Nutritive Profile, Digestibility, and Carbohydrate Fractionation of Three Sugarcane Genotypes Treated with Calcium Oxide
Abstract
1. Introduction
2. Materials and Methods
2.1. Area, Experimental Design, and Tretament Procedure
2.2. Sampling and Chemical Analyses
2.3. Statistical Analyses
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Melo, C.D.; Maduro Dias, C.S.; Wallon, S.; Borba, A.E.; Madruga, J.; Borges, P.A.; Ferreira, M.T.; Elias, R.B. Influence of Climate Variability and Soil Fertility on the Forage Quality and Productivity in Azorean Pastures. Agriculture 2022, 12, 358. [Google Scholar] [CrossRef]
- Rojas-Downing, M.M.; Nejadhashemi, A.P.; Harrigan, T.; Woznicki, S.A. Climate change and livestock: Impacts, adaptation, and mitigation. Clim. Risk Manag. 2017, 16, 145–163. [Google Scholar] [CrossRef]
- Tedeschi, L.O.; Molle, G.; Menendez, H.M.; Cannas, A.; Fonseca, M.A. The assessment of supplementation requirements of grazing ruminants using nutrition models. Transl. Anim. Sci. 2019, 3, 811–828. [Google Scholar] [CrossRef]
- Carvalho, B.F.; Ávila, C.L.S.; Pinto, J.C.; Pereira, M.N.; Schwan, R.F. Effects of propionic acid and Lactobacillus buchneri (UFLA SIL 72) addition on fermentative and microbiological characteristics of sugar cane silage treated with and without calcium oxide. Grass Forage Sci. 2012, 67, 462–471. [Google Scholar] [CrossRef]
- Gunun, N.; Wanapat, M.; Gunun, P.; Cherdthong, A.; Khejornsart, P.; Kang, S. Effect of treating sugarcane bagasse with urea and calcium hydroxide on feed intake, digestibility, and rumen fermentation in beef cattle. Trop. Anim. Health Prod. 2016, 48, 1123–1128. [Google Scholar] [CrossRef]
- Cursi, D.E.; Hoffmann, H.P.; Barbosa, G.V.S.; Bressiani, J.A.; Gazaffi, R.; Chapola, R.G.; Fernandes Junior, A.R.; Balsalobre, T.W.A.; Diniz, C.A.; Santos, J.M.; et al. History and current status of sugarcane breeding, germplasm development and molecular genetics in Brazil. Sugar Tech 2021, 24, 112–133. [Google Scholar] [CrossRef]
- Almeida, G.A.P.; Ferreira, M.A.; Silva, J.L.; Chagas, J.C.C.; Véras, A.S.C.; de Barros, L.J.A.; de Almeida, G.L.P. Sugarcane bagasse as exclusive roughage for dairy cows in smallholder livestock system. Asian Australas. J. Anim. Sci. 2018, 31, 379–385. [Google Scholar] [CrossRef]
- Souza, J.M.; de Sousa, D.O.; de Mesquita, B.S.; Mesquita, L.G.; Silva, L.F.P. Effect of sugarcane fiber digestibility, conservation method and concentrate level on the ruminal ecosystem of beef cattle. AMB Express 2017, 7, 55. [Google Scholar] [CrossRef]
