Abstract
Antibiotics are widely used in livestock and poultry farming for disease prevention and animal growth promotion. Due to the low absorption rate of antibiotics by animals, antibiotics can remain in livestock and poultry manure as parent or metabolites and enter the farm environment with the application of livestock and poultry manure, which has potential effects on crop growth. This study assessed the effects of single and combined contamination of antibiotics in soil on tomato plants. The accumulation of antibiotics in tomato plants and the impacts on crop growth were investigated. A pot experiment with sandy soil was conducted in a greenhouse, and a 100-day tomato growth experiment was conducted by adding different doses of tetracycline and sulfadiazine (0, 25, and 50 mg/kg). The antibiotic contents in various tissues and organs of the tomato were examined, and the changes in photosynthetic intensity and biomass of the tomato were observed to simulate the effects of exogenous antibiotic addition on the growth and quality of the tomato. The results indicated that tomato plants simultaneously absorbed tetracycline and sulfamethazine from soil. Antibiotics were primarily absorbed by tomato roots and were further delivered to plant leaves, stems, and fruits. Antibiotics exhibited the highest concentration in roots, followed by leaves, stems, and fruits. The contents of tetracycline and sulfamethazine in plant tissues elevated with the increase in soil antibiotics, which were higher in the vegetative growth stage than those in the late growth stage. At concentrations of 25 and 50 mg/kg, tetracycline and sulfamethazine combined pollution significantly reduced leaf photosynthetic rates and plant biomass. Notably, high levels of antibiotics led to decreases in tomato yield and quality. Additionally, high concentrations of antibiotic contamination elevated leaf nitrogen, phosphorus, and potassium, but reduced the leaf carbon/nitrogen ratio, as well as reduced the vitamin C content in the fruits. Overall, since antibiotics can accumulate in vegetables and enter the food chain, the findings are crucial for evaluating the impacts of soil antibiotic contamination on the production and food safety of tomato products, and will also add to our knowledge of antibiotic migration into the food chain.
1. Introduction
The usage of antibiotics as veterinary medications and feed additives has grown yearly due to the rapid development of animal husbandry. It is estimated that the total amount of antibiotics used worldwide (including veterinary antibiotics and medical antibiotics) has reached 100,000 to 200,000 T [1]. Among them, veterinary antibiotics accounted for the vast majority of the use. The majority of antibiotics fed to animals are poorly absorbed in the intestines of animals and can be excreted as organic fertilizer through urine or feces [2,3,4]. Therefore, grazing livestock can directly transfer veterinary antibiotics to grassland and farmland soil through feces [5,6,7]. It is reported that more than 70% of veterinary antibiotics are released into the environment through diverse channels, with soil serving as the main pool of antibiotics [8,9,10]. Soil antibiotics can be ingested into the human body through the food chain or drinking water, and even lead to the formation of super bacteria with antibiotic resistance, which constitute a major threat to human health [11,12]. The exposure and adverse reactions of antibiotics in terrestrial ecosystems have attracted great attention in the past 20 years [13]. The effects of antibiotic residues in the environment include antibiotic resistance, the transfer of antibiotic resistance genes, and the effects on human health [12,14,15]. Ecotoxicity effects on non-target organisms and ecological impacts on agroecosystem have been documented [16].
Numerous studies have been conducted on the sources, fate, and ecological risks of antibiotics in the environment [17,18,19]. Tetracycline and sulfamethazine are frequently used in farm animals to treat intestinal and respiratory infections, and they are the most prevalent antibiotics in pork, beef, and animal manure [20]. Tetracycline and sulfonamides are widely distributed worldwide and come from the septic tanks or feces of livestock farms [2]. The concentration of antibiotic residues ranges from a few micrograms to grams per kilogram [14]. Chen et al. found that tetracycline, oxytetracycline, chlortetracycline, and doxycycline were the main pollutants in the feces of eastern China, with the highest concentrations found of 98.2, 354.0, 139.4, 37.2 and 7.1 mg/kg, respectively [21]. Zhang et al. showed that the concentration of oxytetracycline in soil was as high as 50 mg/kg [22]. Priya et al. detected that the concentration of tetracycline in agricultural soil with liquid fertilizer was as high as 0.3 g/kg [23]. Many crops can absorb tetracycline and sulfamethazine from contaminated or artificial soils [24]. It has been found that tetracycline can inhibit the growth of microorganisms in the soil, reduce soil enzyme activity, and significantly reduce the growth of the roots, stems, and leaves of crops [25]. However, until recently, research on the use of antibiotics has mainly focused on the beneficial and adverse effects of single antibiotics on humans and animals. Most studies have focused on the phytotoxicity of antibiotics to young plants, especially the development of seed germination, roots, and the stem [26]. However, there is still lack of studies on the effects of antibiotics on the growth of crops in different growth periods [27]. A few studies have reported on the accumulation of antibiotics in the edible parts of vegetable crops, such as tubers, rhizome crops, fruit, and vegetable crops [28]. Besides, the toxic effects of the combined contamination of several antibiotics on crops also remain largely elusive [29]. Although current studies have shown that chemical fertilizers and livestock manure containing antibiotics as the main sources of contaminants in vegetable fields, the knowledge about the effects of soil antibiotic contamination on vegetable growth and development, the uptake pattern of antibiotics by crops, the migration of antibiotics in crops, and the physiological effects of antibiotics on crops is obviously insufficient so far [30,31]. The research work on the environmental behavior of antibiotics in farmland systems and their physiological effects on crops at home and abroad is still far from providing a comprehensive evaluation of the effects, patterns, and degree of harm of antibiotic residues in the soil environment on farmland ecology and human health [32,33].
In China, aside from food crops, vegetables are the most important crops in terms of economic standing and planting area. Due to the high demand for vegetables in urbanized regions, vegetable farms are mostly located near populated areas. However, the production and quality of vegetables are seriously threatened by the widespread use of chemical fertilizers and animal manure as organic fertilizers [34]. As a major fruit and vegetable crop, the production of the tomato (Lycopersicon esculentum Mill.) has reached 18,000 million tons in 2020 [35]. In this study, to investigate the effects of tetracycline and sulfamethazine on plants, tomato plants were planted in a greenhouse with different concentrations of tetracycline and sulfamethazine. Furthermore, the adverse effects of the combined contamination of the two antibiotics on plants were also studied in different growth stages. We thoroughly determined the accumulation patterns and influencing factors of individual and combined antibiotic contamination in tomato organs. The findings are essential for assessing the impacts of soil antibiotic contamination on the safety, yield, and quality of agricultural products, and will also add to our knowledge of antibiotic migration into the food chain.
2. Materials and Methods
2.1. Collection and Preparation of Soil Samples
The studied soil (Fluvo aquic soil) was collected from Keqiao District, Shaoxing City (29°54′ N, 121°29′ E), and the soil samples were collected at 0–15 cm using a bamboo shovel and mixed thoroughly. The texture was silt loam, with no tetracycline and sulfamethazine detected. The collected soil was air-dried, mixed, and screened by a 5 mm mesh sieve. Part of the soil samples was further sieved through 2 mm and 0.25 mm soil mesh sieves for the analyses of soil physical and chemical properties. The soil properties were as follows: pH 5.67, soil organic carbon (SOC) content 9.23 g/kg, available phosphorus (AP) 7.89 mg/kg, and available potassium (AK) 56 mg/kg. The tetracycline (96% purity) and sulfamethazine (99% purity) were purchased from Sigma, Inc., Germany and stored in an ice chamber at 4 °C. Commercial seeds of tomato (Purchased from the Chinese Academy of Agricultural Sciences, Hangzhou, China.) were used for on-soil tests. They were sterilized and transferred to Petri dishes for germination at 25 °C in the dark. In accordance with the already-used analysis method in Migliore et al. [36], after germination, seedlings were transferred to multipurpose jars on solid Murashige and Skoog (MS) medium (Sigma Inc., Milan, Italy). All reagents in the experiment were purchased from Aladdin (Aladdin Co., Ltd., Shanghai, China) and Sigma (Sigma Inc., Milan, Italy).
2.2. Pot Experiment Design
The pot experiment was carried out in a greenhouse of Zhejiang University, Hangzhou, China. The soil was air-dried and ground to pass a 2 mm sieve. The pot experiment was conducted with 7 antibiotic treatments and 6 replications (Table 1). Each pot contained 25 kg soil with 0.5 g NH4NO3 and 0.2 g KH2PO4 per kilogram of soil added as chemical fertilizer to ensure adequate nutrition during the experiment. To avoid the introduction of other antibiotics from organic fertilizers, animal manure was not applied during the experiment, and antibiotics were added manually according to the dosage in Table 1. After the soil and additives were fully mixed, the soil moisture content was adjusted to 75–80% of the field capacity with deionized water and placed into a bowl with a height of 15 cm and an outer diameter of 30 cm. Tomato seedlings were transplanted at the age of 25 days into each pot, 4 plants in each pot. Soil and plant sampling and related observations were carried out within 100 days after the experiment. The plants were cultured in an artificial climate chamber, where the photoperiod of the plants was 16h light/8 h dark and the diurnal temperature was 25/20 °C. During the culture period, water was added weekly to maintain the field capacity using the weighing method. The experiment lasted from April to September with sufficient illumination.

Table 1.
Dosage of antibiotics for different treatments.
Soil samples were collected 6 times at 1, 5, 10, 20, 50, and 100 days respectively after antibiotic exposure. During the experiment, plant samples were collected at the same time for the determination of antibiotic residues; total N, P, K, and nitrate; vitamin C; and reducing sugars in plants. To facilitate sample collection, the 6 replicate treatments were divided into two groups (each with 3 replicates). On the 20th and 50th days of the experiment, plant sampling was carried out in the first group, and two tomato plants were taken each time. At the end of the experiment (100th day), plant sampling was carried out in the second group, and four tomato plants were collected from each treatment. The harvested plants were first rinsed thoroughly with tap water and deionized water, then dried with toilet paper, and then divided into roots, stems, leaves and fruits, and the fresh weight was recorded immediately. Plant samples were stored at −15 °C before analyses.
