Recent Progress in Genetic Transformation and Gene Editing Technology in Cucurbit Crops
Abstract
1. Introduction
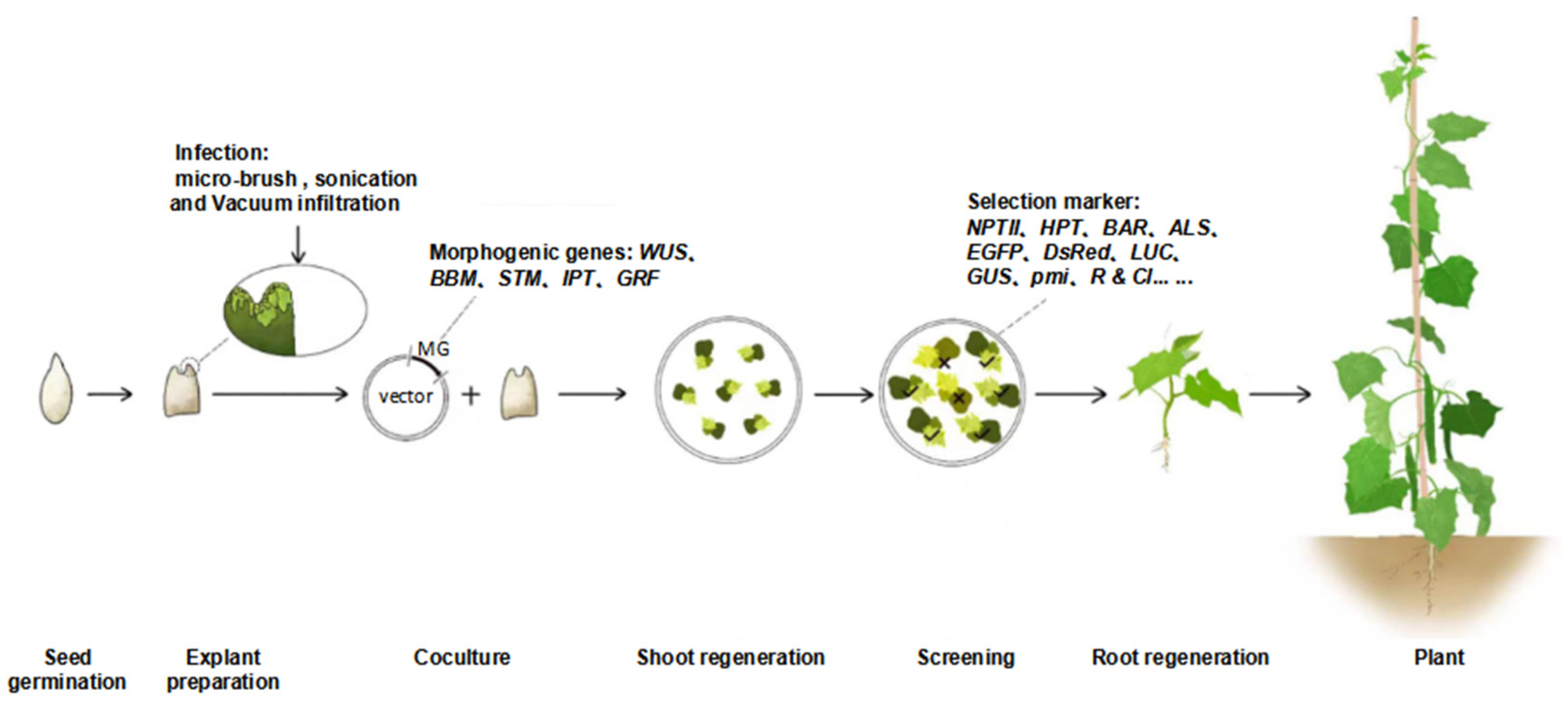
2. Methods to Improve the Genetic Transformation Efficiency of Cucurbit Crops
2.1. Germplasm Influences Transformation Efficiency
2.2. Auxiliary Physical Treatments Significantly Enhance Agrobacterium Infection
2.3. Morphogenic Genes Significantly Increase Transformation Efficiency
2.4. Diversification of the Screening Markers Used in Genetic Transformation
2.5. Other Factors
3. Application of Gene Editing Technology in Cucurbit Crops
3.1. CRISPR/Cas9-Based Gene Knockout
3.2. Precise Editing via Cytosine Base Editors
4. Discussion and Future Prospects
4.1. Establish Genotype-Free Transformation Systems
4.2. Further Development of Gene Editing Technologies in Cucurbit Crops
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, S.; Wang, K.; Li, Z.; Li, Y.; He, J.; Li, H.; Wang, B.; Xin, T.; Tian, H.; Tian, J.; et al. Architecture design of cucurbit crops for enhanced productivity by a natural allele. Nat. Plants 2022, 8, 1394–1407. [Google Scholar] [CrossRef] [PubMed]
- Nanasato, Y.; Tabei, Y. A method of transformation and current progress in transgenic research on cucumbers and Cucurbita species. Plant Biotechnol. (Tokyo Jpn.) 2020, 37, 141–146. [Google Scholar] [CrossRef]
- Li, H.; Yang, X.; Shang, Y.; Zhang, Z.; Huang, S. Vegetable biology and breeding in the genomics era. Sci. China Life Sci. 2023, 66, 226–250. [Google Scholar] [CrossRef]
- Debeaujon, I.; Branchard, M. Somatic embryogenesis in Cucurbitaceae. Plant Cell Tiss. Org. Cult. 1993, 34, 91–100. [Google Scholar] [CrossRef]
- Hooghvorst, I.; Nogués, S. Opportunities and Challenges in Doubled Haploids and Haploid Inducer-Mediated Genome-Editing Systems in Cucurbits. Agronomy 2020, 10, 1441. [Google Scholar] [CrossRef]
- Jarl, C.I.; Bokelmann, G.S.; De Haas, J.M. Protoplast regeneration and fusion in Cucumis: Melon × cucumber. Plant Cell Tiss. Org. Cult. 1995, 43, 259–265. [Google Scholar] [CrossRef]
- Kurtar, E.S.; Seymen, M. Another Culture in Cucurbita Species. Methods Mol. Biol. 2021, 2289, 111–121. [Google Scholar] [CrossRef]
- Trulson, A.J.; Simpson, R.B.; Shahin, E.A. Transformation of cucumber (Cucumis sativus L.) plants with Agrobacterium rhizogenes. Theor. Appl. Genet. 1986, 73, 11–15. [Google Scholar] [CrossRef]
- Fang, G.; Grumet, R. Agrobacterium tumefaciens mediated transformation and regeneration of muskmelon plants. Plant Cell Rep. 1990, 9, 160–164. [Google Scholar] [CrossRef]
- Wyman, C.; Kanaar, R. DNA double-strand break repair: All’s well that ends well. Annu. Rev. Genet. 2006, 40, 363–383. [Google Scholar] [CrossRef]
- Puchta, H.; Fauser, F. Synthetic nucleases for genome engineering in plants: Prospects for a bright future. Plant J. 2014, 78, 727–741. [Google Scholar] [CrossRef]
- Voytas, D.F.; Gao, C. Precision Genome Engineering and Agriculture: Opportunities and Regulatory Challenges. PLoS Biol. 2014, 12, e1001877. [Google Scholar] [CrossRef] [PubMed]
- Zetsche, B.; Gootenberg, J.S.; Abudayyeh, O.O.; Slaymaker, I.M.; Makarova, K.S.; Essletzbichler, P.; Volz, S.E.; Joung, J.; van der Oost, J.; Regev, A.; et al. Cpf1 Is a Single RNA-Guided Endonuclease of a Class 2 CRISPR-Cas System. Cell 2015, 163, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, C.; Wang, X.; Li, X.; You, C. CRISPR/Cas9 technology and its application in horticultural crops. Hortic. Plant J. 2022, 8, 395–407. [Google Scholar] [CrossRef]
- Mao, Y.; Botella, J.R.; Liu, Y.; Zhu, J.-K. Gene editing in plants: Progress and challenges. Natl. Sci. Rev. 2019, 6, 421–437. [Google Scholar] [CrossRef]
