 39.7 | 72.7 | 8.8 | 109.0 | 21.1 |
| Stress | 2.18ab | 0.105 | 0.57ab | 1.96 | 0.33bcd | 40.1 | 65.8 | 7.2 | 125.0 | 21.1 | |
| ANOVA | |||||||||||
| Clone | ns | ns | ** | ns | **** | **** | ** | **** | *** | ns | |
| Treatment | ns | ns | ns | ns | ns | ** | ns | ns | ns | ns | |
| Interaction | * | ns | *** | ns | *** | ns | ns | ns | ns | ns | |
| 3-Year Period (2018–2020) | ||||||||||||
| Clone | Yield (kg vine−1) | Number of clusters | Cluster weight (g) | Number of berries per cluster | Berry weight (g) | WUEyield (kg m−3) | Main shoot length (cm) | TLA (m2 vine−1) | Pruning weight (kg vine−1) | Ravaz index (kg kg−1) | TLA/yield (m2 kg−1) | TLA/Pruning weight (m2 kg−1) |
| 4 | 2.59bc | 13.50bc | 168bc | 121 | 1.71cd | 25.31c | 87c | 2.34bc | 0.25bc | 13.05 | 1.02ab | 9.45ab |
| 94 | 3.47d | 14.92bc | 216d | 128 | 1.79d | 29.99c | 83c | 2.23bc | 0.31c | 12.27 | 0.78a | 8.70a |
| 188 | 3.15cd | 15.40c | 199cd | 132 | 1.78d | 28.78c | 85c | 2.47c | 0.29c | 13.70 | 0.81a | 9.39ab |
| 276 | 2.22b | 12.51bc | 156ab | 112 | 1.60bc | 17.16b | 74b | 2.14bc | 0.26bc | 10.12 | 1.44b | 8.99a |
| 360 | 1.17a | 8.38a | 125a | 112 | 1.19a | 10.02a | 56a | 1.02a | 0.10a | 13.54 | 1.21ab | 9.54ab |
| 372 | 2.32b | 12.84bc | 165bc | 121 | 1.48b | 19.15b | 73b | 1.87b | 0.19b | 14.18 | 0.96ab | 12.12b |
| 373 | 2.23b | 12.21b | 165bc | 109 | 1.60bc | 16.66b | 67b | 1.37a | 0.22bc | 11.61 | 1.21ab | 11.9b |
| Treat. | ||||||||||||
| Control | 2.83 | 13.71 | 185 | 124 | 1.69 | 11.80 | 80 | 2.18 | 0.28 | 13.19 | 0.92 | 9.08 |
| Stress | 2.08 | 11.93 | 156 | 114 | 1.50 | 30.22 | 70 | 1.65 | 0.19 | 12.09 | 1.20 | 10.94 |
| ANOVA | ||||||||||||
| Clone | **** | **** | **** | ns | **** | **** | **** | **** | **** | ns | ** | ** |
| Treat. | **** | ** | *** | ns | **** | **** | **** | **** | **** | ns | ** | ** |
| Interact. | ns | ** | ns | * | **** | **** | *** | ns | ns | ns | *** | **** |
| Year 2021 | ||||||||||||
| Clone | Yield (kg vine−1) | Number of clusters | Cluster weight (g) | Number of berries per cluster | Berry Weight (g) | WUEyield (kg m−3) | Main shoot length (cm) | TLA (m2 vine−1) | TLA/yield (m2 kg−1) | |||
| 4 | 2.04bc | 12.64b | 145cd | 100bcd | 1.57b | 21.53b | 72b | 1.57c | 0.78a | |||
| 94 | 2.62c | 14.41b | 171d | 123d | 1.53b | 24.59b | 56ab | 1.25bc | 0.54a | |||
| 188 | 2.28bc | 13.75b | 150cd | 112cd | 1.46b | 20.72b | 60ab | 1.16abc | 0.75a | |||
| 276 | 1.61abc | 13.44b | 95ab | 65a | 1.53b | 10.14a | 52a | 1.12abc | 1.61b | |||
| 360 | 0.59a | 7.34a | 79a | 76ab | 1.09a | 3.31a | 46a | 0.34a | 0.73a | |||
| 372 | 1.40ab | 10.30ab | 129bc | 81ab | 1.43b | 9.46a | 50a | 0.64ab | 0.49a | |||
| 373 | 1.58abc | 11.46ab | 126bc | 89abc | 1.57b | 11.59a | 60ab | 1.39bc | 0.95a | |||
| Treat. | ||||||||||||
| Control | 2.23 | 13.92 | 142 | 99 | 1.53 | 13.00 | 63 | 1.49 | 0.76 | |||