- Carvalho, M.V.; Rodrigues, P.H.M.; Lima, M.L.P.; dos Anjos, I.A.; de Andrade Landell, M.G.; dos Santos, M.V.; Prada, L.F. Chemical composition and digestibility of sugarcane harvested at two periods of the year. Braz. J. Vet. Res. Anim. Sci. 2010, 47, 298–306. [Google Scholar] [CrossRef]
- Chizzotti, F.H.M.; Pereira, O.G.; Valadares Filho, S.C.; Chizzotti, M.L.; Rodrigues, R.T.S.; Tedeschi, L.O.; Silva, T.C. Does sugar cane ensiled with calcium oxide affect intake, digestibility, performance, and microbial efficiency in beef cattle? Anim. Feed Sci. Technol. 2015, 203, 23–32. [Google Scholar] [CrossRef]
- Bachmann, M.; Martens, S.D.; Le Brech, Y.; Kervern, G.; Bayreuther, R.; Steinhöfel, O.; Zeyner, A. Physicochemical characterisation of barley straw treated with sodium hydroxide or urea and its digestibility and in vitro fermentability in ruminants. Sci. Rep. 2022, 12, 20530. [Google Scholar] [CrossRef] [PubMed]
- Uzatici, A.; Canbolat, O.; Kamalak, A. Effect of Sodium Hydroxide Treatment on Chemical Composition and Feed Value of Common Reed (Phragmites australis) Straw. Fermentation 2022, 8, 749. [Google Scholar] [CrossRef]
- Casperson, B.A.; Wertz-Lutz, A.E.; Dunn, J.L.; Donkin, S.S. Inclusion of calcium hydroxide-treated corn stover as a partial forage replacement in diets for lactating dairy cows. J. Dairy Sci. 2018, 101, 2027–2036. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, B.G.; İstanbullu, H.B.; Schmidt, M.; Logroño, W.; Harms, H.; Nikolausz, M. Effect of Alkaline and Mechanical Pretreatment of Wheat Straw on Enrichment Cultures from Pachnoda marginata Larva Gut. Fermentation 2023, 9, 60. [Google Scholar] [CrossRef]
- Lancaster, N.A.; Muegge, C.R.; Carvalho, J.R.; Lopes, R.C.; Narumiya, R.S.; Pinese, F.; Baird, A.N.; Schoonmaker, J.P. Effect of calcium oxide and soybean hull addition to feedlot diets containing dried distillers grains and corn stover on steer performance, carcass characteristics, and digestibility. Transl. Anim. Sci. 2020, 4, txaa105. [Google Scholar] [CrossRef]
- Stehr, K.R.; Terry, S.A.; Ribeiro, G.O.; Gruninger, R.J.; Penner, G.B.; McKinnon, J.; Gibb, D.; McAllister, T.A. Effect of replacing barley silage with calcium oxide-treated barley straw on rumen fermentation, rumen microbiota, nutrient digestibility, and growth performance of finishing beef cattle. Can. J. Anim. Sci. 2021, 101, 493–506. [Google Scholar] [CrossRef]
- de Pádua, F.T.; Fontes, C.A.; Deminicis, B.B.; de Almeida Carlos, L.; Neto, O.C.; de Oliveira, V.C. Fermentation characteristics of silage of sugar cane treated with calcium oxide, lactobacillus buchneri and their associations. Am. J. Plant Sci. 2014, 5, 636–646. [Google Scholar] [CrossRef]
