Effects of Water Stress on Resveratrol Accumulation and Synthesis in ‘Cabernet Sauvignon’ Grape Berries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Treatments
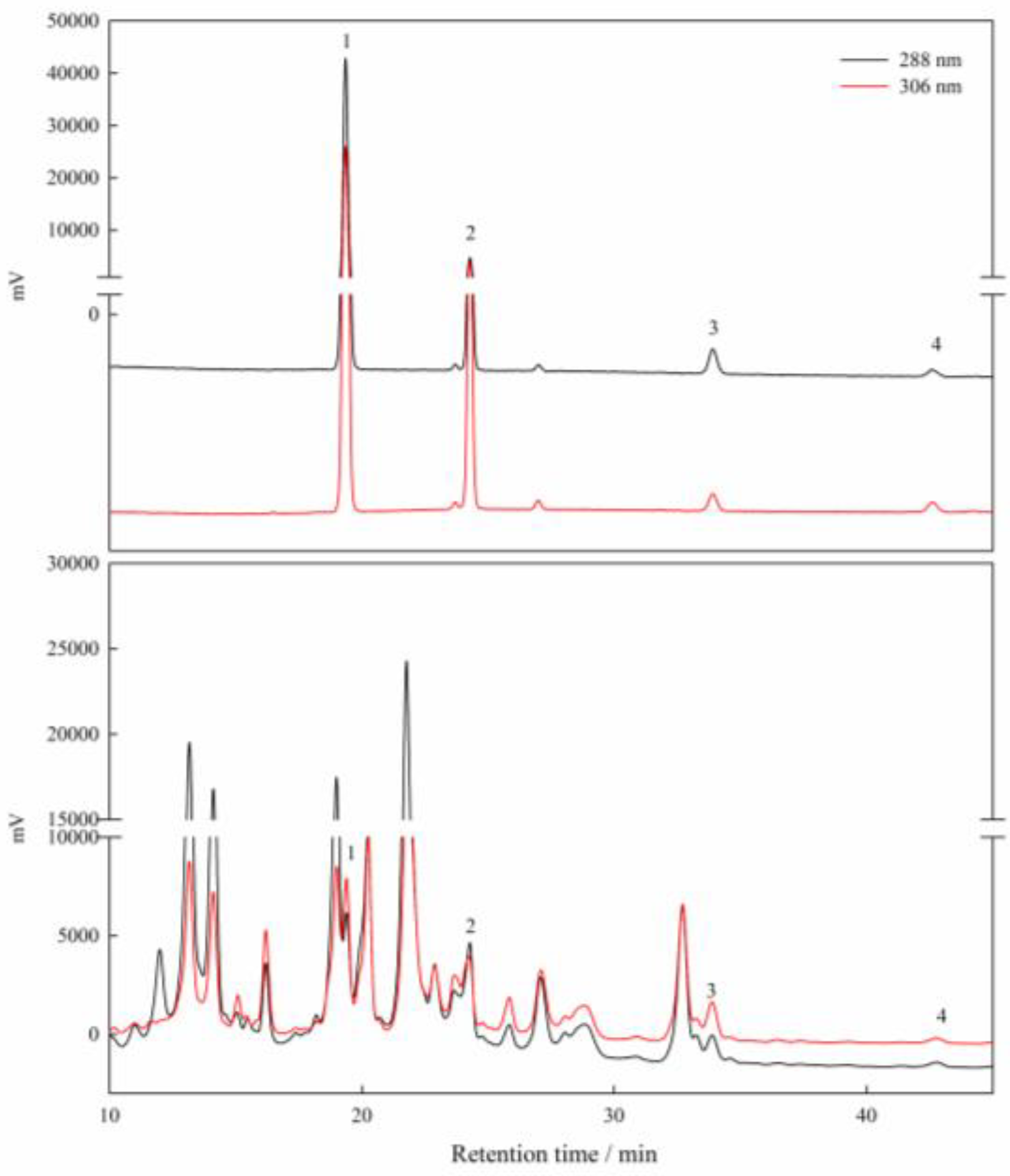
2.2. Measurements of Physiological Data and Stilbenes
2.3. Measurements of Enzyme Activity
2.4. RNA Extraction and Quantification of mRNA
2.5. Statistics
3. Results
3.1. Weather Conditions and Leaf Water Potential
3.2. Physiological Responses to Water Stress
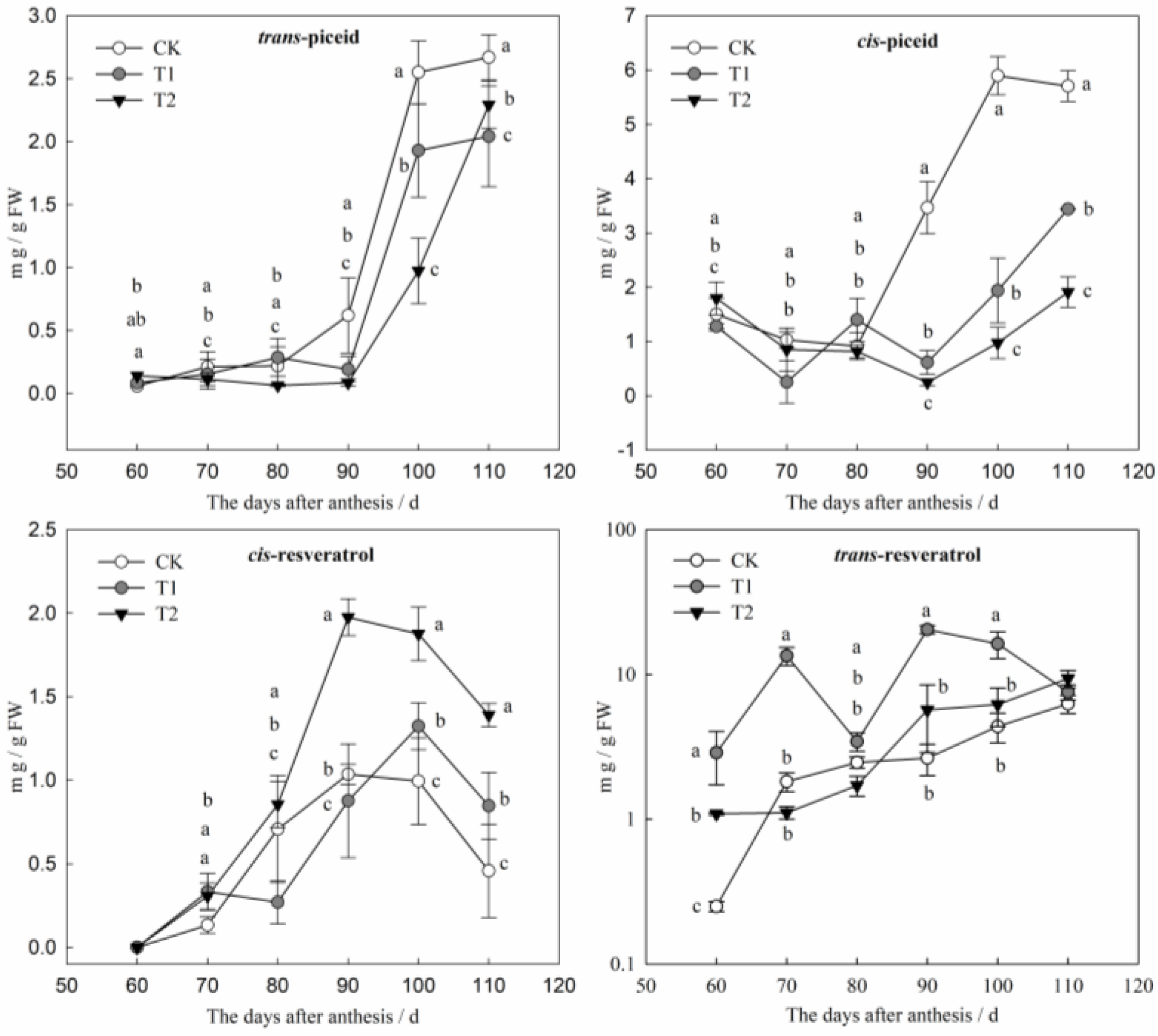
3.3. Effects of Water Stress on the Accumulation of Stilbenes
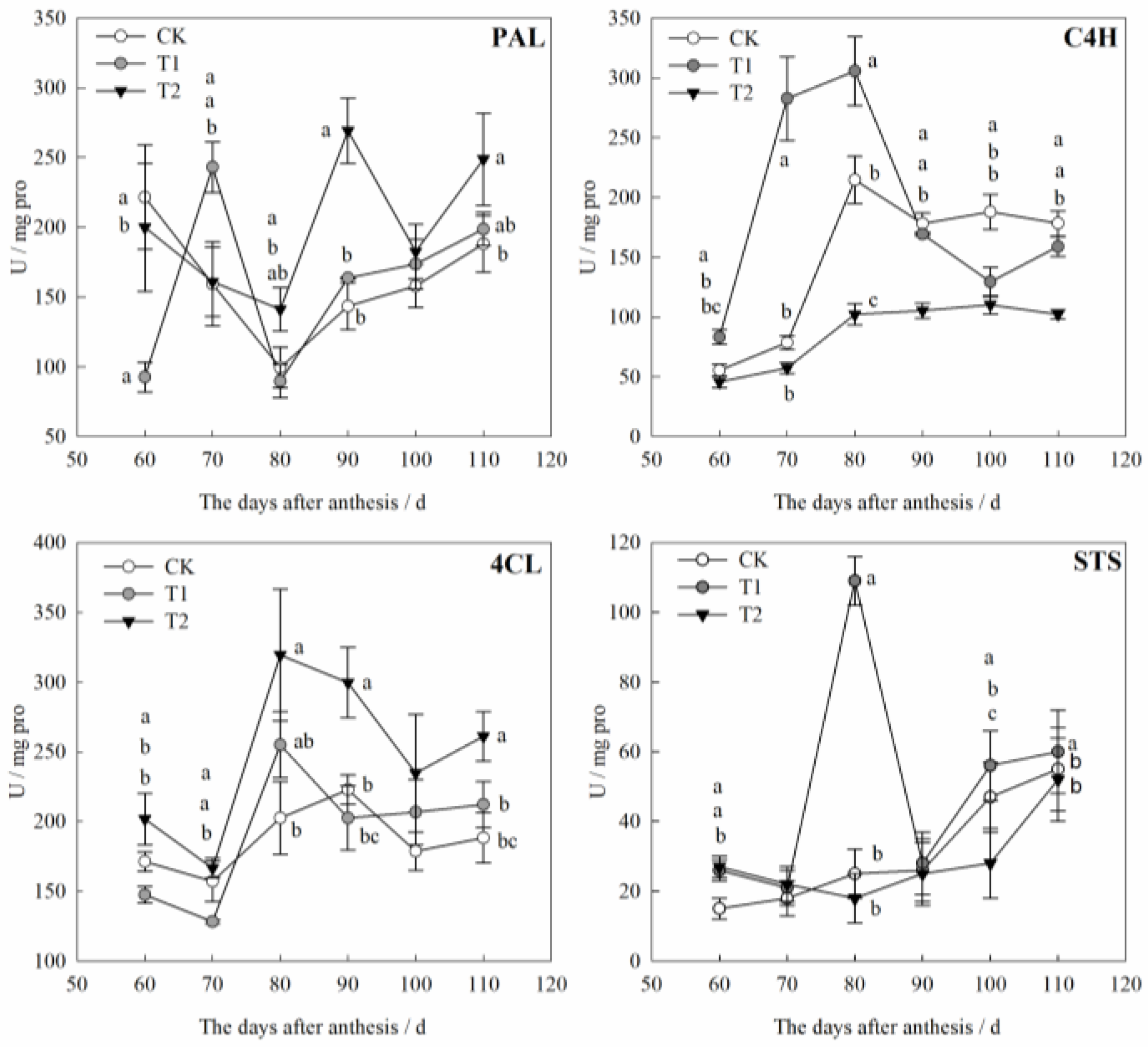
3.4. Effects of Water Stress on Activities of Resveratrol Synthesis-Related Enzymes in Grape Berries
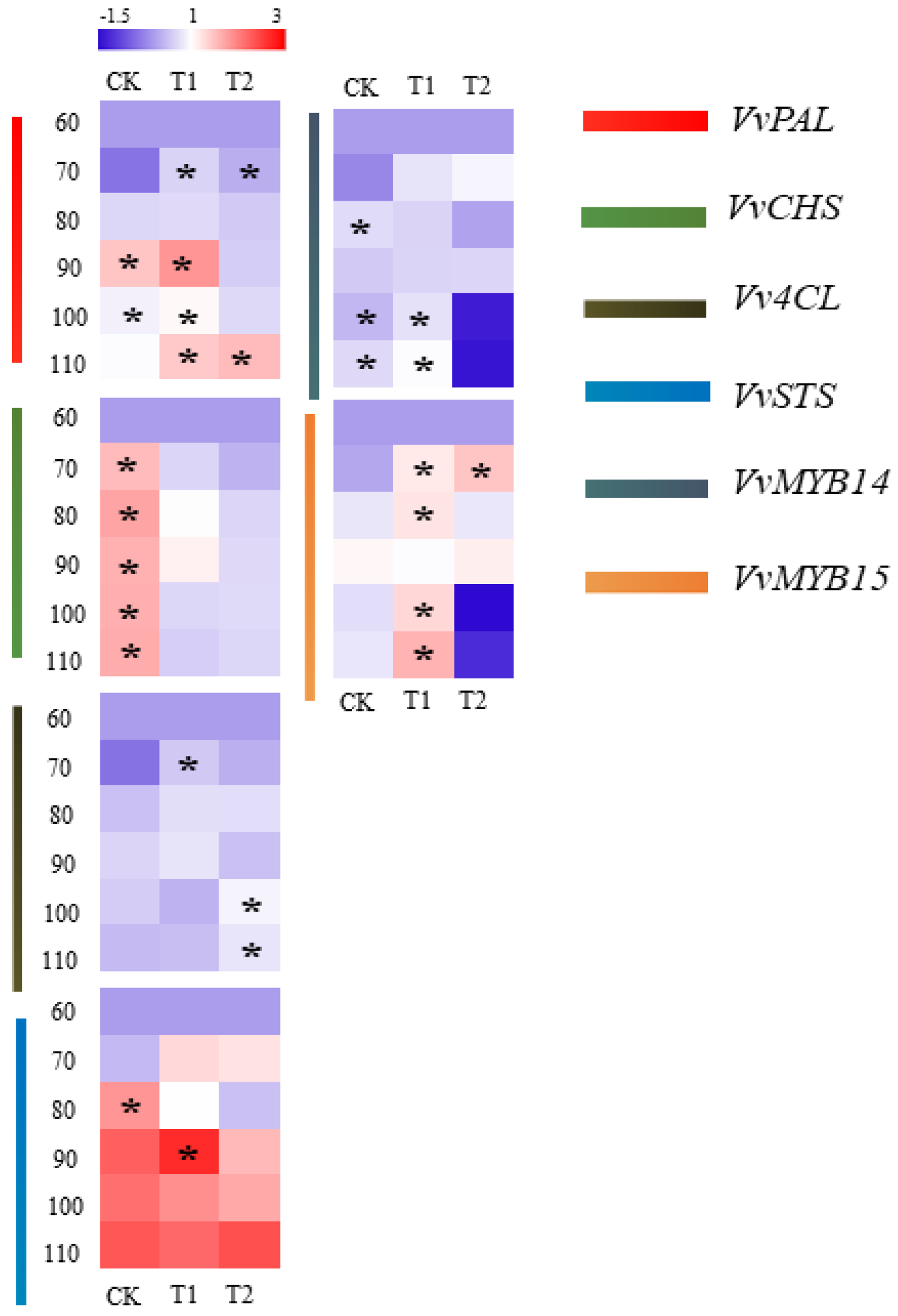
3.5. Effects of Water Stress on the Transcriptional Level of Resveratrol Synthesis-Related Genes in Grape Berries
3.6. Correlation Analysis between Transcriptional Level of Resveratrol Synthesis-Related Genes and Resveratrol Content
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B
| Gene Name | Primer Sequence (5′→3′) | Accession No. | |
|---|---|---|---|
| Forward | Reverse | ||
| PAL | AGTCATCCGAGCATCAACTAAA | CCACCATGTAGAGCCTTGTT | EF192469 |
| 4CL | GGAGAAGGTTTCACCGTCATTA | CGTCGGAGTTGATCGAAAC | JN858959 |
| CHS | GCCAAGGCCATCAAAGAATG | TAGCAGCTTGGTGAGTTGATAG | AF020709 |
| STS | CAGCAGCCCAAACATTTATTCC | GTTCCAATCGCTAATACCAAGTG | KX523623 |
| Myb14 | TCTGAGGCCGGATATCAAAC | GGGACGCATCAAGAGAGTGT | EU181424 |
| Myb15 | CAAGAATGAACAGATGGAGGAG | TCTGCGACTGCTGGGAAA | KX523624 |
