Effects of Harvest Time on the Fruit Quality of Kinnow and Feutrell’s Early Mandarins (Citrus reticulata Blanco)
Abstract
:1. Introduction
2. Materials and Methods
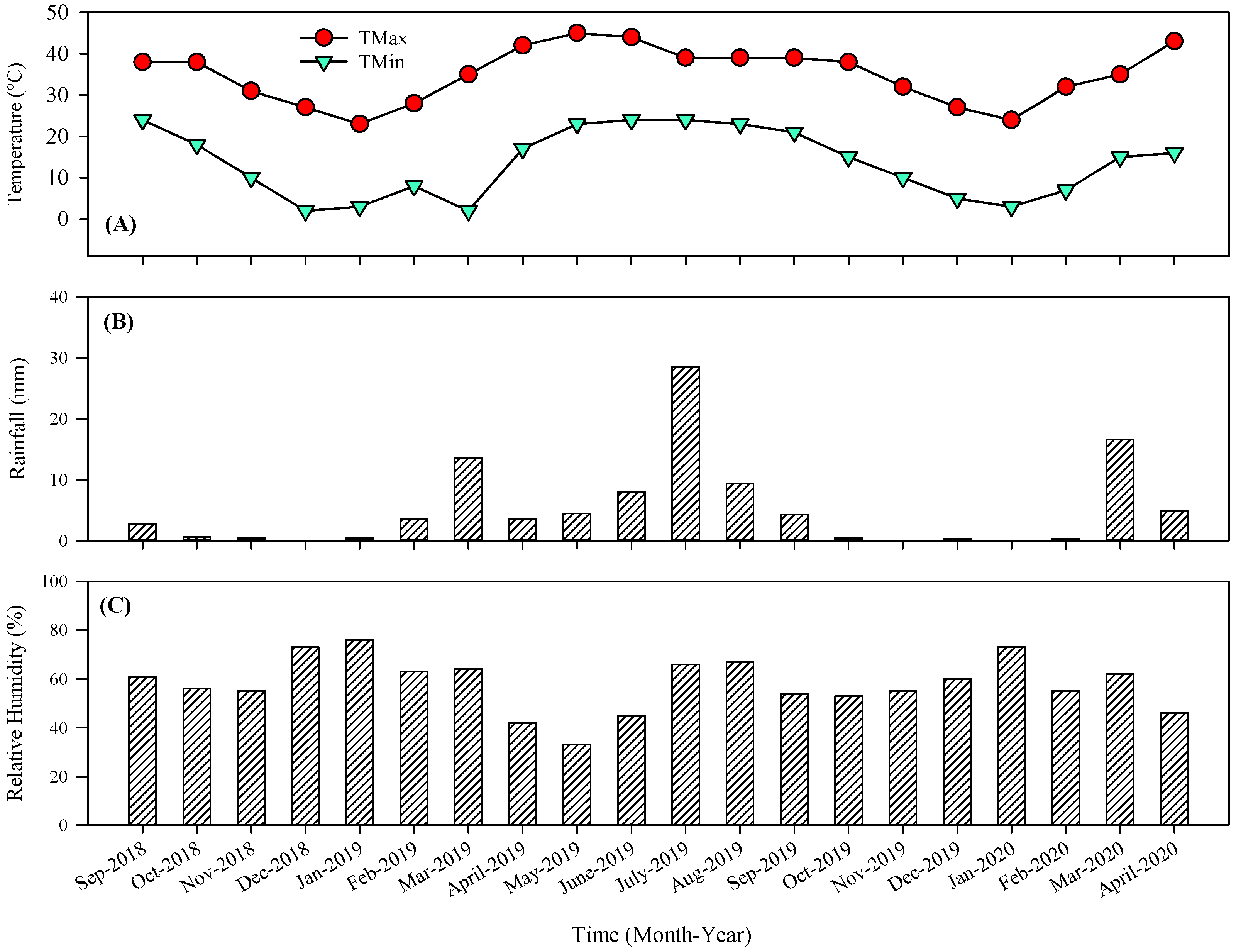
2.1. Plant Materials and Experimental Site
2.2. Measurements
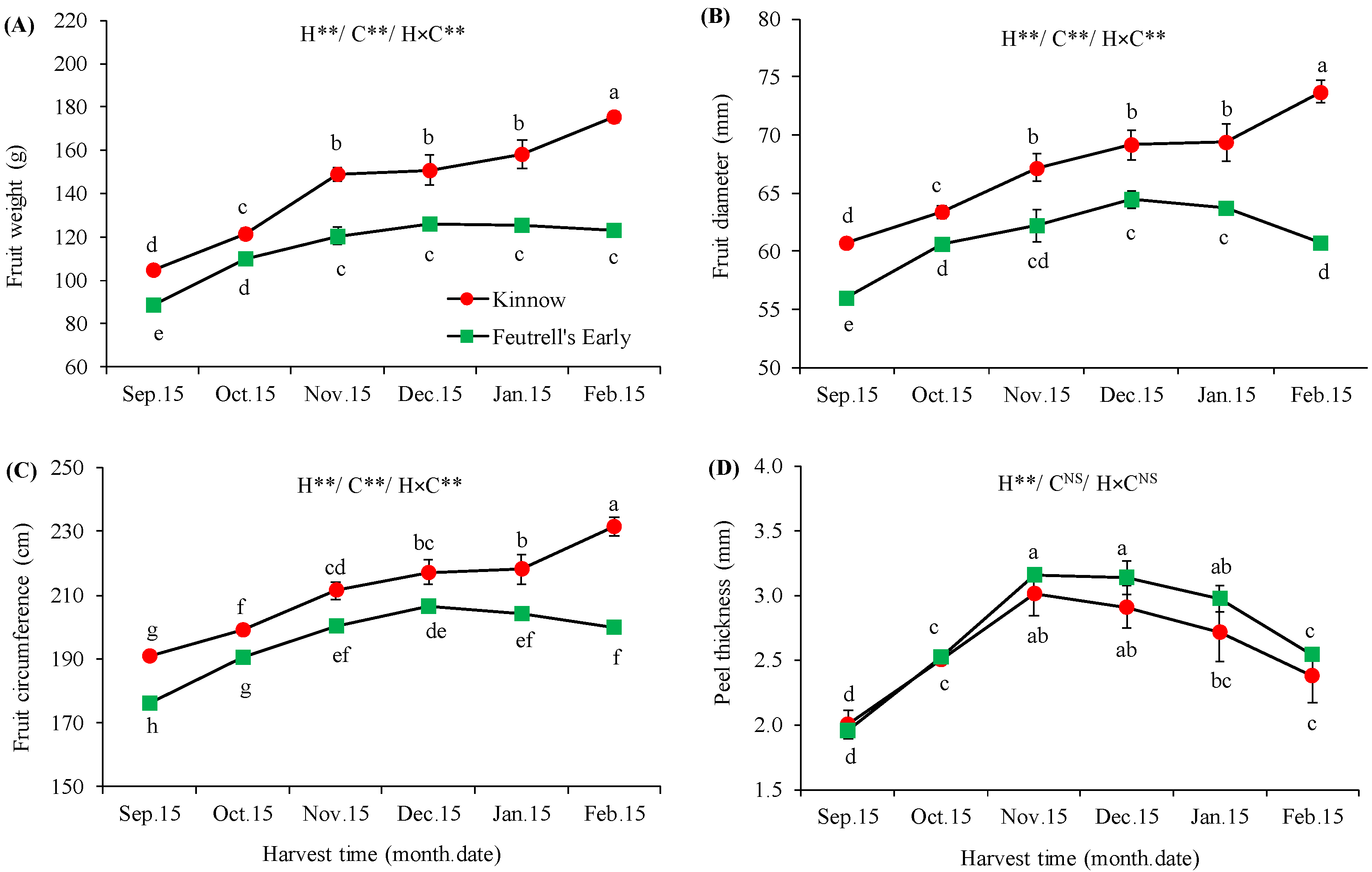
2.2.1. Physical Parameters
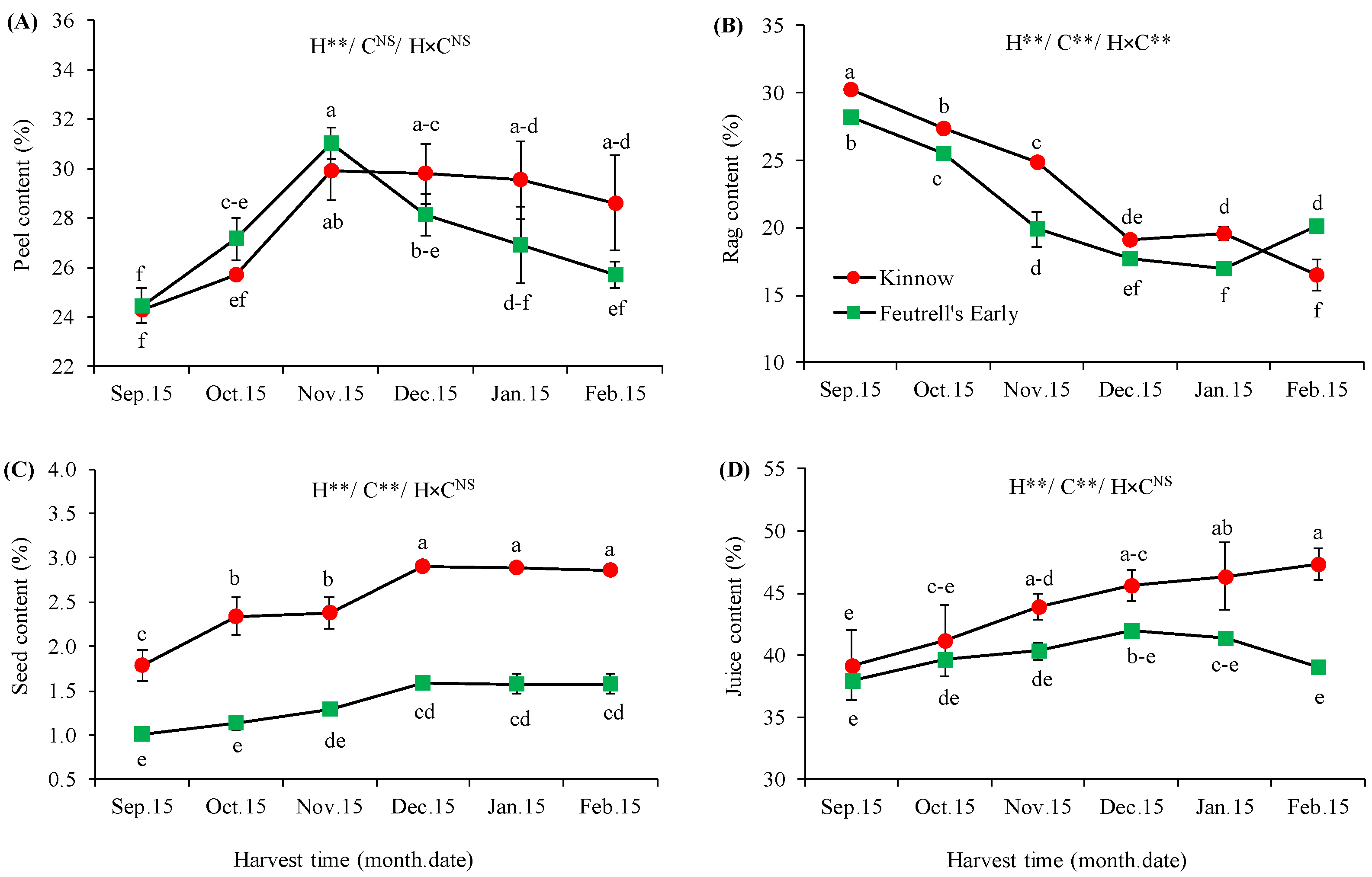
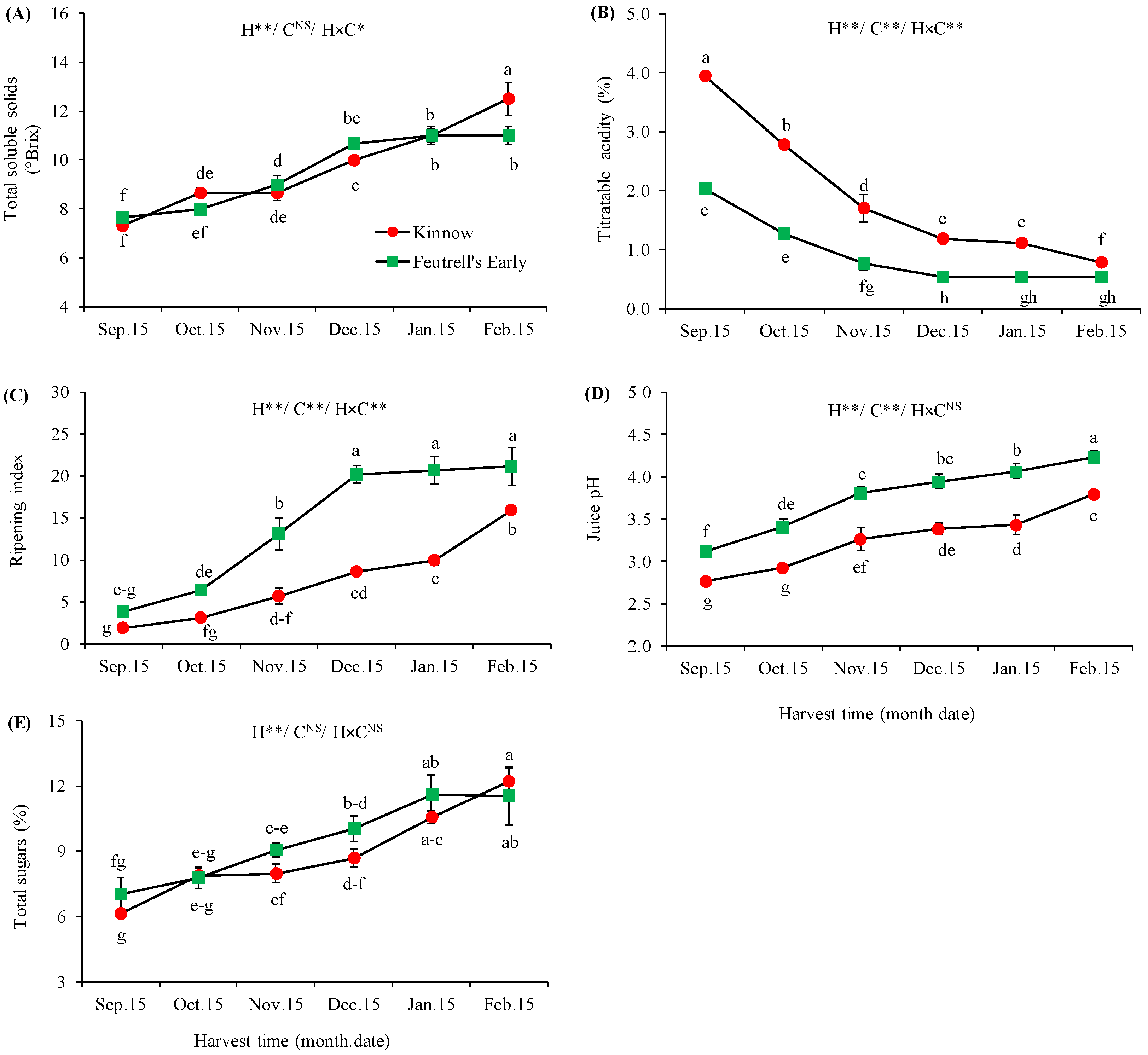
