Effect of Fomesafen on the Nutritional Quality and Amino Acids of Vigna angularis Based on Metabonomics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Main Reagents and Equipment
2.2. Vigna angularis Planting Experiment under Fomesafen Stress
2.3. Sample Pretreatment
2.4. Determination of Quality Indexes of Vigna angularis under FSA Stress
2.5. Untargeted Metabolomic Assays
2.5.1. Untargeted Metabolomics Sample Processing
2.5.2. Untargeted Metabolomics LC-MS Detection
2.6. Targeted Amino Acid Metabolomic Detection
2.6.1. Extraction of Amino Acid Metabolites
2.6.2. LC-MS Detection
2.6.3. Preparation of Amino Acid Standard Solution
2.6.4. Calibration Curve Equation
2.7. Data Processing
3. Results
3.1. Effect of FSA Stress on Appearance and Quality of Vigna angularis
3.2. Non-Targeted Metabolomic Analysis of Vigna angularis under FSA Stress
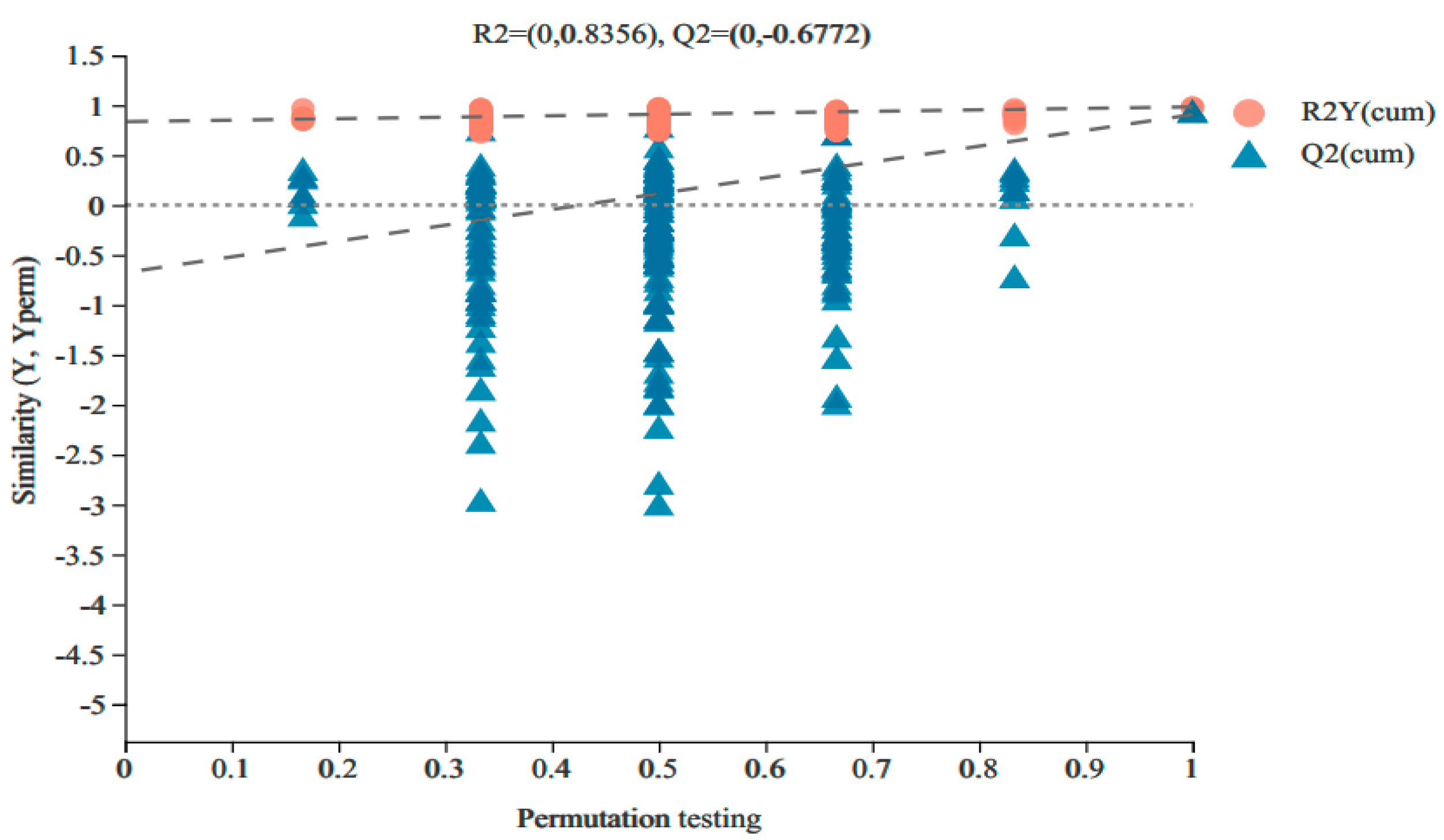
3.2.1. Non-Targeted Metabolomic OPLS-DA Analysis of Vigna angularis under FSA Stress
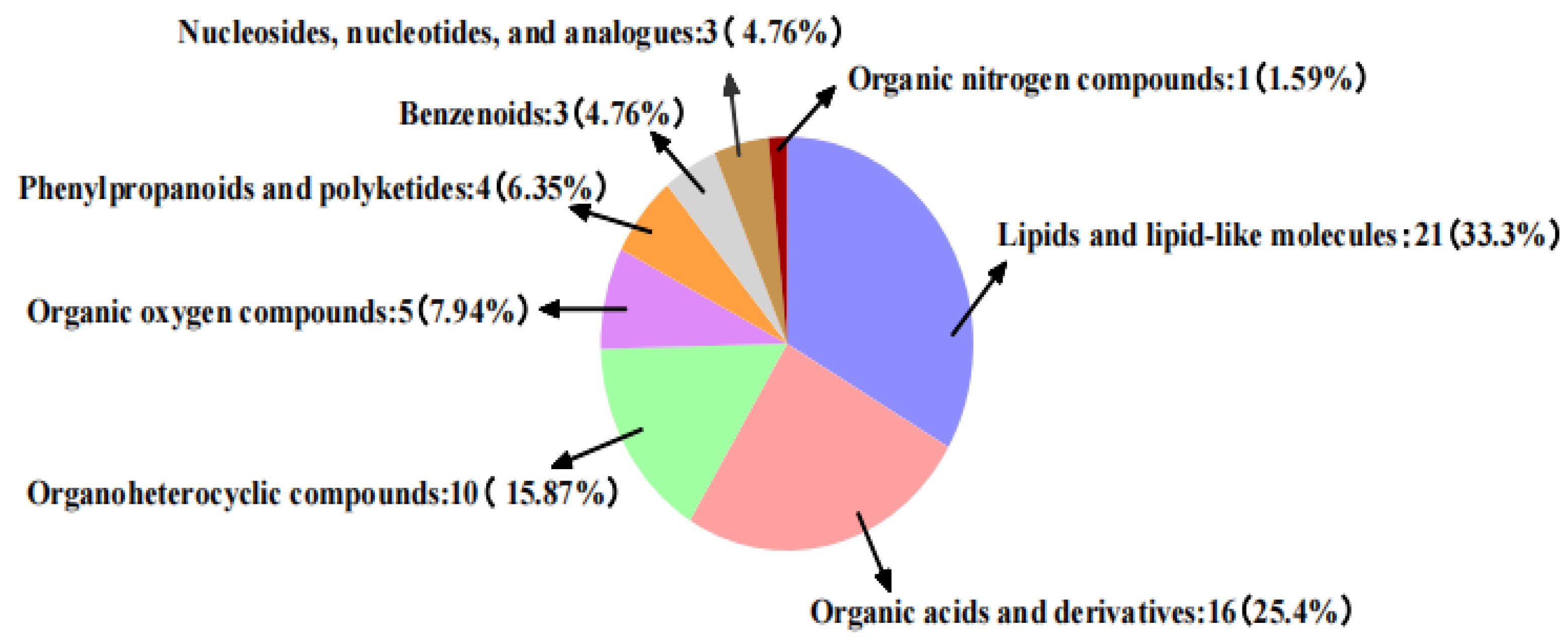
3.2.2. Difference Statistics and Volcano Plots of Non-Targeted Metabolites in Vigna angularis under FSA Stress
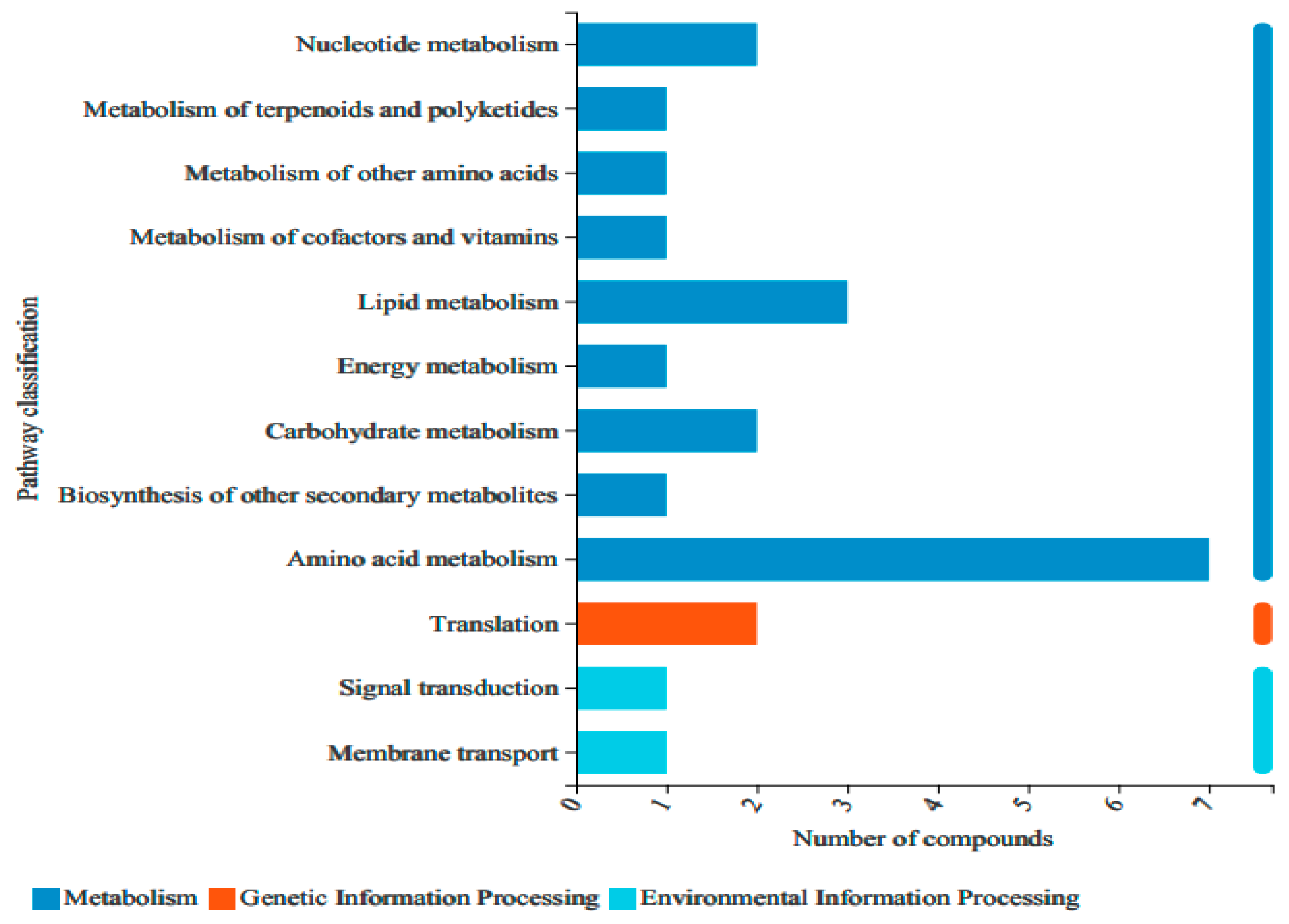
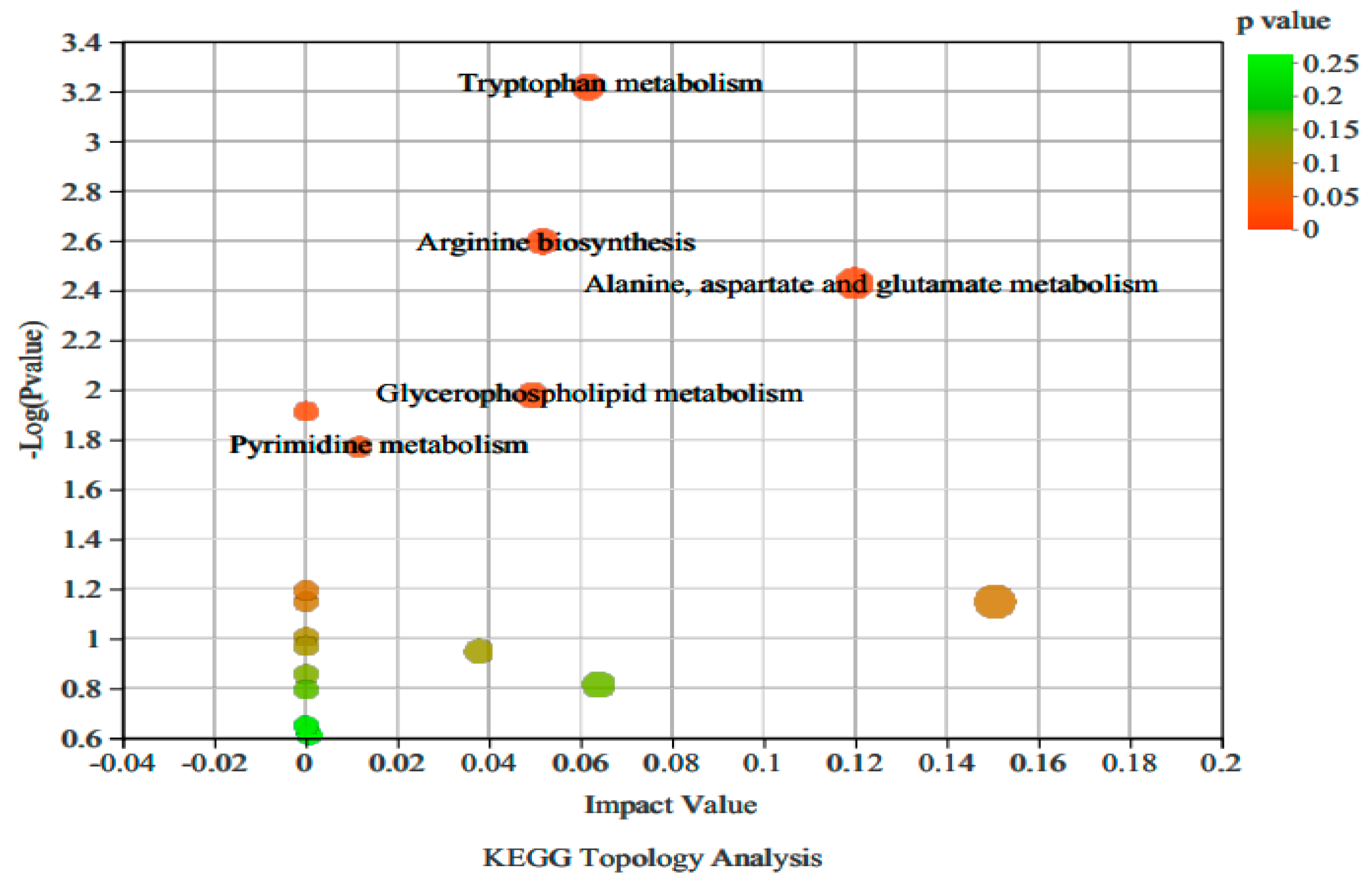
3.2.3. Analysis of Non-Targeted Metabolic Pathways in Vigna angularis under FSA Stress
