Abstract
The exponential growth of agricultural output is a result of the increasing world population. Agro-wastes are now acknowledged as an alternative material for the manufacture of renewable, inexpensive, and sustainable bio-composite-based products. Huge amounts of agricultural produce are often lost owing to a lack of processing facilities or storage space. Agriculture wastes contain a significant concentration of carbohydrates as well as various multifunctional groups and organic substances, such as polymeric proteins. Interestingly, nanotechnology provides better potential to transform agricultural wastes easily into valuable and cost-effective products, removing the need to utilize noxious chemicals, which can create a variety of health and environmental difficulties. Recently, there has been an increase in interest in eco-friendly nanomaterial (NM) production techniques that utilize extracts generated from agricultural waste. Such nanoparticles (NPs) have been extensively studied for numerous uses, including antibacterial agents, water purification, the breakdown of industrial colours, and many others. Consequently, the purpose of this review is to investigate the different sources, characteristics, and nano-management of agro-waste; valuable NMs derived from agro-waste; and their possible applications.
1. Introduction
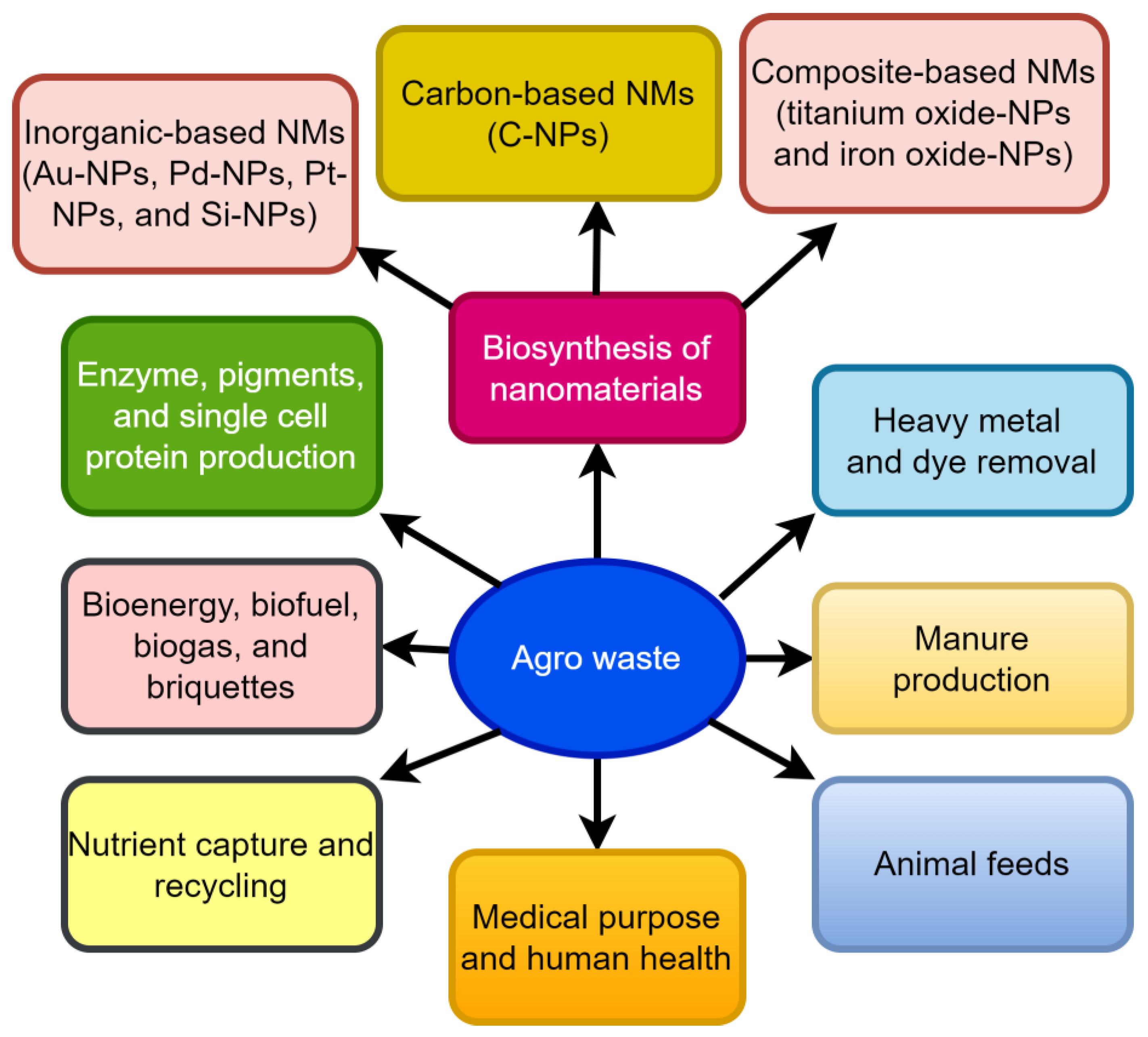
Agriculture makes the biggest contribution to the economy of a resource sector around the world. It is estimated that Canada makes 36.1 metric tonnes of waste each year, which is 10 metric tonnes more per person than the United States. Every year, Canada makes about 1.33 billion metric tonnes of waste, of which 1.12 billion metric tonnes are industrial waste [1]. As of 2020, the United States uses approximately 4532 trillion British thermal units (TBtu), or about 4.9 percent of its total primary energy consumption. Wood and wood-derived biomass accounted for about 2101 TBtu, while biofuels (mostly ethanol) provided 2000 TBtu, and municipal garbage generated 430 TBtu [2]. The United States produced the most biofuels of any country in the world in 2020, with production estimated to reach 1347 petajoules [3]. Agriculture is the primary source of food, trash, greenhouse emissions, and energy in every region of the world. Agriculturally based industries produce a lot of garbage as a result of their operations. Non-consumable plant portions are referred to as agro-waste, while edible parts are the primary source of energy for humans [4]. Biological oxygen demand (BOD), chemical oxygen demand (COD), and suspended solids are all significant in this vast volume of agro-waste [5]. A lack of effective disposal of these types of waste items can harm the earth’s surface and groundwater, which in turn contributes to all forms of pollution. A large portion of garbage is still unutilized and disposed of improperly, which only exacerbates the problem. A variety of agricultural and industrial processes generate low-cost by-products from all over the globe. This aids in the creation of value-added products from those remnants. There is still a lot of untapped potential in many cases, but nothing practical is being made out of it. Burning, dumping, or uncontrolled land filling are the most common disposal methods for agro-industrial waste that hasn’t been processed or repurposed [6]. These unprocessed wastes contribute to global warming by emitting a variety of greenhouse gases. As a result, the usage of fossil fuels generates greenhouse gases (GHG) [7]. There are numerous possible uses, including the creation of biotechnological products, the synthesis of NPs, and the generation of manure and biofuels, among others (Figure 1).
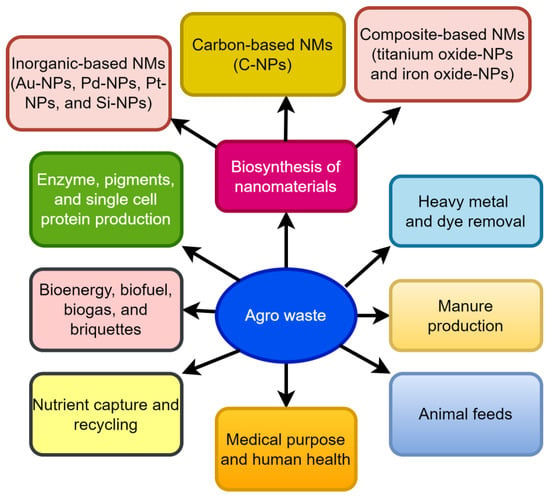
Figure 1.
Management of agro-waste.
Agricultural wastes, which can include horticultural, aquatic, or culinary wastes, contain vital bio-chemicals such aslignin, cellulose, chitin, and polyphenolic compounds [8]. By simple pyrolysis, some of the compounds can produce carbon dots, whereas the constituents of other compounds act as reducing, stabilizing, and capping agents during the creation of NPs [9]. Because of the economical, environmental, and technological advantages, the fabrication of NMs using agro-waste as substrates is quickly gaining popularity [9]. These NPs are prepared using a combination of mechanical and chemical processes, including crushing, grinding, alkali, bleaching, and acid treatments [10]. The morphology of nanoscale biopolymers is studied using AFM, SEM, and TEM [11]. Researchers are examining the functional group modifications and crystallographic structure of nanoscale biopolymers using FTIR and X-ray diffraction, respectively [12].
In recent years, nanotechnology has reached nearly every zone of technology ranging from clinical, commercial, agricultural, and even residence old utilities, and has hugely facilitated the evolution of current technology. Applications of NPs and nanotechnology have been introduced in recent years in the agricultural and food sectors in order to increase overall sector productivity [13,14]. Nanotechnology is a branch of science that deals with materials with sizes between 1 and 100 nm [15]. The role of nanotechnology in agriculture and environmental protection is expanding. Agro-waste, which includes weeds such as Gloriosa superba, Medicago sativa, Cyperus rotundus, and Tinospora cordifolia, as well as agricultural residues including ricehusk, soy, and coconut shell, wheat straw, banana peel, orange peel, and pomegranate peel, has been utilized to create nanostructures. Genetically modified organisms (GMPs), atomically modified organisms (AMOs), animal products, insecticides, farming methods, and many other possibilities to study with significant potential to revolutionize the agriculture scenario globally will all benefit from nanotech research in agriculture [16,17]. One of the primary goals for potential applications has been to control the particle size, shape, and crystalline nature of the NPs that are produced through the synthesis of NPs [18]. NPs serve as a connection between large-scale materials and the atomic or molecular structures that compose them. Because of this, they are excellent candidates for applications in a variety of fields, including medicine, catalysis, electrochemistry, biotechnology, and the detection of trace substances [19]. In order to prepare NPs with a variety of morphologies and sizes, various synthetic methods have been utilized [20]. Although these methods have produced superior NPs, there is still a need for a fundamental understanding of how to improve the manufacturing process [21]. This understanding could be utilized on an industrial and commercial scale to produce products that are better constructed, have a longer lifespan, are cleaner, safer, and more intelligent. Some examples of these types of products include communication technology, medicines, transportation, agriculture, and industrial products. As a result, the primary focus is on the design of NPs using methods that are not harmful to the environment. These provide solutions to the growing problems that are associated with concerns over the environment. This review investigates the characteristics of agro-waste, valuable NMs, and their possible applications.
2. Nanomaterials and Nano-Management of Agro Wastes
Agro-waste comes in various forms. It can be divided into two primary types, namely agricultural residues and residues from the agricultural industry [22]. Further agricultural residues comprise two types, namely process residues (Husk, Bagasse, Molasses, etc.) and field residues (stems, seeds, stalks, etc.). Agricultural industry waste means commercial-level produce waste such as orange-peel, tea, potato-peel, pineapple-peel, groundnut oil cake, sugarcane, soybean oil cake, cotton, coffee, cereal, chocolate, fruits and vegetables, etc., all contribute significantly to waste. These waste materials can be utilized to make biofuels as well as organic solvents like ethanol, acetone, and butanol. Additionally, pineapple leaf fibre is being researched as a fibre glass replacement [23]. Another illustration of lignocellulosic waste that is a sizable representative is waste from the production of wine [24]. There are many examples where agro-wastes are used as a replacement for fossil fuel and the manufacturing of NMs. Agro-waste is also environmentally friendly and sustainable. A further method would involve removing these wastes from the environment by utilizing NPs. NPs can be used, for instance, to eliminate airborne pesticides like Malathion and Chlorpyrifos [25]. There are several classes of nanofillers available in various nations for spot maintenance, such as removing photocatalytic contaminants from water using titanium oxide nanoparticles as a filter [26].
In current decades, the management of agro-waste has become a major concern, and numerous processes have been developed for dealing with organic wastes, including the use of compost and organic fertilizers, biochar, and the removal of environmental pollutants. Some agro-waste cannot be posted, like oils, due to their low economic value and ability to reduce landfill waste. Consequently, the agro-waste management system has evolved since the last century due to the advancement of our civilization [27]. In order to achieve low emissions, low consumption, and high efficiency, agricultural wastes should be managed following the principles of the three Rs (i.e., reduce, reuse, and recycle) [28]. Multiple studies evaluated the utilization and management of agro-waste from various perspectives, including:
- (a)
- Manufacturing of nano-silica or Si-nanoparticles [29,30,31,32];
- (b)
- Nano-cellulose production as a precious pharmaceutical component [33];
- (c)
- Biochar production [34,35,36,37,38,39];
- (d)
- High-performance super-capacitor manufacturing using porous nano-carbon [40] or graphene oxide [41].
On the other hand, numerous studies, including the following publications, have addressed the management strategies for agro-waste:
- (a)
- Waste management in agriculture and food processing [42];
- (b)
- Agro-waste and its environmental impact, as well as management approaches [43];
- (c)
- Applications of antimicrobial/antibiotic resistance to agricultural waste [44];
- (d)
- Producing agro-composites for use in packaging [45];
- (e)
- Using agro-waste ashes to make asphalt binder [46];
- (f)
- The bioconversion of certain agricultural waste products into α- amylase [40], lignocellulolytic enzymes [47,48];
- (g)
- Bioenergy production [49], like the biodiesel from raw nano-sized sugar beet agro-industrial waste [50], biogas [51,52,53], pellets [54], and biofuel [55];
- (h)
- Using an extract from agro-waste, like pineapple peels, sugarcane bagasse, and banana peels for the biogenic platinum [56];
- (i)
- Placing agro-waste in concrete instead of natural aggregate [57];
- (j)
- Adsorbents or nano-adsorbents made from agri-food industry waste for heavy metal removal from the environment [58];
- (k)
- Development of composting [59,60,61];
- (l)
- Utilizing agricultural and horticultural byproducts in biorefining processes [62,63];
- (m)
- Creating bioactive compounds from waste products of the food industry [64,65,66];
- (n)
- Manufacturing of cementitious additives [67];
- (o)
- Creating unbaked earthen blocks [68];
- (p)
- Nano-cellulose production from agricultural waste for cosmetic formulations [69] for ingredients in medicine [33].
3. Different Sources of Agro-Waste
Agro-waste is the leftover output of agricultural activities, such as the processing and manufacture of vegetables, fruits, poultry, meat, and dairy products [70]. There are four main types of agricultural waste: livestock manure, agricultural sector manure, fruit and vegetable manure, and crop residues and byproducts [71].
3.1. Crop Residues
In the field, crop leftovers like stovers, straws, leaves, seed pods, and so on are the primary sources of agro-waste. As the most plentiful and cheap organic waste, these crop wastes can be easily transformed into a wide range of high-value products [72]. The three primary crop by-products that are utilized to produce bioethanol globally are wheat straws, corn stovers, and rice straws, which are available all year long. Only a small portion is used to produce biofuels or as animal feed, and the remainder is burned, leading to significant environmental issues [73]. Among these three primary agricultural leftovers, rice straw is one of the utmost promising and valuable biomasses globally, with an annual production of approximately 732 million tonnes, and Asia being the leading producer [74]. It is estimated that 355.35 million metric tonnes of wheat straws are produced each year in the world, making wheat straw the majority of the waste recovered from wheat [75]. Corn stover is one of the most promising crop residues for making lingo-cellulosic ethanol, with an envisioned manufacturing of four tonnes consistent with acre and a global manufacturing of approximately 128 million tonnes per 12 months. Agro-waste is also made up of crop residues from oats, sorghum, and barley [76].
3.2. Agricultural Industry Wastes
Waste produced during the preparation of crops for industrial use is the second category of agro-waste. This includes waste products from the extraction of oil or juice, such as sugarcane bagasse, fruit and vegetable peels, and fruit marc/pomace. It also includes products from meat processing facilities and slaughterhouses, such as meat, eggs, chicken skin, and animal fat [77]; starch residue from the starch industry; de-oiled seed cakes from the edible oil industry; and molasses/black treacle from the sugar industry. Among many agro-industry wastes, sugarcane bagasse is the most prevalent. This bagasse is the dry pulpy fibrous residue generated by enterprises following the extraction of juice. About 180 million tonnes of sugarcane bagasse are thought to be available in the world [78]. Orange peel, apple pomace, and fruit wastes from the production of fruit juice, cider, and other food processing units are other examples of agro-industry wastes. Products from non-food agro-industries like de-oiled seed cakes from non-edible oil plants like Pongamia pinnata and Jatropha curcas are also included in the category of agro-industry waste [79].
3.3. Livestock Wastes
Millions of rural households around the world rely on livestock and crop production for a living. It is feasible and popular in some communities to use animal dung as a source of energy to produce biogas or to simply make manure cakes for burning [80]. Typical examples include using animal slurry as fuel for combined heat and power generation, burning chicken litter as fuel, and digesting animal slurry. However, in many instances, these projects are only financially viable if a market can be found for the heat produced (e.g., for use at nearby manufacturers) and the by-products (e.g., fertilizers for farms). Waste heat can be used on the farm or in neighboring areas where it can be reticulated or piped [81].
3.4. Fruit and Vegetable Wastes (FVWs)
FVWs are residual fruits and vegetables that have not been processed. Examples include tomatoes, oranges, mangoes, jackfruit, pineapple, bananas, and many more. FVWs produced by wholesale markets and the food processing sectors are huge [70]. These organic wastes are also extremely perishable, which raises serious concerns about environmental pollution. According to statistics, about 15, 32, 6.5, and 1.8 million tonnes of trash are produced from the production, packaging, distribution, and consumption of fruit and vegetables in the US, China, Philippines, and India, respectively [70]. Currently, roughly 5.6 million tonnes of FVWs are manufactured annually in India and dumped outside of cities [82].
4. Composition of Agro-Wastes
The compositional study of feedstock focuses primarily on lignocellulosic content, such as cellulose, hemicellulose, and lignin, and biochemical content, such as total carbohydrates, protein, lipids, etc. Bio-alcohols are produced as a result of the greater cellulose and hemicellulose content. Approximately 30% to 50% of cellulose and 20% to 38% of hemicellulose make up the crop residues. In contrast, the lignin concentration of crops ranges from 7% to 21%. Depending on the varied sources of origin, agro-processing wastes comprise a wide range of cellulose (21% to 45%), hemicellulose (15% to 33%), and lignin (5% to 24%). Rice bran waste contains just 5% lignin and has been demonstrated to be a viable feedstock for bioethanol production. Prior to ethanol production, however, high-lignin-content sugarcane bagasse must be processed for lignin removal [83]. Bio-hydrogen, biogas, and alcohols can be produced from a carbohydrate-rich substrate because of their quick breakdown. Between 40% and 85% of the total solids in industrial wastes are made up of carbohydrate compounds. Similarly, livestock wastes such as cattle manure have a carbohydrate content of 50–60%, making it an excellent fuel for biogas production because of its abundance [84]. Similarly, the protein and lipid levels of various agricultural waste feedstock are critical for determining their biogas potential [85]. Nano-biotechnology has been utilized in the field of antimicrobial research in order to delve into the depths of scientific complexities that are contained within the boundaries of medical practice. It has been demonstrated that NPs possess powerful antimicrobial properties, which allow them to combat superbugs that are highly resistant to antibiotics. In order to produce NPs that are capable of destroying multidrug-resistant bacteria and fungi, scientists have taken advantage of the synergy effect created by the relationship between the precursor (metal) and plants (capping/reducing agent). Table 1 displays the anti-microbial characteristics of NPs that were manufactured from agricultural by-products.

Table 1.
Anti-microbial activities of NPs synthesized from agro-waste.