| Stress | 1.23 | 9.90 | 114 | 86 | 1.39 | 15.95 | 50 | 0.65 | 0.91 | |||
| ANOVA | ||||||||||||
| Clone | *** | ** | **** | *** | **** | **** | ** | * | *** | |||
| Treat. | *** | *** | ** | ns | ** | ns | *** | **** | ns | |||
| Interact. | ns | ns | ns | * | ns | ns | ns | ns | *** | |||
| 3-Year Period (2018–2020) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Clone | Berry Weight | TSS | pH | Total Acidity | IM | Tartaric Acid | Malic Acid | Tartaric/Malic | % Juice | % H2O | |
| 4 | 1.71cd | 22.9ab | 4.04abc | 3.99b | 5.82a | 3.34a | 1.81c | 1.88a | 67.5bc | 76.3 | |
| 94 | 1.79d | 22.5a | 3.96a | 3.74ab | 6.08ab | 3.46a | 1.53b | 2.33b | 69.5c | 75.6 | |
| 188 | 1.74d | 23.3ab | 4.00ab | 3.77ab | 6.23abc | 3.46a | 1.43b | 2.68b | 67.3bc | 74.7 | |
| 276 | 1.54b | 23.1ab | 4.07bc | 3.73a | 6.44bc | 3.50a | 1.15a | 3.27cd | 66.7b | 74.8 | |
| 360 | 1.19a | 23.8b | 4.19d | 3.82ab | 6.12abc | 4.06b | 1.41b | 3.09c | 55.2a | 73.8 | |
| 372 | 1.48b | 23.1ab | 4.00ab | 3.55a | 6.58c | 3.47a | 1.10a | 3.18cd | 66.0b | 74.9 | |
| 373 | 1.60bc | 23.1ab | 4.11cd | 3.60a | 6.46bc | 3.56a | 1.10a | 3.44d | 65.0b | 75.4 | |
| Treat. | |||||||||||
| Control | 1.66 | 22.9 | 4.08 | 3.73 | 6.18 | 3.45 | 1.47 | 2.53 | 66.7 | 75.1 | |
| Stress | 1.49 | 23.3 | 4.03 | 3.75 | 6.32 | 3.65 | 1.25 | 3.15 | 63.9 | 75.0 | |
| Clone × Treat. | |||||||||||
| 4 | Control | 1.66bc | 23.0 | 4.05cd | 3.91 | 5.92 | 3.29 | 1.79d | 1.80a | 68.7 | 77.2 |
| Stress | 1.77bc | 22.9 | 4.03bcd | 4.07 | 5.73 | 3.39 | 1.83d | 1.96ab | 66.3 | 75.5 | |
| 94 | Control | 1.84c | 22.6 | 4.02bc | 3.67 | 6.23 | 3.52 | 1.58bcd | 2.26abc | 70.8 | 75.2 |
| Stress | 1.74bc | 22.4 | 3.91ab | 3.80 | 5.94 | 3.41 | 1.49bc | 2.41bcd | 68.2 | 76.0 | |
| 188 | Control | 1.83c | 23.4 | 4.13cde | 3.83 | 6.08 | 3.36 | 1.73cd | 2.04ab | 67.7 | 74.5 |
| Stress | 1.64bc | 23.1 | 3.88a | 3.71 | 6.38 | 3.55 | 1.13a | 3.32ef | 67.0 | 74.8 | |
| 276 | Control | 1.78bc | 22.9 | 4.05ce | 3.70 | 6.43 | 3.32 | 1.31ab | 2.69cd | 69.5 | 74.4 |
| Stress | 1.31a | 23.4 | 4.10cde | 3.76 | 6.45 | 3.68 | 0.99a | 3.84f | 64.0 | 75.2 | |
| 360 | Control | 1.28a | 23.3 | 4.16de | 3.73 | 5.88 | 3.80 | 1.57cd | 2.75cd | 57.2 | 74.5 |
| Stress | 1.10a | 24.3 | 4.23e | 3.91 | 6.35 | 4.32 | 1.26ab | 3.44ef | 53.1 | 73.0 | |
| 372 | Control | 1.65bc | 22.8 | 4.01abc | 3.62 | 6.42 | 3.35 | 1.21a | 2.89de | 67.7 | 75.1 |
| Stress | 1.30a | 23.3 | 3.99abc | 3.48 | 6.75 | 3.59 | 1.00a | 3.47ef | 64.3 | 74.7 | |
| 373 | Control | 1.61b | 22.6 | 4.13cde | 3.67 | 6.30 | 3.48 | 1.12a | 3.30ef | 65.5 | 75.0 |
| Stress | 1.59b | 23.6 | 4.09cde | 3.54 | 6.62 | 3.64 | 1.08a | 3.59f | 64.4 | 75.8 | |
| ANOVA | |||||||||||
| Clone | **** | * | **** | ** | *** | **** | **** | **** | **** | ns | |