3.3. Metabolomic Analysis of Targeted Amino Acids in Vigna angularis under FSA Stress
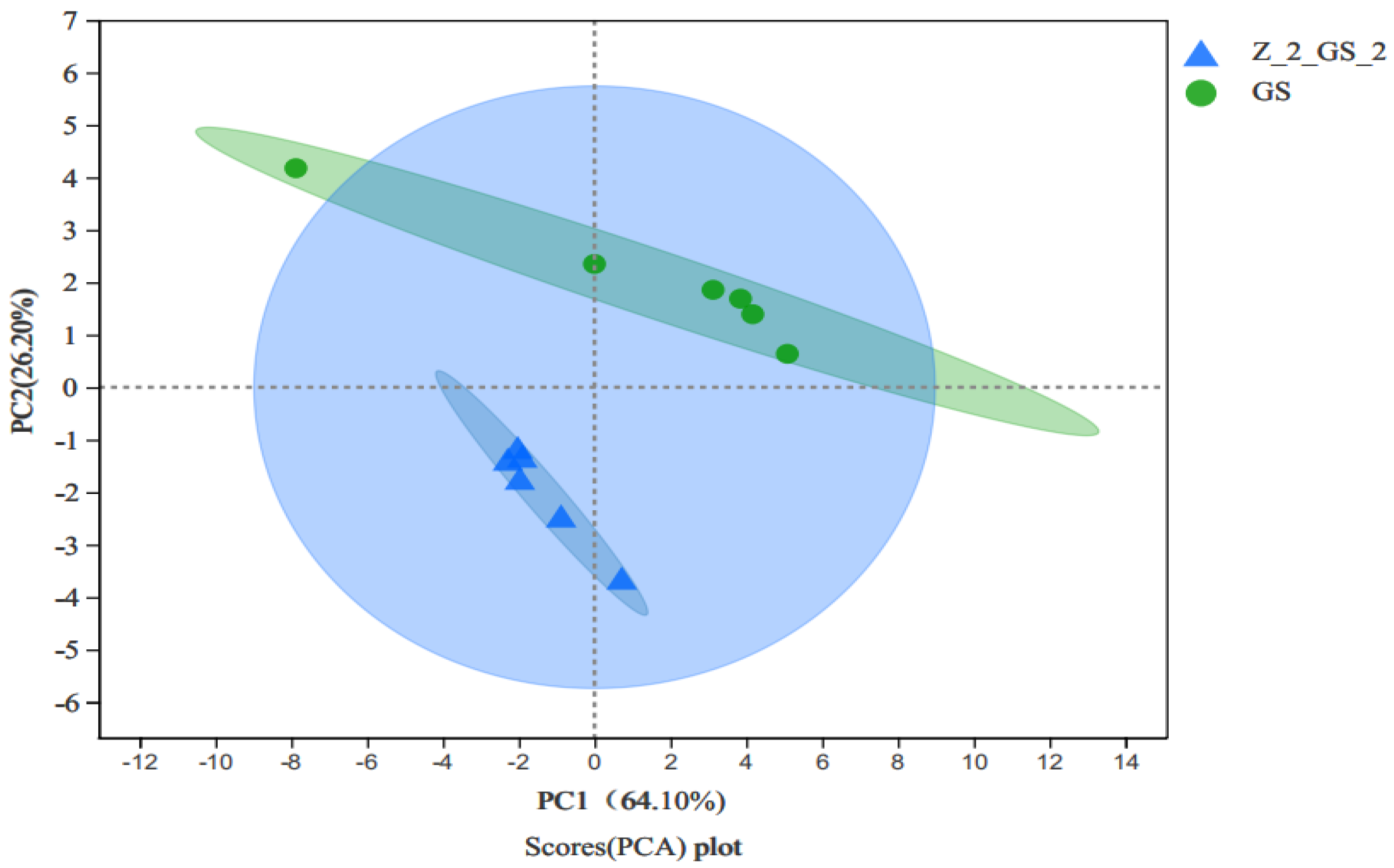
3.3.1. PCA Analysis of Targeted Amino Acids in Vigna angularis under FSA Stress
3.3.2. Univariate Statistical Analysis of Amino Acid Metabolites in Vigna angularis under FSA Stress
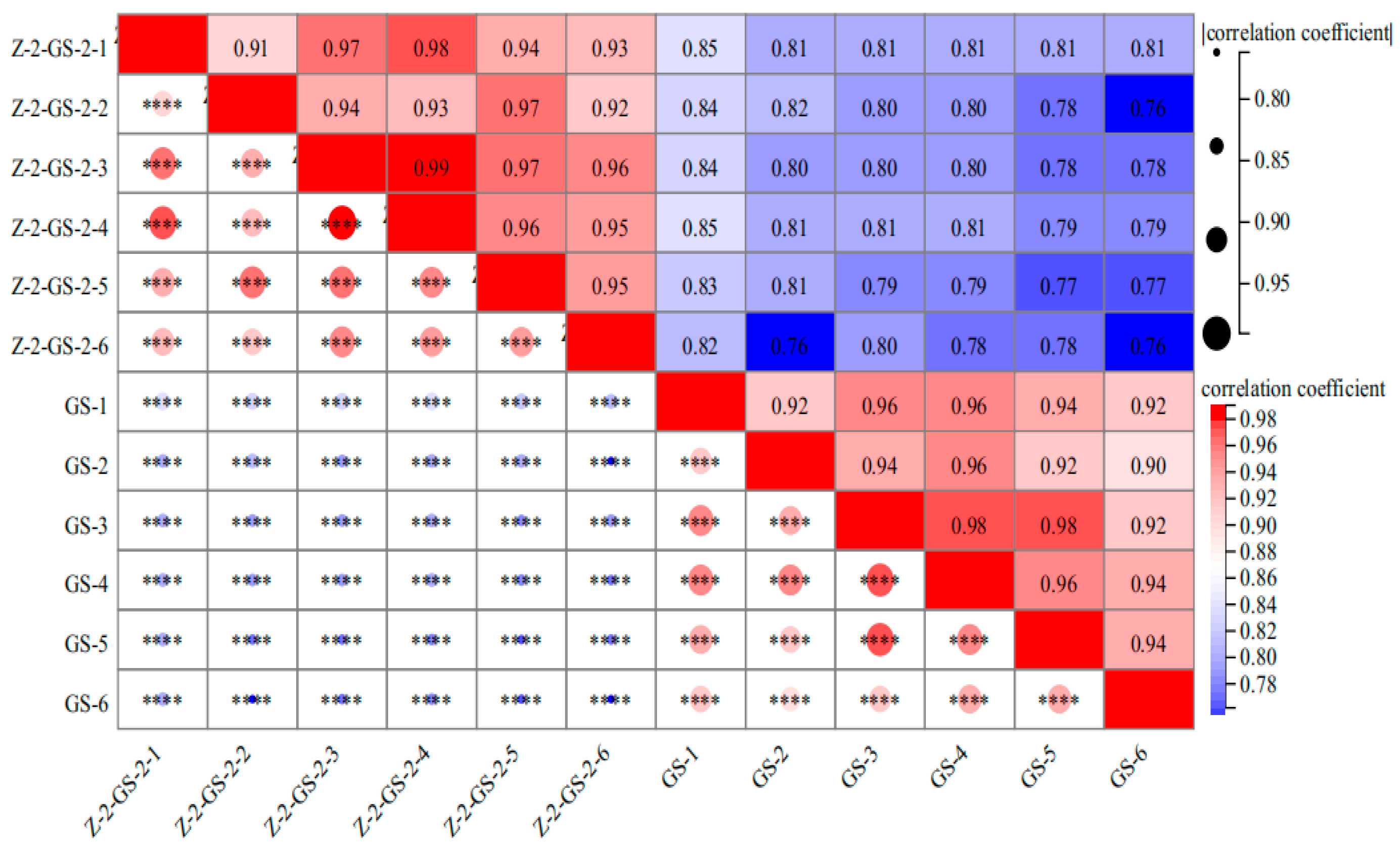
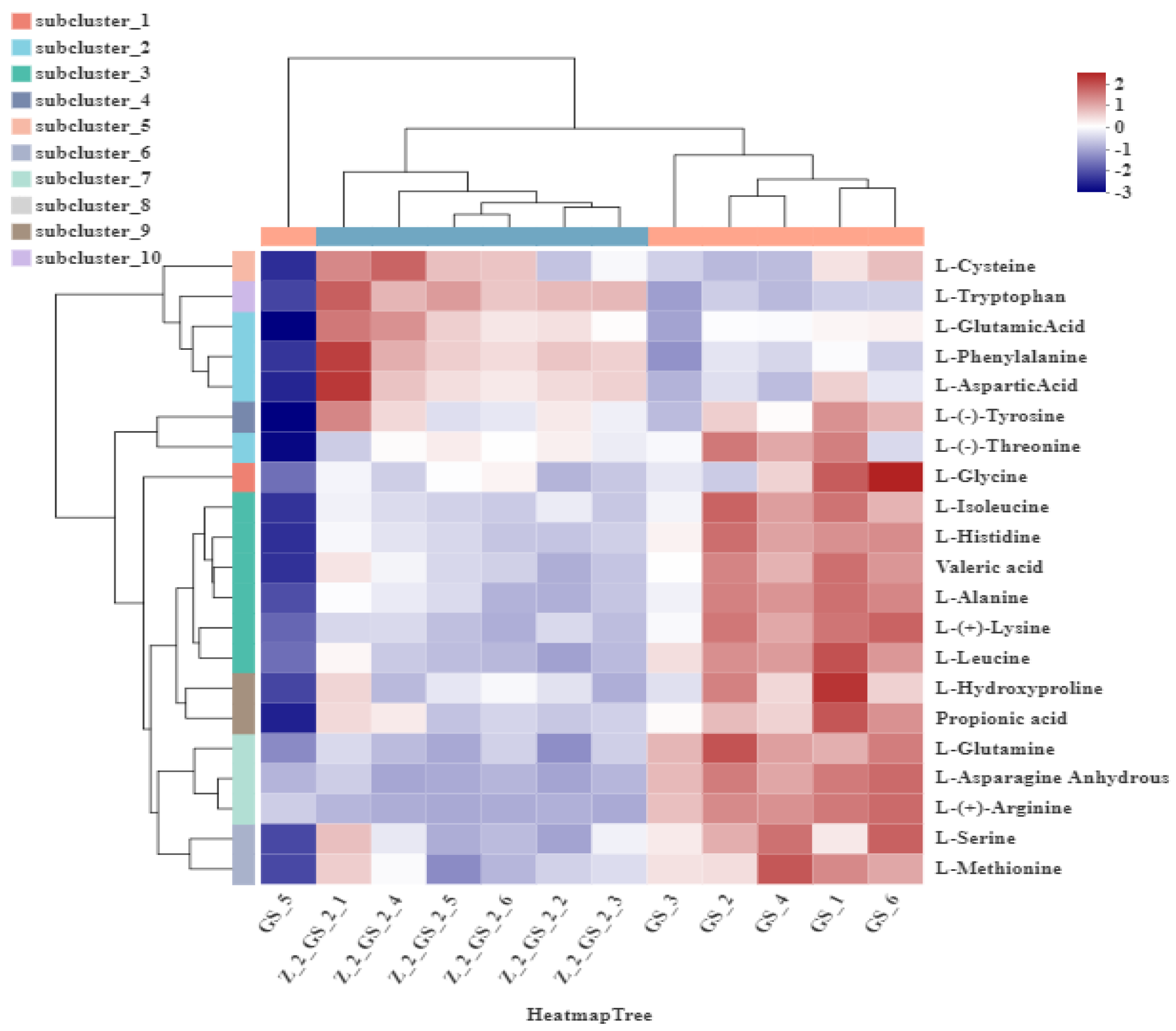
3.3.3. Hierarchical Clustering and Correlation Analysis of Amino Acid Metabolites in Vigna angularis under FSA Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shen, X.H. Common rust and root rot control methods of red bean in Heilongjiang Province. Heilongjiang Agric. Sci. 2017, 7, 132–133. [Google Scholar]
- Liu, J.X.; Liu, H.X.; Wen, R.Y.; Liu, Z.P. Detection and analysis of nutritional components in adzuki bean germplasm resources. J. Shanxi Datong Univ. (Nat. Sci. Ed.) 2017, 33, 54–57+76. [Google Scholar]
- Li, J. High value for both food and medicine. Benefits of eating adzuki beans in spring. Chin. Food 2022, 7, 157–158. [Google Scholar]
- Zhang, B.; Xue, W.T. Research progress on functional characteristics of adzuki bean. Food Sci. 2012, 33, 264–266. [Google Scholar]
- Zhang, H.J.; Jia, D.Y.; Yao, K. Research progress on nutrition and health function of mung bean. Food Ferment. Technol. 2012, 48, 7–10. [Google Scholar]