5. Bio-Synthesis of NMs from Agro-Wastes
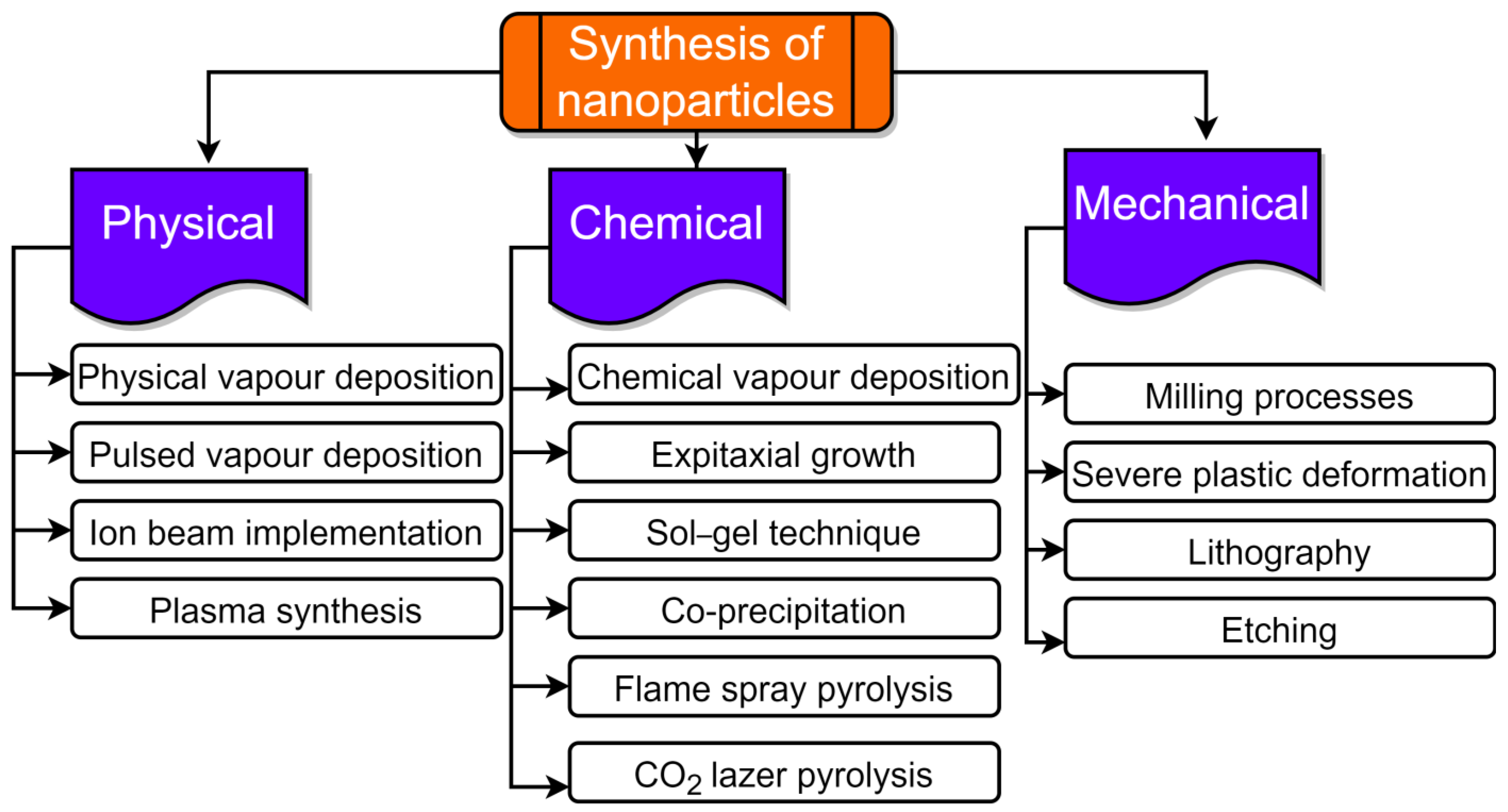
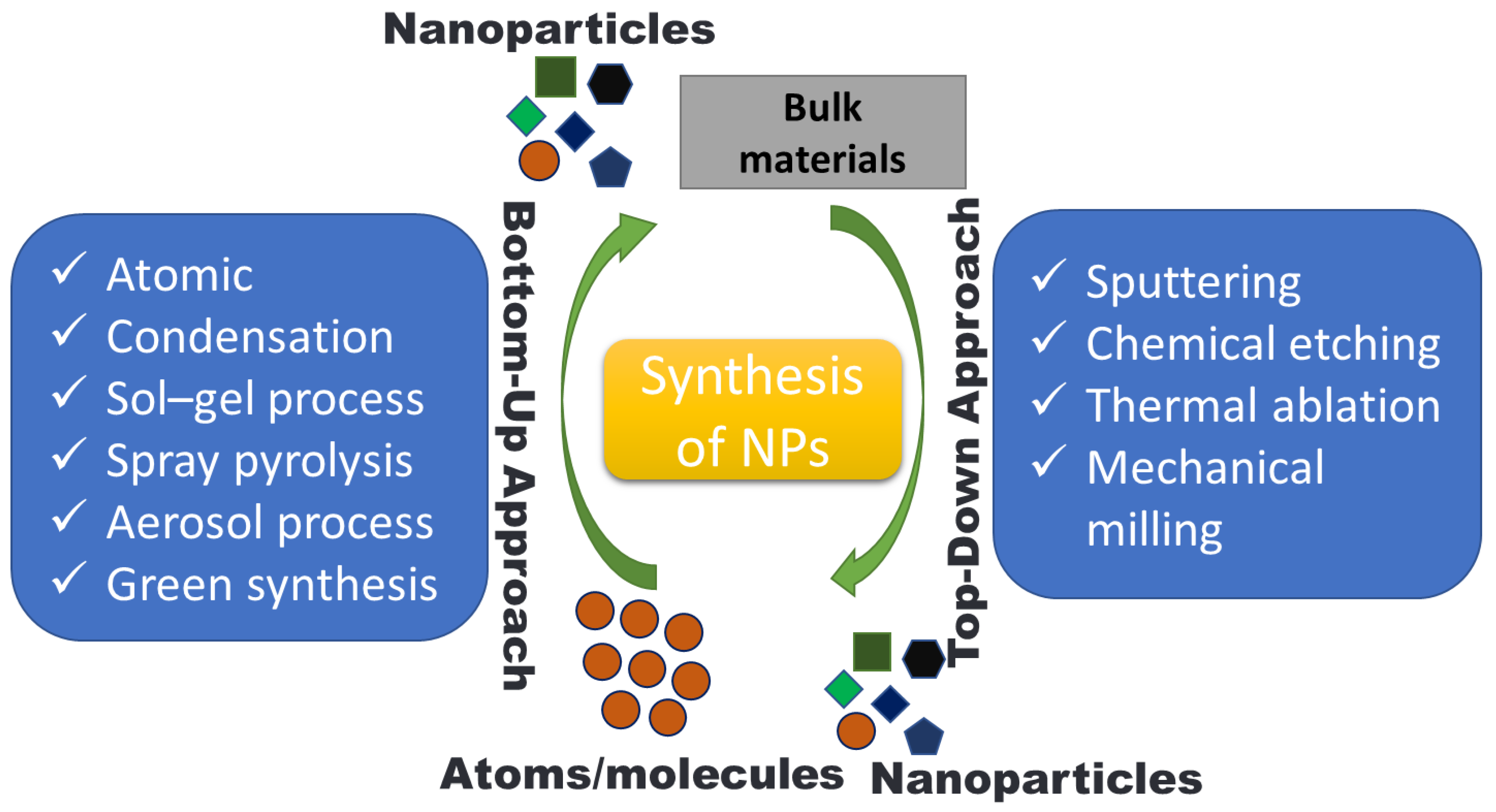
Utilizing agricultural waste resources is a sustainable technique of synthesis that allows for the efficient management and usage of plant wastes and biomass [92]. NPs have been successfully synthesized by varieties of agro-wastes like corn cob, rice bran, fruit seeds, wheat bran, peels, palm oil mill effluent, etc. [16,93,94]. NPs production can occur intracellularly or extracellularly. The size and type of NPs are greatly influenced by the synthesis time, growth temperature, and pH [95]. These agricultural wastes are a rich source of biomolecules like proteins, phenolics, and flavonoids that can act as reductant agents in the environmentally friendly production of various metal NPs. Agro-waste has been used in several biotechnological techniques to enhance the nutritional properties through solid-state fermentation (SSF), the production of enzymes [96], organic acids [97], and as renewable materials for the production of biogas [98]. The synthesis route of NPs from agro-waste is shown in Figure 2. NPs can be created using a wide range of methods as of right now. However, they can be created using the “top-down” and “bottom-up” approaches, which are the two basic techniques. The first method uses a top-to-bottom strategy to break larger particles down into progressively smaller dimensions and a variety of forms; such processes include mechanical milling, laser ablation, chemical etching, electrochemical explosion, lithography, attrition, etc. The second strategy is a bottom-up strategy in which smaller components are used to make larger NPs such as atoms, monomers, and molecules, etc. The constituent atoms and molecules that make up a structure come together and self-assemble precisely where they are needed in the bottom-up method. A variety of processes, including pyrolysis, plasma, chemical vapor deposition, the sol–gel process, spinning, biological methods, atomic condensation, etc., use a bottom-up approach, shown in Figure 2 and Figure 3.
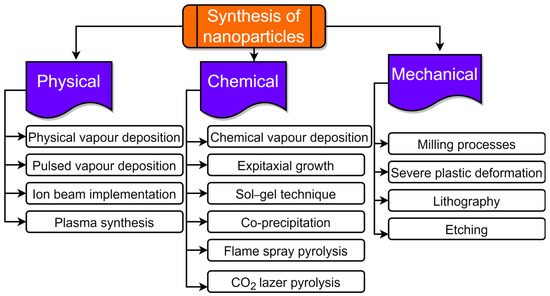
Figure 2.
Synthesis of nanoparticles from agro-waste.
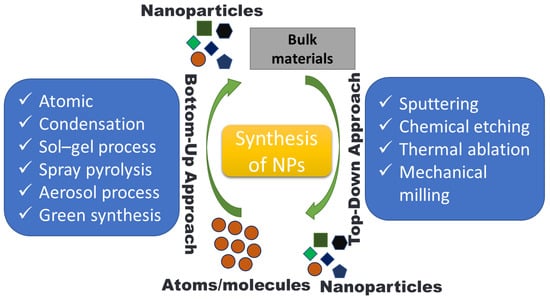
Figure 3.
Nanoparticle synthesis strategies.
5.1. Inorganic-Based NMs
5.1.1. Gold Nanoparticles (Au-NPs)
Although gold has always been a subject of fascination, the development of nano-technology has dramatically increased the number of uses for metal. Au-NPs in particular have high levels of biocompatibility and non-toxicity. Since gold is not oxidized, it can also be used for long-term biomedical applications. It has been discovered that Au-NPs enhance the therapeutic and sensing functions of proteins and antibodies. Au-NPs have been investigated for a variety of uses, including anti-coagulant, bio-imaging, anti-oxidant, thrombolytic, anti-cancer, photo-thermal, fluorescent, catalytic, bio-labelling, anti-bacterial, and bio-sensing. Numerous physical and chemical processes have successfully produced well-defined Au-NPs, but they are expensive and require the use of toxic materials. Additionally, the chemical manufacture of Au-NPs may cause hazardous compounds to adhere to the surface of the particles, which could have detrimental effects in medical applications [86,99]. Therefore, creating NPs from microbes or plant-based materials can be advantageous. Biomolecules released by biomass act as a reducing and capping agent, resulting in the creation of NPs that are more biocompatible and removing the need for abrasive chemical processes. Agro-wastes, such as banana and custard apple peels, which are abundant in phenolic components like lignin and pectin, are currently being investigated for the creation of various NPs [100,101]. As the population grows, so does the demand for agro-industry products to fulfill the rising demand; massive volumes of food products are created, producing significant amounts of agro-industrial waste in the process. To create and characterize Au-NPs, scientists used watermelon rind extract [102]. The produced Au-NPs showed strong synergistic action and anti-bacterial efficacy against food-borne pathogens. Additionally, it demonstrated strong anti-oxidant and proteasome inhibitory activity. The production of Au-NPs using agro-wastes is therefore presented as an environmentally friendly method, making them a possible candidate for usage in biomedical, pharmaceutical, cosmetic, food, and drug delivery applications [103].
5.1.2. Palladium Nanoparticles (Pd-NPs)
Pd-NPs have also been synthesized from Annona squamosa peel extract. In this experiment, 10 mL of aqueous extract and 80 mL of 1 mM palladium acetate were combined and heated to 60 °C for 4 h. XRD, UV-Vis spectrophotometer, and TEM were used to characterize the produced particles. Pd-NPs with an average size of 100 nm and spherical form were confirmed. The watermelon rind aqueous extract was used as a capping and reducing agent for Pd-NPs. The importance of the extract’s polyhydroxyl groups in the synthesis of Pd-NPs was further established. The Pd-NPs with a mean size of 96.4 nm and a polydispersity index of 0.243 exhibited potential industrial catalytic application by the Suzuki coupling of aryl halide with phenylboronic acid at ambient temperature [104,105].
5.1.3. Platinum Nanoparticles (Pt-NPs)
Dauthal and Mukhopadhyay [106] have reported employing Punica granatum peel extract for the production of Pt-NPs with a size between 16 and 23 nm; the produced particles were crystalline and spherical. The particles’ negative zeta potential showed that they were very stable. The capping and stability of the produced particles were due to, according to the FTIR study, peel extract polyphenolic compounds and their quinones, which contain hydroxyl and carbonyl functional groups. By eliminating the man-made contaminant 3-nitrophenol by NaBH4, the particles demonstrated good catalytic activity. The creation of polydispersed, spherical, 23 ± 2 nm-sized TiO2-NPs was another successful application of the Annona squamosa extract [107].
5.1.4. Silicon Nanoparticles (Si-NPs)
The second most common element on Earth, after oxygen, is silicon, and agriculture has begun to place a high value on this element [108]. Si is regarded as a transitional element for plants, falling somewhere between an essential and non-essential category because it is not only necessary for plant existence but also provides adequate benefits when present [109]. These NPs have the ability to interact with plants both directly and indirectly, resulting in morphological and physiological alterations that impart stress resistance. This enhances plant growth, boosts biomass, changes architecture and physiology, modulates tissue differentiation, activates defense mechanisms, and aids in acclimating to stressful situations in plants [110]. When administered at varying concentrations, Si-NPs displayed anti-stress benefits against moderate to severe drought stress in Hawthorns (Crataegus sp.) [111]. The seedlings’ responses varied depending on the concentration. Increased membrane electrolyte leakage; relative water content; improved photosynthetic capacity; and higher concentrations of chlorophylls, carotenoids, and proline are some of these consequences.
The use of nano-SiO2 by accumulating proline increased tolerance in saline environments by maintaining ionic balance, enhancing the antioxidant system, and increasing the level of different phytopropanoids, which in turn caused osmotic adjustments. Furthermore, silicon dioxide nanoparticles are demonstrated to improve water use effectiveness, stomatal conductance, transpiration rate, and lowered chlorophyll degradation during salt stress, resulting in tolerance to external insult [112]. The epicuticular wax layer has undergone major alterations, and the main cause is salt stress [113].
Surprisingly, strawberry plants treated with nano-Si had superior epicuticular wax thickness and structure than salt-stressed plants [114]. Another study that looked at the effects of nano-Si to reduce salt stress on sweet pepper plants found substantial differences between them and their bulk equivalent [115]. The plants received low concentrations of nano-Si (1.0 & 2.0 cm3/L) via irrigation for weeks, which improved the leaves and fresh weight. On potato plants subjected to various salt stresses, the SiO2-NPs concentration-dependent impact was also noted (NaCl, 50 & 100 mM). Both a lower concentration (50 mg per liter) and a greater concentration (100 mg per liter) of the NPs allowed them to demonstrate improved stress reduction [116]. However, smaller concentrations were discovered to be more beneficial. This research significantly provides a better perspective on the positive and negative influence of NPs, challenging the preconceptions that only hazardous elements of NMs exist. As a result, before using NPs in any sector, it is necessary to first investigate their properties [117].
In times of external stress, plant hormones are essential for promoting better adaptability to a variety of shifting environmental conditions. Gibberellin, a plant growth hormone, plays a key role in how plants respond to a variety of abiotic challenges, such as drought, shade, flooding, and cold temperatures, mostly by slowing down plant development and shifting the emphasis to coping with stress. By using NMs, plant hormones can also be delivered to the plant [110]. For instance, the Arabidopsis thaliana plant was tested using Mesoporous Silica-NPs that contained abscisic acid [118]. The prolonged hormone release was clearly demonstrated, and the seedlings’ capacity to withstand drought was also strengthened.
5.1.5. Silver Nanoparticles
Nanotechnology has piqued the curiosity of many people because of its numerous uses in technological domains. Metal NPs such as silver, gold, iron, zinc, cadmium, graphene, and platinum have been employed in optical, catalytic, antibacterial, and biological activities. Because of their unique qualities, such as shape, size, optical, electrical, and magnetic properties, silver nanoparticles are widely used in coated materials, sensingmaterials, electrical components, antimicrobial applications, and composite fibres [119]. NPs have superior physiochemical capabilities because of their high volume ratio to surface area ratio, which can be employed for a number of applications such as antibacterial, antioxidant, and industrial wastewater treatment [120]. Researchers have been inspired to create NPs via physical, chemical, and biological means since the attributes of NPs are mostly based on their structure, size, and biological composition. Physical and chemical methods typically cost money and demand a lot of energy, pressure, and heat [121]. The chemicals used in the process are potentially dangerous to the environment and poisonous. As a result, there is a constant need for the creation of eco-friendly processes for producing NPs without the use of dangerous chemicals. There are now several ways to create NMs, but due to their many uses, particularly in the removal of dangerous and harmful compounds, biological processes are becoming increasingly significant [122]. Plant extract is frequently used to create metal and metal oxide NPs because it has capping and reducing properties [123].
5.2. Carbon-Based NMs
Carbon Nanoparticles (C-NPs)
The production of C-NPs using currently used technology is known to require considerable use of premium feedstock’s in addition to expensive catalysts and required energy to drive C-NPs synthesis. A common technique for producing C-NPs on an industrial scale at a large scale is chemical vapor deposition (CVD), which is known to use a variety of costly supplies of ethylene and carbon monoxide [124]. The majority of developing nations contribute agricultural fibre, and they also use a significant percentage of their agricultural waste as fuel. Goods like rice husk, groundnut shell, bagasse, and coconut fibre are used, and India contributes over 400 tonnes of agricultural waste on its own. It is recognized that the above-mentioned agricultural waste items can produce carbon compounds with nanostructures. C-NPs can be used for a variety of things, such as capacitors and high-performance electrode materials for batteries; they are also well-known for being employed as spectacular photo-luminescent materials [125]. For C-NPs that have a higher surface area, efficient molecule binding is achievable. One of the processes for producing activated carbon has been called carbonization, and it involves raising temperatures to 600–900 °C while using inert gases like argon and nitrogen to produce activated carbon from raw materials like coal, nutshells, and wood. Another method is called “oxidation,” which involves heating the carbonised material at a temperature between 600 and 1200 °C in the presence of oxidizing gases such as oxygen, carbon dioxide, or steam [126,127]. Black carbon might technically be used to describe carbon measured by light absorption [128]. It is recognized that various sources produce various forms of carbon. Diesel engines and the burning of wood and coal on a domestic and industrial scale using heavy oils are the main sources of black carbon emissions. These are made by burning agricultural waste that remains after forest fires. The formation of a point defect in a diamond particle, continuing laser ablation of graphite with oxidation and functionalization, and thermal degradation of organic compounds are all required for the creation of C-NPs using ion beam radiations [129,130,131].
5.3. Composite-Based NMs
5.3.1. Zinc and Nickel Oxide Nanoparticles
Rambutan peels were used to create biogenic zinc oxide nanocrystals in the following ways: zinc nitrate hexahydrate was reacted with Rambutan peel extract at 80 °C for two hours to produce the zinc ellagate complex. The zinc ellagate complex was subsequently oven dried for 8 h at 40 °C and then calcined at 450 °C in a muffle furnace to produce zinc oxide nanocrystals. Standard characterization investigations, including XRD, SEM-EDX, XPS, and FTIR, were used to establish that the p-track conjugation effect of zinc oxide nanocrystals promoted polyphenol production. Because of the growing ZnO nanocrystals’ adsorption on the cotton surface, the cotton fabric treated with zinc oxide nanocrystals showed exceptional anti-bacterial activity against E. coli (18.5 mm) and S. aureus (23 mm) [132].
According to a related study, Yuvakkumar et al. [133] also successfully synthesized nickel oxide (NiO) nanocrystals using rambutan peels to produce nickel–ellagate complexes for the first time. The extract’s polyphenols helped nickel nitrate hexahydrate transform into NiO nanocrystals. The strong antibacterial activity of 25 and 35 mm against E. coli and S. aureus, respectively, were demonstrated using cotton fabric coated with synthetic NiO crystals.
5.3.2. Iron Oxide (Fe3O4) Magnetic Nanoparticles
The extracted rind of watermelon was used in a reaction of sodium acetate and iron chloride with constant stirring at 80 °C for three hours to produce Fe3O4 magnetic nanoparticles [134]. UV-Vis spectrophotometer, AFM, XRD, DLS, FTIR, and TEM were used to describe the spherical-shaped biogenic particles, which ranged in size from 2 to 20 nm and had a strong magnetic response. With extremely good yields of 94%, the particles created by the extract’s polyphenols showed exceptional catalytic activity in the synthesis of 2-oxo-1,2,3,4-tetrahydropyrimidine derivatives. The creation of iron oxide magnetic nanoparticles using plantain peel extract was also reported by Venkateswarlu et al. [135] in a similar manner. Using common analytical methods, the biogenic particles were identified. The particles had a sphere-like form and were evenly scattered. Their size, as determined by TEM, was less than 50 nm. The bio-produced Fe3O4 magnetic nanoparticles had a saturation magnetization of 15.8 emu/g and demonstrated exceptional magnetic properties [136]. The different synthesis of NMs by agro-waste is shown in Table 2.

Table 2.
Synthesis of NMs by agro-waste.
5.3.3. Titanium Dioxide Nanoparticles (TiO2-NPs)
There are other NPs that exert their effects through different pathways including gene regulation but we have only discussed the anti-oxidative actions of those NPs that scavenge reactive oxygen species (ROS) produced in response to stress in plants. As an illustration, TiO2-NPs exposure (0.01%) enhanced the amount of chlorophyll and biomass by inducing anti-oxidant enzyme activities, which reduced malondialdehyde and hydrogen peroxide and boosted the formation of proline and soluble carbohydrates, maintaining the osmotic equilibrium [179,180]. Similar to this, nano-titanium dioxide was able to promote the expression of a certain important non-coding or nonsense RNA thought to be crucial to the mechanism of resistance to abiotic stress [181].
Lack of water poses a serious problem for plant production because it reduces plant vigour and severely harms crops. By raising the movement of the nitrate reductase (NR) enzyme, which in turn promotes the accumulation of osmolytes, nano-TiO2 can help the plant’s hydration status [182]. Nitric oxide (NO) is produced as a result of the enhanced NR enzyme activity, and this in turn triggers the creation of proline and glycine betaine(GB) [183]. TiO2-NPs frequently exhibit a defensive mechanism against stress in plants that is both enzymatic and non-enzymatic. It is interesting to note that TiO2-NPs are recognized for controlling other enzymes like glutamate hydrogenase and glutamine synthase, which results in the accumulation of additional nutrients and the creation of essential oils [184]. In order to assess the impact of nano-titanium dioxide (0, 50, 100, and 200 mg/L) on Moldavian balm (Dracocephalum moldavic) plants cultivated under aggravated salinity stress, Gohari et al. conducted a greenhouse experiment (0, 50, and 100 mM NaCl) [185]. Under standard conditions, the plants treated with TiO2 (100 mg/L) produced 1.19% more of the essential oil’s geranial, zcitral, geranyl acetate, and geraniol. This suggested that aromatic plants could be protected from the stress situation by directly altering the profile and composition of their essential oil production. Another investigation on medicinal plants demonstrated that the effects of drought stress were lessened by adding methyl jasmonate (200 M), salicylic acid (100 M), and titanium dioxide nanoparticles (20 ppm) [186]. The outcomes indicate that this improves water stress tolerance by enzymatic and non-enzymatic anti-oxidant defense systems.
5.3.4. Silica-Nanoparticles (SiNPs)
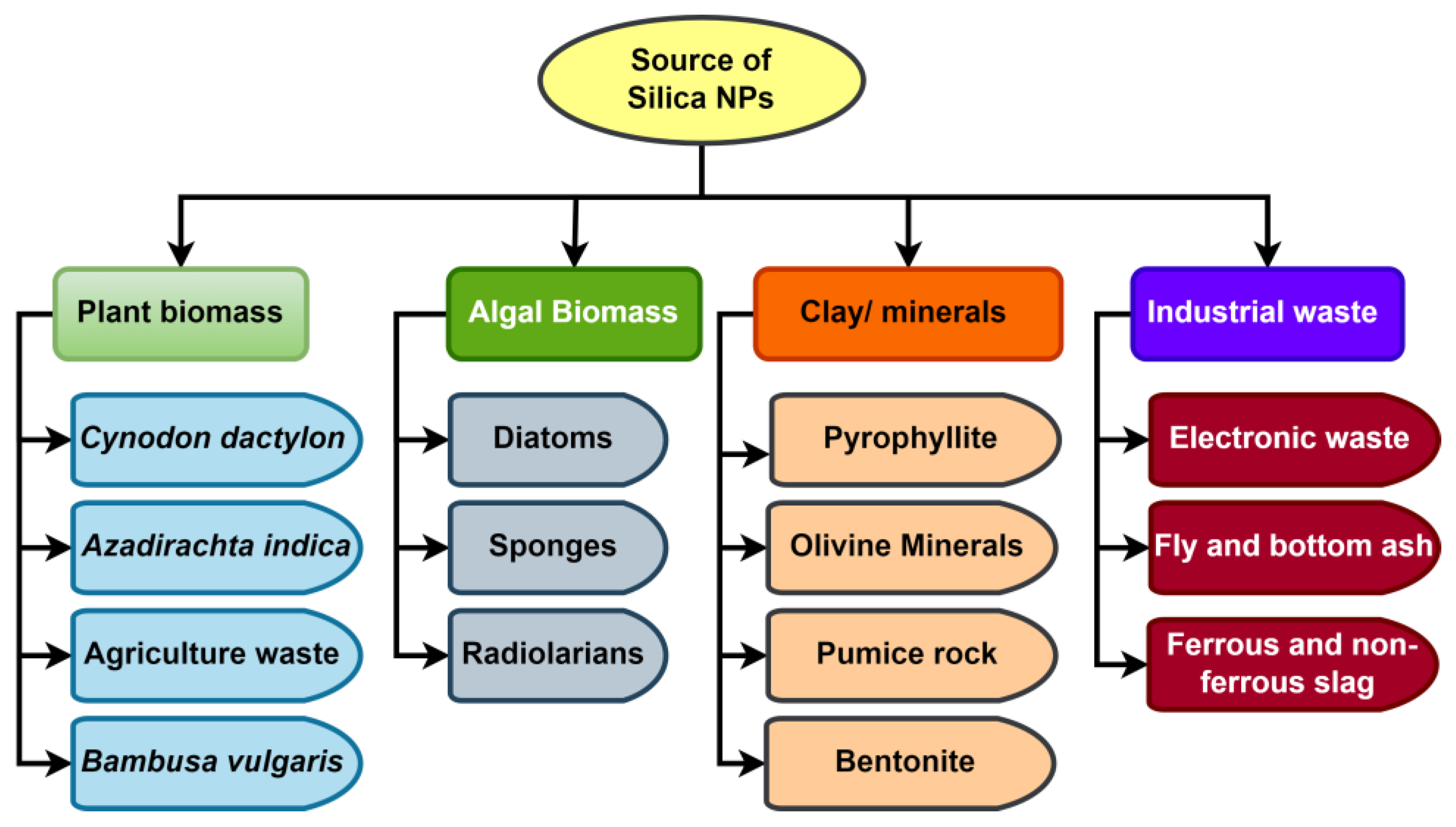
Wastes created by various agricultural activities are one of the cheapest sources for the synthesis of SiNPs, and different agricultural waste comprises around 50–90% SiO2. Because of the lower cost of raw materials and higher silica content, agro-waste has the potential to be used for the large-scale manufacture of SiNPs. For the extraction of bound silica from agricultural waste, several physical, chemical, and biological processes are used. The extraction of silica from its source is required for the commercial manufacturing of SiNPs. Figure 4 depicts various sources that could be employed as precursor materials (clay, industrial waste, algal biomass, and plant biomass, for example) in the production of SiNPs. The conditions maintained at the time of synthesis dictate the size of NPs, and the size of SiNPs typically ranges from 10 to 500 nm [187]. Three different forms of SiNP were found in one study when analyzing the bioavailability of SiNPs in invivo conditions: short rod (NSR), long rod (NLR), and spherical (NS) [188]. In a study conducted by a different team of researchers, NSR and NLR shapes were also confirmed [189]. The additional geometries in which SiNPs can be made include nano-ribbons, nano-helices, nano-tubes, and nano-zigzags [190]. However, in order to achieve the smallest interfacial surface area, amorphous SiNPs often take on the most common spherical shape [191]. In a work conducted by Akhayere et al. [192], spherical magnetic SiNPs with an average size of 162 nm were obtained from barley husk waste and utilised to remove petroleum, and its byproducts from water sources also discovered spherical SiNPs in corn cobs [193]. Because of their spherical form, these NPs tend to agglomerate, allowing pollutants to be effectively absorbed at different layers.
