Capsaicinoid Content in the Pericarp and Placenta of Bolilla Peppers (Capsicum annuum L.) throughout the Ripening of the Fruit at Two Different Stages of Plant Maturation
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Growing and Fertilizing of the Peppers
2.3. Ripening and Harvesting of the Peppers
2.4. Harvesting Conditions and Appearance of Bolilla Peppers at the Two Stages of Plant Maturity (Young and Adult)
2.5. Extraction Procedure
2.6. Identifying the Capsaicinoids through HPLC-MS
2.7. Developing the HPLC-FLR Quantification Method
2.8. Statistical Analysis
3. Results and Discussion
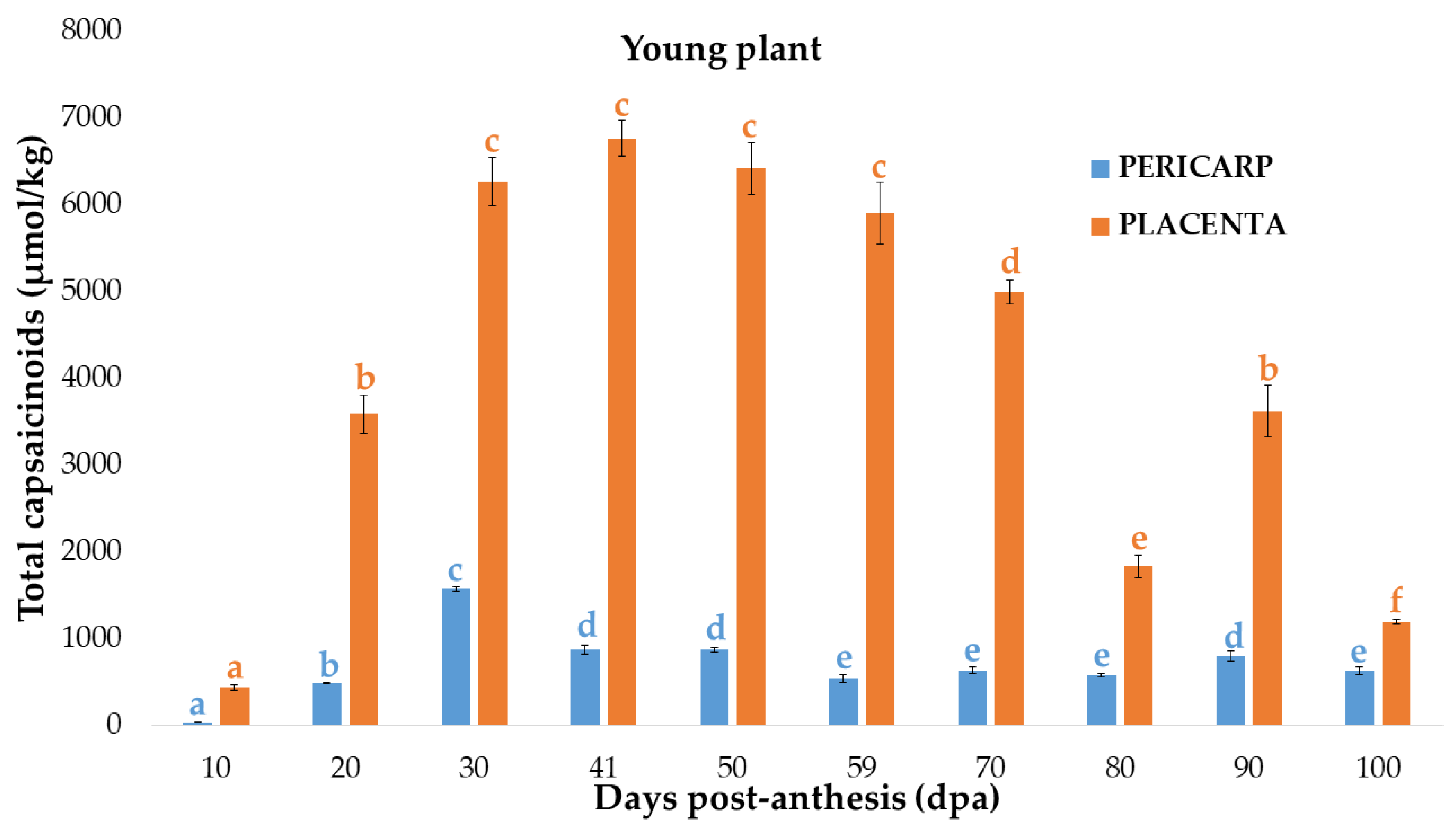
3.1. Changes of Capsaicinoid Content in the Pericarp of Bolilla Pepper at Two Different Plant-Maturity Stages
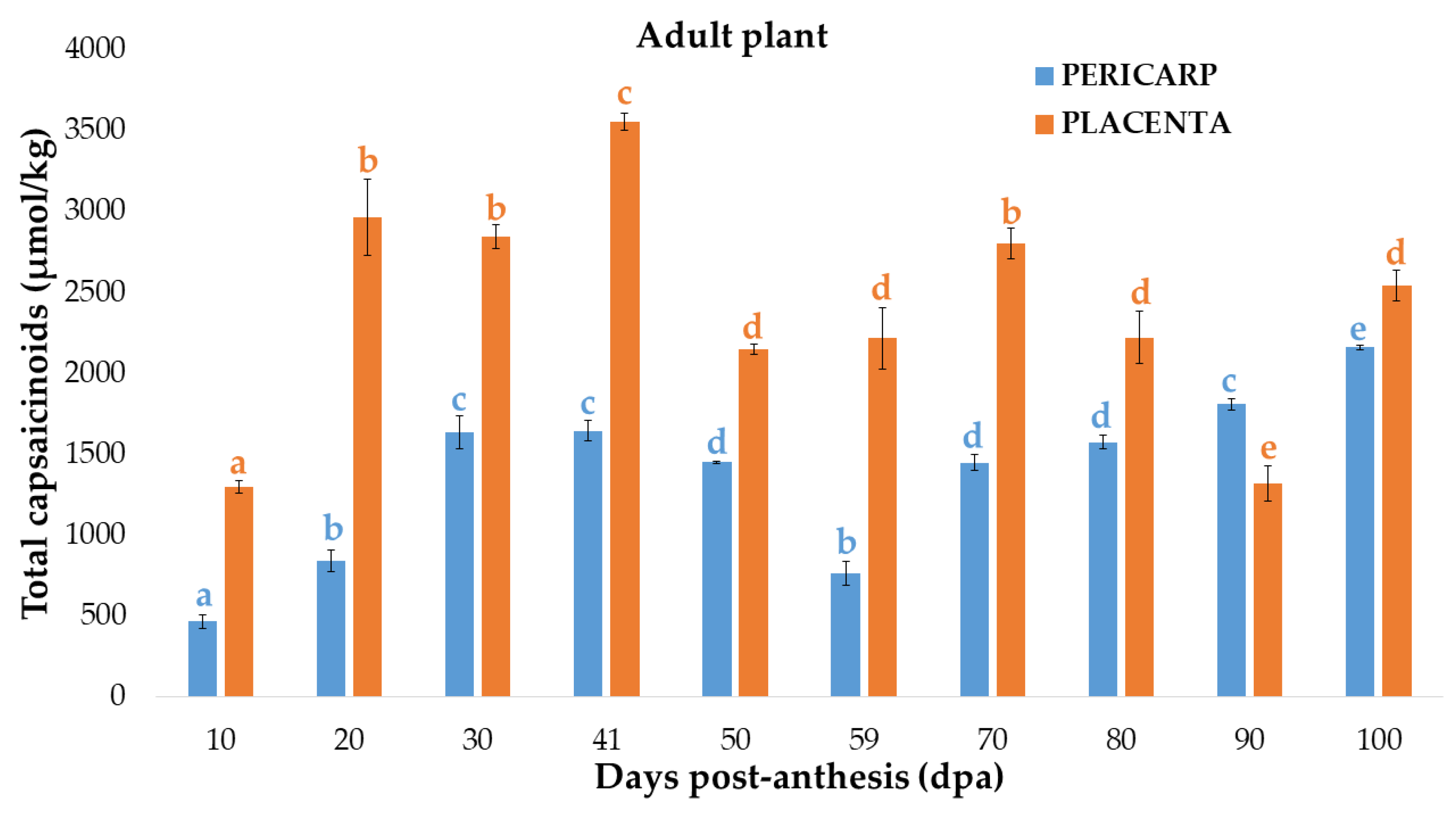
3.2. Changes of Capsaicinoids Content in the Placenta of the Bolilla Pepper at Two Different Plant-Maturity Stages
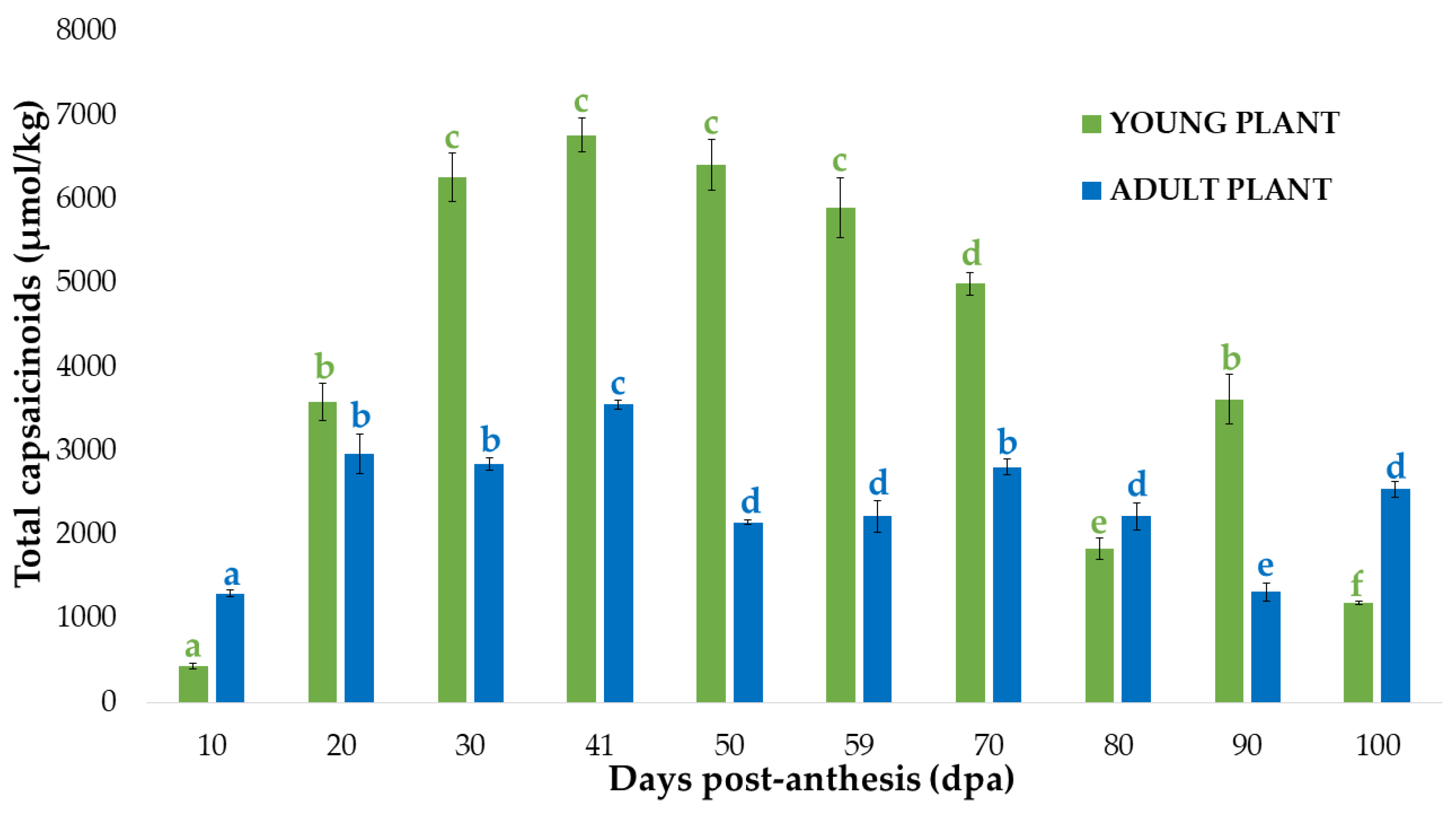
3.3. Comparison of Total Capsaicinoid Content in the Pericarp and Placenta of Bolilla Peppers from Young and Adult Plants
3.4. Individual Capsaicinoid Contents
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sahid, Z.D.; Syukur, M.; Maharijaya, A.; Nurcholis, W. Total Phenolic and Flavonoid Contents, Antioxidant, and α-Glucosidase Inhibitory Activities of Several Big Chili (Capsicum annuum L.) Genotypes. Cienc. Rural 2023, 53, 1–8. [Google Scholar] [CrossRef]
- Pérez-Grajales, M.; Martínez-Damián, M.T.; Cruz-Álvarez, O.; Potrero-Andrade, S.M.; Peña-Lomelí, A.; González-Hernández, V.A.; Villegas-Monter, A. Content of Capsaicinoids and Physicochemical Characteristics of Manzano Hot Pepper Grown in Greenhouse. Not. Bot. Horti. Agrobo. 2019, 47, 119–127. [Google Scholar] [CrossRef]
- Barboza, G.E.; Carrizo García, C.; Leiva González, S.; Scaldaferro, M.; Reyes, X. Four New Species of Capsicum (Solanaceae) from the Tropical Andes and an Update on the Phylogeny of the Genus. PLoS ONE 2019, 16, e0209792. [Google Scholar] [CrossRef] [PubMed]
- Pickersgill, B. Parallel vs. Convergent Evolution in Domestication and Diversification of Crops in the Americas. Front. Ecol. Evol. 2018, 6, 1–15. [Google Scholar] [CrossRef]
- Guo, Y.; Bai, J.; Duan, X.; Wang, J. Accumulation Characteristics of Carotenoids and Adaptive Fruit Color Variation in Ornamental Pepper. Sci. Hortic. 2021, 275, 109699. [Google Scholar] [CrossRef]
