Abstract
The influence of polyamines (PAs), putrescine (Put) and spermidine (Spd) on the efficiency of gynogenesis in ovule cultures of red beet (syn. beetroot) (Beta vulgaris L. vulgaris) cultivar “Czerwona Kula” and breeding accessions no. 3/2010 and no. 7/2008 was investigated. The effect of Put on the process of plant regeneration from gynogenetic embryos was studied. The response to the applied PAs was strongly dependent on the genotype. In “Czerwona Kula”, an increase in the number of obtained embryos was achieved by using each of the two PAs in the B5 medium. The effect of Spd was stronger. Put added to the regeneration medium at the concentration of 0.5 mg L−1 increased the number of obtained plants. All shoots placed on the rooting medium supplemented with 160 mg L−1 Put formed roots. The distribution of ploidy and homozygosity of gynogenetic plants depended on the genotype. Of the tested genotypes, the highest number of haploid plants, 68%, was obtained in red beet “Czerwona Kula”. The highest percentage of homozygotes, 69% for the glucose phosphate isomerase (GPI, E.C.5.3.1.9) isoenzyme and 100% for the aspartate aminotransferase (AAT, E.C.2.6.1.1) isoenzyme, was obtained in the population of gynogenetic plants of cultivar “Czerwona Kula”.
1. Introduction
Polyamines (PAs) regulate many biological processes and are considered plant growth regulators (PGRs) influencing cell division, differentiation, organogenesis, pollen formation, fertilization, gene expression, DNA and protein synthesis, apoptosis, and stress responses [1,2]. These PGRs influence the growth and development of plant organs such as flower buds, fruits, storage organs and roots [3,4,5]. Regla-Marquez et al. [6] reported that the amount of endogenous PAs increased in the developing somatic and gametic embryos of Capsicum chinense Jacq. In the last decade, mutant studies conducted predominantly in Arabidopsis thaliana revealed an obligatory requirement for PAs in zygotic and somatic embryogenesis [7]. The addition of PAs, spermidine (Spd) and putrescine (Put) to regeneration media during the induction of gynogenesis in in vitro conditions resulted in improved development of the gynogenic embryos and haploid plantlets [8]. PAs regulated the tolerance of embryos to stresses [9]. In the process of gynogenesis, stress is a factor that changes the path of reproductive cell development from gametic to somatic embryogenesis. Excessive levels of stress may cause death of the embryo. PAs involved in this process can maintain the non-toxic level of reactive oxygen species (ROS), which are produced under stress conditions in plants [10]. Studies have shown that the exogenous use of PAs can improve the effectiveness of gametic embryogenesis through gynogenesis in onion [11,12] and cucumber [2,13].
Doubled haploid plants are a valuable breeding material that can efficiently speed up the process of production of new cultivars of economically important crops. Among crop plants, red beet, syn. beetroot (Beta vulgaris ssp. Vulgaris convar. crassa var. conditiva) and sugar beet are very important plants. Both of them belong to the same family of Amaranthaceae. Sugar beet (Beta vulgaris ssp. vulgaris convar. crassa var. altissima) has an established position as an industrial plant for the production of sugar. In recent years, there has been a growing interest in the biological activity of red beet and its potential usage as a health-promoting and disease-preventing functional food [14].
The genetic relationship between sugar beet and red beet has been estimated by several researchers, which contributed to the understanding of the genetic basis of both species [15]. This knowledge is used in modern breeding programs in which, by crossing with sugar beet, the following genes are introduced into red beet: sterile cytoplasm self-fertility, monogerm seed and the annual growth habit. However, DNA fingerprinting revealed low genetic similarities (GS) between sugar beet and fodder beet. Therefore, although gynogenesis was confirmed as an effective method of generating doubled haploids in sugar beet, in the literature, there are few reports on the application of this method to induce doubled haploid lines in red beet. Barański [16] managed to obtain several red beet haploid plants in the in vitro cultures of unfertilized ovules. Not until 2021 did two research groups publish the results of their experiments, in which they obtained homozygous red beet plants via gynogenesis. [17,18].
On the basis of numerous reports, it can be concluded that apart from the genotype, the factors strongly influencing the gynogenesis process are the composition of either the induction medium or the medium for regeneration of gynogenetic plants [16,18,19,20]. PGRs are the important components of culture media, enabling the initiation of the above-mentioned processes. Currently, some researchers consider polyamines (PAs) as a group of growth regulators. However, the concentrations at which they show any effect are much higher in comparison to the typical PGRs [1,2,21,22].
The primary goal of the research was to obtain gynogenetic plants of red beet. In the presented study, we examined the effect of PAs, spermidine and putrescine on the induction of gynogenesis and regeneration of plants from gynogenetic embryos.
2. Materials and Methods
2.1. Plant Material
Roots of different genotypes of red beet, which were intended for donor plants (donors) of buds, were planted in a substrate consisting of 1:3 (v/v) sand and soil, then placed in a cold chamber at +4 °C after undergoing through the vernalization process. Then, roots were planted in plastic containers with a capacity of 20 dm3 (two roots per container) and maintained in a growth chamber under strictly controlled growth conditions of +18 °C temperature during the 16 h photoperiod (photosynthetic photon flux density, PPFD at 55 µmol m−2 s−1 by warm-white fluorescent lamps) and +16 °C at night. All plants were grown in a growth chamber for eight months. Inflorescence shoots developing from the roots served as donors of flower buds used for research on gynogenesis.
The red beet cultivar “Czerwona Kula” was used in all of the experiments. In the experiments on the influence of the genotype, apart from the above-mentioned cultivar, the heterozygotic breeding accessions, 3/2010 and 7/2008, provided by cooperating Breeding and Seed Company—POLAN (Limited Liability Company—Cracow, Poland) were used.