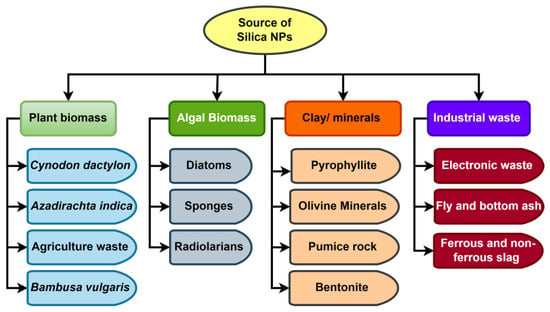
Figure 4.
Different raw materials for commercial production of silica nanoparticles.
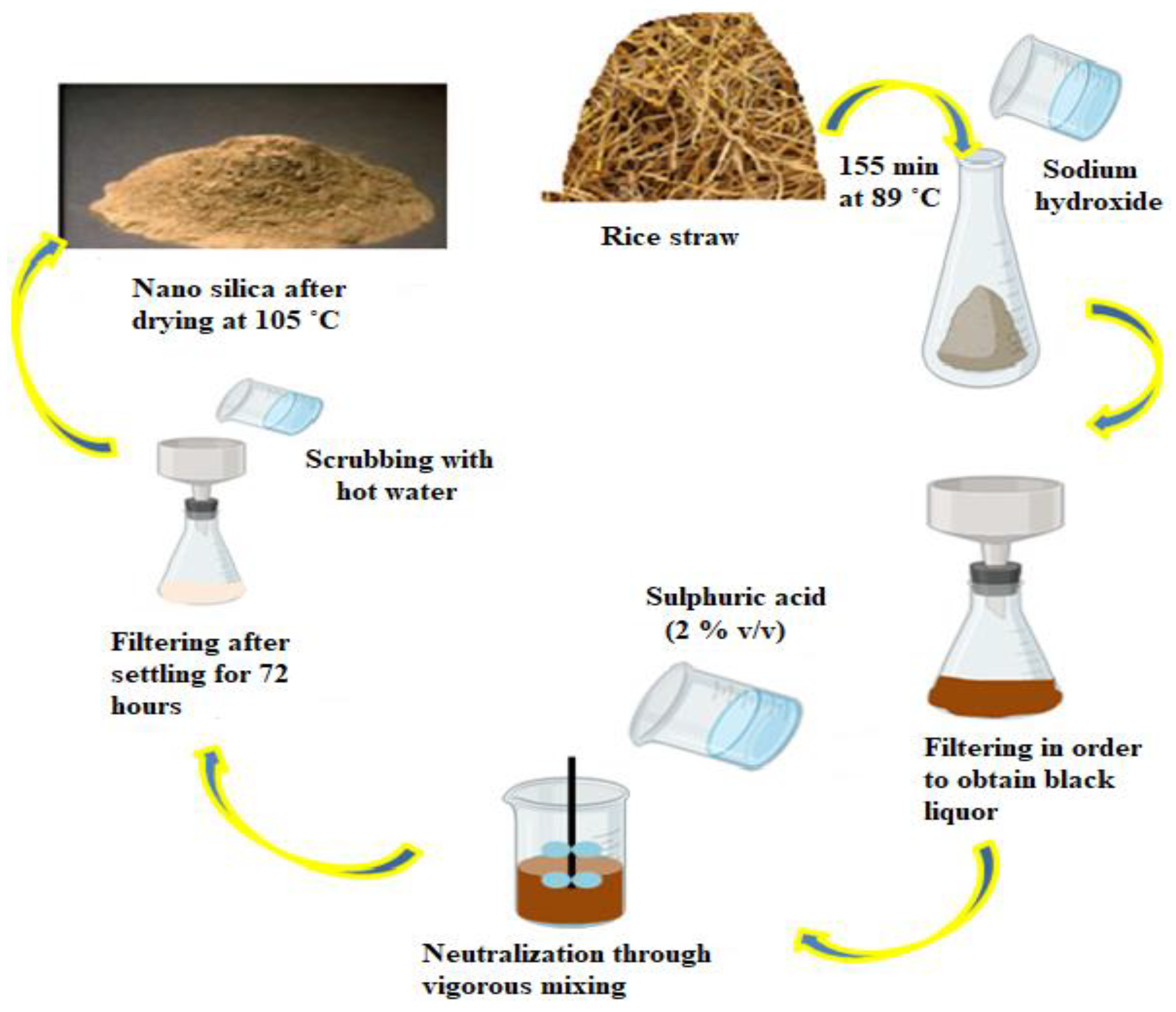
Numerous precursors serve as pollutants on the surface of value-added products derived from biological substrate digestion [194]. Therefore, silica extraction employing biological agents has gained popularity in recent years [195]. Biological approaches for NP extraction are recommended over thermal and chemical procedures due to their reduced toxicity, environmental safety, and speed and ease of isolation [196]. As a result, numerous researchers have concentrated on the biological synthesis of nano-silica as a unique way for the production of different NMs. In one study, California red worms were used to decompose rice husks and create micro silica [197]. The worms were fed rice husks for five months; following which, the humus they expelled was neutralized with calcium carbonate and calcined at various temperatures (500, 600, and 700 °C) prior to being digested with a mixture of HCl and HNO3 in a ratio of 1:3. Depending on the calcination temperature, nano-silica between 55 and 250 nm in size was generated. According to these studies, in order to obtain a single NP, the calcination temperature must be less than 600 °C [195]. To create NPs, they used a combination of biological, chemical, and physical methods. Aside from that, a recent study has indicated that proteins derived from marine diatoms and sponges, such as silaffins, silicatein, and polyamines, are important biomolecular in the biomimetic silicification process and can be used to synthesize SiNP [198]. Silaffins and long-chain polyamines (LCPAs) form the key important factor in the bio-silicification process, converting silicon ions to nano-silica [199]. A stage in the extraction of nano-silica from rice straw is shown in Figure 5.
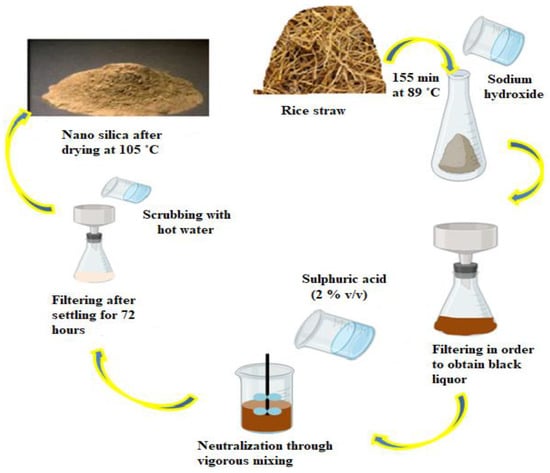
Figure 5.
Different steps in the isolation of nano-silica from rice straw.
6. Characterisation of NMs from Agro-Wastes
6.1. Fourier Transform Infrared Spectroscopy (FTIR)
In FTIR spectroscopy, energy and matter interact with one another. It is feasible to determine the functional groups that are present and describe the sample’s chemical structure by recording the quantity of absorbed energy and comparing it to a database. Non-destructive and highly effective, FTIR spectroscopy can be used to study the chemical and physical characteristics of lignocellulosic biomass [200]. There are three main parts that make up plant fibre: cellulose, lignin, and hemicellulose. Alkanes, esters, aromatics, ketones, and alcohols with diverse functional groups that contain oxygen make up the majority of these materials. FTIR has been extensively used in the literature to confirm the removal of amorphous sections like hemicellulose and lignin from the fibre matrix after chemical treatments like alkali treatment and bleaching or to evaluate if extracted cellulose nanofibres contain any lignin or hemicellulose [201]. In the past, scientists have studied at the infrared spectra of cellulose, hemi-cellulose, and lignin [202,203]. An FTIR spectroscopic examination of isorafibres after being untreated, alkaline-treated, bleached, and acid-treated was reported by Chirayil et al. [204]. They discovered a peak in the spectra of all fibres at 3300 per cm, which corresponded to the OH stretching vibrations of the hydrogen-bonded hydroxyl group and indicated the fibres’ hydrophilic inclination [205].
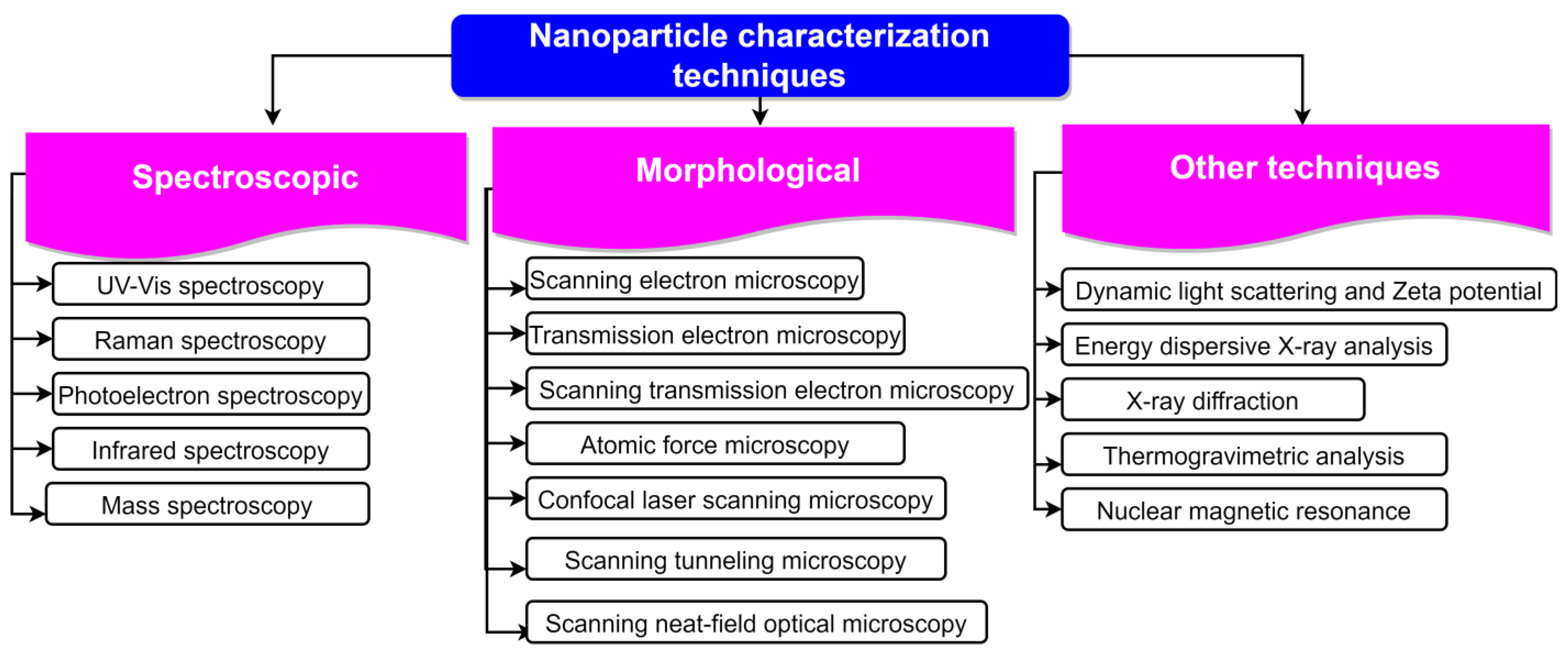
The peak at 1630 per cm was caused by the bending mode vibration of the water [206]. The peak at 1228 per cm in raw fibre was caused by the smelly skeletal vibration of lignin [207]. On the FTIR investigations of the CNs made from banana peel by means of enzymatic treatment, Lima et al. [208] came to similar conclusions. They were able to demonstrate that the enzymatic treatment was successful in removing hemicellulose and lignin from the matrix of the fibres. The bulk of the lignin in the banana peel cellulose nanofibres was reportedly eliminated using bleaching, as reported by Zope et al. [209]. After bleaching, the vibrations at 1525 per cm (aromatic ring vibrations), 1238 per cm (guaiaryl ring breathing with stretching C=O), and 761 per cm (C-H deformations) vanished. Karimi et al. [210] studied the FTIR of unbleached (UBNF) and bleached (BNF) kenaf bast cellulosic fibre samples and found that neither UBNF nor BNF samples contained lignin. Techniques employed for NP characterization are shown in Figure 6.
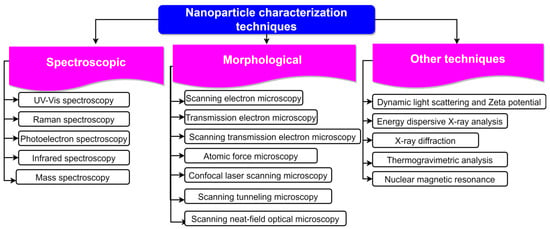
Figure 6.
Different techniques employed for characterization of NPs.
6.2. X-ray Photoelectron Spectroscopy (XPS)
XPS, also referred to as electron spectroscopy, is a technique for chemical study [211]. XPS has been utilized for surface analysis on a wide variety of materials [212]. XPS provides quantitative and chemical state data from the surface of the studied material [213]. The XPS studies of cellulose nanofibres have been reported in very few works. Ahola et al. [214] looked at the chemistry of model films made from cellulose nano-fibrils using XPS. They made skinny and soft native cellulose model films by spin-coating cellulose nano-fibril dispersions on silica substrates. They discovered that the percentage of identified substrate material (nitrogen/silicon) rises in both model films relative to the standard, indicating that the surface has been coated by cellulose nano-fibrils. Zuo et al. [215] also investigated the surface chemical composition of nano-fibrillated cellulose aero-gels and films prior to and after coating with perfluorodecyl trichlorosilane (PFOTS). The CMC fibrils’ surface counterions were determined to be carbon, oxygen, and trace levels of sodium, while fluorine and silicon were present in the modified samples. They determined that PFOTS had interacted with cellulose.
6.3. Measurements of the Zeta-Potential of Agriculture-Based NPs
The zeta-potential is a significant aspect of cellulose nanofibre suspension stability. It denotes the degree of repulsion in dispersion among adjacent, similarly charged particles. The zeta-potential measurements of cellulose nano-fibre (CNs) suspensions obtained from banana peels were reported by Dominic et al. [216]. They expanded the number of CN passages in the high-pressure homogenizer and obtained highly stable CN aqueous suspensions in water with zeta potential values between 16.2 and 44.2 mV. They reported the scale and zeta capability values of CNs obtained with the aid of various quantities of passages in a high stress homogenizer. Similar consequences for CNs removed from banana plant wastes had been suggested [217]. They reported zeta capacity values that ranged from −20 to −40.31 mV. Surattanamal et al. [218] observed that the zeta ability of nanofibres acquired by means of chemical treatment and enzymatic remedy were −16.2 and 25.5 mV, respectively. They observed that enzymatic remedy produced cellulose nanofibres with the best zeta capacity that were greater electrically than the ones produced with the aid of chemical treatment. Li et al. [219] investigated the hydrophilicity of rice, waxy maize, wheat, and potato starch by measuring their water contact angles. The zeta-potential of starch granules was determined to be −20.5, −19.1, −20.4, and −4.2 mV for rice, waxy maize, wheat, and potato starch, respectively. With an increase in dispersion pH from 2.07 to 11.96, the zeta potential of starch nanocrystals studied by Wei et al. [220] was found to decrease from −6.7 to −34.5 mV. They also found that as the pH of the dispersion increased, the distribution peaks of the starch nanocrystals grew wider.
6.4. Thermal Analysis of NPs Obtained from Agro-Waste
Decomposition, melting, polymerization, crystallization, and oxidation are just some of the chemical changes that can be detected through thermal analysis. Thermal analyses are methods that have been developed to determine the kinetics of the thermal degradation and the thermal transformation of agricultural waste NPs, both of which can be used to define the operating conditions for employing these materials in fields as diverse as the chemical and the environmental.
6.4.1. Nanocellulose and Micro Cellulose Derived from Agro-Waste
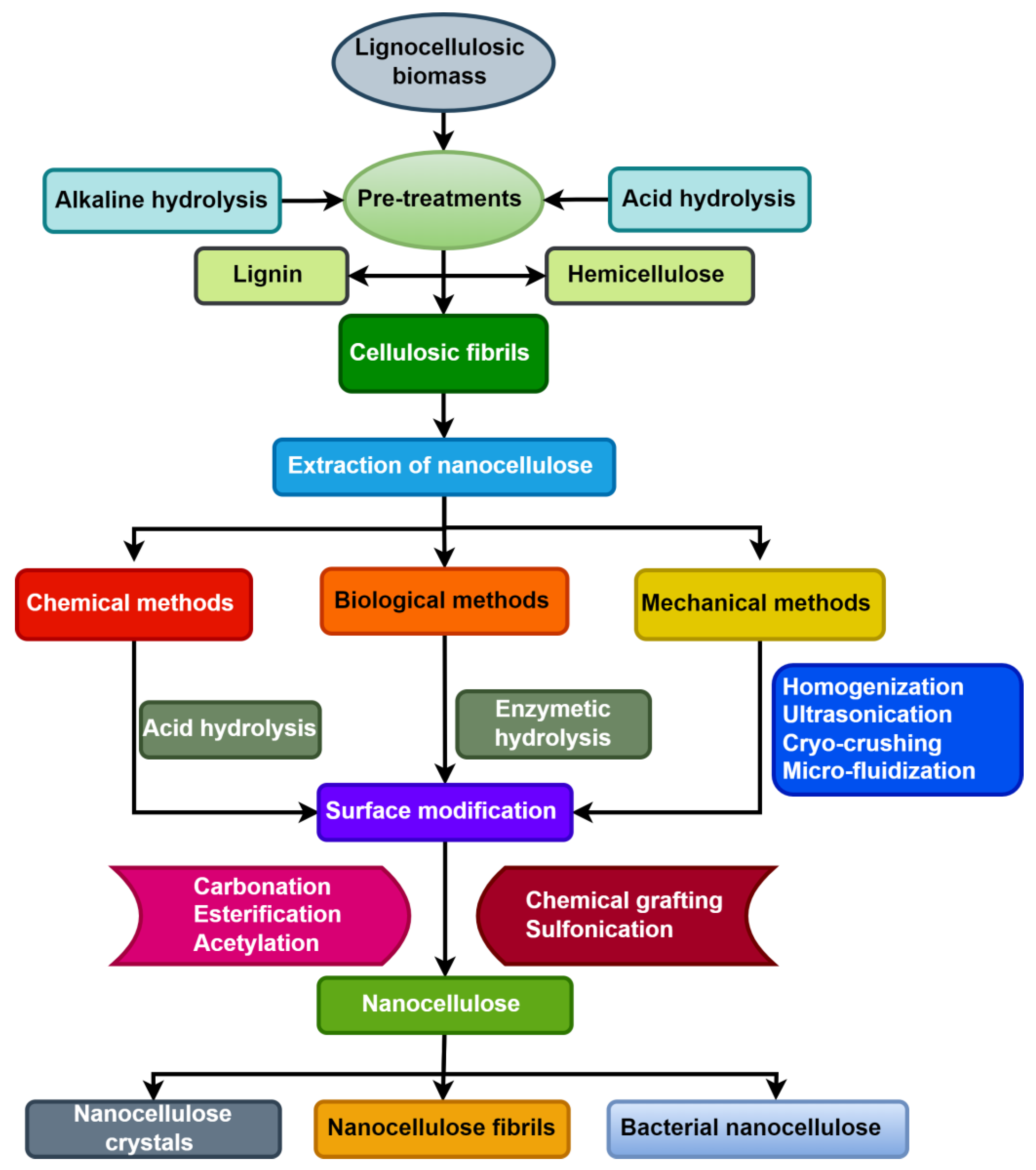
Raw pineapple leaves were subjected to alkali treatment, bleaching, acid treatment, and neutralization to extract micro cellulose. Ogah et al. investigated the thermal stability of various agro-nanofibres (rice hull, corncob, flax shive, walnut shell) in order to determine the decomposition of cellulose, hemicellulose, and lignin [221]. Thermo gravimetric measurements were taken at heating rates between 5 and 20 °C per min. The agricultural fibres’ weight loss and derivative losing weight curves demonstrated that the first step correlates to moisture evaporation, with hemicellulose degradation occurring next between 180 and 300 °C because of its brittle branched-chain structure. Because of its thermally stable linear chain structure, cellulose decomposes between 290 and 360 °C. Lignin is the final decomposition from 300 to 500 °C. The highly cross-linked and complex structure accounts for the wide temperature range of its decomposition. The use of diverse agricultural wastes for the extraction of nanocellulose is regarded as a sustainable strategy, with these wastes classified as lignocellulosic biomass, which mostly contains lignin, cellulose, and hemicellulose [222]. Nanocellulose extraction from lignocellulosic biomass is typically separated into two stages. The first stage involves extracting hemicellulose, lignin, and other non-cellulosic components from biomass. The second step is to remove nanocellulose from cellulosic fibrils using various chemical, biological, and mechanical approaches [223]. Figure 7 depicts a flow chart of a short nanocellulose extraction approach from lignocellulosic biomass.
