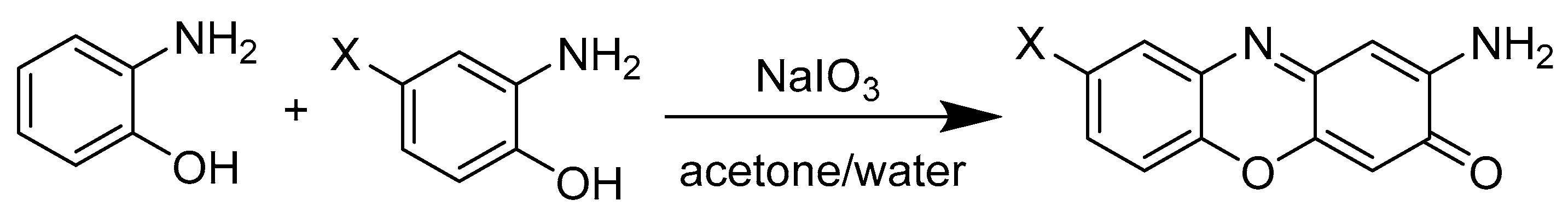Abstract
Natural products have been postulated as an alternative to the use of synthetic herbicides in pest control. The latter compounds have caused numerous problems and these include the appearance of resistance to such herbicides. Aminophenoxazinones are natural products that have shown multiple biological activities, such as pharmacological or phytotoxic effects. In the case of phytotoxicity, the mode of action of aminophenoxazinones has not been widely exploited in agriculture and resistant weeds have not been reported to date. This fact makes aminophenoxazinones promising candidates in the development of herbicides. In the study reported here, seven aminophenoxazinone derivatives have been synthesized and their phytotoxicity has been evaluated on wheat coleoptiles and two important agricultural weeds (Lolium rigidum and Portulaca oleracea). Several derivatives have shown phytotoxic activity, which is similar to the positive control pendimethalin, and even higher in some cases at the highest concentrations tested. The most affected parameter in weeds was root length and the most susceptible weed was P. oleracea. Compound 2, in which nitrogen atoms are present in the heterocycles, was the most active and this was followed by compound 1. Modifications at C-8 led to a reduced activity, with the exception of the nitro compound on the root length of P. oleracea. However, the phytotoxicity also increased on introducing an iodo-substituent at C-4. The results highlight aminophenoxazinones as promising candidates in the development of natural herbicides.
1. Introduction
In agricultural systems, weeds are the primary factor that affects crop yield and quality, and they lead to a decrease in the potential yield for a given crop by up to 45–95% [1,2]. Global economic losses due to weeds have been estimated to exceed USD 100 billion per year [3] and the global cost of weed control is in the billions [4]. In an effort to maximize crop yields, synthetic chemicals are used indiscriminately in modern agriculture to eliminate weeds and this has a negative impact on the soil, water and human and animal health, and it also increases weed resistance [5]. Weeds have now developed resistance to 21 of the 31 modes of action of known herbicides and 165 different herbicides [6]. In Spain, the most abundant weeds already have resistance to the most common modes of action of herbicides, namely the inhibition of acetyl CoA carboxylase (ACCase) and acetolactate synthetase (ALS) [7]. There is, therefore, a need to identify more economical and environmentally friendly weed control strategies.
Given the above, science, agricultural policy, and society need alternative and sustainable methods of weed control. An important and innovative solution to this problem is allelopathy, which is the direct or indirect, harmful or beneficial effect of one plant on another due to the release of chemical compounds (often called allelochemicals) into the environment [8]. The potential applications of allelopathy in weed management are numerous. The most common approach involves selecting active allelochemicals and using them directly as biological herbicides. The use of allelochemicals in sustainable weed management offers several benefits, and these include alternative modes of action, more specific interactions with target weeds, lower concentrations and less damage to the environment [9].
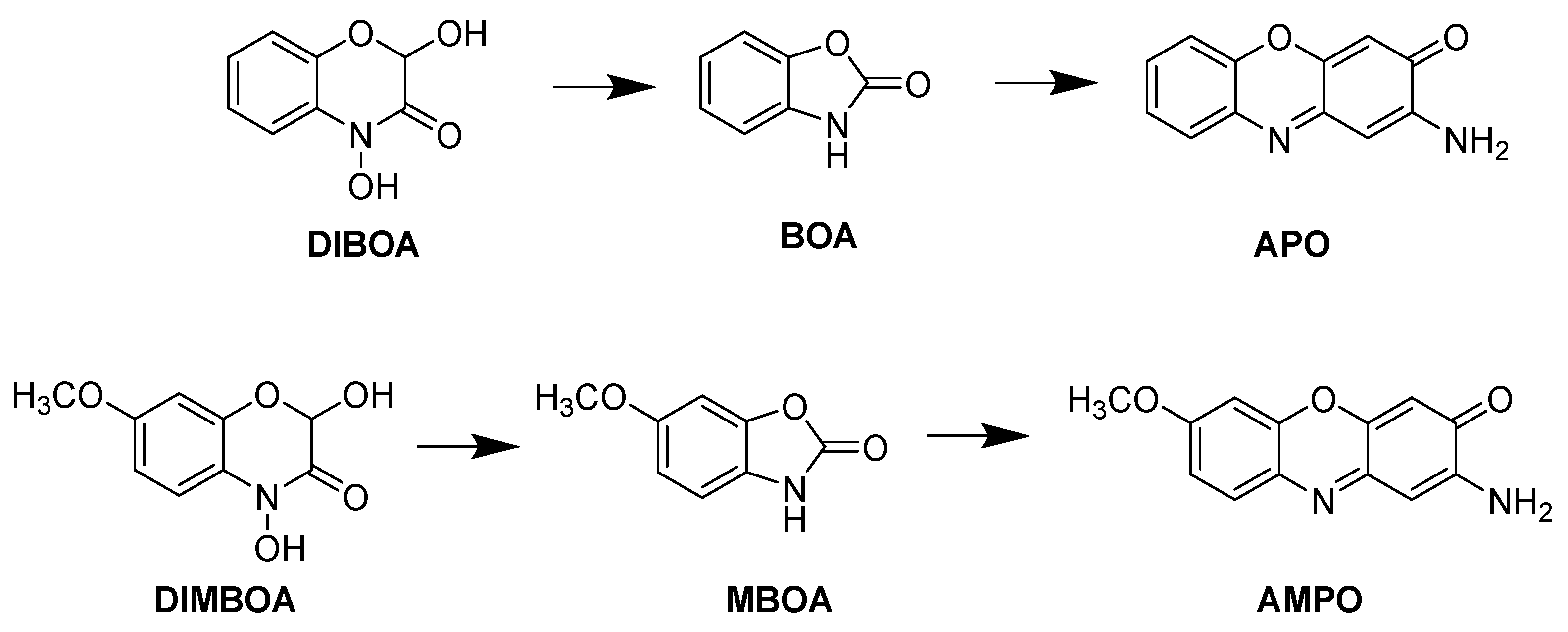
Numerous allelochemicals have been identified, and aminophenoxazinones represent an interesting group of bioactive compounds. These compounds come from the breakdown of benzoxazinones in various plant species and are formed through the transformation into a 2-aminophenol derivative and then dimerization of benzoxazinones, such as the cyclic hydroxamic acids 2,4-dihydroxy-(2H)-1,4-benzoxazin-3(4H)-one (DIBOA) and 2,4-dihydroxy-7-methoxy-(2H)-1,4-benzoxazin-3(4H)-one (DIMBOA), produced and exudated by prominent crops, such as wheat, rye and maize [10]. Once these compounds are exuded into the soil, they are transformed by hydrolysis to benzoxazin-2(3H)-one (BOA) [11] and 6-methoxybenzoxazin-2(3H)-one (MBOA) [12], respectively. These compounds are subsequently degraded by microorganisms to 2-amino-3H-phenoxazin-3-one (APO) from BOA and 2-amino-7-methoxy-3H-phenoxazin-3-one (AMPO) from MBOA (Figure 1) [13].
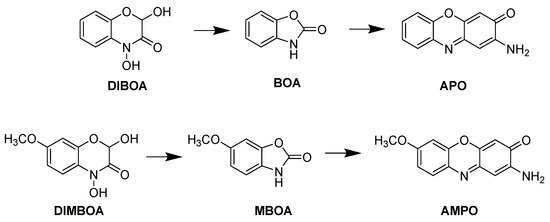
Figure 1.
Degradation processes of DIBOA to APO and DIMBOA to AMPO.
Aminophenoxazinones comprise a family of natural products known for their biological activity. In fact, intense interest in these compounds began more than a century ago, when various industrial and medicinal uses were discovered for them, namely as pigments, pesticides or medicines. Aminophenoxazinones have a tricyclic structure with double bonds in the aromatic system, featuring both oxygen and nitrogen atoms. This enables the creation of synthetic variations with improved properties [14]. Aminophenoxazinones are more stable and easier to extract than their precursors with the benzoxazinone structure. These advantages have led to extensive research being carried out on their biological activities and modes of action, as well as their commercial production [15].