In addition, the height of tomato plants in each treatment was measured on the 20th, 50th, and 100th days of the experiment. On the 50th day of the experiment, the chlorophyll content (SPAD value) and photosynthetic parameters of the largest expanded leaf of each plant were measured, including stomatal conductance, transpiration rate, intercellular carbon dioxide concentration, and photosynthetic rate [37]. SPAD-502 chlorophyll (KONICA MINOLTA, Inc., Tokyo, Japan) was used to detect the leaf SPAD value (chlorophyll content), and the photosynthetic parameters were measured using the LI-6400 portable photosynthesis apparatus (LI-COR, Inc., Lincoln, Nebraska, USA) [37]. The measurements were carried out under daylight conditions between 11 a.m. and 2 p.m.
2.3. Chemical Analyses
All chemicals used in the experiments were of analytical grade. All glassware and vessels used in the analyses were soaked in 10% HNO3 overnight and rinsed thoroughly with deionized water before use. The accuracy of the data was checked using materials that met national standards (GBW-07404), and precision based on duplicate samples was found to be better than ±5%.
Soil pH was determined with a pH meter in the supernatant at the water: soil ratio of 2.5:1 (FE20-FiveEasy™ pH, Mettler Toledo, Giessen, Hesse, Germany). Sand, silt, and clay concentrations in the soil samples were evaluated by the micropipette method: Add dispersant to disperse the particles, then put the suspension in the settling cylinder and measure it by specific gravity meter [36]. SOC was determined by element analyzer after soil grounded through 0.149 mm sieve and wrapped in tin foil (Elementar Vario EL Cube, Langenselbold, Hesse, Germany) [38]. Soil available potassium (AK) was extracted with 1 mol/L ammonium acetate and determined with atomic absorption spectrometry (AAS, Analytik Jena novAA 300, Jena, Germany) [39]. Soil available phosphorus (AP) was determined by 0.5 mol/L NaHCO3 extraction ascorbic acid/molybdate colorimetry [39].
After the fresh plant samples were ground with a small food grinder, 5 g of each sample was weighed and extracted with 20 mL EDTA-Mcilvine (0.05 mol/L EDTA + 0.06 mol/L Na2HPO4 + 0.08 mol/L citric acid, pH 4) for 5 min [40]. After extraction, the supernatant was separated under a centrifugal force of 2000 g for antibiotic determination. The sample was purified by chromatography column and determined by a UV spectrophotometer (UV-5800, Metash Instruments Co., Ltd., Shanghai, China). The chromatographic column was SHIM-PACK.VP-ODS (5 μm, 150 mm × 4.6 mm ID), the mobile phase was 0.01 mol/L oxalic acid +acetonitrile + methanol (76 + 16 + 8), and the flow rate was 1 mL/min. The detection limits of tetracycline and sulfamethazine were 2.5 and 5 μg/kg, and the results were expressed based on fresh weight. Acetonitrile and methanol used in the test were HPLC grade, and citric acid, Na2HPO4, and EDTA were analytical reagent grade [40]. The total carbon (C) and total nitrogen (N) of plant leaves were measured with CHN elemental analyzer after soil grounded and wrapped in tin foil (Flash EA 1112, Thermo Finnigan LLC., California, USA). After digesting 0.5 g dry leaf sample with H2O-HNO3 solution, the phosphorus (P) was determined by 0.5 mol/L NaHCO3 extraction ascorbic acid/molybdate colorimetry; the concentrations of calcium (Ca) and potassium(K) were determined by atomic absorption spectrometry leaching with 1 mol/L ammonium acetate (AAS, Analytik Jena novAA 300, Langenselbold, Hesse, Germany) [39]. At the same time, the contents of nitrate of plant fruits were measured with a UV spectrophotometer (UV-5800, Metash Instruments Co., Ltd., Shanghai, China), Vitamin C was measured with high-performance liquid chromatograph leaching with 20 g/L oxalic acid (HPLC, Agilent-1260, Agilent Technologies Inc., California, USA) [41], and reducing sugar was determined by potassium permanganate titration extraction with ethanol, which was determined by titration with Fehling reagent (national standard method GB5009.7-85) [42], and the results were expressed based on dry mass.
2.4. Data Analysis
Results of the experiments are shown with means and standard deviations. Statistics were carried out in the SPSS v26.0 (SPSS Inc., Chicago, IL, USA). The p values < 0.05 by Tukey’s HSD one-way analysis of variance (ANOVA) were used to analyze the significant differences (p < 0.05). Statistical analysis was performed by OriginPro 2020 software (OriginLab Co., Ltd., Northampton, MA, USA).
3. Results and Discussion
3.1. Changes in Tetracycline and Sulfamethazine in Soil
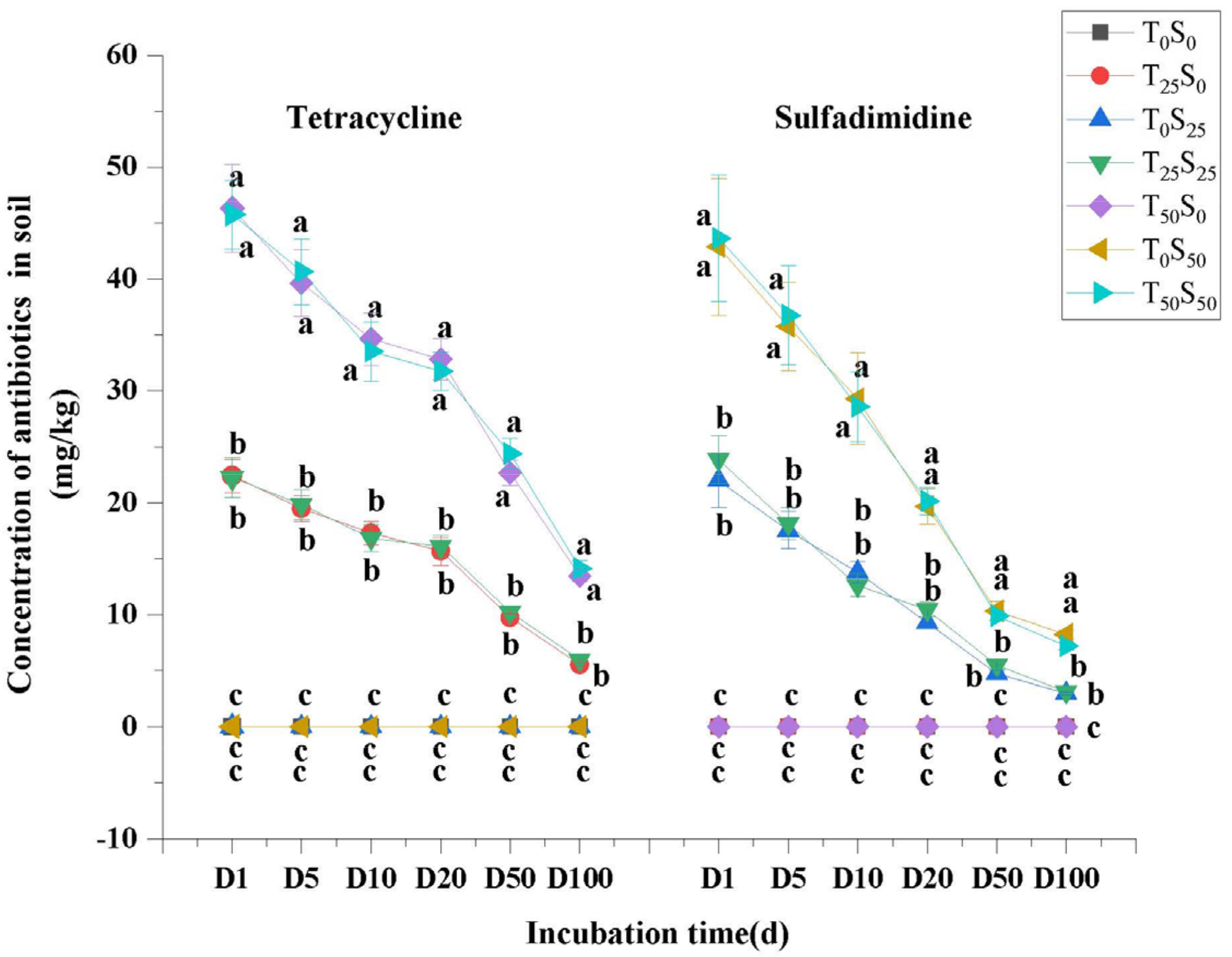
Tetracycline and sulfamethazine were not detected in the soil treated without tetracycline and sulfamethazine. The residues of tetracycline and sulfamethazine in the soil treated with tetracycline and sulfamethazine increased with the addition of antibiotics but decreased with the increase in culture time (Figure 1). On the 100th day of the experiment, the residual rates of tetracycline in treatment T25S0 and treatment T25S25 (25 mg/kg tetracycline treatment) were 22.04% and 23.88%, respectively. The residual rates of tetracycline in T50S0 and T50S50 (50 mg/kg tetracycline treatment) were 26.94% and 28.26%, respectively. Meanwhile, the residual rates of sulfamethazine in the soil of treatmentsT0S25 and treatment T25S25 with 25 mg/kg sulfamethazine were 11.87% and 12.52%, respectively. The residual rates of sulfamethazine in the soil of treatment T0S50 and T50S50 with 50 mg/kg sulfamethazine were 16.48% and 14.42%, respectively. In general, the degradation rate of sulfamethazine in the soil was higher than that of tetracycline. The residual percentage of the two antibiotics in the soil decreased with the increase in the dosage of antibiotics. Compared with the treatment of single antibiotics, the combined contamination of two antibiotics had no significant effect on the degradation of antibiotics in the soil.
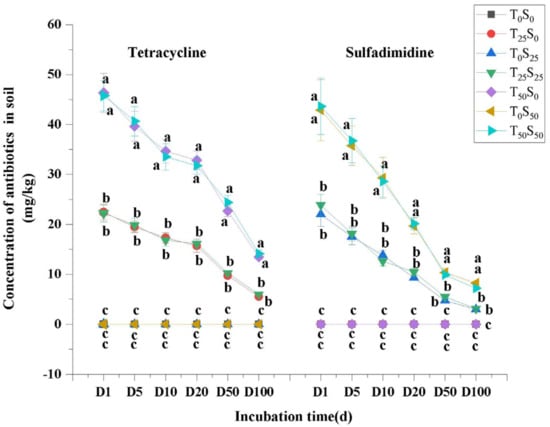
Figure 1.
