Spatially Explicit Soil Acidification under Optimized Fertilizer Use in Sub-Saharan Africa
Abstract
:1. Introduction
2. Materials and Methods
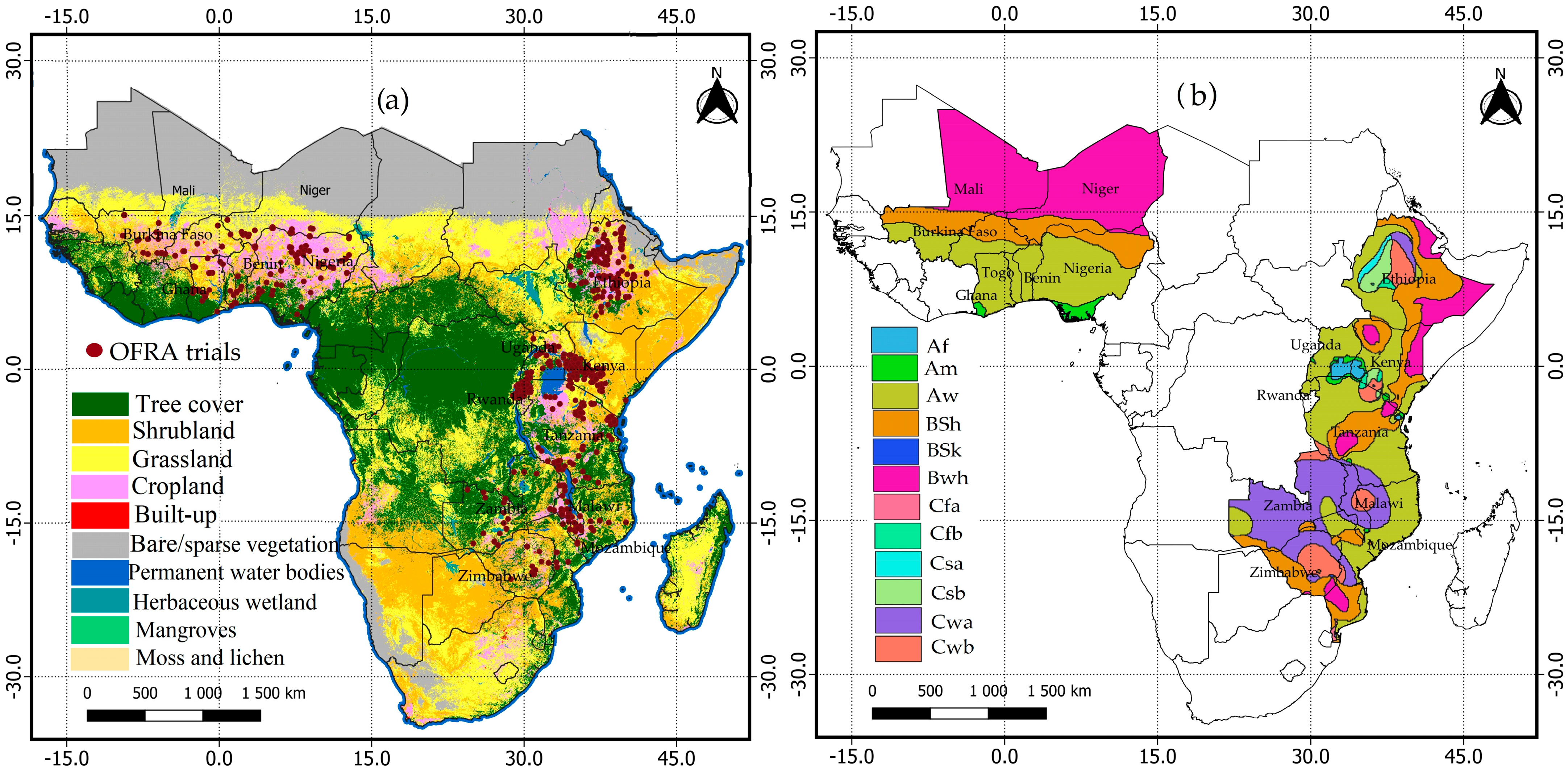
2.1. Site Description
2.2. Calculation of Element Fluxes and Soil Acidification Rates
2.2.1. Assessment of the Nutrient Budget in OFRA Field Trials
2.2.2. Calculation of Soil Acidification (H+) and Change in Total Soil Acid Neutralizing Capacity (ANC)
2.3. Mapping Soil Acidification in Selected Countries in SSA
2.3.1. Covariates Collection and Selection
2.3.2. Model Evaluation, Prediction, and Uncertainties Assessment
2.4. Linear Mixed-Effect Analysis
3. Results
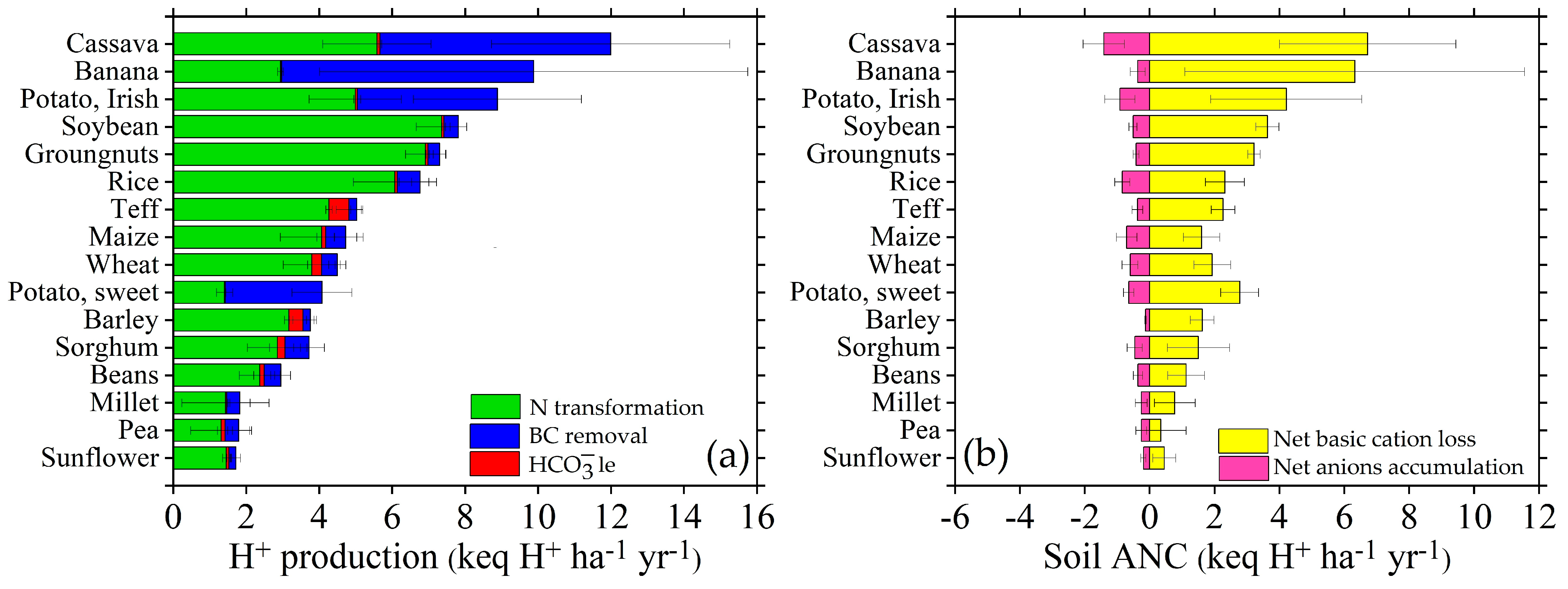
3.1. Descriptive Statistics of Nutrient Fluxes, Soil Acidification, and Total Soil Acid Neutralizing Capacity (ANC) in Different Cropping Systems
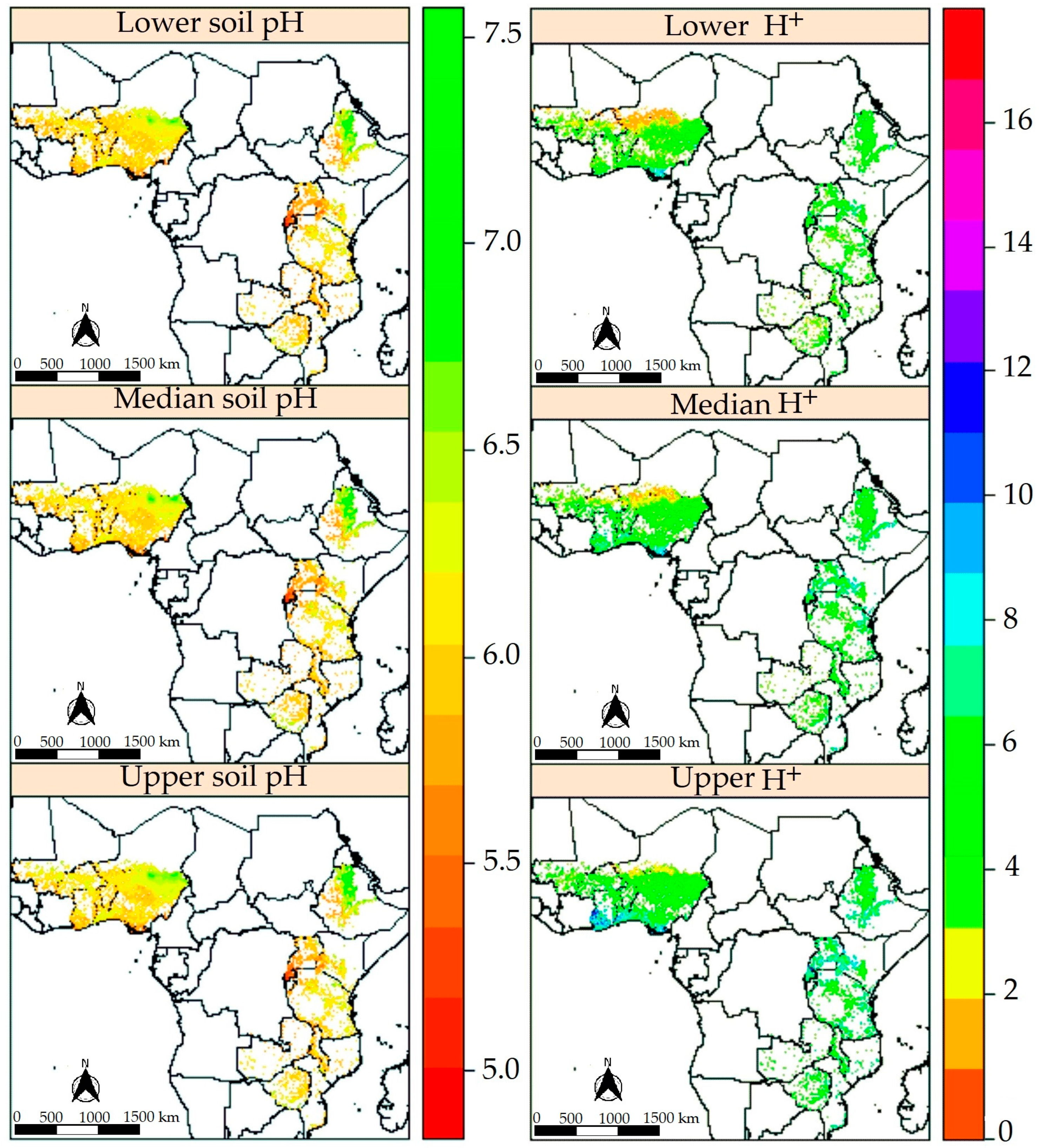
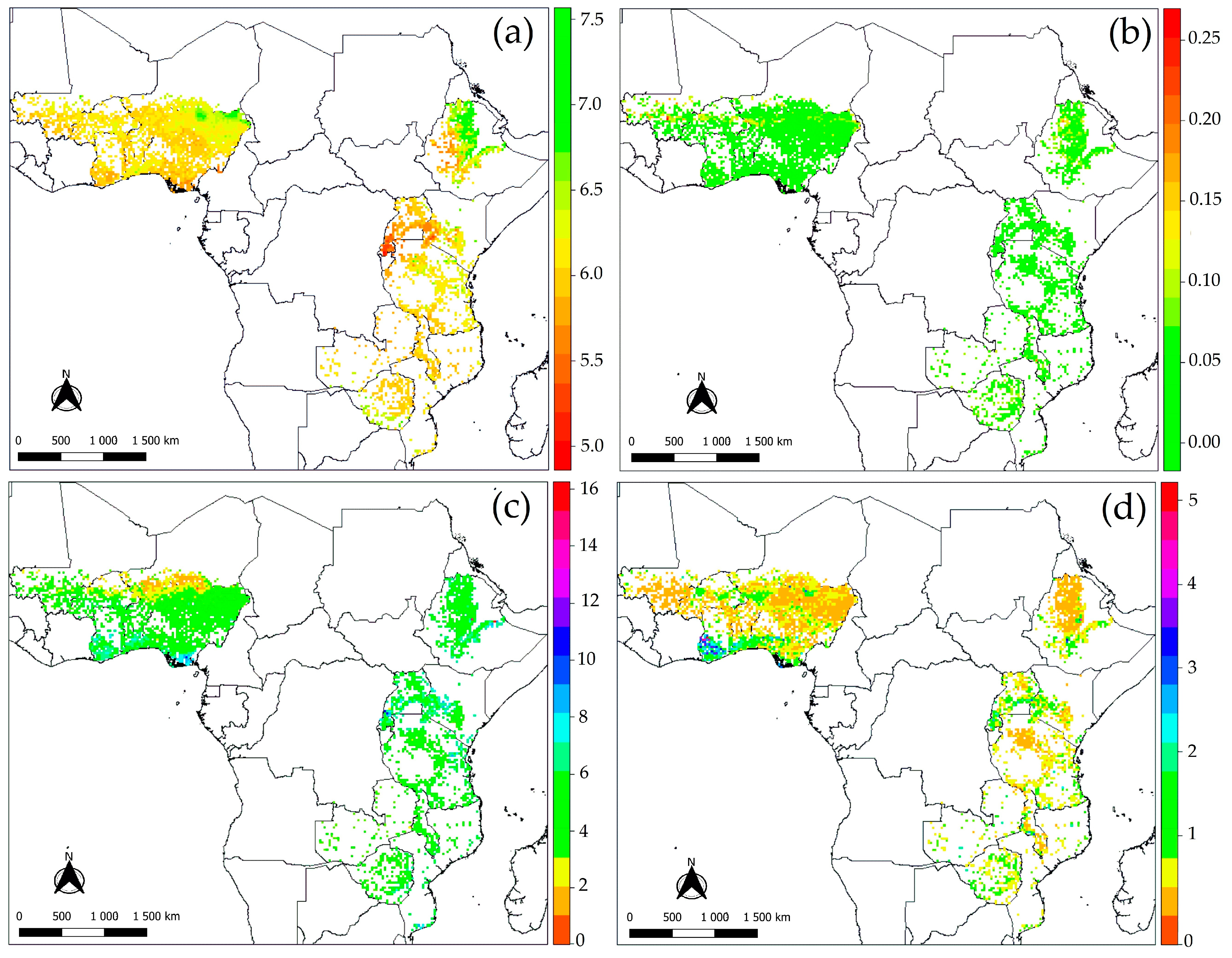
3.2. Spatial Patterns of Soil pH and Soil Acidification
3.2.1. Selected Environmental Covariates and Their Relative Importance
3.2.2. Cross Validation Results
3.2.3. Predictions of Soil pH, Produced Protons (H+), and Their Uncertainties
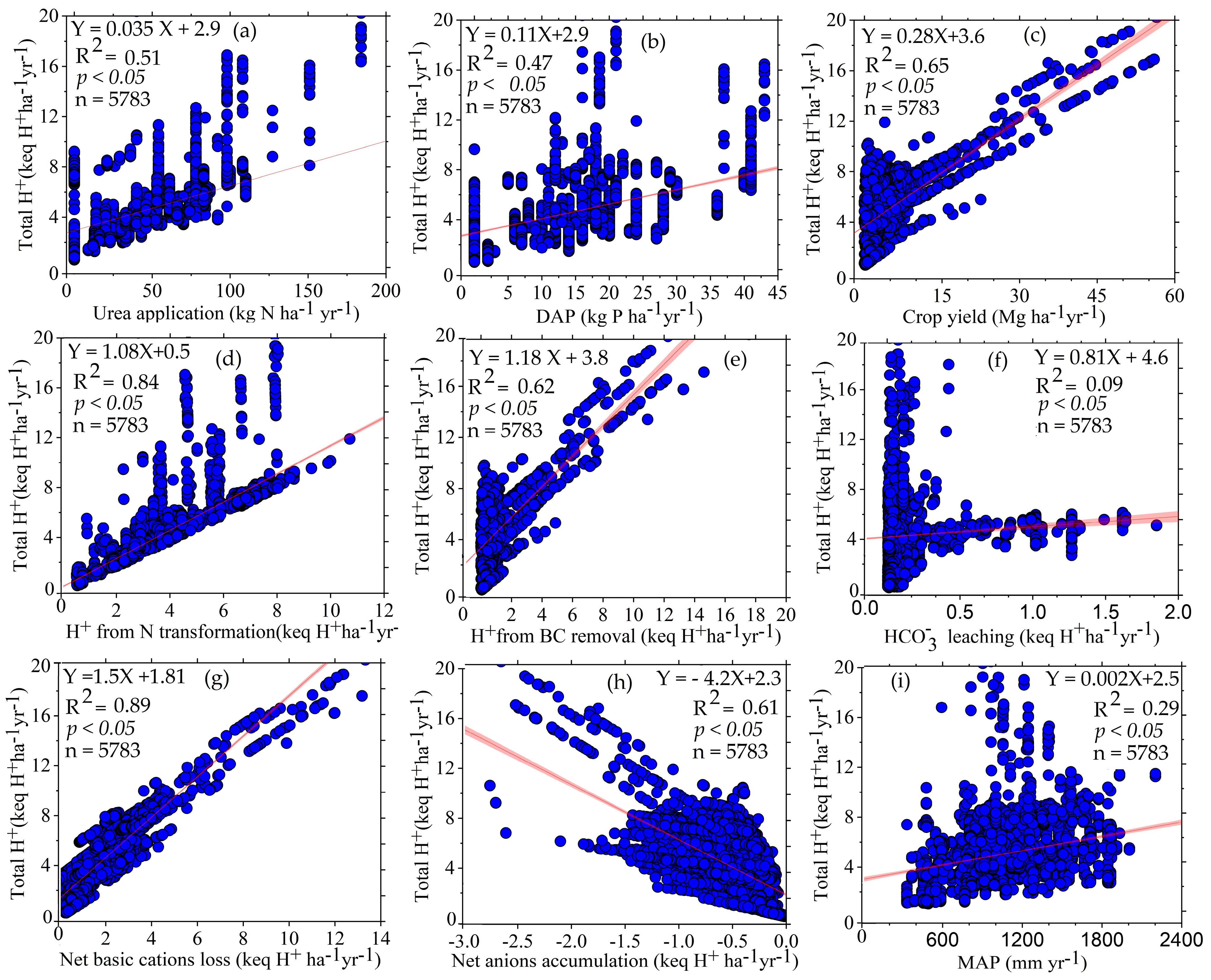
3.3. Relationship between Protons Produced (H+) and Its Factors
4. Discussion
4.1. N Transformation and Basic Cation Budget
4.2. Model Performances, Soil Acidification Patterns, and Uncertainties Assessment
4.3. Implications and Practical Recommendations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- De Pauw, E.F. The Management of Acid Soils in Africa. Outlook Agric. 1994, 23, 11–16. [Google Scholar] [CrossRef]
- Sanchez, P.A. Soils of the Tropics. In Properties and Management of Soils in the Tropics; Cambridge University Press: Cambridge, UK, 2019; pp. 82–119. [Google Scholar] [CrossRef]
- Weil, R.R.; Brady, N.C. The Nature and Properties of Soils, 15th ed.; Pearson: Columbus, OH, USA, 2016; ISBN 978-0-13-325448-8. [Google Scholar]
- Bolan, N.S.; Hedley, M.J.; White, R.E. Processes of soil acidification during nitrogen cycling with emphasis on legume based pastures. Plant Soil 1991, 134, 53–63. [Google Scholar] [CrossRef]
- Helyar, K.; Porter, W. Soil Acidification, its Measurement and the Processes Involved. In Soil Acidity and Plant Growth; Elsevier: Amsterdam, The Netherlands, 1989; pp. 61–101. [Google Scholar] [CrossRef]
- Hedley, M.; Bolan, N. Role of Carbon, Nitrogen, and Sulfur Cycles in Soil Acidification. In Handbook of Soil Acidity; Taylor Francis Group: Abingdon, UK, 2003. [Google Scholar] [CrossRef]
- CaiXan, T.; Rengel, Z. Role of Plant Cation/Anion Uptake Ratio in Soil Acidification. In Handbook of Soil Acidity; Taylor Francis Group: Abingdon, UK, 2003. [Google Scholar]
- Vanlauwe, B.; Kihara, J.; Chivenge, P.; Pypers, P.; Coe, R.; Six, J. Agronomic use efficiency of N fertilizer in maize-based systems in sub-Saharan Africa within the context of integrated soil fertility management. Plant Soil 2010, 339, 35–50. [Google Scholar] [CrossRef]
- Takala, B. Soil Acidity and Its Management Options in Western Ethiopia: Review. J. Environ. Earth Sci. 2019, 9, 27–35. [Google Scholar] [CrossRef]
- Zingore, S.; Mutegi, J.K.; Agesa, B.; Tamene, L.; Kihara, J.; Zingore, S.; Mutegi, J.K.; Agesa, B.; Tamene, L.; Kihara, J.; et al. Soil Degradation in Sub-Saharan Africa and Crop Production Options for Soil Rehabilitation. Better Crops 2015, 99, 24–26. [Google Scholar]
