Phytoactive Aryl Carbamates and Ureas as Cytokinin-like Analogs of EDU
Abstract
:1. Introduction
2. Materials and Methods
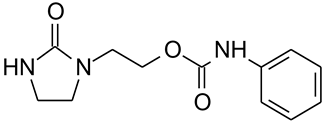
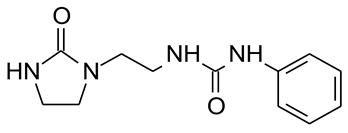
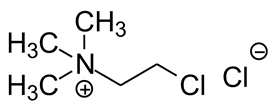
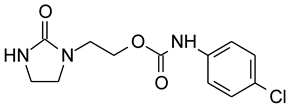
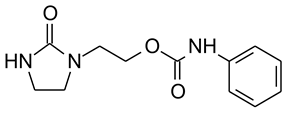
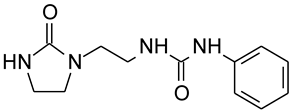
2.1. Chemicals
2.2. Instruments
2.3. Laboratory Experiment
2.4. Field Test
2.4.1. Crude Grain Gluten Content
2.4.2. Protein Mass Percentage
2.5. Statistical Analysis
3. Results and Discussion
3.1. Laboratory Experiments
3.2. Field Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gupta, S.K.; Sharma, M.; Majumder, B.; Maurya, V.K.; Deeba, F.; Zhang, J.-L.; Pandey, V. Effects of Ethylenediurea (EDU) on Regulatory Proteins in Two Maize (Zea mays L.) Varieties under High Tropospheric Ozone Phytotoxicity. Plant Physiol. Biochem. 2020, 154, 675–688. [Google Scholar] [CrossRef]
- Zhang, G.; Kobayashi, K.; Wu, H.; Shang, B.; Wu, R.; Zhang, Z.; Feng, Z. Ethylenediurea (EDU) Protects Inbred but Not Hybrid Cultivars of Rice from Yield Losses Due to Surface Ozone. Environ. Sci. Pollut. Res. 2021, 28, 68946–68956. [Google Scholar] [CrossRef]
- Agathokleous, E.; Kitao, M.; Shi, C.; Masui, N.; Abu-ElEla, S.; Hikino, K.; Satoh, F.; Koike, T. Ethylenediurea (EDU) Spray Effects on Willows (Salix sachalinensis F. Schmid) Grown in Ambient or Ozone-Enriched Air: Implications for Renewable Biomass Production. J. For. Res. 2022, 33, 397–422. [Google Scholar] [CrossRef]
- Singh, A.K.; Mitra, S.; Kar, G. Assessing the Impact of Current Tropospheric Ozone on Yield Loss and Antioxidant Defense of Six Cultivars of Rice Using Ethylenediurea in the Lower Gangetic Plains of India. Environ. Sci. Pollut. Res. 2022, 29, 40146–40156. [Google Scholar] [CrossRef] [PubMed]
- Shang, B.; Fu, R.; Agathokleous, E.; Dai, L.; Zhang, G.; Wu, R.; Feng, Z. Ethylenediurea Offers Moderate Protection against Ozone-Induced Rice Yield Loss under High Ozone Pollution. Sci. Total Environ. 2022, 806, 151341. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, I.J.; Rathore, D. Assessment of Dose–Response Relationship between Ozone Dose and Groundnut (Arachis hypogaea L) Cultivars Using Open Top Chamber (OTC) and Ethylenediurea (EDU). Environ. Technol. Innov. 2021, 22, 101494. [Google Scholar] [CrossRef]
- Jabeen, F.; Ahmed, S. Ethylenediurea Regulates Growth and Physiochemical Responses of Pisum Sativum to Ambient O3. Int. J. Environ. Sci. Technol. 2021, 18, 3571–3580. [Google Scholar] [CrossRef]
- Nigar, S.; Nazneen, S.; Khan, S.; Ali, N.; Sarwar, T. Response of Vigna radiata L. (Mung bean) to Ozone Phytotoxicity Using Ethylenediurea and Magnesium Nitrate. J. Plant Growth Regul. 2023, 42, 121–133. [Google Scholar] [CrossRef]
- Jabeen, F.; Ahmed, S.; Shah, A.A. Impact of Ambient Ozone Pollution on Yield Attributes of Pisum sativum L. Plants by Using Ethylenediurea. Iran. J. Plant Physiol. 2021, 11, 3789–3798. [Google Scholar] [CrossRef]
- Surabhi, S.; Pande, V.; Pandey, V. Ethylenediurea (EDU) Mediated Protection from Ambient Ozone-Induced Oxidative Stress in Wheat (Triticum aestivum L.) under a High CO2 Environment. Atmos. Pollut. Res. 2022, 13, 101503. [Google Scholar] [CrossRef]