- Romão, C.O.; Carvalho, G.G.P.; Leite, V.M.; Santos, A.S.; Chagas, D.M.T.; Ribeiro, O.L.; Oliveira, P.A.; Magalhães, A.F.; Pires, A.J.V. Chemical composition and dry matter digestibility of sugar cane oxide treated with calcium. Arq. Bras. Med. Vet. Zootec. 2014, 66, 529–538. [Google Scholar] [CrossRef]
- Siqueira, G.; Arantes, V.; Saddler, J.N.; Ferraz, A.; Milagres, A.M. Limitation of cellulose accessibility and unproductive binding of cellulases by pretreated sugarcane bagasse lignin. Biotechnol. Biofuels 2017, 10, 176. [Google Scholar] [CrossRef]
- Domingues, F.N.; Oliveira, M.D.S.D.; Siqueira, G.R.; Roth, A.P.D.T.P.; Santos, J.D.; Mota, D.A. Aerobic stability, pH and development dynamic of microorganisms on fresh sugarcane hydrolyzed with whitewash. Rev. Bras. Zootec. 2011, 40, 715–719. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists, 18th ed.; Association of Official Analytical Chemists Inc.: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Mertens, D.R. Gravimetric determination of amylase-treated neutral detergent fiber in feeds with refluxing in beakers or crucibles: Collaborative study. J. AOAC Int. 2002, 85, 1217–1240. [Google Scholar] [PubMed]
- Van Soest, P.J.; Wine, R.H. Use of Detergents in the Analysis of Fibrous Feeds. IV. Determination of Plant Cell-Wall Constituents. J. Assoc. Off. Anal. Chem. 1967, 50, 50–55. [Google Scholar] [CrossRef]
- Goering, H.K.; Van Soest, P.J. Forage Fiber Analysis: Apparatus, Reagents, Procedures, and Some Applications. In Agriculture Handbook; United States Department of Agriculture: Washington, DC, USA, 1970. [Google Scholar]
- Sniffen, C.J.; O’connor, J.D.; Van Soest, P.J.; Fox, D.G.; Russell, J.B. A net carbohydrate and protein system for evaluating cattle diets: II. Carbohydrate and protein availability. J. Anim. Sci. 1992, 70, 3562–3577. [Google Scholar] [CrossRef] [PubMed]
- Métodos Para Análise de Alimentos. INCT, Ciência Animal, 1st ed.; Detmann, E., Souza, M.A., Valadares Filho, S.C., Queiroz, A.C., Berchielle, T.T., Saliba, E.O.S., Cabral, L.S., Pina, D.S., Ladeira, M.M., Azevedo, J.A.G., Eds.; Suprema: Visconde do Rio Branco, Brazil, 2012. [Google Scholar]
- NRC—National Research Council. Nutrient Requirements of Dairy Cattle, 7th ed.; The National Academies Press: Washinton, DC, USA, 2001. [Google Scholar]
- Bataglia, O.C.; Furlani, A.M.C.; Teixeira, J.P.F.; Furlani, P.R.; Gallo, J.R. Métodos de Análise Química de Plantas; Boletim Técnico; Instituto Agronômico: Campinas, Brazil, 1983. [Google Scholar]
- de Souza, N.R.D.; Fracarolli, J.A.; Junqueira, T.L.; Chagas, M.F.; Cardoso, T.F.; Watanabe, M.D.; Cavalett, O.; Venzke Filho, S.P.; Dale, B.E.; Bonomi, A.; et al. Sugarcane ethanol and beef cattle integration in Brazil. Biomass Bioenergy 2019, 120, 448–457. [Google Scholar] [CrossRef]