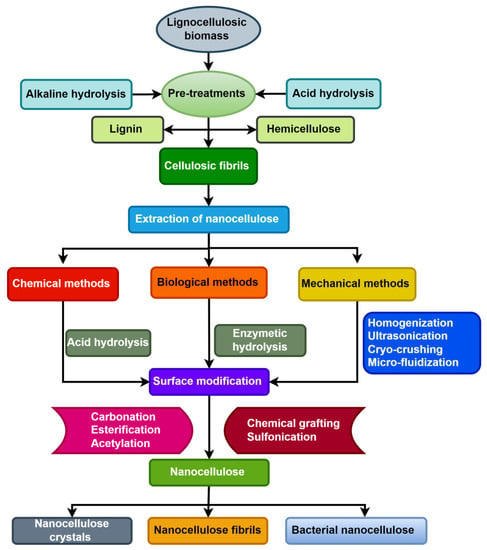
Figure 7.
Flow chart of isolation process of nanocellulose from lignocellulosic biomass.
6.4.2. Cellulose Nanofibrils (CNF)
Microwave liquefaction was used by Xie et al. [224] to study the characteristics of cellulose nanofibrils obtained from bamboo. In a nitrogen atmosphere, 2 mg of the sample were heated to 800 °C at a rate of 20 °C/min. The weight loss curves that were obtained demonstrate typical results of the literature with various steps, as mentioned above in the study by Xu et al. [225]. The maximum decomposition rate temperature can be determined from the weight loss curve’s derivative; this can be computed using a formula called the derivative of time (Tmax). The temperature at which the cellulose in the original bamboo begins to break down is known as the Tmax. For the microwave-liquefied residue sample at 25 °C, the derivative weight loss curve allows for the observation of the decline in this temperature. This decline can be explained by the bamboo’s fragmented structure during microwave treatment. It is essential to employ losing weight curves and their related derivative weight loss curves in order to understand the events that may happen during heat therapy. Endoglucanase pre-treatment enhances the disintegration of cellulosic wood fibre pulp into cellulose nanofibres, according to Padhi et al. [226]. Lima et al. [208] extracted cellulose nanofibres from banana peel bran using chemical treatment (alkaline treatment, bleaching, and acid hydrolysis) and enzymatic treatment (alkaline treatment and xylanase hydrolysis). Chemically and enzymatically treated nanofibres had average diameters of 10.9 and 7.6 nm and lengths of 454.9 and 2889.7 nm, respectively [227]. They demonstrated that the NPs that were separated from both treatments may be used as reinforcement in composites.
6.4.3. Nanocrystals and Nanofibres
Robles et al. [228] present a case study for the nanocrystals of chitin and cellulose. As the weight loss peak moves to elevated temperature, this indicates that the composite’s thermal resistance has improved. Different studies are about the decomposition of cellulose/starch [229,230], but as shown by Sun et al. [231], thermal analyses are also utilized to assess nanocrystals and nanofibres derived from agricultural wastes in order to determine decomposition processes and temperatures.
6.4.4. Parameters of Kinetics
Numerous authors have demonstrated that kinetic variables of agricultural waste NM thermal decomposition can be estimated from thermal analysis [100,102,103,104]. Equation (1) can be used to describe the rate of thermal decomposition.
where t is the period of time required for the reaction to occur, k(T) is a constant, and f(α) is a function that changes depending on the type of material being converted. The current conversion factor is expressed as shown in Equation (2):
with w0 representing the initial sample weight, wt representing the mass sample weight at time t, and wf representing the final sample weight. The mgs are the unit of measure for all sample sizes. The reaction rate constant is determined by the Arrhenius equation and can be expressed as shown in Equation (3):
where A, Ea, and R denote the pre-exponential factor, activation energy, and constant of ideal gas, respectively.
From Equations (1)–(3) and some integration, as Henrique et al. describe, it is possible to calculate the kinetic parameters [232]. The authors employed two approaches, Kissinger and Flynn-Ozawa, to find out the conversion and activation energy of cellulose nanocrystals taken from clean mango seeds, Eucalyptus urograndis, and cellophane. An example of cellulose nanocrystals that were extracted from clean mango seeds shows that the activation energy can be very different for the same rate of conversion, depending on how the extraction was done. The Arrhenius parameters and activation energy of tropical lignocellulosic agro wastes were determined using three iso-conversional techniques, Kissinger–Akahira–Sunose (KAS), Flynn–Wall–Ozawa (FWO), and Friedman [233]. It was also shown that the breakdown of cellulose, hemicellulose, and lignin followed a second order kinetic model. It was described how these models can be applied to the process of wood degradation during pyro-gasification [234].
6.5. Morphological Characterisation of NPs Derived from Agro-Waste
Morphology explores the specifics of material systems into sorts of normal shapes so that it will realize how various materials interact with each other. Morphology is also connected with macroscopic qualities, and the microstructure of polymers is associated with both the conformation and morphology of the polymer. There are numerous techniques for characterizing bio-based NMs that could be used to calculate their volume, shape, and size distribution. TEM and AFM are the typical microscopic techniques.
6.5.1. Atomic Force Microscopy (AFM)
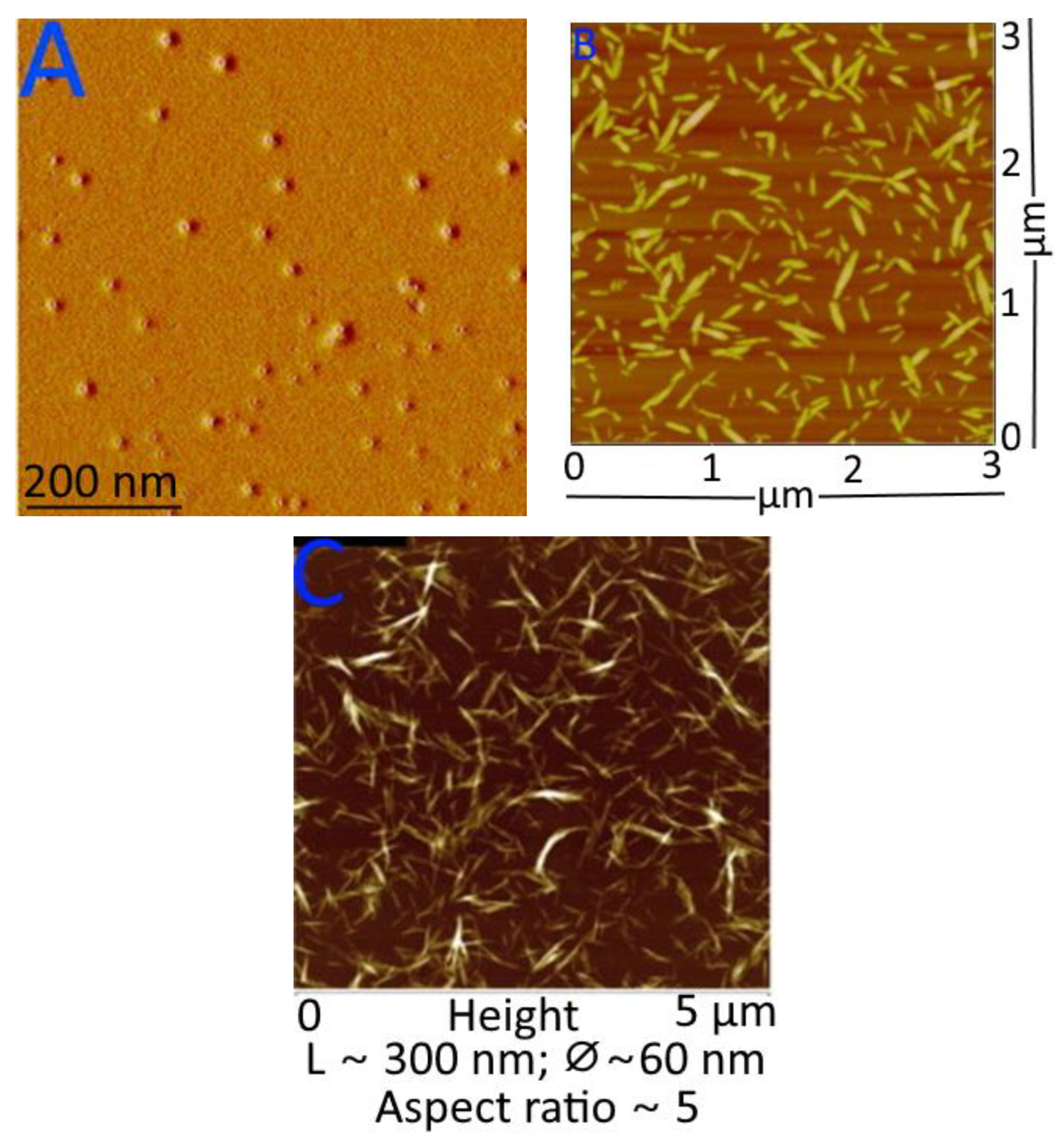
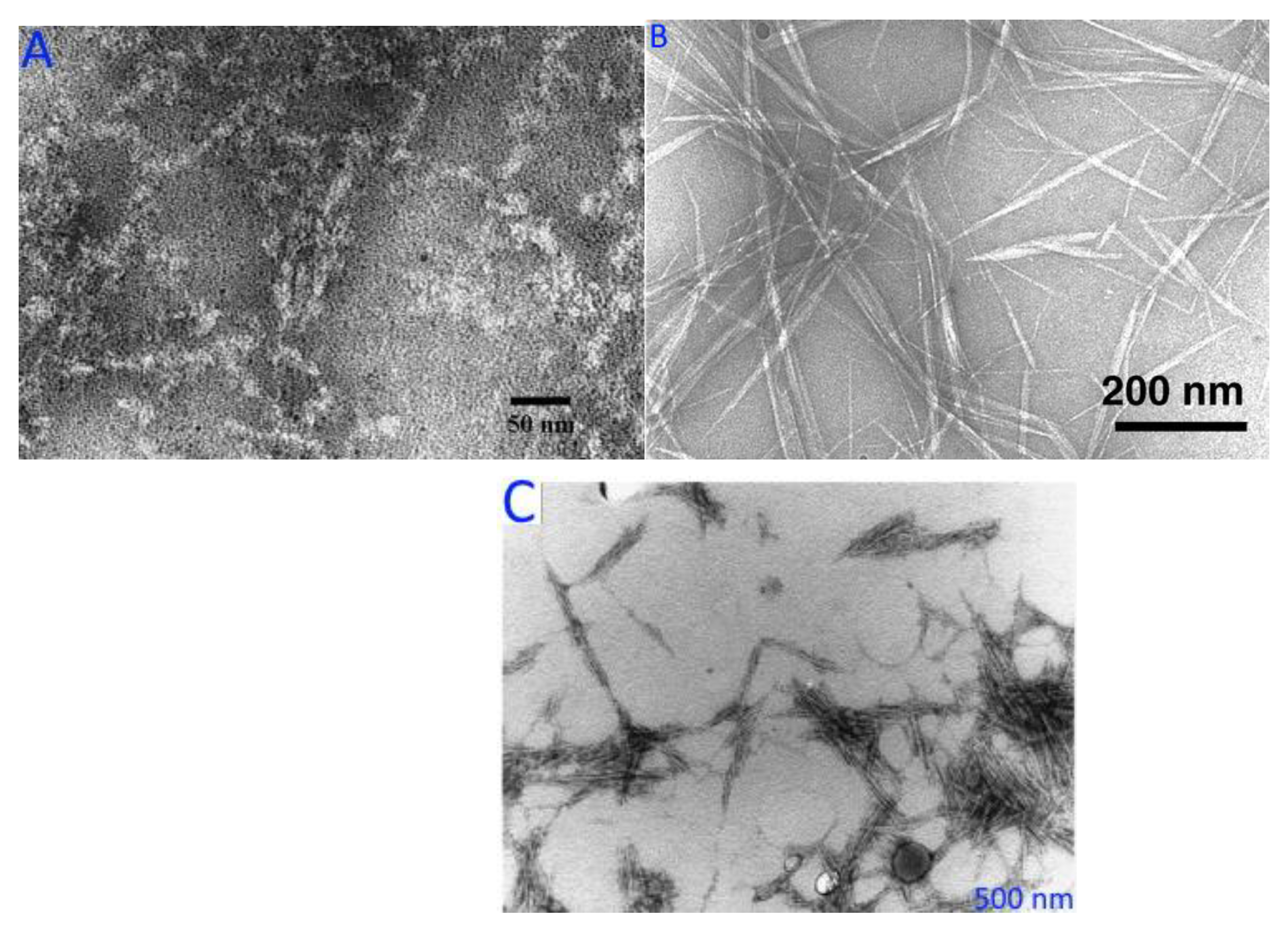
In an AFM, the physico–chemical characteristics of a sample are found by moving a sharp probe on a cantilever over the sample and watching how the cantilever moves as a laser beam reflects off of it. AFM gives accurate and precise information about the size of cellulose nano-fibrils, as well as their morphology, surface topography, mechanical properties, and ability to stick to other things [235,236]. Nano-cellulose particle thickness; lateral connections between nano-fibrils; nano-scale morphology and roughness; porous network structure, mechanical, optical, and permeability properties; and the shape parameter of individual particles may all be determined by this method. It has been found that the roughness of cellulose’s surface affects its flow characteristics contact angle, hydrophilicity, and local mass transfer, as a membrane. AFM may also be used to analyse the nanoscale particle size distribution of cellulose fibres [237,238], and AFM images of nano-cellulose from many resources and the advantages of AFM are used in determining changes caused by various factors. AFM images of (A) starch nanocrystals, (B) cellulose nanocrystals, and (C) chitin nano-whiskers are depicted in Figure 8.
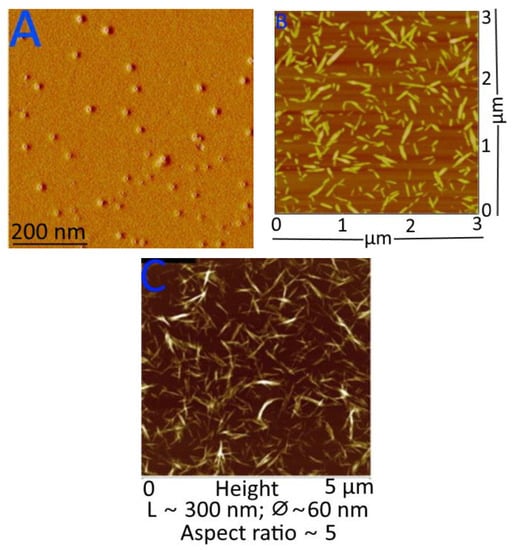
Figure 8.
AFM images of (A) starch nanocrystals, (B) cellulose nanocrystals, and (C) chitin nano-whiskers. Figure 8A–C reprinted with permission (copyright © 2023, 2013, and 2014, Elsevier Ltd., Amsterdam, The Netherlands) from Espino-Pérez et al. [239], Liu et al. [240], and Salaberria et al. [241].
6.5.2. Transmission Electron Microscopy (TEM)
The image of TEM plays a crucial role in cellulose micro-fibril and nanocrystal morphological studies. TEM has traditionally been the method of choice for visualizing the morphology of individual or grouped CMCs [242]. Variation in transmitted and diffracted beam intensity due to microstructural changes on the electron route that alter diffraction conditions is the origin of image contrast. The TEM images of (A) starch nanocrystals, (B) chitin nanocrystals, and (C) cellulose nanocrystals are shown in Figure 9. However, there are a few things to keep in mind when using TEM to characterize NPs. It is critical to check whether a satisfactory dispersion of NPs has been obtained while characterizing TEM.
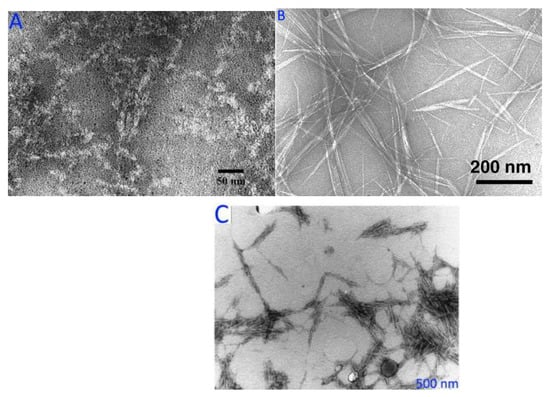
Figure 9.
TEM images of (A) starch nanocrystals, (B) chitin nanocrystals, and (C) cellulose nanocrystals. Figure 9A–C reprinted with permission (copyright © 2023 Elsevier Ltd., Amsterdam, The Netherlands; copyright © 2023 American Chemical Society, Washington, USA; and copyright © 2023 Elsevier B.V., Amsterdam, The Netherlands) from Chen et al. [243], Fan et al. [244], and Neto et al. [245], respectively.
7. Application of NMs Derived from Agro-Wastes
7.1. Nano-Biosensors
Nano-biosensors (NBSs) are analytical tools with a minimum dimension of 100 nm or less [123]. NBSs are created to monitor plant fractions, soil, water, and NPs as well as nanotubes, nanowires, or nanocrystals in the agro-ecosystem [246]. In comparison to current analytical sensors and biosensors that integrate biological element detection with chemical or physical principles, NBSs provide a powerful tool by utilizing the physico–chemical characteristics of NMs. A transducer transforms biological data into a signal that an electrical component can produce. With this capability, an agronomist can accurately and quickly monitor the crops’ nutritional and water needs as well as any early signs of disease [247]. For plant science research, high-resolution crop monitoring with nano-biosensors may be a highly helpful instrument [248]. The real-time continuous measurement of plant metabolites and hormones will provide unprecedented levels of insight into and control over plant biosynthetic pathways [249].
7.2. Use of Nano-Fertilisers
Despite great expectations, the industrial manufacturing of nano-fertilizers on a wide scale and their use has not yet materialized. This is unquestionably a result of the unclear legislative direction. For instance, the European Union is actively working on developing a legislative and regulatory framework. Another contentious point is that, unexpectedly, it is simple to confirm that research has ignored macronutrients in favour of micronutrients when we examine the current literature. This is notable since crop output is actually primarily impacted by N, P, and K nutrition—despite the fact that microelements are crucial to plant metabolism [250].
In conclusion, there are still a lot of high expectations to live up to. According to international and national organizations (FAO, UNEAP, USEPA, and EEA) concerned with sustainable agricultural development and food security, applied research on nanotechnology in agriculture should be reoriented in accordance with clear priorities [251]. One of these priorities is unquestionably the creation of N and P nano-fertilizers.
7.3. Nano-Fungicides
The majority of agricultural damage, including that to important crops such asrice, wheat, barley, groundnuts, and cotton, is caused by fungi [252]. These illnesses have an impact on society since they severely reduce crop productivity and the economy. Fungicides that are commonly used can reduce these losses, but they can harm biodiversity because they target a wide variety of living things. As a result, we must consider other strategies to improve the management of fungal diseases. The creation of NPs as a successful tactic against fungal diseases is one of the greatest methods. Ag-NPs are frequently utilized for disinfection due to their antibacterial characteristics [253]. The efficiency of Ag-NPs and Ag-ions against the plant-pathogenic fungi Magnaporthe grisea and Bipolaris sorokiniana was tested in an experiment. When used for three hours, the ionic and nanoparticulate versions dramatically lessened the severity of the condition and were effective against the fungi [254]. Both Cu-NPs and Ag-NPs have the ability to inhibit the growth of the fungi Alternaria alternata and Botrytis cinerea. Rhizopus stolonifer, Fusarium oxysporum, and Mucor plumbeus are all susceptible to the antifungal effects of ZnO-NPs and MgO-NPs [255]. Two common pesticides, zineb and mancozeb, were enclosed in a MWCNT-g-PCA hybrid material; mancozeb was confirmed to be a more useful fungicide against A. alternate [256].
7.4. Adsorbents
Rice husk, an agricultural by-product, was used as a precursor for the synthesis of silica NPs [257]. XRD and SEM were utilized to investigate the specific characteristics of synthesized NPs [258]. As an adsorbent, Fe3O4@n-SiO2 nanoparticles were used to remove Cr(VI) from an aqueous solution [259]. Results showed that Cr (VI) ion removal was greatest at a pH of 2.0. As the initial concentration of Cr(VI) was increased from 1.36 × 10−2 M to 2.4 × 10−2 M, a corresponding decrease in removal (%) was observed [259]. After regeneration, the adsorbent was found to significantly remove Cr (VI) for up to six cycles (>60 ± 0.8%). However, it loses efficiency with each cycle, and its adsorption efficiency drops from 91.56 to 61.65 ± 0.08% from the first to sixth cycle [259]. Regeneration suggests that using this adsorbent for chromium-rich water/wastewater can result in cost-effective treatment.
7.5. Catalyst
Gupta et al. [260] reported the use of pine needles in the synthesis of nanocomposites of nanocellulose and NiFe2O4 nanoparticles. These nanocomposites were created by first synthesizing carbon nanofibres (CNF) from pine needles, then silanising these nanofibres (SCNF) with tetraethyl orthosilicate. Ni (II) and Fe(III) solutions were mixed into the aqueous CNF or SCNF mixture. After adjusting the pH of this mixture to 7.5 and stirring it for 2 h, it was hydrothermally treated at 160 °C to make nanocellulose/NiFe2O4 nanocomposites. Researchers found that SNCF-supported NiFe2O4 (NiFe2O4@SCNF) works well as a catalyst for the oxidative breakdown of Remazol Black 5 (RB5) and the reduction of nitrophenols. Titanium oxide nanoparticles with a size of 10 nm that were supported on hazelnut shell- or olive residue-activated carbon were recently prepared by Donar and colleagues [261] for use as a photocatalyst in the photodegradation of methylene blue. Honey pomelo peel-derived porous carbon was utilized in the synthesis of NiCo2O4 nanocomposites by Guo et al. [262]. After mixing a solution of Ni(II), Co(II), and urea with porous carbon and then subjecting the mixture to treatment at 120 °C, carbon-supported NiCo2O4 nanosheets were produced.
7.6. Battery
The lower-temperature carbothermal or magnesiothermic reduction of amorphous silica found in rice husks could produce crystal silicon for use in battery materials (such as lithium-ion batteries) [263]. The conventional method of manufacturing silicon results in higher costs than alternative methods. As a result, a significant amount of effort is being put towards the development of a process that is low in cost, high in volume, and commercially viable for producing high-purity silicon for use in electrical applications [264]. Numerous methods have been devised for extracting silicon from rice husk. By making silicon anode materials from biomass resources, the battery industry can make good use of agricultural waste products. As a result, it is believed that silicon anode materials with a nano/micro-hierarchical structure, in which CNTs are tightly intertwined between the RH-derived Si nanoparticles, are the best possible anode materials for lithium-ion batteries [263].
