Combined Morphological and Palynological Classification for Hibiscus syriacus L. (Malvaceae): Construction of the Diagnostic Classification Framework and Implications of Pollen Morphological Variation on Fruiting
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Sources
2.2. Scanning Electron Microscopy (SEM) and Coding the Pollen Qualitative Traits
2.3. Flower and Leaf Morphological Indicators and Their Measurement
2.4. Data Analysis
3. Results
3.1. Floral and Leaf Morphology of 24 H. syriacus Cultivars
3.2. Overall Pollen Morphology of H. syriacus
3.2.1. Quantitative Traits of H. syriacus Pollen Grains
3.2.2. Qualitative Traits of H. syriacus Pollen
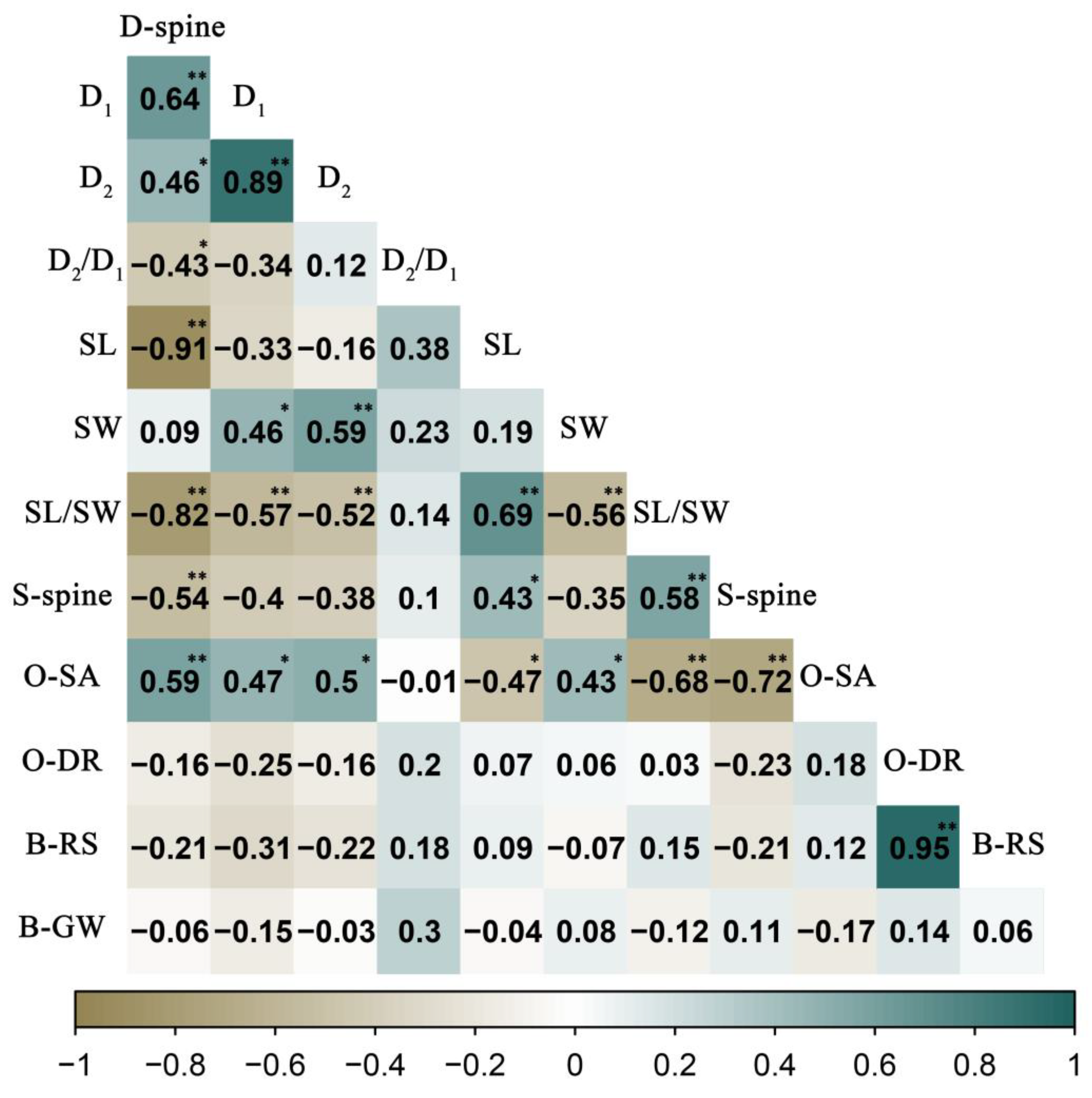
3.3. Variation and Correlation of Pollen Morphology between the 24 H. syriacus Cultivars
3.4. Correlations among Pollen, Flower, and Leaf Morphological Characteristics of H. syriacus Cultivars
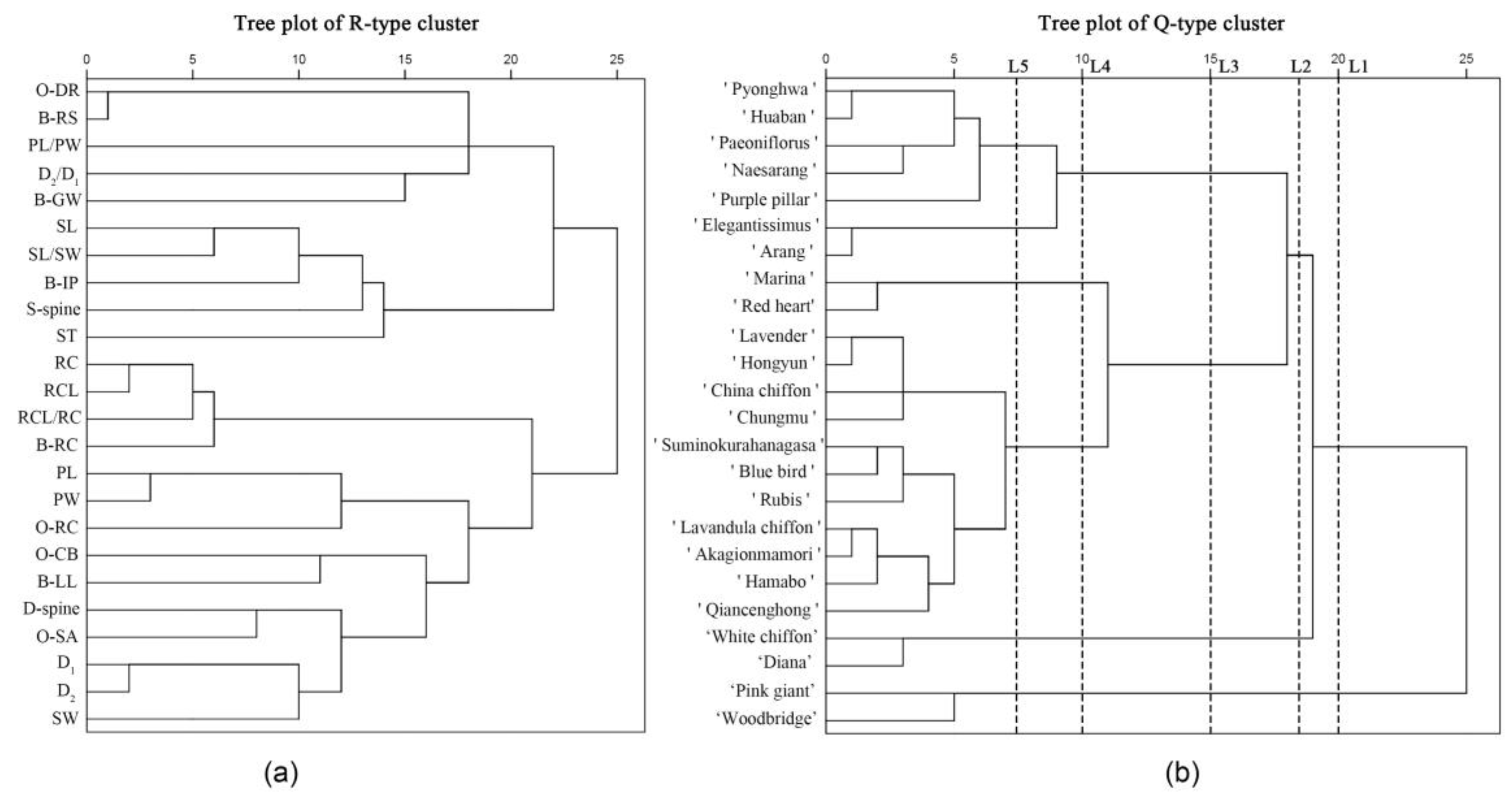
3.5. Clustering Analysis of 24 H. syriacus Cultivars Based on the 24 Combined Morphological Traits
4. Discussion
4.1. Pollen Morphological Variation of H. syriacus Cultivars and Novel Diagnostic Pollen Traits
4.2. Combining Morphological Indicators Helps to Better Distinguish Cultivars of H. syriacus and the Contribution of Qualitative Indicators to Clustering
4.3. Effects of H. syriacus Pollen Traits on Fruiting
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Samples | D1 | D2 | P/E | SL | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Range | cv | Mean | Range | cv | Mean | Range | cv | Mean | Range | cv | |
| 1 | 130.70 | 110.89–145.87 | 7.67% | 124.04 | 104.11–131.79 | 6.47% | 0.95 | 0.82–1.13 | 8.75% | 15.07 | 13.32–17.91 | 8.94% |
| 2 | 123.03 | 110.35–136.40 | 9.52% | 131.06 | 120.92–139.25 | 5.04% | 1.07 | 0.93–1.18 | 7.50% | 16.66 | 13.14–22.76 | 17.09% |
| 3 | 141.09 | 132.97–145.58 | 2.45% | 146.03 | 132.60–158.22 | 6.32% | 1.03 | 0.97–1.13 | 4.91% | 15.65 | 13.25–17.80 | 10.45% |
| 4 | 127.95 | 122.82–134.50 | 3.31% | 129.78 | 123.55–135.09 | 2.88% | 1.01 | 1.00–1.04 | 1.06% | 16.81 | 12.48–20.26 | 14.83% |
| 5 | 127.13 | 119.37–133.47 | 3.60% | 129.30 | 110.46–138.07 | 6.04% | 1.02 | 0.85–1.06 | 6.31% | 23.70 | 20.15–27.10 | 8.82% |
| 6 | 144.34 | 133.46–160.95 | 5.64% | 139.12 | 112.37–148.10 | 9.58% | 0.97 | 0.73–1.05 | 13.09% | 13.42 | 12.09–15.49 | 8.25% |
| 7 | 120.83 | 98.91–135.14 | 13.77% | 117.76 | 103.68–128.76 | 9.53% | 0.98 | 0.93–1.05 | 4.77% | 15.31 | 13.84–17.55 | 8.02% |
| 8 | 123.44 | 112.96–140.53 | 8.66% | 121.44 | 116.96–126.63 | 3.09% | 0.99 | 0.90–1.04 | 5.43% | 15.55 | 12.73–19.05 | 12.33% |
| 9 | 126.29 | 119.89–129.83 | 2.88% | 125.44 | 120.65–129.62 | 2.11% | 0.99 | 0.97–1.03 | 1.90% | 19.56 | 16.64–22.36 | 9.50% |
| 10 | 125.19 | 105.58–133.40 | 9.44% | 129.51 | 105.82–145.29 | 10.70% | 1.03 | 0.99–1.10 | 3.71% | 20.15 | 15.08–23.40 | 11.81% |