Phytotoxicity is one of the most high-profile activities of aminophenoxazinones. Macías et al. reported the inhibitory activity of the natural products DIMBOA and DIBOA and their degradation products, and highlighted the degradation product APO, which shows up to 76% inhibition of seedling growth [16]. Studies have shown that the phytotoxic effects of APO on the growth of certain plants, such as the model species Arabidopsis thaliana, are due to its inhibition of histone deacetylases and histone acetyltransferases. This leads to specific changes in the histone acetylation processes [17] and slow plant growth. These enzymes constitute an evolutionarily conserved class of enzymes and they are present in most organisms. These enzymes are important in processes such as the change from vegetative to reproductive growth, or in rapid responses to internal signals, amongst others [18]. To the best of our knowledge, weeds have not shown resistance to this mode of action to date [6]. For the reasons outlined above, the use of aminophenoxazinones as lead compounds in the development of natural herbicides is, in our opinion, a very interesting approach.
In order to improve the physicochemical properties and activity of aminophenoxazinones, we propose the synthesis of APO derivatives and the evaluation of their activity in phytotoxicity bioassays on etiolated wheat coleoptiles and important weeds in agriculture. This approach will allow for the identification of substituents and positions in the molecule that are involved in the bioactivity. Such changes could also enable improvements in the solubility, thus increasing the opportunities for aminophenoxazinones to become leading compounds in the development of future herbicides.
2. Materials and Methods
2.1. General Experimental Procedures
1H nuclear magnetic resonance (NMR) and 13C NMR spectra were obtained on Bruker spectrometers at 400/100 and 500/125 MHz, respectively, with dymetilsulfoxide (DMSO) (from VWR Chemicals) as the internal reference. The residual solvent peaks were set to δ 2.50 ppm for 1H NMR and δ 39.5 ppm for 13C NMR. The exact masses were measured on an ultra performance liquid chromatography quadruple time of fly-electro spray ionization (UPLC-QTOF-ESI) (Waters Synapt G2) high-resolution mass spectrometer (HRTOFESIMS). The reactions were monitored by thin layer chromatography, using Merck-Kiesegel 60 F254 normal phase plates. The reagents for the synthetic procedures were supplied by Sigma-Aldrich Co. and the solvents used for purification were supplied by VWR International.
2.2. Synthesis of 2-Amino-3H-phenoxazin-3-one Derivatives (Compounds 1–6)
The molecule 2-amino-3H-phenoxazin-3-one and its derivatives were synthesized according to the following scheme (Figure 2), where an oxidative condensation between the two initial phenols take place. [19]
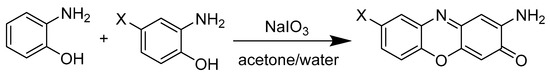
Figure 2.
General procedure for the synthesis of APO and its derivatives.
2.2.1. Synthesis of 2-Amino-3H-phenoxazin-3-one (Compound 1)
A solution of 2-aminophenol (250 mg) in acetone (10 mL) was added to a solution of sodium iodate (430 mg) in deionized water (50 mL). The mixture was stirred for 10 min and a solution of 2-aminophenol (250 mg) in acetone (7 mL) was added. After 2 h, a solution of sodium iodate (950 mg) in deionized water (70 mL) was added. The reaction mixture was stirred at room temperature for 20 h and acetone was evaporated under reduced pressure. The remaining mixture was cooled (ice) and the solid was filtered off and washed with cold water to give 239 mg of crude product. A sample (100 mg) was purified by counter-current chromatography-centrifugal partition chromatography (Gilson PLC 2250), with a 2/3/2/3 mixture of hexane/ethyl acetate/methanol/water (ARIZONA L) [20] in an ascending mode. The procedure consisted of three stages. In the equilibrium step, 100% of the upper phase (UP) was introduced into the rotor at 50 mL/min for 4 min at 500 rpm. A mixture of 5% UP/95% lower phase (LP) was then used for 16 min at 5 mL/min and 1500 rpm. Elution was the second stage, and this took 50 min under the following conditions: 5%UP/95%LW at a rate of 5 mL/min at 1500 rpm. Extrusion was the last stage, and this took 15 min under the following conditions: 100%UP/0%LW at a rate of 50 mL/min at 500 rpm. The 2-Amino-3H-phenoxazin-3-one was obtained after 27 min in the elution step, in a 28% yield (71 mg).
2-Amino-3H-phenoxazin-3-one, dark orange powder. 1H-NMR (DMSO, 400 MHz) δ (ppm) 7.71 (1H, dd, J = 7.8, 1.7 Hz, H-9), 7.50 (1H, dd, J = 8.1, 1.7 Hz, H-6), 7.46 (1H, td, J = 8.1, 1.5 Hz, H-7), 7.39 (1H, td, J = 8.1, 1.7 Hz, H-8), 6.82 (NH2), 6.35 (1H, s, H-4), 6.36 (1H, s, H-1); 13C-NMR (DMSO, 100 MHz) δ (ppm) 180.7 (C, C-3), 149.3 (C, C-2), 148.7 (C, C-4a), 147.8 (C, C-10a), 142.4 (C, C-5a), 134.2 (C, C-9a), 129.3 (CH, C-7), 128.4 (CH, C-9), 125.7 (CH, C-8), 116.4 (CH, C-6), 103.9 (CH, C-4), 98.8 (CH, C-1).
2.2.2. Synthesis of 2-Amino-3H-dipyrido [3,2-b:2′,3′-e][1,4]-oxazin-3-one (Compound 2)
A solution of 2-amino-3-hydroxypiridine (330 mg) in methanol (10 mL) was added to a solution of sodium iodate (356 mg) in deionized water (50 mL). The mixture was stirred for 10 min and a solution of 2-amino-3-hydroxypiridine (330 mg) in methanol (10 mL) was added. After 2 h, a solution of sodium iodate (720 mg) in deionized water (70 mL) was added. The mixture was stirred for 20 h and methanol was removed under reduced pressure. The remaining mixture was cooled (ice) and the solid was filtered off and washed with cold water to give 2-amino-3H-dipyrido [3,2-b:2′,3′-e][1,4]-oxazin-3-one (230 mg, 35% yield).
2-Amino-3H-dipyrido [3,2-b:2′,3′-e][1,4]-oxazin-3-one, black powder. 1H-NMR (DMSO, 400 MHz) δ (ppm) 8.54 (1H, d, J = 8.8 Hz, H-8), 8.28 (2H, NH2), 7.95 (1H, d, J = 8.2 Hz, H-6), 7.45 (1H, dd, J = 8.9, 3.8 Hz), 6.56 (1H, s, H-4); 13C-NMR (DMSO, 125 MHz) δ (ppm) 176.3 (C, C-3), 158.1 (C, C-2), 153.0 (C, C-10a), 151.0 (C, C-4a), 146.8 (CH, C-8), 145.8 (C, C-9a), 138.6 (C, C-5a), 124.1 (CH, C-6), 123.5 (CH, C-7), 105.3 (CH, C-4). HRESIMS m/z 215.0571 [M + H]+ (calcd for C10H6N4O2, 215.0569).
2.2.3. Synthesis of 2-Amino-3-oxo-3H-phenoxazine-8-carboxylic acid (Compound 3)
A solution of 2-aminophenol (280 mg) in methanol (10 mL) was added to sodium iodate (470 mg) in deionized water. The mixture was stirred for 10 min and a solution of 2-amino-3-hydroxybenzoic acid (270 mg) in methanol (10 mL) was added. After 2 h, a solution of sodium iodate (980 mg) in deionized water (70 mL) was added. The mixture was stirred for 20 h and methanol was evaporated under reduced pressure. The remaining mixture was cooled (ice) and the solid was filtered off to give the crude product (286 mg). Counter-current chromatography-centrifugal partition chromatography was used for the purification. The crude product (50 mg) was purified with a 1/9/1/9 mixture of hexane/ethyl acetate/methanol/water (ARIZONA C) [20] in a descending mode. The procedure consisted of three stages. In the equilibrium step, 100%LW was introduced into the rotor for 4 min at a rate of 50 mL/min at 500 rpm. Then, 5%LW/95%UP was used for 16 min at a rate of 5 mL/min at 2000 rpm. Elution was the second step, and the conditions were as follows: 5%LW/95%UP at a rate of 5 mL/min at 2000 rpm. Finally, in the extrusion step, the conditions were as follows: 100%LW at a rate of 50 mL/min at 500 rpm for 15 min. The 2-Amino-3-oxo-3H-phenoxazine-8-carboxylic acid was obtained after 29 min in the elution step (5.8 mg, 5.2% yield).
2-Amino-3-oxo-3H-phenoxazine-8-carboxylic acid, dark yellow powder. 1H-NMR (DMSO, 500 MHz) δ (ppm) 8.18 (1H, d, J = 2.1 Hz, H-9), 7.97 (1H, dd, J = 2.1, 8.6, H-7), 7.55 (1H, d, J = 8.6 Hz, H-6), 6.91 (2H, NH2), 6.41 (1H, s, H-4), 6.37 (1H, s, H-1); 13C-NMR (DMSO, 125 MHz) δ (ppm) 180.4 (C, C-3), 167.1 (C, C-11), 149.0 (C, C-2), 148.7 (C, C-4a), 147.6 (C, C-10a), 144.6 (C, C-5a), 133.4 (C, C-9a), 129.2 (CH, C-7), 128.9 (CH, C-9), 127.6 (C, C-8), 116.2 (CH, C-6), 105.3 (CH, C-4), 98.3 (CH, C-1).