| EF1 | GTTAAGATGATTCCAACCAAGCCC | TCTCCACGCTCTTGATGACTC | XM_002279562 |
| Actin | TCCTTGCCTTGCGTCATCTAT | CACCAATCACTCTCCTGCTACAA | XM_002277287 |
References
- Cantos, E.; Espín, J.C.; Tomás-Barberán, F.A. Postharvest stilbene-enrichment of red and white table grape varieties using UV-C irradiation pulses. J. Agric. Food Chem. 2002, 50, 6322–6329. [Google Scholar] [CrossRef]
- Li, H.G.; Xia, N.; Forstermann, U. Cardiovascular effects and molecular targets of resveratrol. Nitric Oxide -Biol. Chem. 2012, 26, 102–110. [Google Scholar] [CrossRef]
- Akaberi, M.; Hosseinzadeh, H. Grapes (Vitis vinifera) as a Potential Candidate for the Therapy of the Metabolic Syndrome. Phytother. Res. 2016, 30, 540–556. [Google Scholar] [CrossRef]
- Nassiri-Asl, M.; Hosseinzadeh, H. Review of the Pharmacological Effects of Vitis vinifera (Grape) and its Bioactive Constituents: An Update. Phytother. Res. 2016, 30, 1392–1403. [Google Scholar] [CrossRef]
- Delmas, D. Transport, stability, and biological activity of resveratrol. Resveratrol. Health 2011, 1215, 48–59. [Google Scholar] [CrossRef]
- Figueiras, T.S.; Neves-Petersen, M.T.; Petersen, S.B. Activation Energy of Light Induced Isomerization of Resveratrol. J. Fluoresc. 2011, 21, 1897–1906. [Google Scholar] [CrossRef]
- Souid, I. The effect of salt stress on resveratrol and piceid accumulation in two Vitis vinifera L. cultivars. Physiol. Mol. Biol. Plants 2019, 25, 625–635. [Google Scholar] [CrossRef]