| 11 | 119.08 | 90.19–131.66 | 10.38% | 123.24 | 105.56–138.39 | 9.84% | 1.04 | 0.99–1.17 | 5.21% | 19.73 | 17.73–21.13 | 4.98% |
| 12 | 118.66 | 112.55–125.56 | 4.22% | 118.32 | 104.18–127.02 | 5.91% | 1.00 | 0.92–1.07 | 5.27% | 16.73 | 14.02–19.65 | 11.38% |
| 13 | 114.59 | 104.39–128.85 | 5.31% | 119.76 | 111.69–129.57 | 4.43% | 1.05 | 0.97–1.15 | 5.04% | 19.33 | 14.68–23.21 | 13.45% |
| 14 | 123.16 | 113.81–135.99 | 5.95% | 126.94 | 119.62–134.17 | 4.04% | 1.03 | 0.96–1.10 | 4.17% | 14.45 | 11.50–18.30 | 15.58% |
| 15 | 122.76 | 90.72–137.44 | 12.08% | 119.16 | 90.9–135.96 | 12.10% | 0.97 | 0.90–1.05 | 4.50% | 14.20 | 11.32–17.95 | 14.25% |
| 16 | 116.54 | 109.08–127.62 | 5.93% | 119.18 | 111.99–127.62 | 4.35% | 1.02 | 0.92–1.12 | 5.35% | 15.06 | 12.14–19.45 | 16.80% |
| 17 | 117.64 | 108.77–128.85 | 4.93% | 116.95 | 113.16–121.18 | 1.99% | 1.00 | 0.91–1.06 | 4.53% | 20.24 | 13.58–24.71 | 15.39% |
| 18 | 121.06 | 114.61–130.31 | 3.80% | 123.36 | 113.45–138.66 | 4.15% | 1.02 | 0.95–1.10 | 5.40% | 16.13 | 12.71–19.12 | 13.25% |
| 19 | 126.71 | 102.54–144.72 | 8.79% | 126.80 | 103.63–131.22 | 7.64% | 1.00 | 0.95–1.04 | 2.75% | 18.54 | 17.05–22.53 | 8.84% |
| 20 | 125.33 | 117.65–138.44 | 5.55% | 124.52 | 111.99–137.85 | 5.34% | 0.99 | 0.94–1.04 | 3.75% | 16.08 | 10.73–19.42 | 15.35% |
| 21 | 138.20 | 132.71–143.62 | 2.54% | 134.37 | 131.24–138.45 | 1.80% | 0.97 | 0.94–1.00 | 2.00% | 18.47 | 16.41–20.07 | 4.99% |
| 22 | 121.18 | 103.63–125.44 | 5.26% | 121.79 | 99.99–138.45 | 7.65% | 1.00 | 0.96–1.11 | 4.22% | 14.54 | 12.00–19.55 | 16.41% |
| 23 | 114.31 | 102.90–123.26 | 5.79% | 112.39 | 102.9–118.68 | 5.38% | 0.98 | 0.93–1.02 | 3.45% | 17.22 | 14.38–18.61 | 8.40% |
| 24 | 111.33 | 98.55–120.09 | 7.61% | 116.22 | 97.27–126.29 | 9.04% | 1.04 | 0.99–1.10 | 3.58% | 25.04 | 22.89–28.24 | 6.78% |
| Samples | SW | SL/SW | D–spine | S–spine | ||||||||
| Mean | Range | cv | Mean | Range | cv | Mean | Range | cv | Mean | Range | cv | |
| 1 | 8.59 | 6.61–10.15 | 13.85% | 1.79 | 1.31–2.29 | 18.30% | 4.36 | 3.50–5.01 | 10.76% | 20.36 | 16.26–23.51 | 11.20% |
| 2 | 9.01 | 7.13–10.67 | 12.35% | 1.85 | 1.53–2.37 | 11.96% | 3.77 | 2.93–5.19 | 17.18% | 22.09 | 16.52–27.74 | 16.03% |
| 3 | 15.00 | 10.99–18.61 | 16.54% | 1.06 | 0.90–1.37 | 16.21% | 4.56 | 3.78–5.43 | 11.45% | 12.85 | 9.98–19.73 | 20.57% |
| 4 | 8.37 | 6.73–9.86 | 10.84% | 2.01 | 1.78–2.42 | 8.71% | 3.88 | 3.12–4.99 | 15.57% | 19.19 | 14.80–22.74 | 12.66% |
| 5 | 10.43 | 8.99–12.14 | 10.53% | 2.29 | 1.97–2.61 | 10.20% | 2.70 | 2.23–3.24 | 10.59% | 20.49 | 16.14–26.44 | 16.78% |
| 6 | 12.73 | 11.54–14.36 | 6.43% | 1.06 | 0.84–1.28 | 11.54% | 5.40 | 4.72–6.00 | 7.56% | 18.75 | 16.20–23.38 | 12.21% |
| 7 | 9.47 | 8.13–11.56 | 11.07% | 1.63 | 1.36–1.83 | 9.18% | 3.96 | 3.33–4.83 | 13.62% | 20.28 | 14.57–27.85 | 18.96% |