2.2.4. Synthesis of 2-Amino-8-nitro-3H-phenoxazin-3-one (Compound 4)
A solution of 2-aminophenol (280 mg) in acetone (10 mL) was added to a solution of sodium iodate (500 mg) in deionized water (50 mL). The mixture was stirred for 10 min and a solution of 2-amino-4-nitrophenol (480 mg) in acetone (10 mL) was added. After 2 h, a solution of sodium iodate (980 mg) in deionized water (70 mL) was added. The mixture was stirred for 20 h and acetone was removed under reduced pressure. The remaining mixture was cooled (ice) and the solid was filtered off and washed with cold water to give the crude product (441 mg). The sample (97 mg) was purified by column chromatography using a mixture of silica gel (Geduran® Si 60 (0.063–0.020 mm)) and ethyl acetate: hexane 6:4 as the mobile phase, to give 2-amino-8-nitro-3H-phenoxazin-3-one (19 mg, 12.5% yield).
2-Amino-8-nitro-3H-phenoxazin-3-one, dark orange powder. 1H-NMR (DMSO, 500 MHz) δ (ppm) 8.42 (1H, d, J = 2.6 Hz, H-9), 8.24 (1H, dd, J = 2.6, 9.6 Hz, H-7), 7.70 (1H, d, J = 9.6 Hz, H-6), 7.14 (2H, NH2), 6.47 (1H, s, H-4), 6.37 (1H, s, H-1); 13C-NMR (DMSO, 125 MHz) δ (ppm) 181.1 (C, C-3), 150.4 (C, C-2), 149.0 (C, C-4a), 148.6 (C, C-10a), 146.7 (C, C-8), 144.5 (C, C-5a), 133.7 (C, C-9a), 123.3 (CH, C-7), 123.1 (CH, C-9), 117.7 (CH, C-6), 105.1 (CH, C-4), 98.4 (CH, C-1). HRESIMS m/z 258.0518 [M + H]+ (calc. for C12H8N3O4, 258.0515).
2.2.5. Synthesis of 2-Amino-8-chloro-3H-phenoxazin-3-one (Compound 5) and 2-Amino-8-chloro-4-iodo-3H-phenoxazin-3-one (Compound 6)
A solution of 2-aminophenol (280 mg) in acetone (10 mL) was added to a solution of sodium iodate (500 mg) in water (50 mL). The mixture was stirred for 10 min and a solution of 2-amino-4-chlorophenol (370 mg) was added. After 2 h, a solution of sodium iodate (1 g) in deionized water (100 mL) was added. The mixture was stirred for 22 h and acetone was evaporated under reduced pressure. The remaining mixture was cooled (ice) and the solid was filtered off to give the crude product (1.36 g). A sample (30 mg) was purified by preparative layer chromatography (PLC Silica Gel 60 F254 0.5 mm, 20 × 20 cm, Sigma-Aldrich, Canada), using a mixture of acetone: chloroform 8:2 as the mobile phase, to give the products 2-amino-8-chloro-3H-phenoxazin-3-one (3.6 mg, 25.7% yield) and 2-amino-8-chloro-4-iodo-3H-phenoxazin-3-one (4.5 mg, 32.2% yield).
2-Amino-8-chloro-3H-phenoxazin-3-one, orange powder, 1H-NMR (DMSO, 500 MHz) δ (ppm) 7.75 (1H, d, J = 2.6 Hz, H-9), 7.55 (1H, d, J = 8.6 Hz, H-6), 7.48 (1H, dd, J = 2.6, 9.6 Hz, H-7), 6.99 (2H, NH2), 6.39 (1H, s, H-4), 6.35 (1H, s, H-1); 13C-NMR (DMSO, 125 MHz) δ (ppm) 180.3 (C, C-3), 149.1 (C, C-2), 148.8 (C, C-4a), 147.9 (C, C-10a), 140.8 (C, C-5a), 134.7 (C, C-9a), 128.6 (C, C-8), 128.0 (CH, C-7), 126.7 (CH, C-9), 117.6 (CH, C-6), 103.7 (CH, C-4), 98.1 (CH, C-1). HRESIMS m/z 247.0289 [M + H]+ (calc. for C12H8N2O2Cl, 247.0274).
2-Amino-8-chloro-4-iodo-3H-phenoxazin-3-one, orange powder, 1H-NMR (DMSO, 500 MHz) δ (ppm) 7.81 (1H, d, J = 2.4 Hz, H-9), 7.63 (1H, d, J = 8.8 Hz, H-6), 7.52 (1H, dd, J = 8.8, 2.5 Hz, H-7), 7.11 (2H, NH2), 6.36 (1H, s, H-1); 13C-NMR (DMSO, 125 MHz) δ (ppm) 176.2 (C, C-3), 149.8 (C, C-2), 148.4 (C, C-4a), 146.3 (C, C-10a), 141.2 (C, C-5a), 135.0 (C, C-9a), 129.2 (C, C-8), 128.4 (CH, C-7), 126.6 (CH, C-9), 117.8 (CH, C-6), 98.1 (CH, C-1), 81.2 (C, C-4). HRESIMS m/z 372.9247 [M + H]+ (calc. for C12H7N2O2ClI, 372.9241).
2.3. Synthesis of 2,8-Diamino-3H-phenoxazin-3-one (Compound 7)
A solution of 2-amino-8-chloro-3H-phenoxazin-3-one (compound 4) (450 mg) in acetone (50 mL) was added to a solution of ammonium chloride (800 mg) in deionized water (50 mL) and zinc powder (800 mg). The mixture was sonicated for 1 h in an ultrasonic bath. The mixture was filtered through Celite® and dried with anhydrous Na2SO4. The solvent was evaporated under reduced pressure to give the crude product (305.1 mg). 50 mg of the crude product was purified by counter-current chromatography-centrifugal partition chromatography, with a 2/3/2/3 mixture of hexane/ethyl acetate/methanol/water (ARIZONA L) [20] in a descending mode. The procedure consisted of three stages. In the equilibrium step, 100%LW was introduced into the rotor for 4 min at a rate of 50 mL/min at 500 rpm. Then, 5%LW/95%UP was used for 16 min at a rate of 5 mL/min at 1500 rpm. The second step involved elution, and this took 50 min under the following conditions: 5%LW/95%UP at a rate of 5 mL/min at 1500 rpm. Finally, the extrusion step took 15 min under the following conditions: 100%LW with a rate of 50 mL/min at 500 rpm. A mixture of the product and ammonium chloride (47 mg in total) was obtained after 20 min in the elution step. The ammonium chloride was removed by solid-phase extraction (SPE) on 8.7 mg of the mixture, using deionized water to remove NH4Cl and acetone to elute the pure product. The 2,8-diamino-3H-phenoxazin-3-one (1.5 mg, 55% yield) was obtained.
2,8-Diamino-3H-phenoxazin-3-one, brown powder, 1H-NMR (DMSO; 400 MHz) δ (ppm) 7.25 (1H, d, J = 8.7 Hz, H-6), 6.83 (1H, d, J = 2.6 Hz, H-9), 6.77 (1H, dd, J = 8.7, 2.6 Hz, H-7), 6.60 (2H, NH2 (2)), 6.34 (1H, s, H-1), 6.26 (1H, s, H-4), 5.50 (2H, NH2 (8)); 13C-NMR (DMSO, 100 MHz) δ (ppm) 179.9 (C, C-3), 149.6 (C, C-4a), 148.1 (C, C-10a), 147.7 (C, C-2), 147.0 (C, C-8), 135.2 (C, C-5a), 134.3 (C, C-9a), 117.1 (CH, C-7), 116.4 (CH, C-6), 109.9 (CH, C-9), 102.7 (CH, C-4), 98.7 (CH, C-1). HRESIMS m/z 228.0789 [M + H]+ (calc. for C12H9N3O2, 228.0773).
2.4. Wheat Coleoptile Bioassay
Wheat coleoptile bioassays were performed according to the method previously described by Rial et al. (File S1) [21]. All compounds were pre-dissolved in DMSO and diluted to final concentrations (1000, 300, 100, 30 and 10 µM) in a phosphate/citrate buffer at pH 5.6, with 2% sucrose. The active ingredient of the herbicide Stomp Aqua®, pendimethalin, was used as a positive control at the same concentrations. A solution of 0.1% DMSO in the phosphate/citrate buffer was used as a negative control. The assay was carried out in triplicate.
Five coleoptiles were placed in each test tube, with 2.0 mL of the test compound or a positive or negative control. The tubes were rotated at 0.25 rpm using a roller tube device at 25 ± 1 °C for 24 h in the absence of light. Upon completion, the length of the coleoptiles was digitized, and the data were statistically evaluated using a Welch’s test. Results were expressed as the percent difference from the control; negative values indicate inhibition of the elongation of the coleoptiles, positive values indicate stimulation and zero represents the control.