- Chianu, J.N.; Chianu, J.N.; Mairura, F. Mineral fertilizers in the farming systems of sub-Saharan Africa. A review. Agron. Sustain. Dev. 2011, 32, 545–566. [Google Scholar] [CrossRef] [Green Version]
- Wortmann, C.S.; Milner, M.; Kaizzi, K.C.; Nouri, M.; Cyamweshi, A.R.; Dicko, M.K.; Kibunja, C.N.; Macharia, M.; Maria, R.; Nalivata, P.C.; et al. Maize-nutrient response information applied across Sub-Saharan Africa. Nutr. Cycl. Agroecosyst. 2017, 107, 175–186. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, B.; Xu, M.; Zhang, H.; He, X.; Zhang, L.; Gao, S. Intensified soil acidification from chemical N fertilization and prevention by manure in an 18-year field experiment in the red soil of southern China. J. Soils Sediments 2014, 15, 260–270. [Google Scholar] [CrossRef]
- Sanchez, P.A. Properties and Management of Soils in the Tropics. Soil Sci. 1977, 124, 187. [Google Scholar] [CrossRef]
- Abruna, F.; Pearson, R.W.; Elkins, C.B. Quantitative Evaluation of Soil Reaction and Base Status Changes Resulting from Field Application of Residually Acid-Forming Nitrogen Fertilizers. Soil Sci. Soc. Am. J. 1958, 22, 539–542. [Google Scholar] [CrossRef]
- Juo, A.S.R.; Dabiri, A.; Franzluebbers, K. Acidification of a kaolinitic Alfisol under continuous cropping with nitrogen fertilization in West Africa. Plant Soil 1995, 171, 245–253. [Google Scholar] [CrossRef]
- Stumpe, J.M.; Vlek, P.L.G. Acidification Induced by Different Nitrogen Sources in Columns of Selected Tropical Soils. Soil Sci. Soc. Am. J. 1991, 55, 145–151. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, Y. Spatial Variability of Soil Chemical Properties in a Jujube Slope on the Loess Plateau of China. Soil Sci. 2011, 176, 550–558. [Google Scholar] [CrossRef]
- Hengl, T.; Miller, M.A.E.; Križan, J.; Shepherd, K.D.; Sila, A.; Kilibarda, M.; Antonijević, O.; Glušica, L.; Dobermann, A.; Haefele, S.M.; et al. African soil properties and nutrients mapped at 30 m spatial resolution using two-scale ensemble machine learning. Sci. Rep. 2021, 11, 6130. [Google Scholar] [CrossRef]
- Hounkpatin, K.O.; Bossa, A.Y.; Yira, Y.; Igue, M.A.; Sinsin, B.A. Assessment of the soil fertility status in Benin (West Africa)—Digital soil mapping using machine learning. Geoderma Reg. 2021, 28, e00444. [Google Scholar] [CrossRef]
- Kamamia, A.W.; Vogel, C.; Mwangi, H.M.; Feger, K.-H.; Sang, J.; Julich, S. Mapping soil aggregate stability using digital soil mapping: A case study of Ruiru reservoir catchment, Kenya. Geoderma Reg. 2021, 24, e00355. [Google Scholar] [CrossRef]
- Kempen, B.; Dalsgaard, S.; Kaaya, A.K.; Chamuya, N.; Ruipérez-González, M.; Pekkarinen, A.; Walsh, M.G. Mapping topsoil organic carbon concentrations and stocks for Tanzania. Geoderma 2019, 337, 164–180. [Google Scholar] [CrossRef]
- Leenaars, J.; Elias, E.; Wösten, J.; Ruiperez-González, M.; Kempen, B. Mapping the major soil-landscape resources of the Ethiopian Highlands using random forest. Geoderma 2019, 361, 114067. [Google Scholar] [CrossRef]
- Sindayihebura, A.; Ottoy, S.; Dondeyne, S.; Van Meirvenne, M.; Van Orshoven, J. Comparing digital soil mapping techniques for organic carbon and clay content: Case study in Burundi’s central plateaus. Catena 2017, 156, 161–175. [Google Scholar] [CrossRef]