- Averilla, J.N. Improved extraction of resveratrol and antioxidants from grape peel using heat and enzymatic treatments. J. Sci. Food Agric. 2019, 99, 4043–4053. [Google Scholar] [CrossRef]
- Parage, C. Structural, Functional, and Evolutionary Analysis of the Unusually Large Stilbene Synthase Gene Family in Grapevine. Plant Physiol. 2012, 160, 1407–1419. [Google Scholar] [CrossRef]
- Valletta, A.; Iozia, L.M.; Leonelli, F. Impact of Environmental Factors on Stilbene Biosynthesis. Plants 2021, 10, 90. [Google Scholar] [CrossRef]
- Vannozzi, A. Genome-wide analysis of the grapevine stilbene synthase multigenic family: Genomic organization and expression profiles upon biotic and abiotic stresses. BMC Plant Biol. 2012, 12, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Holl, J. The R2R3-MYB transcription factors MYB14 and MYB15 regulate stilbene biosynthesis in Vitis vinifera. Plant Cell 2013, 25, 4135–4149. [Google Scholar] [CrossRef] [PubMed]
- Gatto, P. Ripening and Genotype Control Stilbene Accumulation in Healthy Grapes. J. Agric. Food Chem. 2008, 56, 11773–11785. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, N. Drought stress modulates cuticular wax composition of the grape berry. J. Exp. Bot. 2020, 71, 3126–3141. [Google Scholar] [CrossRef]
- Pannu, N.; Bhatnagar, A. Resveratrol: From enhanced biosynthesis and bioavailability to multitargeting chronic diseases. Biomed. Pharm. 2019, 109, 2237–2251. [Google Scholar] [CrossRef] [PubMed]