2.5. Phytotoxicity Bioassay
The phytotoxicity bioassay was performed according to the procedure previously described by Rial and co-workers (File S2) [21]. The assay was carried out on two agricultural weeds, Portulaca oleracea and Lolium rigidum, which were selected because they belong to—and are representative of—important families of agricultural weeds. Weed seeds were purchased from Semillas Cantueso (Cordoba, Spain). Twenty seeds were placed in Petri dishes on Whatman® paper (d = 50 mm), with 1.0 mL of the buffer solution (10 mM 2-(N-morpholino) ethanesulfonic acid (MES), pH adjusted to 6.0), together with the products, at concentrations of 1000, 300, 100, 30 and 10 µM, with four replicates per concentration tested. The active ingredient of the herbicide Stomp Aqua® (pendimethalin) was used as a positive control at the same concentrations and the buffer solution was used as a negative control.
The dishes with the seeds and the test solutions were kept in the dark at 25 °C in a germination chamber for 7 days. The dishes were then stored in a freezer for 24 h. The elongation of the shoots and roots and the germination ratio of the seeds were measured by placing the seedlings on a plastic film and using a Fitomed® system [22]. Statistical analysis was performed using a Welch’s test, with significance levels set at 0.01 and 0.05. Germination percentage, root length and shoot length are shown as the percent difference from the control. Zero represents control, positive values represent stimulation, and negative values represent inhibition.
2.6. IC50 Calculation
IC50 values were determined by nonlinear regression, using the GraphPadPrism 5 software package (San Diego, CA, USA).
3. Results and Discussion
3.1. Synthesis of APO and Its Derivatives
Several processes have been described for the synthesis of APO and other related compounds. One approach involves the soft reduction of nitrophenols [23] and another concerns the oxidative reactions of 2-aminophenol, for example, using catalysts [24] or using electrochemical processes, such as cyclic voltamperometry [25]. In our case, it was decided to use oxidative cyclocondesantions of 2-aminophenols with a solid oxidant, namely NaIO3. This strategy allowed the introduction of different functional groups into the APO backbone by using different 2-aminophenol derivatives as starting materials (Figure 2) [19].
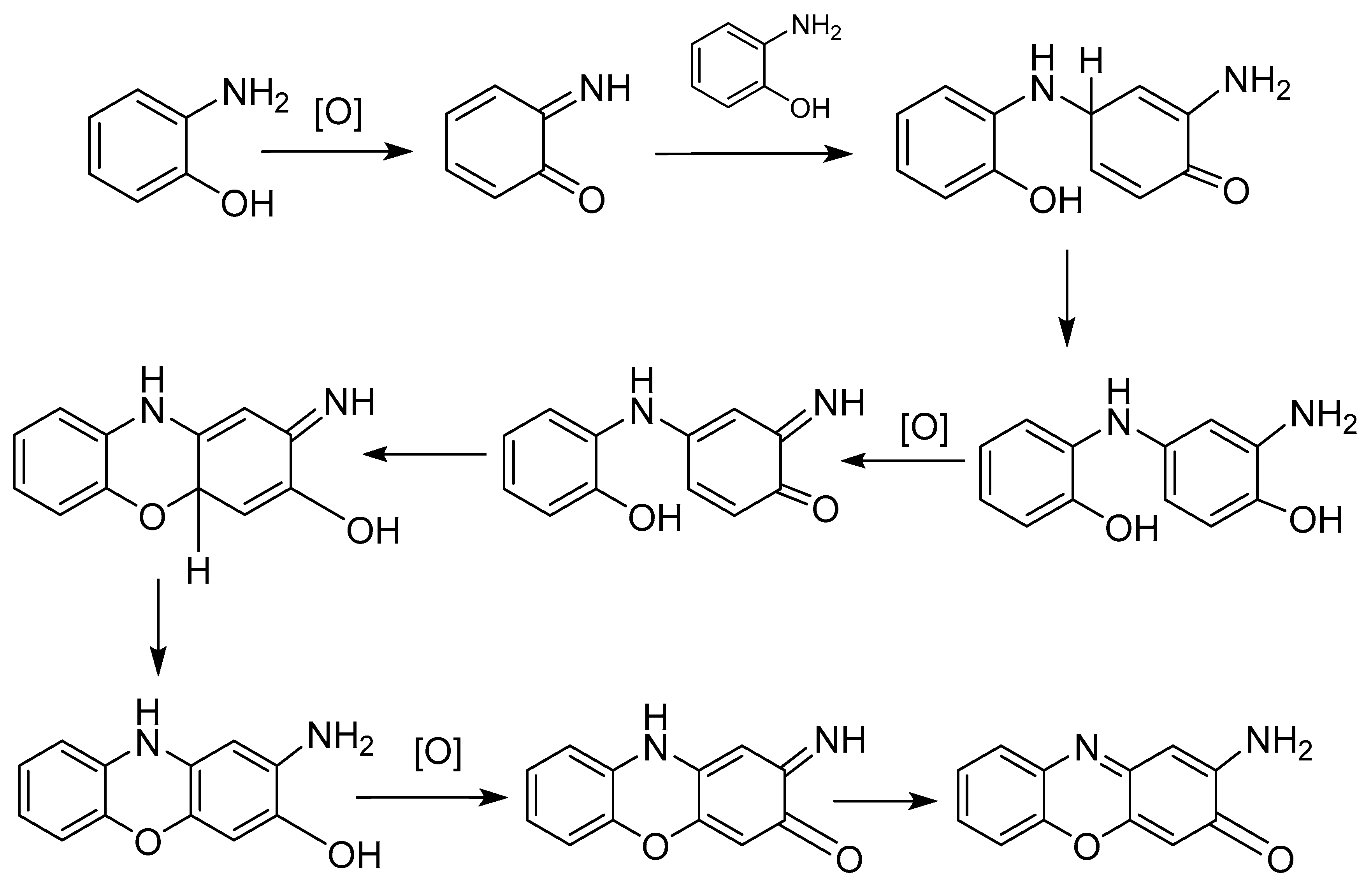
The reaction mechanism was proposed by Giurg et al. in 2006 and is shown in Figure 3 [26].
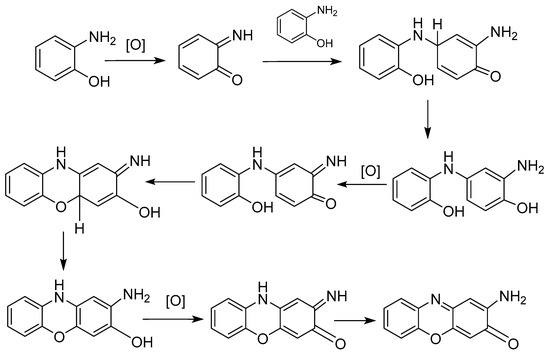
Figure 3.
Reaction mechanism proposed for the synthesis of APO and its derivatives.
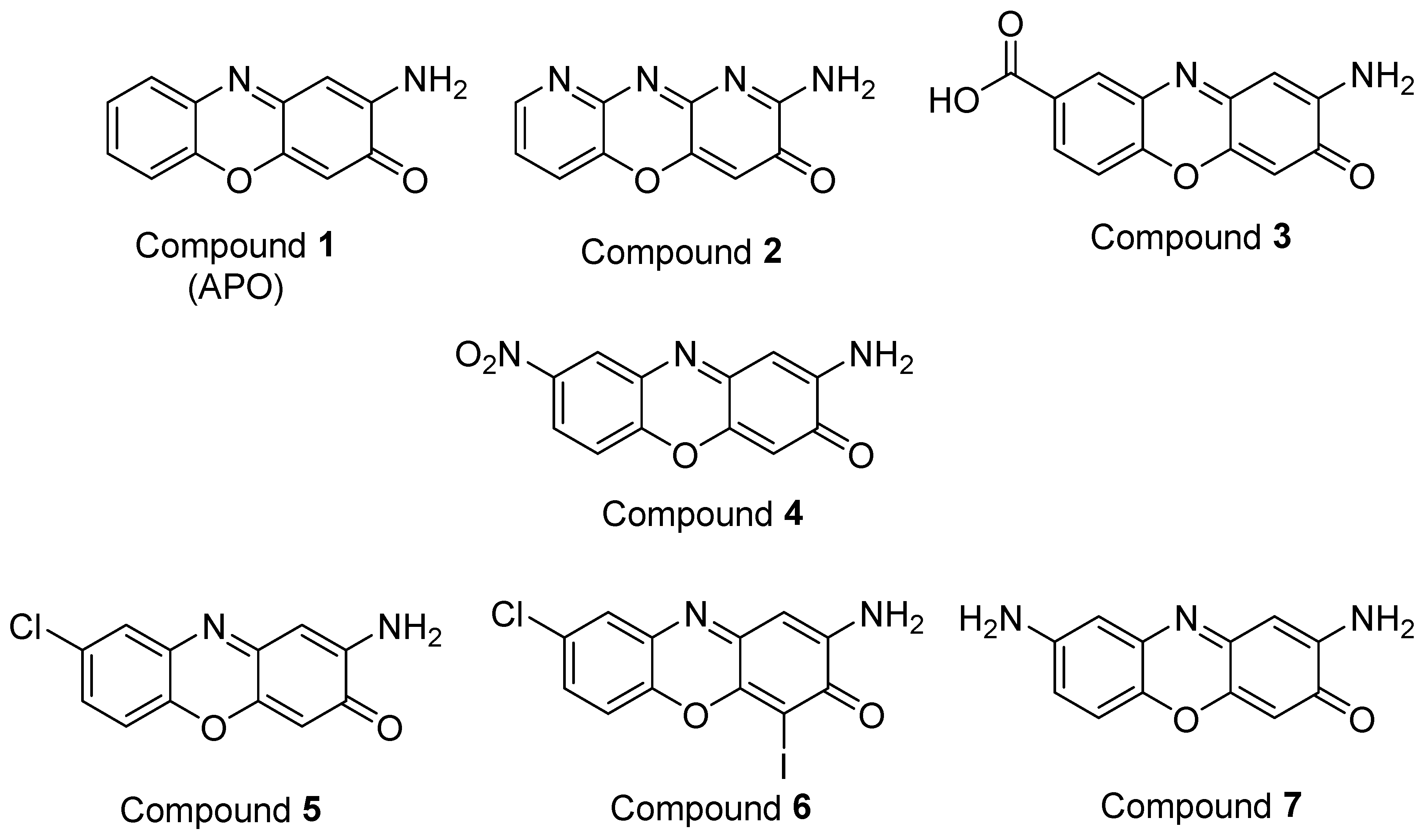
This synthesitic method was employed to obtain APO and six derivatives (Figure 4). The introduction of nitrogen into the molecule was explored because many bioactive compounds contain heterocycles in their structures. This goal was achieved using the pyridine derivative 2-aminopyridin-3-ol instead of 2-aminophenol as the starting material, to obtain compound 2. In the case of compound 3, a carboxylic acid group was introduced at position 8. Polar groups, such as carboxylic acids, increase the solubility of the molecules in water and this could also increase the bioavailability, as indicated by Lipinski’s rules [27]. Nitro groups are present in numerous compounds, with multiple biological activities that include antimicrobial, antiparasitic, tranquilizer, etc. [28,29], and for this reason, compound 4 was synthesized. A number of commercial drugs and agrochemicals contain halogen atoms in their structures [30] and there are numerous halogenated natural products that have biological activities, such as antibiotic properties [31]. As a consequence, compound 5 was synthesized and compound 6 was obtained as a byproduct of the reaction. Finally, the presence of nitrogen groups in molecules with pharmacological activities, such as antibiotic, antidepressant and antimalarial, among others, has been extensively described in the literature [32,33]. For this reason, the reduction of compound 4 was carried out to obtain the diamine derivative (compound 7). The structural characterization of the synthesized compounds was carried out by 1D and 2D NMR experiments (Figures S1–S7).
