Use of Multivariate Analysis in Screening for Drought Tolerance in Ornamental Asteraceae Species
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Drought Treatments and Growth Parameters
2.3. Photosynthetic Pigments
2.4. Quantification of Proline
2.5. Determination of MDA and Antioxidant Compounds
2.6. Statistical Analyses
3. Results
3.1. Effect of Water Stress on Growth Parameters
3.2. Biochemical Analysis
3.3. Multivariate Analysis of Data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lewis Smith, R.I.; Richardson, M. Fuegian plants in Antarctica: Natural or anthropogenically assisted immigrants? Biol. Invasions 2011, 13, 1–5. [Google Scholar] [CrossRef]
- Mandel, J.R.; Dikow, R.B.; Siniscalchi, C.M.; Thapa, R.; Watson, L.E.; Funk, V.A. A fully resolved backbone phylogeny reveals numerous dispersals and explosive diversifications throughout the history of Asteraceae. Proc. Natl. Acad. Sci. USA 2019, 116, 14083–14088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elomaa, P.; Zhao, Y.; Zhang, T. Flower heads in Asteraceae-recruitment of conserved developmental regulators to control the flower-like inflorescence architecture. Hortic. Res. 2018, 5, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikolić, M.; Stevović, S. Family Asteraceae as a sustainable planning tool in phytoremediation and its relevance in urban areas. Urban For. Urban Green. 2015, 14, 782–789. [Google Scholar] [CrossRef]
- Snyder, R. Climate change impacts on water use in horticulture. Horticulturae 2017, 3, 27. [Google Scholar] [CrossRef] [Green Version]
- Dullinger, I.; Wessely, J.; Bossdorf, O.; Dawson, W.; Essl, F.; Gattringer, A.; Klonner, G.; Kreft, H.; Kuttner, M.; Moser, D.; et al. Climate change will increase the naturalization risk from garden plants in Europe. Glob. Ecol. Biogeogr. 2017, 26, 43–53. [Google Scholar] [CrossRef] [Green Version]
- Hayden Reichard, S.; White, P. Horticulture as a pathway of invasive plant introductions in the United States: Most invasive plants have been introduced for horticultural use by nurseries, botanical gardens, and individuals. BioScience 2001, 51, 103–113. [Google Scholar] [CrossRef]
- van Kleunen, M.; Essl, F.; Pergl, J.; Brundu, G.; Carboni, M.; Dullinger, S.; Early, R.; González-Moreno, P.; Groom, Q.J.; Hulme, P.E.; et al. The changing role of ornamental horticulture in alien plant invasions. Biol. Rev. 2018, 93, 1421–1437. [Google Scholar] [CrossRef]
- Hulme, P.E.; Brundu, G.; Carboni, M.; Dehnen-Schmutz, K.; Dullinger, S.; Early, R.; Essl, F.; González-Moreno, P.; Groom, Q.J.; Kueffer, C.; et al. Integrating invasive species policies across ornamental horticulture supply chains to prevent plant invasions. J. Appl. Ecol. 2018, 55, 92–98. [Google Scholar] [CrossRef]
- Haeuser, E.; Dawson, W.; van Kleunen, M. The effects of climate warming and disturbance on the colonization potential of ornamental alien plant species. J. Ecol. 2017, 105, 1698–1708. [Google Scholar] [CrossRef] [Green Version]
- Darras, A.I. Implementation of Sustainable Practices to Ornamental Plant Cultivation Worldwide: A Critical Review. Agronomy 2020, 10, 1570. [Google Scholar] [CrossRef]
- Eftekhari, M.S. Impacts of climate change on agriculture and horticulture. In Climate Change; Springer International Publishing: Cham, Switzerland, 2022; pp. 117–131. [Google Scholar] [CrossRef]
- Caser, M.; Ruffoni, B.; Scariot, V. Screening for drought tolerance in Salvia spp. and Helichrysum petiolare: A way to select low maintenance ornamental plants. Acta Hortic. 2012, 953, 239–246. [Google Scholar] [CrossRef]
- Cicevan, R.; Al Hassan, M.; Sestras, A.F.; Prohens, J.; Vicente, O.; Sestras, R.E.; Boscaiu, M. Screening for drought tolerance in cultivars of the ornamental Genus Tagetes (Asteraceae). PeerJ 2016, 4, e2133. [Google Scholar] [CrossRef] [Green Version]
- Franco, J.A.; Martínez-Sánchez, J.J.; Fernández, J.A.; Bañón, S. Selection and nursery production of ornamental plants for landscaping and xerogardening in semi-arid environments. J. Hortic. Sci. Biotechnol. 2006, 81, 3–17. [Google Scholar] [CrossRef]
- Juan Vicedo, J.; Laguna, E.; Ríos, S.; Casas, J. Ornamental potential of the coastal plant Lapiedra martinezii Lag. (Amaryllidaceae): The role of its revalorization in xero-gardening and ex-situ conservation. Nereis Interdiscip. Ibero-Am. J. Methods Model. Simul. 2021, 13, 211–226. [Google Scholar] [CrossRef]
- Ali, A.A.; El-Shanawani, M.A.; Khalifa, A.A.; Mohammad, M.A. Macro- and micromorphology of the leaves, stems and flowers of Chrysanthemum carinatum L. Bull. Pharm. Sci. 2010, 33, 141–168. [Google Scholar] [CrossRef]
- CABI. Invasive Species Compendium; CAB International: Wallingford, UK, 2022; Available online: www.cabi.org/isc (accessed on 30 November 2022).
- Johnson, M.F. A monograph of the genus Ageratum L. (Compositae-Eupatorieae). Ann. Missouri Bot. Gard. 1971, 58, 6–88. [Google Scholar] [CrossRef]
- SANBI, 2016. South African National Biodiversity Institute (SANBI). Declared Weeds & Alien Invader Plants. Available online: https://www.plantzafrica.com/miscell/aliens1.htm (accessed on 15 December 2022).
- Xie, G.; Chen, W.; Lin, M.; Zheng, Y.; Guo, P.; Zheng, Y. Ecological risk assessment with MCDM of some invasive alien plants in China. In Cutting-Edge Research Topics on Multiple Criteria Decision Making, Proceedings of the 20th International Conference, MCDM 2009, Chengdu, China, June 21–26 2009; Shi, Y., Wang, S., Peng, Y., Li, J., Zeng, Y., Eds.; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar] [CrossRef]
- Halevy, A.B. Handbook of Flowering, 1st ed.; CRC Press: Boca Raton, FL, USA, 1985. [Google Scholar] [CrossRef]
- Almeida, J.D.D.; Freitas, H. Exotic naturalized flora of continental Portugal—A reassessment. Bot. Complut. 2006, 30, 117–130. [Google Scholar]
- Brown, E.A. New South Wales Flora Online: Xerochrysum Bracteatum. Royal Botanic Gardens & Domain Trust: Sydney, Australia. Available online: https://plantnet.rbgsyd.nsw.gov.au/ (accessed on 15 December 2022).
- Baker, M.L.; de Salas, M.F. A Census of the Vascular Plants of Tasmania and Index to the Student’s Flora of Tasmania and Flora of Tasmania Online; Tasmanian Herbarium, Tasmanian Museum and Art Gallery: Hobart, TAS, Australia, 2013; ISBN 978-1-921599-73-6. [Google Scholar]
- Ramasamy, S.; Rajendran, A. Occurrence of major invasives in Nilgiri Biosphere Reserve, India: Perspective and prospective. Aliens Invasive Species Bull. 2012, 32, 42–44. [Google Scholar]
- Missouri Botanical Garden. Plant Finder. Available online: http://www.missouribotanicalgarden.org/plantfinder/plantfindersearch.aspx (accessed on 21 February 2023).
- Wilson, A. Flora of Australia, Volume 37. Asteraceae; ABRS/CSIRO: Melbourne, Australia, 2015; 668p.
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef] [Green Version]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Blainski, A.; Lopes, G.; Mello, J. Application and analysis of the Folin Ciocalteu method for the determination of the total phenolic content from Limonium brasiliense L. Molecules 2013, 18, 6852–6865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, 2013, Vienna, Austria. Available online: https://www.R-project.org/ (accessed on 22 November 2022).
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002; ISBN 0-387-95457-0. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis [Internet]; Springer: New York, NY, USA, 2016; Available online: https://ggplot2.tidyverse.org (accessed on 22 November 2022).
- Pedersen, T. ggforce: Accelerating ‘ggplot2’. R Package Version 0.4.1, 2022. Available online: https://CRAN.R-project.org/package=ggforce (accessed on 22 November 2022).