| 8 | 8.96 | 6.94–10.51 | 12.32% | 1.75 | 1.57–2.11 | 11.10% | 4.00 | 3.62–4.62 | 8.81% | 18.98 | 15.15–22.57 | 15.51% |
| 9 | 9.93 | 8.12–12.70 | 17.00% | 2.02 | 1.38–2.63 | 18.93% | 3.26 | 2.82–3.76 | 10.69% | 25.63 | 20.22–31.54 | 14.08% |
| 10 | 9.66 | 8.73–11.28 | 8.16% | 2.09 | 1.64–2.36 | 9.77% | 3.15 | 2.48–4.38 | 17.25% | 27.37 | 20.57–32.75 | 13.79% |
| 11 | 10.66 | 9.32–11.72 | 7.34% | 1.86 | 1.60–2.12 | 8.49% | 3.03 | 2.23–3.46 | 12.69% | 24.14 | 15.73–30.56 | 19.24% |
| 12 | 9.18 | 6.57–11.33 | 14.99% | 1.84 | 1.40–2.22 | 12.72% | 3.59 | 2.93–4.26 | 11.79% | 18.04 | 11.51–21.48 | 16.09% |
| 13 | 12.00 | 9.19–14.48 | 13.54% | 1.66 | 1.01–2.24 | 23.53% | 3.01 | 2.41–3.56 | 12.51% | 23.74 | 16.28–30.04 | 20.59% |
| 14 | 9.64 | 6.50–12.36 | 19.02% | 1.56 | 0.93–2.42 | 28.34% | 4.36 | 3.17–5.65 | 17.97% | 18.57 | 14.37–26.65 | 21.80% |
| 15 | 9.24 | 7.28–12.56 | 15.69% | 1.56 | 1.21–1.88 | 15.62% | 4.44 | 2.86–5.85 | 22.93% | 22.70 | 11.66–29.09 | 22.96% |
| 16 | 8.25 | 7.27–10.35 | 10.88% | 1.84 | 1.43–2.32 | 16.82% | 3.97 | 3.01–5.26 | 18.22% | 21.90 | 17.93–28.00 | 17.12% |
| 17 | 9.19 | 7.25–11.59 | 15.83% | 2.22 | 1.87–2.72 | 13.02% | 3.00 | 2.20–4.74 | 23.33% | 24.65 | 18.59–31.32 | 15.44% |
| 18 | 8.74 | 5.31–11.31 | 22.37% | 1.91 | 1.40–2.51 | 20.75% | 3.81 | 3.08–4.75 | 13.89% | 20.78 | 17.90–28.74 | 17.10% |
| 19 | 8.50 | 7.02–9.84 | 9.72% | 2.19 | 1.93–2.53 | 8.75% | 3.44 | 2.68–3.87 | 11.56% | 23.45 | 20.04–27.43 | 10.08% |
| 20 | 8.48 | 4.91–10.04 | 17.15% | 1.91 | 1.70–2.19 | 8.55% | 3.99 | 3.26–5.74 | 17.63% | 20.86 | 18.13–23.08 | 8.60% |
| 21 | 10.88 | 6.92–14.54 | 22.89% | 1.78 | 1.38–2.64 | 23.71% | 3.75 | 3.46–4.30 | 5.91% | 20.92 | 14.03–27.63 | 21.24% |
| 22 | 8.43 | 6.57–10.94 | 16.13% | 1.73 | 1.50–2.05 | 10.94% | 4.26 | 3.20–5.13 | 16.02% | 20.23 | 15.00–24.85 | 14.00% |
| 23 | 8.34 | 6.50–10.60 | 17.78% | 2.11 | 1.67–2.58 | 14.32% | 3.34 | 3.00–4.06 | 8.87% | 20.40 | 18.57–23.90 | 7.57% |
| 24 | 11.59 | 9.15–14.28 | 17.70% | 2.23 | 1.76–3.09 | 21.53% | 2.23 | 1.92–2.48 | 8.93% | 21.11 | 14.46–28.10 | 17.76% |
| No. | Cultivar | Fruiting Rate (%) |
|---|---|---|
| 1 | ‘Pink giant’ | 4.4 |
| 2 | ‘Woodbridge’ | 1.5 |
| 3 | ‘Elegantissimus’ | 0.1 |
| 4 | ‘Arang’ | 15.8 |
| 5 | ‘Rubis’ | 13.2 |
| 6 | ‘Chungmu’ | 0.1 |
| 7 | ‘Suminokurahanagasa’ | 2.3 |
| 8 | ‘Blue bird’ | 26.2 |
| 9 | ‘Akagionmamori’ | 0.1 |
| 10 | ‘Red heart’ | 26.0 |
| 11 | ‘Naesarang’ | 1.0 |
References
- Liu, Z.; Gao, L. Ancient Hibiscus syriacus culture of China. Agric. Archaeol. 2010, 4, 228–231. [Google Scholar]
- National Institute of Forest Science. Illustration of Hibiscus syriacus L. cultivars; Seoul Press: Seoul, Republic of Korea, 2014.
- Bae, S.H.; Younis, A.; Hwang, Y.J.; Lim, K.B. Various Pollen Morphology in Hibiscus syriacus. Flower Res. J. 2015, 23, 125–130. [Google Scholar] [CrossRef]