- Ma, R.P.; Ren, S.C. Health function, processing, and utilization of adzuki bean. Grain Sci. Technol. Econ. 2012, 37, 36–37. [Google Scholar]
- Hori, Y.; Sato, S.; Hatai, A. Antibacterial activity of plant extracts from adzuki beans (Vigna angularis) in vitro. Phytother. Res. 2010, 20, 5–9. [Google Scholar]
- Zhou, S.X.; Wei, C.J.; Hu, H.Y.; Gao, B.J.; Li, Z.J. Effects of fomesafen on microorganism and enzyme activity in soybean rhizosphere soil and its degradation in the rhizosphere. J. Plant Nutr. Fert. 2018, 24, 203–211. [Google Scholar]
- Patel, R.; Patidar, J.; Jain, K.K. Effect of different doses of fomesafen + fenoxaprop + chlorimuron-ethyl (ready-mix) against weeds in soybean. Indian J. Weed Sci. 2021, 53, 5–7. [Google Scholar] [CrossRef]
- Pritam, G.; Kalipada, P. Efficacy of fomesafen against broadleaved weeds and productivity improvement in soybean. Plant Cell Biotechnol. Mol. Biol. 2020, 21, 53–60. [Google Scholar]
- Huang, C.Y.; Wang, Y.; Huang, Y.J.; Park, D.W. The efficacy of eight herbicides in controlling weeds in adzuki bean fields and their safety in adzuki bean fields. Weed Sci. 2014, 32, 101–106. [Google Scholar]
- Bao, Q.; Liu, C.Q.; Duan, X.M.; Liu, S.H.; Wang, P.; Song, X.Q. Weeding effect of fomesafen on adzuki bean in different application periods. Mod. Agric. 2008, 6, 44–45. [Google Scholar]