2.2. Induction of Gynogenesis
Green, immature flower buds about 3 mm long with undivided petals were collected. Our earlier microscopic observations showed that buds of this size already contain mature ovules (data not presented). The buds were disinfected with 70% ethanol for 10 min on the laboratory shaker and washed two times in sterile distilled water. Ovules were isolated from flower buds under the Zeiss binocular microscope, model Stemi with 20× magnification. Next, using preparation needles, ovules were placed in Erlenmeyer flasks (100 mL) containing 30 mL of different induction media, solidified with 6.5 g L−1 agar (described below in Table 1). Ovules were cultured in 27 °C under continuous light (PPFD 55 μmol·m−2·s−1). Embryos formed on media within 6 to 14 weeks. The embryos were then subcultured on the regeneration media. The efficiency of the gynogenesis induction was defined by the number of obtained embryos per 100 planted ovules. Twenty-four ovules were placed per Erlenmeyer flask as replication. The number of plated ovules per treatment ranged from 40 to 688 depending on the availability of plant material. The induction media on the basis of N6 [23] and B5 [24] were supplemented with 100 g L−1 sucrose (Sigma-Aldrich Chemie GmbH, Schnelldorf, Germany), solidified with 6.5 g L−1 agar (Sigma-Aldrich Chemie GmbH, Schnelldorf, Germany) and their pH was adjusted to 5.6.

Table 1.
Components of the induction media prepared based on the B5 and N6 medium.
The influence of various PGRs at different combinations (Table 1) in both tested media on the gynogenesis induction was examined in red beet “Czerwona Kula”. The B5 and N6 media supplemented with 0.5 mg L−1 IAA 0.2 mg L−1 BA were used as control media, on which Górecka et al. [25] obtained gynogenetic embryos of red beet in the previous studies. The effect of the genotype on the gynogensis process was examined in all three genotypes on the medium that proved to be the most effective for the androgenesis in “Czerwona Kula” [26], i.e., B5-6 medium supplemented with 0.5 mg L−1 IAA (Sigma-Aldrich Chemie GmbH, Schnelldorf, Germany), 0.2 mg L−1 BA(Sigma-Aldrich Chemie GmbH, Schnelldorf, Germany) and 290 mg L−1 Spd (Sigma-Aldrich Chemie GmbH, Schnelldorf, Germany). A flask containing 24 ovules was a repetition in conducted experiments.
2.3. Regeneration of Gynogenetic Plants
Embryos of “Czerwona Kula” obtained in gynogenesis process were transferred for plant regeneration on MS media [27] with the addition of sucrose 30 g L−1, solidified with 6.5 g L−1 agar and pH adjusted to 5.6, applied by Zayachkovskaya et al. [18] and modified in our studies for the regeneration of plants from the gynogenetic embryos of red beet. The MS media containing 0.2 mg L−1 BA (Sigma-Aldrich Chemie GmbH, Schnelldorf, Germany) and supplemented with one of the auxins or PAs: 1 mg L−1 IAA (Sigma-Aldrich Chemie GmbH, Schnelldorf, Germany), 1 mg L−1 NAA (Sigma-Aldrich Chemie GmbH, Schnelldorf, Germany) or 0.5 mg L−1 Put (Sigma-Aldrich Chemie GmbH, Schnelldorf, Germany) were examined. Embryos were cultured under 16 h photoperiod at PPFD of 55 μmol·m−2·s−1 and temperature of 20 °C. The effectiveness of shoot/plant formation was evaluated after 6-week subculture on the regeneration media by counting the number of obtained regenerants, divided into two categories: plants and shoots.
In order to examine the influence of the genotype on the efficiency of plant regeneration, embryos of all tested genotypes were cultured on MS medium containing 0.2 mg L−1 BA and 0.5 mg L−1 Put with 30 g L−1 sucrose, which proved to be the most efficient for the regeneration based on the previous experiment.
At the multiplication stage, a test tube containing one embryo was a replication; in the experiment regarding further micropropagation stages, five shoots cultured in 330 mL jar constituted a replication.
2.4. Rooting of Shoots of Gynogenetic Regenerants
In order to select the optimal in vitro rooting medium, an experiment was carried out with the “Czerwona Kula” shoots. Shoots above 1 cm in length were rooted on modified MS medium containing ½ MS macronutrients with the addition of 20 g L−1 sucrose, and supplemented with NAA at the concentrations of 1 or 3 mg L−1 and Put at 0.5 or 160 mg L−1.
2.5. Acclimatization
The plants of “Czerwona Kula” were planted ex vitro following shoot rooting in vitro, as described above, whereas the plants of 3/2010 breeding accession were planted ex vitro directly from regeneration medium, because they formed roots spontaneously. The plants were rinsed in distilled water, dipped for a moment in 2% Kaptan solution (UPL Polska Sp. z o.o., Warszawa, Poland) and planted in multipots containing peat and sand substrate (1:3, v/v). Multipots with plants were placed in high-humidity conditions in a plastic tunnel in a growth chamber with a temperature of 20 °C during the day and 18 °C at night, and the PPFD of 55 µmol·m−2·s−1 at 16 h photoperiod. After 3–4 weeks, the plastic tunnel was gradually ventilated to reduce humidity. In the 5th week, the numbers of acclimatized plants were assessed. Then, the plants were transferred to 0.4 dm3 pots and grown in a greenhouse.
2.6. Ploidy Evaluation
Ploidy of the gynogenetic plants grown in a greenhouse was evaluated using flow cytometry (FCM). Young leaf fragments (ca. 0.5 cm2) taken from green parts of a leaf (avoiding red anthocyanin stained leaf sections) were used for analysis. The nuclei were extracted from the chopped plant material in a Petri dish with 0.5 mL nuclei isolation/staining Partec buffer [28] supplemented with 1% polyvinylpyrrolidone (PVP) and fluorescent stain 4′,6-diamidino-2-phenylindole (DAPI) (50 µg mL−1). After addition of 1 mL of the same buffer, the samples were filtered through a 30 µm filter and incubated in ice for 2–3 h in darkness. The fluorescence of the nuclei was measured using a CyFlow Ploidy Analyser “DAPI/PI” (Partec GmbH, Münster, Germany) with UV-LED 365 nm. Samples with at least 2000 nuclei were measured. Diploid donor plants of each genotype (“Czerwona Kula”, breeding accessions 3/2010 and 7/2008) were used as external standards for FCM analysis of gynogenetic plants.