- Kannaujia, R.; Singh, P.; Prasad, V.; Pandey, V. Evaluating Impacts of Biogenic Silver Nanoparticles and Ethylenediurea on Wheat (Triticum aestivum L.) against Ozone-Induced Damages. Environ. Res. 2022, 203, 111857. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Sharma, M.; Maurya, V.K.; Deeba, F.; Pandey, V. Effects of Ethylenediurea (EDU) on Apoplast and Chloroplast Proteome in Two Wheat Varieties under High Ambient Ozone: An Approach to Investigate EDU’s Mode of Action. Protoplasma 2021, 258, 1009–1028. [Google Scholar] [CrossRef] [PubMed]
- Maurya, V.K.; Gupta, S.K.; Sharma, M.; Majumder, B.; Deeba, F.; Pandey, N.; Pandey, V. Growth, Physiological and Proteomic Responses in Field Grown Wheat Varieties Exposed to Elevated CO2 under High Ambient Ozone. Physiol. Mol. Biol. Plants 2020, 26, 1437–1461. [Google Scholar] [CrossRef] [PubMed]
- Agathokleous, E.; Kitao, M.; Wang, X.; Mao, Q.; Harayama, H.; Manning, W.J.; Koike, T. Ethylenediurea (EDU) Effects on Japanese Larch: An One Growing Season Experiment with Simulated Regenerating Communities and a Four Growing Season Application to Individual Saplings. J. For. Res. 2021, 32, 2047–2057. [Google Scholar] [CrossRef]
- Agathokleous, E.; Kitao, M.; Koike, T. Ethylenediurea (EDU) Effects on Hybrid Larch Saplings Exposed to Ambient or Elevated Ozone over Three Growing Seasons. J. For. Res. 2022, 33, 117–135. [Google Scholar] [CrossRef]
- Singh, A.A.; Singh, S.; Agrawal, M.; Agrawal, S.B. Assessment of Ethylene Diurea-Induced Protection in Plants Against Ozone Phytotoxicity. In Reviews of Environmental Contamination and Toxicology; Whitacre, D.M., Ed.; Springer International Publishing: Cham, Switzerland, 2015; Volume 233, pp. 129–184. ISBN 978-3-319-10479-9. [Google Scholar]
- Lee, E.H.; Chen, C.M. Studies on the Mechanisms of Ozone Tolerance: Cytokinin-like Activity of N-[2-(2-Oxo-1-Imidazolidinyl)Ethyl]-N’-Phenylurea, a Compound Protecting against Ozone Injury. Physiol. Plant. 1982, 56, 486–491. [Google Scholar] [CrossRef]
- Oshchepkov, M.S.; Kalistratova, A.V.; Savelieva, E.M.; Romanov, G.A.; Bystrova, N.A.; Kochetkov, K.A. Natural and Synthetic Cytokinins and Their Applications in Biotechnology, Agrochemistry and Medicine. Russ. Chem. Rev. 2020, 89, 787. [Google Scholar] [CrossRef]
- Oshchepkov, M.; Kovalenko, L.; Kalistratova, A.; Ivanova, M.; Sherstyanykh, G.; Dudina, P.; Antonov, A.; Cherkasova, A.; Akimov, M. Anti-Proliferative and Cytoprotective Activity of Aryl Carbamate and Aryl Urea Derivatives with Alkyl Groups and Chlorine as Substituents. Molecules 2022, 27, 3616. [Google Scholar] [CrossRef]
- Kalistratova, A.V.; Kovalenko, L.V.; Oshchepkov, M.S.; Gamisoniya, A.M.; Gerasimova, T.S.; Demidov, Y.A.; Akimov, M.G. Synthesis of New Compounds in the Series of Aryl-Substituted Ureas with Cytotoxic and Antioxidant Activity. Mendeleev Commun. 2020, 30, 153–155. [Google Scholar] [CrossRef]
- State Register of Selection Achievements Approved for Use. V.1. “Varieties of Plants” (Official Edition). FGBNU “Rosinformagrotech 2022, 11. Available online: https://msh.astrobl.ru/documents/document-16g6g-72c9-6g9-55 (accessed on 22 February 2023).