Changes in tetracycline and sulfamethazine concentrations under different treatments with incubation time. Different lowercase letters mean significant differences among different treatments (p < 0.05). T0S0: control, T25S0: tetracycline 25 mg/kg + sulfamethazine 0 mg/kg, T0S25: tetracycline 0 mg/kg + Sulfamethazine 25 mg/kg, T25S25: tetracycline 25 mg/kg + sulfamethazine 25 mg/kg, T50S0: tetracycline 50 mg/kg + sulfamethazine 0 mg/kg, T0S50: tetracycline 0 mg/kg + sulfamethazine 50 mg/kg, T50S50: tetracycline 50 mg/kg + sulfamethazine 50 mg/kg. D1: 1st day, D5: 5th day, D10: 10th day, D25: 25th day, D50: 50th day, D100: 100th day.
3.2. Changes in Tetracycline and Sulfamethazine in Roots and Shoots of Tomato Plants
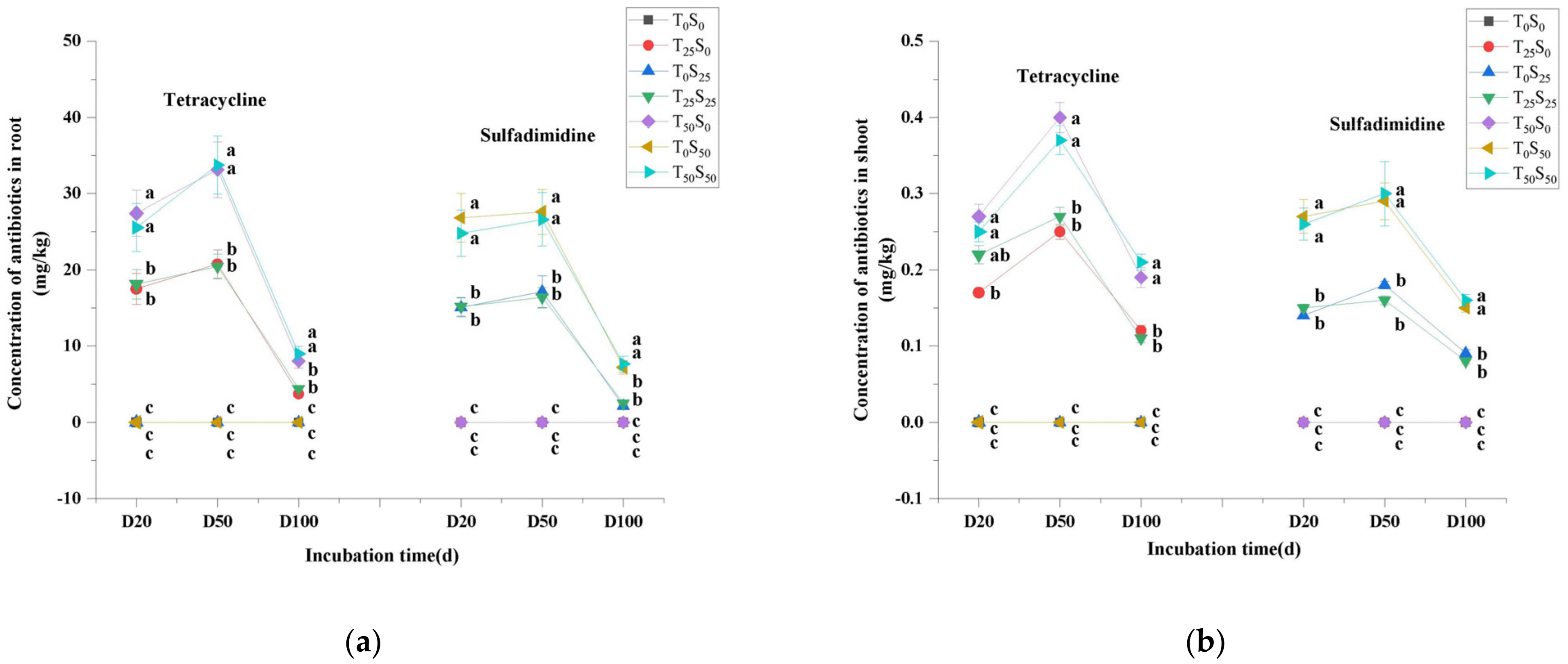
Similar to the changes in tetracycline and sulfamethazine in soil content, antibiotics were not detected in tomato plants growing on soil without antibiotic treatment, while tetracycline and sulfamethazine were detected in plant tissues that grew on soil treated with antibiotics (Figure 2). The levels of antibiotics in plant tissues elevated with the increase in additive antibiotics in the soil, which indicated that tomato plants absorbed more antibiotics with the increase in antibiotic pollution level in the soil. It is worth noting that the contents of tetracycline and sulfamethazine in tomato roots were significantly higher than those in the shoots (Figure 2). The concentrations of tetracycline and sulfamethazine in tomato roots were 31.00–102.88 and 23.56–107.64 times higher than those in the shoots, respectively, which indicated that the concentrations of tetracycline and sulfamethazine in tomato roots were significantly enriched. However, the migration of antibiotics in plants was relatively weak. The difference in antibiotics between roots and shoots decreased with the extension of the experiment time. On the 20th day to the 50th day of the experiment, the contents of tetracycline and sulfamethazine in shoots and roots increased at first and then decreased. After the 50th day of the experiment, the decrease in antibiotics in plants might have been related to the dilution effect caused by the rapid increase of plant biomass, the decrease in the availability of tetracycline in the soil, and reduced absorption of the antibiotics by plants due to the degradation of the antibiotics in the soil. There was no significant difference in the contents of antibiotics in the roots and shoots between the two kinds of combined antibiotic contamination and single antibiotic contamination treatments. The results also showed that the absorption of sulfamethazine was slightly lower than that of tetracycline when antibiotics were added at the same concentration.
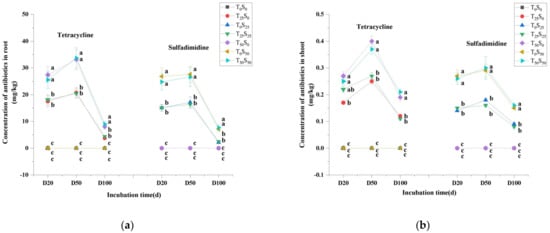
Figure 2.
Changes in tetracycline and sulfamethazine concentration in tomato (a) root and (b) shoot under different treatments with incubation time. Different lowercase letters mean significant differences among different treatments (p < 0.05). T0S0: control, T25S0: tetracycline 25 mg/kg + sulfamethazine 0 mg/kg, T0S25: tetracycline 0 mg/kg + Sulfamethazine 25 mg/kg, T25S25: tetracycline 25 mg/kg + sulfamethazine 25 mg/kg, T50S0: tetracycline 50 mg/kg + sulfamethazine 0 mg/kg, T0S50: tetracycline 0 mg/kg + sulfamethazine 50 mg/kg, T50S50: tetracycline 50 mg/kg + sulfamethazine 50 mg/kg.
3.3. Residues of Tetracycline and Sulfamethazine in Different Tissues of Tomato during Harvest
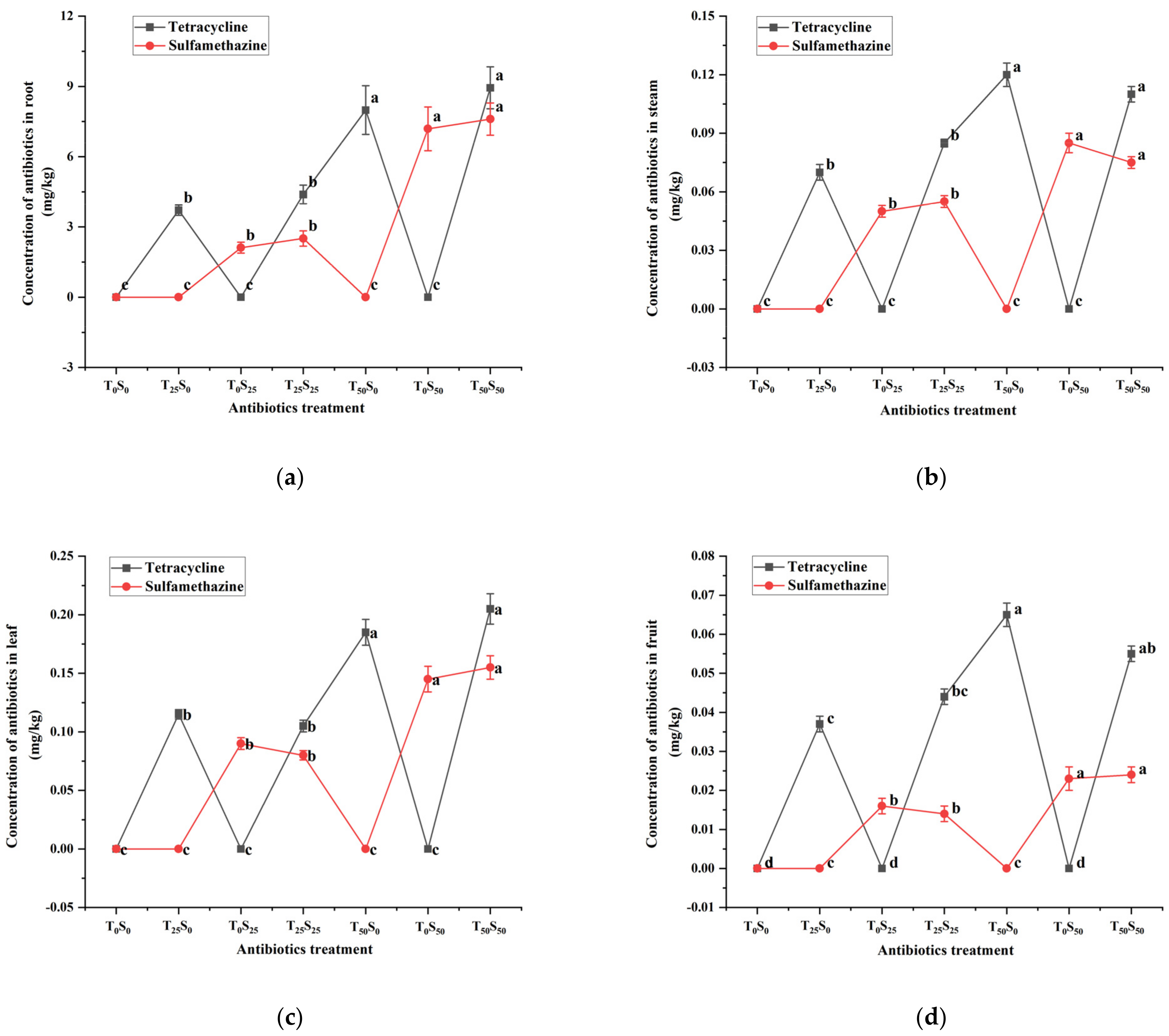
The residues of two antibiotics in various organs of tomatoes at the time of harvest (100th day) are shown (Figure 3). The tetracycline and sulfamethazine were still detected in the roots, leaves, stems, and fruits of the tomatoes grown in the soil treated with antibiotics. The contents of tetracycline and sulfamethazine in plant tissues increased with the increase in antibiotic concentration in the soil. The contents of tetracycline and sulfamethazine were the highest in roots, followed by leaves, stems, and fruits. Most of the antibiotics remained in the roots, and the concentrations of antibiotics in the roots were 42–101, 24–50, and 100–317 times more than that in stems, leaves, and fruits, respectively.