- Leenaars, J.G.; Claessens, L.; Heuvelink, G.B.; Hengl, T.; González, M.R.; van Bussel, L.G.; Guilpart, N.; Yang, H.; Cassman, K.G. Mapping rootable depth and root zone plant-available water holding capacity of the soil of sub-Saharan Africa. Geoderma 2018, 324, 18–36. [Google Scholar] [CrossRef]
- Uwiragiye, Y.; Ngaba, M.J.Y.; Zhao, M.; Elrys, A.S.; Heuvelink, G.B.; Zhou, J. Modelling and mapping soil nutrient depletion in humid highlands of East Africa using ensemble machine learning: A case study from Rwanda. Catena 2022, 217, 106499. [Google Scholar] [CrossRef]
- Dewitte, O.; Jones, A.; Spaargaren, O.; Breuning-Madsen, H.; Brossard, M.; Dampha, A.; Deckers, J.; Gallali, T.; Hallett, S.; Jones, R.; et al. Harmonisation of the soil map of Africa at the continental scale. Geoderma 2013, 211–212, 138–153. [Google Scholar] [CrossRef] [Green Version]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef] [Green Version]
- Meyer, H.; Pebesma, E. Predicting into unknown space? Estimating the area of applicability of spatial prediction models. Methods Ecol. Evol. 2021, 12, 1620–1633. [Google Scholar] [CrossRef]
- Zanaga, D.; van de Kerchove, R.; de Keersmaecker, W.; Souverijns, N.; Brockmann, C.; Quast, R.; Wevers, J.; Grosu, A.C.; Paccini, A.; Vergnaud, S.; et al. ESA WorldCover 10 m 2020 V100; International Institute for Applied Systems Analysis: Laxenburg, Austria, 2021. [Google Scholar]
- Ghimire, R.; Machado, S.; Bista, P. Soil pH, Soil Organic Matter, and Crop Yields in Winter Wheat–Summer Fallow Systems. Agron. J. 2017, 109, 706–717. [Google Scholar] [CrossRef] [Green Version]
- Laouali, D.; Delon, C.; Adon, M.; Ndiaye, O.; Saneh, I.; Gardrat, E.; Dias-Alves, M.; Tagesson, T.; Fensohlt, R.; Galy-Lacaux, C. Source contributions in precipitation chemistry and analysis of atmospheric nitrogen deposition in a Sahelian dry savanna site in West Africa. Atmos. Res. 2021, 251, 105423. [Google Scholar] [CrossRef]
- FAO; Lesschen, J.P.; Stoorvogel, J.; Smaling, E. FAO Scaling Soil Nutrient Balances. Enabling Mesolevel Applications for African Realities; FAO: Rome, Italy, 2004. [Google Scholar]
- Zhu, Q.; de Vries, W.; Liu, X.; Hao, T.; Zeng, M.; Shen, J.; Zhang, F. Enhanced acidification in Chinese croplands as derived from element budgets in the period 1980–2010. Sci. Total. Environ. 2018, 618, 1497–1505. [Google Scholar] [CrossRef] [Green Version]
- Tarkalson, D.D.; Payero, J.O.; Hergert, G.W.; Cassman, K.G. Acidification of Soil in a Dry Land Winter Wheat-sorghum/corn-fallow Rotation in the Semiarid U.S. Great Plains. Plant Soil 2006, 283, 367–379. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Q.; Liu, X.; Hao, T.; Zeng, M.; Shen, J.; Zhang, F.; De Vries, W. Modeling soil acidification in typical Chinese cropping systems. Sci. Total. Environ. 2018, 613–614, 1339–1348. [Google Scholar] [CrossRef] [Green Version]
- Hao, T.; Liu, X.; Zhu, Q.; Zeng, M.; Chen, X.; Yang, L.; Shen, J.; Shi, X.; Zhang, F.; de Vries, W. Quantifying drivers of soil acidification in three Chinese cropping systems. Soil Tillage Res. 2021, 215, 105230. [Google Scholar] [CrossRef]
- De Vries, W.; Breeuwsma, A. The relation between soil acidification and element cycling. Water Air Soil Pollut. 1987, 35, 293–310. [Google Scholar] [CrossRef]
- Dong, Y.; Yang, J.-L.; Zhao, X.-R.; Yang, S.-H.; Zhang, G.-L. Contribution of different proton sources to the acidification of red soil with maize cropping in subtropical China. Geoderma 2021, 392, 114995. [Google Scholar] [CrossRef]