- Carolina, A.; Soldan, F.; Arvelos, S.; Watanabe, E.O.; Hori, C.E. Supercritical Fluid Extraction of Oleoresin from Capsicum annuum Industrial Waste. J. Clean. Prod. 2021, 297, 126593. [Google Scholar] [CrossRef]
- Baenas, N.; Belovic, M.; Ilic, N.; Moreno, D.A.; García-Viguera, C. Industrial Use of Pepper (Capsicum annum L.) Derived Products: Technological Benefits and Biological Advantages. Food Chem. 2019, 274, 872–885. [Google Scholar] [CrossRef]
- Nazneen Chowdhury, M.F.; Rafii, M.Y.; Izera Ismail, S.; Izan Ramlee, S.; Hosen, M.; Karim, K.M.R.; Ferdous Ikbal, M.; Halidu, J.; Sahmsiah Sahmat, S. Growth and Yield Performances, Pathogenicity, Heat Tolerance, Antioxidant Activity, and Pungency Level of Anthracnose Resistant and Heat Tolerant Inbreed Lines and Their F1 Hybrids of Chili (Capsicum annuum L.). Sci. Hortic. 2023, 309, 111606. [Google Scholar] [CrossRef]
- Fayos, O.; Ochoa-Alejo, N.; Martinez de la Vega, O.; Saviron, M.; Orduna, J.; Mallor, C.; Barbero, G.F.; Garcés-Claver, A. Assessment of Capsaicinoid and Capsinoid Accumulation Patterns during Fruit Development in Three Chili Pepper Genotypes (Capsicum Spp.) Carrying Pun1 and PAMT Alleles Related to Pungency. J. Agric. Food Chem. 2019, 67, 12219–12227. [Google Scholar] [CrossRef]
- Daood, H.G.; Halasz, G.; Palotás, G.; Palotás, G.; Bodai, Z.; Helyes, L. HPLC Determination of Capsaicinoids with Cross-Linked C18 Column and Buffer-Free Eluent. J. Chromatogr. Sci. 2015, 23, 135–143. [Google Scholar] [CrossRef]
- Xie, Z.Q.; Li, H.X.; Hou, X.J.; Huang, M.Y.; Zhu, Z.M.; Wei, L.X.; Tang, C.X. Capsaicin Suppresses Hepatocarcinogenesis by Inhibiting the Stemness of Hepatic Progenitor Cells via SIRT1/SOX2 Signaling Pathway. Cancer Med. 2022, 11, 4283–4296. [Google Scholar] [CrossRef] [PubMed]
- Thongin, S.; Den-udom, T.; Uppakara, K.; Sriwantana, T.; Sibmooh, N.; Laolob, T.; Boonthip, C.; Wichai, U.; Muta, K.; Ketsawatsomkron, P. Beneficial Effects of Capsaicin and Dihydrocapsaicin on Endothelial Inflammation, Nitric Oxide Production and Antioxidant Activity. Biomed. Pharmacother. 2022, 154, 113521. [Google Scholar] [CrossRef] [PubMed]
- Cortes-Ferre, H.E.; Antunes-Ricardo, M.; Gutiérrez-Uribe, J.A. Enzyme-Assisted Extraction of Anti-Inflammatory Compounds from Habanero Chili Pepper (Capsicum chinense) Seeds. Front. Nut. 2022, 9, 942805. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Mitra, S.; Tareq, A.M.; Emran, T.B.; Hossain, M.J.; Alqahtani, A.M.; Alghazwani, Y.; Dhama, K.; Simal-Gandara, J. Medicinal Plants Used Against Hepatic Disorders in Bangladesh: A Comprehensive Review. J. Ethnopharmacol. 2022, 282, 114588. [Google Scholar] [CrossRef] [PubMed]