- Tang, S.Y.; Zhang, L.; Tang, J.; Xiang, J.; Chu, N.M.; Yang, J.Y. Study on physicochemical properties and starch properties of several adzuki beans. China Agric. Bull. 2018, 34, 143–148. [Google Scholar]
- Lin, L.M.; Wang, Q.f.; Yu, H.; Xu, H.; Zhang, Z.W. Analysis of freezing characteristics and metabolites of edible cassava root tubers. Sci. Technol. Food Ind. 2022, 43, 1–12. [Google Scholar]
- Wang, Q.Q. Studies on the Fungi of the Genus Bursangium in Black Tea and Their Effects on Tea Quality. Master’s Thesis, Guizhou Normal University, Guiyang, China, 2021. [Google Scholar] [CrossRef]
- A, J.Y.; He, J.; Sun, R.B. Ten key points of metabonomics data processing principal component analysis. J. Pharm. 2018, 53, 929–937. [Google Scholar]
- Luo, S.; Zhang, Q.L.; Yang, F.; Lu, J.J.; Pu, X.X.; Zhang, J.; Wang, L. Analysis of functional components of antibacterial black koji in Maotai flavor Daqu based on non-targeted metabonomics. Food Ferment. Ind. 2022, 48, 16–23. [Google Scholar]
- Kasia, D.; Shelton, B.; Guillaume, P. Update on amino acid transporter functions and possible amino acid sensing mechanisms in plants. Semin. Cell Dev. Biol. 2018, 74, 105–113. [Google Scholar]
- Ananieva, E.A.; Wilkinson, A.C. Branched-chain amino acid metabolism in cancer. Curr. Opin. Clin. Nutr. Metab. Care. 2018, 21, 64–70. [Google Scholar] [CrossRef]