- Taiyun, W.; Viliam, S. R Package ‘Corrplot’: Visualization of a Correlation Matrix Version 0.92. 2021. Available online: https://github.com/taiyun/corrplot (accessed on 22 November 2022).
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [Green Version]
- Gupta, B.; Huang, B. Mechanism of Salinity tolerance in plants: Physiological, biochemical, and molecular characterization. Int. J. Genomics 2014, 2014, 701596. [Google Scholar] [CrossRef] [Green Version]
- Flexas, J.; Medrano, H. Energy dissipation in C3 plants under drought. Funct. Plant Biol. 2002, 29, 1209–1215. [Google Scholar] [CrossRef]
- Alscher, R.G.; Cumming., J.R. Stress Responses in Plants: Adaptation and Acclimation Mechanisms; Wiley-Liss: New York, NY, USA, 1990; p. 407. [Google Scholar]
- Sun, J.; Gu, J.; Zeng, J.; Han, S.; Song, A.; Chen, F.; Fang, W.; Jiang, J.; Chen, S. Changes in leaf morphology, antioxidant activity and photosynthesis capacity in two different drought-tolerant cultivars of Chrysanthemum during and after water stress. Sci. Hortic. 2013, 161, 249–258. [Google Scholar] [CrossRef]
- Singh, B.B.; Mai-Kodomi, Y.; Terao, T. A simple screening method for drought tolerance in cowpea. Indian J. Genet. Plant Breed. 1999, 59, 211–220. [Google Scholar]
- Chloupek, O.; Dostál, V.; Středa, T.; Psota, V.; Dvořáčková, O. Drought tolerance of barley varieties in relation to their root system size. Plant Breed. 2010, 129, 630–636. [Google Scholar] [CrossRef]
- Al Hassan, M.; Chaura, J.; Donat-Torres, M.P.; Boscaiu, M.; Vicente, O. Antioxidant responses under salinity and drought in three closely related wild monocots with different ecological optima. AoB Plants 2017, 9, plx009. [Google Scholar] [CrossRef]
- Al Hassan, M.; Estrelles, E.; Soriano, P.; López-Gresa, M.P.; Bellés, J.M.; Boscaiu, M.; Vicente, O. Unraveling salt tolerance mechanisms in halophytes: A comparative study on four mediterranean Limonium species with different geographic distribution patterns. Front. Plant Sci. 2017, 8, 1438. [Google Scholar] [CrossRef] [Green Version]
- González-Orenga, S.; Plazas, M.; Ribera, E.; Pallotti, C.; Boscaiu, M.; Prohens, J.; Vicente, O.; Fita, A. Transgressive biochemical response to water stress in interspecific eggplant hybrids. Plants 2023, 12, 194. [Google Scholar] [CrossRef]