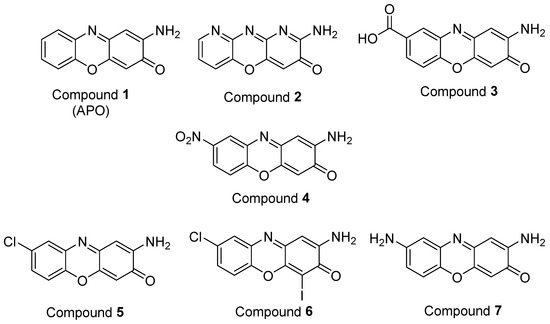
Figure 4.
APO and its derivatives synthesized in this study.
All of the synthesized derivatives are characterized by a significant drawback during purification, as they remained strongly adhered to all commonly used solid supports during chromatography. As a consequence, the CPC-CCC (centrifugal partition chromatography) technique was used in this study [34]. In this technique immiscible liquids were used as the stationary and mobile phases, with the stationary phase being kept static by centrifugal force [35]. The use of the CPC-CCC technique overcame the problems associated with the use of solid supports, as the products could not adhere to the stationary phase and recovery rates of up to 96% were achieved [36]. A range of tests were conducted with different operating conditions and solvent systems from the ARIZONA method to separate the products [20]. This resulted in the efficient purification of compounds 1 and 7 and the partial purification of compounds 3 and 6. However, on using the CPC-CCC technique, it proved difficult to separate molecules of similar polarity and, in these cases, silica gel chromatography was used.
3.2. Phytotoxic Activity on Etiolated Wheat Coleoptiles
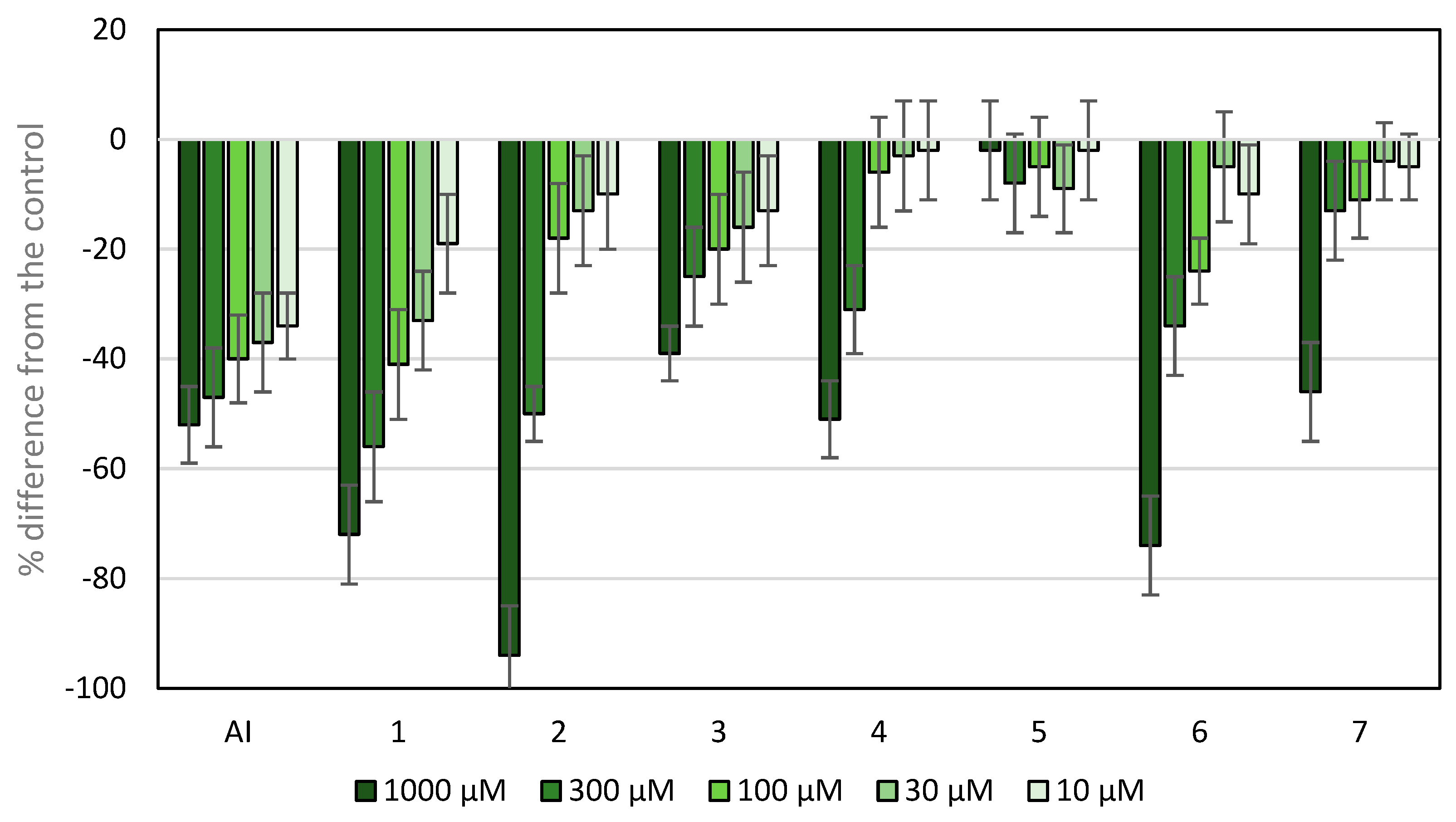
The wheat coleoptile bioassay was used to assess the phytotoxicity of the compounds under study, as it is a rapid test (24 h) and is sensitive to several bioactive substances, including plant growth regulators and herbicide [37]. The results are shown in Figure 5, and the IC50 values are shown in Table 1.
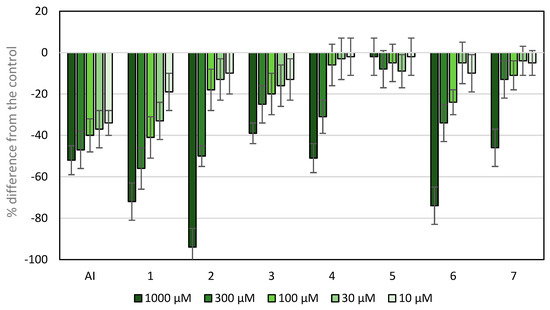
Figure 5.
Effects of active ingredients and synthetic compounds of the herbicide Stomp Aqua® (pendimethalin, AI) on the elongation of wheat coleoptiles. Values are expressed as the percent difference from the control. Each bar is the mean ± standard deviation.

Table 1.
IC50 Values for the active ingredient of the herbicide Stomp Aqua® (pendimethalin, AI) and the synthesized compounds on wheat coleoptile elongation.