- Netto, A.J.; Gama, M.A.S.; Guido, S.I.; Bessa, R.J.B.; Inácio, J.G.; Monteiro, C.C.F.; Melo, G.G.S.; Ribeiro, E.F.; Ferreira, M.A. Replacing corn with full-fat corn germ in a basal diet containing cactus (Opuntia stricta) cladodes and sugarcane as forage sources induces milk fat depression associated with the trans-10 shift in dairy cows. Anim. Feed Sci. Technol. 2022, 288, 115289. [Google Scholar] [CrossRef]
- Moura, R.L.; Oliveira, M.E.; Carvalho, W.F.; Rodrigues, M.M.; Santos, M.S.; Edvan, R.L.; Abdalla, A.L.; Moreira, M.Z.; Silva, E.M. Evaluation of grass and legume tropical mixtures and performance of grazed sheep. S. Afr. J. Anim. Sci. 2022, 52, 25–33. [Google Scholar] [CrossRef]
- Cabral, A.M.D.; de Albuquerque Brasil, L.H.; Marques, D.H.M.; Azevedo, M.; de Carvalho, F.F.R.; Barreto, L.M.G.; de Lima Santos, G.C. Thermoregulatory responses of Saanen goats fed increasing levels of sugarcane in place of corn silage. Small Rumin. Res. 2022, 217, 106845. [Google Scholar] [CrossRef]
- Fukushima, R.S.; Bacha, C.B.; Fuzeto, A.P.; Port, A.C.R.; Herling, V.R.; Velásquez, A.V. Utilization of equations to predict carbohydrate fractions in some tropical grasses. Anim. Feed Sci. Technol. 2015, 208, 12–22. [Google Scholar] [CrossRef]
- Carvalho, B.F.; Ávila, C.L.S.; Pinto, J.C.; Schwan, R.F. Effect of propionic acid and Lactobacillus plantarum UFLA SIL 1 on the sugarcane silage with and without calcium oxide. Afr. J. Microbiol. Res. 2013, 7, 4159–4168. [Google Scholar] [CrossRef]
- Rabelo, C.H.S.; De Rezende, A.V.; Rabelo, F.H.S.; Nogueira, D.A.; Elias, R.F.; de Faria Júnior, D.C. Aerobic stability of in natura sugarcane hydrolyzed with calcium oxide. Ciênc. Anim. Bras. 2011, 12, 257–265. [Google Scholar] [CrossRef]
- De Rezende, A.V.; Silveira Rabelo, C.H.; Andrade, L.P.; Silveira Rabelo, F.H.; Dos Santos, W.B. Characteristics of sugar cane in natura and hydrolyzed with lime in different storage times. Rev. Caatinga 2013, 26, 107–116. [Google Scholar]
- Zhang, J.; Kong, C.; Yang, M.; Zang, L. Comparison of calcium oxide and calcium peroxide pretreatments of wheat straw for improving biohydrogen production. ACS Omega 2020, 5, 9151–9161. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Ge, X.; Zhang, Q.; Zhou, X.; Chen, Z.; Keener, H.; Li, Y. Comparison of sodium hydroxide and calcium hydroxide pretreatments of giant reed for enhanced enzymatic digestibility and methane production. Bioresour. Technol. 2017, 244, 1150–1157. [Google Scholar] [CrossRef] [PubMed]