- Oraee, A.; Tehranifar, A. Evaluating the potential drought tolerance of pansy through its physiological and biochemical responses to drought and recovery periods. Sci. Hortic. 2020, 265, 109225. [Google Scholar] [CrossRef]
- Bahar, E. The effect of extreme water stress on leaf drying limits and possibilities of recovering in three grapevine (Vitis vinifera L.) cultivars. Afr. J. Agric. Res. 2011, 6, 1151–1160. [Google Scholar]
- Castellarin, S.D. Water deficits accelerate ripening and induce changes in gene expression regulating flavonoid biosynthesis in grape berries. Planta 2007, 227, 101–112. [Google Scholar] [CrossRef]
- Lamb, C.J.; Rubery, P.H. A spectrophotometric assay for trans-cinnamic acid 4-hydroxylase activity. Anal. Biochem. 1975, 68, 554–561. [Google Scholar] [CrossRef]
- Knobloch, K.H.; Hahlbrock, K. Isoenzymes of p-coumarate: CoA ligase from cell suspension cultures of Glycine max. Eur. J. Biochem. 1975, 52, 311–320. [Google Scholar] [CrossRef]
- Lister, C.E. Developmental changes in enzymes of flavonoid biosynthesis in the skins of red and green apple cultivars. J. Sci. Food Agric. 1996, 71, 313–320. [Google Scholar] [CrossRef]
- Krzyzaniak, Y. Combined enzymatic and metabolic analysis of grapevine cell responses to elicitors. Plant Physiol. Biochem. 2018, 123, 141–148. [Google Scholar] [CrossRef]
- Ojeda, H. Influence of pre-and postveraison water deficit on synthesis and concentration of skin phenolic compounds during berry growth of Vitis vinifera cv. Shiraz. Am. J. Enol. Vitic. 2002, 53, 261–267. [Google Scholar]