- Chen, K.; Wang, Y.; Zhang, R.; Zhang, H.; Gao, C. CRISPR/Cas Genome Editing and Precision Plant Breeding in Agriculture. Annu. Rev. Plant Biol. 2019, 70, 667–697. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-C.; Chiang, C.-H.; Li, C.-M.; Yu, T.-A. Transgenic watermelon lines expressing the nucleocapsid gene of Watermelon silver mottle virus and the role of thiamine in reducing hyperhydricity in regenerated shoots. Plant Cell Tissue Organ Cult. (PCTOC) 2010, 106, 21–29. [Google Scholar] [CrossRef]
- Yu, T.A.; Chiang, C.H.; Wu, H.W.; Li, C.M.; Yang, C.F.; Chen, J.H.; Chen, Y.W.; Yeh, S.D. Generation of transgenic watermelon resistant to Zucchini yellow mosaic virus and Papaya ringspot virus type W. Plant Cell Rep. 2011, 30, 359–371. [Google Scholar] [CrossRef]
- Vasudevan, V.; Sathish, D.; Ajithan, C.; Sathish, S.; Manickavasagam, M. Efficient Agrobacterium-mediated in planta genetic transformation of watermelon [Citrullus lanatus Thunb.]. Plant Biotechnol. Rep. 2021, 15, 447–457. [Google Scholar] [CrossRef]
- Cao, L.; Wei, W.; Shen, J.; Xu, Z.; Li, Z. Study on the optimization of transformation systems in watermelon. Veg. Res. 2022, 2, 1–9. [Google Scholar] [CrossRef]
- Kim, H.-A.; Min, S.-R.; Choi, D.-W.; Choi, P.-S.; Hong, S.-G. Development of transgenic cucumber expressing TPSP gene and morphological alterations. J. Plant Biotechnol. 2010, 37, 72–76. [Google Scholar] [CrossRef]
- Xin, T.; Tian, H.; Ma, Y.; Wang, S.; Yang, L.; Li, X.; Zhang, M.; Chen, C.; Wang, H.; Li, H.; et al. Targeted creating new mutants with compact plant architecture using CRISPR/Cas9 genome editing by an optimized genetic transformation procedure in cucurbit plants. Hortic. Res. 2022, 9, uhab086. [Google Scholar] [CrossRef]
- Vasudevan, A.; Selvaraj, N.; Ganapathi, A.N.; Choi, C.W. Agrobacterium-mediated Genetic Transformation in Cucumber (Cucumis sativus L.). Am. J. Biochem. Biotechnol. 2007, 3, 24–32. [Google Scholar] [CrossRef]
- Selvaraj, N.; Kasthurirengan, S.; Vasudevan, A.; Manickavasagam, M.; Choi, C.W.; Ganapathi, A. Evaluation of green fluorescent protein as a reporter gene and phosphinothricin as the selective agent for achieving a higher recovery of transformants in cucumber (Cucumis sativus L. cv. Poinsett76) via Agrobacterium tumefaciens. Vitr. Cell. Dev. Biol.-Plant 2010, 46, 329–337. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Shi, B.; Yu, T.; Qi, J.; Meyerowitz, E.M.; Jiao, Y. The Stem Cell Niche in Leaf Axils Is Established by Auxin and Cytokinin in Arabidopsis. Plant Cell 2014, 26, 2055–2067. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Chai, L.a.; Liu, C.; Si, Y.; Fan, H. Improved Agrobacterium tumefaciens-mediated transformation using antibiotics and acetosyringone selection in cucumber. Plant Biotechnol. Rep. 2022, 16, 17–27. [Google Scholar] [CrossRef]
- García-Almodóvar, R.C.; Gosalvez, B.; Aranda, M.A.; Burgos, L. Production of transgenic diploid Cucumis melo plants. Plant Cell Tissue Organ Cult. (PCTOC) 2017, 130, 323–333. [Google Scholar] [CrossRef]
- Pan, W.; Cheng, Z.; Han, Z.; Yang, H.; Zhang, W.; Zhang, H. Efficient genetic transformation and CRISPR/Cas9-mediated genome editing of watermelon assisted by genes encoding developmental regulators. J. Zhejiang Univ. Sci. B 2022, 23, 339–344. [Google Scholar] [CrossRef]
- Feng, Q.; Xiao, L.; He, Y.; Liu, M.; Wang, J.; Tian, S.; Zhang, X.; Yuan, L. Highly efficient, genotype-independent transformation and gene editing in watermelon (Citrullus lanatus) using a chimeric ClGRF4-GIF1 gene. J. Integr. Plant Biol. 2021, 63, 2038–2042. [Google Scholar] [CrossRef]
- Zhao, Z.; Qi, Y.; Yang, Z.; Cheng, L.; Sharif, R.; Raza, A.; Chen, P.; Hou, D.; Li, Y. Exploring the Agrobacterium-mediated transformation with CRISPR/Cas9 in cucumber (Cucumis sativus L.). Mol. Biol. Rep. 2022, 49, 11481–11490. [Google Scholar] [CrossRef]
- Sundar, I.K.; Sakthivel, N. Advances in selectable marker genes for plant transformation. J. Plant Physiol. 2008, 165, 1698–1716. [Google Scholar] [CrossRef]