- Shetty, D.J.; Kshirsagar, P.; Tapadia-Maheshwari, S.; Lanjekar, V.; Singh, S.K.; Dhakephalkar, P.K. Alkali pretreatment at ambient temperature: A promising method to enhance biomethanation of rice straw. Bioresour. Technol. 2017, 226, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Mota, D.A.; Oliveira, M.D.S.D.; Domingues, F.N.; Manzi, G.M.; Ferreira, D.D.S.; Santos, J.D. Hydrolysis of cane sugar with lime or hydrated lime. Rev. Bras. Zootec. 2010, 39, 1186–1190. [Google Scholar] [CrossRef]
- Oliveira, M.D.S.; Barbosa, J.C.; Mota, D.A.; Andrade, A.T. Effect of virgin lime hydrolysis on bromatologic composition of sugarcane. Vet. Notícias 2008, 14, 19–27. [Google Scholar]
- Siqueira, G.R.; Roth, M.D.T.P.; Moretti, M.H.; Benatti, J.M.B.; Resende, F.D.D. Use of sugarcane in ruminant nutrition. Rev. Bras. Saúde Prod. Anim. 2012, 13, 991–1008. [Google Scholar] [CrossRef]
- Carvalho, G.G.P.D.; Garcia, R.; Pires, A.J.V.; Pereira, O.G.; Fernandes, F.É.P.; Obeid, J.A.; Carvalho, B.M.A.D. Carbohydrate fractioning of elephantgrass silage wilted or enriched with cocoa meal. Rev. Bras. Zootec. 2007, 36, 1000–1005. [Google Scholar] [CrossRef]
- Melo, L.J.O.T.D.; Oliveira, F.J.D.; Bastos, G.Q.; Anunciação Filho, C.J.D.; Reis, O.V.D. Sugarcane genotype x harvest cycles interaction in Zona da Mata Norte of Pernambuco. Bragantia 2006, 65, 197–205. [Google Scholar] [CrossRef]
- Ajala, E.O.; Ighalo, J.O.; Ajala, M.A.; Adeniyi, A.G.; Ayanshola, A.M. Sugarcane bagasse: A biomass sufficiently applied for improving global energy, environment and economic sustainability. Bioresour. Bioprocess. 2021, 8, 1–25. [Google Scholar] [CrossRef]
- Chao, Q.; Feng, A. Scientific basis of climate change and its response. Glob. Energy Interconnect. 2018, 1, 420–427. [Google Scholar] [CrossRef]
- Xiao, C.; Feng, Z.; You, Z.; Zheng, F. Population boom in the borderlands globally. J. Clean. Prod. 2022, 371, 133685. [Google Scholar] [CrossRef]
| Level of CaO (% Fresh Matter Basis) | Genotype | Time (Hours) | ||
|---|---|---|---|---|
| 0 | 12 | 24 | ||
| Temperature (°C) | ||||
| IAC-862480 | 26.3 | 26.8 | 34.9 | |
| 0 | SP-791011 | 25.7 | 24.9 | 31.3 |
| CTC-3 | 25.0 | 27.1 | 34.7 | |
| IAC-862480 | 33.3 | 28.8 | 28.8 | |
| 1.5 | SP-791011 | 32.6 | 29.1 | 28.3 |
| CTC-3 | 29.3 | 29.5 | 28.0 | |
| IAC-862480 | 39.9 | 34.5 | 30.8 | |
| 3.0 | SP-791011 | 34.7 | 32.5 | 29.6 |
| CTC-3 | 33.1 | 33.5 | 30.3 | |
| IAC-862480 | 44.0 | 34.7 | 31.4 | |
| 4.5 | SP-791011 | 34.9 | 33.5 | 29.9 |
| CTC-3 | 35.2 | 36.0 | 31.1 | |
| Item 1 | Genotype | CaO Level (%) | SEM | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| IAC-862480 | SP-791011 | CTC-3 | 0 | 1.5 | 3.0 | 4.5 | Genotype | CaO Level | Genotype × CaO Level | ||
| Chemical Composition; g kg−1 (Dry matter basis) | |||||||||||
| Dry matter | 353.8 ab | 375.8 a | 339.7 b | 302.8 | 356.2 | 378.6 | 388.2 | 1.7 | <0.01 | <0.01 | 0.65 |