- Nava-Ochoa, A.E.; Antunes-Ricardo, M.; Guajardo-Flores, D. Nano-Sized Carriers for Capsaicinoids with Topic Analgesic and Anti-Inflammatory Effects. J. Biotechnol. 2021, 333, 77–85. [Google Scholar] [CrossRef]
- Sun, B.; Chen, C.; Song, J.; Zheng, P.; Wang, J.; Wei, J.; Cai, W.; Chen, S.; Cai, Y.; Yuan, Y.; et al. The Capsicum MYB31 Regulates Capsaicinoid Biosynthesis in the Pepper Pericarp. Plant Physiol. Biochem. 2022, 176, 21–30. [Google Scholar] [CrossRef]
- Nwokem, C.O. Evaluation of Capsaicin Content in Parts of Some Peppers Grown in Nigeria. Sci. World J. 2021, 16, 90–93. [Google Scholar]
- Chiaiese, P.; Corrado, G.; Minutolo, M.; Barone, A.; Errico, A. Transcriptional Regulation of Ascorbic Acid During Fruit Ripening in Pepper (Capsicum annuum). Plants 2019, 8, 206. [Google Scholar] [CrossRef]
- Iwai, K.; Suzuki, T.; Fujiwake, H. Formation and Accumulation of Pungent Principle of Hot Pepper Fruits, Capsaicin and Its Analogues, in Capsicum annuum var. annuum cv. Karayatsubusa at Different Growth Stages after Flowering. Agric. Biol. Chem. 1979, 43, 2493–2498. [Google Scholar] [CrossRef]
- Bernal, M.A.; Calderón, A.A.; Pedreño, M.A.; Muñoz, R.; Barceló, A.R.; Merino, F.C. Capsaicin Oxidation by Peroxidase from Capsicum annuum (Var. annuum) Fruits. J. Agric. Food Chem. 1993, 41, 1041–1044. [Google Scholar] [CrossRef]
- Bernal, M.A.; Calderón, A.A.; Pedreño, M.A.; Muñoz, R.; Barceló, A.R.; Merino, F.C. Dihydrocapsaicin Oxidation by Capsicum annuum (var. annuum) Peroxidase. J. Food Sci. 1993, 58, 611–613. [Google Scholar] [CrossRef]
- Lima, M.F.; Carvalho, S.I.C.; Ragassi, C.F.; Bianchetti, L.B.; Faleiro, F.G.; Reifschneider, F.J.B. Characterization of a pepper collection (Capsicum frutescens L.) from Brazil. Genet. Mol. Res. 2017, 16, 16039704. [Google Scholar] [CrossRef] [PubMed]
- Olguín-Rojas, J.A.; Fayos, O.; Vázquez-León, L.A.; Ferreiro-González, M.; Rodrígues-Jimenes, G.C.; Palma, M.; Garcés-Claver, A.; Barbero, G.F. Progression of the Total and Individual Capsaicinoids Content in the Fruits of Three Different Cultivars of Capsicum chinense Jacq. Agronomy 2019, 9, 141. [Google Scholar] [CrossRef]
- Clifton, D.J. Capsicum. The Genus Capsicum. In Medicinal and Aromatic Plants—Industrial Profiles, 1st ed.; CRC Press: Boca Ratón, FL, USA, 2003. [Google Scholar]