- Lin, R.R.; Yuan, H.F.; Zhong, X.Q.; Tang, S.C.; Wu, J.J.; Guo, Z.B. Effects of Different Boiling Processes on Nutrients and Flavor Substances of “Fotiaoqiang”. Food Sci. 2022, 1–12. Available online: http://kns.cnki.net/kcms/detail/11.2206.TS.20220613.1545.159.html (accessed on 26 December 2022).
- Hou, N.; Zhao, L.L.; Wei, A.Z.; Yang, T.X. Amino acid composition and nutritional value evaluation of different germplasm Zanthoxylum bungeanum. Food Sci. 2017, 38, 113–118. [Google Scholar]
- Jiang, Y.G.; Xu, Q.S. Research progress on the role and mechanism of conditionally essential amino acids in wound healing. Amino Acid Biol. Resour. 2002, 3, 59–62. [Google Scholar]
- Luo, H.J. Physiological and Ecological Responses of Different Varieties of Adzuki Bean to Water Stress and Rehydration. Master’s Thesis, Shanxi Normal University, Taiyuan, China, 2015. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?FileName=1015606671.nh&DbName=CMFD2015 (accessed on 26 December 2022).
- Wang, J.Y. Study on the Metabonomic Response of Rice Seedlings under High Concentrations of CO2 and Lead Stress. Master’s Thesis, Shenyang Normal University, Shenyang, China, 2020. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?FileName=1020741446.nh&DbName=CMFD2020 (accessed on 26 December 2022).