2.7. Homozygosity Evaluation
To assess homozygosity of plants obtained in ovule cultures, two isoenzymes were analyzed: GPI (glucose-6-phosphate isomerase E.C.5.3.1.9) and AAT (aspartate aminotransferase E.C.2.6.1.1). Methods described by Westphal and Wricke [29] or Kiszczak et al. [30] were used. Electrophoresis was conducted on a 10% starch gel according to the Gottlieb method [31]. Separation of enzymes was performed according to Selander et al. [32]. Weeden and Gottlieb’s method [33] was used for visualization of polymorphism of tested isoenzymes.
2.8. Statistic
The number of repetitions varied in particular experiments and was dependent on the availability of plant material. Data were analyzed using ANOVA/MANOVA non-parametric analyses such as the Kruskal–Wallis [34] (1952), at an adopted level of significant difference at p ≤ 0.05. Statistical analyses were performed using Statistica 8.0 software package for Windows (StatSoft Inc., Tulsa, OK, USA).
3. Results
3.1. Induction of Gynogensis
Comparing the control B5 and N6 media, significantly more embryos of “Czerwona Kula”, i.e., 2.3 embryos per 100 plated ovules, were obtained on N6 medium (Table 2, Figure 1A). When comparing B5 and N6 media, of which both contained 0.5 mg L−1 IAA, 0.2 mg L−1 BA and supplemented with 322 mg L−1 Put or 290 mg L−1 Spd, the significantly higher numbers of embryos, i.e., 3.0 and 3.3 embryos, were obtained on B5 media, which was significantly different from all other variants of B5 medium, N6-2 and N5-4. On the media variants based on N6, the higher numbers of embryos (1.8 and 2.8) were obtained on the media containing 2,4-D in the presence of Put or Spd. However, on N6 medium without these PAs, no embryo formation was observed.

Table 2.
Influence of the composition of the B5 induction medium on the effectiveness of gynogenesis in red beet “Czerwona Kula”.
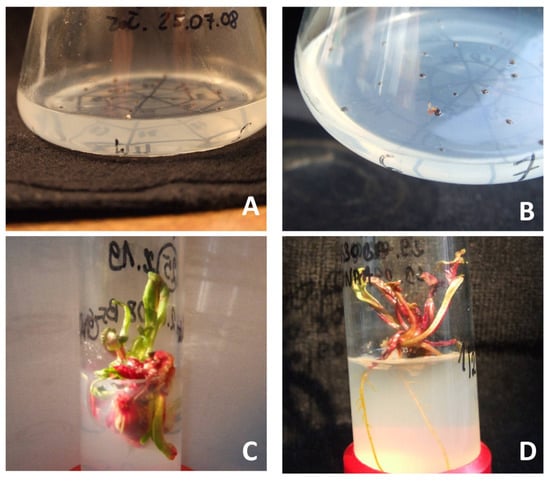
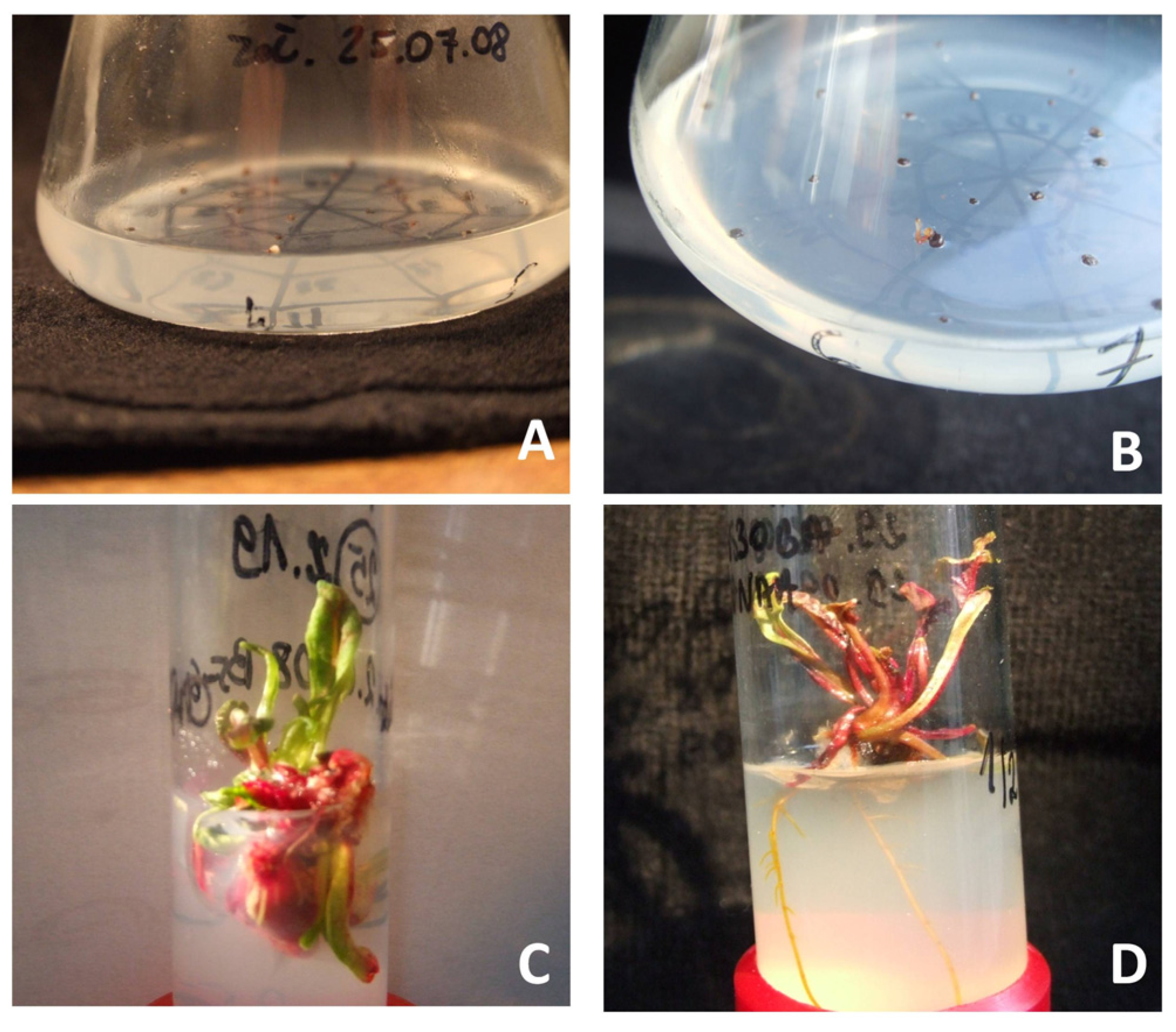
Figure 1.