- Plazas, M.; González-Orenga, S.; Nguyen, H.T.; Morar, I.M.; Fita, A.; Boscaiu, M.; Prohens, J.; Vicente, O. Growth and antioxidant responses triggered by water stress in wild relatives of eggplant. Sci. Hortic. 2022, 293, 110685. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, D.P. Use of physiological indices as a screening technique for drought tolerance in oilseed Brassica species. Ann. Bot. 1998, 81, 413–420. [Google Scholar] [CrossRef] [Green Version]
- Vanhove, A.C.; Vermaelen, W.; Panis, B.; Swennen, R.; Carpentier, S.C. Screening the banana biodiversity for drought tolerance: Can an in vitro growth model and proteomics be used as a tool to discover tolerant varieties and understand homeostasis. Front. Plant Sci. 2012, 3, 176. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Ni, Z.; Chen, Q.; Guo, Z.; Gao, W.; Su, X.; Qu, Y. Proteomic responses of drought-tolerant and drought-sensitive cotton varieties to drought stress. Mol. Genet. Genom. 2016, 291, 1293–1303. [Google Scholar] [CrossRef]
- Belluau, M.; Shipley, B. Predicting habitat affinities of herbaceous dicots to soil wetness based on physiological traits of drought tolerance. Ann. Bot. 2017, 119, 1073–1084. [Google Scholar] [CrossRef] [Green Version]
- Cuimei, Z.; Shangli, S.; Zhen, L.; Fan, Y.; Guoli, Y. Drought tolerance in alfalfa (Medicago sativa L.) varieties is associated with en-hanced antioxidative protection and declined lipid peroxidation. J. Plant Physiol. 2019, 232, 226–240. [Google Scholar] [CrossRef]
- Gambetta, G.A.; Herrera, J.C.; Dayer, S.; Feng, Q.; Hochberg, U.; Castellarin, S.D. The physiology of drought stress in grapevine: Towards an integrative definition of drought tolerance. J. Exper. Bot. 2020, 71, 4658–4676. [Google Scholar] [CrossRef] [PubMed]
- Pipatsitee, P.; Tisarum, R.; Samphumphuang, T.; Kongpugdee, S.; Taota, K.; Eiumnoh, A.; Cha-Um, S. Evaluation of water deficit tolerance in maize genotypes using biochemical, physio-morphological changes and yield traits as multivariate cluster analysis. Not. Bot. Horti Agrobo. 2022, 50, 12572. [Google Scholar] [CrossRef]