- Sandra, Y.M.A.; Maharijaya, A.; Sobir. Screening of Resistance to Geminivirus and Whitefly in Pepper. Euphytica 2022, 218, 155. [Google Scholar] [CrossRef]
- Wang, X.-W.; Li, P.; Liu, S.-S. Whitefly interactions with plants. Curr. Opin. Insect Sci. 2017, 19, 70–75. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, J.; Zhang, H.; Dzah, C.S.; Zandile, M.; Duan, Y.; Ma, H.; Luo, X. Advances in ultrasound assisted extraction of bioactive compounds from cash crops–A review. Ultrason. Sonochem. 2018, 48, 538–549. [Google Scholar] [CrossRef]
- Vázquez-Espinosa, M.; Olguín-Rojas, J.; Fayos, O.; González-De-Peredo, A.V.; Espada-Bellido, E.; Ferreiro-González, M.; Barroso, C.G.; Barbero, G.F.; Garcés-Claver, A.; Palma, M. Influence of Fruit Ripening on the Total and Individual Capsaicinoids and Capsiate Content in Naga Jolokia Peppers (Capsicum chinense Jacq.). Agronomy 2020, 10, 252. [Google Scholar] [CrossRef]
- Barbero, G.F.; Liazid, A.; Palma, M.; Barroso, C.G. Ultrasound-assisted extraction of capsaicinoids from peppers. Talanta 2008, 75, 1332–1337. [Google Scholar] [CrossRef]
- Vázquez-Espinosa, M.; González-de-Peredo, A.V.; Ferreiro-González, M.; Barroso, C.G.; Palma, M.; Barbero, G.F.; Espada-Bellido, E. Optimizing and Comparing Ultrasound-and Microwave-Assisted Extraction of Antioxidant Capsinoids in Peppers. Agronomy 2019, 9, 633. [Google Scholar] [CrossRef]
- Barbero, G.F.; Ruiz, A.G.; Liazid, A.; Palma, M.; Vera, J.C.; Barroso, C.G. Evolution of Total and Individual Capsaicinoids in Peppers during Ripening of the Cayenne Pepper Plant (Capsicum annuum L.). Food Chem. 2014, 153, 200–206. [Google Scholar] [CrossRef]
- Vázquez-Espinosa, M.; González-de-Peredo, A.V.; Espada-Bellido, E.; Ferreiro-González, M.; Barbero, G.F.; Palma, M. The Effect of Ripening on the Capsaicinoids Composition of Jeromin Pepper (Capsicum annuum L.) at Two Different Stages of Plant Maturity. Food Chem. 2023, 399, 133979. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, D.C.; Hertwing, K.M. Peroxidase-catalyzed oxidation of capsaicinoids: Steady-state and transient-state kinetic studies. Arch. Biochem. Biophys. 2003, 417, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Jeeatid, N.; Suriharn, B.; Techawongstien, S.; Chanthai, S.; Bosland, P.W.; Techawongstien, S. Evaluation of the Effect of Genotype-by-Environment Interaction on Capsaicinoid Production in Hot Pepper Hybrids (Capsicum chinense Jacq.) under Controlled Environment. Sci. Hortic. 2018, 235, 334–339. [Google Scholar] [CrossRef]