The following stages of regeneration of red beet plants from gynogenetic embryos in in vitro cultures of cultivar “Czerwona Kula”: (A)—gynogenetic embryo in ovule cultures, (B)—growing gynogenetic embryo in ovule cultures, (C)—shoots multiplication obtained in ovule cultures, (D)—a plant obtained by regeneration from gynogenetic embryos.
In the second experiment, embryos were cultured on the B5-6 medium containing (mg L−1), 0.5 IAA, 0.2 BA and 290 Spd. Embryos were obtained with different frequency depending on the genotype, with significantly higher embryo number (3.2) recorded for “Czerwona Kula” (Table 3) compared to the breeding accessions 3/2010 and 7/2008.

Table 3.
Influence of the genotype on the effectiveness of gynogenesis in red beet on B5-6 medium containing 0.5 mg L−1 IAA, 0.2 mg L−1 BA and 290 mg L−1 Spd.
3.2. Plant Regeneration
After the gynogenetic embryos were placed on the regeneration media, different types of regenerants were obtained (Figure 1B–D). In “Czerwona Kula”, the number of regenerants depended on the medium composition (Table 4). The complete plants, 1.2 per embryo, were recorded only on the MS medium supplemented with 0.2 mg L−1 BA and 1 mg L−1 IAA. On the remaining media, unrooted shoots were observed. The highest number of shoots, 4.5 per embryo, was recorded on MS medium with BA and 0.5 mg L−1 Put.

Table 4.
Effect of PGRs on plant regeneration from gynogenetic embryos of red beet “Czerwona Kula”. Basal MS medium containing 30 g L−1 sucrose was used for plant regeneration.
In the second experiment in which the gynogenetic embryos were placed on the regeneration media, different types of regenerants were observed. In “Czerwona Kula”, the number and type of regenerants depended on the medium composition (Table 5). The highest number of shoots, 70 per embryo, were recorded on the MS medium supplemented with 0.2 mg L−1 BA and 0.5 mg L−1 Put. Most of them were shorter shoots (<1 cm). On the other hand, on the media with the addition of auxins, statistically fewer shoots were observed, but shoots longer than 1 cm constituted a larger share. The largest amount long shoots was obtained on the medium containing IAA 1 mg L−1.

Table 5.
Effect of PGRs on shoot multiplication of “Czerwona Kula”. Basal MS medium containing 30 g L−1 sucrose was used for shoot multiplication.
In the experiment on the influence of the genotype on the efficiency of the plant regeneration, the frequency of regeneration was evaluated using the regeneration medium considered the optimal, based on previous experiments. Thus, the MS medium supplemented with 0.2 mg L−1 BA and 0.5 mg L−1 Put was used. The highest number of regenerants, 1.13 per embryo, was obtained for the breeding line 3/2010, and among them, 10 regenerants were the well-formed shoots (Table 6). In “Czerwona Kula”, fewer shoots were obtained, i.e., 0.65 per embryo, and 9 of them were properly formed shoots without callus. In the 7/2008 breeding line, three shoots were observed, which were formed via callus.

Table 6.
Effect of genotype on the number of regenerants obtained on MS medium containing 30 g L−1 sucrose, 0.2 mg L−1 BA and 0.5 mg L−1 Put.
3.3. Rooting and Acclimatization
The highest efficiency of in vitro shoot rooting was obtained on MS ½ medium containing 3 mg L−1 NAA and 160 mg L−1 Put. On this medium, 100% of shoots formed roots (Table 7).

Table 7.
Shoot rooting of red beet “Czerwona Kula” depending on PGR composition. Rooting medium contained ½ MS mineral salts and 20 g L−1 sucrose.
All plants of the breeding accession 3/2010 survived the process of acclimatization (Table 8). However, in the other two genotypes, much less plants acclimatized. In “Czerwona Kula”, 56.6% of plants continued growth in ex vitro conditions, and only one plant of 7/2008 breeding accession survived. During further growth in a greenhouse, many of the plants died. Therefore, the ploidy level using FCM was evaluated for 91 and 19 plants of “Czerwona Kula” and 3/2010 breeding accession, respectively.

Table 8.
Effect of the genotype on the efficiency of acclimatization.