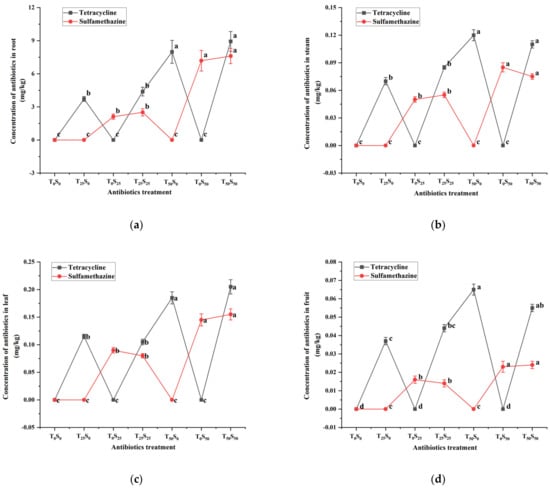
Figure 3.
Changes in tetracycline and sulfamethazine concentrations in tomato (a) root, (b) steam, (c) leaf and (d) friut under different treatments at harvest. Different lowercase letters mean significant differences among different treatments (p < 0.05). T0S0: control, T25S0: tetracycline 25 mg/kg + sulfamethazine 0 mg/kg, T0S25: tetracycline 0 mg/kg + Sulfamethazine 25 mg/kg, T25S25: tetracycline 25 mg/kg + sulfamethazine 25 mg/kg, T50S0: tetracycline 50 mg/kg + sulfamethazine 0 mg/kg, T0S50: tetracycline 0 mg/kg + sulfamethazine 50 mg/kg, T50S50: tetracycline 50 mg/kg + sulfamethazine 50 mg/kg.
3.4. Effects of Antibiotic Pollution on Nutrient Content in Plant Organs
The contents of C, N, P, K, Ca, and other elements in the leaves at the 50th and 100th days of the experiment (vegetative growth period and fruit harvest period) are shown (Table 2). When the concentration of antibiotics was 25 mg/kg, the content of C in leaves was similar to that in the control treatment (without antibiotic treatment), and there was no significant difference in the detection value of C in leaves. However, when the concentration of antibiotics was 50 mg/kg, there was a significant difference in the content of C in the leaves between the plants grown on the soil treated with antibiotics and the control. With the treatment with antibiotics, the content of C in the leaves of the plants decreased significantly.

Table 2.
Changes of C, N, P, K, Ca content in tomato leaves at different growth stages with tetracycline and sulfamethazine pollution.
The N content in the leaves increased after antibiotic treatment, but when the antibiotic dosage was 25 mg/kg, the N content in the leaves was significantly higher than that in the control group at the vegetative stage, but the difference was not significant at the harvest stage (Table 2). However, when the antibiotic dosage was 50 mg/kg, the N content in leaves was significantly higher than that of the control in both the vegetative stage and the harvest stage. The results showed that the nitrogen content in the leaves of the plants under the combined pollution of the two antibiotics was higher than that under the single pollution, indicating that the combined pollution of the two antibiotics affected the N accumulation in the leaves of the plants.
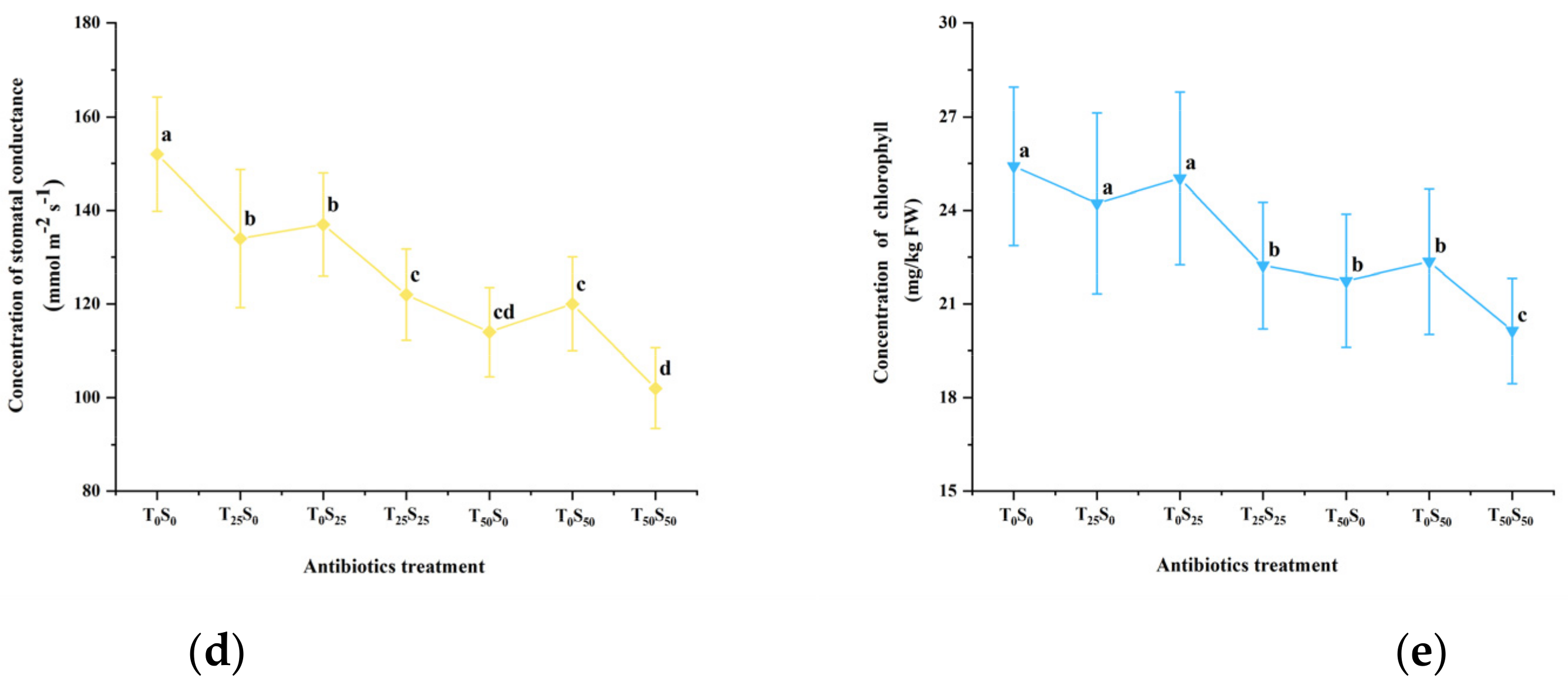
3.5. Effects of Antibiotic Pollution on Photosynthesis
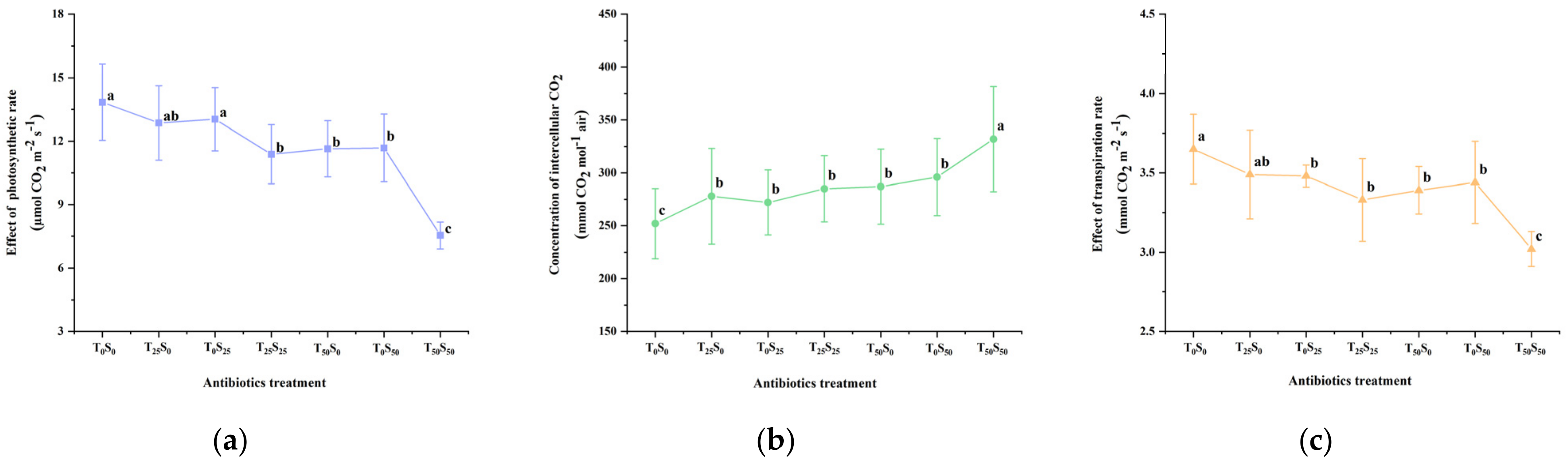
The chlorophyll content (SPAD value) and photosynthetic parameters were determined on the 50th day of the experiment (Figure 4). The results showed that antibiotic pollution led to the increased concentration of intercellular CO2 and decreased photosynthetic rate, transpiration rate, stomatal conductance, and chlorophyll content in the leaves of growing tomato plants. This effect increased with the increase in antibiotic pollution concentrations, especially under the conditions of combined pollutions. Compared with the control, when the single addition concentration of tetracycline and sulfamethazine was 25 mg/kg, it slightly reduced the photosynthetic rate, transpiration rate, and chlorophyll content, but there was no significant difference from the control. However, the addition of tetracycline and sulfadiazine (25 mg/kg) significantly increased the intercellular CO2 concentration and reduced the stomatal conductance. Among them, the single addition of 25 mg/kg of tetracycline and sulfadiazine increased the intercellular CO2 concentration by 10.32% and 7.94% and decreased the stomatal conductance by 11.84% and 9.87% compared with the control, respectively. However, under the combined pollution of tetracycline and sulfamethazine (25 mg/kg), at the same time, the intercellular CO2 concentration was significantly increased by 13.10% compared with the control. Compared with the control, the chlorophyll decreased by 12.51%, the stomatal conductance decreased by 19.74%, the transpiration rate decreased by 8.77%, and the photosynthetic rate decreased by 17.77%.