- Zewdie, Y.; Bosland, P.W. Evaluation of Genotype, Environment, and Genotype-by-Environment Interaction for Capsaicinoids in Capsicum annuum L. Euphytica 2000, 111, 185–190. [Google Scholar] [CrossRef]
- Mueller-Seitz, E.; Hiepler, C.; Petz, M. Chili pepper fruits: Content and Pattern of Capsaicinoids in Single Fruits of Different Ages. J. Agric. Food Chem. 2008, 56, 12114–12121. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.; Jayaprakasha, G.K.; Crosby, K.; Yoo, K.S.; Leskovar, D.I.; Jifon, J.; Patil, B.S. Ascorbic Acid, Capsaicinoid, and Flavonoid Aglycone Concentrations as a Function of Fruit Maturity Stage in Greenhouse-Grown Peppers. J. Food Com. Anal. 2014, 33, 195–202. [Google Scholar] [CrossRef]
- Fayos, O.; De Aguiar, A.C.; Jiménez-Cantizano, A.; Ferreiro-González, M.; Garcés-Claver, A.; Mallor, C.; Ruíz-Rodríguez, A.; Palma, M.; Barroso, C.G.; Barbero, G.F. Ontogenetic Variation of Individual and Total Capsaicinoids in Malagueta Peppers (Capsicum frutescens) during Fruit Maturation. Molecules 2017, 22, 736. [Google Scholar] [CrossRef]
- Estrada, B.; Bernal, M.A.; Díaz, J.; Pomar, F.; Merino, F. Fruit Development in Capsicum annuum: Changes in Capsaicin, Lignin, Free Phenolics, and Peroxidase Patterns. J. Agric. Food Chem. 2000, 48, 6234–6239. [Google Scholar] [CrossRef]
- Wang, D.; Bosland, P.W. The Genes of Capsicum. HortScience 2006, 41, 1169–1187. [Google Scholar] [CrossRef]
- Harvell, K.P.; Bosland, P.W. The Environment Produces a Significant Effect on Pungency of Chiles. HortScience 1997, 32, 1292. [Google Scholar] [CrossRef]
- Fujiwake, H.; Suzuki, T.; Iwai, K. Capsaicinoid Formation in the Protoplast from the Placenta of Capsicum Fruits. Agric. Biol. Chem. 1982, 46, 2591–2592. [Google Scholar] [CrossRef]
- Cervantes-Hernández, F.; Alcalá-González, P.; Martínez, O.; Ordaz-Ortiz, J.J. Placenta, Pericarp, and Seeds of Tabasco Chili Pepper Fruits Show a Contrasting Diversity of Bioactive Metabolites. Metabolites 2019, 9, 206. [Google Scholar] [CrossRef] [PubMed]
- Ananthan, R.; Subhash, K.; Longvah, T. Capsaicinoids, Amino Acid and Fatty Acid Profiles in Different Fruit Components of the World Hottest Naga King Chilli (Capsicum chinense Jacq). Food Chem. 2018, 238, 51–57. [Google Scholar] [CrossRef]
- Barbero, G.F.; Liazid, A.; Azaroual, L.; Palma, M.; Barroso, C.G. Capsaicinoid Contents in Peppers and Pepper-Related Spicy Foods. Int. J. Food Prop. 2016, 19, 485–493. [Google Scholar] [CrossRef]