| Organic matter | 868.7 | 868.9 | 870.1 | 964.1 | 898.7 | 827.0 | 787.1 | 3.0 | 0.98 | <0.01 | 0.77 |
| Crude protein | 44.7 a | 37.8 b | 37.6 b | 45.8 | 40.9 | 38.2 | 35.3 | 0.6 | <0.01 | <0.01 | 0.70 |
| Ether extract | 9.9 | 9.8 | 9.6 | 9.9 | 10.0 | 9.8 | 9.3 | 0.1 | 0.31 | 0.02 | 0.05 |
| NDFa | 325.5 a | 306.0 b | 300.1 b | 401.9 | 338.2 | 270.4 | 231.7 | 1.4 | <0.01 | 0.03 | <0.01 |
| Acid detergent fiber | 269.0 a | 272.7 a | 242.8 b | 305.4 | 271.2 | 244.7 | 224.6 | 3.2 | <0.01 | <0.01 | 0.13 |
| Hemicellulose | 116.0 | 112.0 | 115.6 | 122.0 | 98.5 | 93.9 | 143.9 | 3.1 | 0.84 | <0.01 | 0.82 |
| Lignin | 47.3 | 47.2 | 46.8 | 59.8 | 49.9 | 42.0 | 36.6 | 1.0 | 0.98 | <0.01 | 0.09 |
| Cellulose | 214.8 ab | 221.8 a | 192.2 b | 236.4 | 216.1 | 201.2 | 184.8 | 2.8 | <0.01 | <0.01 | 0.41 |
| Non-fibrous carbohydrates | 429.6 | 451.0 | 469.3 | 491.7 | 477.8 | 438.2 | 392.2 | 6.6 | 0.06 | <0.01 | 0.32 |
| Total digestible nutrients | 630.4 | 644.1 | 638.1 | 696.7 | 657.5 | 606.2 | 589.7 | 5.2 | 0.56 | <0.01 | 0.56 |
| Total carbohydrates | 814.0 | 834.2 | 822.8 | 908.2 | 847.6 | 778.9 | 759.9 | 5.3 | 0.31 | <0.01 | 0.41 |
| Phosphorus | 3.3 b | 4.3 a | 3.4 b | 3.2 | 3.8 | 4.1 | 3.6 | 0.2 | 0.05 | 0.43 | 0.20 |
| Calcium | 424.1 | 459.8 | 424.8 | 41.4 | 374.0 | 554.8 | 774.7 | 10.2 | 0.27 | <0.01 | 0.36 |
| In vitro digestibility; % | |||||||||||
| Dry matter | 71.6 | 69.1 | 70.4 | 66.5 | 69.8 | 71.8 | 73.5 | 6.1 | 0.27 | <0.01 | 0.39 |
| Carbohydrate fractionation, % | |||||||||||
| A + B1 | 52.6 b | 53.9 ab | 57.0 a | 54.1 | 56.4 | 56.2 | 51.2 | 5.0 | <0.01 | 0.05 | 0.09 |
| B2 | 33.5 b | 32.5 ab | 29.6 a | 30.1 | 29.5 | 30.8 | 37.1 | 3.6 | 0.01 | <0.01 | 0.15 |
| C | 13.8 | 13.6 | 13.5 | 15.8 | 14.1 | 13.0 | 11.6 | 3.0 | 0.78 | <0.01 | 0.25 |
| Item | Regression Equation | Determination Coefficient (R2) | p-Value | |
|---|---|---|---|---|
| Linear | Quadratic | |||
| Dry matter | Ŷ = 30.379 + 4.0414 (CaO level) − 0.4853 (CaO level)2 | 0.99 | <0.01 | <0.01 |
| Organic matter | Ŷ = 96.6015 − 5.29233 (CaO level) + 0.283636 (CaO level)2 | 0.99 | <0.01 | 0.04 |
| Crude protein | Ŷ = 4.52347 − 0.228542 (CaO level) | 0.98 | <0.01 | 0.42 |
| Ether extract | Ŷ = 1.01219 − 1.29942 (CaO level) | 0.66 | 0.01 | 0.06 |
| NDFa 1 | Ŷ = 40.3573 − 5.10634 (CaO level) + 0.278224 (CaO level)2 | 0.99 | <0.01 | 0.03 |
| Acid detergent fiber | Ŷ = 30.1849 − 1.79220 (CaO level) | 0.99 | <0.01 | 0.28 |
| Hemicellulose | Ŷ = 12.3792 − 3.26628 (CaO level) + 0.816358 (CaO level)2 | 0.96 | 0.03 | <0.01 |
| Lignin | Ŷ = 5.8729 − 5.15095 (CaO level) | 0.98 | <0.01 | 0.28 |
| Cellulose | Ŷ = 23.5123 − 1.13127 (CaO level) | 0.99 | <0.01 | 0.73 |