Except compound 5, all tested compounds showed inhibitory activity. At the highest concentrations tested, compounds 1, 2 and 6 showed higher activity and lower IC50 values than the active ingredient (AI) of the herbicide Stomp Aqua® used as a positive control. The presence of an additional nitrogen heteroatom in the framework of APO (compound 2) enhanced the phytotoxic activity, with values higher than those of APO at the highest concentration (compound 1), namely 94 and 72%, respectively. However, the IC50 value of compound 1 was slightly lower than that of compound 2, because at the second concentration, the inhibition values were 56 and 50%, respectively, with more marked differences at the subsequent concentrations. A comparison between compounds 5 and 6 shows that the introduction of an iodo-substituent at the C-4 position improved inhibition from 2 to 74%, respectively, at the highest concentration. Nevertheless, other modifications at carbon 8 reduced the phytotoxicity of APO.
In brief, the results described above show that the introduction of nitrogen into the scaffold of APO (compound 2) improves the inhibitory activity. In contrast, the introduction of a chloro-substituent at position C-8 (compound 5) decreased the APO inhibitory activity. This trend is reversed at the highest concentration upon the introduction of a second halogen atom, iodine, at the C-4 position (compound 6). The remaining groups introduced in the C-8 position of the APO scaffold, e.g., an acid group (compound 3), nitro group (compound 4) and amino group (compound 7), did not improve the inhibitory activity shown by APO (compound 1) in this bioassay.
3.3. Phytotoxic Activity on Weed Seeds
Given the inhibitory activity of several synthesized compounds in a wheat coleoptile bioassay, they were also evaluated in a more specific bioassay testing their phytotoxic potential against two important agricultural weeds, P. oleracea and L. rigidum. Almost all of the compounds tested did not show activity on the germination of the weeds used in this bioassay. In the case of P. oleracea, even the positive control did not show inhibitory activity, although APO did show inhibition at the highest concentration, with a value of 30% (data are not shown).
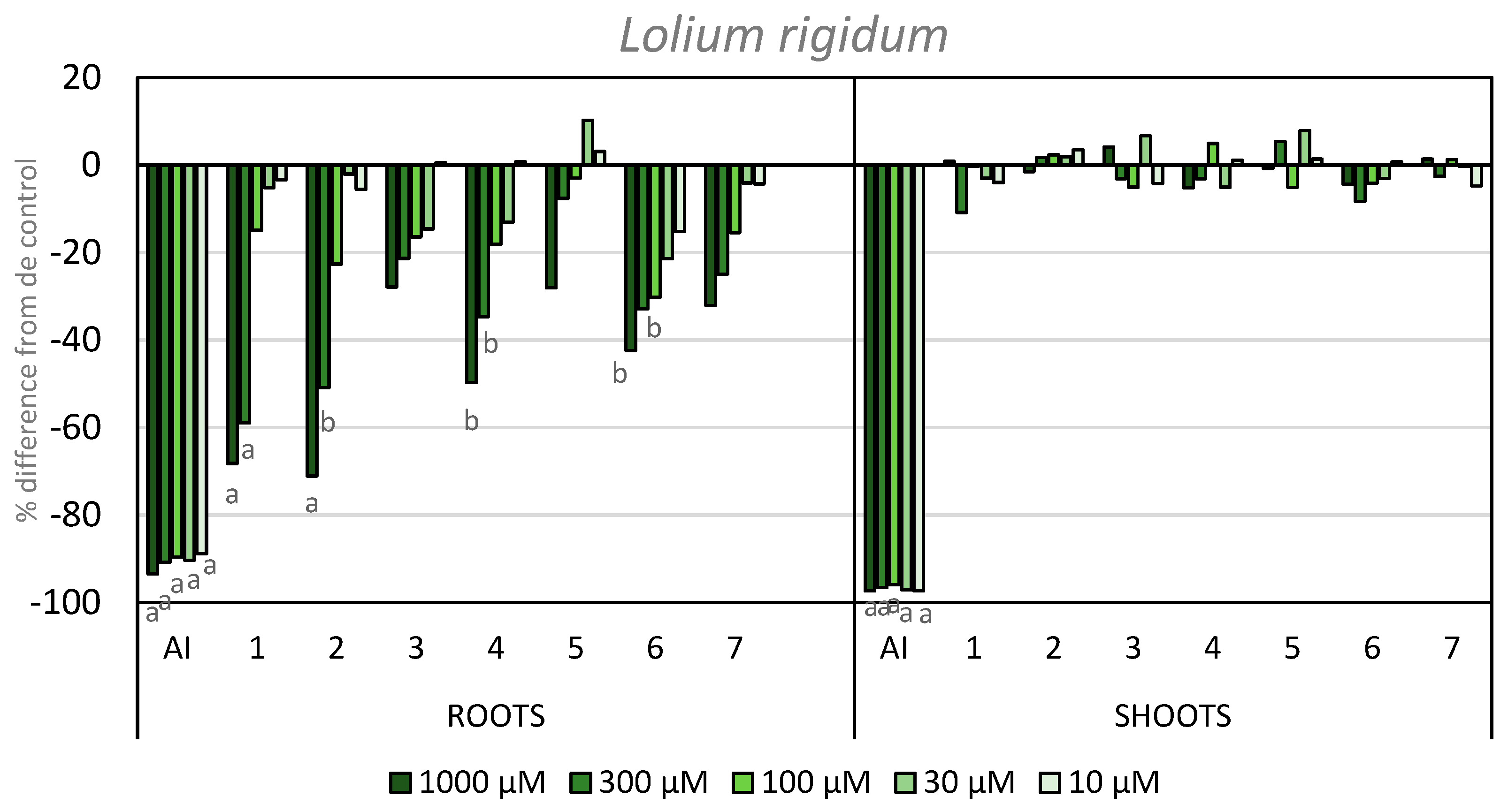
Regarding the activity on the elongation of L. rigidum roots and shoots, none of the tested compounds showed inhibitory activity on the shoot length. In contrast, several compounds did show inhibitory activity on root growth. It is worth highlighting the activity shown by compounds 1 and 2, with 68 and 71% inhibition at the highest concentration, respectively. Furthermore, both of these compounds showed inhibition above 50% at the second concentration tested. Compound 4 also gave an inhibition higher than 50% at its highest concentration. The introduction of an iodo-substituent at the C-4 position improved the inhibition (cf., compounds 6 and 5), with −42% activity at 1000 µM. In a similar way to the coleoptile bioassay, the introduction of different functional groups at the C-8 position did not improve the initial inhibitory activity when compared to APO, although the incorporation of nitrogen heteroatoms into the scaffold of APO did improve activity (Figure 6).
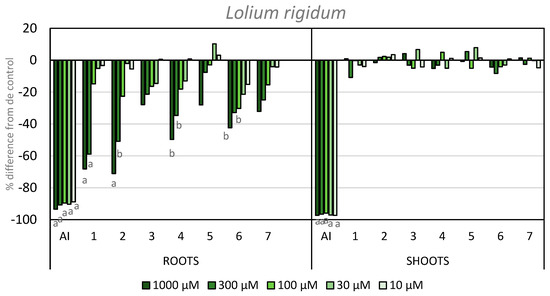
Figure 6.
Effect of the active ingredient of the herbicide Stomp Aqua® (pendimethalin, AI) and the synthesized compounds on the root and shoot length of L. rigidum. Values are expressed as the percent difference from the control. Significance levels p < 0.01 (a) or 0.01 < p< 0.05 (b). There are letters a and b on the graph under the bars. It means the significance levels of our study.