- Yan, F.; Wen, Z.S.; Wang, R.; Luo, W.L.; Du, Y.F.; Wang, W.J.; Chen, X.Y. Identification of the lipid biomarkers from plasma in idiopathic pulmonary fibrosis by Lipidomics. BMC Pulm. Med. 2017, 17, 173–174. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Y.; Yang, F.; Mao, S.; Li, S.X.; Lin, H.J.; Yan, X.F.; Lin, J.X. Research progress on physiological functions of plant lipids in response to stress. J. Biol. Eng. 2021, 37, 2658–2667. [Google Scholar]
- Li, Y.J.; Ma, P.J.; Long, Z.F.; Shu, J.H.; Chen, Y.; Wang, X.L. Metabonomic analysis of Pulsatilla Chinensis under low phosphorus and drought stress. J. Gra. Sci. 2022, 30, 329–338. [Google Scholar]
- Atteya, A.K.; El-Serafy, R.S.; El-Zabalawy, K.M.; Elhakem, A.; Genaidy, E.A. Exogenously Supplemented Proline and Phenylalanine Improve Growth, Productivity, and Oil Composition of Salted Moringa by Up-Regulating Osmoprotectants and Stimulating Antioxidant Machinery. Plants 2022, 11, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Ham, Y.-H.; Jason Chan, K.K.; Chan, W. Thioproline Serves as an Efficient Antioxidant, Protecting Human Cells from Oxidative Stress and Improves Cell Viability. Chem. Res. Toxicol. 2020, 33, 37–39. [Google Scholar] [CrossRef]
- Liang, X.; He, J.; Zhang, N.; Muhammad, A.; Lu, X.; Shao, Y. Probiotic potentials of the silkworm gut symbiont Enterococcus casseliflavus ECB140, a promising L-tryptophan producer is living inside the host. J. Appl. Microbiol. 2022, 133, 47–51. [Google Scholar] [CrossRef]
- Cauli, O.; Rodrigo, R.; Llansola, M.; Montoliu, C.; Monfort, P.; Piedrafita, B.; Felipo, V. Glutamatergic and gabaergic neurotransmission and neuronal circuits in hepatic encephalopathy. Metab. Brain Dis. 2009, 24, 69–80. [Google Scholar] [CrossRef]
- Tong, B.C.; Barbul, A. Cellular and physiological effects of arginine. Mini-Rev. Med. Chem. 2004, 4, 823–832. [Google Scholar] [CrossRef]
- Pascual, M.B.; El-Azaz, J.; de la Torre, F.N.; Cañas, R.A.; Avila, C.; Cánovas, F.M.l. Biosynthesis and Metabolic Fate of Phenylalanine in Conifers. Front. Recent Dev. Plant Sci. 2016, 7, 1030–1042. [Google Scholar]
- Mckee, T.; Mckee, J.R. Biochemistry, 2nd ed.; Mc Graw-Hill Education: Boston, MA, USA, 1998; pp. 370–375. [Google Scholar]
- Zhang, X.M.; Guo, C.J.; Li, J.X.; Xu, Q.S. Research progress on the application of genetic engineering in L-tryptophan production. J. Acad. Mil. Med. Sci. 2005, 4, 379–382. [Google Scholar]
- Cheng, G.; Xu, J.Z.; Zhang, W.G. Research progress on the biosynthesis mechanism of L-arginine and its metabolic engineering breeding. Bul. Mikrobiyol. 2016, 43, 1379–1387. [Google Scholar]
- Liu, J.X.; Wang, J.C.; Liu, X.L. Response of arginine metabolism of oat seedlings to exogenous NO under lanthanum stress. J. Chin. Soc. Rare Earths. 2018, 36, 236–246. [Google Scholar]

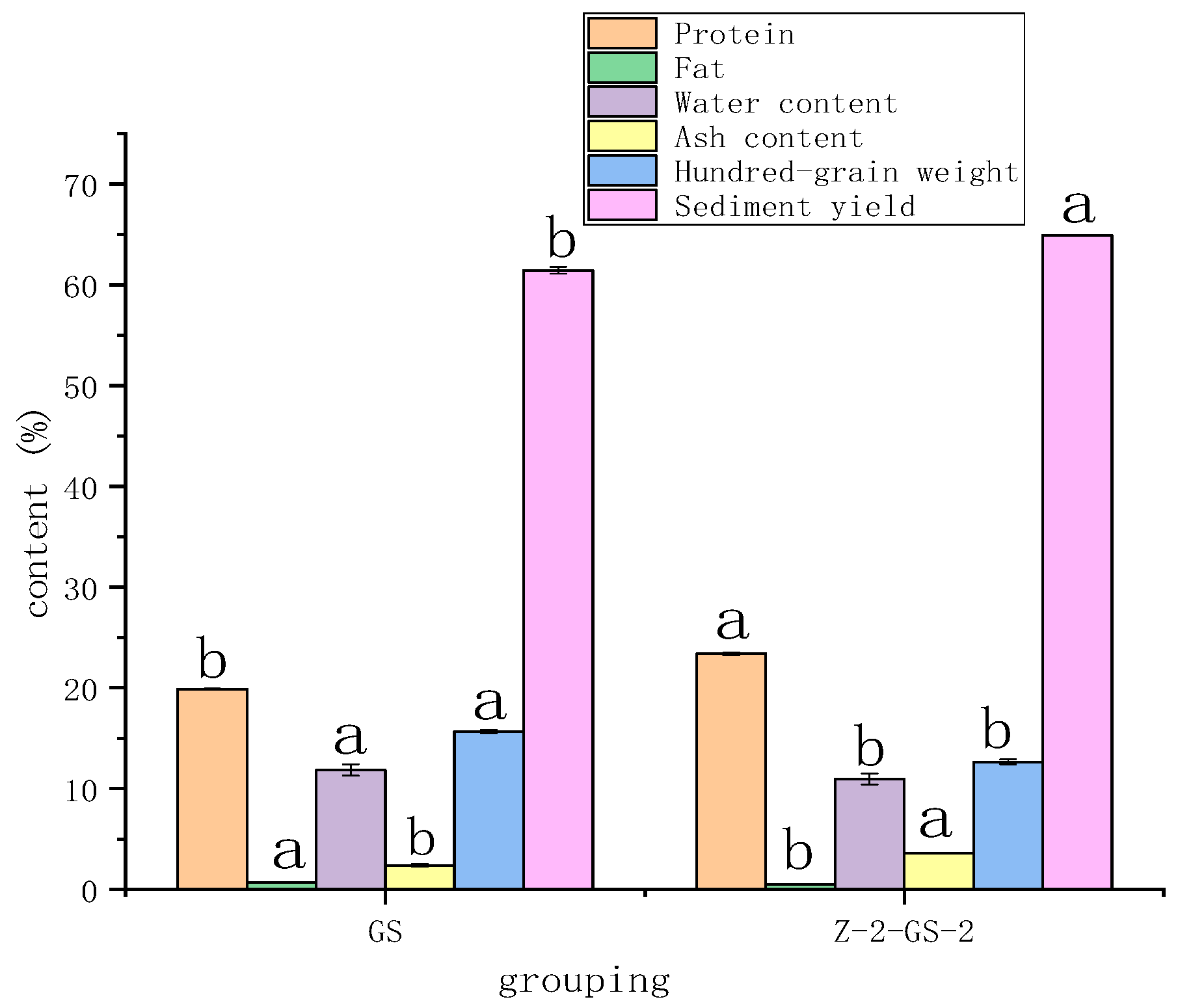

| Amino Acid | Curvilinear Equation | R2 | Stability RSD |
|---|---|---|---|
| L-Alanine | y = 3.62534 × 104x + 2745.21904 | 0.99 | 6.71 |
| L-(+)-Arginine | y = 3.34990 × 105x + 22,361.20942 | 0.99 | 2.26 |
| L-Asparagine Anhydrous | y = 11,190.20535x + 362.17558 | 0.99 | 1.41 |
| L-Aspartic Acid | y = 14,381.55174x + 2544.17691 | 1.00 | 0.59 |
| L-Glutamine | y = 8.80826 × 104x − 2141.00556 | 0.99 | 9.28 |
| L-Glutamic Acid | y = 6.18543 × 104x − 5738.94582 | 0.99 | 3.14 |
| Glycine | y = 1283.62469x + 387.78725 | 1.00 | 7.21 |
| L-Histidine | y = 5.99474 × 105x + 20,541.52719 | 0.99 | 4.27 |
| L-Isoleucine | y = 4.26940 × 105x + 4177.43684 | 0.99 | 5.68 |
| L-Cysteine | y = 11603.86832x + 114.70251 | 0.99 | 6.41 |
| L-Leucine | y = 5.91002 × 105x + 10,046.23662 | 0.99 | 2.02 |
| L-Hydroxyproline | y = 3.53570 × 105x − 381.01868 | 1.00 | 4.30 |
| L-Tryptophan | y = 3.04138 × 105x − 3702.86927 | 0.99 | 1.94 |
| L-(+)-Lysine | y = 1.32331 × 105x + 4482.19554 | 0.99 | 2.43 |
| L-Methionine | y = 1.65349 × 105x − 916.81109 | 0.99 | 2.81 |
| L-Phenylalanine | y = 9.77658 × 105x − 451.91207 | 0.99 | 1.65 |
| Propionic acid | y = 2.65314 × 106x + 4.11763 × 104 | 0.99 | 2.50 |
| L-Serine | y = 15,677.23525x + 3640.87777 | 0.99 | 3.12 |
| L-(−)-Threonine | y = 6.81196 × 104x + 2645.97560 | 0.99 | 0.42 |