- Badina, L.E. Guidelines for Performing Laboratory Work on the Discipline “Chemical Growth Regulators and Their Application”; House of Michurinsk State Agrarian University: Michurinsk, Russia, 2006. [Google Scholar]
- Zhang, G.; Cao, R.; Risalat, H.; Hu, Q.; Pan, X.; Hu, Y.; Shang, B.; Wu, H.; Zhang, Z.; Feng, Z. Ethylenediurea Reduces Grain Nitrogen but Enhances Protein and Carbon Yield in Rice Cultivars. Agronomy 2022, 12, 1988. [Google Scholar] [CrossRef]
- Kochetkov, K.A.; Gorunova, O.N.; Bystrova, N.A. Biologically Oriented Hybrids of Indole and Hydantoin Derivatives. Molecules 2023, 28, 602. [Google Scholar] [CrossRef]
- Guo, Q.; Meng, Y.; Qu, G.; Wang, T.; Yang, F.; Liang, D.; Hu, S. Improvement of Wheat Seed Vitality by Dielectric Barrier Discharge Plasma Treatment. Bioelectromagnetics 2018, 39, 120–131. [Google Scholar] [CrossRef]
- Chapter 1: ISTA Certificates. Int. Rules Seed Test. 2023, 2023, i-1–14. [CrossRef]
- Mullan, D.; Pietragalla, J. Leaf Relative Water Content in Physiological Breeding II: A Field Guide to Wheat Phenotyping. In The International Maize and Wheat Improvement Center; Pask, A., Pietragalla, J., Mullan, D., Reynolds, M., Eds.; CIMMYT: MéxicoVeracruz, Mexico, 2012; Chapter 5; Volume 25, ISBN 978-970-648-182-5. [Google Scholar]
- Step-by-Step Recommendations for the Correct Use of Growth Regulators in Cereal Crops. Available online: https://Www.Agro-by.Basf.Com (accessed on 22 February 2023).
- Stucky, B.J.; Guralnick, R.; Deck, J.; Denny, E.G.; Bolmgren, K.; Walls, R. The Plant Phenology Ontology: A New Informatics Resource for Large-Scale Integration of Plant Phenology Data. Front. Plant Sci. 2018, 9, 517. [Google Scholar] [CrossRef] [Green Version]
- Balamurugan, V.S.; Sivabalan, S.; Thamizhselvan, I. Determination of Gluten Level on Traditionally Treated Wheat. Int. J. Res. Anal. Rev. 2018, 5, 426–431. [Google Scholar]