7.7. Health Risks and the Toxicological Impact of NPs in Agriculture
There has been a lot of study into the risks and health effects of NM, but we still don’t understand them very well. There is currently no uniform set of standards for the management of risks associated with the use of NPs in the agri-food industry. To ensure nano-safety, unified regulatory frameworks have been developed with a particular emphasis on safety-by-design synthesis methods [265]. To transfer information among diverse nanotechnology sectors, fields such as risk communication, risk management, policymaking, and risk assessment among many stakeholders are required. Generally, nano-safety researchers use a two-way strategy. The first strategy is to comprehend toxicity to human health, which is primarily directed at organisms, tissues, and cells. Secondly, groups worry about potential threats to their health from the environment. These communities are mostly concerned with different elements of the tree of life [266]. After that, research on the safety of NMs in plants has focused on the direct or intentional contact of food crops with specific NMs (like nano-insecticides, nano-fertilizers, and nano-herbicides), as well as indirect or unintentional exposure [267]. The fundamental consideration before utilizing NPs in any field is their physical and chemical characteristics, since these may directly affect their potential environmental and health dangers, hence limiting their actual potential as a useful entity. The size and concentration of the NPs are the most essential aspects from the standpoint of toxicity [268]. In light of the extensive research showing that NPs as small as 5 nm can easily pass through cell wall pores and as small as 20 nm can pass through plasmodesmata, it is reasonable to assume that NPs in the lower size range can enter plants more quickly and cause greater phytotoxicity even at lower concentrations [269]. The potential transfer from roots to shoots is hampered, as in the case of bigger size graphene oxide, and the bioaccumulation is lowered [270].
Recent studies have also demonstrated that phytohormones are crucial to the signaling of the external insult response. A hormone imbalance in plants is suggested by the NPs’ reduction of auxins, cytokinins, and salicylic acid, which has an impact on the metabolism as a whole [271]. When NP concentrations are extremely high, photosynthesis may be substantially hampered, maybe even leading to plant death. According to numerous research, exposure to NP has significantly reduced or inhibited plant development [272].
In light of the above-mentioned data, it is apparent that a number of parameters must be taken into account before realizing the beneficial exploitation of NPs in the agricultural system. The interaction between plants and soil is what drives agricultural productivity; hence it is crucial to understand the physicochemical properties of the soil system. It is thought to be the primary sink for NPs; therefore, any interactions between NPs and the soil’s constituents could have a significant effect on how they turn out [273]. It has been discovered that increasing toxicity is caused by pH, organic content, and cation exchange capacity. For example, pH 5.5 loamy clay soil showed negligible toxicity to ZnO-NPs even at 2000 mg/kg concentration, whereas loamy clay soil with pH 7.34 showed considerable toxicity at 45.45 mg/kg concentration [274]. Furthermore, soil organic matter has an important role in changing the safety concerns of the NPs, as evidenced by the lowered toxic effects of the zinc oxide nanoparticles when given with Alginate at a dosage of 400 to 800 mg/kg [275]. The biomass of plants is significantly reduced when ZnO-NPs are used in isolation, though. In addition, NPs have a direct effect on the structure of the microbial community of soil in a dose-dependent way, as seen by DNA fingerprinting analysis, which revealed declining taxa of Bradyrhizobium, Bradyrhizobiaceae, and Rhizobiales [276]. However, favourable benefits for Sphingomonadaceae and Streptomycetaceae were also predicted. It is noteworthy to see that NPs are increasing the taxa of the community linked to the breakdown process of organic pollutants and biopolymers while decreasing the taxa of the community linked to nitrogen fixation [277]. Ag-NPs also showed a dose-dependent effect on Rhizobium’s and Azotobacter’s nitrate-reduction activity, with a 0.3 ppm increase in Azotobacter’s activity [278]. The majority of environmental-based nano safety research has focused on preventing unintentional NP releases into the environment during the production, use, and disposal of agriculture-related nanoproducts. Studies comparing the relative toxicity of NPs-dialuminium dioxide at ZnO (15 nm), bare-ZnO (10 nm), and ZnO at KH550 (20 nm) on grown green peas have been conducted (Pisum sativum). Zn levels in seeds and roots were greater in plants treated with Al2O3 at ZnO-NP (1000 mg/kg for 65 days) compared to bulk and other Zn-NP exposure [279]. Photosynthetic pigments (Chl-a and carotenoid) rose considerably, but protein and carbohydrate content remained essentially constant throughout all treatments, with the notable exception of Al2O3 at ZnO-NP [280]. Similarly, a very high concentration of ZnO-NPs (1000 mg/L) had no effect on seed germination or root elongation. Cu and Ag-NPs, on the other hand, hindered zucchini root growth (Cucurbita pepo) [281]. Plant biomass and transpiration rate were drastically reduced when exposed to Ag-NPs at concentrations of 500 and 100 mg/L, respectively [282].
Indeed, the direct release of NMs into the environment through agro-nanotechnologies and the incorporation into the human exposure pathway via the food supply chain is theoretically possible. Because of the potential movement of NMs via the food chain, which is the present problem with agricultural nano-chemical application, safety assessment is critically needed. There are several ways for humans to be exposed to nano-agrochemicals, including increased requirement for food crops, huge food waste in affluent sectors, and other agricultural operations (food crops are harvested both before and after) [283]. Furthermore, because plants are widely consumed, they provide a substantial opportunity to ease the transfer of NPs to different tropic levels and, ultimately, they are important in the food chain since they are mostly ingested by different trophic microorganisms, animals, and humans [284].
It is critical to comprehend the single health notion, particularly in the context of agricultural nanotechnology. Previous studies and reports focused on the need for seeing modern health concerns via a single health concept cannot be minimized; this must be understated. Furthermore, many peer-reviewed journals portray their study findings as evidence of the need for a unified health approach. We must also recognize the outstanding efforts of various international organizations (FAO and WHO, etc.) devoted to promoting the concept of one health and raising awareness of its significance [285].
8. Future Perspectives
NMs have shown a growing interest in agricultural activities, likely as a direct result of the expanding research and innovations in science and engineering. NMs have also been shown to be useful in anaerobic digestion, and as such, they have the potential to be useful in the conversion of agro-biomass. In general, nanotechnology has the potential to promote and drive the next revolutions in agricultural structures; however, there is a paucity of data on the effects that these NM-enhanced agricultural structures may have on human health and the environment. Concerns have been raised regarding the effects that may result from the interactions of these NMs with foods that have been packaged. Additional research is required to address these concerns about people’s health.
9. Conclusions
The disposal of agro-industrial and other solid wastes is another major concern in developing nations. It has been discovered that one method for effectively managing waste is to make use of agro-waste products as a source for the synthesis of nanostructured materials. It is imperative that the contemporary strategies for the synthesis of agro-waste-based, cost-effective, and environmentally friendly graphene oxide compounds receive adequate emphasis. Emerging techniques for the utilization of agro-waste and biopigments have the potential to play a significant role in the environmentally friendly synthesis of various NPs. In the applications of NMs for water treatment, bioimaging, and medication delivery, it is always essential to utilize clean materials rather than trash that could introduce hazardous chemicals. In other words, biomass wastes that may release toxins are not appropriate sources for NMs or substrates, particularly in nanotechnologies related to health and the environment. This article summarizes the various sources, characteristics, and nano-management of agro-waste. Additionally, it discusses the bio-synthesis, morphological characterization techniques, and applications of various agro-based NPs.
Author Contributions
Conceptualization, P.K.N. and B.S.I.; methodology, P.C.N., A.O. and S.D.; software, A.O. and M.S.; validation, P.K.N. and B.S.I.; formal analysis, P.C.N. and S.D.; investigation, P.C.N. and S.D.; resources, M.S., K.S., P.K.N. and B.S.I.; data curation, A.O., P.C.N. and S.D.; writing—original draft preparation, P.C.N., M.S. and S.D.; writing—review and editing, M.S., P.K.N., K.S. and B.S.I.; visualization, M.S., P.K.N., K.S. and B.S.I.; supervision, P.K.N. and B.S.I.; project administration, P.K.N.; funding acquisition, P.K.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are included in the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Weisz, U.; Pichler, P.-P.; Jaccard, I.S.; Haas, W.; Matej, S.; Bachner, F.; Nowak, P.; Weisz, H. Carbon emission trends and sustainability options in Austrian health care. Resour. Conserv. Recycl. 2020, 160, 104862. [Google Scholar] [CrossRef]
- Edodi, S. Managing the environment: Issues and priority actions for sustainable waste management in Uganda. Afr. Geogr. Rev. 2022, 1–15. [Google Scholar] [CrossRef]
- Dey, T.; Bhattacharjee, T.; Nag, P.; Ghati, A.; Kuila, A. Valorization of agro-waste into value added products for sustainable development. Bioresour. Technol. Rep. 2021, 16, 100834. [Google Scholar] [CrossRef]
- Yuan, N.; Zhang, X.; Zhao, A.; Tan, K.; Cui, Y. High-alumina fly ash as sustainable aluminum sources for the in situ preparation of Al-based eco-MOFs. Colloids Surf. A Physicochem. Eng. Asp. 2022, 640, 128421. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, A.K.; Bilal, M.; Prasad, S.; Rameshwari, K.T.; Chandra, R. Paper and pulp mill wastewater: Characterization, microbial-mediated degradation, and challenges. In Nanotechnology in Paper and Wood Engineering; Elsevier: Amsterdam, The Netherlands, 2022; pp. 371–387. [Google Scholar]
- Yuan, N.; Tan, K.; Zhang, X.; Zhao, A.; Guo, R. Synthesis and adsorption performance of ultra-low silica-to-alumina ratio and hierarchical porous ZSM-5 zeolites prepared from coal gasification fine slag. Chemosphere 2022, 303, 134839. [Google Scholar] [CrossRef]
- Mert, M. Fossil & renewable energy consumption, GHGs (greenhouse gases) and economic growth: Evidence from a panel of EU (European Union) countries. Energy 2014, 74, 439–446. [Google Scholar]
- Lu, H.; Yadav, V.; Bilal, M.; Iqbal, H.M. Bioprospecting microbial hosts to valorize lignocellulose biomass–Environmental perspectives and value-added bioproducts. Chemosphere 2022, 288, 132574. [Google Scholar] [CrossRef]
- Elemike, E.E.; Ekennia, A.C.; Onwudiwe, D.C.; Ezeani, R.O. Agro-waste materials: Sustainable substrates in nanotechnology. In Agri-Waste and Microbes for Production of Sustainable Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2022; pp. 187–214. [Google Scholar]
- Farooq, A.; Patoary, M.K.; Zhang, M.; Mussana, H.; Li, M.; Naeem, M.A.; Mushtaq, M.; Farooq, A.; Liu, L. Cellulose from sources to nanocellulose and an overview of synthesis and properties of nanocellulose/zinc oxide nanocomposite materials. Int. J. Biol. Macromol. 2020, 154, 1050–1073. [Google Scholar] [CrossRef]
- Venkateshaiah, A.; Padil, V.V.; Nagalakshmaiah, M.; Waclawek, S.; Černík, M.; Varma, R.S. Microscopic techniques for the analysis of micro and nanostructures of biopolymers and their derivatives. Polymers 2020, 12, 512. [Google Scholar] [CrossRef]
- Khan, S.A.; Khan, S.B.; Khan, L.U.; Farooq, A.; Akhtar, K.; Asiri, A.M. Fourier transform infrared spectroscopy: Fundamentals and application in functional groups and nanomaterials characterization. In Handbook of Materials Characterization; Springer: Berlin/Heidelberg, Germany, 2018; pp. 317–344. [Google Scholar]
- Guleria, G.; Thakur, S.; Shandilya, M.; Sharma, S.; Thakur, S.; Kalia, S. Nanotechnology for sustainable agro-food systems: The need and role of nanoparticles in protecting plants and improving crop productivity. Plant Physiol. Biochem. 2022, 194, 533–549. [Google Scholar] [CrossRef]
- Yasmine, R.; Ahmad, J.; Qamar, S.; Qureshi, M.I. Engineered nanomaterials for sustainable agricultural production, soil improvement, and stress management: An overview. Eng. Nanomater. Sustain. Agric. Prod. Soil Improv. Stress Manag. 2023, 1–23. [Google Scholar] [CrossRef]
- Afolalu, S.A.; Ikumapayi, O.M.; Oloyede, O.R.; Ogedengbe, T.S.; Ogundipe, A.T. Advances in Nanotechnology and Nanoparticles in the 21st Century–An Overview. In Proceedings of the 3rd African International Conference on Industrial Engineering and Operations Management, Nsukka, Nigeria, 5–7 April 2022. [Google Scholar]
- Yadav, M.; Dwibedi, V.; Sharma, S.; George, N. Biogenic silica nanoparticles from agro-waste: Properties, mechanism of extraction and applications in environmental sustainability. J. Environ. Chem. Eng. 2022, 10, 108550. [Google Scholar] [CrossRef]
- Choudhary, R.; Kaur, P.; Adholeya, A. Synthesis of Agro-waste-mediated Silica Nanoparticles: An Approach Towards Sustainable Agriculture. In Nanotechnology in Agriculture and Environmental Science; CRC Press: Boca Raton, FL, USA, 2022; pp. 278–291. [Google Scholar]
- Sharma, D.; Kanchi, S.; Bisetty, K. Biogenic synthesis of nanoparticles: A review. Arab. J. Chem. 2019, 12, 3576–3600. [Google Scholar] [CrossRef]
- Eivazzadeh-Keihan, R.; Noruzi, E.B.; Chidar, E.; Jafari, M.; Davoodi, F.; Kashtiaray, A.; Gorab, M.G.; Hashemi, S.M.; Javanshir, S.; Cohan, R.A. Applications of carbon-based conductive nanomaterials in biosensors. Chem. Eng. J. 2022, 442, 136183. [Google Scholar] [CrossRef]
- Rahman, A.; Chowdhury, M.A.; Hossain, N. Green synthesis of hybrid nanoparticles for biomedical applications: A review. Appl. Surf. Sci. Adv. 2022, 11, 100296. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Bahmid, N.A.; Taha, A.; Abdel-Moneim, A.-M.E.; Shehata, A.M.; Tan, C.; Kharazmi, M.S.; Li, Y.; Assadpour, E.; Castro-Muñoz, R. Bioactive-loaded nanodelivery systems for the feed and drugs of livestock; purposes, techniques and applications. Adv. Colloid Interface Sci. 2022, 308, 102772. [Google Scholar] [CrossRef] [PubMed]
- Birniwa, A.H.; Abubakar, A.S.; Mahmud, H.N.M.E.; Kutty, S.R.M.; Jagaba, A.H.; Abdullahi, S.S.A.; Zango, Z.U. Application of agricultural wastes for cationic dyes removal from wastewater. In Textile Wastewater Treatment; Springer: Berlin/Heidelberg, Germany, 2022; pp. 239–274. [Google Scholar]
- Ahmed ES, J.; Ganesh, G.M. A Comprehensive Overview on Corrosion in RCC and Its Prevention Using Various Green Corrosion Inhibitors. Buildings 2022, 12, 1682. [Google Scholar] [CrossRef]
- Esquivel-Hernández, D.A.; García-Pérez, J.S.; López-Pacheco, I.Y.; Iqbal, H.M.; Parra-Saldívar, R. Resource recovery of lignocellulosic biomass waste into lactic acid-Trends to sustain cleaner production. J. Environ. Manag. 2022, 301, 113925. [Google Scholar] [CrossRef]
- Singh, G.; Ramadass, K.; Sooriyakumar, P.; Hettithanthri, O.; Vithange, M.; Bolan, N.; Tavakkoli, E.; Van Zwieten, L.; Vinu, A. Nanoporous materials for pesticide formulation and delivery in the agricultural sector. J. Control. Release 2022, 343, 187–206. [Google Scholar] [CrossRef]
- Song, C.; Chen, K.; Chen, M.; Jin, X.; Liu, G.; Du, X.; Chen, D.; Huang, Q. Sequential combined adsorption and solid-phase photocatalysis to remove aqueous organic pollutants by H3PO4-modified TiO2 nanoparticles anchored on biochar. J. Water Process Eng. 2022, 45, 102467. [Google Scholar] [CrossRef]
- Elbasiouny, H.; Elbanna, B.A.; Al-Najoli, E.; Alsherief, A.; Negm, S.; El-Nour, A.; Nofal, A.; Sharabash, S. Agricultural waste management for climate change mitigation: Some implications to Egypt. In Waste Management in MENA Regions; Springer: Berlin/Heidelberg, Germany, 2020; pp. 149–169. [Google Scholar]
- Shaaban, S.; Nasr, M. Toward three R’s agricultural waste in MENA: Reduce, reuse, and recycle. In Waste Management in MENA Regions; Springer: Berlin/Heidelberg, Germany, 2020; pp. 337–353. [Google Scholar]
- Adebisi, J.; Agunsoye, J.; Ahmed, I.; Bello, S.; Haris, M.; Ramakokovhu, M.; Hassan, S. Production of silicon nanoparticles from selected agricultural wastes. Mater. Today Proc. 2021, 38, 669–674. [Google Scholar] [CrossRef]
- Singh, S.P.; Endley, N. Fabrication of nano-silica from agricultural residue and their application. In Nanomaterials for Agriculture and Forestry Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 107–134. [Google Scholar]
- Peerzada, J.G.; Chidambaram, R. A statistical approach for biogenic synthesis of nano-silica from different agro-wastes. Silicon 2021, 13, 2089–2101. [Google Scholar] [CrossRef]
- Athinarayanan, J.; Jaafari, S.A.A.H.; Periasamy, V.S.; Almanaa, T.N.A.; Alshatwi, A.A. Fabrication of biogenic silica nanostructures from Sorghum bicolor leaves for food industry applications. Silicon 2020, 12, 2829–2836. [Google Scholar] [CrossRef]
- Kamel, R.; El-Wakil, N.A.; Dufresne, A.; Elkasabgy, N.A. Nanocellulose: From an agricultural waste to a valuable pharmaceutical ingredient. Int. J. Biol. Macromol. 2020, 163, 1579–1590. [Google Scholar] [CrossRef] [PubMed]
- Gabhane, J.W.; Bhange, V.P.; Patil, P.D.; Bankar, S.T.; Kumar, S. Recent trends in biochar production methods and its application as a soil health conditioner: A review. SN Appl. Sci. 2020, 2, 1307. [Google Scholar] [CrossRef]
- Sakhiya, A.K.; Anand, A.; Kaushal, P. Production, activation, and applications of biochar in recent times. Biochar 2020, 2, 253–285. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Filho, J.F.L.; Melo, L.C.A.; de Assis, I.R.; de Oliveira, T.S. Influence of pyrolysis temperature and feedstock on the properties of biochars produced from agricultural and industrial wastes. J. Anal. Appl. Pyrolysis 2020, 149, 104839. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, D.; Xu, H.; Lu, W.; Ren, X.; Cai, H.; Lei, H.; Huo, E.; Zhao, Y.; Qian, M. Biochar filled high-density polyethylene composites with excellent properties: Towards maximizing the utilization of agricultural wastes. Ind. Crops Prod. 2020, 146, 112185. [Google Scholar] [CrossRef]
- El-Bassi, L.; Azzaz, A.A.; Jellali, S.; Akrout, H.; Marks, E.A.; Ghimbeu, C.M.; Jeguirim, M. Application of olive mill waste-based biochars in agriculture: Impact on soil properties, enzymatic activities and tomato growth. Sci. Total Environ. 2021, 755, 142531. [Google Scholar] [CrossRef]
- Kwoczynski, Z.; Čmelík, J. Characterization of biomass wastes and its possibility of agriculture utilization due to biochar production by torrefaction process. J. Clean. Prod. 2021, 280, 124302. [Google Scholar] [CrossRef]
- Bhatt, K.; Lal, S.; Srinivasan, R.; Joshi, B. Bioconversion of agriculture wastes to produce α-amylase from Bacillus velezensis KB 2216: Purification and characterization. Biocatal. Agric. Biotechnol. 2020, 28, 101703. [Google Scholar] [CrossRef]
- Tamilselvi, R.; Ramesh, M.; Lekshmi, G.; Bazaka, O.; Levchenko, I.; Bazaka, K.; Mandhakini, M. Graphene oxide–Based supercapacitors from agricultural wastes: A step to mass production of highly efficient electrodes for electrical transportation systems. Renew. Energy 2020, 151, 731–739. [Google Scholar] [CrossRef]