| Treatment | **** | ns | ** | ns | ns | ** | **** | **** | *** | ns | |
| Interaction | **** | ns | ** | ns | ns | ns | ** | *** | ns | ns | |
| 3-Year Period (2018–2020) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Clone | Colour Intensity | Tone | Total Anthocyanins | Extractable Anthocyanins | Polyphenols Content | TPI | AE Index | SM Index | Tannins (mg L−1) | |
| 4 | 5.0a | 0.94 | 740 | 341 | 39.6ab | 19.9 | 52.9 | 65.4ab | 3.39ab | |
| 94 | 5.4ab | 0.97 | 704 | 340 | 36.8a | 18.1 | 51.3 | 62.7a | 3.51ab | |
| 188 | 5.3ab | 0.95 | 761 | 335 | 45.3b | 21.4 | 56.1 | 68.4bc | 4.34b | |
| 276 | 6.7d | 0.97 | 682 | 288 | 38.2ab | 17.7 | 56.9 | 64.2ab | 3.55ab | |
| 360 | 6.2cd | 1.06 | 671 | 321 | 42.2ab | 21.0 | 53.4 | 72.0c | 7.39c | |
| 372 | 5.8bc | 0.94 | 607 | 343 | 34.0a | 19.8 | 55.3 | 67.0abc | 3.58ab | |
| 373 | 6.9d | 0.95 | 606 | 347 | 38.6ab | 20.6 | 55.0 | 67.9abc | 2.72a | |
| Treat. | ||||||||||
| Control | 5.7 | 0.99 | 621 | 307 | 38.1 | 19.2 | 54.2 | 66.9 | 3.98 | |
| Stress | 6.0 | 0.94 | 745 | 355 | 40.4 | 20.3 | 54.6 | 66.7 | 4.16 | |
| Clone × Treat. | ||||||||||
| 4 | Control | 4.7 | 0.94 | 680abc | 351 | 43.8bcd | 19.0 | 51.4 | 65.4 | 3.66 |
| Stress | 5.2 | 0.95 | 799bc | 330 | 35.4abc | 20.8 | 54.3 | 65.5 | 3.12 | |
| 94 | Control | 5.7 | 1.01 | 535ab | 277 | 34.2ab | 19.0 | 50.1 | 66.9 | 3.24 |
| Stress | 5.0 | 0.93 | 872c | 404 | 39.4abcd | 17.2 | 52.6 | 58.6 | 3.78 | |
| 188 | Control | 5.3 | 0.99 | 714bc | 335 | 44.6cd | 21.0 | 54.8 | 67.6 | 4.55 |
| Stress | 5.2 | 0.91 | 808bc | 335 | 46.0cd | 21.8 | 57.4 | 69.2 | 4.13 | |
| 276 | Control | 5.8 | 1.01 | 733bc | 261 | 35.2abc | 15.7 | 58.0 | 62.6 | 3.61 |
| Stress | 7.5 | 0.93 | 631abc | 315 | 41.3abcd | 19.7 | 55.8 | 65.7 | 3.50 | |
| 360 | Control | 6.0 | 1.00 | 594abc | 274 | 34.4abc | 22.8 | 52.8 | 73.2 | 6.85 |
| Stress | 6.5 | 1.12 | 767bc | 368 | 50.0d | 19.2 | 54.1 | 70.7 | 7.93 | |
| 372 | Control | 5.6 | 0.99 | 405a | 306 | 37.7abcd | 19.2 | 52.7 | 65.8 | 2.99 |
| Stress | 6.0 | 0.88 | 809bc | 379 | 30.4a | 20.5 | 57.8 | 68.1 | 4.17 | |
| 373 | Control | 6.8 | 1.01 | 683abc | 343 | 36.9abcd | 18.1 | 59.8 | 66.9 | 2.98 |
| Stress | 7.0 | 0.89 | 528ab | 351 | 40.3abcd | 23.1 | 50.2 | 68.9 | 2.46 | |
| ANOVA | ||||||||||
| Clone | **** | ns | ns | ns | ** | ns | ns | *** | **** | |
| Treatment | ns | ns | ** | * | ns | ns | ns | ns | ns | |
| Interaction | ns | ns | ** | ns | ** | ns | ns | ns | ns | |
| 3-Year Period (2018–2020) | Year 2021 | ||||||
|---|---|---|---|---|---|---|---|
| Clone | QItechnological berry | QIphenolic berry | QIoverall berry | QItechnological berry | QIphenolic berry | QIoverall berry | |
| 4 | 6.2 | 5.2 | 11.5 | 5.3 | 4.8abc | 10.1ab | |
| 94 | 6.1 | 5.2 | 11.3 | 3.6 | 4.1ab | 7.8a | |
| 188 | 6.7 | 5.4 | 12.1 | 3.8 | 3.7a | 7.5a | |
| 276 | 6.7 | 5.5 | 12.2 | 5.3 | 6.2bc | 11.5b | |