- McBratney, A.; Santos, M.M.; Minasny, B. On digital soil mapping. Geoderma 2003, 117, 3–52. [Google Scholar] [CrossRef]
- Meyer, H.; Reudenbach, C.; Hengl, T.; Katurji, M.; Nauss, T. Improving performance of spatio-temporal machine learning models using forward feature selection and target-oriented validation. Environ. Model. Softw. 2018, 101, 1–9. [Google Scholar] [CrossRef]
- Deane-Mayer, Z.A.; Knowles, J. Ensembles of Caret Models, R Package CaretEnsemble, Version 2.0.1. 2019. Available online: https://cran.r-project.org/web/packages/caretEnsemble/caretEnsemble.pdf (accessed on 26 January 2023).
- Arrouays, D.; Grundy, M.G.; Hartemink, A.E.; Hempel, J.W.; Heuvelink, G.B.; Hong, S.Y.; Lagacherie, P.; Lelyk, G.; McBratney, A.B.; McKenzie, N.J.; et al. GlobalSoilMap. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2014; pp. 93–134. [Google Scholar] [CrossRef]
- Hengl, T.; Nussbaum, M.; Wright, M.N.; Heuvelink, G.B.M.; Gräler, B. Random forest as a generic framework for predictive modeling of spatial and spatio-temporal variables. PeerJ 2018, 6, e5518. [Google Scholar] [CrossRef] [Green Version]
- Poggio, L.; de Sousa, L.M.; Batjes, N.H.; Heuvelink, G.B.M.; Kempen, B.; Ribeiro, E.; Rossiter, D. SoilGrids 2.0: Producing soil information for the globe with quantified spatial uncertainty. Soil 2021, 7, 217–240. [Google Scholar] [CrossRef]
- Berge, H.T.; Hijbeek, R.; van Loon, M.; Rurinda, J.; Tesfaye, K.; Zingore, S.; Craufurd, P.; van Heerwaarden, J.; Brentrup, F.; Schröder, J.; et al. Maize crop nutrient input requirements for food security in sub-Saharan Africa. Glob. Food Secur. 2019, 23, 9–21. [Google Scholar] [CrossRef]
- Guo, J.H.; Liu, X.J.; Zhang, Y.; Shen, J.L.; Han, W.X.; Zhang, W.F.; Christie, P.; Goulding, K.W.T.; Vitousek, P.M.; Zhang, F.S. Significant Acidification in Major Chinese Croplands. Science 2010, 327, 1008–1010. [Google Scholar] [CrossRef] [Green Version]
- Bolan, N.; Sarmah, A.K.; Bordoloi, S.; Bolan, S.; Padhye, L.P.; Van Zwieten, L.; Sooriyakumar, P.; Khan, B.A.; Ahmad, M.; Solaiman, Z.M.; et al. Soil acidification and the liming potential of biochar. Environ. Pollut. 2023, 317, 120632. [Google Scholar] [CrossRef]
- Elrys, A.S.; Uwiragiye, Y.; Zhang, Y.; Abdel-Fattah, M.K.; Chen, Z.-X.; Zhang, H.-M.; Meng, L.; Wang, J.; Zhu, T.-B.; Cheng, Y.; et al. Expanding agroforestry can increase nitrate retention and mitigate the global impact of a leaky nitrogen cycle in croplands. Nat. Food 2022, 4, 109–121. [Google Scholar] [CrossRef]
- Dong, Y.; Yang, J.-L.; Zhao, X.-R.; Yang, S.-H.; Mulder, J.; Dörsch, P.; Peng, X.-H.; Zhang, G.-L. Soil acidification and loss of base cations in a subtropical agricultural watershed. Sci. Total Environ. 2022, 827, 154338. [Google Scholar] [CrossRef]
- Randall, P.J.; Abaidoo, R.C.; Hocking, P.J.; Sanginga, N. Mineral Nutrient Uptake and Removal By Cowpea, Soybean and Maize Cultivars in West Africa, and Implications for Carbon Cycle Effects on Soil Acidification. Exp. Agric. 2006, 42, 475–494. [Google Scholar] [CrossRef]
- Poss, R.; Saragoni, H. Leaching of nitrate, calcium and magnesium under maize cultivation on an oxisol in Togo. Nutr. Cycl. Agroecosyst. 1992, 33, 123–133. [Google Scholar] [CrossRef]
- Oikeh, S.O.; Chude, V.O.; Carsky, R.J.; Weber, G.K.; Horst, W.J. Legume Rotation in The Moist Tropical Savanna: Managing Soil Nitrogen Dynamics and Cereal Yields in Farmers’ Fields. Exp. Agric. 1998, 34, 73–83. [Google Scholar] [CrossRef]