- Rajakaruna, N.; Bradfield, G.E.; Bohm, B.A.; Whitton, J. Adaptive differentiation in response to water stress by edaphic races of Lasthenia californica (Asteraceae). Int. J. Plant Sci. 2003, 164, 371–376. [Google Scholar] [CrossRef] [Green Version]
- Mráz, P.; Tarbush, E.; Müller-Schärer, H. Drought tolerance and plasticity in the invasive knapweed Centaurea stoebe s.l. (Asteraceae): Effect of populations stronger than those of cytotype and range. Ann. Bot. 2014, 114, 289–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hair, F.F.; Anderson, R.E.; Tatham, R.L.; Black, W.C. Multivariate Data Analysis, 5th ed.; Prentice-Hall Inc.: Upple Saddle River, NJ, USA, 1998; pp. 239–325. [Google Scholar]
- Nielsen, J.P.; Munck, L. Evaluation of malting barley quality using exploratory data analysis. I. Extraction of information from micromalting data of spring and winter barley. J. Cereal Sci. 2003, 38, 173–180. [Google Scholar] [CrossRef]
- Ahmed, K.; Shabbir, G.; Ahmed, M.; Shah, K.N. Phenotyping for drought resistance in bread wheat using physiological and biochemical traits. Sci. Total Environ. 2020, 729, 139082. [Google Scholar] [CrossRef]
- Arteaga, S.; Yabor, L.; Díez, M.J.; Prohens, J.; Boscaiu, M.; Vicente, O. The use of proline in screening for tolerance to drought and salinity in common bean (Phaseolus vulgaris L.) genotypes. Agronomy 2020, 10, 817. [Google Scholar] [CrossRef]
- Zou, J.; Hu, W.; Li, Y.; He, J.; Zhu, H.; Zhou, Z. Screening of drought resistance indices and evaluation of drought resistance in cotton (Gossypium hirsutum L.). J. Integr. Agric. 2020, 19, 495–508. [Google Scholar] [CrossRef]
- Conti, V.; Romi, M.; Parri, S.; Aloisi, I.; Marino, G.; Cai, G.; Cantini, C. Morpho-physiological classification of italian tomato cultivars (Solanum lycopersicum L.) according to drought tolerance during vegetative and reproductive growth. Plants 2021, 10, 1826. [Google Scholar] [CrossRef]
- Aminzadeh, B.; Sani, B.; Alizadeh, B.; Mozaffari, H. Identification of drought tolerant oilseed rape genotypes using multivariate analysis. J. Crop Breed. 2002, 14, 75–84. [Google Scholar]