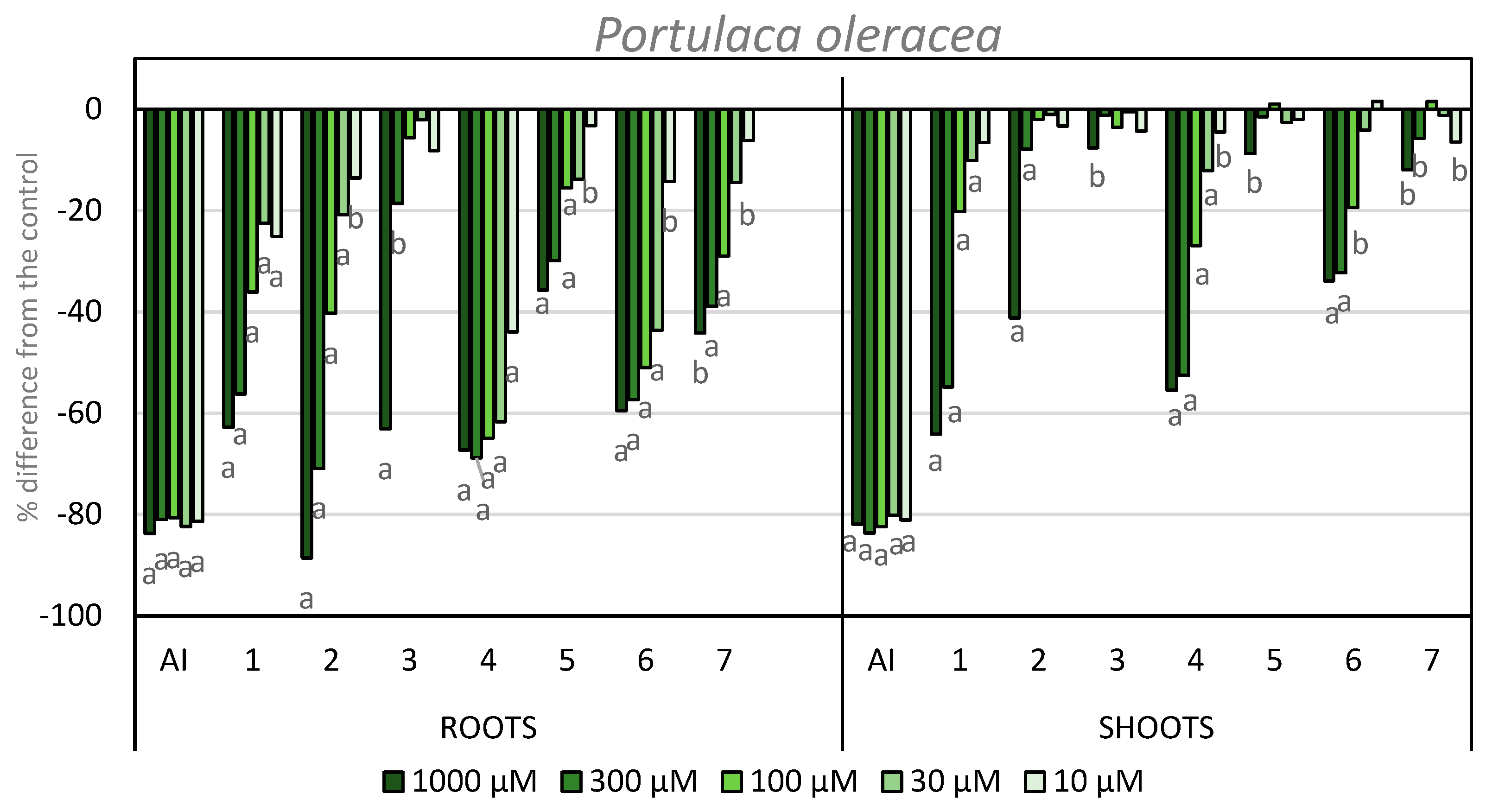
The results for root and shoot length of P. oleracea are shown in Figure 7. The inhibitory activity on the shoots for the products evaluated are higher than on L. rigidum. In this case, the highest activity was found for compounds 1 and 4, which gave inhibition of more than 50% at the two highest concentrations tested. Compound 2 showed −41% activity at its maximum concentration. In the case of compound 5, the same trend was observed as in previous bioassays, i.e., the introduction of an iodo-substituent at the C-4 position (compound 6) imparts a higher activity to the compound. Compounds 5 and 7 were once again inactive.
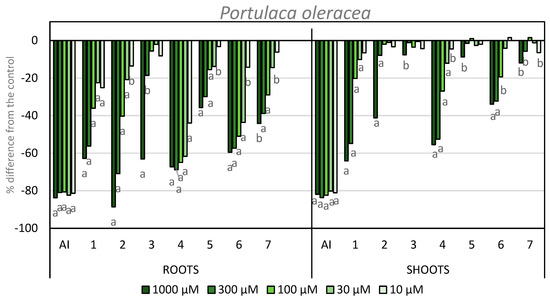
Figure 7.
Effect of the active ingredient of the herbicide Stomp Aqua® (pendimethalin, AI) and the synthesized compounds on the root and shoot length of P. oleracea. Values are expressed as the percent difference from the control. Significance levels p < 0.01 (a) or 0.01 < p< 0.05 (b). There are letters a and b on the graph under the bars. It means the significance levels of our study.
Regarding root growth, the inhibitory activity of compound 2 should be highlighted, as it showed 88% inhibition at the highest concentration tested. Furthermore, this compound showed a higher inhibition than APO (compound 1), which showed 62% inhibition at the highest concentration. This activity was even higher than the activity shown by the positive control. The inhibitory activity of compound 3 at its highest concentration is 63% and this is the highest activity presented by this compound in any bioassays described here. Once again, the inhibition values for compound 6 are higher than those for compound 5. The inhibitory activity exhibited by compound 4 stands out, since these values are maintained at 60% inhibition at the four highest concentrations, and it shows activity even at the lowest concentration. Compound 7 also showed inhibition of around 40% at the first two concentrations tested.
The phytotoxic activity obtained on both weeds showed that compound 2 is the most active example reported here, with an inhibitory activity on the root length higher than that of APO and even higher than the positive control on P. oleracea. It is also worth noting the profile shown by compounds 4 and 6 on P. oleracea, with higher activities at lower concentrations when compared to APO.
4. Conclusions
Regarding the structure–activity relationship (SAR), the results show that the most prominent structural modification is the introduction of nitrogen heteroatoms into the scaffold of the APO molecule (compound 2). It should also be noted that it would be very interesting to introduce other functional groups in the C-4 position, without modifications at the C-8 position of APO in view of the results obtained for compounds 5 and 6. As far as the C-8 position is concerned, it is worth noting that the introduction of an amino group (compound 7) or an acid group (compound 3) did not increase the phytotoxic effects. However, when a nitro group (compound 4) was introduced, the activity on P. oleracea improved at lower concentrations with respect to APO.
In summary, aminophenoxazinones have shown phytotoxic activity on wheat coleoptiles and on two important weeds, L. rigidum and P. oleracea. The most affected parameter on weeds was root length, and P. oleracea showed to be more susceptible to the tested compounds than L. rigidum. The results indicate that the introduction of nitrogen atoms into the heterocycle provides an interesting model scaffold for further research. Modifications at C-4 should also be explored without substitution on C-8. The results described here show that further research should be carried out on the phytotoxic activity of aminophenoxazinones and highlight this family of natural products as lead compounds in the development of natural herbicides.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy13020568/s1, File S1: Wheat coleoptile bioassay protocol; File S2: Phytotoxicity bioassay protocol; Figure S1: Structural characterization of 2-amino-3H-phenoxazin-3-one; Figure S2: Structural characterization of 2-amino-3H-dipyrido [3,2-b:2’,3’-e][1,4]-oxazin-3-one; Figure S3: Structural characterization of 2-amino-3-oxo-3H-phenoxazine-8-carboxylic acid; Figure S4: Structural characterization of 2-amino-8-nitro-3H-phenoxazin-3-one; Figure S5: Structural characterization of 2-amino-8-chloro-3H-phenoxazin-3-one; Figure S6: Structural characterization of 2-amino-8-chloro-4-iodo-3H-phenoxazin-3-one; Figure S7: Structural characterization of 2,8-diamino-3H-phenoxazin-3-one.
Author Contributions
Conceptualization, C.D.-F., C.R. and J.M.G.M.; methodology, C.D.-F., C.R. and R.M.V.; validation, C.D.-F., C.R. and R.M.V.; formal analysis, C.D.-F., C.R. and R.M.V.; investigation, C.D.-F.; resources, F.A.M.; data curation, C.D.-F.; writing—original draft preparation, C.D.-F. and C.R.; writing—review and editing, R.M.V. and J.M.G.M.; visualization, C.D.-F. and C.R.; supervision, R.M.V. and J.M.G.M.; project administration, R.M.V. and J.M.G.M.; funding acquisition, R.M.V. and F.A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Spanish Agencia Estatal de Investigación, grant number (PID2020-115747RB-I00/AEI/10.13039/501100011033) and Conserjería de Universidades, Investigación e Innovación, Junta de Andalucia, grant number (2021-073/PAI/PAIDI/PR).
Acknowledgments
The authors thank FITÓ S.A. (Barcelona, Spain) for supplying wheat seeds.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Scavo, A.; Mauromicale, G. Crop Allelopathy for Sustainable Weed Management in Agroecosystems: Knowing the Present with a View to the Future. Agronomy 2021, 11, 2104. [Google Scholar] [CrossRef]
- Oerke, E.C. Crop Losses to Pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Appleby, A.P.; Muller, F.; Carpy, S. Weed Control. In Agrochemicals; Muller, F., Ed.; Wiley: New York, NY, USA, 2000; pp. 687–707. [Google Scholar]
- Kraehmer, H.; Baur, P. Weed Anatomy; Wiley-Blackwell: Chichester, UK, 2013. [Google Scholar]
- Scavo, A.; Restuccia, A.; Mauromicale, G. Allelopathy: Principles and Basic Aspects for Agroecosystem Control. In Sustainable Agriculture Reviews; Gaba, S., Smith, B., Lichtfouse, E., Eds.; Springer: Cham, Switzerland, 2018; Volume 28, pp. 47–101. [Google Scholar]
- Heap, I. The International Herbicide-Resistant Weed Database [Online]. Available online: www.weedscience.org (accessed on 21 December 2022).