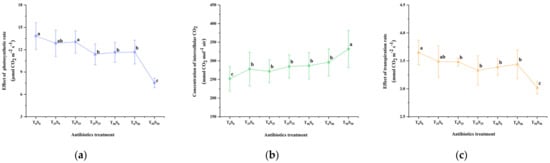
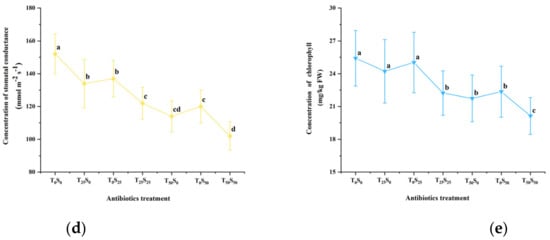
Figure 4.
Effects of tetracycline and sulfamethazine pollution on (a) photosynthetic rate, (b) concentration of intercellular CO2, (c) transpiration rate, (d) stomatal conductance, and (e) chlorophyll content of tomato plants. Different lowercase letters mean significant differences among different treatments (p < 0.05). T0S0: control, T25S0: tetracycline 25 mg/kg + sulfamethazine 0 mg/kg, T0S25: tetracycline 0 mg/kg + Sulfamethazine 25 mg/kg, T25S25: tetracycline 25 mg/kg + sulfamethazine 25 mg/kg, T50S0: tetracycline 50 mg/kg + sulfamethazine 0 mg/kg, T0S50: tetracycline 0 mg/kg + sulfamethazine 50 mg/kg, T50S50: tetracycline 50 mg/kg + sulfamethazine 50 mg/kg.
When the concentration of tetracycline was increased to 50 mg/kg, compared with the control, the chlorophyll, stomatal conductance, transpiration rate and photosynthetic rate decreased by 15.98%, 25.00%, 7.12%, and 15.90%, respectively. Accordingly, when 50 mg/kg of sulfadiazine was added, the above four parameters decreased by 12.04%, 21.05%, 5.75%, and 15.60%, respectively.
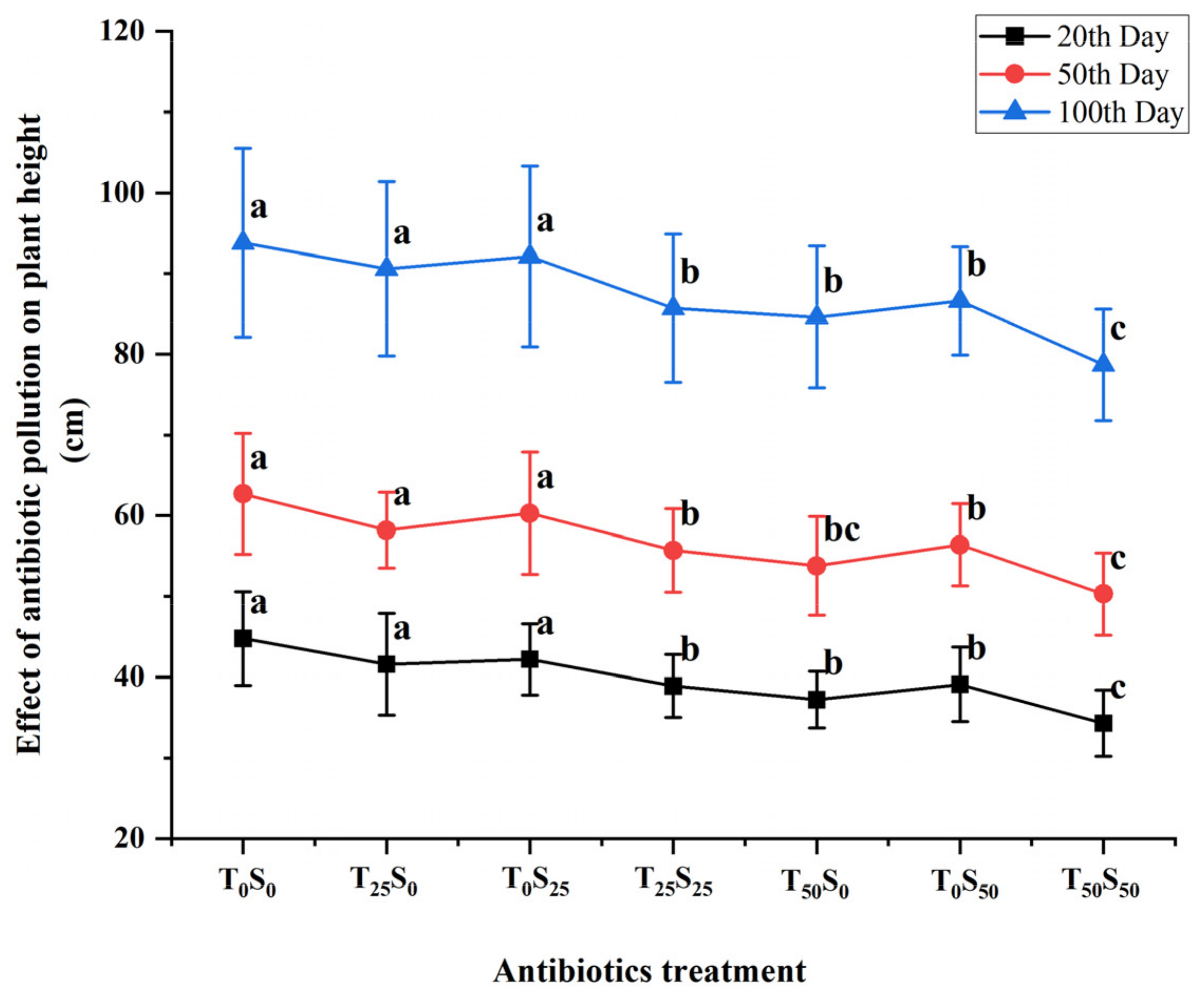
3.6. Effects of Antibiotic Pollution on Tomato Plant Height and Length
The variation in tomato plant height was examined under different antibiotic treatments (Figure 5). When individual treatments of 25 mg/kg of antibiotics were added, tetracycline and sulfamethazine had no significant effect on the plant height in the three growth stages, and the height was similar to that of the control. However, when 25 mg/kg of tetracycline and sulfamethazine were added at the same time, the plant height in each growth stage was significantly lower than that of the control treatment. The plant heights on the 20th, 50th, and 100th days of the experiment were decreased by 13.17%, 11.16%, and 8.64%, respectively.
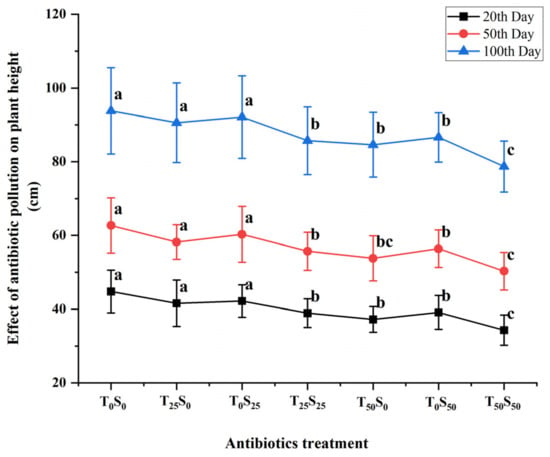
Figure 5.
Effects of tetracycline and sulfamethazine pollution on tomato plants height at different growth stages. Different lowercase letters mean significant differences among different treatments (p < 0.05). T0S0: control, T25S0: tetracycline 25 mg/kg + sulfamethazine 0 mg/kg, T0S25: tetracycline 0 mg/kg + Sulfamethazine 25 mg/kg, T25S25: tetracycline 25 mg/kg + sulfamethazine 25 mg/kg, T50S0: tetracycline 50 mg/kg + sulfamethazine 0 mg/kg, T0S50: tetracycline 0 mg/kg + sulfamethazine 50 mg/kg, T50S50: tetracycline 50 mg/kg + sulfamethazine 50 mg/kg.
When a single antibiotic was added, tetracycline and sulfamethazine (50 mg/kg) had significant effects on the plant height in three growth stages. Among them, when 50 mg/kg of tetracycline was added on the 20th, 50th, and 100th days, plant height was decreased by 16.96%, 14.19%, and 9.81%, respectively, compared with the control. The plant height decreased by 12.72%, 10.05%, and 7.68%, respectively, compared with the control on the 20th, 50th, and 100th days after adding 50 mg/kg of sulfamethazine. At the same time, the plant height of 50 mg/kg of tetracycline and sulfadiazine was significantly lower than that of the control, and decreased by 23.44%, 19.78%, and 16.10% on the 20th, 50th, and 100th days, respectively, compared with the control. In general, the effect of tetracycline was slightly greater than that of sulfamethazine, and the effect of the combined pollution was greater than that of single pollution. The degree of influence decreased with the increase in tomato growth period.