- De Aguiar, A.C.; Sales, L.P.; Coutinho, J.P.; Barbero, G.F.; Godoy, H.T.; Martínez, J. Supercritical Carbon Dioxide Extraction of Capsicum Peppers: Global Yield and Capsaicinoid Content. J. Supercrit. Fluids 2013, 81, 210–216. [Google Scholar] [CrossRef]
- De Aguiar, A.C.; Coutinho, J.P.; Barbero, G.F.; Godoy, H.T.; Martínez, J. Comparative Study of Capsaicinoid Composition in Capsicum Peppers Grown in Brazil. Int. J. Food Prop. 2016, 19, 1292–1302. [Google Scholar] [CrossRef]

| Code | Fruit Sprouting Date | Days Post-Anthesis (dpa) | Visual State |
|---|---|---|---|
| M-10 | 17 April | 100 | Over-ripening |
| M-9 | 07 April | 90 | Over-ripening |
| M-8 | 28 March | 80 | Red color |
| M-7 | 17 March | 70 | Red color |
| M-6 | 08 March | 59 | Red color |
| M-5 | 27 February | 50 | Red color |
| M-4 | 17 February | 41 | Green-Red color |
| M-3 | 06 February | 30 | Green color |
| M-2 | 27 January | 20 | Green color |
| M-1 | 17 January | 10 | Green color |
| Code | Fruit Sprouting Date | Days Post-Anthesis (dpa) | Visual State |
|---|---|---|---|
| M-10 | 26 June | 100 | Over-ripening |
| M-9 | 16 June | 90 | Over-ripening |
| M-8 | 6 June | 80 | Red color |
| M-7 | 26 May | 70 | Red color |
| M-6 | 17 May | 59 | Red color |
| M-5 | 08 May | 50 | Red color |
| M-4 | 28 April | 41 | Green-Red color |
| M-3 | 17 April | 30 | Green color |
| M-2 | 07 April | 20 | Green color |
| M-1 | 28 March | 10 | Green color |
| Concentration (µmol/kg) | n-DHC | C | DHC | h-C | h-DHC | |
|---|---|---|---|---|---|---|
| Young plant | Pericarp | |||||
| M-1 | 5.05 ± 0.46 a | 10.26 ± 0.36 a | 9.97 ± 0.44 a | 2.09 ± 0.04 a | 2.23 ± 0.16 a | |
| M-2 | 49.42 ± 0.41 b | 217.52 ± 3.11 b | 186.08 ± 2.63 b | 8.23 ± 0.11 b | 14.43 ± 0.34 b | |
| M-3 | 197.52 ± 2.86 c | 677.98 ± 14.75 c | 622.03 ± 10.66 c | 19.15 ± 1.07 c | 49.31 ± 0.47 c | |
| M-4 | 117.51 ± 6.35 d | 434.80 ± 15.61 d | 361.95 ± 23.77 d | 10.16 ± 1.13 d | 21.79 ± 1.12 d | |
| M-5 | 106.30 ± 3.68 d | 390.72 ± 10.96 e | 327.02 ± 10.00 d | 11.61 ± 2.56 d | 28.51 ± 1.29 e | |
| M-6 | 59.01 ± 7.44 e | 272.88 ± 37.30 f | 176.19 ± 22.87 e | 8.52 ± 0.47 e | 18.30 ± 1.96 f | |
| M-7 | 76.17 ± 9.53 f | 301.92 ± 36.33 f | 206.06 ± 28.48 e | 9.72 ± 0.16 f | 23.56 ± 3.29 g | |
| M-8 | 71.78 ± 3.17 f | 286.55 ± 13.48 f | 183.87 ± 6.76 e | 9.37 ± 0.20 f | 22.81 ± 0.47 g | |