2.2.2. Total Soluble Solids, Titratable Acidity, Ripening Index, and Juice pH
2.2.3. Total Sugars
2.2.4. Vitamin C Content
2.2.5. Determination of Antioxidant Profile
2.2.6. Total Phenolic Contents
2.3. Statistical Analysis
3. Results
3.1. Variation in Physical Attributes of Mandarin Fruits at Different Harvesting Dates
3.2. Variations in Biochemical Attributes at Different Harvesting Dates
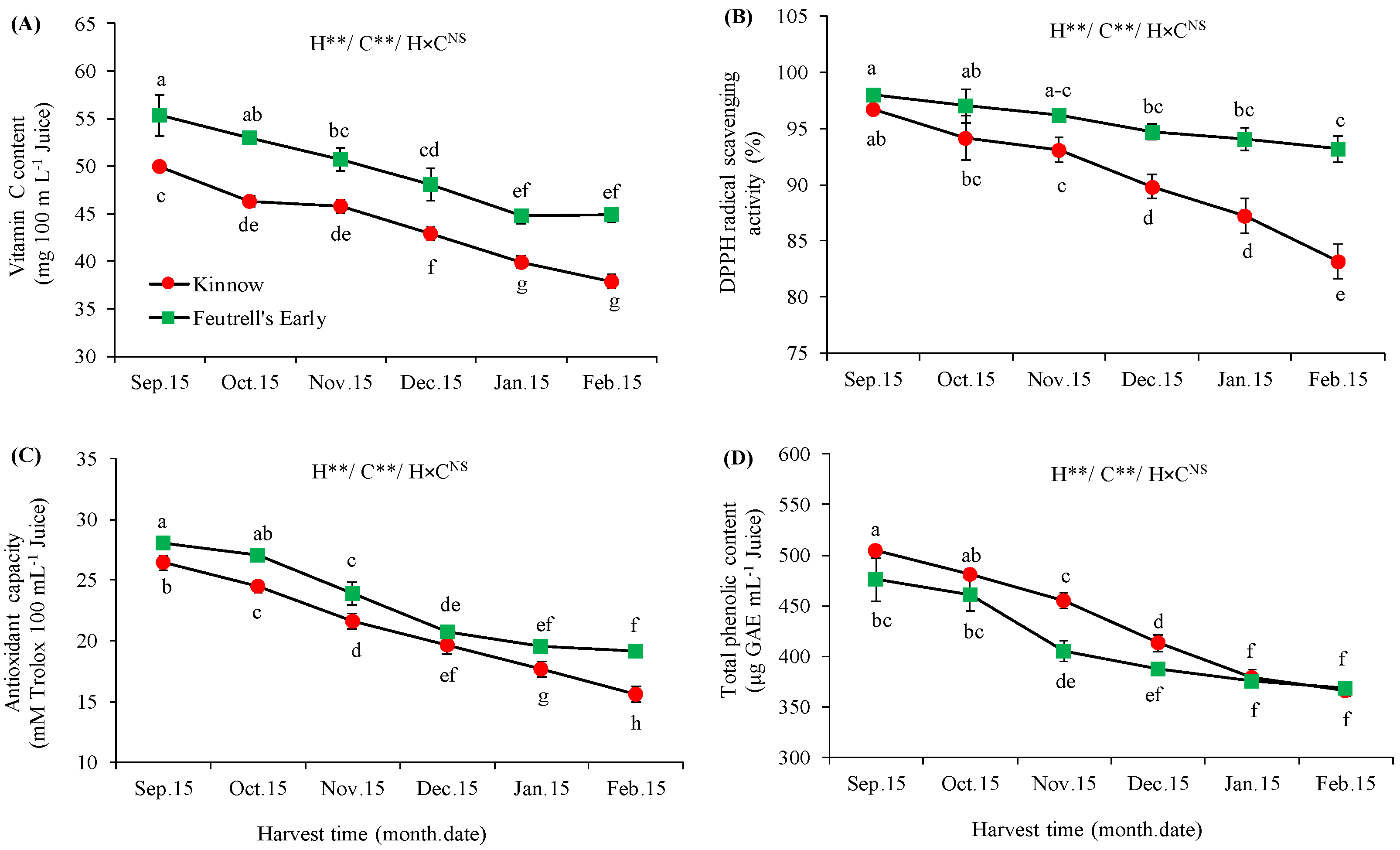
3.3. Variations in Bioactive Compounds at Different Harvesting Dates
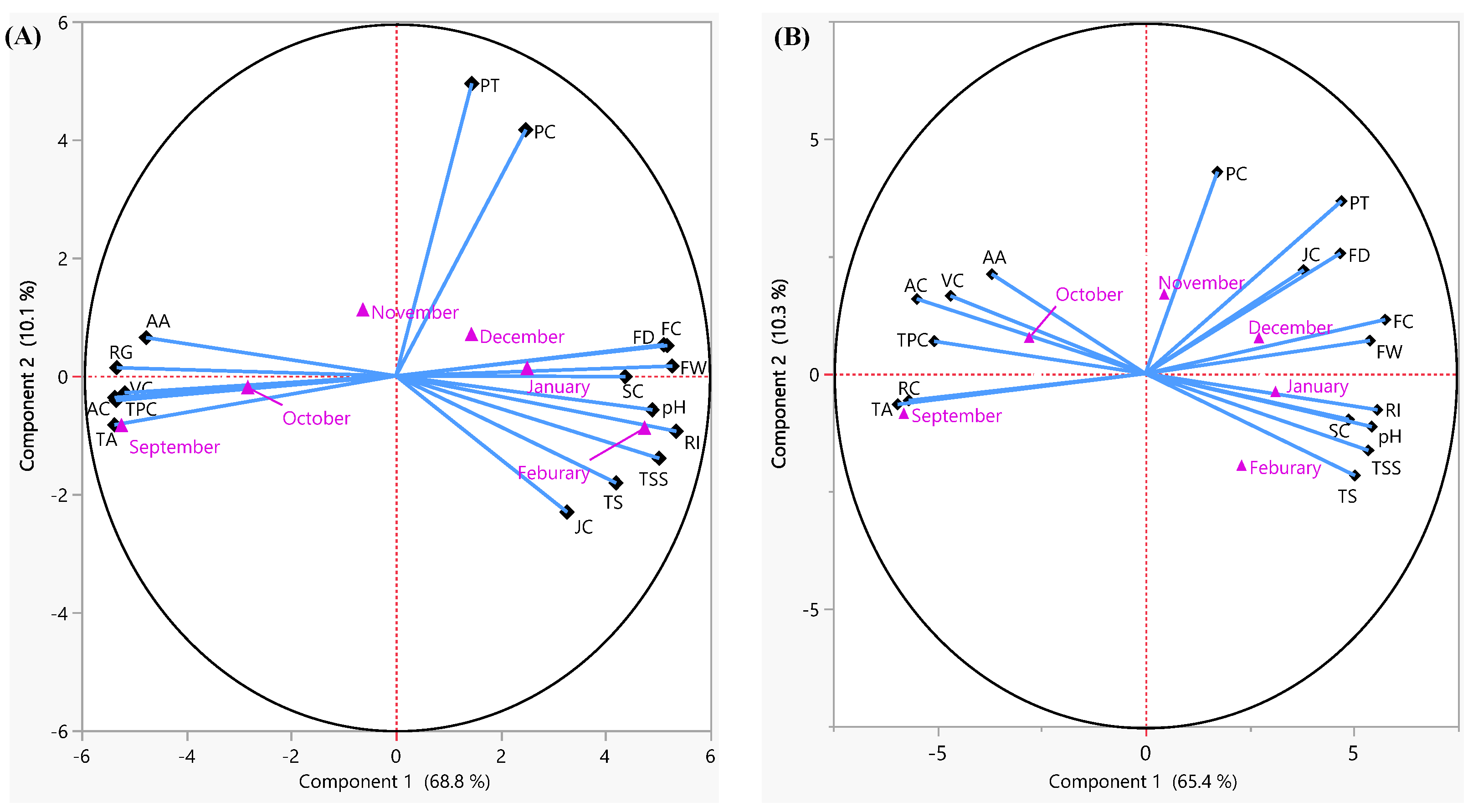
3.4. Principal Component Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lado, J.; Cuellar, F.; Rodrigo, M.J.; Zacarías, L. Nutritional composition of mandarins. In Nutritional Composition of Fruit Cultivars; Simmonds, M., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 419–443. [Google Scholar]
- Goldenberg, L.; Yaniv, Y.; Porat, R.; Carmi, N. Mandarin fruit quality: A review. J. Sci. Food Agric. 2018, 98, 18–26. [Google Scholar]
- Lv, X.; Zhao, S.; Ning, Z.; Zeng, H.; Shu, Y.; Tao, O.; Xiao, C.; Lu, C.; Liu, Y. Citrus fruits as a treasure trove of active natural metabolites that potentially provide benefits for human health. Chem. Cent. J. 2015, 9, 68. [Google Scholar] [PubMed] [Green Version]
- FAOSTAT. Food and Agriculture Organization of the United Nations (FAO). FAOSTAT Database. Available online: http://www.fao.org/faostat/ (accessed on 3 September 2022).
- MNFSR. Fruit, Vegetable, and Condiments Statistics of Pakistan (2019–2020); Ministry of National Food Security & Research Economic Wing Islamabad: Islamabad, Pakistan, 2020.