| L-(−)-Tyrosine | y = 1.79224 × 105x + 9042.08487 | 1.00 | 2.20 |
| Valeric acid | y = 6.85992 × 104x + 482.11990 | 0.99 | 2.41 |
| Numbers | Metabolite | VIP | FC | p_Value | FDR | Retention Time | M/Z | Content Change |
|---|---|---|---|---|---|---|---|---|
| 1 | 3,4,5-trihydroxy-6-[(3-methylbut-2-enoyl)oxy]oxane-2-carboxylic acid | 1.56 | 0.90 | 0.04 | 0.18 | 1.28 | 318.00 | − |
| 2 | Cytarabine | 1.91 | 0.90 | 0.00 | 0.03 | 0.76 | 244.00 | − |
| 3 | Cynaroside A | 2.90 | 1.17 | 0.00 | 0.00 | 2.71 | 462.00 | + |
| 4 | (7b,10a)-3-Hydroxy-1,3,5-cadinatrien-9-one | 1.32 | 0.95 | 0.01 | 0.09 | 4.29 | 503.00 | − |
| 5 | Isobornyl 2-methylbutyrate | 1.38 | 1.05 | 0.00 | 0.02 | 5.41 | 221.00 | + |
| 6 | 19-Oxotestosterone | 1.21 | 0.97 | 0.01 | 0.08 | 4.28 | 285.00 | − |
| 7 | 3-Hydroxyadipic acid 3,6-lactone | 1.82 | 1.07 | 0.00 | 0.03 | 1.33 | 162.00 | + |
| 8 | P-cresol | 1.01 | 1.02 | 0.00 | 0.04 | 1.84 | 91.00 | + |
| 9 | 2’-O-Methyladenosine | 1.23 | 0.96 | 0.01 | 0.09 | 2.14 | 282.00 | − |
| 10 | 4-Hydroxy-5-(phenyl)-valeric acid-O-glucuronide | 1.86 | 0.92 | 0.01 | 0.09 | 4.77 | 388.00 | − |
| 11 | Sterebin B | 1.44 | 1.04 | 0.00 | 0.01 | 6.05 | 743.00 | + |
| 12 | 1-Lyso-2-arachidonoyl-phosphatidate | 1.95 | 0.91 | 0.01 | 0.08 | 8.37 | 476.00 | − |
| 13 | Muricatenol | 2.14 | 1.14 | 0.00 | 0.06 | 6.57 | 631.00 | + |
| 14 | Squamotacin | 1.88 | 1.10 | 0.01 | 0.11 | 6.55 | 645.00 | + |
| 15 | 6alpha-Hydroxyphaseollin | 2.22 | 1.19 | 0.02 | 0.13 | 5.98 | 321.00 | + |
| 16 | Porric acid B | 1.24 | 0.96 | 0.02 | 0.14 | 3.42 | 289.00 | − |
| 17 | (+)-Abscisic Acid | 2.16 | 1.09 | 0.00 | 0.00 | 2.86 | 265.00 | + |
| 18 | Glu-Val | 1.42 | 1.07 | 0.04 | 0.18 | 2.18 | 247.00 | + |
| 19 | Molybdopterin precursor Z | 1.45 | 1.08 | 0.01 | 0.09 | 0.50 | 310.00 | + |
| 20 | L-NMMA | 1.69 | 1.08 | 0.01 | 0.09 | 0.59 | 189.00 | + |
| 21 | Niacinamide | 1.51 | 0.94 | 0.02 | 0.12 | 1.19 | 123.00 | − |
| 22 | 3-Methyl-3-butenyl apiosyl-(1->6)-glucoside | 2.10 | 0.85 | 0.01 | 0.09 | 2.39 | 381.00 | − |
| 23 | Captopril-cysteine disulfide | 1.59 | 0.93 | 0.02 | 0.12 | 3.76 | 319.00 | − |
| 24 | Salsoline-1-carboxylate | 2.96 | 0.71 | 0.03 | 0.16 | 5.56 | 260.00 | − |
| 25 | Dehydroabietic acid | 1.33 | 0.96 | 0.01 | 0.10 | 5.94 | 301.00 | − |
| 26 | Soyasaponin V | 1.57 | 1.04 | 0.00 | 0.01 | 6.04 | 923.00 | + |
| 27 | Momordin Ia | 1.58 | 1.04 | 0.00 | 0.01 | 6.19 | 743.00 | + |
| 28 | LysoPC(18:1(11Z)) | 1.34 | 0.96 | 0.02 | 0.13 | 6.46 | 522.00 | − |
| 29 | 1-(4Z,7Z,10Z,13Z,16Z,19Z-docosahexaenoyl)-glycero-3-phosphate | 2.12 | 0.87 | 0.00 | 0.07 | 7.61 | 500.00 | − |
| 30 | 1-Linoleoylglycerophosphocholine | 1.07 | 0.98 | 0.02 | 0.13 | 6.22 | 520.00 | − |
| 31 | Sphinganine | 1.12 | 1.03 | 0.02 | 0.12 | 6.13 | 302.00 | + |
| 32 | Arnidenediol | 1.12 | 1.02 | 0.00 | 0.04 | 6.05 | 407.00 | + |
| 33 | Eremopetasitenin C2 | 2.69 | 0.84 | 0.00 | 0.02 | 5.82 | 443.00 | − |
| 34 | (all-E)-Crocetin | 1.62 | 0.95 | 0.00 | 0.02 | 5.74 | 329.00 | − |
| 35 | Physalin I | 1.33 | 0.92 | 0.02 | 0.15 | 5.02 | 559.00 | − |
| 36 | Dihydrogenistein | 1.63 | 0.93 | 0.01 | 0.07 | 4.72 | 273.00 | − |
| 37 | Indolylacryloylglycine | 1.86 | 0.90 | 0.01 | 0.09 | 4.59 | 227.00 | − |
| 38 | Luteoloside | 1.74 | 0.92 | 0.01 | 0.11 | 4.30 | 449.00 | − |
| 39 | (3E)-4-phenylbuta-1,3-dien-2-ol | 1.28 | 0.95 | 0.02 | 0.14 | 4.28 | 129.00 | − |
| 40 | 5-Hydroxyindoleacetylglycine | 1.75 | 0.90 | 0.03 | 0.17 | 4.17 | 231.00 | − |
| 41 | Arginyl-Serine | 2.19 | 0.83 | 0.02 | 0.14 | 4.04 | 561.00 | − |
| 42 | 5-Methoxyindoleacetate | 1.27 | 0.95 | 0.04 | 0.19 | 3.87 | 206.00 | − |
| 43 | Gamma-Glutamyl-S-(1-propenyl)cysteine sulfoxide | 2.26 | 0.86 | 0.01 | 0.08 | 3.22 | 271.00 | − |
| 44 | Norharman | 1.25 | 1.05 | 0.00 | 0.04 | 3.15 | 169.00 | + |
| 45 | P-Coumaroyl 3-hydroxytyrosine | 2.78 | 0.79 | 0.00 | 0.02 | 3.08 | 361.00 | − |
| 46 | Benzaldehyde | 1.14 | 0.96 | 0.03 | 0.16 | 2.75 | 107.00 | − |
| 47 | (+/−)-Taxifolin | 2.03 | 0.92 | 0.00 | 0.04 | 2.60 | 305.00 | − |
| 48 | Kinetin-7-N-glucoside | 2.05 | 1.14 | 0.02 | 0.12 | 2.43 | 360.00 | + |
| 49 | Succinoadenosine | 1.67 | 0.94 | 0.00 | 0.04 | 2.31 | 384.00 | − |