- Pasynkov, A.V.; Pasynkova, E.N. Method for Prediction of Raw Gluten Content in Wheat Grain. IOP Conf. Ser. Earth Environ. Sci. 2021, 858, 012013. [Google Scholar] [CrossRef]
- Beljkaš, B.; Matić, J.; Milovanović, I.; Jovanov, P.; Mišan, A.; Šarić, L. Rapid Method for Determination of Protein Content in Cereals and Oilseeds: Validation, Measurement Uncertainty and Comparison with the Kjeldahl Method. Accred. Qual. Assur. 2010, 15, 555–561. [Google Scholar] [CrossRef]
- Kozin, A.V.; Abramova, L.S.; Guseva, E.S.; Derunets, I.V. Establishment of Metrological Parameters of the Method for Measuring the Protein Mass Fraction in Fish Food Products by the Kjeldahl Method. Food Syst. 2022, 4, 239–245. [Google Scholar] [CrossRef]
- Agathokleous, E. Environmental Hormesis, a Fundamental Non-Monotonic Biological Phenomenon with Implications in Ecotoxicology and Environmental Safety. Ecotoxicol. Environ. Saf. 2018, 148, 1042–1053. [Google Scholar] [CrossRef] [Green Version]
- Agathokleous, E.; Belz, R.G.; Kitao, M.; Koike, T.; Calabrese, E.J. Does the Root to Shoot Ratio Show a Hormetic Response to Stress? An Ecological and Environmental Perspective. J. For. Res. 2019, 30, 1569–1580. [Google Scholar] [CrossRef] [Green Version]
- Dinani, E.T.; Shukla, M.R.; Turi, C.E.; Sullivan, J.A.; Saxena, P.K. Thidiazuron: Modulator of Morphogenesis In Vitro. In Thidiazuron: From Urea Derivative to Plant Growth Regulator; Ahmad, N., Faisal, M., Eds.; Springer: Singapore, 2018; pp. 1–36. ISBN 978-981-10-8004-3. [Google Scholar]
- van Voorthuizen, M.J.; Song, J.; Novák, O.; Jameson, P.E. Plant Growth Regulators INCYDE and TD-K Underperform in Cereal Field Trials. Plants 2021, 10, 2309. [Google Scholar] [CrossRef]