3.4. Ploidy Evaluation
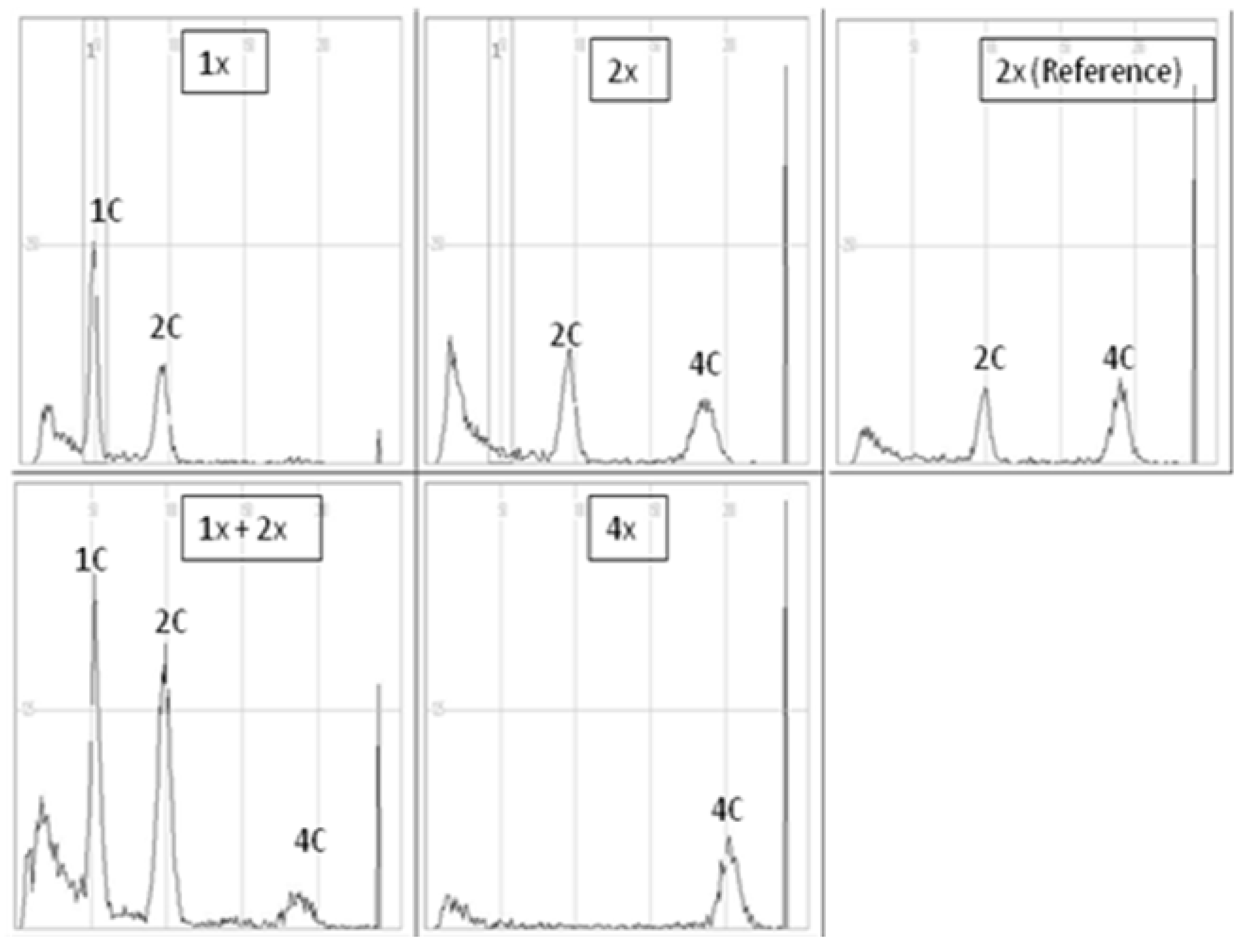
The evaluation of the ploidy level of gynogenetic plants was performed in relation to diploid reference (donor) plants of each of the analyzed genotypes. In all diploid reference plants, there were two distinct peaks, 2C DNA and 4C DNA (location on the X-axis ca. 100 and 200, respectively), which indicated strong endoreplication often found in red beet and sugar beet [35] (Figure 2). When peaks 1C and 2C appeared on the FCM histograms of a gynogenetic plant (X-axis peak positions ca. 50 and 100, respectively), the plant was considered haploid (1x). When the histogram was similar to that of the reference diploid plant, the analyzed plants were considered diploid (2x), while when a distinct third 4C peak appeared, the plants were assessed as 1x + 2x mixoploids. In turn, tetraploidy was attributed to plants for which a clear first peak appeared on the X-axis in a place characteristic of 4C DNA (peak position on the X-axis, ca. 200). In the case of the triploid (3x), the first clear peak was on the X-axis at about 150.
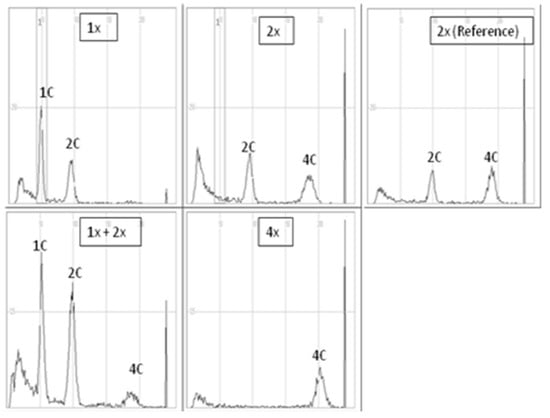
Figure 2.
Sample histograms of cytometric analysis of haploid (1x), diploid (2x), mixoploid plants with a haploid and diploid genomes (1x + 2x), tetraploid (4x) plant of the red beet accession 3/2010 and diploid reference plant (donor plant “Czerwona Kula”).
In “Czerwona Kula”, 11 and 28 out of 40 plants were haploid and diploid, respectively, and 1 plant showed mixoploid status (2x + 1x) (Table 9). In 3/2010 breeding accession out of 19 plants, 8, 5, 3 and 3 plants turned out to be haploid, diploid, triploid and tetraploid, respectively.

Table 9.
Ploidy of red beet gynogenetic plants in red beet “Czerwona Kula” and 3/2010 breeding accession.
3.5. Homozygosity Evaluation
The homozygosity evaluation showed that the 11 gynogenetic red beet plants of “Czerwona Kula” were homozygous for GPI. In breeding accession 3/2010, six homozygotes were found (Table 10). The remaining plants for both genotypes were GPI heterozygotes. In terms of the AAT isoenzyme, all of the plants of “Czerwona Kula” were homozygous, and in 3/2010 breeding accession, 10 plants were homozygous.

Table 10.
Homozygosity evaluation using analysis with isoenzymes GPI and ATT of gynogenetic doubled haploids of red beet “Czerwona Kula” and 3/2010 breeding accession.