- Ali, M. Cluster Development-Based Agriculture Transformation Plan Vision-2025. Project 2020, 131, 434. [Google Scholar]
- Qureshi, M.A.; Jaskani, M.J.; Khan, A.S.; Ahmad, R. Influence of Endogenous Plant Hormones on Physiological and Growth Attributes of Kinnow Mandarin Grafted on Nine Rootstocks. J. Plant Growth Regul. 2022, 41, 1254–1264. [Google Scholar]
- Opara, U.L.; Pathare, P.B. Bruise damage measurement and analysis of fresh horticultural produce—A review. Postharvest Biol. Technol. 2014, 91, 9–24. [Google Scholar]
- Magwaza, L.S.; Opara, U.L. Analytical methods for determination of sugars and sweetness of horticultural products—A review. Sci. Hortic. 2015, 184, 179–192. [Google Scholar]
- Bermejo, A.; Llosá, M.J.; Cano, A. Analysis of Bioactive Compounds in Seven Citrus Cultivars. Food Sci. Technol. Int. 2011, 17, 55–62. [Google Scholar] [CrossRef]
- Sun, C.; Aernouts, B.; Saeys, W. Effects of harvest time, fruit size and cultivar on the bulk optical properties of Satsuma mandarin. Postharvest Biol. Technol. 2021, 175, 111412. [Google Scholar]
- da Cruz, M.A.; Neves, C.; de Carvalho, D.U.; Colombo, R.C.; Bai, J.; Yada, I.F.U.; Leite Junior, R.P.; Tazima, Z.H. Five Rootstocks for "Emperor" Mandarin Under Subtropical Climate in Southern Brazil. Front. Plant Sci. 2021, 12, 777871. [Google Scholar] [CrossRef]
- Cronje, P.J.; Barry, G.H.; Huysamer, M. Canopy position affects pigment expression and accumulation of flavedo carbohydrates of ‘Nules Clementine’ mandarin fruit, thereby affecting rind condition. J. Am. Soc. Hortic. Sci. 2013, 138, 217–224. [Google Scholar]
- Olarewaju, O.O.; Magwaza, L.S.; Fawole, O.A.; Tesfay, S.Z.; Opara, U.L. Comparative effects of canopy position on physicochemical properties of ‘Marsh’ grapefruit during non-chilling postharvest cold storage. Sci. Hortic. 2018, 241, 1–7. [Google Scholar]
- Alam, S.M.; Liu, D.-H.; Liu, Y.-Z.; Han, H.; Hussain, S.B.; Ateeq, M. Molecular elucidation for the variance of organic acid profile between citrus top and bottom canopy fruits. Sci. Hortic. 2022, 302, 111181. [Google Scholar]
- Sun, Y.; Singh, Z.; Tokala, V.Y.; Heather, B. Harvest maturity stage and cold storage period influence lemon fruit quality. Sci. Hortic. 2019, 249, 322–328. [Google Scholar]
- Hijaz, F.; Gmitter, F.G., Jr.; Bai, J.; Baldwin, E.; Biotteau, A.; Leclair, C.; McCollum, T.G.; Plotto, A. Effect of fruit maturity on volatiles and sensory descriptors of four mandarin hybrids. J. Food Sci. 2020, 85, 1548–1564. [Google Scholar]
- Zhang, J.; Zhang, J.-y.; Shan, Y.-x.; Can, G.; Lian, H.; Zhang, L.-y.; Wei, L.; Liang, Y.; Zhong, B.-l. Effect of harvest time on the chemical composition and antioxidant capacity of Gannan navel orange Citrus sinensis L. Osbeck ‘Newhall’ juice. J. Integr. Agric. 2022, 21, 261–272. [Google Scholar]
- Hussain, S.B.; Anjum, M.A.; Hussain, S.; Ejaz, S.; Ahmed, M. Physico-chemical profiling of promising sweet orange cultivars grown under different agro-climatic conditions of Pakistan. Erwerbs Obstbau 2017, 59, 315–324. [Google Scholar]
- Kim, M.; Yun, S.K.; Kim, S.S.; Park, Y.; Joa, J.; Han, S.; Shin, K.; Song, K.J. Response of citrus to freezing tolerance differs depending on genotypes and growing conditions. Hortic. Environ. Biotechnol. 2021, 62, 181–189. [Google Scholar]
- Lee, S.K.; Kader, A.A. Preharvest and postharvest factors influencing vitamin C content of horticultural crops. Postharvest Biol. Technol. 2000, 20, 207–220. [Google Scholar]
- Magwaza, L.S.; Mditshwa, A.; Tesfay, S.Z.; Opara, U.L. An overview of preharvest factors affecting vitamin C content of citrus fruit. Sci. Hortic. 2017, 216, 12–21. [Google Scholar]
- Rekha, C.; Poornima, G.; Manasa, M.; Abhipsa, V.; Devi, J.P.; Kumar, H.T.V.; Kekuda, T.R.P. Ascorbic acid, total phenol content and antioxidant activity of fresh juices of four ripe and unripe citrus fruits. Chem. Sci. Trans. 2012, 1, 303–310. [Google Scholar] [CrossRef]
- Ahmed, W.; Azmat, R.; Akram, J.; Mehmood, A.; Ahmed, R.; Qayyum, A.; Khan, S.U.; Masaud, S.; Husain, I.; Liaquat, M. Effects of various harvesting times on bioactive compound in Citrus paradisi (shamber). Pak. J. Bot. 2020, 52, 949–954. [Google Scholar]
- Bai, J.; Baldwin, E.; Plotto, A.; Manthey, J.A.; McCollum, G.; Irey, M.; Luzio, G. Influence of harvest time on quality of ‘Valencia’ oranges and juice. Proc. Fla. State Hortic. Soc. 2009, 122, 308–342. [Google Scholar]
- Hervalejo, A.; Arjona-López, J.M.; Ordóñez-Díaz, J.L.; Romero-Rodríguez, E.; Calero-Velázquez, R.; Moreno-Rojas, J.M.; Arenas-Arenas, F.J. Influence of Harvesting Season on Morphological and Sensory Quality, Bioactive Compounds and Antioxidant Activity of Three Late-Season Orange Cultivars ‘Barberina’, ‘Valencia Midknight’ and ‘Valencia Delta Seedless’. Agronomy 2021, 11, 673. [Google Scholar]
- Muhtaseb, J. Effect of harvesting date on fruit quality of grapefruit cv. ‘Red Blush’ under Jordan Valley conditions. Fruits 2007, 62, 107–113. [Google Scholar] [CrossRef] [Green Version]
- Bai, J.; Baldwin, E.A.; McCollum, G.; Plotto, A.; Manthey, J.A.; Widmer, W.W.; Luzio, G.; Cameron, R. Changes in volatile and non-volatile flavor chemicals of “Valencia” orange juice over the harvest seasons. Foods 2016, 5, 4. [Google Scholar]
- Kumar, R.; Vijay, S.; Khan, N. Comparative Nutritional Analysis and Antioxidant Activity of Fruit Juices of some Citrus spp. Octa J. Biosci. 2013, 1, 44–53. [Google Scholar]
- Goldenberg, L.; Yaniv, Y.; Kaplunov, T.; Doron-Faigenboim, A.; Porat, R.; Carmi, N. Genetic diversity among mandarins in fruit-quality traits. J. Agric. Food Chem. 2014, 62, 4938–4946. [Google Scholar]
- Costanzo, G.; Iesce, M.R.; Naviglio, D.; Ciaravolo, M.; Vitale, E.; Arena, C. Comparative studies on different citrus cultivars: A revaluation of waste mandarin components. Antioxidants 2020, 9, 517. [Google Scholar]
- Lado, J.; Gambetta, G.; Zacarias, L. Key determinants of citrus fruit quality: Metabolites and main changes during maturation. Sci. Hortic. 2018, 233, 238–248. [Google Scholar]
- Khalid, S.; Malik, A.U.; Saleem, B.A.; Khan, A.S.; Khalid, M.S.; Amin, M. Tree age and canopy position affect rind quality, fruit quality and rind nutrient content of ‘Kinnow’ mandarin (Citrus nobilis Lour× Citrus deliciosa Tenora). Sci. Hortic. 2012, 135, 137–144. [Google Scholar]
- Ruck, J. Chemical Methods for Analysis of Fruits and Vegetables; Canada Department of Agriculture: Ottawa, ON, Canada, 1963.