| M-9 | 66.75 ± 4.59 f | 266.79 ± 15.26 f | 177.04 ± 4.18 e | 8.52 ± 0.64 g | 22.32 ± 1.93 g | |
| M-10 | 67.26 ± 2.56 f | 347.82 ± 6.35 g | 252.35 ± 24.88 f | 11.01 ± 2.19 g | 30.62 ± 2.44 h | |
| Placenta | ||||||
| M-1 | 53.13 ± 7.34 a | 180.50 ± 22.42 a | 164.22 ± 17.55 a | 16.80 ± 1.48 a | 13.89 ± 1.28 a | |
| M-2 | 364.47 ± 41.67 b | 1640.18 ± 185.05 b | 1399.41 ± 172.50 b | 81.46 ± 9.32 b | 96.87 ± 12.09 b | |
| M-3 | 908.80 ± 20.81 c | 3164.30 ± 99.76 c | 2597.84 ± 342.46 c | 96.09 ± 5.68 b | 85.17 ± 5.63 b | |
| M-4 | 750.74 ± 54.64 d | 2580.06 ± 240.86 d | 2279.49 ± 128.16 c | 107.09 ± 1.30 c | 180.74±13.01 c | |
| M-5 | 804.13 ± 35.86 d | 2900.02 ± 135.09 e | 2408.40 ± 118.93 c | 102.33 ± 5.66 c | 196.49 ± 6.56 c | |
| M-6 | 716.12 ± 16.51 d | 3189.83 ± 203.06 e | 2249.87 ± 151.32 c | 99.22 ± 2.32 c | 178.74±24.12 c | |
| M-7 | 640.04 ± 7.98 e | 2477.59 ± 22.20 d | 1608.74 ± 94.22 d | 85.67 ± 4.74 b | 177.08 ± 2.53 c | |
| M-8 | 240.98 ± 8.34 f | 882.03 ± 78.04 f | 597.29 ± 35.21 e | 35.41 ± 1.54 d | 71.77 ± 3.41 d | |
| M-9 | 259.03 ± 14,46 f | 1179.06 ± 243.45 f | 782.38 ± 29.57 f | 39.02 ± 4.82 d | 88.48 ± 5.88 e | |
| M-10 | 164.89 ± 12.73 g | 461.78 ± 53.74 g | 449.26 ± 2.12 g | 14.61 ± 1.41 e | 51.04 ± 1.41 f | |
| Adult plant | Pericarp | |||||
| M-1 | 28.92 ± 3.43 a | 270.11 ± 33.92 a | 139.69 ± 9.29 a | 7.00 ± 0.07 a | 8.77 ± 0.43 a | |
| M-2 | 65.14 ± 6.33 b | 462.23 ± 37.79 b | 280.16 ± 22.09 b | 13.87 ± 1.62 b | 19.67 ± 1.18 b | |
| M-3 | 136.36 ± 17.36 c | 836.05 ± 105.30 c | 603.80 ± 70.93 c | 18.30 ± 2.27 c | 41.13 ± 3.94 c | |
| M-4 | 176.62 ± 7.89 d | 750.46 ± 25.63 d | 649.78 ± 25.92 c | 20.49 ± 1.06 c | 47.71 ± 2.66 c | |
| M-5 | 134.11 ± 0.36 c | 768.15 ± 2.31 d | 486.84 ± 5.71 d | 19.95 ± 1.08 c | 39.72 ± 0.15 c | |
| M-6 | 76.71 ± 9.09 b | 371.38 ± 37.67 b | 254.60 ± 18.04 b | 10.67 ± 1.04 d | 23.24 ± 3.96 b | |
| M-7 | 153.42 ± 4.63 c | 700.31 ± 24.35 d | 525.54 ± 18.18 d | 23.93 ± 1.44 e | 44.68 ± 1.09 c | |
| M-8 | 199.35 ± 4.72 e | 629.41 ± 16.19 e | 662.81 ± 19.64 c | 26.69 ± 1.20 e | 54.58 ± 1.81 d | |
| M-9 | 243.58 ± 6.95 f | 722.09 ± 8.97 d | 741.26 ± 13.55 e | 30.35 ± 0.28 f | 69.60 ± 2.13 e | |
| M-10 | 309.40 ± 6.60 g | 851.42 ± 17.63 c | 874.30 ± 2.33 f | 41.81 ± 0.21 g | 81.89 ± 0.02 f | |
| Placenta | ||||||
| M-1 | 75.79 ± 7.43 a | 410.16 ± 62.24 a | 9.97 ± 20.77 a | 21.50 ± 2.46 a | 39.57 ± 1.39 a | |
| M-2 | 230.39 ± 16.90 b | 1020.43 ± 129.85 b | 186.08 ± 80.98 b | 46.97 ± 2.62 b | 68.29 ± 4.66 b | |