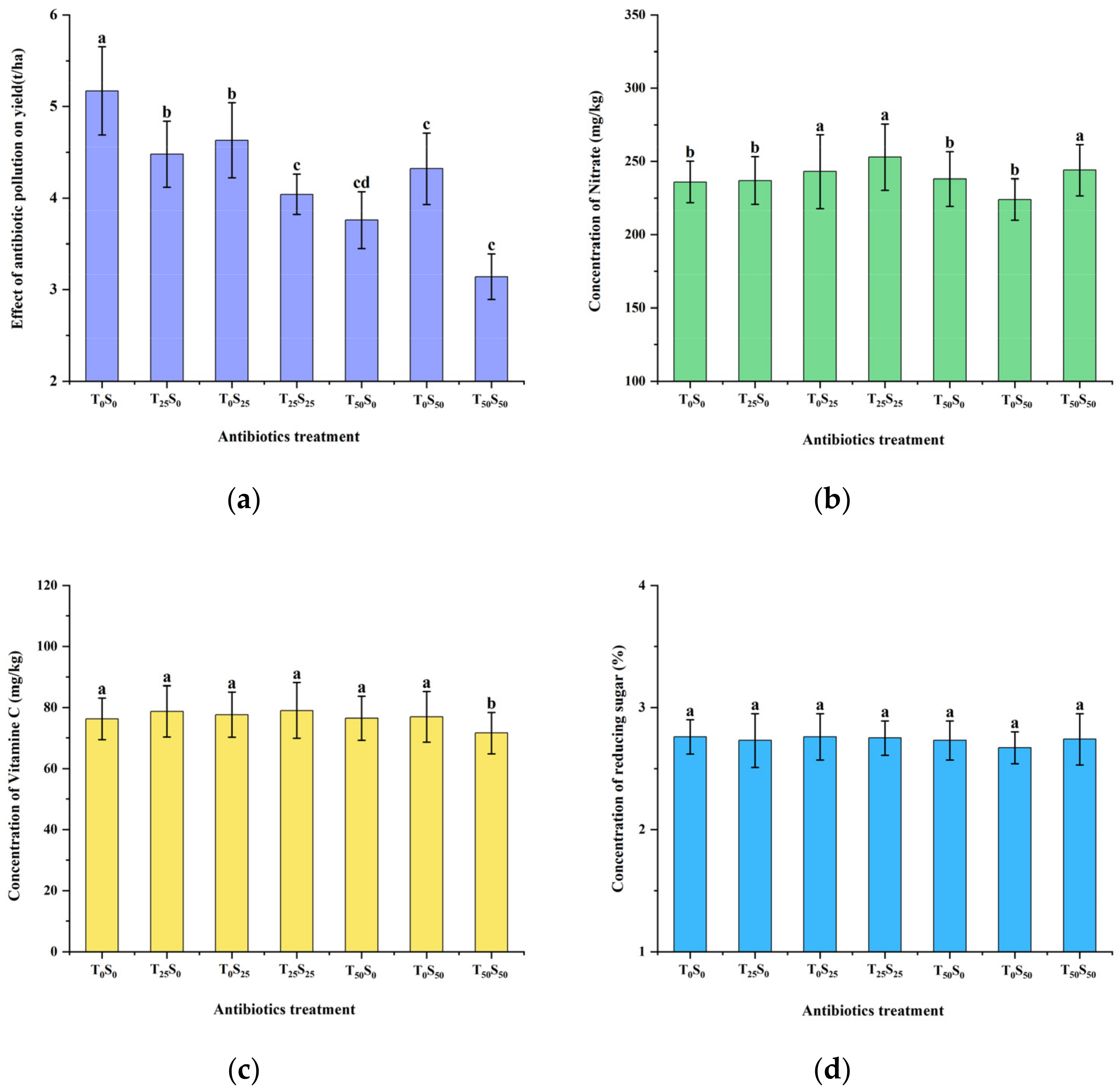
3.7. Effects of Antibiotic Contamination on Yield and Quality
Soil antibiotic pollution reduced tomato yield but had little impact on quality indicators (Figure 6). When 25 mg/kg of antibiotics were added alone, tetracycline and sulfamethazine reduced tomato yield by 13.35% and 10.45%, respectively, compared with the control. When 50 mg/kg of antibiotics were added alone, tetracycline and sulfamethazine reduced tomato yield by 27.27% and 16.44%, respectively, compared with the control. Similar to the effect on plant height, the effect of tetracycline was slightly greater than that of sulfamethazine. The effect of combined pollution was greater than that of single pollution. When 25 mg/kg of tetracycline and sulfamethazine were added at the same time, the tomato yield significantly decreased by 21.86% compared with the control. When 50 mg/kg of tetracycline and sulfamethazine were added at the same time, the yield of tomatoes conspicuously decreased by 39.26% compared with the control.
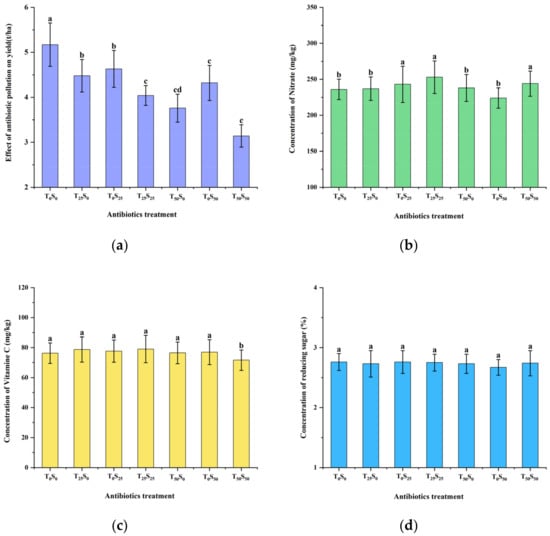
Figure 6.
Effects of tetracycline and sulfamethazine pollution on (a) yield, (b) concentrations of nitrate, (c) vitamin C, and (d) reducing sugar of tomato plants. Different lowercase letters mean significant differences among different treatments (p < 0.05). T0S0: control, T25S0: tetracycline 25 mg/kg + sulfamethazine 0 mg/kg, T0S25: tetracycline 0 mg/kg + Sulfamethazine 25 mg/kg, T25S25: tetracycline 25 mg/kg + sulfamethazine 25 mg/kg, T50S0: tetracycline 50 mg/kg + sulfamethazine 0 mg/kg, T0S50: tetracycline 0 mg/kg + sulfamethazine 50 mg/kg, T50S50: tetracycline 50 mg/kg + sulfamethazine 50 mg/kg.
The effects of antibiotic contamination on the accumulation of nitrate in tomatoes were only observed in three treatments: in the addition of 25 mg/kg of sulfamethazine, 25 mg/kg of tetracycline combined with 25 mg/kg of sulfamethazine, 50 mg/kg of tetracycline combined with 50 mg/kg of sulfamethazine significantly increased the accumulation of nitrate by 2.97%, 7.20%, and 3.38% compared with the control, respectively. The effect of antibiotic pollution on vitamin C content in tomatoes only appeared in the treatment of adding 50 mg/kg of tetracycline and sulfamethazine at the same time, which significantly reduced vitamin C content by 6.10% compared with the control. The effect of each treatment on reducing sugar content in tomatoes was not obvious.
4. Discussion
Previous studies have shown that antibiotics accumulated in the soil can be absorbed and highly enriched in plants (e.g., barley, corn, potatoes, peas, etc.), and this can have certain effects on plant growth. Therefore, antibiotics are likely to enter the human body via the food chain and induce the production of drug-resistant bacteria in humans. It has been pointed out that the presence of antibiotics can have important effects on the growth of the above-ground parts of plants, the development of the root system, and the activity of chloroplast systems [43,44]. Different types of plants vary greatly in the accumulation and uptake of antibiotics, and some plants can accumulate high levels of antibiotics, even higher than the concentration of antibiotics in the culture substrate [44,45]. Our results indicate that high concentrations of antibiotic pollution can affect the growth and photosynthesis of tomatoes. These findings should raise more concern in tomato production, especially in areas confronting combined antibiotic pollution.
Some plants can actively absorb antibiotics and deliver them to the stems, leaves, and fruits. Roots are the main site of antibiotic accumulation in plants [46]. Consistently in our study, antibiotics mainly accumulated in tomato root systems, suggesting their major role in the absorption and accumulation of antibiotics. Only a small proportion of antibiotics are transported to the shoot of the plant [47]. Compared with shoots, the antibiotic concentration in the roots is much higher. Therefore, the roots are more sensitive to soil antibiotic pollution, and the responses are more direct.
It has been shown that the uptake of contaminants by crop plants increases with the increase in contaminant concentrations in the soil. Additionally, the uptake of soil contaminants is greater in crops at the peak growth stage [48]. Our results showed that the accumulation of tetracycline and sulfamethazine in tomato leaves first increased and then decreased with the added time of tetracycline and sulfamethazine in the soil. These results may be related to the varied absorption abilities of tomato plants in different growth stages, as well as the dynamics of antibiotic residues in the soil. Additionally, along with plant growth, the crop biomass rose and plants were more resilient to external environment challenges. Therefore, the resistance of plants to the adverse effects of antibiotic pollutants in the soil may have also increased [49].
Similar to the combined pollution of heavy metals in soil, the inhibition effects of the two antibiotics in soil on vegetable growth significantly increased, which may be related to the cumulative effects of different antibiotics on plants [49,50]. Plants may respond to different environmental challenges through common mechanisms. For instance, photosynthesis is fundamental to all plants in terms of plant growth, nutrition, and resistance to abiotic or biotic stresses [51]. The effect of antibiotics on the photosynthesis of tomato plants is similar to the stress of salt, alkalinity, and heavy metals [52].
High concentrations of tetracycline and sulfamethazine significantly decreased leaf photosynthetic rate, transpiration rate, and stomatal conductance, as well as significantly increased intercellular CO2 concentration. In this study, the decrease in tomato biomass exposed to high concentrations of tetracycline and sulfamethazine in the soil may be related to the negative effects of tetracycline and sulfamethazine on photosynthesis [52,53].