- He, L. Comparision in Cytology and Molecular Biology of Simple and Double Flower on Hibiscus syriacus. Ph.D. Thesis, Beijing Forestry University, Beijing, China, 2006. [Google Scholar]
- Zhao, Y.; Feng, Q.; Tian, L.; Zhang, J.; Wang, X.; Liu, Y.; Liu, D. Pollen morphology and numerical taxonomy of 22 Hibiscus syriacus. Guihaia 2021, 41, 103–113. [Google Scholar] [CrossRef]
- Oliveira, P.P.; dos Santos, F.d.A.R. Morfologia polínica de Hibiscus pernambucensis Arruda e Hibiscus tiliaceus L. (Malvaceae). Acta Biol. Leopoldensia 2004, 26, 203–211. [Google Scholar]
- EI, N.S. Pollen morphology of Egyptian Malvaceae: An assessment of taxonomic value. Turk. J. Bot. 2004, 28, 227–240. [Google Scholar]
- Yuan, T.; Wang, L. Pollen Morphology of Several Tree Peony Wild Species and Discussion on Its Evolution and Taxonomy. J. Beijing For. Univ. 1999, 21, 18–21. [Google Scholar]
- Palazzesi, L.; Pujana, R.R.; Burrieza, H.P.; Steinhardt, A.P. Pollen grain morphology of selected allergenic species native to Southern South America. J. Torrey Botanic. Soc. 2007, 134, 527–533. [Google Scholar] [CrossRef]
- Korszun, S.; Klimko, M. Microsporangia and pollen morphology of Ginkgo biloba cultivars. Dendrobiology 2014, 71, 83–92. [Google Scholar] [CrossRef]
- Ma, S.L.; Lu, Y.M. Classification and phylogenetic analysis of Chinese hawthorn assessed by plant and pollen morphology. Genet. Mol. Res. Gmr 2016, 15, gmr.15038739. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.H.; Glc, B.; Zhang, D.X. Pollen morphology of Callicarpa L. (Lamiaceae) from China and its systematic implications. Plant Syst. Evol. 2016, 302, 67–88. [Google Scholar] [CrossRef]
- Xiao, F.; Wang, X.H.; Wang, Y.Q.; Liu, C.L.; Xie, L.S.; Ren, X.Y. Numerical Classification and Principle Component Analysis of 27 Hibiscus Cultivars. J. Cent. South Univ. For. Technol. 2019, 39, 59–64. [Google Scholar]
- Xiao, F.; She, Y.; She, J.; Wang, Y.; Wu, F.; Xie, P.; Chen, Q. Intraspecific Pollen Morphology Variation and Its Responses to Environmental Factors of Wild Cathaya argyrophylla Chun Et Kuang Endemic to China. Forests 2022, 13, 651. [Google Scholar] [CrossRef]
- Minnaar, C.; Anderson, B.; de Jager, M.L.; Karron, J.D. Plant–pollinator interactions along the pathway to paternity. Ann. Bot. 2018, 123, 225–245. [Google Scholar] [CrossRef] [PubMed]
- Phillips, B.B.; Williams, A.; Osborne, J.L.; Shaw, R.F. Shared traits make flies and bees effective pollinators of oilseed rape (Brassica napus L.). Basic Appl. Ecol. 2018, 32, 66–76. [Google Scholar] [CrossRef]
- Lynn, A.; Piotter, E.; Harrison, E.; Galen, C. Sexual and natural selection on pollen morphology in Taraxacum. Am. J. Bot. 2020, 107, 364–374. [Google Scholar] [CrossRef]
- Xu, K.X. Numerical Taxonomy; Science Publishing: Beijing, China, 1994. [Google Scholar]