| M-3 | 272.81 ± 5.79 c | 1089.28 ± 37.61 b | 622.03 ± 26.35 c | 35.08 ± 0.67 c | 82.83 ± 2.07 c | |
| M-4 | 418.52 ± 5.69 d | 1422.40 ± 41.36 c | 361.95 ± 18.59 d | 39.46 ± 0.65 d | 107.34 ± 2.24 d | |
| M-5 | 212.59 ± 1.44 b | 749.23 ± 18.89 d | 327.02 ± 9.55 d | 28.12 ± 1.88 e | 60.57 ± 0.71 e | |
| M-6 | 233.76 ± 26.69 b | 816.22 ± 89.56 d | 176.19 ± 61.86 b | 37.78 ± 4.87 d | 70.46 ± 7.66 b | |
| M-7 | 340.63 ± 2.81 e | 1046.50 ± 58.31 b | 206.06 ± 29.57 b | 45.31 ± 2.08 b | 99.08 ± 1.32 f | |
| M-8 | 278.97 ± 32.90 f | 954.76 ± 31.94 b | 183.87 ± 83.62 b | 37.67 ± 3.45 d | 77.16 ± 9.43 b | |
| M-9 | 108.55 ± 1.55 g | 326.66 ± 4.29 e | 177.04 ± 6.59 b | 15.89 ± 0.15 f | 34.69 ± 0.95 g | |
| M-10 | 322.98 ± 12.72 h | 971.29 ± 125.28 b | 252.35 ± 102.96 b | 47.60 ± 15.08 b | 93.16 ± 2.42 h | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vázquez-Espinosa, M.; Álvarez-Romero, M.; González-de-Peredo, A.V.; Ruíz-Rodríguez, A.; Ferreiro-González, M.; Barbero, G.F.; Palma, M. Capsaicinoid Content in the Pericarp and Placenta of Bolilla Peppers (Capsicum annuum L.) throughout the Ripening of the Fruit at Two Different Stages of Plant Maturation. Agronomy 2023, 13, 435. https://doi.org/10.3390/agronomy13020435
Vázquez-Espinosa M, Álvarez-Romero M, González-de-Peredo AV, Ruíz-Rodríguez A, Ferreiro-González M, Barbero GF, Palma M. Capsaicinoid Content in the Pericarp and Placenta of Bolilla Peppers (Capsicum annuum L.) throughout the Ripening of the Fruit at Two Different Stages of Plant Maturation. Agronomy. 2023; 13(2):435. https://doi.org/10.3390/agronomy13020435
Chicago/Turabian StyleVázquez-Espinosa, Mercedes, María Álvarez-Romero, Ana V. González-de-Peredo, Ana Ruíz-Rodríguez, Marta Ferreiro-González, Gerardo F. Barbero, and Miguel Palma. 2023. "Capsaicinoid Content in the Pericarp and Placenta of Bolilla Peppers (Capsicum annuum L.) throughout the Ripening of the Fruit at Two Different Stages of Plant Maturation" Agronomy 13, no. 2: 435. https://doi.org/10.3390/agronomy13020435
APA StyleVázquez-Espinosa, M., Álvarez-Romero, M., González-de-Peredo, A. V., Ruíz-Rodríguez, A., Ferreiro-González, M., Barbero, G. F., & Palma, M. (2023). Capsaicinoid Content in the Pericarp and Placenta of Bolilla Peppers (Capsicum annuum L.) throughout the Ripening of the Fruit at Two Different Stages of Plant Maturation. Agronomy, 13(2), 435. https://doi.org/10.3390/agronomy13020435