In addition, the results of this study also showed that antibiotic pollution could also cause changes in plant nutrients, which mainly manifested in the decrease in total carbon in leaf tissue and the increase in total nitrogen, Ca, P and K contents [40,41].
The decrease in total carbon may be related to the impact of antibiotics on the photosynthesis of plants, that is, with the increase in antibiotic pollution, the concentration of intercellular CO2 in plant leaves increases (accumulates), thus reducing the photosynthetic rate, transpiration rate, stomatal conductance, and chlorophyll content, thus resulting in the photosynthetic efficiency of plant leaves being decreased and the accumulation of organic carbon being decreased. This effect may also be the main reason for the decline in the height and yield of tomato plants caused by antibiotic pollution [46,52,53].
The increase in N, Ca, P and K content in leaves may also be related to changes in photosynthesis. Antibiotics can inhibit the synthesis of folic acid, that is, antibiotics hinder photosynthesis and plant biomass decreases, thereby relatively increasing the content of other nutrient elements in leaves [37,41]. In addition, high concentrations of antibiotic pollution can affect the absorption of soil water by plants, which can also promote the relative concentration of nutrients in leaves and reduce the migration of nutrients to other organs to a certain extent, but this claim needs further experimental verification.
5. Conclusions
Under the condition of combined antibiotic pollution, tomatoes can simultaneously absorb tetracycline and sulfamethazine from soil and transfer them to leaves, stems, fruits, and other organs. The contents of tetracycline and sulfamethazine in plant tissues increased with the increase in antibiotic pollution levels in the soil and were higher at the early growth stage than at the late growth stage. The content of antibiotics in different organs was highest in roots, followed by leaves, stems, and fruits. When the concentrations of tetracycline and sulfamethazine in the soil were 25 and 50 mg/kg, both tetracycline and sulfamethazine could affect tomato growth, thereby reducing photosynthetic rate, chlorophyll content, plant height, and biomass. High concentrations of antibiotic contamination could lead to the accumulation of N, P, and K in plant leaves, reduce the C/N ratio in leaves, reduce the vitamin C content in fruits, and slightly increase the accumulation of nitrate, but their concentrations had no significant effect on reducing sugars. The results showed that there was no significant difference in the contents of antibiotics in roots and shoots between two kinds of combined antibiotic contamination and single antibiotic contamination treatments; therefore, the combined pollution of antibiotics in the soil had no interaction with the accumulation of tetracycline and sulfamethazine in plants and the degradation of antibiotics in the soil, but both of them could enhance the impact on plant growth and biomass. From the results of this study, the accumulation of antibiotics in vegetables should not be underestimated. Eating fresh vegetables grown in soil rich in antibiotics may cause potential risks to human health. At present, the toxicological research of antibiotics and other drugs mainly involves the direct effects on animals, plants, and microorganisms; the ecotoxicological study of antibiotics on plants is mainly focused on the toxicological study of aquatic plants and terrestrial plants under the conditions of hydroponic culture under experimental simulation conditions. The toxicological study of soil as a substrate and of antibiotics on major types of vegetables in China is limited. The mechanism of absorption and enrichment of antibiotics by plants and the influencing factors need further study. Since the activity of antibiotics can change greatly after they enter the soil environment, it is necessary to study the dynamic absorption characteristics of residual antibiotics in soil and their relationship with antibiotic residues in soil that grows vegetables and other crops.
Author Contributions
Conceptualization and methodology, Q.X.; experiment, Q.X.; software: Q.X.; data curation, Q.X.; writing—original draft preparation, Q.X.; writing—review and editing, M.Z.; visualization, Q.X.; supervision, M.Z.; project administration, M.Z. All authors have read and agreed to the published version of the manuscript.
Funding
The research was supported by the Natural Science Foundation of Zhejiang Province, China (No. R306011), and the National Natural Science Foundation of China (No. 21177108).
Data Availability Statement
The datasets used and/or analyses during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest or personal relationships that could have appeared to influence the work reported in this paper.
References
- Azanu, D.; Mortey, C.; Darko, G.; Weisser, J.J.; Styrishave, B.; Abaidoo, R.C. Uptake of Antibiotics from Irrigation Water by Plants. Chemosphere 2016, 157, 107–114. [Google Scholar] [CrossRef]
- Aust, M.-O.; Godlinski, F.; Travis, G.R.; Hao, X.; McAllister, T.A.; Leinweber, P.; Thiele-Bruhn, S. Distribution of Sulfamethazine, Chlortetracycline and Tylosin in Manure and Soil of Canadian Feedlots after Subtherapeutic Use in Cattle. Environ. Pollut. 2008, 156, 1243–1251. [Google Scholar] [CrossRef] [PubMed]
- Quaik, S.; Embrandiri, A.; Ravindran, B.; Hossain, K.; Al-Dhabi, N.A.; Arasu, M.V.; Ignacimuthu, S.; Ismail, N. Veterinary Antibiotics in Animal Manure and Manure Laden Soil: Scenario and Challenges in Asian Countries. J. King Saud Univ.-Sci. 2020, 32, 1300–1305. [Google Scholar] [CrossRef]
- Zhao, L.; Dong, Y.H.; Wang, H. Residues of Veterinary Antibiotics in Manures from Feedlot Livestock in Eight Provinces of China. Sci. Total Environ. 2010, 408, 1069–1075. [Google Scholar] [CrossRef]
- Metcalfe, C.D.; Bayen, S.; Desrosiers, M.; Muñoz, G.; Sauvé, S.; Yargeau, V. An Introduction to the Sources, Fate, Occurrence and Effects of Endocrine Disrupting Chemicals Released into the Environment. Environ. Res. 2022, 207, 112658. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Li, X.-W.; Wang, J.-F.; Christakos, G.; Hu, M.-G.; An, L.-H.; Li, F.-S. Spatial Estimation of Antibiotic Residues in Surface Soils in a Typical Intensive Vegetable Cultivation Area in China. Sci. Total Environ. 2012, 430, 126–131. [Google Scholar] [CrossRef]
- Shi, W.; Yue, T.; Du, Z.; Wang, Z.; Li, X. Surface Modeling of Soil Antibiotics. Sci. Total Environ. 2016, 543, 609–619. [Google Scholar] [CrossRef]
- De Voogt, P. Reviews of Environmental Contamination and Toxicology; Springer International Publishing: Cham, Switzerland, 2017; Volume 243, p. 7233. ISBN 9783319587233. [Google Scholar]
- Tasho, R.P.; Cho, J.Y. Veterinary Antibiotics in Animal Waste, Its Distribution in Soil and Uptake by Plants: A Review. Sci. Total Environ. 2016, 563–564, 366–376. [Google Scholar] [CrossRef]
- Huygens, J.; Rasschaert, G.; Heyndrickx, M.; Dewulf, J.; Van Coillie, E.; Quataert, P.; Daeseleire, E.; Becue, I. Impact of Fertilization with Pig or Calf Slurry on Antibiotic Residues and Resistance Genes in the Soil. Sci. Total Environ. 2022, 822, 153518. [Google Scholar] [CrossRef] [PubMed]
- Onalenna, O.; Rahube, T.O. Assessing Bacterial Diversity and Antibiotic Resistance Dynamics in Wastewater Effluent-Irrigated Soil and Vegetables in a Microcosm Setting. Heliyon 2022, 8, e09089. [Google Scholar] [CrossRef]
- Rocha, D.C.; da Silva Rocha, C.; Tavares, D.S.; de Morais Calado, S.L.; Gomes, M.P. Veterinary Antibiotics and Plant Physiology: An Overview. Sci. Total Environ. 2021, 767, 144902. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Yang, L.; Li, G.; Fang, L.; Yu, X.; Tang, Y.-T.; Li, M.; Chen, L. Veterinary Antibiotics Can Reduce Crop Yields by Modifying Soil Bacterial Community and Earthworm Population in Agro-Ecosystems. Sci. Total Environ. 2022, 808, 152056. [Google Scholar] [CrossRef] [PubMed]
- Camotti Bastos, M.; Soubrand, M.; Le Guet, T.; Le Floch, É.; Joussein, E.; Baudu, M.; Casellas, M. Occurrence, Fate and Environmental Risk Assessment of Pharmaceutical Compounds in Soils Amended with Organic Wastes. Geoderma 2020, 375, 114498. [Google Scholar] [CrossRef]
- Schauss, K.; Focks, A.; Heuer, H.; Kotzerke, A.; Schmitt, H.; Thiele-Bruhn, S.; Smalla, K.; Wilke, B.-M.; Matthies, M.; Amelung, W.; et al. Analysis, Fate and Effects of the Antibiotic Sulfadiazine in Soil Ecosystems. TrAC Trends Anal. Chem. 2009, 28, 612–618. [Google Scholar] [CrossRef]
- Hembach, N.; Bierbaum, G.; Schreiber, C.; Schwartz, T. Facultative Pathogenic Bacteria and Antibiotic Resistance Genes in Swine Livestock Manure and Clinical Wastewater: A Molecular Biology Comparison. Environ. Pollut. 2022, 313, 120128. [Google Scholar] [CrossRef]