- Kunhikrishnan, A.; Thangarajan, R.; Bolan, N.S.; Xu, Y.; Mandal, S.; Gleeson, D.B.; Seshadri, B.; Zaman, M.; Barton, L.; Tang, C.; et al. Chapter One—Functional Relationships of Soil Acidification, Liming, and Greenhouse Gas Flux. In Advances in Agronomy; Sparks, D.L., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 139, pp. 1–71. [Google Scholar]
- Rowell, D.L.; Wild, A. Causes of soil acidification: A summary. Soil Use Manag. 1985, 1, 32–33. [Google Scholar] [CrossRef]
- Hao, T.; Zhu, Q.; Zeng, M.; Shen, J.; Shi, X.; Liu, X.; Zhang, F.; de Vries, W. Impacts of nitrogen fertilizer type and application rate on soil acidification rate under a wheat-maize double cropping system. J. Environ. Manag. 2020, 270, 110888. [Google Scholar] [CrossRef]
- Zhou, T.; Geng, Y.; Chen, J.; Pan, J.; Haase, D.; Lausch, A. High-resolution digital mapping of soil organic carbon and soil total nitrogen using DEM derivatives, Sentinel-1 and Sentinel-2 data based on machine learning algorithms. Sci. Total Environ. 2020, 729, 138244. [Google Scholar] [CrossRef]
- Hengl, T.; De Jesus, J.M.; Heuvelink, G.B.M.; Gonzalez, M.R.; Kilibarda, M.; Blagotić, A.; Shangguan, W.; Wright, M.N.; Geng, X.; Bauer-Marschallinger, B.; et al. SoilGrids250m: Global gridded soil information based on machine learning. PLoS ONE 2017, 12, e0169748. [Google Scholar] [CrossRef] [Green Version]
- Hounkpatin, O.K.; de Hipt, F.O.; Bossa, A.Y.; Welp, G.; Amelung, W. Soil organic carbon stocks and their determining factors in the Dano catchment (Southwest Burkina Faso). Catena 2018, 166, 298–309. [Google Scholar] [CrossRef]
- Mponela, P.; Snapp, S.; Villamor, G.B.; Tamene, L.; Le, Q.B.; Borgemeister, C. Digital soil mapping of nitrogen, phosphorus, potassium, organic carbon and their crop response thresholds in smallholder managed escarpments of Malawi. Appl. Geogr. 2020, 124, 102299. [Google Scholar] [CrossRef]
- Heuvelink, G.B.M.; Angelini, M.E.; Poggio, L.; Bai, Z.; Batjes, N.H.; Van den Bosch, R.; Bossio, D.; Estella, S.; Lehmann, J.; Olmedo, G.F.; et al. Machine learning in space and time for modelling soil organic carbon change. Eur. J. Soil Sci. 2020, 72, 1607–1623. [Google Scholar] [CrossRef]
- Raza, S.; Zamanian, K.; Ullah, S.; Kuzyakov, Y.; Virto, I.; Zhou, J. Inorganic carbon losses by soil acidification jeopardize global efforts on carbon sequestration and climate change mitigation. J. Clean. Prod. 2021, 315, 128036. [Google Scholar] [CrossRef]
- Rengel, Z.; Tang, C.; Raphael, C.; Bowden, J.W. Understanding subsoil acidification: Effect of nitrogen transformation and nitrate leaching. Soil Res. 2000, 38, 837–849. [Google Scholar] [CrossRef]
- Adams, F.; Pearson, R.W. Neutralizing Soil Acidity under Bermudagrass Sod. Soil Sci. Soc. Am. J. 1969, 33, 737–742. [Google Scholar] [CrossRef]
- Noble, A.D.; Bramley, R.G.V.; Wood, A.W.; Hurney, A.P. Sugarcane and Soil Acidity—Why We Should Be Worried? In Proceedings of the ASSCT—Australian Society of Sugar Cane Technologists, Cairns, QL, Australia, 29 April–2 May 1997. [Google Scholar]
- Wang, N.; Xu, R.-K.; Li, J.-Y. Amelioration of an acid ultisol by agricultural by-products. Land Degrad. Dev. 2010, 22, 513–518. [Google Scholar] [CrossRef]
- Kochian, L.V.; Piñeros, M.A.; Liu, J.; Magalhaes, J.V. Plant Adaptation to Acid Soils: The Molecular Basis for Crop Aluminum Resistance. Annu. Rev. Plant Biol. 2015, 66, 571–598. [Google Scholar] [CrossRef]