- Erdtman, G.; Vishnu-Mittre. On Terminology in Pollen and Spore Morphology. Grana Palynol. 1958, 1, 6–9. [Google Scholar] [CrossRef]
- Wang, F.X.; Qian, N.F.; Zhang, Y.L.; Yang, H.Q. Pollen Flora of China, 2nd ed.; Science Publishing: Beijing, China, 1995; pp. 5–13. [Google Scholar]
- Punt, W.; Hoen, P.P.; Blackmore, S.; Nilsson, S.; Le Thomas, A. Glossary of pollen and spore terminology. Rev. Palaeobot. Palynol. 2007, 143, 1–81. [Google Scholar] [CrossRef]
- Erdtman, G. The Acetolysis Method. A Revised Description. Sven. Bot. Tidskr. 1960, 54, 561–564. [Google Scholar]
- Abdi, H.; Williams, L.J. Principal Component Analysis. WIREs Comput. Stat. 2010, 2, 433–459. [Google Scholar] [CrossRef]
- Martinović, S.; Vlahović, M.; Gajić-Kvaščev, M.; Vuksanović, M.; Glišić, D.; Volkov-Husović, T. Principal component analysis of morphological descriptors for monitoring surface defects induced by thermal shock. J. Eur. Ceram. Soc. 2021, 41, 426–429. [Google Scholar] [CrossRef]
- Fukunaga, K. Introduction to Statistical Pattern Recognition; Academic Press: Cambridge, MA, USA, 1990. [Google Scholar] [CrossRef]
- Parks, J.M. Part 2: Applications of Multivariate Statistics in Geology||Cluster Analysis Applied to Multivariate Geologic Problems. J. Geol. 1966, 74, 703–715. [Google Scholar] [CrossRef]
- Mccallum, B.; Chang, S.M. Pollen competition in style: Effects of pollen size on siring success in the hermaphroditic common morning glory, Ipomoea purpurea. Am. J. Bot. 2016, 103, 466–468. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; She, Y.; She, J.; Zhang, J.; Zhang, X.; Luo, C. Assessing habitat suitability and selecting optimal habitats for relict tree Cathaya argyrophylla in Hunan, China: Integrating pollen size, environmental factors, and niche modeling for conservation. Ecol. Indic. 2022, 145, 109669. [Google Scholar] [CrossRef]
- EI, N.S.; Sawady, N. Pollen morphology of Malvaceae and its taxonomic significance in Yemen. Flora Medit 2008, 18, 431–439. [Google Scholar]
- Andrade, K.; Guerra, S.; Debut, A. Fullerene-Based Symmetry in Hibiscus rosa-sinensis Pollen. PLoS ONE 2014, 9, e102123. [Google Scholar] [CrossRef]
- Shaheen, N.; Khan, M.A.; Hayat, M.Q.; Yasmin, G. Pollen morphology of 14 species of Abutilon and Hibiscus of the family Malvaceae (sensu stricto). J. Med. Plants Res. 2009, 3, 921–929. [Google Scholar]
- Halbritter, H.; Heigl, H. Hibiscus syriacus. In PalDat—A Palynological Database; Available online: https://www.paldat.org/pub/Hibiscus_syriacus/304207 (accessed on 7 March 2023).
- Hao, M.; Cao, F.; Zhang, W.; Wang, G. Variation of pollen morphology among male trees of Ginkgo. J. For. Eng. 2006, 20, 53–55. [Google Scholar]
- Meo, A.A.; Khan, M.A. Pollen morphology of invasive species Parthenium hysterophorus L. (Heliantheae—Asteracea) from Islamabad and Rawalpindi, Pakistan. J. Agric. 2005, 21, 227–230. [Google Scholar]