- Özgen, M.; Saraçoğlu, O.; Geçer, E.N. Antioxidant capacity and chemical properties of selected barberry (Berberis vulgaris L.) fruits. Hortic. Environ. Biotechnol. 2012, 53, 447–451. [Google Scholar]
- Iqbal, M.; Khan, M.; Zafar, M.; Munir, M. Effect of harvesting date on fruit size, fruit weight and total soluble solids of feutrell’s early and kinnow cultivars of Mardan (Citrus reticulata) on the economic conditions of farming community of Faisalabad. Sarhad J. Agric. 2012, 28, 19–21. [Google Scholar]
- Rokaya, P.R.; Baral, D.R.; Gautam, D.M.; Shrestha, A.K.; Paudyal, K.P. Effect of altitude and maturity stages on quality attributes of mandarin (Citrus reticulata Blanco). Am. J. Plant Sci. 2016, 7, 958. [Google Scholar] [CrossRef] [Green Version]
- Nawaz, R.; Abbasi, N.A.; Hafiz, I.A.; Khalid, A. Impact of climate variables on growth and development of Kinnow fruit (Citrus nobilis Lour x Citrus deliciosa Tenora) grown at different ecological zones under climate change scenario. Sci. Hortic. 2020, 260, 108868. [Google Scholar]
- Bal, J.; Chohan, G. Studies on Fruit Quality at Maturity and Ripening of Kinnow Mandarin on Different Rootstocks. Indian J. Hortic. 1987, 44, 45–51. [Google Scholar]
- Grewal, A.; Hafiz, I.; Chaudhary, A.; Khan, M.; Chaudhary, M. Quality estimation during marketing of kinnow and feutrell’s early. Int. J. Agric. Biol. 2000, 2, 328–330. [Google Scholar]
- Khan, M.; Mumtaz, S.; Ahmad, S.; Abbas, M.; Khan, I. Some studies on the morphology of Kinnow mandarin and Feutrell’s early. Pak. J. Agric. Sci. 2008, 42, 424–431. [Google Scholar]
- Hussain, S.B.; Shi, C.-Y.; Guo, L.-X.; Kamran, H.M.; Sadka, A.; Liu, Y.-Z. Recent advances in the regulation of citric acid metabolism in citrus fruit. Crit. Rev. Plant Sci. 2017, 36, 241–256. [Google Scholar]
- Hussain, S.B.; Naseer, M.; Manzoor, M.; Akbar, A.; Hayyat, S.; Sabir, S. Maturity Indices and Harvesting Methods for Citrus Fruit. In Citrus Production; CRC Press: Boca Raton, FL, USA, 2022; pp. 311–318. [Google Scholar] [CrossRef]
- Riaz, M.; Zamir, T.; Rashid, N.; Jamil, N.; Masood, Z.; Jabeen, U.; Mandokhel, F.; Behlil, F.; Mengal, F.; Khan, M. Quality assessment in different stages of maturity of fruits, mandarins kinnow and feutrell’s early collected from the fruit market of Quetta city at in relation to their benefits for human health. Am. Euras. J. Toxicol. Sci. 2015, 7, 203–238. [Google Scholar]
- Nawaz, R.; Abbasi, N.A.; Hafiz, I.A.; Khalid, A. Impact of climate variables on fruit internal quality of Kinnow mandarin (Citrus nobilis Lour x Citrus deliciosa Tenora) in ripening phase grown under varying environmental conditions. Sci. Hortic. 2020, 265, 109235. [Google Scholar]
- Anwar, S.; Ahmad, B.; Surfarz, M.; Hussain, K.; Bhutti, K.; Saqib, M. Effect of picking time on physical and chemical characteristics of sweet orange. Int. J. Agric. Biol. 1999, 1, 59–61. [Google Scholar]
- Tsai, H.-L.; Chang, S.K.; Chang, S.-J. Antioxidant content and free radical scavenging ability of fresh red pummelo [Citrus grandis (L.) Osbeck] juice and freeze-dried products. J. Agric. Food Chem. 2007, 55, 2867–2872. [Google Scholar] [CrossRef] [PubMed]
- Moulehi, I.; Bourgou, S.; Ourghemmi, I.; Tounsi, M.S. Variety and ripening impact on phenolic composition and antioxidant activity of mandarin (Citrus reticulate Blanco) and bitter orange (Citrus aurantium L.) seeds extracts. Ind. Crops Prod. 2012, 39, 74–80. [Google Scholar] [CrossRef]