- Pandey, S.; Dwivedi, N. Utilisation and management of agriculture and food processing waste. In Innovations in Food Technology; Springer: Berlin/Heidelberg, Germany, 2020; pp. 269–288. [Google Scholar]
- Maji, S.; Dwivedi, D.H.; Singh, N.; Kishor, S.; Gond, M. Agricultural waste: Its impact on environment and management approaches. In Emerging Eco-Friendly Green Technologies for Wastewater Treatment; Springer: Berlin/Heidelberg, Germany, 2020; pp. 329–351. [Google Scholar]
- Zalewska, M.; Popowska, M. Antimicrobial/antibiotic resistance genes due to manure and agricultural waste applications. In Antibiotics and Antimicrobial Resistance Genes; Springer: Berlin/Heidelberg, Germany, 2020; pp. 139–161. [Google Scholar]
- Suffo, M.; De La Mata, M.; Molina, S. A sugar-beet waste based thermoplastic agro-composite as substitute for raw materials. J. Clean. Prod. 2020, 257, 120382. [Google Scholar] [CrossRef]
- Fareed, A.; Zaidi, S.B.A.; Ahmad, N.; Hafeez, I.; Ali, A.; Ahmad, M.F. Use of agricultural waste ashes in asphalt binder and mixture: A sustainable solution to waste management. Constr. Build. Mater. 2020, 259, 120575. [Google Scholar] [CrossRef]
- Lateef, A.; Nazir, R.; Jamil, N.; Alam, S.; Shah, R.; Khan, M.N.; Saleem, M. Synthesis and characterization of environmental friendly corncob biochar based nano-composite—A potential slow release nano-fertilizer for sustainable agriculture. Environ. Nanotechnol. Monit. Manag. 2019, 11, 100212. [Google Scholar] [CrossRef]
- Naidu, Y.; Siddiqui, Y.; Idris, A.S. Comprehensive studies on optimization of ligno-hemicellulolytic enzymes by indigenous white rot hymenomycetes under solid-state cultivation using agro-industrial wastes. J. Environ. Manag. 2020, 259, 110056. [Google Scholar] [CrossRef] [PubMed]
- Donner, M.; Gohier, R.; de Vries, H. A new circular business model typology for creating value from agro-waste. Sci. Total Environ. 2020, 716, 137065. [Google Scholar] [CrossRef]
- Abdelhady, H.H.; Elazab, H.A.; Ewais, E.M.; Saber, M.; El-Deab, M.S. Efficient catalytic production of biodiesel using nano-sized sugar beet agro-industrial waste. Fuel 2020, 261, 116481. [Google Scholar] [CrossRef]
- Bolzonella, D.; Battista, F.; Mattioli, A.; Nicolato, C.; Frison, N.; Lampis, S. Biological thermophilic post hydrolysis of digestate enhances the biogas production in the anaerobic digestion of agro-waste. Renew. Sustain. Energy Rev. 2020, 134, 110174. [Google Scholar] [CrossRef]
- Dalpaz, R.; Konrad, O.; da Silva Cyrne, C.C.; Barzotto, H.P.; Hasan, C.; Guerini Filho, M. Using biogas for energy cogeneration: An analysis of electric and thermal energy generation from agro-industrial waste. Sustain. Energy Technol. Assess. 2020, 40, 100774. [Google Scholar] [CrossRef]
- Kapoor, R.; Ghosh, P.; Kumar, M.; Sengupta, S.; Gupta, A.; Kumar, S.S.; Vijay, V.; Kumar, V.; Vijay, V.K.; Pant, D. Valorization of agricultural waste for biogas based circular economy in India: A research outlook. Bioresour. Technol. 2020, 304, 123036. [Google Scholar] [CrossRef]
- Thapa, S.; Engelken, R. Optimization of pelleting parameters for producing composite pellets using agricultural and agro-processing wastes by Taguchi-Grey relational analysis. Carbon Resour. Convers. 2020, 3, 104–111. [Google Scholar] [CrossRef]
- Ardebili, S.M.S.; Khademalrasoul, A. An assessment of feasibility and potential of gaseous biofuel production from agricultural/animal wastes: A case study. Biomass Convers. Biorefinery 2020, 12, 5105–5114. [Google Scholar] [CrossRef]
- Ishak, N.; Kamarudin, S.; Timmiati, S.; Karim, N.; Basri, S. Biogenic platinum from agricultural wastes extract for improved methanol oxidation reaction in direct methanol fuel cell. J. Adv. Res. 2021, 28, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Mo, K.H.; Thomas, B.S.; Yap, S.P.; Abutaha, F.; Tan, C.G. Viability of agricultural wastes as substitute of natural aggregate in concrete: A review on the durability-related properties. J. Clean. Prod. 2020, 275, 123062. [Google Scholar] [CrossRef]
- Landin-Sandoval, V.; Mendoza-Castillo, D.; Bonilla-Petriciolet, A.; Aguayo-Villarreal, I.; Reynel-Avila, H.; Gonzalez-Ponce, H. Valorization of agri-food industry wastes to prepare adsorbents for heavy metal removal from water. J. Environ. Chem. Eng. 2020, 8, 104067. [Google Scholar] [CrossRef]
- Gebremikael, M.T.; Ranasinghe, A.; Hosseini, P.; Laboan, B.; Sonneveld, E.; Pipan, M.; Oni, F.E.; Montemurro, F.; Höfte, M.; Sleutel, S. How do novel and conventional agri-food wastes, co-products and by-products improve soil functions and soil quality? Waste Manag. 2020, 113, 132–144. [Google Scholar] [CrossRef]
- Khan, H.N.; Faisal, M. Planning and engineering strategies of agricultural wastes and their remediation strategies. In Contaminants in Agriculture; Springer: Berlin/Heidelberg, Germany, 2020; pp. 219–232. [Google Scholar]
- Siles-Castellano, A.B.; López, M.J.; Jurado, M.M.; Suárez-Estrella, F.; López-González, J.A.; Estrella-González, M.J.; Moreno, J. Industrial composting of low carbon/nitrogen ratio mixtures of agri-food waste and impact on compost quality. Bioresour. Technol. 2020, 316, 123946. [Google Scholar] [CrossRef]
- Kammoun, M.; Ayeb, H.; Bettaieb, T.; Richel, A. Chemical characterisation and technical assessment of agri-food residues, marine matrices, and wild grasses in the South Mediterranean area: A considerable inflow for biorefineries. Waste Manag. 2020, 118, 247–257. [Google Scholar] [CrossRef]
- Koutra, E.; Mastropetros, S.G.; Ali, S.S.; Tsigkou, K.; Kornaros, M. Assessing the potential of Chlorella vulgaris for valorization of liquid digestates from agro-industrial and municipal organic wastes in a biorefinery approach. J. Clean. Prod. 2021, 280, 124352. [Google Scholar] [CrossRef]
- Arun, K.; Madhavan, A.; Sindhu, R.; Binod, P.; Pandey, A.; Reshmy, R.; Sirohi, R. Remodeling agro-industrial and food wastes into value-added bioactives and biopolymers. Ind. Crops Prod. 2020, 154, 112621. [Google Scholar] [CrossRef]
- Ben-Othman, S.; Jõudu, I.; Bhat, R. Bioactives from agri-food wastes: Present insights and future challenges. Molecules 2020, 25, 510. [Google Scholar] [CrossRef] [PubMed]
- Gullón, P.; Gullón, B.; Romaní, A.; Rocchetti, G.; Lorenzo, J.M. Smart advanced solvents for bioactive compounds recovery from agri-food by-products: A review. Trends Food Sci. Technol. 2020, 101, 182–197. [Google Scholar] [CrossRef]
- Banu, S.S.; Karthikeyan, J.; Jayabalan, P. Effect of agro-waste on strength and durability properties of concrete. Constr. Build. Mater. 2020, 258, 120322. [Google Scholar] [CrossRef]
- Jannat, N.; Hussien, A.; Abdullah, B.; Cotgrave, A. Application of agro and non-agro waste materials for unfired earth blocks construction: A review. Constr. Build. Mater. 2020, 254, 119346. [Google Scholar] [CrossRef]
- Bongao, H.C.; Gabatino, R.R.A.; Arias, C.F.H.; Magdaluyo, E.R., Jr. Micro/nanocellulose from waste Pili (Canarium ovatum) pulp as a potential anti-ageing ingredient for cosmetic formulations. Mater. Today Proc. 2020, 22, 275–280. [Google Scholar] [CrossRef]
- Pattanaik, L.; Pattnaik, F.; Saxena, D.K.; Naik, S.N. Biofuels from agricultural wastes. In Second and Third Generation of Feedstocks; Elsevier: Amsterdam, The Netherlands, 2019; pp. 103–142. [Google Scholar]
- Saini, J.K.; Saini, R.; Tewari, L. Lignocellulosic agriculture wastes as biomass feedstocks for second-generation bioethanol production: Concepts and recent developments. 3 Biotech 2015, 5, 337–353. [Google Scholar] [CrossRef]
- Beuchat, L. Oncom (fermented peanut press cake). In Legume-Based Fermented Foods; CRC Press: Boca Raton, Florida, 1986; pp. 135–144. [Google Scholar]
- Chang, C.C.; Li, R. Agricultural waste. Water Environ. Res. 2019, 91, 1150–1167. [Google Scholar] [CrossRef]
- Afolalu, S.A.; Oladipupo, S.; Bose, M.E.; Abioye, A.A.; Adejuyigbe, S.B.; Ajayi, O.O.; Ongbali, S.O. Agro waste A sustainable source for steel reinforcement-review. J. Phys. Conf. Ser. 2019, 1378, 032032. [Google Scholar] [CrossRef]
- Sarkar, N.; Ghosh, S.K.; Bannerjee, S.; Aikat, K. Bioethanol production from agricultural wastes: An overview. Renew. Energy 2012, 37, 19–27. [Google Scholar] [CrossRef]
- Jahirul, M.I.; Rasul, M.G.; Chowdhury, A.A.; Ashwath, N. Biofuels production through biomass pyrolysis—A technological review. Energies 2012, 5, 4952–5001. [Google Scholar] [CrossRef]
- Jogi, K.; Bhat, R. Valorization of food processing wastes and by-products for bioplastic production. Sustain. Chem. Pharm. 2020, 18, 100326. [Google Scholar] [CrossRef]
- Putri, D.N.; Sahlan, M.; Montastruc, L.; Meyer, M.; Negny, S.; Hermansyah, H. Progress of fermentation methods for bio-succinic acid production using agro-industrial waste by Actinobacillus succinogenes. Energy Rep. 2020, 6, 234–239. [Google Scholar] [CrossRef]
- Duran, M.; Aday, M.S.; Zorba, N.N.D.; Temizkan, R.; Büyükcan, M.B.; Caner, C. Potential of antimicrobial active packaging ‘containing natamycin, nisin, pomegranate and grape seed extract in chitosan coating’ to extend shelf life of fresh strawberry. Food Bioprod. Process. 2016, 98, 354–363. [Google Scholar] [CrossRef]
- Canete-Rodriguez, A.M.; Santos-Duenas, I.M.; Jimenez-Hornero, J.E.; Ehrenreich, A.; Liebl, W.; Garcia-Garcia, I. Gluconic acid: Properties, production methods and applications—An excellent opportunity for agro-industrial by-products and waste bio-valorization. Process Biochem. 2016, 51, 1891–1903. [Google Scholar] [CrossRef]
- Chel, A.; Kaushik, G. Renewable energy for sustainable agriculture. Agron. Sustain. Dev. 2011, 31, 91–118. [Google Scholar] [CrossRef]
- Kosseva, M. Processing of food wastes; Chapter 3. Adv. Food Nutr. Res. 2009, 58, 57–136. [Google Scholar]
- Sun, Y.; Cheng, J. Hydrolysis of lignocellulosic materials for ethanol production: A review. Bioresour. Technol. 2002, 83, 1–11. [Google Scholar] [CrossRef]
- Møller, H.; Sommer, S.; Ahring, B.K. Methane productivity of manure, straw and solid fractions of manure. Biomass Bioenergy 2004, 26, 485–495. [Google Scholar] [CrossRef]
- Garcia, N.H.; Mattioli, A.; Gil, A.; Frison, N.; Battista, F.; Bolzonella, D. Evaluation of the methane potential of different agricultural and food processing substrates for improved biogas production in rural areas. Renew. Sustain. Energy Rev. 2019, 112, 1–10. [Google Scholar] [CrossRef]
- Thangadurai, D.; Naik, J.; Sangeetha, J.; Al-Tawaha, A.R.M.S.; Adetunji, C.O.; Islam, S.; David, M.; Shettar, A.K.; Adetunji, J.B. Nanomaterials from agrowastes: Past, present, and the future. In Handbook of Nanomaterials and Nanocomposites for Energy and Environmental Applications; Springer: Berlin/Heidelberg, Germany, 2021; pp. 471–487. [Google Scholar]
- Wasilewska, A.; Klekotka, U.; Zambrzycka, M.; Zambrowski, G.; Święcicka, I.; Kalska-Szostko, B. Physico-chemical properties and antimicrobial activity of silver nanoparticles fabricated by green synthesis. Food Chem. 2023, 400, 133960. [Google Scholar] [CrossRef] [PubMed]
- Al-Ogaidi, I.A.Z. Camellia sinensis (green tea) mediated synthesis of zinc oxide nanoparticles and detect its antibacterial activity against Escherichia coli, Staphylococcus aureus and Acinetobacter baumannii. J. Biotechnol. Res. Cent. 2017, 11, 34–40. [Google Scholar] [CrossRef]
- Azari, B.; Pourahmad, A.; Sadeghi, B.; Mokhtary, M. Incorporation of Zinc Oxide Nanoparticles in RHA-MTW Zeolite and its Application for Degradation of Dye. J. Nanoanalysis 2020, 7, 179–189. [Google Scholar]
- Kotp, A.A.; Farghali, A.A.; Amin, R.M.; bdel Moaty, S.A.; El-Deen, A.G.; Gadelhak, Y.M.; Younes, H.A.; Syame, S.M.; Mahmoud, R.K. Green-synthesis of Ag nanoparticles and its composite with PVA nanofiber as a promising Cd2+ adsorbent and antimicrobial agent. J. Environ. Chem. Eng. 2019, 7, 102977. [Google Scholar] [CrossRef]
- Ahmadieh, R.; Mohseni, S. Evaluation of Antibacterial Properties of Magnetic Iron Oxide Nanoparticles Synthesized using EchinopsPersicus Extract Coated with Chloramphenicol. J. Ilam Univ. Med. Sci. 2021, 29, 59–71. [Google Scholar]
- Devadiga, A.; Shetty, K.V.; Saidutta, M. Timber industry waste-teak (Tectona grandis Linn.) leaf extract mediated synthesis of antibacterial silver nanoparticles. Int. Nano Lett. 2015, 5, 205–214. [Google Scholar] [CrossRef]
- Meral, R.; Kose, Y.E.; Ceylan, Z.; Cavidoglu, İ. The potential use of agro-industrial by-products as sources of bioactive compounds: A nanotechnological approach. Stud. Nat. Prod. Chem. 2022, 73, 435–466. [Google Scholar]
- Mohamed, H.; Shah, A.M.; Song, Y. Conversion of Agro-industrial Wastes into Value-Added Products. In Conversion of Renewable Biomass into Bioproducts; ACS Publications: Washington, DC, USA, 2021; pp. 197–217. [Google Scholar]
- Zamare, D.; Vutukuru, S.; Babu, R. Biosynthesis of nanoparticles from agro-waste: A sustainable approach. Int. J. Eng. Appl. Sci. Technol. 2016, 1, 85–92. [Google Scholar]
- Lateef, A.; Oloke, J.; Gueguim-Kana, E.; Raimi, O. Production of fructosyltransferase by a local isolate of Aspergillus niger in both submerged and solid substrate media. Acta Aliment. 2012, 41, 100–117. [Google Scholar] [CrossRef]
- Adeoye, A.; Lateef, A.; Gueguim-Kana, E. Optimization of citric acid production using a mutant strain of Aspergillus niger on cassava peel substrate. Biocatal. Agric. Biotechnol. 2015, 4, 568–574. [Google Scholar] [CrossRef]
- Kana, E.G.; Oloke, J.; Lateef, A.; Adesiyan, M. Modeling and optimization of biogas production on saw dust and other co-substrates using artificial neural network and genetic algorithm. Renew. Energy 2012, 46, 276–281. [Google Scholar] [CrossRef]
- Elegbede, J.A.; Lateef, A. Green synthesis of silver (Ag), gold (Au), and silver–gold (Ag–Au) alloy nanoparticles: A review on recent advances, trends, and biomedical applications. In Nanotechnology and Nanomaterial Applications in Food, Health, and Biomedical Sciences; Apple Academic Press: Palm Bay, FL, USA, 2019; pp. 3–89. [Google Scholar]
- Ortega, F.; Versino, F.; López, O.V.; García, M.A. Biobased composites from agro-industrial wastes and by-products. Emergent Mater. 2022, 5, 873–921. [Google Scholar] [CrossRef] [PubMed]
- Jampílek, J.; Kráľová, K. Preparation of nanocomposites from agricultural waste and their versatile applications. In Multifunctional Hybrid Nanomaterials for Sustainable Agri-Food and Ecosystems; Elsevier: Amsterdam, The Netherlands, 2020; pp. 51–98. [Google Scholar]
- Tassoni, A.; Tedeschi, T.; Zurlini, C.; Cigognini, I.M.; Petrusan, J.-I.; Rodríguez, Ó.; Neri, S.; Celli, A.; Sisti, L.; Cinelli, P. State-of-the-art production chains for peas, beans and chickpeas—Valorization of agro-industrial residues and applications of derived extracts. Molecules 2020, 25, 1383. [Google Scholar] [PubMed]
- Muniyappan, N.; Pandeeswaran, M.; Amalraj, A. Green synthesis of gold nanoparticles using Curcuma pseudomontana isolated curcumin: Its characterization, antimicrobial, antioxidant and anti-inflammatory activities. Environ. Chem. Ecotoxicol. 2021, 3, 117–124. [Google Scholar] [CrossRef]
- Lakshmipathy, R.; Reddy, B.P.; Sarada, N.; Chidambaram, K.; Pasha, S.K. Watermelon rind-mediated green synthesis of noble palladium nanoparticles: Catalytic application. Appl. Nanosci. 2015, 5, 223–228. [Google Scholar] [CrossRef]
- Roopan, S.M.; Bharathi, A.; Kumar, R.; Khanna, V.G.; Prabhakarn, A. Acaricidal, insecticidal, and larvicidal efficacy of aqueous extract of Annona squamosa L peel as biomaterial for the reduction of palladium salts into nanoparticles. Colloids Surf. B Biointerfaces 2012, 92, 209–212. [Google Scholar] [CrossRef]
- Dauthal, P.; Mukhopadhyay, M. Biofabrication, characterization, and possible bio-reduction mechanism of platinum nanoparticles mediated by agro-industrial waste and their catalytic activity. J. Ind. Eng. Chem. 2015, 22, 185–191. [Google Scholar] [CrossRef]
- Roopan, S.M.; Bharathi, A.; Prabhakarn, A.; Rahuman, A.A.; Velayutham, K.; Rajakumar, G.; Padmaja, R.; Lekshmi, M.; Madhumitha, G. Efficient phyto-synthesis and structural characterization of rutile TiO2 nanoparticles using Annona squamosa peel extract. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 98, 86–90. [Google Scholar] [CrossRef]
- Attia, E.A.; Elhawat, N. Combined foliar and soil application of silica nanoparticles enhances the growth, flowering period and flower characteristics of marigold (Tagetes erecta L.). Sci. Hortic. 2021, 282, 110015. [Google Scholar] [CrossRef]
- Maret, W. The quintessence of metallomics: A harbinger of a different life science based on the periodic table of the bioelements. Metallomics 2022, 14, mfac051. [Google Scholar] [CrossRef]
- Wahab, A.; Abdi, G.; Saleem, M.H.; Ali, B.; Ullah, S.; Shah, W.; Mumtaz, S.; Yasin, G.; Muresan, C.C.; Marc, R.A. Plants’ physio-biochemical and phyto-hormonal responses to alleviate the adverse effects of drought stress: A comprehensive review. Plants 2022, 11, 1620. [Google Scholar] [CrossRef]
- Qayyum, A.; Nadeem, F.; Bibi, Y.; Ullah, R.; Raza, H.; Bajwa, H.J.; Sher, A. 24 Role of Nanoparticles in Improving Stress Tolerance in Crop Plants. In Biostimulants for Crop Production and Sustainable Agriculture; CABI: Oxfordshire, UK, 2022; p. 386. [Google Scholar]
- Wang, Q. Chronic Impact of Cerium Oxide Nanoparticles on Solanum lycopersicum L. and Brassica rapa L.; Southern Illinois University at Carbondale: Carbondale, IL, USA, 2014. [Google Scholar]
- Avestan, S.; Ghasemnezhad, M.; Esfahani, M.; Byrt, C.S. Application of nano-silicon dioxide improves salt stress tolerance in strawberry plants. Agronomy 2019, 9, 246. [Google Scholar] [CrossRef]
- Guru, A.; Sahoo, S.K.; Saha, P.; Dwivedi, P. Sustainable Agriculture Reviews 53: Nanoparticles: A New Tool to Enhance Stress Tolerance; Springer Nature: London, UK, 2022; Volume 53, p. 185. [Google Scholar]
- Siddiqui, H.; Ahmed, K.B.M.; Sami, F.; Hayat, S. Silicon nanoparticles and plants: Current knowledge and future perspectives. In Sustainable Agriculture Reviews 41; Springer: Cham, Switzerland, 2020; pp. 129–142. [Google Scholar]
- El-Saadony, M.T.; Saad, A.M.; Soliman, S.M.; Salem, H.M.; Desoky, E.-S.M.; Babalghith, A.O.; El-Tahan, A.M.; Ibrahim, O.M.; Ebrahim, A.A.; Abd El-Mageed, T.A. Role of Nanoparticles in Enhancing Crop Tolerance to Abiotic Stress: A Comprehensive Review. Front. Plant Sci. 2022, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Campagnolo, L.; Chavatte-Palmer, P.; Tarrade, A.; Rousseau-Ralliard, D.; Valentino, S.; Park, M.V.; de Jong, W.H.; Wolterink, G.; Piersma, A.H.; Ross, B.L. A perspective on the developmental toxicity of inhaled nanoparticles. Reprod. Toxicol. 2015, 56, 118–140. [Google Scholar]
- Zhu, J.; Li, J.; Shen, Y.; Liu, S.; Zeng, N.; Zhan, X.; White, J.C.; Gardea-Torresdey, J.; Xing, B. Mechanism of zinc oxide nanoparticle entry into wheat seedling leaves. Environ. Sci. Nano 2020, 7, 3901–3913. [Google Scholar] [CrossRef]
- Güzel, R.; Erdal, G. Synthesis of Silver Nanoparticles; IntechOpen: London, UK, 2018. [Google Scholar]
- Salem, S.S.; Fouda, A. Green synthesis of metallic nanoparticles and their prospective biotechnological applications: An overview. Biol. Trace Elem. Res. 2021, 199, 344–370. [Google Scholar] [CrossRef]
- Mughal, S.S.; Hassan, S.M. Comparative study of AgO nanoparticles synthesize via biological, chemical and physical methods: A review. Am. J. Mater. Synth. Process. 2022, 7, 15–28. [Google Scholar]
- Khan, S.; Naushad, M.; Al-Gheethi, A.; Iqbal, J. Engineered nanoparticles for removal of pollutants from wastewater: Current status and future prospects of nanotechnology for remediation strategies. J. Environ. Chem. Eng. 2021, 9, 106160. [Google Scholar] [CrossRef]
- El Shafey, A.M. Green synthesis of metal and metal oxide nanoparticles from plant leaf extracts and their applications: A review. Green Process. Synth. 2020, 9, 304–339. [Google Scholar]
- Tessonnier, J.-P.; Rosenthal, D.; Hansen, T.W.; Hess, C.; Schuster, M.E.; Blume, R.; Girgsdies, F.; Pfänder, N.; Timpe, O.; Su, D.S. Analysis of the structure and chemical properties of some commercial carbon nanostructures. Carbon 2009, 47, 1779–1798. [Google Scholar]
- Li, H.; He, X.; Liu, Y.; Huang, H.; Lian, S.; Lee, S.-T.; Kang, Z. One-step ultrasonic synthesis of water-soluble carbon nanoparticles with excellent photoluminescent properties. Carbon 2011, 49, 605–609. [Google Scholar] [CrossRef]