- Vissenberg, K.; Claeijs, N.; Balcerowicz, D.; Schoenaers, S. Hormonal Regulation of Root Hair Growth and Responses to the Environment in Arabidopsis. J. Exp. Bot. 2020, 71, 2412–2427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Zhang, T.; Wang, R.; Zhao, Y. Recent Advances in Auxin Research in Rice and Their Implications for Crop Improvement. J. Exp. Bot. 2018, 69, 255–263. [Google Scholar] [CrossRef]
- Xu, D.; Watahiki, M.K.; Xu, D.; Watahiki, M.K. Phytohormone-Mediated Homeostasis of Root System Architecture; Intech Open: London, UK, 2020; ISBN 978-1-78984-747-5. [Google Scholar]
- Kieber, J.J.; Schaller, G.E. Cytokinin Signaling in Plant Development. Development 2018, 145, dev149344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nisler, J.; Kopečný, D.; Pěkná, Z.; Končitíková, R.; Koprna, R.; Murvanidze, N.; Werbrouck, S.P.O.; Havlíček, L.; De Diego, N.; Kopečná, M.; et al. Diphenylurea-Derived Cytokinin Oxidase/Dehydrogenase Inhibitors for Biotechnology and Agriculture. J. Exp. Bot. 2021, 72, 355–370. [Google Scholar] [CrossRef]
- Soltys-Kalina, D.; Plich, J.; Strzelczyk-Żyta, D.; Śliwka, J.; Marczewski, W. The Effect of Drought Stress on the Leaf Relative Water Content and Tuber Yield of a Half-Sib Family of ‘Katahdin’-Derived Potato Cultivars. Breed. Sci. 2016, 66, 328–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lenton, J.R.; Hedden, P.; Gale, M.D. Gibberellin Insensitivity and Depletion in Wheat-Consequences for Development. In Hormone Action in Plant Development—A Critical Appraisal; Hoad, G.V., Lenton, J.R., Jackson, M.B., Atkin, R.K., Eds.; Butterworths: London, UK, 1987; pp. 145–160. [Google Scholar]
- Appleford, N.E.J.; Lenton, J.R. Gibberellins and Leaf Expansion in Near-Isogenic Wheat Lines Containing Rht1 and Rht3 Dwarfing Alleles. Planta 1991, 183, 229–236. [Google Scholar] [CrossRef] [PubMed]
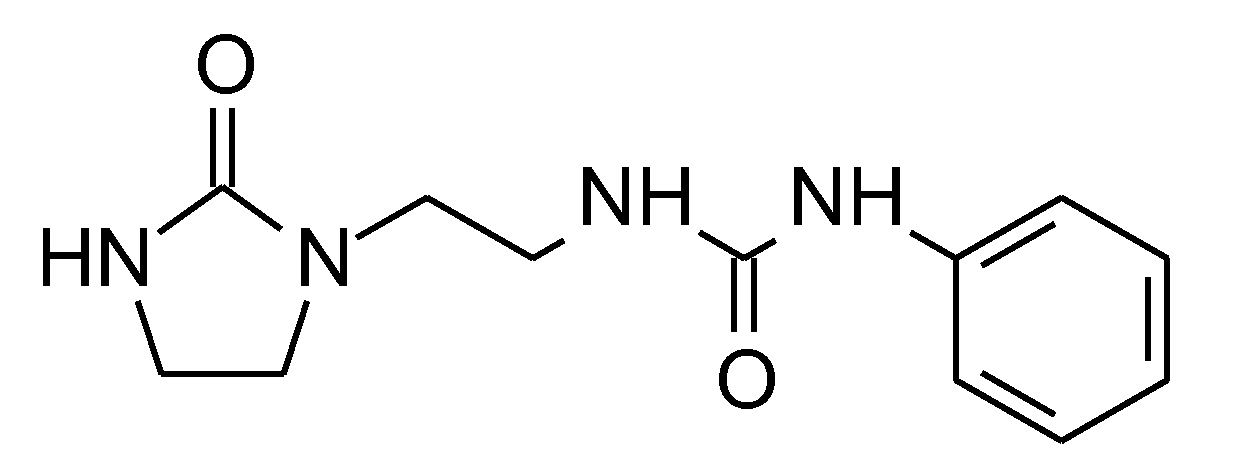
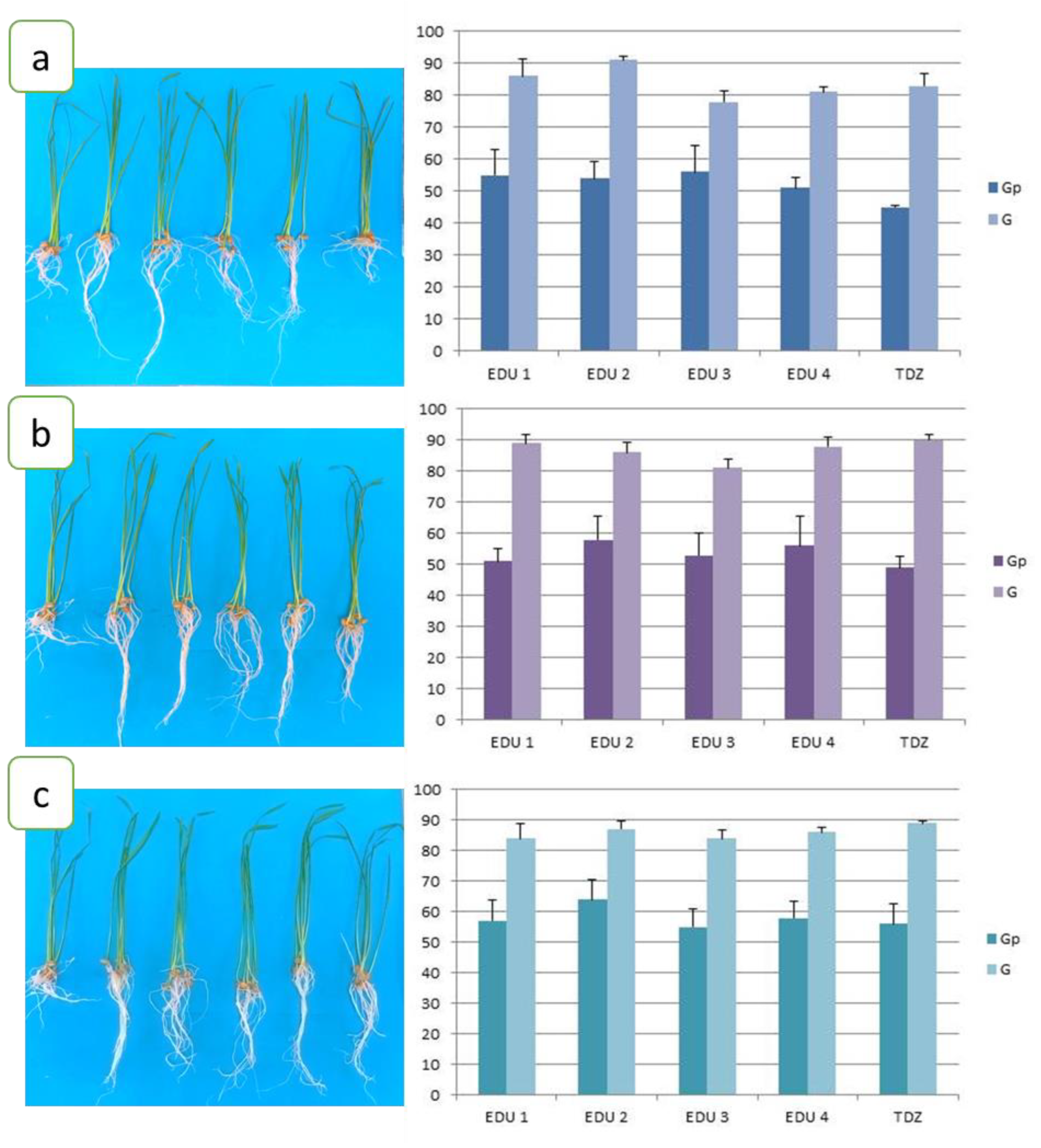

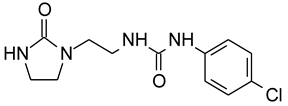
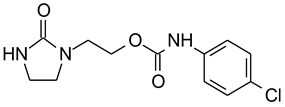