- Mendoza, J.E.; Balanza, V.; Cifuentes, D.; Bielza, P. Selection for larger body size in Orius laevigatus: Intraspecific variability and effects on reproductive parameters. Biol. Control 2020, 148, 104310. [Google Scholar] [CrossRef]
- Xia, J.; Lu, J.; Wang, Z.; Hao, B.; Wang, H.; Liu, G. Pollen limitation and Allee effect related to population size and sex ratio in the endangered Ottelia acuminata (Hydrocharitaceae): Implications for conservation and reintroduction. Plant Biol. 2013, 15, 376–383. [Google Scholar] [CrossRef]
- Satil, F.; Kaya, A.; Ünal, M. Fruit, seed and pollen morphology of Chorispora DC. Species (Brassicaceae) of Turkey. Bangladesh J. Bot. 2018, 47, 459–466. [Google Scholar] [CrossRef]
- Doi, K.; Inoue, R.; Iwasaki, N. Seed weight mediates effects of pollen on berry weight, ripening, and anthocyanin content in highbush blueberry. Sci. Hortic. 2021, 288, 110313. [Google Scholar] [CrossRef]
- Maryam; Jaskani, M.J.; Ahmad, S.; Awan, F.S. Metaxenial Effects on Morphological Attributes in Date Palm cvs. Hillawi and Khadrawy. Pak. J. Agric. Sci. 2015, 52, 387–393. [Google Scholar]
- Konzmann, S.; Koethe, S.; Lunau, K. Pollen grain morphology is not exclusively responsible for pollen collectability in bumble bees. Sci. Rep. 2019, 9, 4705. [Google Scholar] [CrossRef] [PubMed]
- Schlindwein, C.; Pick, R.A.; Martins, C.F. Evaluation of oligolecty in the Brazilian bee Ptilothrix plumata (Hymenoptera, Apidae, Emphorini). Apidologie 2009, 40, 106–116. [Google Scholar] [CrossRef]
- Michener, C.D. The bees of the world. In Systematic Entomology; J.H.U. Press: Baltimore, MD, USA, 2010; Volume 26, pp. 255–256. [Google Scholar]
- Aguilar, C.; Azucena, M.; Parra-Tabla, V. Importance of conserving alternative pollinators: Assessing the pollination efficiency of the squash bee, Peponapis limitaris in Cucurbita moschata (Cucurbitaceae). J. Insect Conserv. 2000, 4, 201–208. [Google Scholar] [CrossRef]
- Ballantyne, G.A.; Baldock, K.; Willmer, P.G. Constructing more informative plant-pollinator networks: Visitation and pollen deposition networks in a heathland plant community. Proc. R. Soc. Biol. Sci. 2015, 282, 20151130. [Google Scholar] [CrossRef]
- Jung, J.-K.; Kim, M.; Lee, C.Y.; Jang, B.-J.; Kim, D.; Kwon, H.Y.; Park, Y. Comparison of Insect Pest Communities on 30 Cultivars of Hibiscus syriacus. J. Korean Soc. For. Sci. 2021, 110, 116–127. [Google Scholar]
- Kim, Y.; Cho, Y.; Kang, Y.K.; Choi, M.; Nam, S.H. A study of the major insect pest communities associated with Hibiscus syriacus (Columniferae, Malvaceae). J. Ecol. Environ. 2013, 36, 125–129. [Google Scholar] [CrossRef]
- You, X.; Zhang, D.; Chi, J.; Wu, H. ComparatiVe study on three Hibiscus syriacus cultivars’ characteristics of nectar secretion, and pollen presentation and entomophilous pollination. Acta Agric. Shanghai 2009, 25, 81–86. [Google Scholar]