- Tang, C.; Diatloff, E.; Rengel, Z.; McGann, B. Growth response to subsurface soil acidity of wheat genotypes differing in aluminium tolerance. Plant Soil 2001, 236, 1–10. [Google Scholar] [CrossRef]
- Sánchez, P.A.; Salinas, J.G. Low-Input Technology for Managing Oxisols and Ultisols in Tropical America. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 1981; Volume 34, pp. 279–406. [Google Scholar] [CrossRef]
- Ghorbani, M.; Amirahmadi, E.; Zamanian, K. In-situ biochar production associated with paddies: Direct involvement of farmers in greenhouse gases reduction policies besides increasing nutrients availability and rice production. Land Degrad. Dev. 2021, 32, 3893–3904. [Google Scholar] [CrossRef]
- Shi, R.-Y.; Ni, N.; Nkoh, J.N.; Li, J.-Y.; Xu, R.-K.; Qian, W. Beneficial dual role of biochars in inhibiting soil acidification resulting from nitrification. Chemosphere 2019, 234, 43–51. [Google Scholar] [CrossRef]
- Hengl, T.; Heuvelink, G.B.M.; Kempen, B.; Leenaars, J.G.B.; Walsh, M.G.; Shepherd, K.D.; Sila, A.; MacMillan, R.A.; De Jesus, J.M.; Tamene, L.; et al. Mapping Soil Properties of Africa at 250 m Resolution: Random Forests Significantly Improve Current Predictions. PLoS ONE 2015, 10, e0125814. [Google Scholar] [CrossRef]
- Zomer, R.J.; Xu, J.; Trabucco, A. Version 3 of the Global Aridity Index and Potential Evapotranspiration Database. Sci. Data 2022, 9, 409. [Google Scholar] [CrossRef]
- Hartmann, J.; Moosdorf, N. Global Lithological Map Database v1.0 (Gridded to 0.5° Spatial Resolution). PANGAEA 2012. [Google Scholar] [CrossRef]
- Nguyen, P.; Shearer, E.J.; Tran, H.; Ombadi, M.; Hayatbini, N.; Palacios, T.; Huynh, P.; Braithwaite, D.; Updegraff, G.; Hsu, K.; et al. The CHRS Data Portal, an Easily Accessible Public Repository for PERSIANN Global Satellite Precipitation Data. Sci. Data 2019, 6, 180296. [Google Scholar] [CrossRef] [Green Version]
- Bouwman, A.F. Direct Emission of Nitrous Oxide from Agricultural Soils. Nutr. Cycl. Agroecosystems 1996, 46, 53–70. [Google Scholar] [CrossRef]
- Galloway, J.N.; Dentener, F.J.; Capone, D.G.; Boyer, E.W.; Howarth, R.W.; Seitzinger, S.P.; Asner, G.P.; Cleveland, C.C.; Green, P.A.; Holland, E.; et al. Nitrogen Cycles: Past, Present, and Future. Biogeochemistry 2004, 70, 153–226. [Google Scholar] [CrossRef]


| Parameters (n = 5783) | Mean | Std | Min | Max |
|---|---|---|---|---|
| pH | 6.02 | 0.54 | 4.90 | 8.00 |
| Exchangeable acidity (Al3+ + H+) (cmol kg−1) | 1.12 | 1.41 | 0.00 | 7.21 |
| Exchangeable base (cmol kg−1) | 13.44 | 8.79 | 2.00 | 51.00 |
| Cation exchange capacity CEC (cmol kg−1) | 19.31 | 12.02 | 3.00 | 65.00 |
| Bulk density (kg m3) | 1289.93 | 150.15 | 895.00 | 1720.00 |
| Clay (%) | 29.72 | 12.47 | 4.00 | 61.00 |
| Sand (%) | 47.15 | 16.67 | 17.00 | 87.00 |
| Silt (%) | 23.14 | 7.23 | 7.00 | 42.00 |
| Soil organic carbon (%) | 2.19 | 1.46 | 0.20 | 10.70 |
| Calcium carbonate (cmol kg−1) | 3.54 | 5.90 | 0.00 | 46.67 |
| Mean annual precipitation (mm yr−1) | 1093.02 | 359.24 | 331.00 | 2202.00 |
| Crop evapotranspiration (mm yr−1) | 158.81 | 38.18 | 78.67 | 255.75 |
| Cropping Systems | Urea (kg ha−1yr−1) | DAP (kg ha−1yr−1) | KCl (kg ha−1yr−1) | Manure (kg ha−1yr−1) | BC Dep (kg ha−1yr−1) | N Dep (kg ha−1yr−1) | S Dep (kg ha−1yr−1) | N Fix (kg ha−1yr−1) | Yield (Mg ha−1yr−1) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Std | Mean | Std | Mean | Std | Mean | Std | Mean | Std | Mean | Std | Mean | Std | Mean | Std | Mean | Std | |