- Zhang, H.J.; Gao, P.; Wang, X.Z.; Luan, F.S. An efficient regeneration protocol for Agrobacterium-mediated transformation of melon (Cucumis melo L.). Genet Mol. Res. 2014, 13, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Bezirganoglu, I.; Hwang, S.Y.; Shaw, J.F.; Fang, T.J. Efficient production of transgenic melon via Agrobacterium-mediated transformation. Genet Mol. Res. 2014, 13, 3218–3227. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Luan, F. Overexpression of the CmACS-3 gene in melon causes abnormal pollen development. Genet Mol. Res. 2015, 14, 10433–10443. [Google Scholar] [CrossRef]
- Hooghvorst, I.; López-Cristoffanini, C.; Nogués, S. Efficient knockout of phytoene desaturase gene using CRISPR/Cas9 in melon. Sci. Rep. 2019, 9, 17077. [Google Scholar] [CrossRef]
- Liu, L.; Gu, Q.; Ijaz, R.; Zhang, J.; Ye, Z. Generation of transgenic watermelon resistance to Cucumber mosaic virus facilitated by an effective Agrobacterium-mediated transformation method. Sci. Hortic. 2016, 205, 32–38. [Google Scholar] [CrossRef]
- Tian, S.; Jiang, L.; Gao, Q.; Zhang, J.; Zong, M.; Zhang, H.; Ren, Y.; Guo, S.; Gong, G.; Liu, F.; et al. Efficient CRISPR/Cas9-based gene knockout in watermelon. Plant Cell Rep. 2017, 36, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Jiang, L.; Cui, X.; Zhang, J.; Guo, S.; Li, M.; Zhang, H.; Ren, Y.; Gong, G.; Zong, M.; et al. Engineering herbicide-resistant watermelon variety through CRISPR/Cas9-mediated base-editing. Plant Cell Rep. 2018, 37, 1353–1356. [Google Scholar] [CrossRef]
- Jang, H.-A.; Kim, H.-A.; Kwon, S.-Y.; Choi, D.-W.; Choi, P.-S. The use of cotyledonary-node explants in Agrobacterium tumefaciensmediated transformation of cucumber (Cucumis sativus L.). J. Plant Biotechnol. 2011, 38, 198–202. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, S.; Wang, X.; Wang, L.; Xu, H.; Wang, X.; Shi, Q.; Wei, M.; Yang, F. Agrobacterium-mediated transformation of cucumber (Cucumis sativus L.) using a sense mitogen-activated protein kinase gene (CsNMAPK). Plant Cell Tissue Organ Cult. (PCTOC) 2012, 113, 269–277. [Google Scholar] [CrossRef]
- Nanasato, Y.; Okuzaki, A.; Tabei, Y. Improving the transformation efficiency of Cucurbita species: Factors and strategy for practical application. Plant Biotechnol. 2013, 30, 287–294. [Google Scholar] [CrossRef]
- Chandrasekaran, J.; Brumin, M.; Wolf, D.; Leibman, D.; Klap, C.; Pearlsman, M.; Sherman, A.; Arazi, T.; Gal-On, A. Development of broad virus resistance in non-transgenic cucumber using CRISPR/Cas9 technology. Mol. Plant Pathol. 2016, 17, 1140–1153. [Google Scholar] [CrossRef]
- Hu, B.; Li, D.; Liu, X.; Qi, J.; Gao, D.; Zhao, S.; Huang, S.; Sun, J.; Yang, L. Engineering Non-transgenic Gynoecious Cucumber Using an Improved Transformation Protocol and Optimized CRISPR/Cas9 System. Mol. Plant 2017, 10, 1575–1578. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.W.; Yu, T.A.; Raja, J.A.J.; Christopher, S.J.; Wang, S.L.; Yeh, S.D. Double-Virus Resistance of Transgenic Oriental Melon Conferred by Untranslatable Chimeric Construct Carrying Partial Coat Protein Genes of Two Viruses. Plant Dis. 2010, 94, 1341–1347. [Google Scholar] [CrossRef]
- Hao, J.; Niu, Y.; Yang, B.; Gao, F.; Zhang, L.; Wang, J.; Hasi, A. Transformation of a marker-free and vector-free antisense ACC oxidase gene cassette into melon via the pollen-tube pathway. Biotechnol. Lett. 2011, 33, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Chovelon, V.; Restier, V.; Giovinazzo, N.; Dogimont, C.; Aarrouf, J. Histological study of organogenesis in Cucumis melo L. after genetic transformation: Why is it difficult to obtain transgenic plants? Plant Cell Rep. 2011, 30, 2001–2011. [Google Scholar] [CrossRef] [PubMed]
- Shan, W.; Zhao, C.; Fan, J.; Cong, H.; Liang, S.; Yu, X. Antisense suppression of alcohol acetyltransferase gene in ripening melon fruit alters volatile composition. Sci. Hortic. 2012, 139, 96–101. [Google Scholar] [CrossRef]