4. Discussion
The available literature does not contain much information on the influence of PAs on the efficiency of gynogenesis in plants. Most of the reports on this topic concern the effects of two PAs, spermidine and putrescine. Martinez et al. [11] applied PAs for induction and regeneration of gynogenetic onion (Allium cepa L.) plants. The authors reported that the addition of putrescine alone, with a few exceptions, did not have any significant effect on either embryo induction or haploid plantlet production. Only combination of 17.6 mg L−1 putrescine and 14 mg L−1 spermidine increased the efficiency of gynogenesis induction. Similar studies on several onion genotypes were conducted by Ebrahimi i Zamani [12]. They applied both polyamines for the induction of gynogenetic embryos in onion. In their experiments, Spd application resulted in the highest production of gynogenic embryos in all onion cultivars in comparison to the medium supplemented with both PAs. In our experiments, the highest number of embryos was obtained on the medium supplemented with Spd. Slightly less embryos were obtained on the medium with Put. In our study, Put was used at the concentration of 322 mg L−1 in the induction media. Such concentration proved to be the most effective in our earlier studies on the induction of gametic embryogenesis via androgenesis in anther culture of carrot [36]. Kiełkowska et al. [37] applied 8.81 mg L−1 of Put for the induction of gynogenetic embryos in carrot. The authors demonstrated that the effect of Put on the increased efficiency of gynogenesis was dependent the type of the medium and presence of other PGRs. In our experiments, the combination of Put with IAA and BA in the B5 medium helped to obtain gynogenetic embryos, while when this polyamine was combined with NAA and 2,4-D in the same medium, no improvement in the efficiency of the induction compared to the control was obtained. Embryos were not obtained on the N6-based medium supplemented with Put in combination with IAA and BA. This medium did not contain myo-inositol, which plays an important role at the level of physiological processes in plant. Therefore, it seems that putrescine has a positive effect on gametic embryogenesis, but only in the presence of myo-inositol. This is a thesis that needs to be verified in further research. Barański et al. [16] obtained ovule development into embryos in red beet on the N6 medium without application of PAs.
Results obtained in our studies indicated that the genotype of the donor plant was an important factor for the effectiveness of gynogenesis induction. Chen et al. [8] believed that the donor genotype had a huge impact on the successful course of the gynogenesis induced from unfertilized ovary or ovule. This was confirmed in studies on gynogenesis of several vegetable species conducted by other authors [11,38,39].
The dominating impact of genotype was also a very important factor influencing the ability of plants to regenerate plants from gynogenetic structures. This was demonstrated in onion by Ponce et al. [40]. In their studies, gynogenetic embryos of three cultivars regenerated plants with a different frequency on BDS medium supplemented with 320 or 640 mg L−1 Put. In our experiments, Put was added to the culture media at much lower concentration (0.5 mg L−1), but results obtained for “Czerwona Kula” cultivar were satisfactory, and 70 regenerated shoots from embryonic structures were recorded. Our results were comparable to these obtained for onion, in which 1.85, 0.00 and 0.51% regenerants were obtained for three genotypes. Experiments of Ponce et al. [40] also showed that the effect of putrescine used at high concentration (160 mg L−1) seemed to be detrimental for plant regeneration in both genotypes. In our studies, we also observed that embryos plated for regeneration did not die on the medium supplemented with low putrescine concentration (0.5 mg L−1), and they regenerated into shoots in the highest number in comparison to other tested media. Our observation may indicate the positive role of this PA in reducing the negative effects of stress on plant tissues. This could be confirmed by the research conducted by Kiełkowska and Adamus [41], who in the summary of their research stated that exogenously applied PAs maintained the viability of B.oleracea L. var. capitata protoplasts by alleviating oxidative stress and stimulating mitotic activity, which further affected the plant regeneration process.
Barański [16] observed differences in rhizogenic ability between the studied breeding accession of red beet. This caused the necessity of conducting plant regeneration in two stages and using special rooting media. In our experiments, the addition of 160 mg L−1 putrescine turned out to be the most effective. All shoots placed on this medium formed roots. Such a high efficiency of shoot rooting in the presence of exogenous putrescine results from the fact that, as reported by Soh and Bhojwani [42], exogenous Put itself, but not other PAs, promoted root formation from shoots grown on auxin-free medium.
Acclimatization ability of the red beet gynogenetic plants was strongly dependent on genotype. Gynogenetic plants of 3/2010 breeding accession acclimatized in 100%, while in other genotypes, much less plants adapted to ex vitro conditions. These results correspond to the research of other authors who also, depending on the genotype, obtained a different percentage of acclimatized plants of cucumber DH line [13].
Successful acclimatization helped to investigate the ploidy of plants that survived this stage. In two examined genotypes, the haploid plants were obtained with different frequency. In addition, plants with ploidy 2x, 3x, 4x and one mixoploid (1x + 2x) were found. Obtaining plants with a higher number of chromosomes than the haploid can be attributed, according to Lukaszewska et al. [43], to the effect of NAA used in the culture media. In their research on hormonal control of endoreduplication in sugar beet, they showed that NAA added to the media in the same amount as in our experiments (1 mg L−1) causes chromosomal duplication.
The appearance of a heterozygotic pattern of bands for both isoenzymes among the tested plants may be due to the reasons suggested by Levites et al. [44]. The authors demonstrated that spontaneous polyploidization caused by their prolonged culturing occurs in the haploid tissues of sugar beet under in vitro conditions. According to their results, the emergence of heterozygotes in polymorphic populations with respect to isocitrate dehydrogenase and 6-phosphogluconate dehydrogenase isoenzymes in combination with the simultaneous homozygotic profile for the other isoenzymes in the same plants indicate the occurrence of spontaneous polyploidization, and the process of chromosome doubling is per se mutagenic, causing genetic and epigenetic changes that were confirmed in many studies [45,46,47,48].