- Stavert, J.R.; Linan-Cembrano, G.; Beggs, J.R.; Howlett, B.G.; Pattemore, D.E.; Bartomeus, I. Hairiness: The missing link between pollinators and pollination. Peerj 2016, 4, e2779. [Google Scholar] [CrossRef] [PubMed]
- Lunau, K.; Piorek, V.; Krohn, O.; Pacini, E. Just spines—Mechanical defense of malvaceous pollen against collection by corbiculate bees. Apidologie 2015, 46, 144–149. [Google Scholar] [CrossRef]

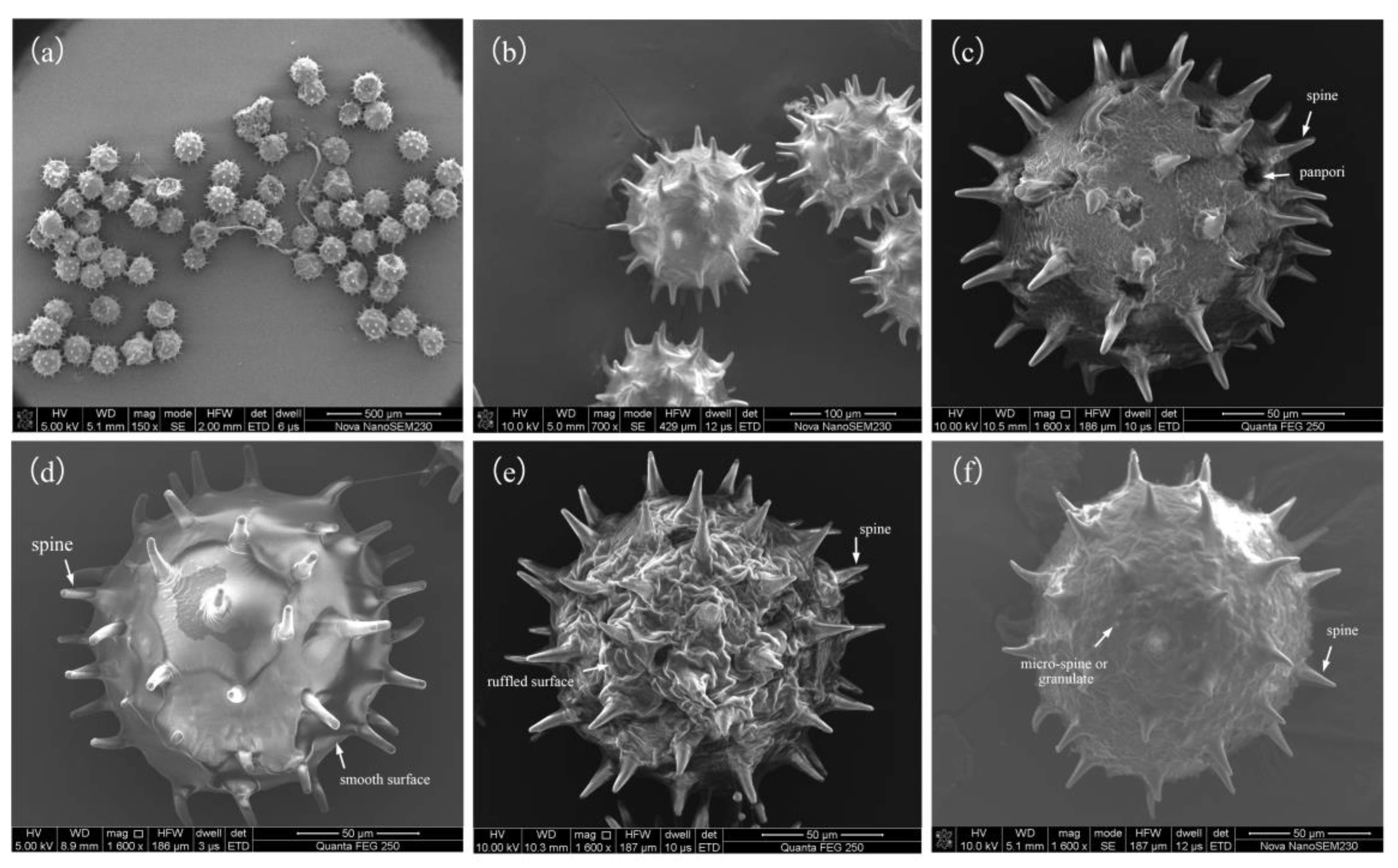
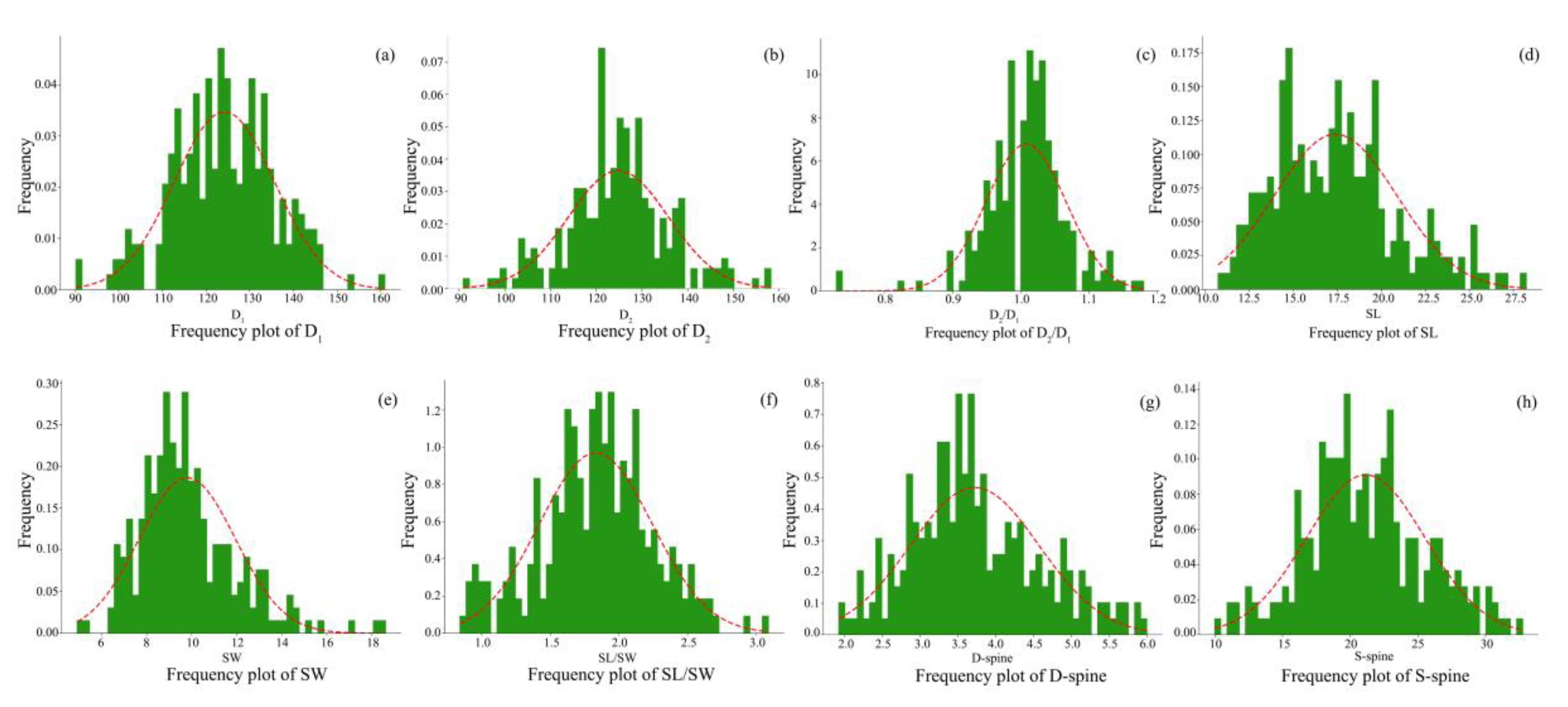
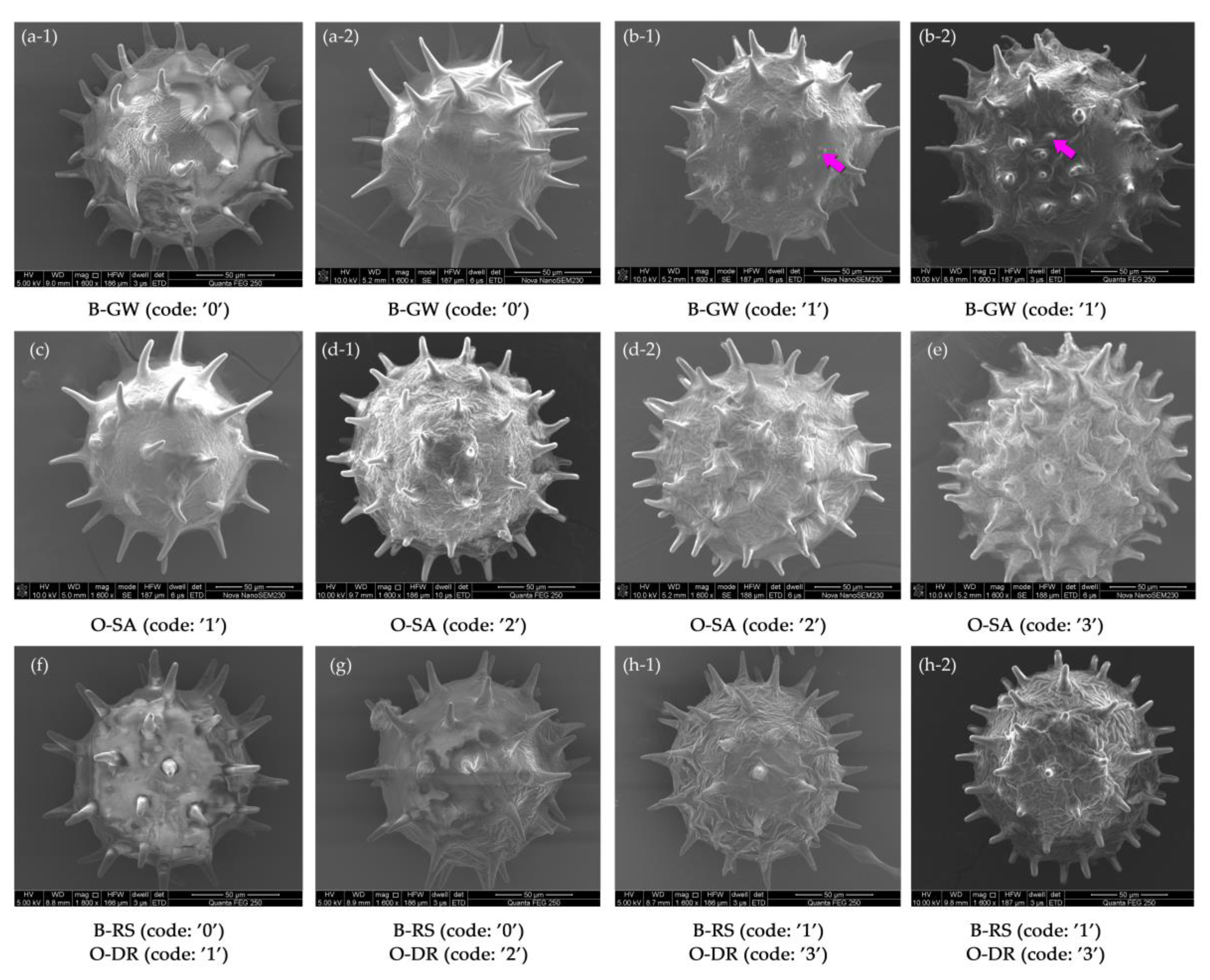
| No. | Cultivar | Source | No. | Cultivar | Source |
|---|---|---|---|---|---|
| 1 | Marina | US | 13 | Purple pillar | US |
| 2 | White chiffon | SH | 14 | Rubis | KR |
| 3 | Pink giant | SH | 15 | Chungmu | KR |
| 4 | China chiffon | SH | 16 | Suminokurahanagasa | KR |
| 5 | Paeoniflorus | HN | 17 | Pyonghwa | KR |
| 6 | Woodbridge | SH | 18 | Blue bird | US |
| 7 | Diana | SH | 19 | Akagionmamori | KR |
| 8 | Lavender | SH | 20 | Red heart | SH |
| 9 | Hamabo | SH | 21 | Qiancenghong | HEN |
| 10 | Elegantissimus | SH | 22 | Hongyun | HEN |
| 11 | Arang | HN | 23 | Huaban | HEN |
| 12 | Lavandula chiffon | SH | 24 | Naesarang | KR |
| No. | Characteristic | Code Type | Code Details |
|---|---|---|---|
| 1 | Pollen diameter parallel to the X-axis (D1) | N | / |
| 2 | Pollen diameter parallel to the Y-axis (D2) | N | / |
| 3 | Pollen shape ratio (D2/D1) | N | / |
| 4 | Length of the spine (SL) | N | / |
| 5 | Width of the spine base (SW) | N | / |
| 6 | Spine index (SL/SW) | N | / |
| 7 | Radius of the pollen grain and spine length, averaged (D-spine) | N | / |
| 8 | Spacing between spines (S-spine) | N | / |
| 9 | Whether the pollen surface has micro-spines or granular verrucae (B-GW) | B | Yes, 1; No, 0 |
| 10 | Whether the pollen surface ruffles strongly (B-RS) | B | Yes, 1; No, 0 |
| 11 | Number of pollen surface spines (O-SA) | O | Few (<40), 1; Medium (40–50), 2; Many (≥50), 3 |
| 12 | Degree of pollen surface ruffling (O-DR) | O | Smooth and largely unruffled, 1; Light ruffles, 2; Strong ruffles, 3 |
| No. | Characteristic | Investigation Method | Code Type | Code Details |
|---|---|---|---|---|
| 1 | Stalk length (ST) | MS | N | / |
| 2 | Petal length (PL) | MS | N | / |
| 3 | Petal width (PW) | MS | N | / |
| 4 | Petals index (PL/PW) | MS | N | / |
| 5 | Red center length (RC) | MS | N | / |
| 6 | Length of red center line (RCL) | MS | N | / |
| 7 | Red center index (RCL/RC) | MS | N | / |
| 8 | Whether the stamens are heterogeneous in terms of inner petals (B-IP) | VS | B | Yes, 1; No, 0 |
| 9 | Whether the petals have a red center (B-RC) | VS | B | Yes, 1; No, 0 |
| 10 | Relationship of the calyx with the bract (O-CB) | VS | O | Shorter, 1; Near Equal Length, 2; Beyond, 3 |