- Ren, Y.; Bang, H.; Curtis, I.S.; Gould, J.; Patil, B.S.; Crosby, K.M. Agrobacterium-mediated transformation and shoot regeneration in elite breeding lines of western shipper cantaloupe and honeydew melons (Cucumis melo L.). Plant Cell Tissue Organ Cult. (PCTOC) 2011, 108, 147–158. [Google Scholar] [CrossRef]
- Choi, J.Y.; Shin, J.S.; Chung, Y.S.; Hyung, N.-I. An efficient selection and regeneration protocol for Agrobacterium-mediated transformation of oriental melon (Cucumis melo L. var. makuwa). Plant Cell Tissue Organ Cult. (PCTOC) 2012, 110, 133–140. [Google Scholar] [CrossRef]
- Bezirganoglu, I.; Hwang, S.-Y.; Fang, T.J.; Shaw, J.-F. Transgenic lines of melon (Cucumis melo L. var. makuwa cv. ‘Silver Light’) expressing antifungal protein and chitinase genes exhibit enhanced resistance to fungal pathogens. Plant Cell Tissue Organ Cult. (PCTOC) 2013, 112, 227–237. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, P.; Wang, X.; Luan, F. An improved method of Agrobacterium tumefaciens-mediated genetic transformation system of melon (Cucumis melo L.). J. Plant Biochem. Biotechnol. 2013, 23, 278–283. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, X.; Ma, S.; Shan, N.; Zhang, X.; Sui, X.; Zhang, Z.; Sui, X. A Protocol for Agrobacterium-mediated Transformation of Cucumber (Cucumis sativus L.) from cotyledon explants. Protoc. Exch. 2017. [Google Scholar] [CrossRef]
- Mendez-Hernandez, H.A.; Ledezma-Rodriguez, M.; Avilez-Montalvo, R.N.; Juarez-Gomez, Y.L.; Skeete, A.; Avilez-Montalvo, J.; De-la-Pena, C.; Loyola-Vargas, V.M. Signaling Overview of Plant Somatic Embryogenesis. Front. Plant Sci. 2019, 10, 77. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, G.; Cheng, C.; Lei, L.; Sun, J.; Xu, Y.; Deng, C.; Dai, Z.; Yang, Z.; Chen, X.; et al. Establishment of an Agrobacterium-mediated genetic transformation and CRISPR/Cas9-mediated targeted mutagenesis in Hemp (Cannabis Sativa L.). Plant Biotechnol. J. 2021, 19, 1979–1987. [Google Scholar] [CrossRef] [PubMed]
- Maren, N.A.; Duan, H.; Da, K.; Yencho, G.C.; Ranney, T.G.; Liu, W. Genotype-independent plant transformation. Hortic. Res. 2022, 9, uhac047. [Google Scholar] [CrossRef]
- Lowe, K.; Wu, E.; Wang, N.; Hoerster, G.; Hastings, C.; Cho, M.J.; Scelonge, C.; Lenderts, B.; Chamberlin, M.; Cushatt, J.; et al. Morphogenic Regulators Baby boom and Wuschel Improve Monocot Transformation. Plant Cell 2016, 28, 1998–2015. [Google Scholar] [CrossRef]
- Maher, M.F.; Nasti, R.A.; Vollbrecht, M.; Starker, C.G.; Clark, M.D.; Voytas, D.F. Plant gene editing through de novo induction of meristems. Nat. Biotechnol. 2020, 38, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Martin-Ortigosa, S.; Finer, J.; Orchard, N.; Gunadi, A.; Batts, L.A.; Thakare, D.; Rush, B.; Schmitz, O.; Stuiver, M.; et al. Overexpression of the Transcription Factor GROWTH-REGULATING FACTOR5 Improves Transformation of Dicot and Monocot Species. Front. Plant Sci. 2020, 11, 572319. [Google Scholar] [CrossRef]
- Debernardi, J.M.; Tricoli, D.M.; Ercoli, M.F.; Hayta, S.; Ronald, P.; Palatnik, J.F.; Dubcovsky, J. A GRF-GIF chimeric protein improves the regeneration efficiency of transgenic plants. Nat Biotechnol 2020, 38, 1274–1279. [Google Scholar] [CrossRef] [PubMed]
- Miki, B.; McHugh, S. Selectable marker genes in transgenic plants: Applications, alternatives and biosafety. J. Biotechnol. 2004, 107, 193–232. [Google Scholar] [CrossRef]
- He, Z.; Duan, Z.; Liang, W.; Chen, F.; Yao, W.; Liang, H.; Yue, C.; Sun, Z.; Chen, F.; Dai, J. Mannose selection system used for cucumber transformation. Plant Cell Rep. 2006, 25, 953–958. [Google Scholar] [CrossRef]
- Hesami, M.; Tohidfar, M.; Alizadeh, M.; Daneshvar, M.H. Effects of sodium nitroprusside on callus browning of Ficus religiosa: An important medicinal plant. J. For. Res. 2020, 31, 789–796. [Google Scholar] [CrossRef]