- Budzinski, I.G.F.; de Moraes, F.E.; Cataldi, T.R.; Franceschini, L.M.; Labate, C.A. Network analyses and data integration of proteomics and metabolomics from leaves of two contrasting varieties of sugarcane in response to drought. Front. Plant Sci. 2019, 10, 1524. [Google Scholar] [CrossRef] [Green Version]
- Kamphorst, S.H.; Gonçalves, G.M.B.; Do Amaral, A.T.; De Lima, V.J.; Leite, J.T.; Schmitt, K.F.M.; Dos Santos, D.R.; Santos, J.S.; De Oliveira, F.T.; Corrêa, C.C.G.; et al. Screening of popcorn genotypes for drought tolerance using canonical correlations. Agronomy 2020, 10, 1519. [Google Scholar] [CrossRef]
- Legendre, P.; Birks, H.J.B. From Classical to canonical ordination. In Tracking Environmental Change Using Lake Sediments; Springer: Dordrecht, The Netherlands, 2012; pp. 201–248. [Google Scholar]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef]
- Kaur, G.; Asthir, B. Proline: A key player in plant abiotic stress tolerance. Biol. Plant. 2015, 59, 609–619. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, U.K.; Islam, M.N.; Siddiqui, M.N.; Cao, X.; Khan, M.A.R. Proline, a multifaceted signalling molecule in plant responses to abiotic stress: Understanding the physiological mechanisms. Plant Biol. 2022, 24, 227–239. [Google Scholar] [CrossRef]
- Mafakheri, A.; Siosemardeh, A.; Bahramnejad, B.; Struik, P.C.; Sohrabi, Y. Effect of drought stress on yield, proline and chloro-phyll contents in three chickpea cultivars. Aust. J. Crop Sci. 2010, 8, 580–585. [Google Scholar]
- Mwadzingeni, L.; Shimelis, H.; Tesfay, S.; Tsilo, T.J. Screening of bread wheat genotypes for drought tolerance using phenotypic and proline analyses. Front. Plant Sci. 2016, 7, 1276. [Google Scholar] [CrossRef] [Green Version]
- Al Hassan, M.; López-Gresa, M.P.; Boscaiu, M.; Vicente, O. Stress tolerance mechanisms in Juncus: Responses to salinity and drought in three Juncus species adapted to different natural environments. Funct. Plant Biol. 2016, 43, 949. [Google Scholar] [CrossRef]