| Concentration, M | Germination Potential % | Germination, % | Primary Root Length, cm | Number of Roots | Lateral Roots Length, cm | Shoot Height, cm | Relative Water Content RWC, % | |
|---|---|---|---|---|---|---|---|---|
| “Control” | 0 | 56 | 75 | - | 4 | 6.5 | 12.4 | 17.62 |
 E1 | 4 × 10−3 | 55 | 86 | 9.9 | 5 | 6.2 * | 13.1 | 31.86 |
| 4 × 10−5 | 51 | 89 | 10.8 | 5 | 7.4 ** | 12.6 | 24.00 | |
| 4 × 10−7 | 57 | 84 | 11.6 | 5 | 7.5 | 13.4 | 23.07 | |
 E2 | 4 × 10−3 | 54 | 91 | 10.3 | 5 | 7.2 * | 12.6 | 20.23 |
| 4 × 10−5 | 58 | 86 | 10.2 | 5 | 7.2 ** | 13.6 | 31.64 | |
| 4 × 10−7 | 64 ** | 87 | 9.1 | 6 | 6.4 | 13.7 | 19.37 | |
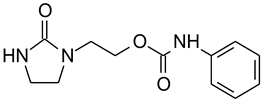 E3 | 4 × 10−3 | 56 | 78 | 10.6 | 5 | 6.7 * | 13.9 | 19.54 |
| 4 × 10−5 | 53 | 81 | 10.0 | 6 | 7.0 ** | 12.6 | 20,54 | |
| 4 × 10−7 | 55 | 84 | 11.3 | 6 | 7.2 | 14.5 | 25.39 | |
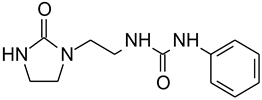 E4 | 4 × 10−3 | 51 | 81 | 10.1 | 5 | 6.5 | 12.5 | 18.59 |
| 4 × 10−5 | 56 | 88 | 10.3 | 6 | 6.4 ** | 13.6 | 19.76 | |
| 4 × 10−7 | 58 | 86 | 10.8 | 5 | 7.0 | 13.6 | 31.50 | |
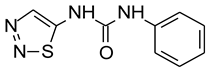 TDZ | 4 × 10−3 | 45 | 83 | - | 4 | 4.0 | 12.3 | 23.85 |
| 4 × 10−5 | 49 | 90 | - | 4 | 5.1 | 13.1 | 33.55 | |
| 4 × 10−7 | 56 | 89 | - | 4 | 5.8 | 11.7 | 23.63 |
| Plant Height, cm | Number of Plants, pcs/m2 | Number of Productive Stems, pcs/m2 | Spike Length, cm | Quantity Per Ear, pcs. | Weight of Grain per Ear, g | ||
|---|---|---|---|---|---|---|---|
| Spikelets | Grains | ||||||
| Control | 54.6 ±2.79 | 238 ±10.5 | 274 ±6.95 | 8.6 ±0.31 | 23.0 ±1.41 | 23.7 ±1.7 | 0.85 ±0.028 |
 E1 | 56.7 ±1.81 | 256 ** ±8.44 | 343 ** ±9.17 | 8.1 ±0.12 | 22.7 ±0.51 | 25.5 ±2.08 | 0.87 ±0.043 |
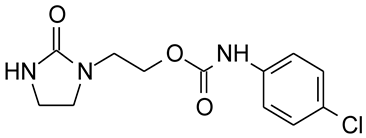 E2 | 52.5 ±4.11 | 245 * ±10.5 | 304 ** ±7.59 | 8.4 ±0.48 | 22.3 ± 0.18 | 20.4 ± 1.91 | 0.86 ± 0.022 |