- Albrigo, L.G. Impact of late harvest on citrus crop losses and juice quality. Proc. Fla. State Hort. Soc. 2006, 119, 199–202. [Google Scholar]
| Soil Depth (cm) | Texture | Saturation (%) | pH | SAR | EC (ds m−1) | Total N (%) | Available P (ppm) | Available K (ppm) |
|---|---|---|---|---|---|---|---|---|
| 30 | Loam | 42 | 7.9 | 0.6 | 1.82 | 0.048 | 8 | 153 |
| Kinnow | Feutrell’s Early | |||||
|---|---|---|---|---|---|---|
| Number | Eigenvalue | Percent Variability | Cumulative | Eigenvalue | Percent Variability | Cumulative |
| 1 | 11.702 | 68.835 | 68.835 | 11.1228 | 65.428 | 65.428 |
| 2 | 1.7232 | 10.137 | 78.972 | 1.7561 | 10.33 | 75.758 |
| 3 | 0.9374 | 5.514 | 84.486 | 1.1022 | 6.483 | 82.242 |
| 4 | 0.6415 | 3.773 | 88.259 | 0.7151 | 4.206 | 86.448 |
| 5 | 0.4985 | 2.933 | 91.192 | 0.5069 | 2.982 | 89.43 |
| 6 | 0.4311 | 2.536 | 93.727 | 0.3977 | 2.34 | 91.769 |
| 7 | 0.2981 | 1.754 | 95.481 | 0.3319 | 1.953 | 93.722 |
| 8 | 0.2347 | 1.381 | 96.862 | 0.2631 | 1.548 | 95.269 |
| 9 | 0.1862 | 1.095 | 97.957 | 0.2227 | 1.31 | 96.579 |
| 10 | 0.1077 | 0.634 | 98.591 | 0.1821 | 1.071 | 97.651 |
| Quality Variables | Kinnow | Feutrell’s Early | ||
|---|---|---|---|---|
| PC1 | PC1 | PC2 | PC2 | |
| FW | 0.92719 | 0.92719 | 0.87547 | 0.11457 |
| FD | 0.89935 | 0.89935 | 0.76053 | 0.4167 |
| FC | 0.91049 | 0.91049 | 0.93586 | 0.18699 |
| PT | 0.25507 | 0.25507 | 0.76519 | 0.59784 |
| SC | 0.77023 | 0.77023 | 0.79339 | −0.15869 |
| RG | −0.93687 | −0.93687 | −0.92394 | −0.09251 |
| PC | 0.43634 | 0.43634 | 0.28065 | 0.69848 |
| JC | 0.57468 | 0.57468 | 0.6174 | 0.35912 |
| pH | 0.86065 | 0.86065 | 0.88308 | −0.18336 |
| TSS | 0.8837 | 0.8837 | 0.87001 | −0.26575 |
| TA | −0.94433 | −0.94433 | −0.96712 | −0.10611 |
| RI | 0.94103 | 0.94103 | 0.90487 | −0.12532 |
| TS | 0.73905 | 0.73905 | 0.81721 | −0.35272 |
| VC | −0.90967 | −0.90967 | −0.76098 | 0.26897 |
| AA | −0.83843 | −0.83843 | −0.59941 | 0.3449 |
| AC | −0.93873 | −0.93873 | −0.89263 | 0.25852 |
| TPC | −0.94396 | −0.94396 | −0.82438 | 0.11242 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manzoor, M.; Hussain, S.B.; Anjum, M.A.; Naseer, M.; Ahmad, R.; Ziogas, V. Effects of Harvest Time on the Fruit Quality of Kinnow and Feutrell’s Early Mandarins (Citrus reticulata Blanco). Agronomy 2023, 13, 802. https://doi.org/10.3390/agronomy13030802
Manzoor M, Hussain SB, Anjum MA, Naseer M, Ahmad R, Ziogas V. Effects of Harvest Time on the Fruit Quality of Kinnow and Feutrell’s Early Mandarins (Citrus reticulata Blanco). Agronomy. 2023; 13(3):802. https://doi.org/10.3390/agronomy13030802
Chicago/Turabian StyleManzoor, Meryam, Syed Bilal Hussain, Muhammad Akbar Anjum, Mudassar Naseer, Riaz Ahmad, and Vasileios Ziogas. 2023. "Effects of Harvest Time on the Fruit Quality of Kinnow and Feutrell’s Early Mandarins (Citrus reticulata Blanco)" Agronomy 13, no. 3: 802. https://doi.org/10.3390/agronomy13030802
APA StyleManzoor, M., Hussain, S. B., Anjum, M. A., Naseer, M., Ahmad, R., & Ziogas, V. (2023). Effects of Harvest Time on the Fruit Quality of Kinnow and Feutrell’s Early Mandarins (Citrus reticulata Blanco). Agronomy, 13(3), 802. https://doi.org/10.3390/agronomy13030802