- Soda, N.; Verma, L.; Giri, J. CRISPR-Cas9 based plant genome editing: Significance, opportunities and recent advances. Plant Physiol. Biochem. 2018, 131, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, J.; Guo, S.; Tian, S.; Zhang, J.; Ren, Y.; Li, M.; Gong, G.; Zhang, H.; Xu, Y. CRISPR/Cas9-mediated mutagenesis of ClBG1 decreased seed size and promoted seed germination in watermelon. Hortic. Res. 2021, 8, 70. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Zhang, J.; Ren, Y.; Li, M.; Tian, S.; Yu, Y.; Zuo, Y.; Gong, G.; Zhang, H.; et al. The NAC transcription factor ClNAC68 positively regulates sugar content and seed development in watermelon by repressing ClINV and ClGH3.6. Hortic. Res. 2021, 8, 214. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Guo, S.; Ji, G.; Zhao, H.; Sun, H.; Ren, Y.; Tian, S.; Li, M.; Gong, G.; Zhang, H.; et al. A unique chromosome translocation disrupting ClWIP1 leads to gynoecy in watermelon. Plant J. Cell Mol. Biol. 2020, 101, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Sun, H.; Zong, M.; Guo, S.; Ren, Z.; Zhao, J.; Li, M.; Zhang, J.; Tian, S.; Wang, J.; et al. Localization shift of a sugar transporter contributes to phloem unloading in sweet watermelons. New Phytol. 2020, 227, 1858–1871. [Google Scholar] [CrossRef]
- Ren, Y.; Li, M.; Guo, S.; Sun, H.; Zhao, J.; Zhang, J.; Liu, G.; He, H.; Tian, S.; Yu, Y.; et al. Evolutionary gain of oligosaccharide hydrolysis and sugar transport enhanced carbohydrate partitioning in sweet watermelon fruits. Plant Cell 2021, 33, 1554–1573. [Google Scholar] [CrossRef]
- Cao, L.; Li, C.; Li, H.; Wang, Z.; Jiang, Y.; Guo, Y.; Sun, P.; Chen, X.; Li, Q.; Tian, H.; et al. Disruption of REC8 in Meiosis I led to watermelon seedless. Plant Sci. 2022, 323, 111394. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Guo, Y.; Yan, J.; Zhang, Z.; Yuan, L.; Wei, C.; Zhang, Y.; Ma, J.; Yang, J.; Zhang, X.; et al. The role of watermelon caffeic acid O-methyltransferase (ClCOMT1) in melatonin biosynthesis and abiotic stress tolerance. Hortic. Res. 2021, 8, 210. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Chang, J.; Li, J.; Lan, G.; Xuan, C.; Li, H.; Ma, J.; Zhang, Y.; Yang, J.; Tian, S.; et al. Disruption of the bHLH transcription factor Abnormal Tapetum 1 causes male sterility in watermelon. Hortic. Res. 2021, 8, 258. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Liu, X.; Yan, S.; Liu, B.; Zhong, Y.; Song, W.; Chen, J.; Wang, Z.; Che, G.; Liu, L.; et al. Pollen tube emergence is mediated by ovary-expressed ALCATRAZ in cucumber. Nat. Commun. 2023, 14, 258. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ning, K.; Che, G.; Yan, S.; Han, L.; Gu, R.; Li, Z.; Weng, Y.; Zhang, X. CsSPL functions as an adaptor between HD-ZIP III and CsWUS transcription factors regulating anther and ovule development in Cucumis sativus (cucumber). Plant J. 2018, 94, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Xin, T.; Zhang, Z.; Li, S.; Zhang, S.; Li, Q.; Zhang, Z.H.; Huang, S.; Yang, X. Genetic Regulation of Ethylene Dosage for Cucumber Fruit Elongation. Plant Cell 2019, 31, 1063–1076. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, B.; Wang, S.; Lin, T.; Yang, L.; Zhao, Z.; Zhang, Z.; Huang, S.; Yang, X. Genome-wide Target Mapping Shows Histone Deacetylase Complex1 Regulates Cell Proliferation in Cucumber Fruit. Plant Physiol. 2020, 182, 167–184. [Google Scholar] [CrossRef]
- Yang, X.; Yan, J.; Zhang, Z.; Lin, T.; Xin, T.; Wang, B.; Wang, S.; Zhao, J.; Zhang, Z.; Lucas, W.J.; et al. Regulation of plant architecture by a new histone acetyltransferase targeting gene bodies. Nat. Plants 2020, 6, 809–822. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, L.; Han, L.; Cheng, Z.; Liu, X.; Wang, S.; Liu, L.; Chen, J.; Song, W.; Zhao, J.; et al. HECATE2 acts with GLABROUS3 and Tu to boost cytokinin biosynthesis and regulate cucumber fruit wart formation. Plant Physiol. 2021, 187, 1619–1635. [Google Scholar] [CrossRef]