| 360 | 6.5 | 7.1 | 13.6 | 4.4 | 7.1c | 11.5b | |
| 372 | 6.8 | 5.6 | 12.4 | 4.6 | 4.7abc | 9.3ab | |
| 373 | 6.7 | 6.0 | 12.7 | 4.2 | 5.1abc | 9.3ab | |
| Treat. | |||||||
| Control | 5.9 | 5.1 | 11.1 | 4.2 | 4.3 | 8.5 | |
| Stress | 7.1 | 6.3 | 13.4 | 4.7 | 5.9 | 10.5 | |
| Clone × Treat. | |||||||
| 4 | Control | 5.9 | 5.0 | 10.9 | 6.20b | 4.4 | 10.6bcde |
| Stress | 6.5 | 5.5 | 12.0 | 4.40ab | 5.2 | 9.6abcde | |
| 94 | Control | 5.7 | 4.5 | 10.3 | 3.75ab | 3.3 | 7.2abc |
| Stress | 6.4 | 5.9 | 12.3 | 3.50ab | 4.8 | 8.3abcde | |
| 188 | Control | 5.8 | 5.2 | 11.0 | 4.40ab | 4.0 | 8.4abcde |
| Stress | 7.5 | 5.6 | 13.2 | 3.20a | 3.3 | 6.6a | |
| 276 | Control | 5.9 | 4.3 | 10.2 | 3.60ab | 4.4 | 8.0abcd |
| Stress | 7.5 | 6.7 | 14.2 | 7.00b | 8.0 | 15.0e | |
| 360 | Control | 5.8 | 6.4 | 12.2 | 4.75ab | 7.0 | 11.8de |
| Stress | 7.3 | 7.8 | 15.1 | 4.00ab | 7.3 | 11.3cde | |
| 372 | Control | 6.3 | 4.7 | 11.0 | 3.60ab | 3.0 | 6.6a |
| Stress | 7.3 | 6.4 | 13.7 | 5.60b | 6.4 | 12.0e | |
| 373 | Control | 6.1 | 5.8 | 11.9 | 2.80a | 4.0 | 6.8ab |
| Stress | 7.3 | 6.1 | 13.4 | 5.50b | 6.3 | 11.8de | |
| ANOVA | |||||||
| Clone | ns | ns | ns | ns | * | ** | |
| Treatment | *** | *** | **** | ns | ** | *** | |
| Interaction | ns | ns | ns | ** | ns | *** | |
| 3-Year Period (2018–2020) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Clone | K | Ca | Mg | P | B | Cu | Mn | Zn | |
| 4 | 1951c | 69.4b | 90.9bc | 83.1ab | 10.6a | 0.286 | 0.84b | 0.212ab | |
| 94 | 1604a | 58.6a | 85.7ab | 75.7a | 10.0a | 0.257 | 0.77ab | 0.193a | |
| 188 | 1744ab | 66.3ab | 87.6ab | 80.3ab | 10.3a | 0.264 | 0.65a | 0.177a | |
| 276 | 1791bc | 64.9ab | 83.4a | 111.4c | 10.3a | 0.278 | 0.86b | 0.222ab | |
| 360 | 2325d | 83.1c | 96.4c | 112.6c | 13.8b | 0.318 | 1.17c | 0.246c | |
| 372 | 1744ab | 64.1ab | 87.3ab | 92.0b | 10.5a | 0.256 | 1.09c | 0.211ab | |
| 373 | 1823bc | 62.8ab | 84.3a | 93.9b | 10.4a | 0.243 | 0.83b | 0.182a | |
| Treat. | |||||||||
| Control | 1889 | 65.7 | 88.4 | 88.2 | 9.9 | 0.289 | 0.89 | 0.197 | |
| Stress | 1821 | 68.4 | 87.5 | 97.3 | 11.8 | 0.255 | 0.89 | 0.216 | |
| Clone × Treat. | |||||||||
| 4 | Control | 1938de | 71.8de | 93.2cd | 76.1ab | 8.7a | 0.277bc | 0.97de | 0.217bc |
| Stress | 1965e | 66.9cd | 88.5abc | 90.0bcd | 12.5d | 0.295bc | 0.72abc | 0.208abc | |
| 94 | Control | 1707abcde | 62.7abcd | 90.2abc | 87.5bc | 9.7ab | 0.268bc | 0.87bcde | 0.182ab |
| Stress | 1500a | 54.4a | 81.3a | 64.0a | 10.2bc | 0.247abc | 0.67ab | 0.204abc | |
| 188 | Control | 1922de | 64.3abcd | 86.6abc | 83.9bc | 9.5ab | 0.291bc | 0.73abc | 0.184ab |
| Stress | 1566ab | 68.4cd | 88.6abc | 76.7ab | 11.1bcd | 0.237ab | 0.58a | 0.170ab | |
| 276 | Control | 1687abcd | 55.3ab | 84.1ab | 80.7abc | 9.8ab | 0.262abc | 0.68ab | 0.143a |