Author Contributions
Conceptualization, K.G. and W.K.; methodology, K.G. and W.K.; validation, K.G., W.K. and M.P.; formal analysis, K.G., W.K., U.K., M.B. and M.P.; data curation, K.G., W.K. and M.P.; writing—original draft, K.G. and W.K.; writing—review and editing, W.K., M.B. and M.P.; visualization, M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Polish Ministry of Agriculture and Rural Development, task no 102.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AAT | aspartate aminotransferase E.C.2.6.1.1 |
| BA | 6-benzyladenine |
| IAA | indole-3-acetic acid |
| IBA | indole-3-butyric acid |
| NAA | 1-naphthylacetic acid |
| PAs | polyamines |
| Put | putrescine |
| PGRs | plant growth regulators |
| Spd | spermidine |
| GPI | glucose-6-phosphate isomerase E.C.5.3.1.9 |
References
- Anwar, R.; Mattoo, A.K.; Handa, A.K. Polyamine Interactions with Plant Hormones: Crosstalk at Several Levels. In Polyamines; Kusano, T., Suzuki, H., Eds.; Springer: Tokyo, Japan, 2015; pp. 267–302. [Google Scholar] [CrossRef]
- Ebrahimzadeh, H.; Shariatpanahi, M.E.; Ahmadi, B.; Soltanloo, H.; Lotfi, M.; Zarifi, E. Efficient parthenogenesis induction and in vitro haploid plant regeneration in cucumber(Cucumis sativus L.) using putrescine, spermidine, and cycocel. J. Plant Growth Regul. 2018, 37, 1127–1134. [Google Scholar] [CrossRef]
- Hussain, S.S.; Ali, M.; Ahmad, M.; Siddique, K.H. Polyamines: Natural and engineered abiotic and biotic stress tolerance in plants. Biotechnol. Adv. 2011, 29, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Podwyszyńska, M.; Kosson, R.; Treder, J. Polyamines and methyl jasmonate in bulb formation of in vitro propagated tulips. Plant Cell Tissue Organ Cult. 2015, 123, 591–605. [Google Scholar] [CrossRef]
- Podwyszyńska, M.; Cieślińska, M. Rooting shoots of apple varieties and their tetraploids obtained by the in vitro technique. Acta Sci. Pol. Hortorum Cultus 2018, 17, 49–62. [Google Scholar] [CrossRef]
- Regla-Márquez, C.F.; Canto-Flick, A.; Avilés-Viñas, S.A.; Valle-Gough, R.E.; Pérez-Pastrana, J.; García-Villalobos, F.J.; Santana-Buzzy, N. Cadaverine: A common PA in zygotic embryos and somatic embryos of the species Capsicum chinense Jacq. Plant Cell Tissue Organ Cult. 2016, 124, 253–264. [Google Scholar] [CrossRef]
- Baron, K.; Stasolla, C. The role of PAs during in vivo and in vitro development. Vitr. Cell. Dev. Biol. Plant 2008, 44, 384–395. [Google Scholar] [CrossRef]
- Chen, J.-F.; Cui, L.; Malik, A.A.; Mbira, K.G. In vitro haploid and dihaploid production via unfertilized ovule culture. Plant Cell Tissue Organ Cult. 2011, 104, 311–319. [Google Scholar] [CrossRef]
- Niazian, M.; Shariatpanahi, M.E. In vitro-based doubled haploid production: Recent improvements. Euphyt 2020, 216, 69. [Google Scholar] [CrossRef]
- Zieliński, K.; Krzewska, M.; Żur, I.; Juzoń, K.; Kopeć, P.; Nowicka, A.; Moravcikova, J.; Skrzypek, E.; Dubas, E. The effect of glutathione and mannitol on androgenesis in anther and isolated microspore cultures of rye (Secale cereale L.). Plant Cell Tissue Organ Cult. 2020, 140, 577. [Google Scholar] [CrossRef]
- Martinez, L.E.; Agüero, C.B.; López, M.E.; Galmarini, C.R. Improvement of in vitro gynogenesis induction in onion (Allium cepa L.) using PAs. Plant Sci. 2000, 156, 221–226. [Google Scholar] [CrossRef]
- Ebrahimi, R.; Zamani, Z. Effect of PAs on in vitro gynogenesis of onion (Allium cepa L.). Am. Eurasian J. Sustain. Agric. 2009, 1, 71–74. [Google Scholar]
- Gałązka, J.; Słomnicka, R.; Góral-Radziszewska, K.; Niemirowicz-Szczytt, K. From pollination to dh lines—Verification and optimization of protocol for production of doubled haploids in cucumber. Acta Sci. Pol. Hortorum Cultus 2015, 14, 81–92. [Google Scholar]
- Clifford, T.; Howatson, G.; West, D.J.; Stevenson, E.J. The potential benefits of red beetroot supplementation in health and disease. Nutrients 2015, 14, 2801–2822. [Google Scholar] [CrossRef]
- Wang, M.; Goldman, I.L. Genetic distance and diversity in table beet and sugar beet accessions measured by randomly amplified polymorphic DNA. J. Am. Soc. Hort. 1999, 124, 630–635. [Google Scholar] [CrossRef]
- Barański, R. In vitro gynogenesis efficiency in red beet (Beta vulgaris L.) Effect of ovule culture conditions. Acta Soc. Bot. Pol. 1996, 65, 57–60. [Google Scholar] [CrossRef]
- Kiszczak, W.; Burian, M.; Kowalska, U.; Górecka, K.; Podwyszyńska, M. Production of homozygous red beet (Beta vulgaris L. subsp. vulgaris) plants by ovule culture. Methods Mol. Biol. 2021, 2289, 301–312. [Google Scholar] [CrossRef]
- Zayachkovskaya, T.; Domblides, E.; Zayachkovsky, V.; Kan, L.; Domblides, A.; Soldatenko, A. Production of gynogenic plants of red beet (Beta vulgaris L.) in unpollinated ovule culture in vitro. Plants 2021, 10, 2703. [Google Scholar] [CrossRef]
- Doctrinal, M.; Sangwan, R.S.; Sangwan-Norreel, B.S. In vitro gynogenesis in Beta vulgaris L. Effects of plant growth regulators, temperature, genotypes and season. Plant Cell Tissue Organ Cult. 1989, 17, 1–12. [Google Scholar] [CrossRef]
- Gürel, E.; Gürel, S. Plant regeneration from unfertilized ovaries of sugar beet (Beta vulgaris L.) cultured In Vitro. Turk. J. Bot. 1998, 22, 233–238. [Google Scholar]
- Pazuki, A.; Aflaki, F.; Gürel, E.; Ergül, A. Gynogenesis induction in sugar beet (Beta vulgaris) improved by 6-benzylaminopurine (BAP) and synergized with cold pretreatment. Sugar Tech. 2017, 20, 69–77. [Google Scholar] [CrossRef]