 E3 | 51.0 ±3.44 | 248 ** ±1.02 | 332 ** ±4.52 | 8.9 ±0.17 | 22.2 ±0.25 | 24.7 ±3.32 | 0.88 ±0.031 |
 E4 | 48.1 ±0.80 | 240 ±8.13 | 299 ** ±9.43 | 8.9 * ±0.39 | 22.5 ±0.57 | 19.6 ±3.39 | 0.82 ±0.058 |
 CCC | 53.8 ±4.66 | 254 ±9.29 | 307 ±7.02 | 8.55 ±0.42 | 22.0 ±0.81 | 16.7 ±1.97 | 0.83 ±0.069 |
| Option | Germination from the Site,% | Productivity, kg/m2 | Increase, kg/m2 | Weight of 1000 Grains, g | Yield kg/4 m2 | Crude Gluten Content in Grain, % | Protein Content in Grain, % |
|---|---|---|---|---|---|---|---|
| Control | 78 ±3.57 | 0.22 ±0.002 | - | 28.85 ±0.72 | 0.88 | 25.0 | 12.33 |
 E1 | 85 ** ±3.16 | 0.335 ** ±0.002 | 0.115 | 31.45 ±1.91 | 1.34 | 26.5 | 12.56 |
 E2 | 81 * ±3.53 | 0.272 ** ±0.003 | 0.052 | 29.93 ±2.09 | 1.088 | 25.7 | 12.43 |
 E3 | 82 * ±3.51 | 0.271 ** ±0.002 | 0.051 | 29.36 ±2.02 | 1.084 | 26.0 | 12.47 |
 E4 | 80 ±2.58 | 0.222 ±0.003 | 0.002 | 29.01 ±1.81 | 0.88 | 25.2 | 12.33 |
 CCC | 84 ±10.04 | 0.223 ±0.004 | 0.003 | 31.85 * ±1.48 | 0.89 | 25.4 | 12.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oshchepkov, M.S.; Kovalenko, L.V.; Kalistratova, A.V.; Solovieva, I.N.; Tsvetikova, M.A.; Gorunova, O.N.; Bystrova, N.A.; Kochetkov, K.A. Phytoactive Aryl Carbamates and Ureas as Cytokinin-like Analogs of EDU. Agronomy 2023, 13, 778. https://doi.org/10.3390/agronomy13030778
Oshchepkov MS, Kovalenko LV, Kalistratova AV, Solovieva IN, Tsvetikova MA, Gorunova ON, Bystrova NA, Kochetkov KA. Phytoactive Aryl Carbamates and Ureas as Cytokinin-like Analogs of EDU. Agronomy. 2023; 13(3):778. https://doi.org/10.3390/agronomy13030778
Chicago/Turabian StyleOshchepkov, Maxim S., Leonid V. Kovalenko, Antonida V. Kalistratova, Inna N. Solovieva, Marina A. Tsvetikova, Olga N. Gorunova, Nataliya A. Bystrova, and Konstantin A. Kochetkov. 2023. "Phytoactive Aryl Carbamates and Ureas as Cytokinin-like Analogs of EDU" Agronomy 13, no. 3: 778. https://doi.org/10.3390/agronomy13030778