| Stress | 1895cde | 74.5def | 82.7ab | 142.0f | 10.7abcd | 0.294bc | 1.03def | 0.300d | |
| 360 | Control | 2329f | 85.3f | 99.5d | 111.0e | 12.1cd | 0.330c | 1.26f | 0.256cd |
| Stress | 2321f | 80.9ef | 93.3bcd | 114.2e | 15.5e | 0.305bc | 1.09ef | 0.236bcd | |
| 372 | Control | 1823bcde | 62.1abcd | 81.0a | 98.0cde | 9.0ab | 0.332c | 0.92cde | 0.223bc |
| Stress | 1665abc | 66.0bcd | 93.5cd | 86.0bc | 12.0cd | 0.179a | 1.27f | 0.200abc | |
| 373 | Control | 1815bcde | 58.3abc | 84.0ab | 79.9abc | 10.2abc | 0.261abc | 0.78abcd | 0.172ab |
| Stress | 1832bcde | 67.4cd | 84.7abc | 108.0de | 10.6abcd | 0.225ab | 0.88bcde | 0.192abc | |
| ANOVA | |||||||||
| Clone | **** | **** | **** | **** | **** | ns | **** | ** | |
| Treatment | ns | ns | ns | *** | **** | ** | ns | ns | |
| Interaction | ** | *** | ** | **** | **** | ** | **** | ** | |
| Year 2021 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clone | Berry Weight | TSS | pH | Total Acidity | IM | Tartaric Acid | Malic Acid | Tartaric/Malic | % Juice | % H2O | YAN (mg L−1) | |
| 4 | 1.57b | 22.4cd | 3.95 | 3.36 | 6.5 | 2.77ab | 2.18abc | 1.22a | 55.5 | 70.6bc | 112a | |
| 94 | 1.53b | 20.8a | 4.16 | 3.30 | 6.4 | 2.66a | 1.96abc | 1.39ab | 58.9 | 70.9c | 115a | |
| 188 | 1.46b | 21.2ab | 4.07 | 3.21 | 6.5 | 3.12b | 2.22bc | 1.35ab | 55.3 | 70.2bc | 135a | |
| 276 | 1.53b | 22.6d | 4.01 | 3.05 | 7.4 | 2.68a | 1.96abc | 1.41ab | 54.0 | 68.1a | 90a | |
| 360 | 1.09a | 22.1cd | 4.19 | 3.26 | 6.9 | 3.63c | 2.36c | 1.61b | 52.4 | 67.8a | 196b | |
| 372 | 1.43b | 22.0abc | 4.09 | 3.23 | 7.0 | 2.95ab | 1.78a | 1.58b | 56.9 | 69.4abc | 110a | |
| 373 | 1.57b | 22.2cd | 4.13 | 3.26 | 6.9 | 2.99ab | 1.82ab | 1.59b | 57.2 | 69.0ab | 120a | |
| Treat. | ||||||||||||
| Control | 1.53 | 21.7 | 4.09 | 3.28 | 6.6 | 2.95 | 2.09 | 1.42 | 57.1 | 70.0 | 123 | |
| Stress | 1.39 | 22.1 | 4.08 | 3.20 | 7.0 | 3.00 | 1.98 | 1.48 | 54.3 | 68.8 | 128 | |
| Clone × Treat. | ||||||||||||
| 4 | Control | 1.62 | 23.1 | 3.91 | 3.29 | 6.7 | 2.91 | 2.35cd | 1.26 | 56.8ab | 70.2cde | 110 |
| Stress | 1.52 | 21.7 | 4.00 | 3.43 | 6.4 | 2.63 | 2.00abcd | 1.18 | 54.2ab | 71.0de | 114 | |
| 94 | Control | 1.63 | 20.8 | 4.20 | 3.39 | 6.2 | 2.73 | 2.07abcd | 1.29 | 58.7ab | 70.7de | 107 |
| Stress | 1.44 | 20.7 | 4.12 | 3.21 | 6.6 | 2.58 | 1.84abc | 1.49 | 59.0b | 71.2e | 122 | |
| 188 | Control | 1.52 | 21.4 | 4.10 | 3.41 | 6.0 | 3.02 | 2.28bcd | 1.25 | 53.3ab | 70.4cde | 114 |
| Stress | 1.40 | 21.0 | 4.03 | 3.02 | 7.1 | 3.22 | 2.17abcd | 1.44 | 57.2ab | 70.0bcde | 154 | |
| 276 | Control | 1.55 | 21.4 | 4.12 | 3.04 | 6.9 | 2.75 | 2.06abcd | 1.42 | 60.8b | 70.8de | 105 |
| Stress | 1.51 | 23.8 | 3.91 | 3.05 | 7.8 | 2.60 | 1.86abcd | 1.40 | 47.2a | 65.4a | 75 | |
| 360 | Control | 1.26 | 22.8 | 4.11 | 3.23 | 7.2 | 3.49 | 1.97abcd | 1.80 | 55.3ab | 67.4ab | 196 |