- Wang, Z.P.; Deloire, A.; Carbonneau, A.; Federspiel, B.; Lopez, F. An in vivo experimental system to study sugar phloem unloading in ripening grape berries during water deficiency stress. Ann. Bot. 2003, 92, 523–528. [Google Scholar] [CrossRef]
- Versari, A. Stilbene Compounds and Stilbene Synthase Expression during Ripening, Wilting, and UV Treatment in Grape cv. Corvina. J. Agric. Food Chem. 2001, 49, 5531–5536. [Google Scholar] [CrossRef]
- Borie, B. Resveratrol and stilbene synthase mRNA production in grapevine leaves treated with biotic and abiotic phytoalexin elicitors. Am. J. Enol. Vitic. 2004, 55, 60–64. [Google Scholar] [CrossRef]
- Vezzulli, S. Methyl jasmonate treatment as a trigger of resveratrol synthesis in cultivated grapevine. Am. J. Enol. Vitic. 2007, 58, 530–533. [Google Scholar] [CrossRef]
- Wang, L. Resveratrols in grape berry skins and leaves in Vitis germplasm. PLoS ONE 2013, 8, e61642. [Google Scholar] [CrossRef]
- Villangó, S. The effect of postveraison water deficit on the phenolic composition and concentration of the Kékfrankos (Vitis vinifera L.) berry. Sci. Hortic. 2016, 209, 113–116. [Google Scholar] [CrossRef]
- Hura, T.; Hura, K.; Grzesiak, S. Contents of total phenolics and ferulic acid, and PAL activity during water potential changes in leaves of maize single-cross hybrids of different drought tolerance. J. Agron. Crop Sci. 2008, 194, 104–112. [Google Scholar] [CrossRef]
- Huntley, S.K. Significant increases in pulping efficiency in C4H-F5H-transformed poplars: Improved chemical savings and reduced environmental toxins. J. Agric. Food Chem. 2003, 51, 6178–6183. [Google Scholar] [CrossRef]