- Al Hassan, M.; Pacurar, A.; López-Gresa, M.P.; Donat-Torres, M.P.; Llinares, J.V.; Boscaiu, M.; Vicente, O. Effects of salt stress on three ecologically distinct Plantago species. PLoS ONE 2016, 11, e0160236. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Cuin, T.; Zhou, M.; Twomei, A.; Naidu, B.; Shabala, S. Compatible solute accumulation and stress-mitigating effects in barley genotypes contrasting in their salt tolerance. J. Exp. Bot. 2007, 58, 4245–4255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozminska, A.; Al Hassan, M.; Hanus-Fajerska, E.; Naranjo, M.A.; Vicente, O.; Boscaiu, M. Comparative analysis of water deficit and salt tolerance mechanisms in Silene. S. Afr. J. Bot. 2018, 117, 193–206. [Google Scholar] [CrossRef]
- Baker, H.G. The evolution of weeds. Annu. Rev. Ecol. Syst. 1974, 5, 1–24. [Google Scholar] [CrossRef]
- Davidson, A.M.; Jennions, M.; Nicotra, A.B. Do invasive species show higher phenotypic plasticity than native species and, if so, is it adaptive? A meta-analysis. Ecol. Lett. 2011, 14, 419–431. [Google Scholar] [CrossRef]
- Granata, M.U.; Bracco, F.; Catoni, R. Phenotypic plasticity of two invasive alien plant species inside a deciduous forest in a strict nature reserve in Italy. J. Sustain. For. 2020, 39, 346–364. [Google Scholar] [CrossRef]
- Fakhr, M.A.; Mazrou, Y.S.A.; Ellmouni, F.Y.; ElSaied, A.; Elhady, M.; Elkelish, A.; Nour, I.H. Investigating the phenotypic plasticity of the invasive weed Trianthema portulacastrum L. Plants 2021, 11, 77. [Google Scholar] [CrossRef]
- Griffith, A.B.; Andonian, K.; Weiss, C.P.; Loik, M.E. Variation in phenotypic plasticity for native and invasive populations of Bromus tectorum. Biol. Invasions 2014, 16, 2627–2638. [Google Scholar] [CrossRef] [Green Version]

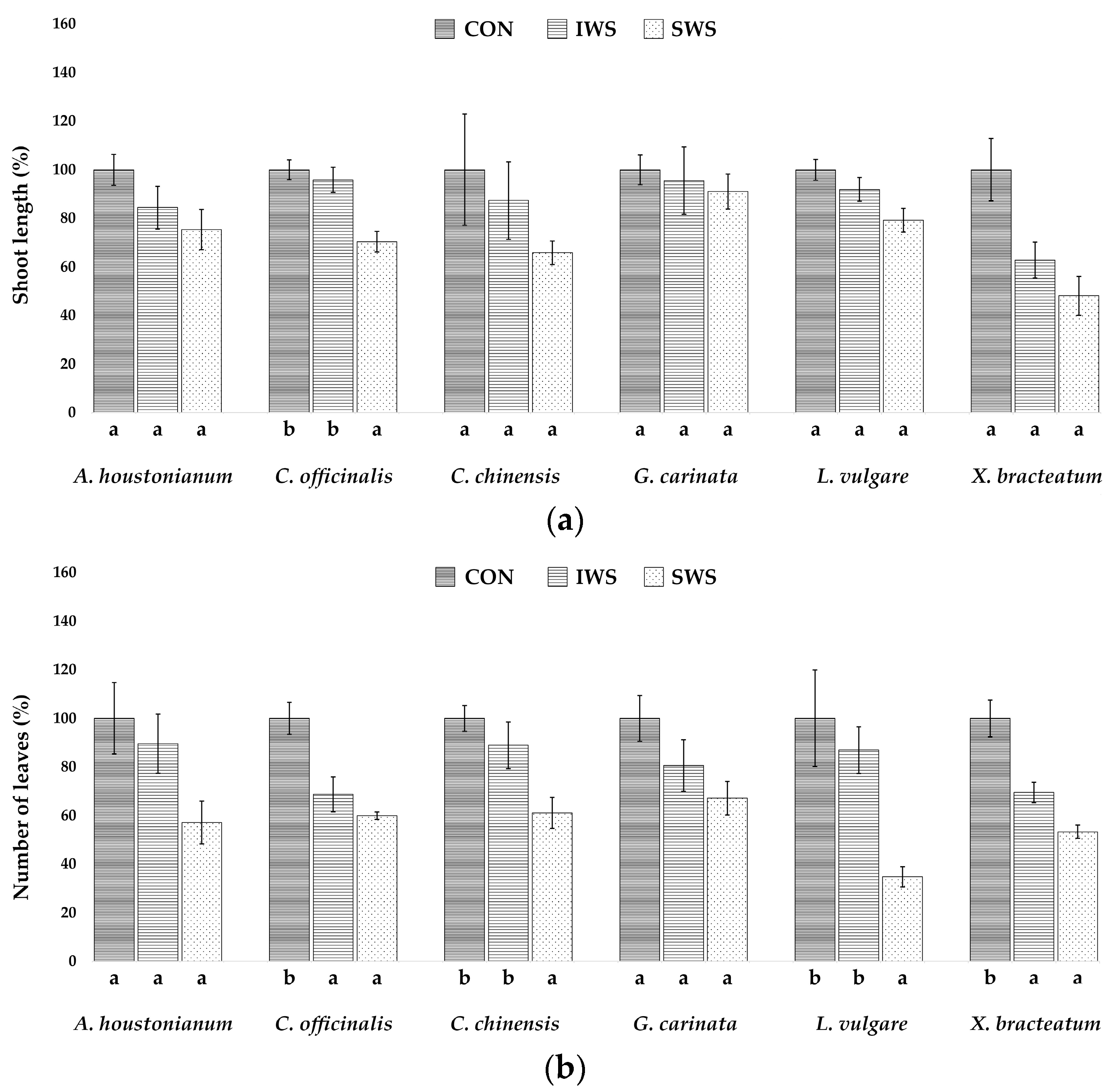
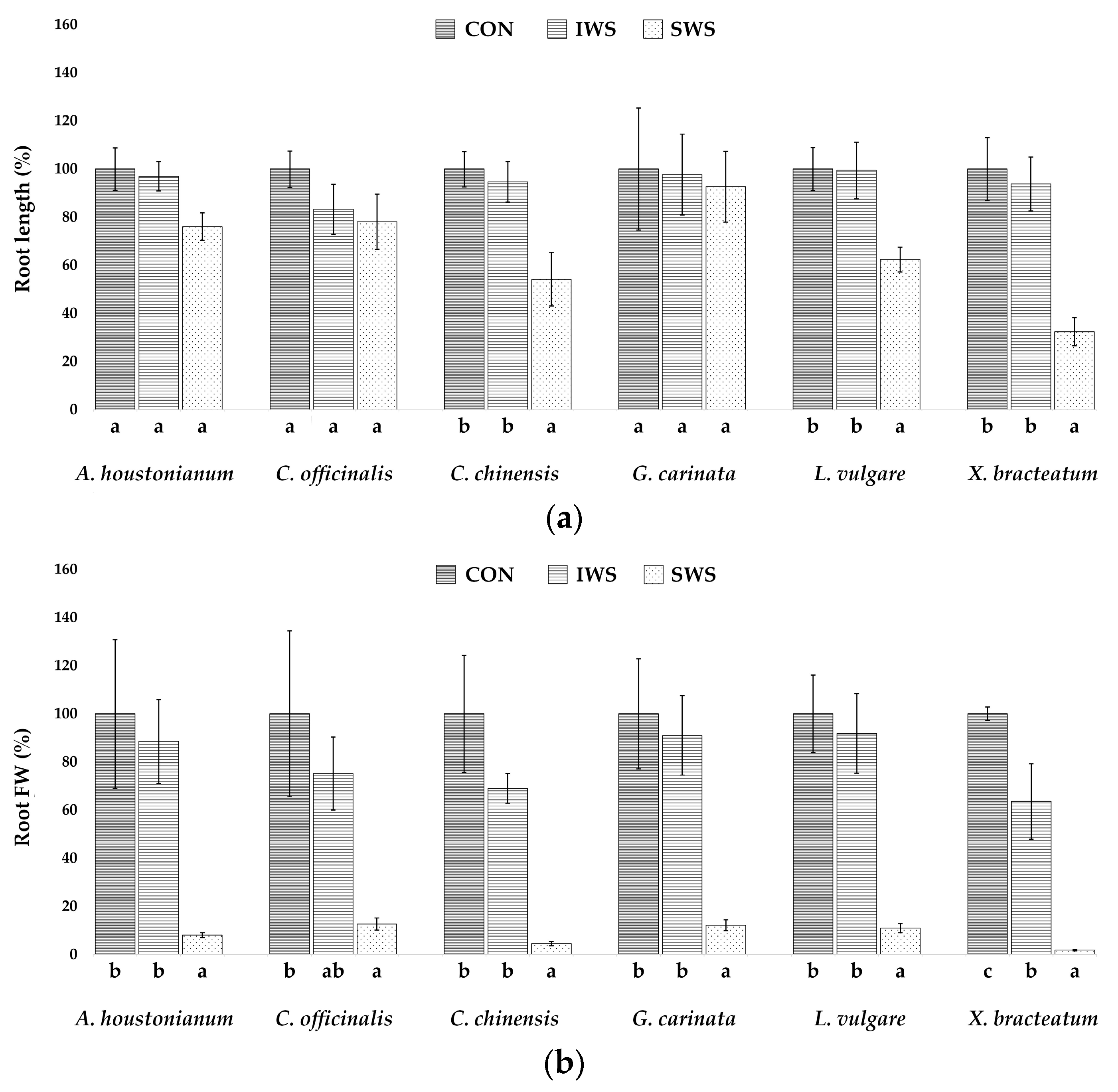
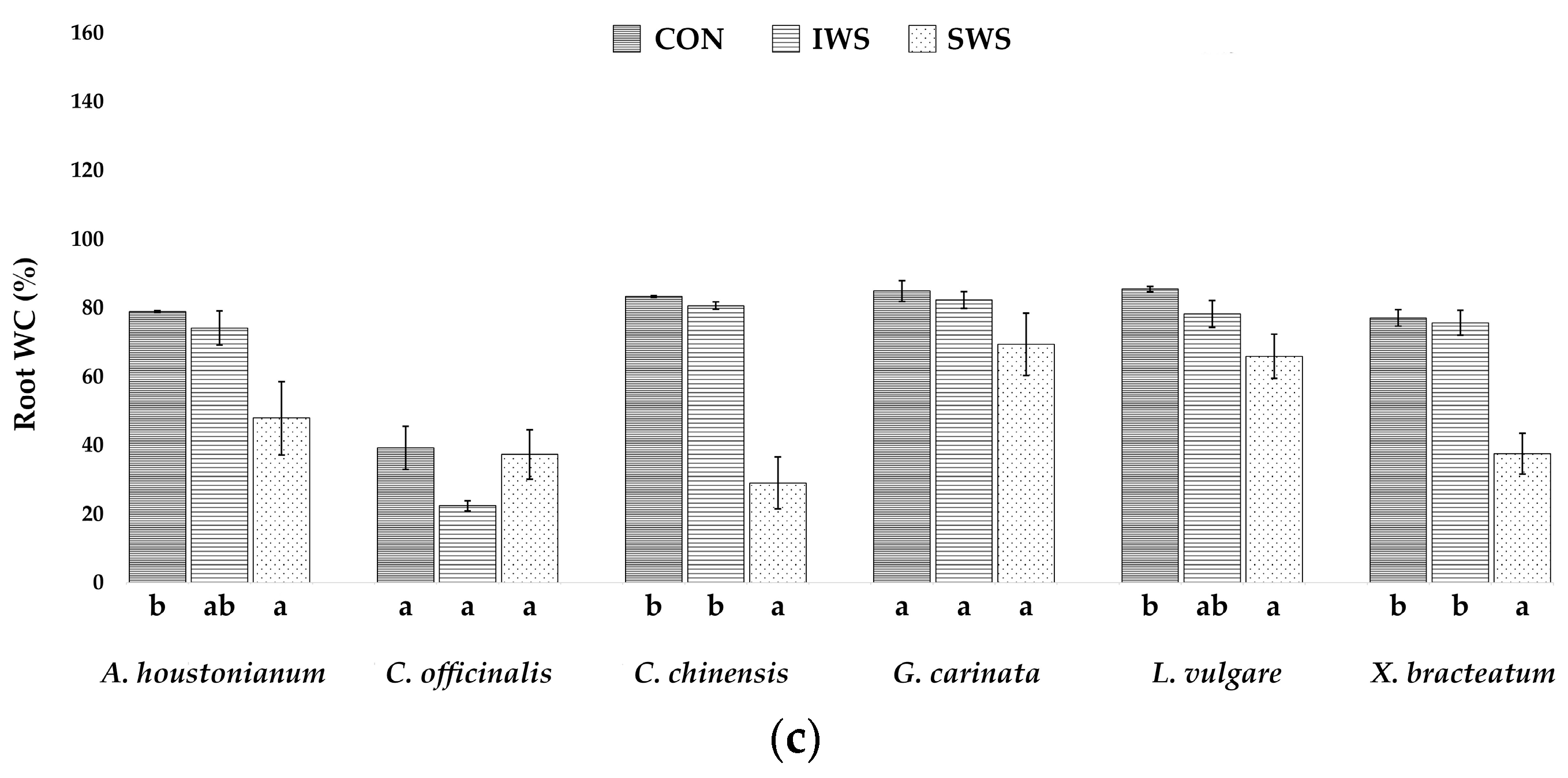
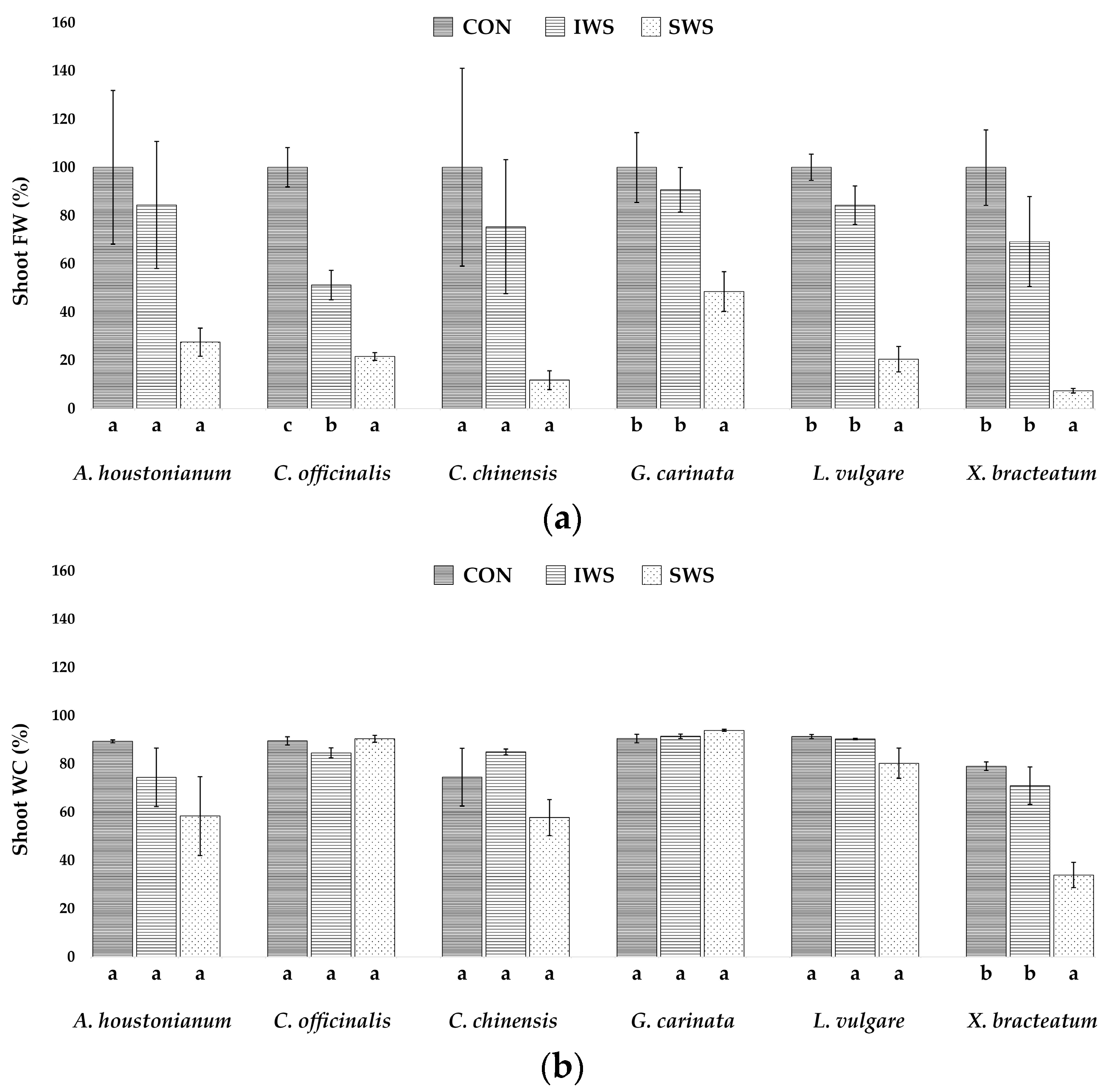
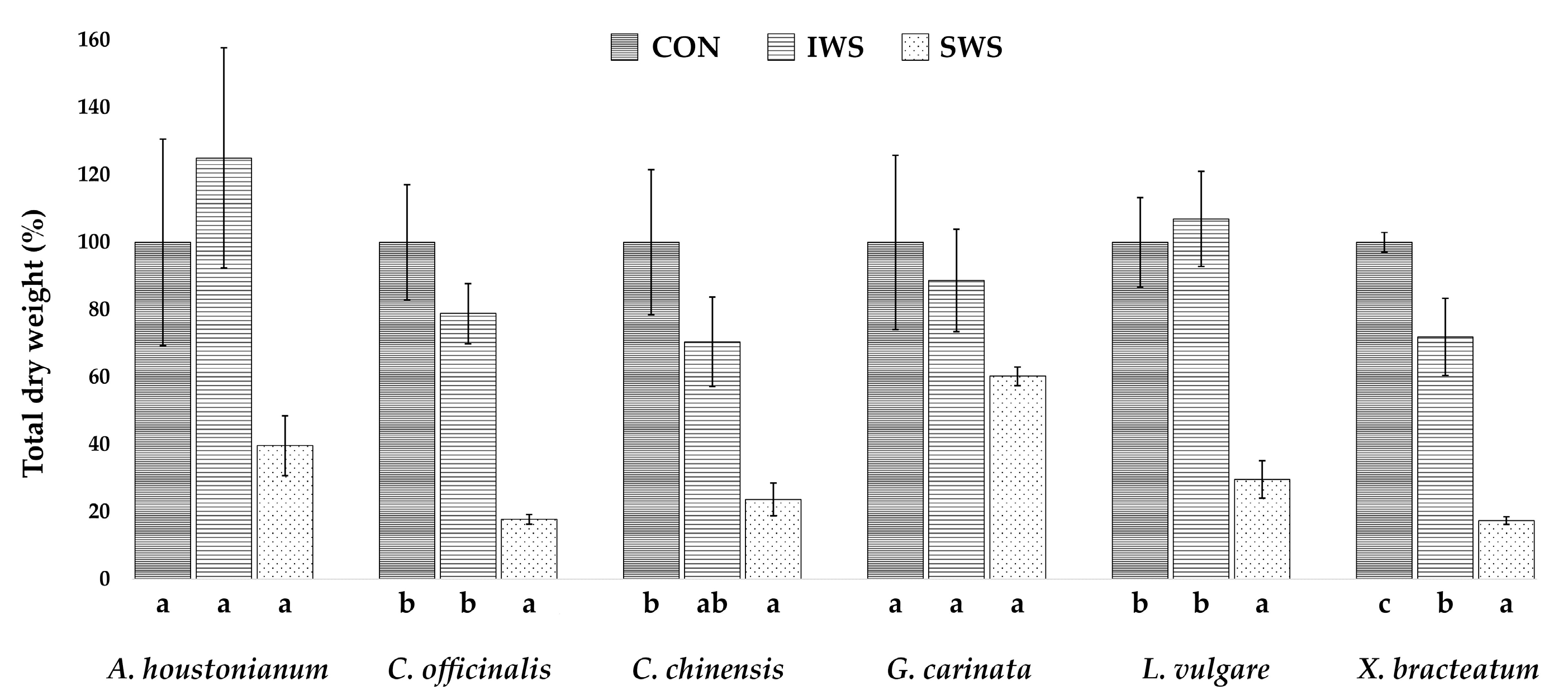

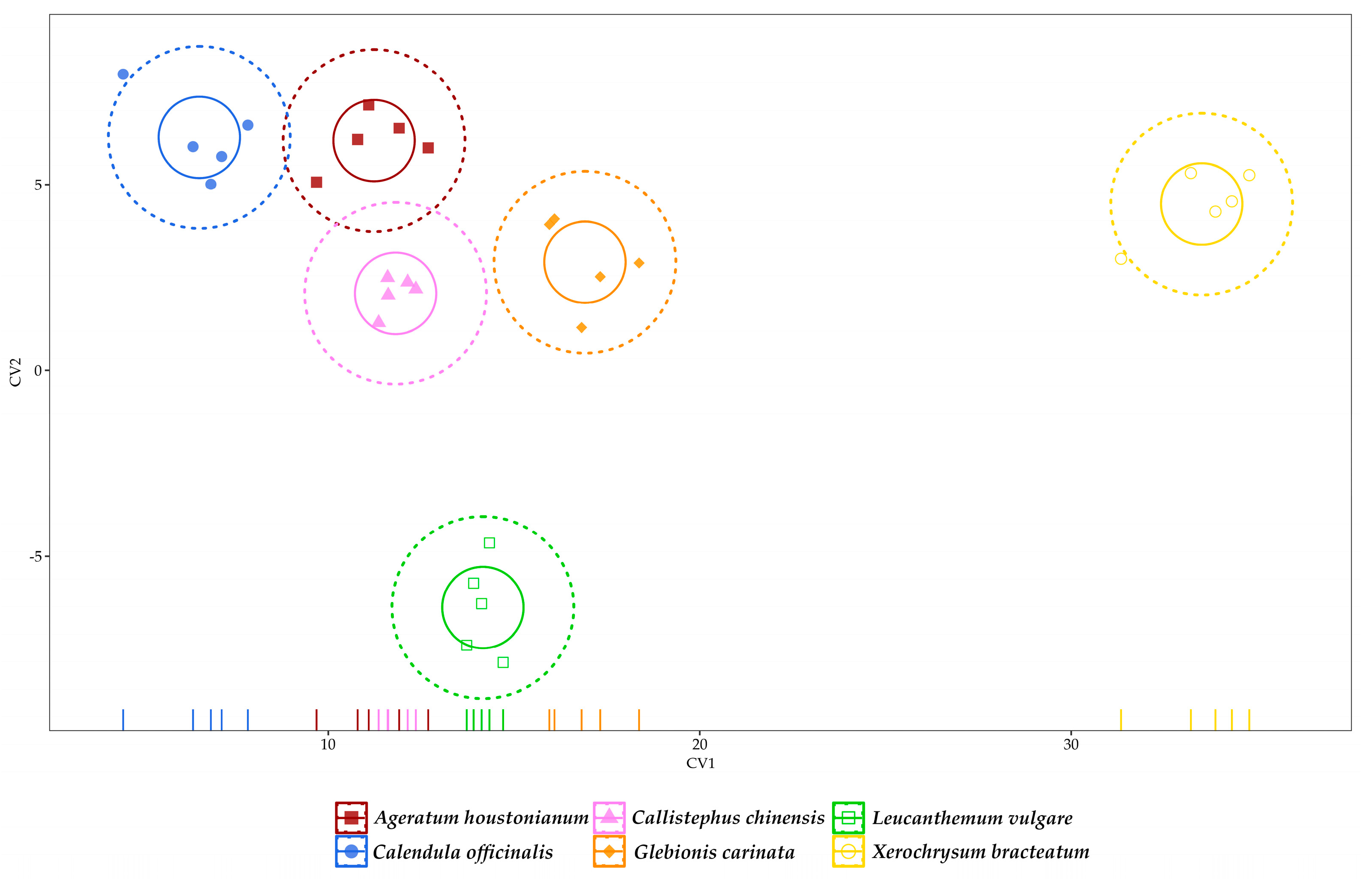
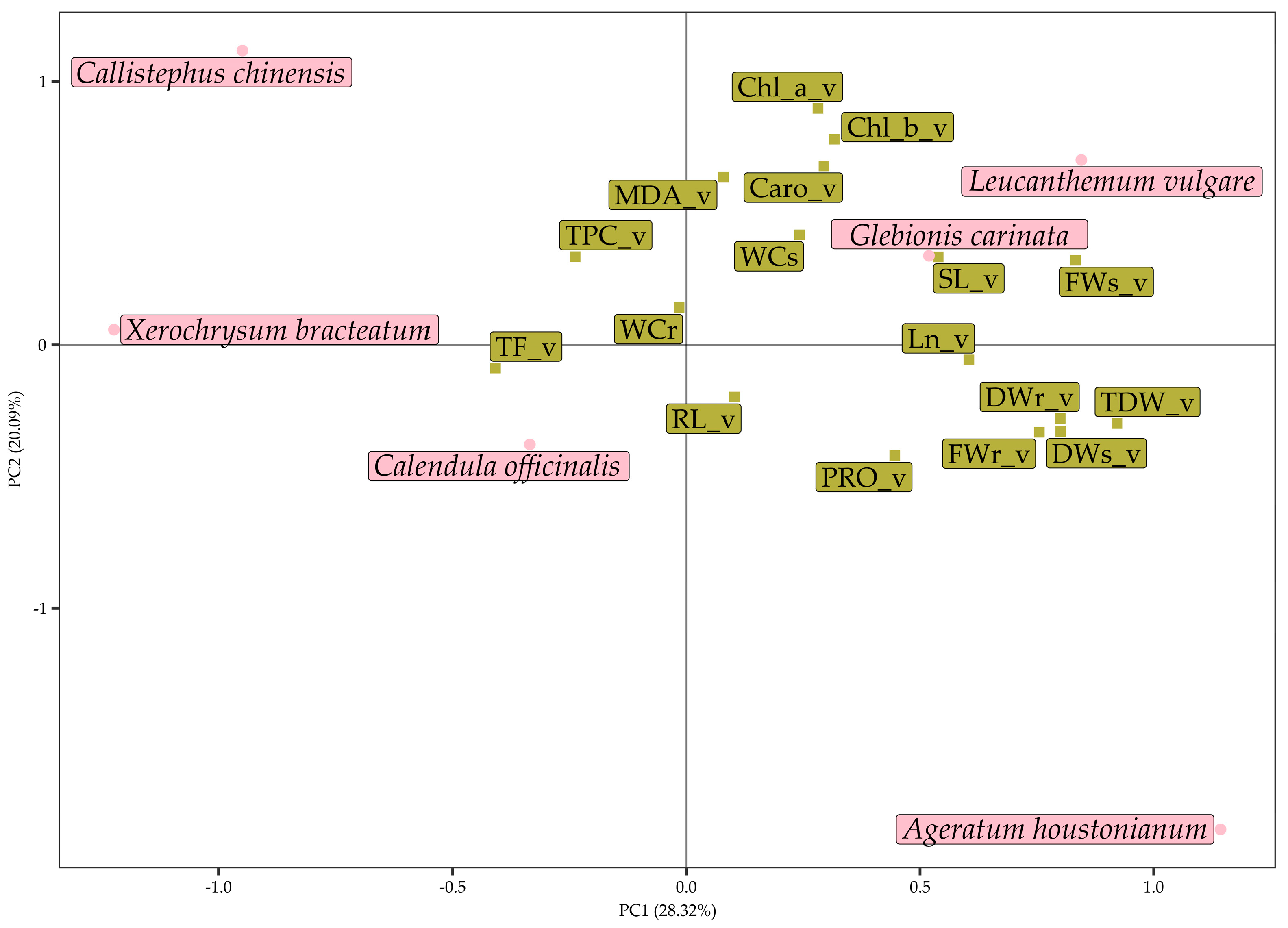

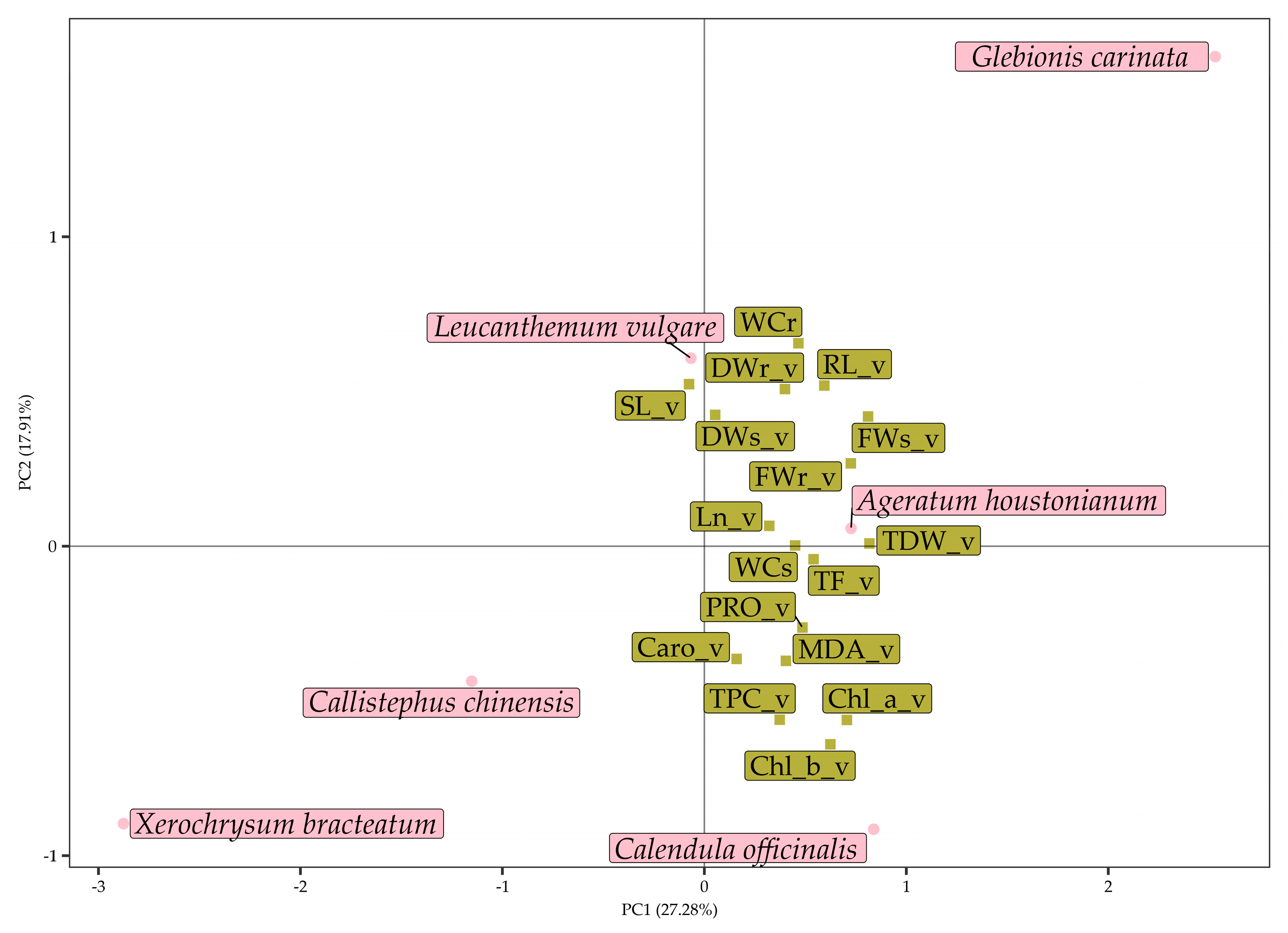
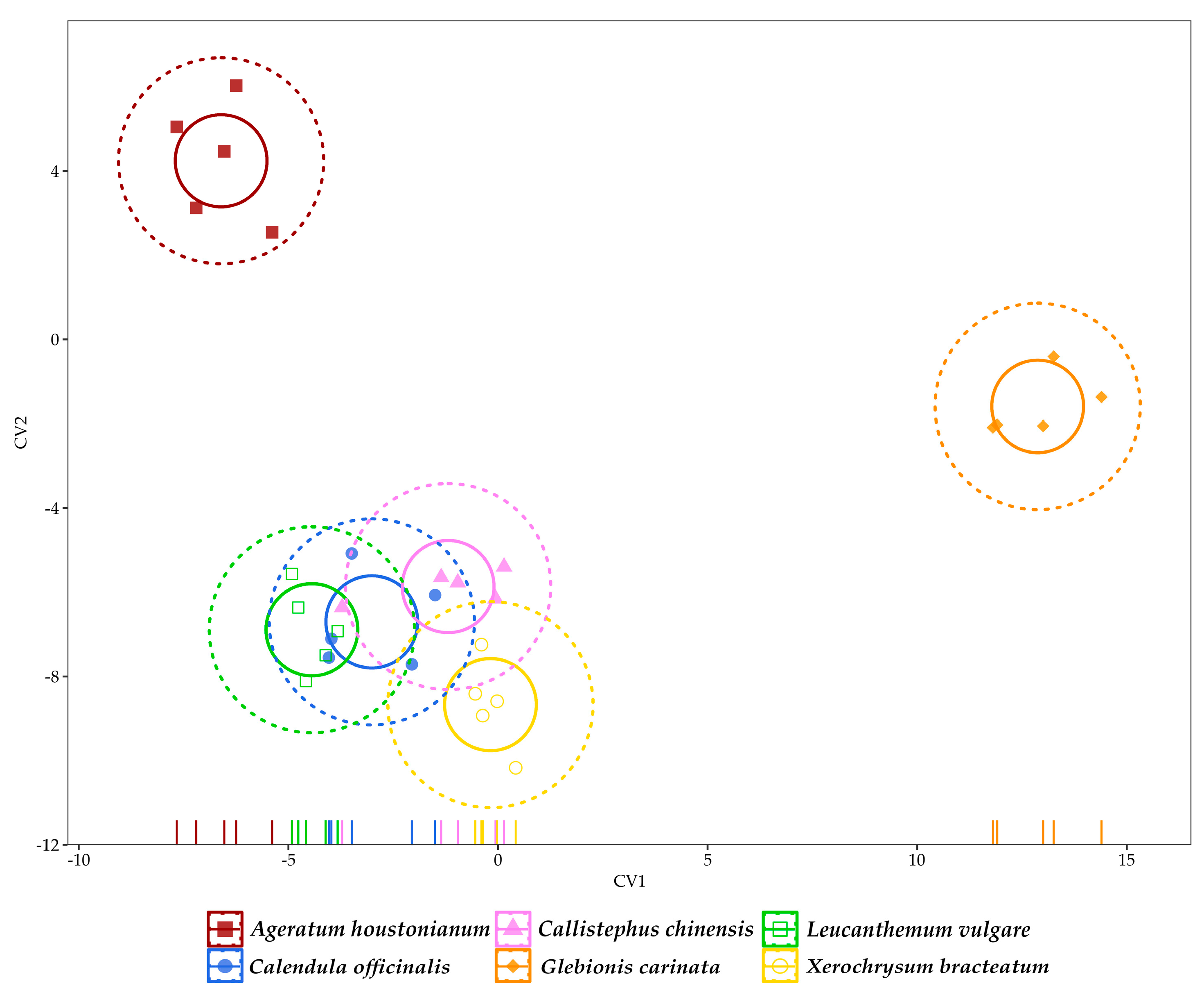
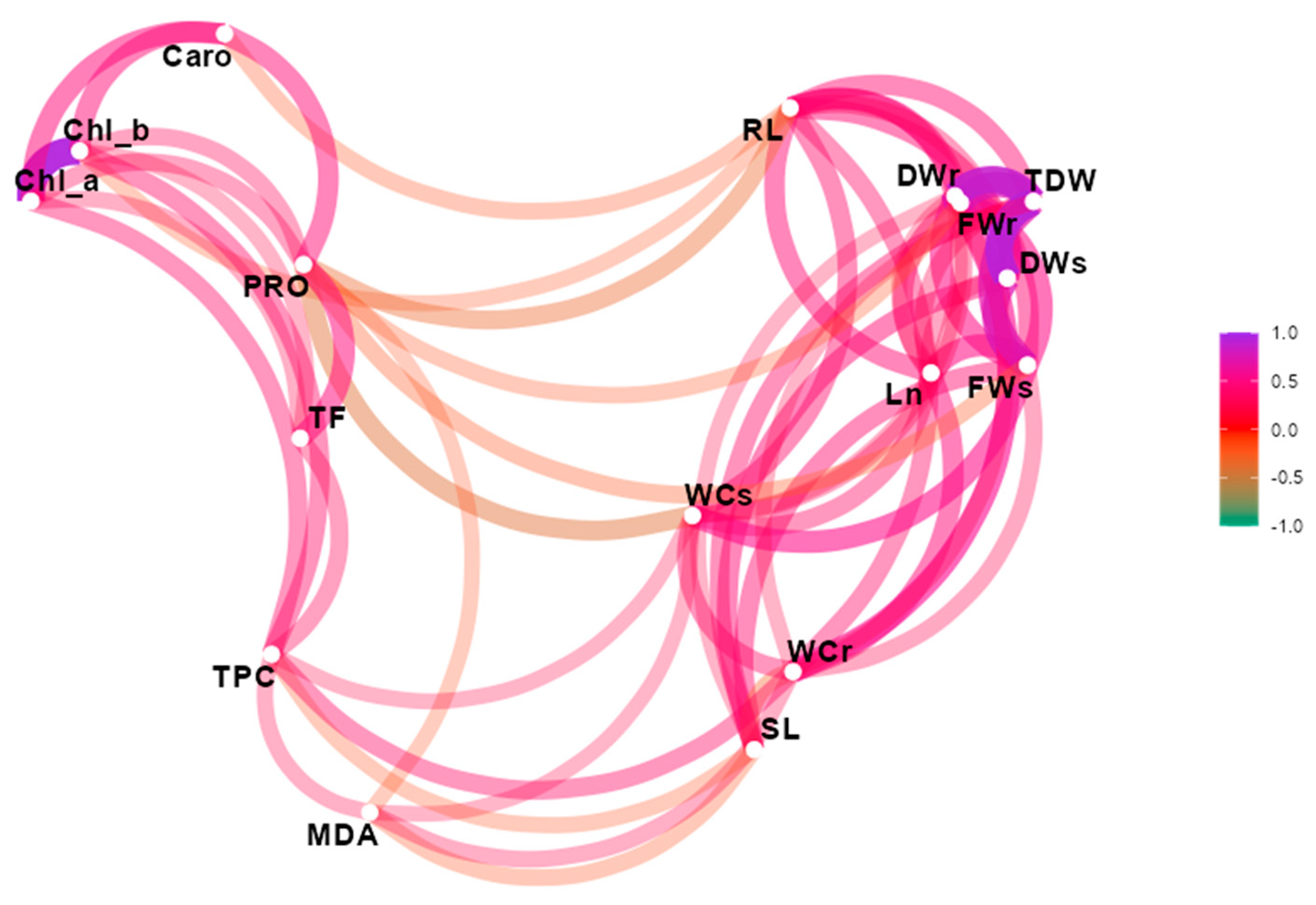
| Parameter | A. houstonianum | C. officinalis | C. chinensis | G. carinata | L. vulgare | X. bracteatum |
|---|---|---|---|---|---|---|
| SL (cm) | 16.6 ± 1.0 | 24.8 ± 0.9 | 14.3 ± 3.2 | 15.5 ± 0.9 | 10.1 ± 0.4 | 11.3 ± 1.4 |