| Stress | 0.93 | 21.5 | 4.27 | 3.30 | 6.5 | 3.77 | 2.75d | 1.42 | 49.5a | 68.1abc | 196 | |
| 372 | Control | 1.60 | 21.2 | 4.10 | 3.28 | 6.6 | 2.67 | 2.00abcd | 1.46 | 59.0ab | 71.0e | 108 |
| Stress | 1.27 | 22.8 | 4.09 | 3.17 | 7.3 | 3.24 | 1.56a | 1.70 | 54.8ab | 67.7ab | 113 | |
| 373 | Control | 1.50 | 21.4 | 4.10 | 3.31 | 6.5 | 3.06 | 1.92abcd | 1.46 | 55.9ab | 69.7bcde | 118 |
| Stress | 1.64 | 23.0 | 4.15 | 3.21 | 7.3 | 2.93 | 1.71ab | 1.72 | 58.5ab | 68.3abcd | 122 | |
| ANOVA | ||||||||||||
| Clone | **** | ** | ns | ns | ns | **** | ** | ** | ns | *** | ** | |
| Treatment | ** | ns | ns | ns | ns | ns | ns | ns | ** | *** | ns | |
| Interaction | ns | ns | ns | ns | ns | ns | ** | ns | ** | ** | ns | |
| Year 2021 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Clone | Colour Intensity | Tone | Total Anthocyanins | Extractable Anthocyanins | Polyphenols Content | TPI | AE Index | SM Index | Tannins (mg L−1) | |
| 4 | 4.72 | 1.18ab | 699a | 459 | 43.8ab | 16.1a | 37.8 | 57.3a | 4.77ab | |
| 94 | 5.09 | 1.31c | 647a | 353 | 39.8a | 14.7a | 44.2 | 63.1ab | 3.31a | |
| 188 | 4.18 | 1.21abc | 652a | 364 | 46.1ab | 16.3a | 44.4 | 66.6bc | 5.30ab | |
| 276 | 5.86 | 1.10a | 1090b | 495 | 54.6b | 18.8a | 52.9 | 61.9ab | 5.10ab | |
| 360 | 5.05 | 1.15ab | 756a | 512 | 67.7c | 22.9b | 44.5 | 70.0c | 7.59c | |
| 372 | 5.21 | 1.13a | 724a | 430 | 44.2ab | 16.0a | 39.3 | 64.7bc | 5.53b | |
| 373 | 5.82 | 1.26bc | 748a | 486 | 49.1ab | 17.7a | 41.7 | 60.9ab | 4.32ab | |
| Treat. | ||||||||||
| Control | 5.10 | 1.26 | 672 | 399 | 44.2 | 16.0 | 42.9 | 63.4 | 4.30 | |
| DI | 5.16 | 1.13 | 846 | 486 | 54.5 | 19.0 | 44.2 | 63.6 | 5.96 | |
| Clone × Treat. | ||||||||||
| 4 | Control | 4.81 | 1.17bcd | 693ab | 487 | 45.8 | 16.8 | 33.5 | 57.1 | 2.98 |
| stress | 4.63 | 1.15abc | 704ab | 431 | 41.8 | 15.3 | 42.1 | 57.4 | 6.56 | |
| 94 | Control | 5.19 | 1.31d | 578a | 320 | 40.2 | 14.7 | 43.6 | 65.6 | 3.09 |
| stress | 4.99 | 1.27bcd | 715ab | 386 | 39.4 | 14.7 | 44.8 | 60.6 | 3.52 | |
| 188 | Control | 4.38 | 1.29cd | 687ab | 348 | 44.6 | 15.5 | 49.5 | 67.0 | 4.88 |
| stress | 3.99 | 1.13ab | 618a | 380 | 47.6 | 17.0 | 39.3 | 66.2 | 5.71 | |
| 276 | Control | 5.02 | 1.29cd | 703ab | 349 | 40.3 | 15.3 | 49.0 | 61.0 | 3.27 |
| stress | 6.69 | 0.91a | 1477c | 642 | 69.0 | 22.2 | 56.8 | 62.8 | 6.92 | |
| 360 | Control | 5.78 | 1.15abc | 784ab | 507 | 58.8 | 20.8 | 41.9 | 66.1 | 5.65 |
| stress | 4.33 | 1.15abc | 727ab | 518 | 76.7 | 25.0 | 47.2 | 73.9 | 9.54 | |
| 372 | Control | 4.94 | 1.21bcd | 642ab | 327 | 33.1 | 12.4 | 46.0 | 64.0 | 5.33 |
| stress | 5.47 | 1.06a | 806ab | 532 | 55.3 | 19.7 | 32.5 | 65.5 | 5.72 | |
| 373 | Control | 5.60 | 1.29cd | 619ab | 456 | 46.5 | 16.4 | 36.8 | 62.8 | 4.87 |
| stress | 6.04 | 1.22bcd | 876b | 516 | 51.8 | 19.0 | 46.6 | 58.9 | 3.77 | |