- Guo, H. Comparison of stilbene synthase from different plant sources for resveratrol biosynthesis. Sheng Wu Gong Cheng Xue Bao Chin. J. Biotechnol. 2014, 30, 1622–1633. [Google Scholar]
- Chong, J.; Poutaraud, A.; Hugueney, P. Metabolism and roles of stilbenes in plants. Plant Sci. 2009, 177, 143–155. [Google Scholar] [CrossRef]
- Lu, D.; Zhao, W.; Zhao, S. Relevant Enzymes, Genes and Regulation Mechanisms in Biosynthesis Pathway of Stilbenes. Open J. Med. Chem. 2012, 2, 15–23. [Google Scholar]
- Chaves, M.M. Deficit irrigation in grapevine improves water-use efficiency while controlling vigour and production quality. Ann. Appl. Biol. 2007, 150, 237–252. [Google Scholar] [CrossRef]
- Castellarin, S.D. Transcriptional regulation of anthocyanin biosynthesis in ripening fruits of grapevine under seasonal water deficit. Plant Cell 2007, 30, 1381–1399. [Google Scholar] [CrossRef]

| Stages | Treatments | Berry Characteristics | Berry Compositions | |||||
|---|---|---|---|---|---|---|---|---|
| Berry Weight | Berry Volume | Total Soluble Solids | Sugar | Titratable Acid | Total Polyphenols | Total Anthocyanin | ||
| g | mm3 | % | mg/g | g·L−1 | mg·g−1 | mg·g−1 | ||
| Green fruit (30 DAA) | CK | 0.46 a | 154.56 a | 4.33 b | 11.60 | 40.15 a | 18.23 | 0.00 |
| T1 | 0.39 b | 131.98 a | 5.20 a,b | 11.40 | 38.25 b | 18.25 | 0.00 | |
| T2 | 0.26 c | 90.88 b | 6.20 a | 11.75 | 37.48 b | 18.86 | 0.00 | |
| ANOVA | *** | *** | ** | ns | *** | ns | ns | |