- Wen, X.; Chen, X.; Tian, N.; Gong, J.; Liu, J.; Rümmeli, M.H.; Chu, P.K.; Mijiwska, E.; Tang, T. Nanosized carbon black combined with Ni2O3 as “universal” catalysts for synergistically catalyzing carbonization of polyolefin wastes to synthesize carbon nanotubes and application for supercapacitors. Environ. Sci. Technol. 2014, 48, 4048–4055. [Google Scholar] [CrossRef]
- Marschilok, A.; Lee, C.-Y.; Subramanian, A.; Takeuchi, K.J.; Takeuchi, E.S. Carbon nanotube substrate electrodes for lightweight, long-life rechargeable batteries. Energy Environ. Sci. 2011, 4, 2943–2951. [Google Scholar] [CrossRef]
- Huang, M.-R.; Li, S.; Li, X.-G. Longan shell as novel biomacromolecular sorbent for highly selective removal of lead and mercury ions. J. Phys. Chem. B 2010, 114, 3534–3542. [Google Scholar] [CrossRef]
- Batalov, A.; Jacques, V.; Kaiser, F.; Siyushev, P.; Neumann, P.; Rogers, L.; McMurtrie, R.; Manson, N.; Jelezko, F.; Wrachtrup, J. Low temperature studies of the excited-state structure of negatively charged nitrogen-vacancy color centers in diamond. Phys. Rev. Lett. 2009, 102, 195506. [Google Scholar] [CrossRef]
- Li, R.; Fei, J.; Cai, Y.; Li, Y.; Feng, J.; Yao, J. Cellulose whiskers extracted from mulberry: A novel biomass production. Carbohydr. Polym. 2009, 76, 94–99. [Google Scholar] [CrossRef]
- Sangeetha, J.; Thangadurai, D.; Hospet, R.; Purushotham, P.; Manowade, K.R.; Mujeeb, M.A.; Mundaragi, A.C.; Jogaiah, S.; David, M.; Thimmappa, S.C. Production of bionanomaterials from agricultural wastes. In Nanotechnology; Springer: Berlin/Heidelberg, Germany, 2017; pp. 33–58. [Google Scholar]
- Yuvakkumar, R.; Suresh, J.; Nathanael, A.J.; Sundrarajan, M.; Hong, S. Rambutan (Nephelium lappaceum L.) peel extract assisted biomimetic synthesis of nickel oxide nanocrystals. Mater. Lett. 2014, 128, 170–174. [Google Scholar] [CrossRef]
- Yuvakkumar, R.; Suresh, J.; Nathanael, A.J.; Sundrarajan, M.; Hong, S. Novel green synthetic strategy to prepare ZnO nanocrystals using rambutan (Nephelium lappaceum L.) peel extract and its antibacterial applications. Mater. Sci. Eng. C 2014, 41, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Prasad, C.; Gangadhara, S.; Venkateswarlu, P. Bio-inspired green synthesis of Fe3O4 magnetic nanoparticles using watermelon rinds and their catalytic activity. Appl. Nanosci. 2016, 6, 797–802. [Google Scholar] [CrossRef]
- Venkateswarlu, S.; Rao, Y.S.; Balaji, T.; Prathima, B.; Jyothi, N. Biogenic synthesis of Fe3O4 magnetic nanoparticles using plantain peel extract. Mater. Lett. 2013, 100, 241–244. [Google Scholar] [CrossRef]
- Adelere, I.A.; Lateef, A. A novel approach to the green synthesis of metallic nanoparticles: The use of agro-wastes, enzymes, and pigments. Nanotechnol. Rev. 2016, 5, 567–587. [Google Scholar] [CrossRef]
- Kumar, R.; Roopan, S.M.; Prabhakarn, A.; Khanna, V.G.; Chakroborty, S. Agricultural waste Annona squamosa peel extract: Biosynthesis of silver nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 90, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.; Iqbal, M.A.; Malik, M.; Raza, M.A.; Shahid, W.; Choi, J.R.; Pham, P.V. Biosynthesis and Characterizations of Silver Nanoparticles from Annona squamosa Leaf and Fruit Extracts for Size-Dependent Biomedical Applications. Nanomaterials 2022, 12, 616. [Google Scholar] [CrossRef] [PubMed]
- Phan, K.S.; Nguyen, T.M.; To, X.T.; Le, T.T.H.; Nguyen, T.T.; Pham, K.D.; Hoang, P.H.; Dong, T.N.; Dang, D.K.; Phan, T.H.T. Allium sativum@ AgNPs and Phyllanthus urinaria@ AgNPs: A comparative analysis for antibacterial application. RSC Adv. 2022, 12, 35730–35743. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Baek, K.-H. Phyto-mediated biosynthesis of silver nanoparticles using the rind extract of watermelon (Citrullus lanatus) under photo-catalyzed condition and investigation of its antibacterial, anticandidal and antioxidant efficacy. J. Photochem. Photobiol. B Biol. 2016, 161, 200–210. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Vo, T.N.N.; Nguyen, N.T.; Ching, Y.C.; Thi, T.T.H. Comparison of biogenic silver nanoparticles formed by Momordica charantia and Psidium guajava leaf extract and antifungal evaluation. PLoS ONE 2020, 15, e0239360. [Google Scholar] [CrossRef]
- Ramesh, P.; Kokila, T.; Geetha, D. Plant mediated green synthesis and antibacterial activity of silver nanoparticles using Emblica officinalis fruit extract. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 142, 339–343. [Google Scholar] [CrossRef]
- Basavegowda, N.; Lee, Y.R. Synthesis of silver nanoparticles using Satsuma mandarin (Citrus unshiu) peel extract: A novel approach towards waste utilization. Mater. Lett. 2013, 109, 31–33. [Google Scholar]
- Das, G.; Patra, J.K.; Debnath, T.; Ansari, A.; Shin, H.-S. Investigation of antioxidant, antibacterial, antidiabetic, and cytotoxicity potential of silver nanoparticles synthesized using the outer peel extract of Ananas comosus (L.). PloS ONE 2019, 14, e0220950. [Google Scholar] [CrossRef]
- Mohamed, Y.M.A.; Elshahawy, I.E. Antifungal activity of photo-biosynthesized silver nanoparticles (AgNPs) from organic constituents in orange peel extract against phytopathogenic Macrophomina phaseolina. Eur. J. Plant Pathol. 2022, 162, 725–738. [Google Scholar]
- Balavijayalakshmi, J.; Ramalakshmi, V. Carica papaya peel mediated synthesis of silver nanoparticles and its antibacterial activity against human pathogens. J. Appl. Res. Technol. 2017, 15, 413–422. [Google Scholar] [CrossRef]
- Aina, A.; Owolo, O.; Adeoye-Isijola, M.; Olukanni, O.; Lateef, A.; Egbe, T.; Aina, F.; Asafa, T.; Abbas, S. Ecofriendly production of silver nanoparticles from the seeds of Carica papaya and its larvicidal and antibacterial efficacy against some selected bacterial pathogens. In IOP Conference Series: Materials Science and Engineering, Proceedings of the Nanotechnology Applications in Africa: Opportunities and Constraints, Ogbomoso, Nigeria, 22–24 October 2019; IOP Publishing Ltd.: Bristol, UK, 2020; p. 012038. [Google Scholar]
- Abubakar, A.S.; Salisu, I.B.; Chahal, S.; Sahni, G.; Pudake, R.N. Biosynthesis and characterization of silver nano particles using black carrot root extract. Int. J. Curr. Res. Rev. 2014, 6, 5. [Google Scholar]
- Lateef, A.; Azeez, M.A.; Asafa, T.B.; Yekeen, T.A.; Akinboro, A.; Oladipo, I.C.; Azeez, L.; Ojo, S.A.; Gueguim-Kana, E.B.; Beukes, L.S. Cocoa pod husk extract-mediated biosynthesis of silver nanoparticles: Its antimicrobial, antioxidant and larvicidal activities. J. Nanostructure Chem. 2016, 6, 159–169. [Google Scholar] [CrossRef]
- Roopan, S.M.; Madhumitha, G.; Rahuman, A.A.; Kamaraj, C.; Bharathi, A.; Surendra, T. Low-cost and eco-friendly phyto-synthesis of silver nanoparticles using Cocos nucifera coir extract and its larvicidal activity. Ind. Crops Prod. 2013, 43, 631–635. [Google Scholar] [CrossRef]
- Lateef, A.; Azeez, M.; Asafa, T.; Yekeen, T.; Akinboro, A.; Oladipo, I.; Ajetomobi, F.; Gueguim-Kana, E.; Beukes, L. Cola nitida-mediated biogenic synthesis of silver nanoparticles using seed and seed shell extracts and evaluation of antibacterial activities. Bionanoscience 2015, 5, 196–205. [Google Scholar] [CrossRef]
- Lateef, A.; Azeez, M.A.; Asafa, T.B.; Yekeen, T.A.; Akinboro, A.; Oladipo, I.C.; Azeez, L.; Ajibade, S.E.; Ojo, S.A.; Gueguim-Kana, E.B. Biogenic synthesis of silver nanoparticles using a pod extract of Cola nitida: Antibacterial and antioxidant activities and application as a paint additive. J. Taibah Univ. Sci. 2016, 10, 551–562. [Google Scholar] [CrossRef]
- Velu, K.; Elumalai, D.; Hemalatha, P.; Janaki, A.; Babu, M.; Hemavathi, M.; Kaleena, P.K. Evaluation of silver nanoparticles toxicity of Arachis hypogaea peel extracts and its larvicidal activity against malaria and dengue vectors. Environ. Sci. Pollut. Res. 2015, 22, 17769–17779. [Google Scholar] [CrossRef]
- Nisha, S.N.; Aysha, O.; Rahaman, J.S.N.; Kumar, P.V.; Valli, S.; Nirmala, P.; Reena, A. Lemon peels mediated synthesis of silver nanoparticles and its antidermatophytic activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 124, 194–198. [Google Scholar] [CrossRef]
- Heydari, R.; Rashidipour, M. Green synthesis of silver nanoparticles using extract of oak fruit hull (Jaft): Synthesis and in vitro cytotoxic effect on MCF-7 cells. Int. J. Breast Cancer 2015, 2015, 846743. [Google Scholar] [CrossRef]
- Shanmugavadivu, M.; Kuppusamy, S.; Ranjithkumar, R. Synthesis of pomegranate peel extract mediated silver nanoparticles and its antibacterial activity. Am. J. Adv. Drug. Deliv. 2014, 2, 174–182. [Google Scholar]
- Kumar, B.; Smita, K.; Cumbal, L.; Angulo, Y. Fabrication of silver nanoplates using Nephelium lappaceum (Rambutan) peel: A sustainable approach. J. Mol. Liq. 2015, 211, 476–480. [Google Scholar] [CrossRef]
- Harish, B.; Uppuluri, K.B.; Anbazhagan, V. Synthesis of fibrinolytic active silver nanoparticle using wheat bran xylan as a reducing and stabilizing agent. Carbohydr. Polym. 2015, 132, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Qian, L.; Yuan, H.; Xiao, D.; Yang, X.; Paau, M.C.; Choi, M.M. Preparation of gold nanoparticles on eggshell membrane and their biosensing application. Talanta 2010, 82, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Devi, P.S.; Banerjee, S.; Chowdhury, S.R.; Kumar, G.S. Eggshell membrane: A natural biotemplate to synthesize fluorescent gold nanoparticles. RSC Adv. 2012, 2, 11578–11585. [Google Scholar] [CrossRef]
- Krishnaswamy, K.; Vali, H.; Orsat, V. Value-adding to grape waste: Green synthesis of gold nanoparticles. J. Food Eng. 2014, 142, 210–220. [Google Scholar] [CrossRef]
- Kanchi, S.; Kumar, G.; Lo, A.-Y.; Tseng, C.-M.; Chen, S.-K.; Lin, C.-Y.; Chin, T.-S. Exploitation of de-oiled jatropha waste for gold nanoparticles synthesis: A green approach. Arab. J. Chem. 2018, 11, 247–255. [Google Scholar] [CrossRef]
- Suriyakala, G.; Sathiyaraj, S.; Babujanarthanam, R.; Alarjani, K.M.; Hussein, D.S.; Rasheed, R.A.; Kanimozhi, K. Green synthesis of gold nanoparticles using Jatropha integerrima Jacq. flower extract and their antibacterial activity. J. King Saud Univ.-Sci. 2022, 34, 101830. [Google Scholar] [CrossRef]
- Vasantharaj, S.; Sripriya, N.; Shanmugavel, M.; Manikandan, E.; Gnanamani, A.; Senthilkumar, P. Surface active gold nanoparticles biosynthesis by new approach for bionanocatalytic activity. J. Photochem. Photobiol. B Biol. 2018, 179, 119–125. [Google Scholar] [CrossRef]
- Ghosh, S.; Sarkar, B. Crop-mediated synthesis of nanoparticles and their applications. In Agricultural Nanobiotechnology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 23–54. [Google Scholar]
- Patra, J.K.; Baek, K.-H. Novel green synthesis of gold nanoparticles using Citrullus lanatus rind and investigation of proteasome inhibitory activity, antibacterial, and antioxidant potential. Int. J. Nanomed. 2015, 10, 7253. [Google Scholar]
- Averilla, J.N.; Oh, J.; Kim, H.J.; Kim, J.S.; Kim, J.-S. Potential health benefits of phenolic compounds in grape processing by-products. Food Sci. Biotechnol. 2019, 28, 1607–1615. [Google Scholar] [CrossRef]
- Harby, A.G.; El-Borady, O.M.; El-Kemary, M. The exploitation of rice husk biomass for the bio-inspired synthesis of gold nanoparticles as a multifunctional material for various biological and photocatalytic applications. Bioprocess Biosyst. Eng. 2022, 45, 61–74. [Google Scholar] [CrossRef]
- Chávez-Sandoval, B.E.; Balderas-López, J.A.; García-Franco, F.; Galindo-Pérez, E.J.; Martínez-Jiménez, A.; Ibáñez-Hernández, M.Á.A. The pH role about synthesis, distribution and potential applications of gold nanoparticles. Int. J. Biomed. Nanosci. Nanotechnol. 2020, 4, 120–137. [Google Scholar] [CrossRef]
- Anand, K.; Gengan, R.; Phulukdaree, A.; Chuturgoon, A. Agroforestry waste Moringa oleifera petals mediated green synthesis of gold nanoparticles and their anti-cancer and catalytic activity. J. Ind. Eng. Chem. 2015, 21, 1105–1111. [Google Scholar] [CrossRef]
- Kiran, M.; Kumar, C.R.; Shwetha, U.; Onkarappa, H.; Betageri, V.; Latha, M. Green synthesis and characterization of gold nanoparticles from Moringa oleifera leaves and assessment of antioxidant, antidiabetic and anticancer properties. Chem. Data Collect. 2021, 33, 100714. [Google Scholar] [CrossRef]
- Lateef, A.; Ojo, S.; Folarin, B.; Gueguim-Kana, E.; Beukes, L. Kolanut (Cola nitida) mediated synthesis of silver–gold alloy nanoparticles: Antifungal, catalytic, larvicidal and thrombolytic applications. J. Clust. Sci. 2016, 27, 1561–1577. [Google Scholar] [CrossRef]
- Kumar, S.; Sabuj, A.; RP, N.; Lijo Joseph, O.; George, A.M.; Jane, I.C. Utilization of Agro Waste for Energy Engineering Applications: Toward the Manufacturing of Batteries and Super Capacitors. In Smart and Sustainable Approaches for Optimizing Performance of Wireless Networks: Real-time Applications; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2022; pp. 189–200. [Google Scholar]
- Jeyaraj, M.; Gurunathan, S.; Qasim, M.; Kang, M.-H.; Kim, J.-H. A comprehensive review on the synthesis, characterization, and biomedical application of platinum nanoparticles. Nanomaterials 2019, 9, 1719. [Google Scholar] [CrossRef] [PubMed]
- Palacios-Ponce, S.; Ilyina, A.; Ramos-González, R.; Ruiz, H.A.; Martínez-Hernández, J.L.; Segura-Ceniceros, E.P.; Aguilar, M.A.; Sánchez, O.; Aguilar, C.N. Bioproducts obtained from the bioprocessing of the banana peel waste: An Overview. In Applied Chemistry and Chemical Engineering; Apple Academic Press: Palm Bay, FL, USA, 2017; pp. 223–250. [Google Scholar]
- Chandna, S.; Thakur, N.S.; Reddy, Y.N.; Kaur, R.; Bhaumik, J. Engineering lignin stabilized bimetallic nanocomplexes: Structure, mechanistic elucidation, antioxidant, and antimicrobial potential. ACS Biomater. Sci. Eng. 2019, 5, 3212–3227. [Google Scholar] [CrossRef] [PubMed]
- Kamran, U.; Bhatti, H.N.; Iqbal, M.; Jamil, S.; Zahid, M. Biogenic synthesis, characterization and investigation of photocatalytic and antimicrobial activity of manganese nanoparticles synthesized from Cinnamomum verum bark extract. J. Mol. Struct. 2019, 1179, 532–539. [Google Scholar] [CrossRef]
- Donmez, S.; Keyvan, E. Green synthesis of zinc oxide nanoparticles using grape seed extract and evaluation of their antibacterial and antioxidant activities. Inorg. Nano-Met. Chem. 2023, 1–8. [Google Scholar] [CrossRef]
- Sakina, B. The Impact of TiO2 Nanoparticles Treatment, Developmental, and Environmental Factors on Phenolic Content and Antioxidant Capacity of Grapevine (Vitis vinifera L.) Leaves. Ph.D. Thesis, University of Pécs, Pécs, Hungary, 2022; pp. 1–95. Available online: http://pea.lib.pte.hu/handle/pea/34475 (accessed on 16 January 2023).
- Abbas, Q.; Yousaf, B.; Ullah, H.; Ali, M.U.; Ok, Y.S.; Rinklebe, J. Environmental transformation and nano-toxicity of engineered nano-particles (ENPs) in aquatic and terrestrial organisms. Crit. Rev. Environ. Sci. Technol. 2020, 50, 2523–2581. [Google Scholar] [CrossRef]
- Choudhury, S.R. Genome-wide alterations of epigenomic landscape in plants by engineered nanomaterial toxicants. In Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2019; Volume 84, pp. 199–223. [Google Scholar]
- Kandhol, N.; Jain, M.; Tripathi, D.K. Nanoparticles as potential hallmarks of drought stress tolerance in plants. Physiol. Plant. 2022, 174, e13665. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.N.; AlSolami, M.A.; Basahi, R.A.; Siddiqui, M.H.; Al-Huqail, A.A.; Abbas, Z.K.; Siddiqui, Z.H.; Ali, H.M.; Khan, F. Nitric oxide is involved in nano-titanium dioxide-induced activation of antioxidant defense system and accumulation of osmolytes under water-deficit stress in Vicia faba L. Ecotoxicol. Environ. Saf. 2020, 190, 110152. [Google Scholar] [CrossRef] [PubMed]
- Sharma, Y.; Manro, B.; Thakur, P.; Khera, A.; Dey, P.; Gupta, D.; Khajuria, A.; Jaiswal, P.K.; Barnwal, R.P.; Singh, G. Nanotechnology as a vital science in accelerating biofuel production, a boon or bane. Biofuels Bioprod. Biorefining 2022. [Google Scholar] [CrossRef]
- Gohari, G.; Mohammadi, A.; Akbari, A.; Panahirad, S.; Dadpour, M.R.; Fotopoulos, V.; Kimura, S. Titanium dioxide nanoparticles (TiO2 NPs) promote growth and ameliorate salinity stress effects on essential oil profile and biochemical attributes of Dracocephalum moldavica. Sci. Rep. 2020, 10, 912. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, H.; Ilyas, N.; Akhtar, N.; Raja, N.I.; Zainab, T.; Shah, T.; Ahmad, A.; Ahmad, P. Biosynthesis and characterization of titanium dioxide nanoparticles and its effects along with calcium phosphate on physicochemical attributes of wheat under drought stress. Ecotoxicol. Environ. Saf. 2021, 223, 112519. [Google Scholar] [CrossRef]
- Chen, F.; Hong, H.; Shi, S.; Goel, S.; Valdovinos, H.F.; Hernandez, R.; Theuer, C.P.; Barnhart, T.E.; Cai, W. Engineering of hollow mesoporous silica nanoparticles for remarkably enhanced tumor active targeting efficacy. Sci. Rep. 2014, 4, 5080. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.; Ran, F.; Cui, Y.; Liu, C.; Zhao, Q.; Gao, Y.; Wang, D.; Wang, S. A comparison between sphere and rod nanoparticles regarding their in vivo biological behavior and pharmacokinetics. Sci. Rep. 2017, 7, 4131. [Google Scholar] [CrossRef]
- Selvarajan, V.; Obuobi, S.; Ee, P.L.R. Silica nanoparticles—A versatile tool for the treatment of bacterial infections. Front. Chem. 2020, 8, 602. [Google Scholar] [CrossRef]
- Abbasi, M.; Ghoran, S.H.; Niakan, M.H.; Jamali, K.; Moeini, Z.; Jangjou, A.; Izadpanah, P.; Amani, A.M. Mesoporous silica nanoparticle: Heralding a brighter future in cancer nanomedicine. Microporous Mesoporous Mater. 2021, 319, 110967. [Google Scholar] [CrossRef]
- Napierska, D.; Thomassen, L.C.; Lison, D.; Martens, J.A.; Hoet, P.H. The nanosilica hazard: Another variable entity. Part. FibreToxicol. 2010, 7, 39. [Google Scholar] [CrossRef]
- Akhayere, E.; Vaseashta, A.; Kavaz, D. Novel magnetic nano silica synthesis using barley husk waste for removing petroleum from polluted water for environmental sustainability. Sustainability 2020, 12, 10646. [Google Scholar] [CrossRef]
- Pieła, A.; Żymańczyk-Duda, E.; Brzezińska-Rodak, M.; Duda, M.; Grzesiak, J.; Saeid, A.; Mironiuk, M.; Klimek-Ochab, M. Biogenic synthesis of silica nanoparticles from corn cobs husks. Dependence of the productivity on the method of raw material processing. Bioorganic Chem. 2020, 99, 103773. [Google Scholar] [CrossRef] [PubMed]
- Sankar, S.; Sharma, S.K.; Kaur, N.; Lee, B.; Kim, D.Y.; Lee, S.; Jung, H. Biogenerated silica nanoparticles synthesized from sticky, red, and brown rice husk ashes by a chemical method. Ceram. Int. 2016, 42, 4875–4885. [Google Scholar] [CrossRef]
- Estevez, M.; Vargas, S.; Castano, V.; Rodriguez, R. Silica nano-particles produced by worms through a bio-digestion process of rice husk. J. Non-Cryst. Solids 2009, 355, 844–850. [Google Scholar] [CrossRef]
- Sarkar, J.; Mridha, D.; Sarkar, J.; Orasugh, J.T.; Gangopadhyay, B.; Chattopadhyay, D.; Roychowdhury, T.; Acharya, K. Synthesis of nanosilica from agricultural wastes and its multifaceted applications: A review. Biocatal. Agric. Biotechnol. 2021, 37, 102175. [Google Scholar] [CrossRef]
- Kaviyarasu, K.; Maaza, M.M.; Manikandan, E.; Kennedy, J. Rice husks as a sustainable source of high quality nanostructured silica for high performance Li-ion battery requital by sol-gel method—A review. Adv. Mater. Lett. 2016, 7, 684–696. [Google Scholar] [CrossRef]
- Ehrlich, H.; Demadis, K.D.; Pokrovsky, O.S.; Koutsoukos, P.G. Modern views on desilicification: Biosilica and abiotic silica dissolution in natural and artificial environments. Chem. Rev. 2010, 110, 4656–4689. [Google Scholar] [CrossRef]
- Luckarift, H.R.; Spain, J.C.; Naik, R.R.; Stone, M.O. Enzyme immobilization in a biomimetic silica support. Nat. Biotechnol. 2004, 22, 211–213. [Google Scholar] [CrossRef]
- El-Azazy, M.; El-Shafie, A.S.; Al-Saad, K. Application of Infrared Spectroscopy in the Characterization of Lignocellulosic Biomasses Utilized in Wastewater Treatment. In Infrared Spectroscopy-Perspectives and Applications; IntechOpen: London, UK, 2022. [Google Scholar]
- Singh, P.P.; Nath, G. Ultrasonic Processing and Thermo-acoustic Analysis of Orange Peel Waste as Smart Acoustic Material: Waste and Biomass Valorization. Waste Biomass Valorization 2022, 13, 2905–2916. [Google Scholar] [CrossRef]
- Seth, R.; Meena, A.; Meena, R. Enzyme-based green synthesis, characterisation, and toxicity studies of cellulose nanocrystals/fibres produced from the Vetiveriazizanioides roots agro-waste. Environ. Sci. Pollut. Res. 2022, 1–16. [Google Scholar] [CrossRef]