| Ln | 15.4 ± 2.2 | 16.0 ± 1.0 | 18.0 ± 0.9 | 40.2 ± 3.7 | 23.0 ± 4.5 | 21.0 ± 1.5 |
| RL (cm) | 29.7 ± 2.6 | 27.9 ± 2.1 | 35.0 ± 2.5 | 34.0 ± 8.6 | 32.3 ± 2.9 | 29.3 ± 3.8 |
| FWr (g) | 6.3 ± 1.9 | 1.4 ± 0.4 | 5.1 ± 1.2 | 4.5 ± 1.0 | 5.4 ± 0.8 | 4.6 ± 0.1 |
| DWr (g) | 1.3 ± 0.3 | 0.8 ± 0.2 | 0.8 ± 0.1 | 0.6 ± 0.2 | 0.8 ± 0.1 | 1.0 ± 0.1 |
| WCr (%) | 78.9 ± 0.2 | 39.2 ± 6.2 | 83.3 ± 0.2 | 84.8 ± 3.0 | 85.4 ± 0.7 | 77.0 ± 2.3 |
| FWs (g) | 8.0 ± 2.5 | 19.4 ± 1.5 | 8.5 ± 3.5 | 14.0 ± 2.0 | 17.3 ± 0.9 | 10.3 ± 1.6 |
| DWs (g) | 0.8 ± 0.2 | 2.0 ± 0.3 | 1.3 ± 0.3 | 1.3 ± 0.3 | 1.5 ± 0.1 | 2.0 ± 0.1 |
| WCs (%) | 89.3 ± 0.5 | 89.5 ± 1.6 | 74.5 ± 11.8 | 90.4 ± 1.7 | 91.2 ± 0.8 | 79.0 ± 1.7 |
| TDW (g) | 2.1 ± 0.6 | 2.8 ± 0.4 | 2.2 ± 0.4 | 2.0 ± 0.5 | 2.3 ± 0.3 | 3.1 ± 0.0 |
| Parameter | Treatment | A. houstonianum | C. officinalis | C. chinensis | G. carinata | L. vulgare | X. bracteatum |
|---|---|---|---|---|---|---|---|
| Chl a (mg g−1 DW) | CON | 6.5 ± 0.8 a | 5.6 ± 1.2 a | 4.4 ± 1.1 a | 4.3 ± 1.0 a | 6.3 ± 0.3 a | 9.0 ± 2.6 a |
| IWS | 4.4 ± 0.5 a | 4.6 ± 0.6 a | 4.5 ± 1.2 a | 4.5 ± 0.4 a | 6.1 ± 0.5 a | 7.2 ± 0.8 a | |
| SWS | 8.7 ± 4.5 a | 9.4 ± 2.1 a | 3.8 ± 0.2 a | 6.2 ± 0.3 a | 4.5 ± 0.7 a | 7.5 ± 0.3 a | |
| Chl b (mg g−1 DW) | CON | 2.0 ± 0.1 a | 1.6 ± 0.2 a | 1.4 ± 0.4 a | 1.7 ± 0.5 a | 2.4 ± 0.1 a | 4.2 ± 1.9 a |
| IWS | 1.4 ± 0.0 a | 1.6 ± 0.2 a | 1.3 ± 0.3 a | 1.8 ± 0.2 a | 2.2 ± 0.2 a | 2.5 ± 0.3 a | |
| SWS | 4.6 ± 3.1 a | 3.7 ± 0.7 b | 1.3 ± 0.1 a | 2.6 ± 0.1 a | 1.8 ± 0.2 a | 3.5 ± 0.1 a | |
| Caro (mg g−1 DW) | CON | 1.2 ± 0.1 a | 0.7 ± 0.1 a | 0.6 ± 0.1 a | 0.9 ± 0.2 a | 0.8 ± 0.1 a | 1.3 ± 0.3 a |
| IWS | 0.8 ± 0.1 a | 0.7 ± 0.1 a | 0.7 ± 0.1 a | 0.8 ± 0.1 a | 1.1 ± 0.2 a | 1.3 ± 0.1 a | |
| SWS | 1.3 ± 0.8 a | 1.2 ± 0.2 a | 0.8 ± 0.0 a | 1.8 ± 0.6 a | 0.7 ± 0.1 a | 3.1 ± 1.7 a | |
| PRO (µmol g−1 DW) | CON | 3.2 ± 1.3 a | 6.5 ± 2.9 a | 2.5 ± 0.3 a | 8.0 ± 2.3 a | 3.2 ± 1.0 a | 98.0 ± 13.6 a |
| IWS | 14.5 ± 3.6 a | 31.9 ± 5.9 b | 1.2 ± 0.3 a | 28.0 ± 8.9 ab | 2.1 ± 0.5 a | 137.8 ± 12.0 ab | |
| SWS | 53.9 ± 11.7 b | 40.9 ± 5.1 b | 13.3 ± 2.6 b | 43.0 ± 3.8 b | 9.4 ± 2.1 b | 154.9 ± 12.4 b | |