| ANOVA | ||||||||||
| Clone | ns | *** | *** | ns | *** | *** | ns | **** | ** | |
| Treatment | ns | **** | *** | ** | *** | ** | ns | ns | *** | |
| Interaction | ns | ** | *** | ns | ns | ns | ns | ns | ns | |
| Parameter | 4 | 94 | 188 | 276 | 372 | ANOVA |
| Technological parameters | ||||||
| Alcohol degree (°) | 12.45c | 10.9a | 11.25ab | 11.2ab | 11.55b | *** |
| pH | 3.32a | 3.44b | 3.57d | 3.56d | 3.48c | **** |
| Glycerol (mg L−1) | 9079 | 9630 | 8791 | 11015 | 10615 | ns |
| Chromatic characteristics | ||||||
| Colour intensity | 3.66a | 3.99c | 4.02c | 3.8b | 4.16d | **** |
| L* | 50.5d | 49.1b | 48.8a | 49.3c | 48.7a | **** |
| a* | 31.85ab | 31.62a | 31.76ab | 31.78ab | 32.41b | ns |
| b* | 7.84c | 5.68a | 6.42b | 9.53d | 7.94c | **** |
| C* | 32.8 | 32.13 | 32.40 | 33.18 | 33.05 | ns |
| h | 13.83c | 10.51a | 11.42b | 16.69d | 13.90c | **** |
| Polyphenol content | 4 | 94 | 188 | 276 | 372 | ANOVA |
| Total phenol index (TPI) | 26.70 | 24.90 | 25.81 | 25.95 | 29.48 | ns |
| Total Anthocyanins (mg L−1) | 55.7a | 58.3abc | 62.5c | 56.5ab | 61.4bc | ** |
| Antioxidant activity (mM trolox) | 13.75 | 13.20 | 14.49 | 16.36 | 14.63 | ns |
| Total tannins (mg catequin L−1) | 970a | 1007ab | 1101bc | 1092b | 1190c | *** |
| Anth./tan. ratio | 0.058b | 0.058b | 0.057b | 0.052a | 0.052a | *** |
| Quercetin 3-galactoside (mg L−1) | 0.22b | 0.19a | 0.24b | 0.29c | 0.36d | **** |
| Miricetin 3-glucoside+ Miricetin 3-glucuronide (mg L−1) | 2.73c | 2.0a | 2.17a | 2.43b | 2.53b | **** |
| Quercetin 3-glucuronide+ Quercetin 3-glucoside (mg L−1) | 2.7d | 1.7a | 2.03b | 2.5cd | 2.43c | **** |
| Resveratrol (mg L−1) | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | - |
| Amino acid profile (μmol ml−1) | 4 | 94 | 188 | 276 | 372 | ANOVA |
| His | 28.1 | 31.5 | 28.2 | 24.8 | 25.2 | ns |
| Ser | 5.31b | 5.32b | 4.67b | 1.77a | 4.14b | *** |
| Arg | 1.64 | 2.65 | 2.27 | 1.42 | 1.44 | ns |
| Gly | 39.1 | 37.8 | 54.4 | 36.6 | 68.6 | ns |
| Asp | 1.71 | 1.76 | 2.06 | 1.27 | 1.72 | ns |
| Glu | 61.9 | 58.0 | 59.6 | 49.4 | 55.5 | ns |
| Thr | 28.9 | 26.2 | 27.9 | 22.8 | 25.9 | ns |
| Ala | 9.9 | 13.3 | 11.7 | 9.6 | 12.9 | ns |
| Pro | 79.9 | 79.2 | 82.8 | 64.2 | 75.2 | ns |
| Cys | 6.3 | 27.4 | 7.6 | 22.0 | 8.4 | ns |
| Lys | 42.6 | 37.4 | 43.1 | 35.3 | 42.1 | ns |
| Tyr | 2.61a | 14.93c | 11.48b | 3.05a | 4.23a | **** |
| Met | 5.08a | 8.43b | 5.16a | 4.42a | 4.06a | ** |
| Val | 0.50a | 2.50b | 1.83b | 0.33a | 0.83a | **** |
| Ile | 0a | 1.67c | 1.00b | 0a | 0.33a | **** |
| Leu | 0.33a | 1.67b | 1.33b | 0.33a | 0.33a | *** |
| Phe | 0a | 1.33c | 1.0b | 0a | 0a | **** |
| Total | 314 | 351 | 346 | 277 | 331 | ns |
| Aromatic Compounds (mg L−1) | 4 | 94 | 188 | 276 | 372 | ANOVA |
|---|---|---|---|---|---|---|
| Ethyl acetate | 103a | 102a | 117b | 119b | 139c | **** |