- Zhang, H.; Li, S.; Yang, L.; Cai, G.; Chen, H.; Gao, D.; Lin, T.; Cui, Q.; Wang, D.; Li, Z.; et al. Gain-of-function of the 1-aminocyclopropane-1-carboxylate synthase gene ACS1G induces female flower development in cucumber gynoecy. Plant Cell 2021, 33, 306–321. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, Z.; Wang, L.; Yan, S.; Cheng, Z.; Liu, X.; Han, L.; Chen, G.; Wang, S.; Song, W.; et al. The CsHEC1-CsOVATE module contributes to fruit neck length variation via modulating auxin biosynthesis in cucumber. Proc. Natl. Acad. Sci. USA 2022, 119, e2209717119. [Google Scholar] [CrossRef]
- Liu, P.; Li, Q.; Wang, H.; Lu, T.; Li, Y.; Sui, X.; Yu, H.; Jiang, W. Ethylene signaling plays an important role in UV-B-induced ascorbic acid accumulation in Cucumis sativus leaves. Authorea 2022. [Google Scholar] [CrossRef]
- Peng, Y.; Chen, L.; Zhu, L.; Cui, L.; Yang, L.; Wu, H.; Bie, Z. CsAKT1 is a key gene for the CeO2 nanoparticle's improved cucumber salt tolerance: A validation from CRISPR-Cas9 lines. Environ. Sci. Nano 2022, 9, 4367–4381. [Google Scholar] [CrossRef]
- Liu, B.; Santo Domingo, M.; Mayobre, C.; Martín-Hernández, A.M.; Pujol, M.; Garcia-Mas, J. Knock-Out of CmNAC-NOR Affects Melon Climacteric Fruit Ripening. Front. Plant Sci. 2022, 13, 878037. [Google Scholar] [CrossRef]
- Wang, J.; Tian, S.; Yu, Y.; Ren, Y.; Guo, S.; Zhang, J.; Li, M.; Zhang, H.; Gong, G.; Wang, M.; et al. Natural variation in the NAC transcription factor NONRIPENING contributes to melon fruit ripening. J. Integr. Plant Biol. 2022, 64, 1448–1461. [Google Scholar] [CrossRef] [PubMed]
- Pechar, G.S.; Donaire, L.; Gosalvez, B.; García-Almodovar, C.; Sánchez-Pina, M.A.; Truniger, V.; Aranda, M.A. Editing melon eIF4E associates with virus resistance and male sterility. Plant Biotechnol. J. 2022, 20, 2006–2022. [Google Scholar] [CrossRef]
- Sohail, H.; Noor, I.; Nawaz, M.A.; Ma, M.; Shireen, F.; Huang, Y.; Yang, L.; Bie, Z. Genome-wide identification of plasma-membrane intrinsic proteins in pumpkin and functional characterization of CmoPIP1-4 under salinity stress. Environ. Exp. Bot. 2022, 202. [Google Scholar] [CrossRef]
- Geng, S.; Sohail, H.; Cao, H.; Sun, J.; Chen, Z.; Zhou, L.; Wang, W.; Ye, R.; Yang, L.; Bie, Z. An efficient root transformation system for CRISPR/Cas9-based analyses of shoot-root communication in cucurbit crops. Hortic. Res. 2022, 9. [Google Scholar] [CrossRef] [PubMed]
- Garneau, J.E.; Dupuis, M.-È.; Villion, M.; Romero, D.A.; Barrangou, R.; Boyaval, P.; Fremaux, C.; Horvath, P.; Magadán, A.H.; Moineau, S. The CRISPR/Cas bacterial immune system cleaves bacteriophage and plasmid DNA. Nature 2010, 468, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Sorek, R.; Lawrence, C.M.; Wiedenheft, B. CRISPR-mediated adaptive immune systems in bacteria and archaea. Annu. Rev. Biochem. 2013, 82, 237–266. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yu, X.; Zhang, C.; Li, N.; Zhao, J. The application of CRISPR/Cas technologies to Brassica crops: Current progress and future perspectives. Abiotech 2022, 3, 146–161. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Tomes, S.; Gleave, A.P.; Hall, W.; Luo, Z.; Xu, J.; Yao, J.L. Significant improvement of apple (Malus domestica Borkh.) transgenic plant production by pre-transformation with a Baby boom transcription factor. Hortic. Res. 2022, 9. [Google Scholar] [CrossRef]
- Luo, W.; Tan, J.; Li, T.; Feng, Z.; Ding, Z.; Xie, X.; Chen, Y.; Chen, L.; Liu, Y.G.; Zhu, Q.; et al. Overexpression of maize GOLDEN2 in rice and maize calli improves regeneration by activating chloroplast development. Sci. China Life Sci. 2023, 66, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Hyun, Y.; Kim, J.; Cho, S.W.; Choi, Y.; Kim, J.-S.; Coupland, G. Site-directed mutagenesis in Arabidopsis thaliana using dividing tissue-targeted RGEN of the CRISPR/Cas system to generate heritable null alleles. Planta 2015, 241, 271–284. [Google Scholar] [CrossRef]