| MDA (nmol g−1 DW) | CON | 427.0 ± 56.6 a | 395 ± 41.6 a | 502.9 ± 131.3 a | 458.9 ± 142.5 a | 1281.3 ± 184.4 a | 428.7 ± 53.6 a |
| IWS | 372.9 ± 82.4 a | 308.8 ± 42.2 a | 537.0 ± 137.1 a | 421.4 ± 124.2 a | 1283.6 ± 226.8 a | 499.1 ± 100.4 a | |
| SWS | 716.9 ± 216.3 a | 347.8 ± 48.9 a | 249.2 ± 52.2 a | 326.0 ± 124.4 a | 2033.7 ± 479.9 a | 244.9 ± 24.7 a | |
| TF (mg eq. C g−1 DW) | CON | 22.5 ± 5.5 a | 5.6 ± 1.0 a | 7.1 ± 2.4 a | 3.0 ± 0.6 a | 8.7 ± 1.6 a | 53.3 ± 14.4 a |
| IWS | 10.8 ± 3.0 a | 3.7 ± 0.5 a | 19.4 ± 7.3 a | 4.4 ± 0.9 a | 7.6 ± 1.3 a | 54.9 ± 11.8 a | |
| SWS | 7.6 ± 5.0 a | 5.2 ± 0.6 a | 8.4 ± 2.7 a | 5.2 ± 0.7 a | 5.7 ± 0.6 a | 23.8 ± 4.1 a | |
| TPC (mg eq. GA g−1 DW) | CON | 26.8 ± 5.0 a | 8.1 ± 1.1 a | 13.5 ± 3.3 a | 13.9 ± 4.2 a | 11.4 ± 1.2 a | 14.0 ± 4.2 a |
| IWS | 16.3 ± 4.6 a | 6.5 ± 0.2 a | 18.9 ± 5.2 a | 11.3 ± 1.3 a | 14.3 ± 2.3 a | 16.6 ± 1.4 a | |
| SWS | 16.9 ± 8.0 a | 7.4 ± 0.9 a | 12.6 ± 1.8 a | 10.5 ± 3.5 a | 12.6 ± 2.4 a | 9.4 ± 1.5 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mircea, D.M.; Calone, R.; Shakya, R.; Saavedra, M.F.; Sestras, R.E.; Boscaiu, M.; Sestras, A.F.; Vicente, O. Use of Multivariate Analysis in Screening for Drought Tolerance in Ornamental Asteraceae Species. Agronomy 2023, 13, 687. https://doi.org/10.3390/agronomy13030687
Mircea DM, Calone R, Shakya R, Saavedra MF, Sestras RE, Boscaiu M, Sestras AF, Vicente O. Use of Multivariate Analysis in Screening for Drought Tolerance in Ornamental Asteraceae Species. Agronomy. 2023; 13(3):687. https://doi.org/10.3390/agronomy13030687
Chicago/Turabian StyleMircea, Diana M., Roberta Calone, Rashmi Shakya, Martín Flores Saavedra, Radu E. Sestras, Monica Boscaiu, Adriana F. Sestras, and Oscar Vicente. 2023. "Use of Multivariate Analysis in Screening for Drought Tolerance in Ornamental Asteraceae Species" Agronomy 13, no. 3: 687. https://doi.org/10.3390/agronomy13030687
APA StyleMircea, D. M., Calone, R., Shakya, R., Saavedra, M. F., Sestras, R. E., Boscaiu, M., Sestras, A. F., & Vicente, O. (2023). Use of Multivariate Analysis in Screening for Drought Tolerance in Ornamental Asteraceae Species. Agronomy, 13(3), 687. https://doi.org/10.3390/agronomy13030687