| Veraison (70 DAA) | CK | 1.00 a | 215.95 a | 15.00 | 91.56 b | 19.56 a | 11.44 | 0.11 b |
| T1 | 0.86 b | 235.06 a | 14.60 | 116.93 a | 18.32 b | 11.4 | 0.34 a | |
| T2 | 0.64 c | 167.33 b | 14.07 | 93.60 b | 16.16 c | 12.58 | 0.15 b | |
| ANOVA | *** | ** | ns | ** | *** | ns | *** | |
| Maturity (110 DAA) | CK | 1.16 a | 275.54 a | 20.64 a | 204.35 a | 7.36 a | 6.29 b | 0.77 b |
| T1 | 1.09 b | 273.45 a | 20.09 a | 199.96 a | 6.19 b | 8.49 a | 0.82 b | |
| T2 | 0.83 c | 173.64 b | 19.16 b | 191.23 b | 7.32 a | 7.66 a | 0.95 a | |
| ANOVA | *** | *** | ** | ** | *** | ** | *** | |
| PAL | 4CL | STS | CHS | MYB14 | MYB15 | |
|---|---|---|---|---|---|---|
| CK | 0.240 | 0.415 | 0.823 * | 0.671 | 0.589 | 0.335 |
| T1 | 0.667 | 0.331 | 0.700 | 0.446 | 0.153 | −0.033 |
| T2 | 0.788 * | 0.626 | 0.852 * | 0.706 | 0.387 | −0.440 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Xi, B.; Dai, H. Effects of Water Stress on Resveratrol Accumulation and Synthesis in ‘Cabernet Sauvignon’ Grape Berries. Agronomy 2023, 13, 633. https://doi.org/10.3390/agronomy13030633
Sun Y, Xi B, Dai H. Effects of Water Stress on Resveratrol Accumulation and Synthesis in ‘Cabernet Sauvignon’ Grape Berries. Agronomy. 2023; 13(3):633. https://doi.org/10.3390/agronomy13030633
Chicago/Turabian StyleSun, Yanli, Ben Xi, and Hongjun Dai. 2023. "Effects of Water Stress on Resveratrol Accumulation and Synthesis in ‘Cabernet Sauvignon’ Grape Berries" Agronomy 13, no. 3: 633. https://doi.org/10.3390/agronomy13030633