| Banana | 40.00 | 4.50 | 16.00 | 2.50 | 39.00 | 2.40 | 228.30 | 20.50 | 19.80 | 2.58 | 5.03 | 0.66 | 10.68 | 1.39 | 5.00 | 0.00 | 20.82 | 17.69 |
| Barley | 40.31 | 1.91 | 19.87 | 0.80 | 0.00 | 0.00 | 172.03 | 20.46 | 17.79 | 6.14 | 4.52 | 1.56 | 9.59 | 3.31 | 5.00 | 0.00 | 2.82 | 1.42 |
| Beans | 35.62 | 33.68 | 16.20 | 4.72 | 11.27 | 12.89 | 272.15 | 73.30 | 22.31 | 6.66 | 5.67 | 1.69 | 12.03 | 3.59 | 5.00 | 0.00 | 1.35 | 0.93 |
| Cassava | 45.72 | 13.91 | 18.94 | 11.28 | 28.56 | 9.02 | 170.12 | 74.01 | 22.54 | 4.33 | 5.73 | 1.10 | 12.16 | 2.33 | 5.00 | 0.00 | 29.14 | 15.04 |
| Groundnut | 4.22 | 17.57 | 11.47 | 8.45 | 10.08 | 8.78 | 146.49 | 87.26 | 16.56 | 5.27 | 4.21 | 1.34 | 8.93 | 2.84 | 80.00 | 0.00 | 1.07 | 0.58 |
| Maize | 68.79 | 21.52 | 16.03 | 11.93 | 14.86 | 13.13 | 240.78 | 161.53 | 21.22 | 5.53 | 5.39 | 1.41 | 11.44 | 2.98 | 5.00 | 0.00 | 3.82 | 1.92 |
| Millet | 17.51 | 24.22 | 6.84 | 6.35 | 0.00 | 0.00 | 127.22 | 96.29 | 14.99 | 8.33 | 3.81 | 2.12 | 8.09 | 4.49 | 5.00 | 0.00 | 1.42 | 0.91 |
| Peas | 10.66 | 19.86 | 5.37 | 7.61 | 10.14 | 8.68 | 143.78 | 176.40 | 13.41 | 5.19 | 3.41 | 1.32 | 7.23 | 2.80 | 5.00 | 0.00 | 0.98 | 0.93 |
| Potato, Irish | 68.29 | 33.06 | 26.97 | 14.54 | 32.86 | 14.86 | 336.44 | 72.68 | 22.85 | 5.00 | 5.81 | 1.27 | 12.32 | 2.70 | 5.00 | 0.00 | 17.06 | 10.21 |
| Rice | 80.90 | 22.83 | 20.66 | 9.56 | 12.88 | 17.31 | 226.72 | 108.83 | 19.29 | 5.11 | 4.90 | 1.30 | 10.40 | 2.76 | 25.00 | 0.00 | 4.15 | 1.56 |
| Sorghum | 40.11 | 16.65 | 14.61 | 7.89 | 6.86 | 7.45 | 170.83 | 85.08 | 17.25 | 5.98 | 4.39 | 1.52 | 9.30 | 3.23 | 5.00 | 0.00 | 2.23 | 1.41 |
| Soybean | 6.81 | 13.75 | 13.82 | 5.50 | 2.68 | 4.28 | 232.58 | 183.27 | 21.56 | 4.75 | 5.48 | 1.21 | 11.63 | 2.56 | 74.78 | 19.13 | 1.31 | 0.76 |
| Teff | 61.00 | 0.00 | 28.00 | 0.00 | 0.00 | 0.00 | 164.69 | 50.00 | 15.53 | 4.70 | 3.95 | 1.19 | 8.38 | 2.53 | 5.00 | 0.00 | 1.42 | 1.09 |
| Wheat | 65.79 | 23.23 | 15.08 | 2.87 | 2.87 | 5.12 | 242.86 | 99.22 | 19.14 | 5.02 | 4.87 | 1.28 | 10.32 | 2.71 | 5.00 | 0.00 | 2.87 | 1.54 |
| Mean SSA | 46.62 | 18.33 | 16.42 | 6.71 | 12.29 | 7.42 | 205.35 | 93.49 | 18.87 | 5.33 | 4.80 | 1.35 | 10.18 | 2.87 | 16.77 | 1.37 | 6.46 | 4.00 |
| Different Sources and Neutralizing Processes of Protons (keq H+ ha−1 yr−1) in Different Cropping Systems in SSA | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cropping Systems | n | N Trans | BC Inputs | Anions Inputs | HCO−3le | BC Uptake | Anions Uptake | BCle | Nle | ANCAn | ANCCat | Total H+ | Soil ANC | ||||||||||||
| Mean | StD | Mean | StD | Mean | StD | Mean | StD | Mean | StD | Mean | StD | Mean | StD | Mean | StD | Mean | StD | Mean | StD | Mean | StD | Mean | StD | ||
| Banana | 27 | 2.94 | 0.08 | 1.46 | 0.04 | 0.11 | 0.01 | 0.03 | 0.01 | 7.24 | 6.15 | 0.33 | 0.28 | 0.87 | 0.65 | 0.05 | 0.05 | −0.22 | 0.15 | 6.65 | 2.28 | 9.88 | 0.05 | 6.43 | 0.11 |
| Barley | 78 | 3.16 | 0.12 | 0.43 | 0.1 | 0.05 | 0.02 | 0.39 | 0.38 | 0.2 | 0.1 | 0.2 | 0.12 | 1.85 | 0.32 | 0.1 | 0.01 | −0.15 | 0.07 | 1.62 | 0.17 | 3.55 | 0.25 | 1.47 | 0.05 |
| Beans | 509 | 2.37 | 0.56 | 0.82 | 0.38 | 0.08 | 0.02 | 0.12 | 0.29 | 0.69 | 0.41 | 0.24 | 0.14 | 1.49 | 0.34 | 0.09 | 0.02 | −0.16 | 0.08 | 1.36 | 0.38 | 2.94 | 0.43 | 1.2 | 0.06 |