| 1-Propanol | 52d | 38a | 42b | 37a | 47c | **** |
| 2-Methyl 1-propanol | 103b | 106b | 88a | 126c | 138d | **** |
| 3-Methyl 1-Butanol acetate | 0.66a | 0.88b | 0.63a | 0.61a | 0.81b | **** |
| 3-Methyl 1-butanol | 160c | 162c | 119a | 136b | 156c | **** |
| Ethyl hexanoate | 2.49 | 2.42 | 2.48 | 2.40 | 2.37 | ns |
| 1-Hexanol | 3.99d | 3.28b | 2.57a | 3.90d | 3.59c | **** |
| Ethyl octanoate | 1.19d | 0.54b | 1.14c | 0.58b | 0.44a | **** |
| Linalool | 0.013b | 0.014b | 0.014b | 0.011a | 0.013b | **** |
| 3-Methyl tio 1-propanol | 3.49d | 2.84b | 2.85b | 2.51a | 3.18c | **** |
| β-Damascenone | 0a | 2.18c | 2.42d | 2.09b | 2.04b | **** |
| Citronellol | 0.019a | 0.023b | 0.025b | 0.019a | 0.023b | **** |
| Ethyl dodecanoate | 0.085c | 0.063b | 0.096d | 0.059ab | 0.054a | **** |
| Hexanoic acid | 9.09c | 8.41a | 9.25d | 8.70b | 8.46a | **** |
| 2-Phenyl ethanol | 53b | 44a | 45a | 46a | 52b | **** |
| Ethyl tetradecanoate | 0.019 | 0.017 | 0.019 | 0.017 | 0.015 | ns |
| Nerolidol | 0.075c | 0.041a | 0.059b | 0.062b | 0.058b | **** |
| Octanoic acid | 1.90c | 0.88a | 1.46b | 0.83a | 0.97a | **** |
| Ethyl hexadecanoate | 8.29c | 5.28a | 8.45c | 5.87b | 5.61ab | **** |
| Decanoic acid | 0.14b | 0.099a | 0.13b | 0.10a | 0.11a | **** |
| 9-Decenoic acid | 4.01d | 4.00cd | 3.99b | 0a | 3.99bc | **** |
| ΣTotal (mg L−1) | 499c | 479b | 438a | 486bc | 560d | **** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero, P.; Botía, P.; Gil-Muñoz, R.; del Amor, F.M.; Navarro, J.M. Evaluation of the Effect of Water Stress on Clonal Variations of Cv. Monastrell (Vitis vinifera L.) in South-Eastern Spain: Physiology, Nutrition, Yield, Berry, and Wine-Quality Responses. Agronomy 2023, 13, 433. https://doi.org/10.3390/agronomy13020433
Romero P, Botía P, Gil-Muñoz R, del Amor FM, Navarro JM. Evaluation of the Effect of Water Stress on Clonal Variations of Cv. Monastrell (Vitis vinifera L.) in South-Eastern Spain: Physiology, Nutrition, Yield, Berry, and Wine-Quality Responses. Agronomy. 2023; 13(2):433. https://doi.org/10.3390/agronomy13020433
Chicago/Turabian StyleRomero, Pascual, Pablo Botía, Rocío Gil-Muñoz, Francisco M. del Amor, and Josefa María Navarro. 2023. "Evaluation of the Effect of Water Stress on Clonal Variations of Cv. Monastrell (Vitis vinifera L.) in South-Eastern Spain: Physiology, Nutrition, Yield, Berry, and Wine-Quality Responses" Agronomy 13, no. 2: 433. https://doi.org/10.3390/agronomy13020433
APA StyleRomero, P., Botía, P., Gil-Muñoz, R., del Amor, F. M., & Navarro, J. M. (2023). Evaluation of the Effect of Water Stress on Clonal Variations of Cv. Monastrell (Vitis vinifera L.) in South-Eastern Spain: Physiology, Nutrition, Yield, Berry, and Wine-Quality Responses. Agronomy, 13(2), 433. https://doi.org/10.3390/agronomy13020433