- Fatma, N.; Al-Shemy, M.T.; Hagag, K.H.; Adel, A.M. Fabrication of microwave silicified oxidized cellulose nanocrystals (SOCN) from Agro waste for sustainable multifunctional wool fabric coloration. J. Clean. Prod. 2022, 386, 135800. [Google Scholar]
- Chirayil, C.J.; Joy, J.; Mathew, L.; Mozetic, M.; Koetz, J.; Thomas, S. Isolation and characterization of cellulose nanofibrils from Helicteresisora plant. Ind. Crops Prod. 2014, 59, 27–34. [Google Scholar] [CrossRef]
- Sahu, S.; Nayak, S.; Sahu, S.B.; Roul, M.K. Influence of Various Surface Treatments on Mechanical, Thermal, Morphological, and Water Absorption Properties of Rattan (Calamus beccarii) Fiber. J. Nat. Fibers 2023, 20, 2125924. [Google Scholar] [CrossRef]
- Luo, X.; Zhang, S.; Xia, Z.; Tan, R.; Li, Q.; Qiao, L.; He, Y.; Zhang, G.; Xu, Z. A combined surface-enhanced Raman spectroscopy (SERS)/colorimetric approach for the sensitive detection of malondialdehyde in biological samples. Anal. Chim. Acta 2023, 1241, 340803. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Zhang, L.; Zhao, X.; Zhai, S.; Wang, Q.; Li, C.; Zhang, X. Utilization of lignin upon successive fractionation and esterification in polylactic acid (PLA)/lignin biocomposite. Int. J. Biol. Macromol. 2022, 203, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.R.; Cristofoli, N.L.; da Costa, A.M.R.; Saraiva, J.A.; Vieira, M.C. Comparative study of the production of cellulose nanofibers from agro-industrial waste streams of Salicornia ramosissima by acid and enzymatic treatment. Food Bioprod. Process. 2023, 137, 214–225. [Google Scholar] [CrossRef]
- Zope, G.; Goswami, A.; Kulkarni, S. Isolation and Characterization of Cellulose Nanocrystals Produced by Acid Hydrolysis from Banana Pseudostem. Bionanoscience 2022, 12, 463–471. [Google Scholar] [CrossRef]
- Karimi, S.; Tahir, P.M.; Karimi, A.; Dufresne, A.; Abdulkhani, A. Kenaf bast cellulosic fibers hierarchy: A comprehensive approach from micro to nano. Carbohydr. Polym. 2014, 101, 878–885. [Google Scholar] [CrossRef]
- Baer, D.R.; Artyushkova, K.; Brundle, C.R.; Castle, J.E.; Engelhard, M.H.; Gaskell, K.J.; Grant, J.T.; Haasch, R.T.; Linford, M.R.; Powell, C.J. Practical guides for x-ray photoelectron spectroscopy: First steps in planning, conducting, and reporting XPS measurements. J. Vac. Sci. Technol. A Vac. Surf. Film. 2019, 37, 031401. [Google Scholar] [CrossRef]
- Tokula, B.; Dada, A.; Inyinbor, A.; Obayomi, K.; Bello, O.; Pal, U. Agro-waste based adsorbents as sustainable materials for effective adsorption of Bisphenol A from the environment: A review. J. Clean. Prod. 2023, 388, 135819. [Google Scholar] [CrossRef]
- Uda, M.; Gopinath, S.C.; Hashim, U.; Halim, N.; Parmin, N.; Uda, M.A.; Anbu, P. Production and characterization of silica nanoparticles from fly ash: Conversion of agro-waste into resource. Prep. Biochem. Biotechnol. 2021, 51, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Ahola, S.; Salmi, J.; Johansson, L.-S.; Laine, J.; Österberg, M. Model films from native cellulose nanofibrils. Preparation, swelling, and surface interactions. Biomacromolecules 2008, 9, 1273–1282. [Google Scholar] [CrossRef] [PubMed]
- Zuo, K.; Wu, J.; Chen, S.; Ji, X.; Wu, W. Superamphiphobic nanocellulose aerogels loaded with silica nanoparticles. Cellulose 2019, 26, 9661–9671. [Google Scholar] [CrossRef]
- Dominic, C.M.; Raj, V.; Neenu, K.; Begum, P.S.; Formela, K.; Saeb, M.R.; Prabhu, D.D.; Vijayan, P.P.; Ajithkumar, T.; Parameswaranpillai, J. Chlorine-free extraction and structural characterization of cellulose nanofibers from waste husk of millet (Pennisetum glaucum). Int. J. Biol. Macromol. 2022, 206, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Neenu, K.; Dominic, C.M.; Begum, P.S.; Parameswaranpillai, J.; Kanoth, B.P.; David, D.A.; Sajadi, S.M.; Dhanyasree, P.; Ajithkumar, T.; Badawi, M. Effect of oxalic acid and sulphuric acid hydrolysis on the preparation and properties of pineapple pomace derived cellulose nanofibers and nanopapers. Int. J. Biol. Macromol. 2022, 209, 1745–1759. [Google Scholar] [CrossRef]
- Surattanamal, F.; Sulong, S.; Waloh, N.; Sohsansa, B.; Dahlan, W.; Suksuwan, A. Physicochemical Properties of Cellulose extracted From Hom Thong Banana Peels. In Proceedings of the International Halal Science and Technology Conference, Bangkok, Thailand, 15–16 December 2022; pp. 194–201. [Google Scholar]
- Li, C.; Li, Y.; Sun, P.; Yang, C. Pickering emulsions stabilized by native starch granules. Colloids Surf. A Physicochem. Eng. Asp. 2013, 431, 142–149. [Google Scholar] [CrossRef]
- Wei, B.; Hu, X.; Li, H.; Wu, C.; Xu, X.; Jin, Z.; Tian, Y. Effect of pHs on dispersity of maize starch nanocrystals in aqueous medium. Food Hydrocoll. 2014, 36, 369–373. [Google Scholar] [CrossRef]
- Ogah, A.O.; Afiukwa, J.N.; Englund, K. Characterization and comparison of thermal stability of agro waste fibers in bio-composites application. J. Chem. Eng. Chem. Res. 2014, 1, 84–93. [Google Scholar]
- Rathour, R.K.; Devi, M.; Dahiya, P.; Sharma, N.; Kaushik, N.; Kumari, D.; Kumar, P.; Baadhe, R.R.; Walia, A.; Bhatt, A.K. Recent Trends, Opportunities and Challenges in Sustainable Management of Rice Straw Waste Biomass for Green Biorefinery. Energies 2023, 16, 1429. [Google Scholar] [CrossRef]
- Szymańska-Chargot, M.; Chylińska, M.; Gdula, K.; Kozioł, A.; Zdunek, A. Isolation and characterization of cellulose from different fruit and vegetable pomaces. Polymers 2017, 9, 495. [Google Scholar] [CrossRef]
- Xie, J.; Hse, C.-Y.; Cornelis, F.; Hu, T.; Qi, J.; Shupe, T.F. Isolation and characterization of cellulose nanofibers from bamboo using microwave liquefaction combined with chemical treatment and ultrasonication. Carbohydr. Polym. 2016, 151, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhu, S.; Xing, C.; Li, D.; Zhu, N.; Zhou, H. Isolation and properties of cellulose nanofibrils from coconut palm petioles by different mechanical process. PLoS ONE 2015, 10, e0122123. [Google Scholar] [CrossRef] [PubMed]
- Padhi, S.; Singh, A.; Routray, W. Nanocellulose from agro-waste: A comprehensive review of extraction methods and applications. Rev. Environ. Sci. Bio/Technol. 2023, 1–27. [Google Scholar] [CrossRef]
- Hozman-Manrique, A.S.; Garcia-Brand, A.J.; Hernández-Carrión, M.; Porras, A. Isolation and Characterization of Cellulose Microfibers from Colombian Cocoa Pod Husk via Chemical Treatment with Pressure Effects. Polymers 2023, 15, 664. [Google Scholar] [CrossRef] [PubMed]
- Robles, E.; Salaberria, A.M.; Herrera, R.; Fernandes, S.C.; Labidi, J. Self-bonded composite films based on cellulose nanofibers and chitin nanocrystals as antifungal materials. Carbohydr. Polym. 2016, 144, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.; Fonseca, A.F.; Pereira, F.V.; Druzian, J.I. Extraction and characterization of cellulose nanocrystals from corn stover. Cell. Chem. Technol. 2015, 49, 127–133. [Google Scholar]
- Sofla, M.R.K.; Brown, R.; Tsuzuki, T.; Rainey, T. A comparison of cellulose nanocrystals and cellulose nanofibres extracted from bagasse using acid and ball milling methods. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 035004. [Google Scholar] [CrossRef]
- Sun, Q.; Fan, H.; Xiong, L. Preparation and characterization of starch nanoparticles through ultrasonic-assisted oxidation methods. Carbohydr. Polym. 2014, 106, 359–364. [Google Scholar] [CrossRef]
- Henrique, M.A.; Neto, W.P.F.; Silvério, H.A.; Martins, D.F.; Gurgel, L.V.A.; da Silva Barud, H.; de Morais, L.C.; Pasquini, D. Kinetic study of the thermal decomposition of cellulose nanocrystals with different polymorphs, cellulose I and II, extracted from different sources and using different types of acids. Ind. Crops Prod. 2015, 76, 128–140. [Google Scholar] [CrossRef]
- Romero Millán, L.M.; Sierra Vargas, F.E.; Nzihou, A. Kinetic analysis of tropical lignocellulosic agrowaste pyrolysis. BioEnergy Res. 2017, 10, 832–845. [Google Scholar] [CrossRef]
- Lopez, G.; Artetxe, M.; Amutio, M.; Alvarez, J.; Bilbao, J.; Olazar, M. Recent advances in the gasification of waste plastics. A critical overview. Renew. Sustain. Energy Rev. 2018, 82, 576–596. [Google Scholar] [CrossRef]
- Dube, A.M. Isolation and characterization of cellulose nanocrystals from Enseteventricosum pseudo-stem fiber using acid hydrolysis. Biomass Convers. Biorefinery 2022, 1–16. [Google Scholar] [CrossRef]
- Le Normand, M.; Moriana, R.; Ek, M. Isolation and characterization of cellulose nanocrystals from spruce bark in a biorefinery perspective. Carbohydr. Polym. 2014, 111, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Abbasi Moud, A. Cellulose Nanocrystals Examined by Atomic Force Microscopy: Applications and Fundamentals. ACS Food Sci. Technol. 2022, 2, 1789–1818. [Google Scholar] [CrossRef]
- Meda, R.S.; Jain, S.; Singh, S.; Verma, C.; Nandi, U.; Maji, P.K. Novel Lagenaria siceraria peel waste based cellulose nanocrystals: Isolation and rationalizing H-bonding interactions. Ind. Crops Prod. 2022, 186, 115197. [Google Scholar] [CrossRef]
- Espino-Pérez, E.; Gilbert, R.G.; Domenek, S.; Brochier-Salon, M.-C.; Belgacem, M.N.; Bras, J. Nanocomposites with functionalised polysaccharide nanocrystals through aqueous free radical polymerisation promoted by ozonolysis. Carbohydr. Polym. 2016, 135, 256–266. [Google Scholar] [CrossRef]
- Liu, H.; Cui, S.; Shang, S.; Wang, D.; Song, J. Properties of rosin-based waterborne polyurethanes/cellulose nanocrystals composites. Carbohydr. Polym. 2013, 96, 510–515. [Google Scholar] [CrossRef]
- Salaberria, A.M.; Diaz, R.H.; Labidi, J.; Fernandes, S.C. Role of chitin nanocrystals and nanofibers on physical, mechanical and functional properties in thermoplastic starch films. Food Hydrocoll. 2015, 46, 93–102. [Google Scholar] [CrossRef]
- Sadare, O.O.; Mabunda, N.; Ikegwu, U.M.; Keitemoge, M.K.; Daramola, M.O.; Moothi, K. Parametric optimization of the production of cellulose nanocrystals (CNCs) from South African corncobs via an empirical modelling approach. Sci. Rep. 2022, 12, 18665. [Google Scholar] [CrossRef]
- Chen, G.; Wei, M.; Chen, J.; Huang, J.; Dufresne, A.; Chang, P.R. Simultaneous reinforcing and toughening: New nanocomposites of waterborne polyurethane filled with low loading level of starch nanocrystals. Polymer 2008, 49, 1860–1870. [Google Scholar] [CrossRef]
- Fan, Y.; Saito, T.; Isogai, A. Chitin nanocrystals prepared by TEMPO-mediated oxidation of α-chitin. Biomacromolecules 2008, 9, 192–198. [Google Scholar] [CrossRef]
- Neto, W.P.F.; Silvério, H.A.; Dantas, N.O.; Pasquini, D. Extraction and characterization of cellulose nanocrystals from agro-industrial residue–Soy hulls. Ind. Crops Prod. 2013, 42, 480–488. [Google Scholar] [CrossRef]
- Sahu, V.K.; Mishra, N.; Tiwari, P.N.; Singh, Y. Chapter-2 Prospectives of Nano Technology in Agriculture. Recent Trends Mol. Biol. Biotechnol. 2020, 27–54. [Google Scholar]
- Mendes, J.; Pinho, T.M.; dos Santos, F.N.; Sousa, J.J.; Peres, E.; Boaventura-Cunha, J.; Cunha, M.; Morais, R. Smartphone applications targeting precision agriculture practices—A systematic review. Agronomy 2020, 10, 855. [Google Scholar] [CrossRef]
- Chandna, S.; Gogde, K.; Kaur, R.; Sagar, V.; Bhaumik, J. Nano-biosensors for Plant Biomass: Concept and Applications. In The Role of Nanoparticles in Plant Nutrition under Soil Pollution; Springer: Berlin/Heidelberg, Germany, 2022; pp. 199–221. [Google Scholar]
- Huang, M.-R.; Li, X.-G. Highly sensing and transducing materials for potentiometric ion sensors with versatile applicability. Prog. Mater. Sci. 2022, 125, 100885. [Google Scholar] [CrossRef]
- Soares, J.C.; Santos, C.S.; Carvalho, S.M.; Pintado, M.M.; Vasconcelos, M.W. Preserving the nutritional quality of crop plants under a changing climate: Importance and strategies. Plant Soil 2019, 443, 1–26. [Google Scholar] [CrossRef]
- Marchiol, L. Nanotechnology in agriculture: New opportunities and perspectives. New Vis. Plant Sci. 2018, 9, 161. [Google Scholar]
- Neme, K.; Mohammed, A. Mycotoxin occurrence in grains and the role of postharvest management as a mitigation strategies. A review. Food Control. 2017, 78, 412–425. [Google Scholar] [CrossRef]
- Eid, A.M.; Fouda, A.; Niedbała, G.; Hassan, S.E.-D.; Salem, S.S.; Abdo, A.M.; Hetta, H.F.; Shaheen, T.I. Endophytic Streptomyces laurentii mediated green synthesis of Ag-NPs with antibacterial and anticancer properties for developing functional textile fabric properties. Antibiotics 2020, 9, 641. [Google Scholar] [CrossRef]
- Bhattacharjee, R.; Kumar, L.; Mukerjee, N.; Anand, U.; Dhasmana, A.; Preetam, S.; Bhaumik, S.; Sihi, S.; Pal, S.; Khare, T. The emergence of metal oxide nanoparticles (NPs) as a phytomedicine: A two-facet role in plant growth, nano-toxicity and anti-phyto-microbial activity. Biomed. Pharmacother. 2022, 155, 113658. [Google Scholar] [CrossRef]
- Huang, T.; Li, X.; Maier, M.; O’Brien-Simpson, N.M.; Heath, D.E.; O’Connor, A.J. Using inorganic nanoparticles to fight fungal infections in the antimicrobial resistant era. Acta Biomater. 2023, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.; Singh, M.; Chavan, A.; Wagh, N.S.; Lakkakula, J. Micro-and nanoencapsulation techniques in agriculture. In Agricultural Nanobiotechnology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 297–323. [Google Scholar]
- Athinarayanan, J.; Periasamy, V.S.; Alhazmi, M.; Alatiah, K.A.; Alshatwi, A.A. Synthesis of biogenic silica nanoparticles from rice husks for biomedical applications. Ceram. Int. 2015, 41, 275–281. [Google Scholar] [CrossRef]
- Subhapriya, S.; Gomathipriya, P. Green synthesis of titanium dioxide (TiO2) nanoparticles by Trigonella foenum-graecum extract and its antimicrobial properties. Microb. Pathog. 2018, 116, 215–220. [Google Scholar] [CrossRef]
- Srivastava, V.; Sharma, Y. Synthesis and characterization of Fe3O4@ n-SiO2 nanoparticles from an agrowaste material and its application for the removal of Cr (VI) from aqueous solutions. Water Air Soil Pollut. 2014, 225, 1776. [Google Scholar] [CrossRef]
- Gupta, K.; Kaushik, A.; Tikoo, K.; Kumar, V.; Singhal, S. Enhanced catalytic activity of composites of NiFe2O4 and nano cellulose derived from waste biomass for the mitigation of organic pollutants. Arab. J. Chem. 2020, 13, 783–798. [Google Scholar] [CrossRef]
- Donar, Y.O.; Bilge, S.; Sınağ, A.; Pliekhov, O. TiO2/Carbon materials derived from hydrothermal carbonization of waste biomass: A highly efficient, low-cost visible-light-driven photocatalyst. ChemCatChem 2018, 10, 1134–1139. [Google Scholar] [CrossRef]
- Guo, D.; Zhang, L.; Song, X.; Tan, L.; Ma, H.; Jiao, J.; Zhu, D.; Li, F. NiCo2O4 nanosheets grown on interconnected honeycomb-like porous biomass carbon for high performance asymmetric supercapacitors. New J. Chem. 2018, 42, 8478–8484. [Google Scholar] [CrossRef]
- Shen, Y. Rice husk silica-derived nanomaterials for battery applications: A literature review. J. Agric. Food Chem. 2017, 65, 995–1004. [Google Scholar] [CrossRef]
- Shen, Y. Rice husk silica derived nanomaterials for sustainable applications. Renew. Sustain. Energy Rev. 2017, 80, 453–466. [Google Scholar] [CrossRef]
- Roy, P. Nothing is ‘Waste’in Agriculture: From Nanotechnology and Bioprocesses Perspectives. In Agriculture Waste Management and Bioresource: The Circular Economy Perspective; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2023; pp. 131–148. [Google Scholar]
- Zhang, X.; Huang, Y.; Chen, W.-J.; Wu, S.; Lei, Q.; Zhou, Z.; Zhang, W.; Mishra, S.; Bhatt, P.; Chen, S. Environmental occurrence, toxicity concerns, and biodegradation of neonicotinoid insecticides. Environ. Res. 2023, 218, 114953. [Google Scholar] [CrossRef] [PubMed]
- Mittal, D.; Kaur, G.; Singh, P.; Yadav, K.; Ali, S.A. Nanoparticle-based sustainable agriculture and food science: Recent advances and future outlook. Front. Nanotechnol. 2020, 2, 10. [Google Scholar] [CrossRef]
- Sharma, R.; Garg, R.; Kumari, A. A review on biogenic synthesis, applications and toxicity aspects of zinc oxide nanoparticles. EXCLI J. 2020, 19, 1325. [Google Scholar] [PubMed]
- El-Shetehy, M.; Moradi, A.; Maceroni, M.; Reinhardt, D.; Petri-Fink, A.; Rothen-Rutishauser, B.; Mauch, F.; Schwab, F. Silica nanoparticles enhance disease resistance in Arabidopsis plants. Nat. Nanotechnol. 2021, 16, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Guan, X.; Yu, B.; Ding, N.; Liu, X.; Ma, Q.; Yang, S.; Yilihamu, A.; Yang, S.-T. Carboxylated graphene oxide-chitosan spheres immobilize Cu2+ in soil and reduce its bioaccumulation in wheat plants. Environ. Int. 2019, 133, 105208. [Google Scholar] [CrossRef]
- Tripathi, D.; Singh, M.; Pandey-Rai, S. Crosstalk of nanoparticles and phytohormones regulate plant growth and metabolism under abiotic and biotic stress. Plant Stress 2022, 6, 100107. [Google Scholar] [CrossRef]
- Cai, L.; Cai, L.; Jia, H.; Liu, C.; Wang, D.; Sun, X. Foliar exposure of Fe3O4 nanoparticles on Nicotiana benthamiana: Evidence for nanoparticles uptake, plant growth promoter and defense response elicitor against plant virus. J. Hazard. Mater. 2020, 393, 122415. [Google Scholar] [CrossRef]
- Shaniv, D.; Dror, I.; Berkowitz, B. Effects of particle size and surface chemistry on plastic nanoparticle transport in saturated natural porous media. Chemosphere 2021, 262, 127854. [Google Scholar] [CrossRef]
- Husen, A. Natural product-based fabrication of zinc-oxide nanoparticles and their applications. In Nanomaterials and Plant Potential; Springer: Berlin/Heidelberg, Germany, 2019; pp. 193–219. [Google Scholar]
- Mishra, S.; Keswani, C.; Abhilash, P.; Fraceto, L.F.; Singh, H.B. Integrated approach of agri-nanotechnology: Challenges and future trends. Front. Plant Sci. 2017, 8, 471. [Google Scholar] [CrossRef]
- Wang, F.; Wang, F.; Zhang, H.; Qin, F.; Xiang, W.; Wu, C.; Yan, C.; Zhu, Z.; Chen, J.; Ge, T. Deciphering differences in microbial community composition and multifunctionality between healthy and Alternaria solani-infected potato rhizosphere soils. Plant Soil 2022, 1–16. [Google Scholar] [CrossRef]
- Rodriguez, V.; Moskwa, L.-M.; Oses, R.; Kühn, P.; Riveras-Muñoz, N.; Seguel, O.; Scholten, T.; Wagner, D. Impact of Climate and Slope Aspects on the Composition of Soil Bacterial Communities Involved in Pedogenetic Processes along the Chilean Coastal Cordillera. Microorganisms 2022, 10, 847. [Google Scholar] [CrossRef]
- Sarkar, M. Effect of silver nanoparticles on nitrogen-cycling bacteria in constructed wetlands. Nanotechnol. Environ. Eng. 2022, 7, 537–559. [Google Scholar] [CrossRef]
- Yusefi-Tanha, E.; Fallah, S.; Rostamnejadi, A.; Pokhrel, L.R. Responses of soybean (Glycine max [L.] Merr.) to zinc oxide nanoparticles: Understanding changes in root system architecture, zinc tissue partitioning and soil characteristics. Sci. Total Environ. 2022, 835, 155348. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Naushad, M.; Iqbal, J.; Bathula, C.; Ala’a, H. Challenges and perspectives on innovative technologies for biofuel production and sustainable environmental management. Fuel 2022, 325, 124845. [Google Scholar] [CrossRef]
- Agrawal, S.; Kumar, V.; Kumar, S.; Shahi, S.K. Plant development and crop protection using phytonanotechnology: A new window for sustainable agriculture. Chemosphere 2022, 299, 134465. [Google Scholar] [CrossRef] [PubMed]
- Tymoszuk, A.; Sławkowska, N.; Szałaj, U.; Kulus, D.; Antkowiak, M.; Wojnarowicz, J. Synthesis, Characteristics, and Effect of Zinc Oxide and Silver Nanoparticles on the In Vitro Regeneration and Biochemical Profile of Chrysanthemum Adventitious Shoots. Materials 2022, 15, 8192. [Google Scholar] [CrossRef]
- Ghosh, S.; Sarkar, B.; Kumar, A.; Thongmee, S. Regulatory affairs, commercialization, and economic aspects of nanomaterials used for agriculture. In Agricultural Nanobiotechnology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 479–502. [Google Scholar]
- Wang, W.; Do, A.T.N.; Kwon, J.-H. Ecotoxicological effects of micro-and nanoplastics on terrestrial food web from plants to human beings. Sci. Total Environ. 2022, 834, 155333. [Google Scholar] [CrossRef]
- Obidovna, D.Z.; Sulaymonovich, D.S. The concept of “healthy lifestyle” in psychological research. Res. J. Anal. Invent. 2022, 3, 53–64. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).