| Cassava | 72 | 5.58 | 1.48 | 1.2 | 0.33 | 0.1 | 0.02 | 0.08 | 0.04 | 7.63 | 3.94 | 1.31 | 0.68 | 1.6 | 0.86 | 0.1 | 0.06 | −1.21 | 0.35 | 8.03 | 1.71 | 12.0 | 0.76 | 6.82 | 0.25 |
| Groundnuts | 247 | 6.91 | 0.55 | 0.65 | 0.32 | 0.06 | 0.03 | 0.07 | 0.04 | 0.49 | 0.27 | 0.17 | 0.09 | 3.55 | 0.28 | 0.24 | 0.02 | −0.11 | 0.06 | 3.39 | 0.29 | 7.3 | 0.3 | 3.28 | 0.05 |
| Maize | 2120 | 4.07 | 1.14 | 0.96 | 0.44 | 0.08 | 0.03 | 0.11 | 0.24 | 1.11 | 0.62 | 0.56 | 0.32 | 2.01 | 0.55 | 0.13 | 0.04 | −0.48 | 0.18 | 2.16 | 0.54 | 4.73 | 0.69 | 1.68 | 0.13 |
| Millet | 520 | 1.43 | 1.19 | 0.38 | 0.37 | 0.04 | 0.02 | 0.04 | 0.03 | 0.55 | 0.42 | 0.19 | 0.15 | 0.8 | 0.6 | 0.05 | 0.04 | −0.15 | 0.09 | 0.97 | 0.46 | 1.83 | 0.61 | 0.82 | 0.07 |
| Pea | 215 | 1.31 | 0.83 | 0.97 | 0.55 | 0.06 | 0.02 | 0.11 | 0.21 | 0.58 | 0.46 | 0.2 | 0.16 | 0.93 | 0.57 | 0.05 | 0.04 | −0.14 | 0.09 | 0.54 | 0.53 | 1.80 | 0.52 | 0.4 | 0.07 |
| Potato, I | 214 | 4.99 | 1.27 | 1.56 | 0.28 | 0.12 | 0.02 | 0.06 | 0.09 | 4.63 | 2.78 | 0.79 | 0.48 | 1.93 | 0.42 | 0.13 | 0.03 | −0.67 | 0.25 | 5.03 | 1.16 | 8.89 | 0.68 | 4.33 | 0.18 |
| Potato, S | 28 | 1.41 | 0.22 | 0.68 | 0.07 | 0.06 | 0.01 | 0.02 | 0.02 | 3.2 | 0.99 | 0.55 | 0.17 | 0.81 | 0.29 | 0.05 | 0.02 | −0.49 | 0.09 | 3.33 | 0.45 | 4.08 | 0.12 | 2.84 | 0.07 |
| Rice | 404 | 6.08 | 1.14 | 0.94 | 0.62 | 0.07 | 0.03 | 0.06 | 0.06 | 1.28 | 0.48 | 0.65 | 0.24 | 2.63 | 0.49 | 0.18 | 0.03 | −0.58 | 0.14 | 2.97 | 0.53 | 6.77 | 0.6 | 2.39 | 0.1 |
| Sorghum | 510 | 2.85 | 0.82 | 0.73 | 0.45 | 0.06 | 0.02 | 0.21 | 0.43 | 1.01 | 0.64 | 0.35 | 0.22 | 1.57 | 0.61 | 0.09 | 0.03 | −0.29 | 0.12 | 1.85 | 0.57 | 3.72 | 0.63 | 1.56 | 0.09 |
| Soybean | 227 | 7.35 | 0.7 | 0.63 | 0.22 | 0.07 | 0.01 | 0.06 | 0.05 | 0.61 | 0.35 | 0.21 | 0.12 | 3.86 | 0.38 | 0.26 | 0.03 | −0.14 | 0.07 | 3.84 | 0.32 | 7.81 | 0.38 | 3.7 | 0.05 |
| Sunflower | 17 | 1.45 | 0.1 | 0.61 | 0.19 | 0.05 | 0.02 | 0.07 | 0.05 | 0.31 | 0.19 | 0.11 | 0.07 | 0.86 | 0.13 | 0.05 | 0 | −0.06 | 0.05 | 0.56 | 0.17 | 1.72 | 0.08 | 0.5 | 0.03 |
| Teff | 171 | 4.27 | 0.09 | 0.39 | 0.07 | 0.04 | 0.01 | 0.54 | 0.34 | 0.44 | 0.34 | 0.22 | 0.17 | 2.43 | 0.33 | 0.13 | 0.01 | −0.18 | 0.09 | 2.48 | 0.25 | 5.03 | 0.22 | 2.3 | 0.06 |
| Wheat | 423 | 3.79 | 0.78 | 0.59 | 0.24 | 0.06 | 0.02 | 0.27 | 0.38 | 0.88 | 0.47 | 0.45 | 0.24 | 2.08 | 0.5 | 0.12 | 0.02 | −0.39 | 0.13 | 2.37 | 0.4 | 4.49 | 0.58 | 1.98 | 0.09 |
| Mean | 5782 | 3.70 | 0.69 | 0.81 | 0.29 | 0.07 | 0.02 | 0.14 | 0.17 | 1.93 | 1.16 | 0.41 | 0.23 | 1.83 | 0.46 | 0.11 | 0.03 | −0.34 | 0.13 | 3.76 | 0.64 | 5.41 | 0.43 | 2.5 | 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uwiragiye, Y.; Ngaba, M.J.Y.; Yang, M.; Elrys, A.S.; Chen, Z.; Zhou, J. Spatially Explicit Soil Acidification under Optimized Fertilizer Use in Sub-Saharan Africa. Agronomy 2023, 13, 632. https://doi.org/10.3390/agronomy13030632
Uwiragiye Y, Ngaba MJY, Yang M, Elrys AS, Chen Z, Zhou J. Spatially Explicit Soil Acidification under Optimized Fertilizer Use in Sub-Saharan Africa. Agronomy. 2023; 13(3):632. https://doi.org/10.3390/agronomy13030632
Chicago/Turabian StyleUwiragiye, Yves, Mbezele Junior Yannick Ngaba, Mingxia Yang, Ahmed S. Elrys, Zhujun Chen, and Jianbin Zhou. 2023. "Spatially Explicit Soil Acidification under Optimized Fertilizer Use in Sub-Saharan Africa" Agronomy 13, no. 3: 632. https://doi.org/10.3390/agronomy13030632