- Gómez de Barreda, D.; Pardo, G.; Osca, J.M.; Catala-Forner, M.; Consola, S.; Garnica, I.; López-Martínez, N.; Palmerín, J.A.; Osuna, M.D. An Overview of Rice Cultivation in Spain and the Management of Herbicide-Resistant Weeds. Agronomy 2021, 11, 1095. [Google Scholar] [CrossRef]
- Macías, F.A.; Durán, A.G.; Molinillo, J.M.G. Allelopathy: The Chemical Language of Plants. In Progress in the Chemistry of Organic Natural Products 112; Kinghorn, A.D., Falk, H., Gibbons, S., Kobayashi, J., Asakawa, Y., Liu, J.K., Eds.; Springer: Cham, Switzerland, 2020; pp. 1–84. [Google Scholar]
- Macías, F.A.; Mejías, F.J.; Molinillo, J.M. Recent Advances in Allelopathy for Weed Control: From Knowledge to Applications. Pest Manag. Sci. 2019, 75, 2413–2436. [Google Scholar] [CrossRef]
- Macías, F.A.; Marín, D.; Oliveros-Bastidas, A.; Molinillo, J.M.G. Rediscovering the Bioactivity and Ecological Role of 1,4-Benzoxazinones. Nat. Prod. Rep. 2009, 26, 478–489. [Google Scholar] [CrossRef]
- Macías, F.A.; Oliveros-Bastidas, A.; Marín, D.; Castellano, D.; Simonet, A.M.; Molinillo, J.M.G. Degradation Studies on Benzoxazinoids. Soil Degradation Dynamics of (2R)-2-O-β-d-Glucopyranosyl-4-Hydroxy-(2H)-1,4-Benzoxazin-3(4H)-One (DIBOA-Glc) and Its Degradation Products, Phytotoxic Allelochemicals from Gramineae. J. Agric. Food Chem. 2005, 53, 554–561. [Google Scholar] [CrossRef]
- Macías, F.A.; Oliveros-Bastidas, A.; Marín, D.; Castellano, D.; Simonet, A.M.; Molinillo, J.M.G. Degradation Studies on Benzoxazinoids. Soil Degradation Dynamics of 2,4-Dihydroxy-7-Methoxy-(2H)-1,4-Benzoxazin-3(4H)-One (DIMBOA) and Its Degradation Products, Phytotoxic Allelochemicals from Gramineae. J. Agric. Food Chem. 2004, 52, 6402–6413. [Google Scholar] [CrossRef]
- Fomsgaard, I.S.; Mortensen, A.G.; Carlsen, S.C.K. Microbial Transformation Products of Benzoxazolinone and Benzoxazinone Allelochemicals––A Review. Chemosphere 2004, 54, 1025–1038. [Google Scholar] [CrossRef]
- Zorrilla, J.G.; Rial, C.; Cabrera, D.; Molinillo, J.M.G.; Varela, R.M.; Macías, F.A. Pharmacological Activities of Aminophenoxazinones. Molecules 2021, 26, 3453. [Google Scholar] [CrossRef]
- Sánchez-Moreiras, A.M.; Coba de la Peña, T.; Martínez, A.; González, L.; Pellisier, F.; Reigosa, M.J. Mode of Action of the Hydroxamic Acid BOA and Other Related Compounds. In Allelopathy; Macías, F.A., Galindo, J.C.G., Molinillo, J.M.G., Cutler, H.G., Eds.; CRC Press LLC: Boca Raton, FL, USA, 2004; pp. 239–252. [Google Scholar]
- Macías, F.A.; Marín, D.; Oliveros-Bastidas, A.; Castellano, D.; Simonet, A.M.; Molinillo, J.M.G. Structure−Activity Relationships (SAR) Studies of Benzoxazinones, Their Degradation Products and Analogues. Phytotoxicity on Standard Target Species (STS). J. Agric. Food Chem. 2005, 53, 538–548. [Google Scholar] [CrossRef]
- Venturelli, S.; Belz, R.G.; Kämper, A.; Berger, A.; von Horn, K.; Wegner, A.; Böcker, A.; Zabulon, G.; Langenecker, T.; Kohlbacher, O.; et al. Plants Release Precursors of Histone Deacetylase Inhibitors to Suppress Growth of Competitors. Plant Cell 2015, 27, 3175–3189. [Google Scholar] [CrossRef]
- He, Y.; Michaels, S.D.; Amasino, R.M. Regulation of Flowering Time by Histone Acetylation in Arabidopsis. Science (1979) 2003, 302, 1751–1754. [Google Scholar] [CrossRef]
- Pasceri, R.; Siegel, D.; Ross, D.; Moody, C.J. Aminophenoxazinones as Inhibitors of Indoleamine 2,3-Dioxygenase (IDO). Synthesis of Exfoliazone and Chandrananimycin A. J. Med. Chem. 2013, 56, 3310–3317. [Google Scholar] [CrossRef]
- Wang, X.; Liu, C.; Ma, Q.-Y.; Tian, Z.-H.; Jiang, H.-Q.; Lv, Q.-T.; Rong, R. A Rapid and Practical Prediction Method for the Arizona Solvent System Family Used in High Speed Countercurrent Chromatography. J. Chromatogr. A 2020, 1629, 461426. [Google Scholar] [CrossRef]
- Rial, C.; Varela, R.M.; Molinillo, J.M.G.; Bautista, E.; Hernández, A.O.; Macías, F.A. Phytotoxicity Evaluation of Sesquiterpene Lactones and Diterpenes from Species of the Decachaeta, Salvia and Podachaenium Genera. Phytochem. Lett. 2016, 18, 68–76. [Google Scholar] [CrossRef]
- Castellano, D.; Macías, F.A.; Castellano, M.; Cambronero, R.M. FITOMED (Automated System for Measurement of Variable Lenghts). Spain Patent P9901565, 15 June 2001. [Google Scholar]
- Macías, F.A.; Marín, D.; Oliveros-Bastidas, A.; Chinchilla, D.; Simonet, A.M.; Molinillo, J.M.G. Isolation and Synthesis of Allelochemicals from Gramineae: Benzoxazinones and Related Compounds. J. Agric. Food Chem. 2006, 54, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Granda, J.; Piekielska, K.; Wąsińska, M.; Kawecka, N.; Giurg, M. Synthesis of 7- and 8-Functionalized 2-Aminophenoxazinones via Cyclocondensation of 2-Aminophenols. Synthesis 2015, 47, 3321–3332. [Google Scholar] [CrossRef]
- Gonçalves, D.; Faria, R.C.; Yonashiro, M.; Bulhões, L.O.S. Electrochemical Oxidation of O-Aminophenol in Aqueous Acidic Medium: Formation of Film and Soluble Products. J. Electroanal. Chem. 2000, 487, 90–99. [Google Scholar] [CrossRef]
- Giurg, M.; Wiech, E.; Piekielska, K.; Gebala, M.; Mlochowski, J.; Wolanski, M.; Ditkowski, B.; Peczynska-Czoch, W. A New Approach to Synthesis of Questiomycin A: Oxidative Cyclocondensation of Ortho-Aminophenol. Pol. J. Chern. 2006, 80, 297–306. [Google Scholar] [CrossRef]
- Tice, C.M. Selecting the Right Compounds for Screening: Does Lipinski’s Rule of 5 for Pharmaceuticals Apply to Agrochemicals? Pest Manag. Sci. 2001, 57, 3–16. [Google Scholar] [CrossRef]
- Noriega, S.; Cardoso-Ortiz, J.; López-Luna, A.; Cuevas-Flores, M.D.R.; Flores De La Torre, J.A. The Diverse Biological Activity of Recently Synthesized Nitro Compounds. Pharmaceuticals 2022, 15, 717. [Google Scholar] [CrossRef]
- Paulai, F.R.; Serrano, S.H.P.; Tavares, L.C. Aspectos Mecanísticos Da Bioatividade e Toxicidade de Nitrocompostos. Quim. Nova 2009, 32, 1013–1020. [Google Scholar] [CrossRef]
- Murphy, C.D. New Frontiers in Biological Halogenation. J. Appl. Microbiol. 2003, 94, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, B.T.; Girish, K.; Channu, B.C.; Thimmaiah, K.N.; Kumara, M.N. Antibacterial Activity of Phenoxazine Derivatives. J. Chem. Pharm. Res. 2015, 7, 1074–1079. [Google Scholar]
- Moloudizargari, M.; Mikaili, P.; Aghajanshakeri, S.; Asghari, M.; Shayegh, J. Pharmacological and Therapeutic Effects of Peganum harmala and Its Main Alkaloids. Pharmacogn. Rev. 2013, 7, 199. [Google Scholar] [CrossRef] [PubMed]
- Uzor, P.F. Alkaloids from Plants with Antimalarial Activity: A Review of Recent Studies. Evid.-Based Complement. Altern. Med. 2020, 2020, 8749083. [Google Scholar] [CrossRef] [PubMed]
- Berthod, A. Countercurrent Chromatography. The Support-Free Liquid Stationary Phase; Barcelo, D., Ed.; Elsevier: Amsterdam, The Netherlands, 2002; Volume 38. [Google Scholar]
- Berthod, A.; Maryutina, T.; Spivakov, B.; Shpigun, O.; Sutherland, I.A. Countercurrent Chromatography in Analytical Chemistry (IUPAC Technical Report). Pure Appl. Chem. 2009, 81, 355–387. [Google Scholar] [CrossRef]
- Marchal, L.; Legrand, J.; Foucault, A. Centrifugal Partition Chromatography: A Survey of Its History, and Our Recent Advances in the Field. Chem. Rec. 2003, 3, 133–143. [Google Scholar] [CrossRef]
- Cutler, S.J.; Hoagland, R.E.; Cutler, H.G. Evaluation of Selected Pharmaceuticals as Potential Herbicides: Bridging the Gap between Agrochemicals and Pharmaceuticals. In Allelopathy in Ecological Agriculture and Forestry; Springer: Dordrecht, The Netherlands, 2000; pp. 129–137. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).