- Wang, Z.-P.; Xing, H.-L.; Dong, L.; Zhang, H.-Y.; Han, C.-Y.; Wang, X.-C.; Chen, Q.-J. Egg cell-specific promoter-controlled CRISPR/Cas9 efficiently generates homozygous mutants for multiple target genes in Arabidopsis in a single generation. Genome Biol. 2015, 16, 144. [Google Scholar] [CrossRef] [PubMed]
- Belhaj, K.; Chaparro-Garcia, A.; Kamoun, S.; Nekrasov, V. Plant genome editing made easy: Targeted mutagenesis in model and crop plants using the CRISPR/Cas system. Plant Methods 2013, 9, 39. [Google Scholar] [CrossRef]
- Abbott, A. Europe’s genetically edited plants stuck in legal limbo. Nature 2015, 528, 319–320. [Google Scholar] [CrossRef] [PubMed]
| Species | Germplasm | Stability | Agrobacterium Strains | Transformation Efficiency (%) | T1 | Reference |
|---|---|---|---|---|---|---|
| Watermelon | Feeling, China baby, Quality | Kanamycin | LBA4404 | 8.5–10%, 1–2.1%, 0–1.3% (PCR) | Reported | [18] |
| Watermelon | Feeling, China baby, Quality | Kanamycin | LBA4404 | 7%, 2.22%, 1.25% (PCR and Southern blot) | NR | [17] |
| Watermelon | the female parent line of Zhengkang No. 6 | Kanamycin/cefotaxime | LBA4404 | 3 plantlets (PCR and Southern blot) | Reported | [36] |
| Watermelon | PI179878 | Hygromycin B | EHA105 | 1.67% (shoots) | NR | [37] |
| Watermelon | ZG94 | Bialaphos resistance | EHA105 | 23% (PCR) | Reported | [38] |
| Watermelon | Arka manik, Sugar baby, Arka muthu, IIHR-14 | BASTA | EHA105 | 17.33%, 12.33%, 16%, 14.66% (GUS, PCR, Southern hybridization) | NR | [19] |
| Watermelon | TC | NR | EHA105 | 6.5% (GFP, PCR) | NR | [29] |
| Watermelon | YL | Glufosinate-ammonium | EHA105 | 12.5% (PCR) | NR | [20] |
| Cucumber | Eunchim | Phospinotricin | EHA101 | 1.7% (Northern blot) | NR | [21] |
| Cucumber | Poinsett 76 | Phosphinothricin | EHA105 LBA4404 | 21.0%, 8.5% (shoots) | Reported | [24] |
| Cucumber | Eunsung | Paromomycin | EHA101 | 4.01% (Southern blot) | Reported | [39] |
| Cucumber | Xintaimici | Kanamycin | LBA4404 | 4.8% (PCR, Southern blot, Western blot) | NR | [40] |
| Cucumber | Shinhokusei 1 | Kanamycin | EHA105 | 11.9 ± 3.5% (plants) | Reported | [41] |
| Cucumber | Ilan | Kanamycin | EHA105 | 3 plants (PCR) | Reported | [42] |
| Cucumber | Cu2 | GFP | EHA105 | 1.32% (plantlets) | Reported | [43] |
| Cucumber | Xintaimici | Kanamycin | GV3101 | 8.10% (PCR) | NR | [26] |
| Cucumber | CCMC | GUS/Hygromycin B | EHA105 | 0.24% (PCR) | NR | [30] |
| Cucumber | Cu2, Xintaimici, 404, Eu1 | GFP | EHA105 | 5.18%, 2.20%, 1.97%, 2.46% (GFP) | Reported | [22] |
| Melon | Silver Light | Kanamycin | LBA4404 | Unspecified (2 transgenic lines) | Reported | [44] |
| Melon | Hetao | Transformation via the pollen-tube pathway | 4.3% (PCR) | Reported | [45] | |
| Melon | Védrantis | Kanamycin | GV2260 | 0.35–3.0% (PCR and GUS histochemical analysis) | NR | [46] |
| Melon | Hybrid line M01–3 | Ovary-injection transformation | 2.7% (PCR) | Reported | [47] | |
| Melon | Cantaloupe F39 Honeydew 150 | Kanamycin/GUS | EHA105 | 0.3%, 0.5% (PCR, GUS and Southern blot) | NR | [48] |
| Melon | Geumnodajieunchun | Kanamycin/geneticin | LBA4404 | 2.9%, 7.1% (PCR) | NR | [49] |
| Melon | Silver Light | Kanamycin | LBA4404 | 11 transgenic lines (PCR and Southern blot) | Reported | [50] |
| Melon | Silver Light | Kanamycin | LBA4404 | 0.8% (PCR) | Reported | [33] |
| Melon | CM-23 | Kanamycin | EHA105 | 4% (PCR, Southern hybridization) | NR | [51] |
| Melon | M-15 | Kanamycin | EHA105 | 13% (PCR) | Reported | [32] |
| Melon | CM-15 | Kanamycin | EHA105 | 4 plants (PCR) | Reported | [34] |
| Melon | Charentais, Galia, Piel de sapo, Blanco | Kanamycin | EHA105 | 1.3%, 1.6%, 3.8%, 2.5% (plants) | Reported | [27] |
| Melon | Charentais | Hygromycin B | EHA105 | 5.68% (shoots) | NR | [35] |
| Melon | m1 | GFP | EHA105 | 3.95% (GFP) | Reported | [22] |
| Pumpkin | JingXinZhen No. 4 | GFP | EHA105 | 3.56% (GFP) | Reported | [22] |