- Galston, A.W.; Sawhey, R.K. PA and plant physiology. Plant Physiol. 1990, 94, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.C.; Wang, C.C.; Sun, C.S.; Hsu, K.C.; Yin, K.C.; Chu, C.Y.; Bi, F.Y. Establishment of an efficient medium for anther culture of rice through comparative experiments on the nitrogen sources. Sci. Sinica. 1975, 18, 659–668. [Google Scholar]
- Gamborg, O.L.; Miller, R.A.; Ojima, K. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 1968, 50, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Górecka, K.; Kowalska, U.; Kiszczak, W.; Krzyżanowska, D.; Górecki, R.; Fornal, L. Induction of androgenetic embriogenesis in red beet. In Proceedings of the 56th Congress of the PBS in Olsztyn, Interdisciplinary and Application Significance of Botanical Sciences, Olsztyn, Poland, 24–30 June 2013. Poster number: K205. [Google Scholar]
- Górecka, K.; Krzyżanowska, D.; Kowalska, U.; Kiszczak, W.; Podwyszyńska, M. Development of embryoids by microspore and anther cultures of red beet (Beta vulgaris L. subsp. vulgaris). J. Cent. Eur. Agric. 2017, 18, 185–195. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Sliwinska, E. Zastosowanie cytometrii przepływowej do oznaczania zawarto’sci DNA u roślin. (Estimation of DNA content in plants using flow cytometry). Adv. Cell Biol. Suppl. 2008, 24, 165–176. [Google Scholar]
- Westphal, L.; Wricke, G. Genetic analysis of DIA, GOT and PGI isozyme loci in Daucus carota L. ssp. sativus. Plan Breed. 1989, 102, 51–57. [Google Scholar] [CrossRef]
- Kiszczak, W.; Krzyżanowska, D.; Strycharczuk, K.; Kowalska, U.; Wolko, B.; Górecka, K. Determination of ploidy and homozygosity of carrot plants obtained from anther cultures. Acta Physiol. Plant 2011, 33, 401–407. [Google Scholar] [CrossRef]
- Gottlieb, L.D. Enzyme differentiation and phylogeny in Clarkia franciscana, C. rubicunda and C. amoena. Evolution 1973, 27, 205–214. [Google Scholar] [CrossRef]
- Selander, R.K.; Smith, M.H.; Yang, S.Y.; Johnson, W.E.; Gentry, J.B. Biochemical polymorphism and systematics in the genus Peromyscus. Variation in the old-field mouse (Peromyscus polionotus). Stud. Genet. 1971, 7103, 49–90. [Google Scholar]
- Weeden, F.N.; Gottlieb, L.D. Isolation of cytoplasmic enzymes from pollen. Plant Physiol. 1980, 66, 400–403. [Google Scholar] [CrossRef]
- Kruskal, W.H.; Wallis, W.A. Use of ranks in one-criterion variance analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- Lukaszewska, E.; Sliwinska, E. Most organs of sugar-beet (Beta vulgaris L.) plants at the vegetative and reproductive stages of development are polysomatic. Sex. Plant Reprod. 2007, 20, 99–107. [Google Scholar] [CrossRef]
- Górecka, K.; Kiszczak, W.; Krzyżanowska, D.; Kowalska, U.; Kapuścińska, A. Effect of PAs on in vitro anther cultures of carrot (Daucus carota L.). Turk. J. Biol. 2014, 38, 593–600. [Google Scholar] [CrossRef]
- Kiełkowska, A.; Adamus, A. Exogenously applied PAs reduce reactive oxygen species, enhancing cell division and the shoot regeneration from Brassica oleracea L. var. Capitata protoplasts. Agron. J. 2021, 11, 735. [Google Scholar] [CrossRef]
- Bohanec, B.; Jakse, M. Variation of gynogenic response among long-day onion (Allium cepa L.) accessions. Plant Cell Rep. 1999, 18, 737–742. [Google Scholar] [CrossRef]
- Tantasawat, P.A.; Sorntip, A.; Poolsawat, O.; Chaowiset, W.; Pornbungkerd, P. Evaluation of factors affecting embryo-like structure and callus formation in unpollinated ovary culture of cucumber (Cucumis sativus). Int. J. Agric. Biol. 2015, 17, 613–618. [Google Scholar] [CrossRef]
- Ponce, M.; Martínez, L.; Galmarini, C. Influence of CCC, putrescine and gellam gum concentration on gynogenic embryo induction in Allium cepa. Biol. Planta 2006, 50, 425–428. [Google Scholar] [CrossRef]
- Kiełkowska, A.; Adamus, A. In vitro culture of unfertilized ovules in carrot (Daucus carrota L.). Plant Cell Tissue Organ Cult. 2010, 102, 309–319. [Google Scholar] [CrossRef]
- Soh, W.Y.; Bhojwani, S.S.; Lee, S. Developmental and structural aspects of root organogenesis. In Morphogenesis in Plant Tissue Cultures; Soh, W.Y., Bhojwani, S.S., Eds.; Springer: Dordrecht, The Netherlands, 1999. [Google Scholar] [CrossRef]
- Lukaszewska, E.; Virden, R.; Sliwinska, E. Hormonal control of endoreduplication in sugar beet (Beta vulgaris L.) seedlings growing in vitro. Plant Biol. 2012, 14, 216–222. [Google Scholar] [CrossRef]
- Levites, E.V.; Svirshchevskaya, A.M.; Kirikovichi, S.S.; Mil’ko, L.V. Variation at isozyme loci in cultured in vitro sugar beet regenerants of gynogenetic origin. Sugar Tech. 2005, 7, 71–75. [Google Scholar] [CrossRef]
- Parisod, C.; Holderegger, R.; Brochmann, C. Evolutionary consequences of autopolyploidy. New Phytol. 2010, 186, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Aversano, R.; Ercolano, M.R.; Caruso, I.; Fasano, C.; Rosellini, D.; Carputo, D. Molecular tools for exploring polyploid genomes in plants. Int. J. Mol. Sci. 2012, 13, 10316–10335. [Google Scholar] [CrossRef] [PubMed]
- Wendel, J.; Lisch, D.; Hu, G.; Mason, A.S. The long and short of doubling down: Polyploidy, epigenetics, and the temporal dynamics of genome fractionation. Curr. Opin. Genet. Dev. 2018, 49, 1–7. [Google Scholar] [CrossRef]
- Podwyszyńska, M.; Markiewicz, M.; Broniarek-Niemiec, A.; Matysiak, B.; Marasek-Ciolakowska, A. Apple autotetraploids with enhanced resistance to apple scab (Venturia inaequalis) due to genome duplication-phenotypic and genetic evaluation. Int. J. Mol. Sci. 2021, 22, 527. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).