| Non-fibrous carbohydrates | Ŷ = 50.0751 − 2.25374 (CaO level) | 0.95 | <0.01 | 0.23 |
| Total digestible nutrients | Ŷ = 69.3432 − 2.48284 (CaO level) | 0.97 | <0.01 | 0.28 |
| Total carbohydrates | Ŷ = 90.0787 − 3.42543 (CaO level) | 0.96 | <0.01 | 0.06 |
| Calcium | Ŷ = 7.91433 + 15.8718 (CaO level) | 0.98 | <0.01 | 0.01 |
| In vitro dry matter digestibility | Ŷ = 33.0472 + 1.5262 (CaO level) | 0.97 | <0.01 | 0.51 |
| A + B1 | Ŷ = 54.0081 + 3.02645 (CaO level) + 0.803745 (CaO level)2 | 0.98 | <0.01 | 0.23 |
| B2 | Ŷ = 30.2280 − 1.94961 (CaO level) + 0.766652 (CaO level)2 | 0.99 | 0.77 | <0.01 |
| C | Ŷ = 15.6804 − 0.909924 (CaO level) | 0.99 | <0.01 | 0.28 |
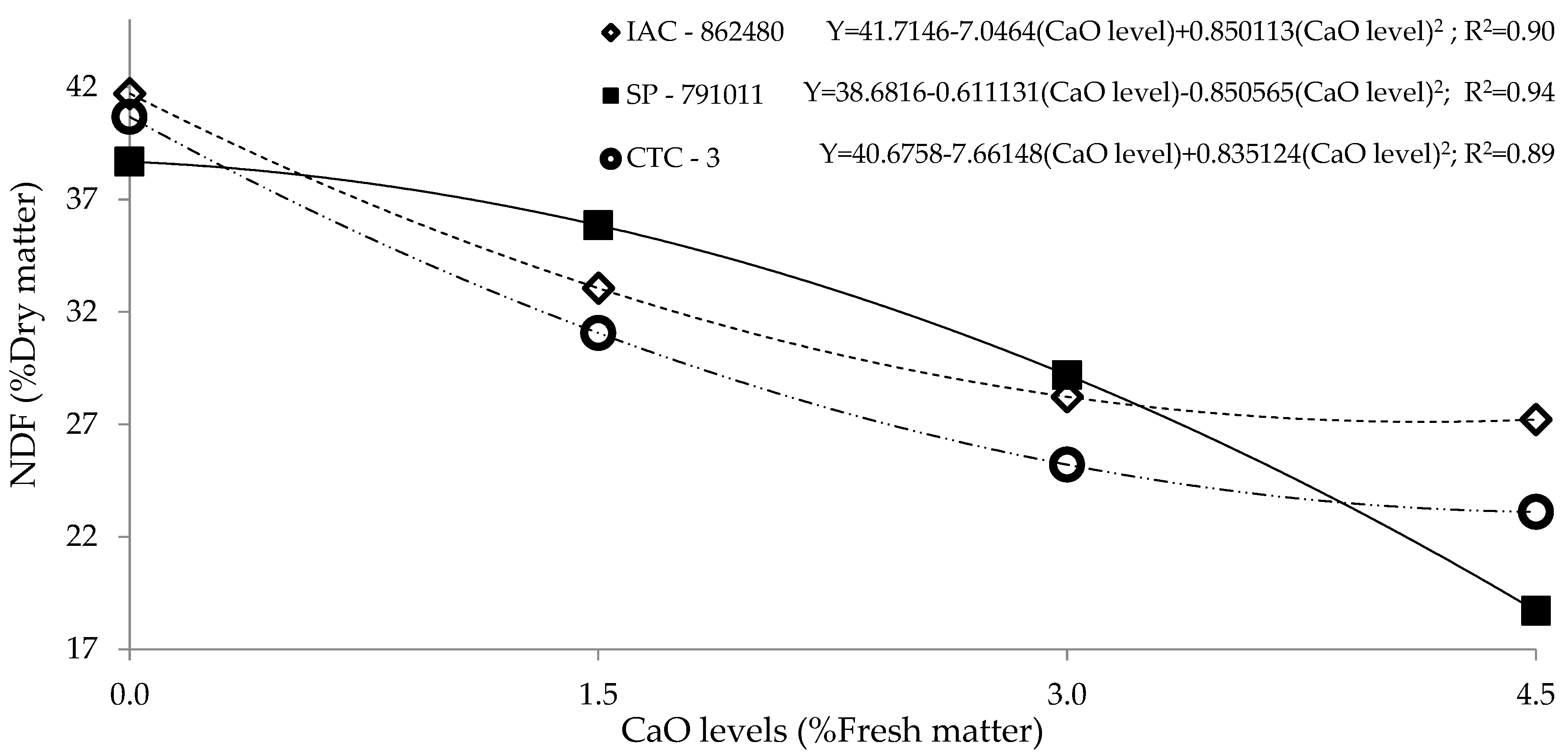
| Level of CaO (% Fresh Matter) | IAC-862480 | SP-791011 | CTC-3 |
|---|---|---|---|
| Neutral Detergent Fiber 1 (% DM) | |||
| 0 | 41.1 | 39.3 | 40.2 |
| 1.5 | 35.0 | 33.9 | 32.5 |
| 3.0 | 26.3 b | 31.1 a | 23.7 b |
| 4.5 | 27.9 a | 18.1 c | 23.6 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Oliveira Romão, C.; Tosto, M.S.L.; Santos, S.A.; Pires, A.J.V.; Ribeiro, O.L.; de Albuquerque Maranhão, C.M.; de Almeida Rufino, L.M.; Correia, G.S.; Alba, H.D.R.; de Carvalho, G.G.P. Nutritive Profile, Digestibility, and Carbohydrate Fractionation of Three Sugarcane Genotypes Treated with Calcium Oxide. Agronomy 2023, 13, 733. https://doi.org/10.3390/agronomy13030733
de Oliveira Romão C, Tosto MSL, Santos SA, Pires AJV, Ribeiro OL, de Albuquerque Maranhão CM, de Almeida Rufino LM, Correia GS, Alba HDR, de Carvalho GGP. Nutritive Profile, Digestibility, and Carbohydrate Fractionation of Three Sugarcane Genotypes Treated with Calcium Oxide. Agronomy. 2023; 13(3):733. https://doi.org/10.3390/agronomy13030733
Chicago/Turabian Stylede Oliveira Romão, Claudio, Manuela Silva Libânio Tosto, Stefanie Alvarenga Santos, Aureliano José Vieira Pires, Ossival Lolato Ribeiro, Camila Maida de Albuquerque Maranhão, Luana Marta de Almeida Rufino, George Soares Correia, Henry Daniel Ruiz Alba, and Gleidson Giordano Pinto de Carvalho. 2023. "Nutritive Profile, Digestibility, and Carbohydrate Fractionation of Three Sugarcane Genotypes Treated with Calcium Oxide" Agronomy 13, no. 3: 733. https://doi.org/10.3390/agronomy13030733
APA Stylede Oliveira Romão, C., Tosto, M. S. L., Santos, S. A., Pires, A. J. V., Ribeiro, O. L., de Albuquerque Maranhão, C. M., de Almeida Rufino, L. M., Correia, G. S., Alba, H. D. R., & de Carvalho, G. G. P. (2023). Nutritive Profile, Digestibility, and Carbohydrate Fractionation of Three Sugarcane Genotypes Treated with Calcium Oxide. Agronomy, 13(3), 733. https://doi.org/10.3390/agronomy13030733