- Chen, Y.; Hammer, E.E.; Richards, V.P. Phylogenetic Signature of Lateral Exchange of Genes for Antibiotic Production and Resistance among Bacteria Highlights a Pattern of Global Transmission of Pathogens between Humans and Livestock. Mol. Phylogenet. Evol. 2018, 125, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Karcı, A.; Balcıoğlu, I.A. Investigation of the Tetracycline, Sulfonamide, and Fluoroquinolone Antimicrobial Compounds in Animal Manure and Agricultural Soils in Turkey. Sci. Total Environ. 2009, 407, 4652–4664. [Google Scholar] [CrossRef]
- Selvam, A.; Xu, D.; Zhao, Z.; Wong, J.W.C. Fate of Tetracycline, Sulfonamide and Fluoroquinolone Resistance Genes and the Changes in Bacterial Diversity during Composting of Swine Manure. Bioresour. Technol. 2012, 126, 383–390. [Google Scholar] [CrossRef]
- Van den Meersche, T.; Pamel, E.V.; Poucke, C.V.; Herman, L.; Heyndrickx, M.; Rasschaert, G.; Daeseleire, E. Development, Validation and Application of an Ultra High Performance Liquid Chromatographic-Tandem Mass Spectrometric Method for the Simultaneous Detection and Quantification of Five Different Classes of Veterinary Antibiotics in Swine Manure. J. Chromatogr. A 2016, 1429, 248–257. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, H.; Luo, Y.; Song, J. Occurrence and Assessment of Veterinary Antibiotics in Swine Manures: A Case Study in East China. Chin. Sci. Bull. 2012, 57, 606–614. [Google Scholar] [CrossRef]
- Zhang, Y.-L.; Lin, S.-S.; Dai, C.-M.; Shi, L.; Zhou, X.-F. Sorption–Desorption and Transport of Trimethoprim and Sulfonamide Antibiotics in Agricultural Soil: Effect of Soil Type, Dissolved Organic Matter, and PH. Environ. Sci. Pollut. Res. 2014, 21, 5827–5835. [Google Scholar] [CrossRef]
- Priya, A.K.; Antony, S.; Kumar, G.M.S.; Sivamoorthi, S.; Vineesh, S. Anthropogenic Impacts on the Contamination in the Coastal Region: A Review. Mater. Today Proc. 2021, 37, 2236–2238. [Google Scholar] [CrossRef]
- Hamscher, G.; Sczesny, S.; Höper, H.; Nau, H. Determination of Persistent Tetracycline Residues in Soil Fertilized with Liquid Manure by High-Performance Liquid Chromatography with Electrospray Ionization Tandem Mass Spectrometry. Anal. Chem. 2002, 74, 1509–1518. [Google Scholar] [CrossRef] [PubMed]
- Carter, L.J.; Harris, E.; Williams, M.; Ryan, J.J.; Kookana, R.S.; Boxall, A.B.A. Fate and Uptake of Pharmaceuticals in Soil–Plant Systems. J. Agric. Food Chem. 2014, 62, 816–825. [Google Scholar] [CrossRef]
- Daghrir, R.; Drogui, P. Tetracycline Antibiotics in the Environment: A Review. Environ. Chem. Lett. 2013, 11, 209–227. [Google Scholar] [CrossRef]
- Pan, M.; Chu, L.M. Phytotoxicity of Veterinary Antibiotics to Seed Germination and Root Elongation of Crops. Ecotoxicol. Environ. Saf. 2016, 126, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Matamoros, V.; Casas, M.E.; Mansilla, S.; Tadić, Đ.; Cañameras, N.; Carazo, N.; Portugal, J.; Piña, B.; Díez, S.; Bayona, J.M. Occurrence of Antibiotics in Lettuce (Lactuca sativa L.) and Radish (Raphanus sativus L.) Following Organic Soil Fertilisation under Plot-Scale Conditions: Crop and Human Health Implications. J. Hazard. Mater. 2022, 436, 129044. [Google Scholar] [CrossRef]
- Hu, W.; Huang, B.; Shi, X.; Chen, W.; Zhao, Y.; Jiao, W. Accumulation and Health Risk of Heavy Metals in a Plot-Scale Vegetable Production System in a Peri-Urban Vegetable Farm near Nanjing, China. Ecotoxicol. Environ. Saf. 2013, 98, 303–309. [Google Scholar] [CrossRef]
- Yu, Z.; Yediler, A.; Yang, M.; Schulte-Hostede, S. Leaching Behavior of Enrofloxacin in Three Different Soils and the Influence of a Surfactant on Its Mobility. J. Environ. Sci. 2012, 24, 435–439. [Google Scholar] [CrossRef]
- Migliore, L.; Godeas, F.; De Filippis, S.P.; Mantovi, P.; Barchi, D.; Testa, C.; Rubattu, N.; Brambilla, G. Hormetic Effect(s) of Tetracyclines as Environmental Contaminant on Zea mays. Environ. Pollut. 2010, 158, 129–134. [Google Scholar] [CrossRef]
- Kang, D.H.; Gupta, S.; Rosen, C.; Fritz, V.; Singh, A.; Chander, Y.; Murray, H.; Rohwer, C. Antibiotic Uptake by Vegetable Crops from Manure-Applied Soils. J. Agric. Food Chem. 2013, 61, 9992–10001. [Google Scholar] [CrossRef]
- Li, X.; Yu, H.; Xu, S.; Hua, R. Uptake of Three Sulfonamides from Contaminated Soil by Pakchoi Cabbage. Ecotoxicol. Environ. Saf. 2013, 92, 297–302. [Google Scholar] [CrossRef]
- Pan, M.; Chu, L.M. Transfer of Antibiotics from Wastewater or Animal Manure to Soil and Edible Crops. Environ. Pollut. 2017, 231, 829–836. [Google Scholar] [CrossRef]
- Chand, J.B.; Hewa, G.; Hassanli, A.; Myers, B. Deficit Irrigation on Tomato Production in a Greenhouse Environment: A Review. J. Irrig. Drain Eng. 2021, 147, 04020041. [Google Scholar] [CrossRef]
- Rajapaksha, A.U.; Vithanage, M.; Lim, J.E.; Ahmed, M.B.M.; Zhang, M.; Lee, S.S.; Ok, Y.S. Invasive Plant-Derived Biochar Inhibits Sulfamethazine Uptake by Lettuce in Soil. Chemosphere 2014, 111, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Aristilde, L.; Melis, A.; Sposito, G. Inhibition of Photosynthesis by a Fluoroquinolone Antibiotic. Environ. Sci. Technol. 2010, 44, 1444–1450. [Google Scholar] [CrossRef]
- Michelini, L.; La Rocca, N.; Rascio, N.; Ghisi, R. Structural and Functional Alterations Induced by Two Sulfonamide Antibiotics on Barley Plants. Plant Physiol. Biochem. 2013, 67, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Kurwadkar, S.; Struckhoff, G.; Pugh, K.; Singh, O. Uptake and Translocation of Sulfamethazine by Alfalfa Grown under Hydroponic Conditions. J. Environ. Sci. 2017, 53, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Madikizela, L.M.; Ncube, S.; Chimuka, L. Uptake of Pharmaceuticals by Plants Grown under Hydroponic Conditions and Natural Occurring Plant Species: A Review. Sci. Total Environ. 2018, 636, 477–486. [Google Scholar] [CrossRef]
- Dorival-García, N.; Zafra-Gómez, A.; Cantarero, S.; Navalón, A.; Vílchez, J.L. Simultaneous Determination of 13 Quinolone Antibiotic Derivatives in Wastewater Samples Using Solid-phase Extraction and Ultra Performance Liquid Chromatography–Tandem Mass Spectrometry. Microchem. J. 2013, 106, 323–333. [Google Scholar] [CrossRef]
- Lindqvist, D.N.; Pedersen, H.Æ.; Rasmussen, L.H. A Novel Technique for Determination of the Fructose, Glucose and Sucrose Distribution in Nectar from Orchids by HPLC-ELSD. J. Chromatogr. B 2018, 1081–1082, 126–130. [Google Scholar] [CrossRef]
- Ahmed, M.B.M.; Rajapaksha, A.U.; Lim, J.E.; Vu, N.T.; Kim, I.S.; Kang, H.M.; Lee, S.S.; Ok, Y.S. Distribution and Accumulative Pattern of Tetracyclines and Sulfonamides in Edible Vegetables of Cucumber, Tomato, and Lettuce. J. Agric. Food Chem. 2015, 63, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Hillis, D.G.; Fletcher, J.; Solomon, K.R.; Sibley, P.K. Effects of Ten Antibiotics on Seed Germination and Root Elongation in Three Plant Species. Arch. Environ. Contam. Toxicol. 2011, 60, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Eggen, T.; Asp, T.N.; Grave, K.; Hormazabal, V. Uptake and Translocation of Metformin, Ciprofloxacin and Narasin in Forage- and Crop Plants. Chemosphere 2011, 85, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wu, W.; Zhang, J.; Lv, P.; Xu, L.; Yan, Y. Progress of Research on the Toxicology of Antibiotic Pollution in Aquatic Organisms. Acta Ecol. Sin. 2018, 38, 36–41. [Google Scholar] [CrossRef]
- Jones-Lepp, T.L.; Sanchez, C.A.; Moy, T.; Kazemi, R. Method Development and Application to Determine Potential Plant Uptake of Antibiotics and Other Drugs in Irrigated Crop Production Systems. J. Agric. Food Chem. 2010, 58, 11568–11573. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, C.; Dang, Z.; Huang, W. Sorption of Tylosin on Agricultural Soils. Soil Sci. 2011, 176, 407–412. [Google Scholar] [CrossRef]
- Ding, C.; He, J. Effect of Antibiotics in the Environment on Microbial Populations. Appl. Microbiol. Biotechnol. 2010, 87, 925–941. [Google Scholar] [CrossRef]
- Chung, H.S.; Lee, Y.-J.; Rahman, M.M.; Abd El-Aty, A.M.; Lee, H.S.; Kabir, M.H.; Kim, S.W.; Park, B.-J.; Kim, J.-E.; Hacımüftüoğlu, F.; et al. Uptake of the Veterinary Antibiotics Chlortetracycline, Enrofloxacin, and Sulphathiazole from Soil by Radish. Sci. Total Environ. 2017, 605–606, 322–331. [Google Scholar] [CrossRef]
- Lin, J.; Wang, Y.; Sun, S.; Mu, C.; Yan, X. Effects of Arbuscular Mycorrhizal Fungi on the Growth, Photosynthesis and Photosynthetic Pigments of Leymus chinensis Seedlings under Salt-Alkali Stress and Nitrogen Deposition. Sci. Total Environ. 2017, 576, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Wang, Z.; Xu, C.; Li, L.; Yang, C. Multiomics Analysis Provides Insights into Alkali Stress Tolerance of Sunflower (Helianthus annuus L.). Plant Physiol. Biochem. 2021, 166, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-R.; Rairat, T.; Loh, S.-H.; Wu, Y.-C.; Vickroy, T.W.; Chou, C.-C. Assessment of Veterinary Drugs in Plants Using Pharmacokinetic Approaches: The Absorption, Distribution and Elimination of Tetracycline and Sulfamethoxazole in Ephemeral Vegetables. PLoS ONE 2017, 12, e0183087. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).