| 11 | Relationship of the red center line with the red center (O-RC) | VS | O | Near Equal Length, 1; Beyond, 2; Beyond Obvious, 3 |
| 12 | Whether the leaf lobing is strong (B-LL) | VG | B | Yes, 1; No, 0 |
| Morphological Traits | Pollen Quantitative Traits | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| D1 | D2 | D2/D1 | SL | SW | SL/SW | S-Spine | D-Spine | ||
| ST | Flower traits | −0.008 | 0.111 | 0.247 | 0.496 * | 0.137 | 0.265 | 0.336 | −0.387 |
| PL | 0.279 | 0.171 | −0.233 | −0.410 * | 0.330 | −0.565 ** | −0.383 | 0.483 * | |
| PW | 0.179 | 0.081 | −0.197 | −0.080 | 0.324 | −0.292 | −0.257 | 0.173 | |
| PL/PW | −0.020 | 0.002 | 0.029 | −0.342 | −0.213 | −0.120 | 0.025 | 0.246 | |
| RC | 0.006 | 0.012 | −0.128 | 0.060 | 0.379 | −0.210 | 0.049 | 0.037 | |
| RCL | −0.089 | −0.151 | −0.115 | 0.094 | 0.309 | −0.149 | 0.063 | −0.053 | |
| RCL/RC | −0.033 | −0.152 | −0.256 | 0.121 | 0.077 | 0.060 | −0.014 | −0.072 | |
| B-IP | −0.301 | −0.198 | 0.237 | 0.434 * | −0.302 | 0.587 ** | 0.204 | −0.495 * | |
| B-RC | 0.086 | 0.018 | −0.190 | 0.148 | 0.105 | 0.089 | −0.004 | −0.068 | |
| O-CB | 0.550 ** | 0.381 | −0.376 | −0.403 | 0.219 | −0.485 * | −0.194 | 0.561 ** | |
| O-RC | −0.136 | −0.182 | −0.038 | −0.164 | −0.001 | −0.177 | −0.094 | 0.123 | |
| B-LL | Leaf trait | 0.223 | 0.062 | −0.338 | −0.478 * | −0.239 | −0.220 | −0.110 | 0.506 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, F.; Wang, X.; Jiang, Y.; Chen, C.; Chen, J.; Zhang, J.; Wen, Y. Combined Morphological and Palynological Classification for Hibiscus syriacus L. (Malvaceae): Construction of the Diagnostic Classification Framework and Implications of Pollen Morphological Variation on Fruiting. Agronomy 2023, 13, 828. https://doi.org/10.3390/agronomy13030828
Xiao F, Wang X, Jiang Y, Chen C, Chen J, Zhang J, Wen Y. Combined Morphological and Palynological Classification for Hibiscus syriacus L. (Malvaceae): Construction of the Diagnostic Classification Framework and Implications of Pollen Morphological Variation on Fruiting. Agronomy. 2023; 13(3):828. https://doi.org/10.3390/agronomy13030828
Chicago/Turabian StyleXiao, Fen, Xiaohong Wang, Yun Jiang, Chulin Chen, Jiajia Chen, Jingwen Zhang, and Yafeng Wen. 2023. "Combined Morphological and Palynological Classification for Hibiscus syriacus L. (Malvaceae): Construction of the Diagnostic Classification Framework and Implications of Pollen Morphological Variation on Fruiting" Agronomy 13, no. 3: 828. https://doi.org/10.3390/agronomy13030828
APA StyleXiao, F., Wang, X., Jiang, Y., Chen, C., Chen, J., Zhang, J., & Wen, Y. (2023). Combined Morphological and Palynological Classification for Hibiscus syriacus L. (Malvaceae): Construction of the Diagnostic Classification Framework and Implications of Pollen Morphological Variation on Fruiting. Agronomy, 13(3), 828. https://doi.org/10.3390/agronomy13030828