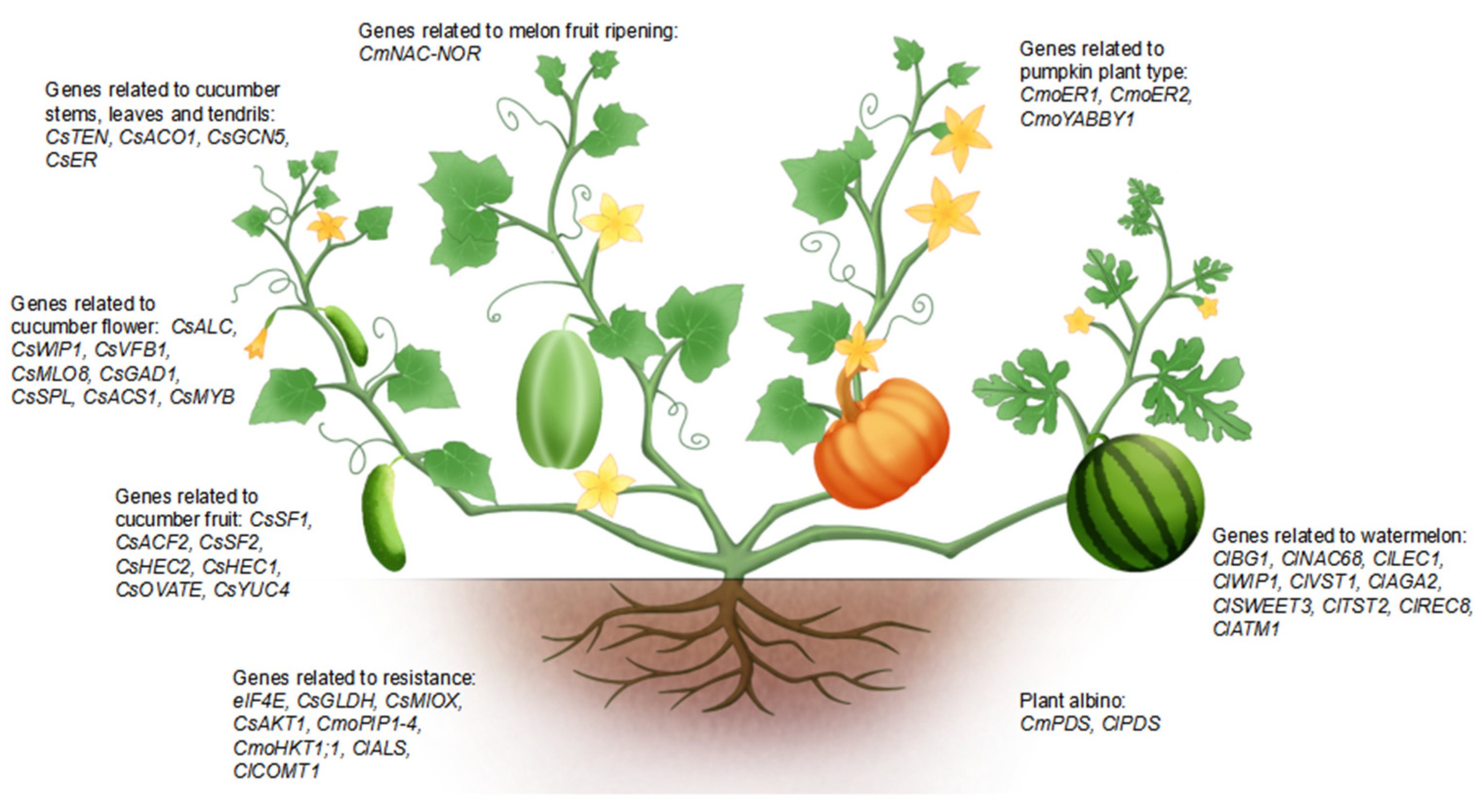
| Species | Gene | ID | Gene Function or Phenotype | References |
|---|---|---|---|---|
| Watermelon | ClALS | Cla019277 | Herbicide resistance | [38] |
| Watermelon | ClPDS | Cla97C07G142100 | Albino plants | [28] |
| Watermelon | ClPDS | Cla010898 | Albino plants | [37] |
| Watermelon | ClBG1 | Cla97C08G153160 | Regulation of seed size and seed germination | [64] |
| Watermelon | ClNAC68 | Cla97C03G059250 | Regulation of fruit sugar content and seed development | [65] |
| Watermelon | ClLEC1 | Cla97C10G188900 | Reproductive development | [29] |
| Watermelon | ClWIP1 | Cla008537 | Sex determination | [66] |
| Watermelon | ClVST1 | Cla97C02G031010 | Vacuolar sugar transporter | [67] |
| Watermelon | ClAGA2, ClSWEET3, ClTST2 | Cla97C04G070460, Cla97C01G000640, Cla97C02G036390 | Carbohydrate partitioning | [68] |
| Watermelon | ClREC8 | Cla97C07G132920 | Seedless watermelon | [69] |
| Watermelon | ClCOMT1 | Cla97C10G188660 | Tolerance to abiotic stresses | [70] |
| Watermelon | ClATM1 | Cla010576 | Male sterility | [71] |
| Cucumber | CsALC | Csa2G356640.1 | Pollen tube emergence | [72] |
| Cucumber | eIF4E | XM_004147349 | Antivirus | [42] |
| Cucumber | CsWIP1, CsVFB1, CsMLO8, CsGAD1 | Csa4M290830, Csa4M641640, Csa5M623470, Csa5M348050 | Gynoecious inbred lines | [43] |
| Cucumber | CsSPL | Csa3M850670 | Male and female fertility and ovule development | [73] |
| Cucumber | CsSF1, CsACF2 | Csa2G174140, Csa1G580750 | Cucurbit-specific RING-type E3 ligase, Rate-limiting enzyme for ethylene biosynthesis | [74] |
| Cucumber | CsSF2 | Csa2G337260 | Fruit elongation | [75] |
| Cucumber | CsTEN, CsACO1 | Csa5P644520.1, Csa6G160180 | Identity and mobility of tendrils | [76] |
| Cucumber | CsHEC2 | Csa2G285890 | Fruit wart | [77] |
| Cucumber | CsACS1, CsMYB | CsaV3_6G044400, CsaV3_6G044410 | Female floral development | [78] |
| Cucumber | CsHEC1, CsOVATE, CsYUC4 | Csa4G639900, Csa4G038760, Csa2G379350 | Fruit neck length | [79] |
| Cucumber | CsGCN5 | Csa6G527060 | Gene transcription and plant development | [30] |
| Cucumber | CsER | CsaV3_4G036080 | Compact plant architecture | [22] |
| Cucumber | CsGLDH, CsMIOX | Csa4M236360.1, Csa2M000640 | Ascorbic acid biosynthesis | [80] |
| Cucumber | CsAKT1 | CsaV3_1G029650 | Salt tolerance | [81] |
| Melon | CmPDS | MELO3C017772.2 | Albino plants | [35] |
| Melon | CmNAC-NOR | MELO3C016540.2 | Fruit ripening | [82] |
| Melon | CmNAC-NOR | MELO3C016540 | Fruit coloring and ripening | [83] |
| Melon | eIF4E | MELO3C002698.2 | Antivirus | [84] |
| Pumpkin | CmoER1, CmoER2 | CmoCh09G003660, CmoCh01G017570 | Compact plant architecture | [22] |
| Pumpkin | CmoPIP1-4 | CmoCh04G011950 | Salt stress | [85] |
| Pumpkin | CmoHKT1;1 | CmoCh10G003830 | High-affinity K+ transporter1 | [86] |
| Pumpkin | CmoYABBY1 | CmoCh15G012090 | Architecture regulation | [1] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, J.; Wang, N.; Li, Y.; Wang, H.; Zhang, W.; Wang, H.; Chai, S. Recent Progress in Genetic Transformation and Gene Editing Technology in Cucurbit Crops. Agronomy 2023, 13, 755. https://doi.org/10.3390/agronomy13030755
Feng J, Wang N, Li Y, Wang H, Zhang W, Wang H, Chai S. Recent Progress in Genetic Transformation and Gene Editing Technology in Cucurbit Crops. Agronomy. 2023; 13(3):755. https://doi.org/10.3390/agronomy13030755
Chicago/Turabian StyleFeng, Jing, Naonao Wang, Yang Li, Huihui Wang, Wenna Zhang, Huasen Wang, and Sen Chai. 2023. "Recent Progress in Genetic Transformation and Gene Editing Technology in Cucurbit Crops" Agronomy 13, no. 3: 755. https://doi.org/10.3390/agronomy13030755
APA StyleFeng, J., Wang, N., Li, Y., Wang, H., Zhang, W., Wang, H., & Chai, S. (2023). Recent Progress in Genetic Transformation and Gene Editing Technology in Cucurbit Crops. Agronomy, 13(3), 755. https://doi.org/10.3390/agronomy13030755