| 50 | Indicaxanthin | 2.78 | 0.79 | 0.00 | 0.03 | 2.14 | 372.00 | − |
| 51 | Valyl-Hydroxyproline | 2.30 | 0.85 | 0.00 | 0.06 | 1.95 | 231.00 | − |
| 52 | Gamma-L-Glutamyl-gamma-L-glutamyl-L-methionine | 2.89 | 0.80 | 0.00 | 0.01 | 1.79 | 440.00 | − |
| 53 | N-Acetylornithine | 3.80 | 1.42 | 0.00 | 0.01 | 0.76 | 157.00 | + |
| 54 | Ascorbalamic acid | 1.46 | 0.96 | 0.00 | 0.03 | 0.74 | 264.00 | − |
| 55 | Cytosine | 1.77 | 0.90 | 0.01 | 0.07 | 0.69 | 112.00 | − |
| 56 | Gamma-Glutamylproline | 1.04 | 1.02 | 0.00 | 0.03 | 0.63 | 286.00 | + |
| 57 | Choline Glycerophosphate | 1.06 | 1.02 | 0.00 | 0.04 | 0.63 | 258.00 | + |
| 58 | L-Glutamine | 2.47 | 1.18 | 0.00 | 0.06 | 0.62 | 147.00 | + |
| 59 | L-Asparagine | 2.02 | 1.09 | 0.00 | 0.04 | 0.60 | 133.00 | + |
| 60 | 2-Pyrrolidinone | 1.53 | 1.06 | 0.00 | 0.01 | 0.59 | 86.00 | + |
| 61 | Indole | 1.30 | 0.97 | 0.00 | 0.05 | 2.49 | 118.00 | − |
| 62 | Kynurenine | 2.95 | 1.20 | 0.00 | 0.00 | 2.06 | 209.00 | + |
| 63 | Levan | 1.50 | 1.05 | 0.00 | 0.01 | 0.87 | 527.00 | + |
| Number | Pathway Description | Pathway ID | Total | Hits | Impact _Value | p_ Value | Metabolite | KEGG Compound ID |
|---|---|---|---|---|---|---|---|---|
| 1 | Alanine, aspartate and glutamate metabolism | map00250 | 28 | 2 | 0.12 | 0.00 | L-Glutamine; L-Glutamine | C00064; C00152 |
| 2 | Tryptophan metabolism | map00380 | 56 | 3 | 0.06 | 0.00 | 5-Methoxyindoleacetate; Indole; Kynurenine | C05660; C00463; C00328 |
| 3 | Arginine biosynthesis | map00220 | 23 | 2 | 0.05 | 0.00 | N-Acetylornithine; L-Glutamine | C00437; C00064 |
| 4 | Glycerophospholipid metabolism | map00564 | 48 | 2 | 0.05 | 0.01 | L-Glutamine; Choline Glycerophosphate | C04230; C00670 |
| 5 | Pyrimidine metabolism | map00240 | 62 | 2 | 0.01 | 0.02 | Cytosine; L-Glutamine | C00380; C00064 |
| Number | Metabolite | VIP | Retention Time | p_Value | FDR | FC | Content Change |
|---|---|---|---|---|---|---|---|
| 1 | L-Alanine | 0.39 | 2.83 | 0.10 | 0.20 | 0.82 | − |
| 2 | L-(+)-Arginine | 3.50 | 3.95 | 0.00 | 0.00 | 0.41 | − |
| 3 | L-Asparagine Anhydrous | 1.57 | 3.44 | 0.00 | 0.01 | 0.57 | − |
| 4 | L-Aspartic Acid | 1.08 | 3.70 | 0.02 | 0.06 | 1.45 | + |
| 5 | L-Glutamine | 0.42 | 3.43 | 0.01 | 0.04 | 0.71 | − |
| 6 | L-Glutamic Acid | 1.88 | 3.39 | 0.04 | 0.11 | 1.24 | + |
| 7 | L-Glycine | 0.18 | 3.17 | 0.30 | 0.37 | 0.88 | − |
| 8 | L-Histidine | 0.35 | 4.00 | 0.16 | 0.28 | 0.86 | − |
| 9 | L-Isoleucine | 0.11 | 1.93 | 0.19 | 0.29 | 0.88 | − |
| 10 | L-Cysteine | 0.17 | 2.93 | 0.07 | 0.14 | 1.25 | + |
| 11 | L-Leucine | 0.24 | 2.02 | 0.03 | 0.10 | 0.77 | − |
| 12 | L-Hydroxyproline | 0.04 | 2.88 | 0.33 | 0.38 | 0.89 | − |
| 13 | L-Tryptophan | 0.78 | 1.91 | 0.00 | 0.00 | 1.55 | + |
| 14 | L-(+)-Lysine | 0.36 | 4.01 | 0.05 | 0.11 | 0.76 | − |
| 15 | L-Methionine | 0.06 | 2.20 | 0.19 | 0.29 | 0.87 | − |
| 16 | L-Phenylalanine | 0.43 | 1.90 | 0.00 | 0.02 | 1.40 | + |
| 17 | Propionic acid | 0.14 | 2.29 | 0.40 | 0.44 | 0.91 | − |
| 18 | L-Serine | 0.14 | 3.43 | 0.21 | 0.29 | 0.86 | − |
| 19 | L-(−)-Threonine | 0.05 | 3.10 | 0.85 | 0.85 | 0.98 | − |
| 20 | L-(−)-Tyrosine | 0.07 | 2.36 | 0.59 | 0.61 | 1.05 | + |
| 21 | Valeric acid | 0.20 | 2.24 | 0.22 | 0.29 | 0.87 | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, H.; Yang, J.; Cao, D. Effect of Fomesafen on the Nutritional Quality and Amino Acids of Vigna angularis Based on Metabonomics. Agronomy 2023, 13, 452. https://doi.org/10.3390/agronomy13020452
Tang H, Yang J, Cao D. Effect of Fomesafen on the Nutritional Quality and Amino Acids of Vigna angularis Based on Metabonomics. Agronomy. 2023; 13(2):452. https://doi.org/10.3390/agronomy13020452
Chicago/Turabian StyleTang, Huacheng, Jian Yang, and Dongmei Cao. 2023. "Effect of Fomesafen on the Nutritional Quality and Amino Acids of Vigna angularis Based on Metabonomics" Agronomy 13, no. 2: 452. https://doi.org/10.3390/agronomy13020452
APA StyleTang, H., Yang, J., & Cao, D. (2023). Effect of Fomesafen on the Nutritional Quality and Amino Acids of Vigna angularis Based on Metabonomics. Agronomy, 13(2), 452. https://doi.org/10.3390/agronomy13020452






